Abstract
β-Blockers have been reported to exhibit potential anticancer effects in cancer cell lines and animal models. However, clinical studies have yielded inconsistent results regarding cancer outcomes and cancer risk when β-blockers were used. This study investigated the association between propranolol and cancer risk.
Between January 1, 2000 and December 31, 2011, a patient cohort was extracted from the Longitudinal Health Insurance Database 2000, a subset of the Taiwan National Health Insurance Research Database. A propranolol cohort (propranolol usage >6 months) and nonpropranolol cohort were matched using a propensity score. Cox proportional hazard models were used to estimate the hazard ratio (HR) and 95% confidence intervals (CIs) of cancer associated with propranolol treatment.
The study sample comprised 24,238 patients. After a 12-year follow-up period, the cumulative incidence for developing cancer was low in the propranolol cohort (HR: 0.75; 95% CI: 0.67–0.85; P < 0.001). Patients with propranolol treatment exhibited significantly lower risks of cancers in head and neck (HR: 0.58; 95% CI: 0.35–0.95), esophagus (HR: 0.35; 95% CI: 0.13–0.96), stomach (HR: 0.54; 95% CI: 0.30–0.98), colon (HR: 0.68; 95% CI: 0.49–0.93), and prostate cancers (HR: 0.52; 95% CI: 0.33–0.83). The protective effect of propranolol for head and neck, stomach, colon, and prostate cancers was most substantial when exposure duration exceeded 1000 days.
This study supports the proposition that propranolol can reduce the risk of head and neck, esophagus, stomach, colon, and prostate cancers. Further prospective study is necessary to confirm these findings.
INTRODUCTION
The β-adrenergic receptor (β-AR) plays an essential role in normal physiologic functions and consists of catecholamines and their corresponding receptors, including the α- and β-AR families. The sympathetic nervous system regulates the body's “fight or flee” response through the β-adrenergic pathway.1 Increasing evidence suggests that β-AR signaling is crucial in cancer progression and metastasis and regulates tumor growth, invasiveness, migration, angiogenesis, apoptosis, and anoikis.2–5 The nonselective β-AR blocker propranolol has exhibited anticancer effects in cancer cell lines and animal models.6–9
The association between β-AR-blocker usage and cancer outcomes has been widely studied in breast cancer,10–14 prostate cancer,15,16 ovarian cancer,17,18 melanoma,19–22 and colon cancer.23 The use of β-AR-blockers has been demonstrated to reduce the recurrence of metastasis and mortality in most studies; however, several population-based cohort studies have yielded inconsistent findings.13–15,18,19,22–24
Whether a β-adrenergic pathway is involved in the initiation of cancer remains unclear. Several retrospective studies have demonstrated that a β-AR blocker can reduce cancer risk,25–28 whereas other studies have yielded conflicting results.29–32 To clarify the association between the nonselective β-AR blocker propranolol and cancer incidence, we conducted a nationwide population-based cohort study, using a substantial dataset available in Taiwan.
METHODS
Data Source
The data were extracted from the National Health Insurance Research Database (NHIRD), which is maintained by the National Health Research Institute of Taiwan. Taiwan, which initiated the National Health Insurance (NHI) program in 1995; it covers approximately 99% of the 23.72 million Taiwanese inhabitants (http://www.nhi.gov.tw/english/index.aspx). The data in this study was obtained from the Longitudinal Health Insurance Database 2000 (LHID2000), a subset of the NHIRD. The LHID2000, which contains all original medical claims and registration files for 1,000,000 enrollees, is derived from the medical claims records of the NHI program. The Taiwan National Health Research Institute reported that no statistically significant differences were found in the distributions of age, gender, or healthcare costs between the sample group of the LHID and all enrollees. The LHID2000 includes comprehensive information such as demographic data, dates of clinical visits, and disease diagnoses of insured people. The diagnostic codes are based on the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). This study was exempt from full review by the Institutional Review Board of China Medical University (CMU-REC-101-012) because the LHID2000 comprises deidentified secondary data released to the public for research purposes.
Study Population
Between January 1, 2000 and December 31, 2011, we extracted data from the LHID2000 for patients who were 20 years of age and older with complete age and sex information and without a history of cancer (ICD-9-CM code 140–208). Patients were divided into 2 cohorts according to propranolol use: a propranolol cohort, consisting of patients who underwent propranolol therapy for at least 6 months; and a nonpropranolol cohort, consisting of patients who did not undergo propranolol therapy. We used the date on which propranolol treatment was initialized as the index date. For treatment comparison, patients taking propranolol and patients not taking propranolol were selected according to a 1:1 matching on a propensity score. The propensity score was calculated using a logistic regression to estimate the probability of the treatment assignment according to the baseline variables, including the year of receiving propranolol treatment, age, sex, Charlson comorbidity index score (CCI score), comorbidities of angina pectoris (ICD-9-CM Code 413), paroxysmal supraventricular tachycardia (ICD-9-CM Code 427.0), hypertensive renal disease (ICD-9-CM Code 403), essential tremor (ICD-9-CM Code 333.1), anxiety (ICD-9-CM Code 300.00), thyrotoxicosis without mention of goiter or other cause (ICD-9-CM Code 242.9), migraine (ICD-9-CM Code 346.90), hypertension (ICD-9-CM Codes 401-405), and medications of metformin, statin, aspirin, α-blockers as well as other β-blockers. Metformin, statin, and aspirin have been reported to have an impact on cancer development.33–35
Outcome Measurements and Comorbidities
The main outcome was that cancer occurred. The confirmation of cancer (ICD-9-CM codes 140–195 and 200–208) events was based on the Registry of Catastrophic Illness Patient Database, a subset of the NHIRD. Histological and pathological confirmation of cancer was required for each case. All subjects were followed from the index date until cancer occurred, the date of withdrawal from the insurance system, or the end of 2011.
Statistical Analysis
The propranolol and nonpropranolol cohorts were matched according to the propensity score. To estimate the propensity score, a logistic regression model was used, in which the propranolol status was regressed on the baseline characteristics listed in Table 1. The standardized difference was used to quantify differences in means or prevalence between the 2 cohorts for continuous and categorical-matching variables. The incidence densities were calculated using sex, age, subdivision cancer, CCI score, and comorbidity for each cohort. Cox proportional hazard models stratifying the matched pairs were used to estimate the hazard ratio (HR) and 95% confidence intervals (CIs) of cancers associated with propranolol treatment in the propranolol cohort; the results were compared with those of the nonpropranolol cohort. All statistical analyses were performed using the SAS statistical package (Version 9.2 for Windows; SAS Institute, Inc., Cary, NC). A 2-tailed P value of <0.05 was considered statistically significant.
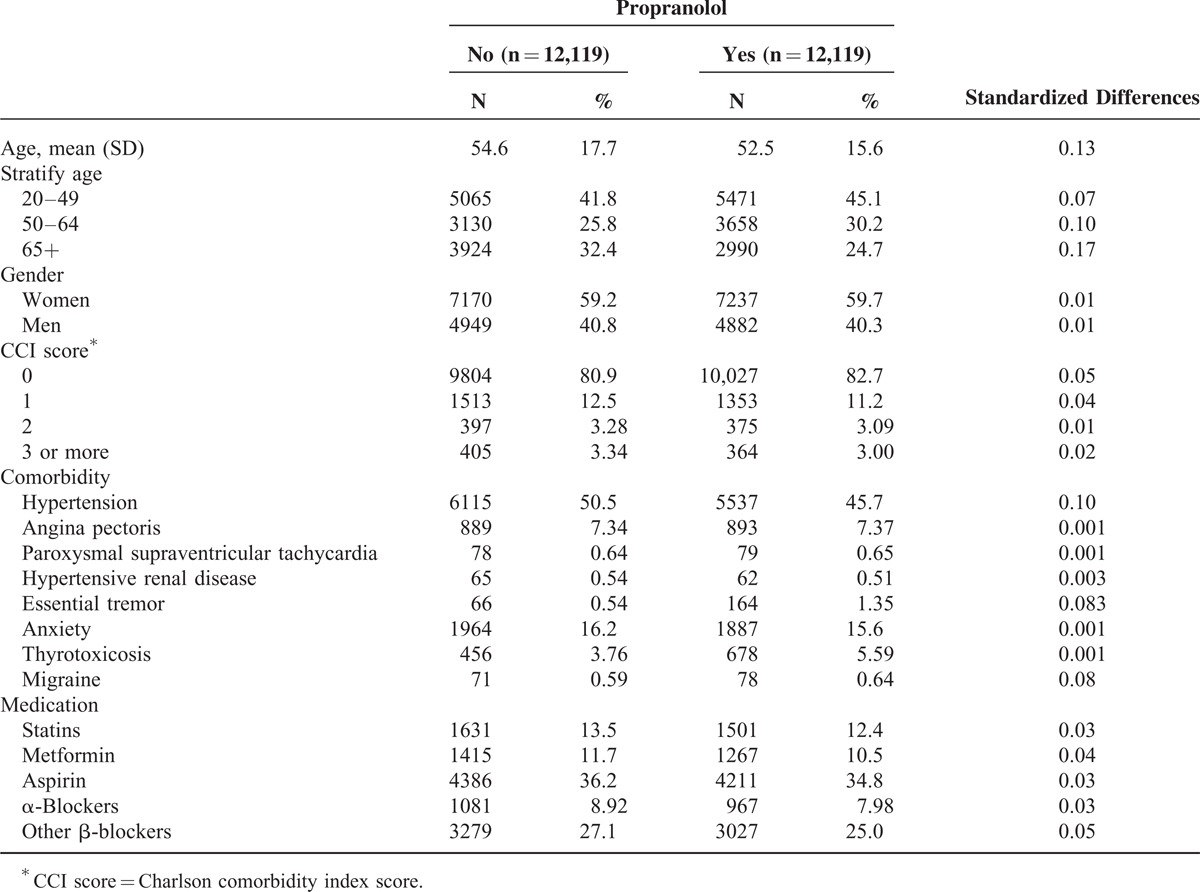
TABLE 1.
Demographic Characteristics of Study Subjects Among Medicine in the Propensity Score-Matched Sample
RESULTS
Among the 24,238 patients who were observed in this study, 12,119 had used propranolol regularly over a period of 6 months, and 12,119 had never used propranolol. The mean ages of the nonpropranolol and propranolol cohorts were 54.6 (±17.7) and 52.5 years (±15.6), respectively (Table 1). The mean follow-up years were 6.96 (SD = 3.20) and 6.50 (SD = 3.33) for the propranolol and the nonpropranolol cohorts, respectively (data not shown). The cumulative incidence of developing cancer was lower in the propranolol cohort than it was in the nonpropranolol cohort (log-rank test: P < 0.01). Table 2 shows the overall, sex-, and age-specific incidences and HRs of the 2 cohorts. The overall incidence density of cancer was significantly higher in the nonpropranolol than in the propranolol cohort (7.47 vs 5.31 per 1000 person-years). Patients using propranolol exhibited a 25% reduction in the risk of cancer compared with patients not using propranolol (95% CI: 0.67–0.85). We selected patients who were 20 years of age and older from the LHID2000 as a cohort representing the general population and calculated the cancer incidence. The incidence rates of cancer in the general population, propranolol, and nonpropranolol cohort were 3.85, 5.31, and 7.47 per 1000 person-years, respectively. Compared with the general population, the incidence rate ratios of the propranolol and nonpropranolol cohorts were 1.38 (95% CI: 1.32–1.44) and 1.94 (95% CI: 1.87–2.01).
TABLE 2.
Comparison of Incidence and Hazard Ratio of Cancer in the Matched Cohorts With Propranolol Treatment and Without Propranolol Treatment Stratified by Sex and Age
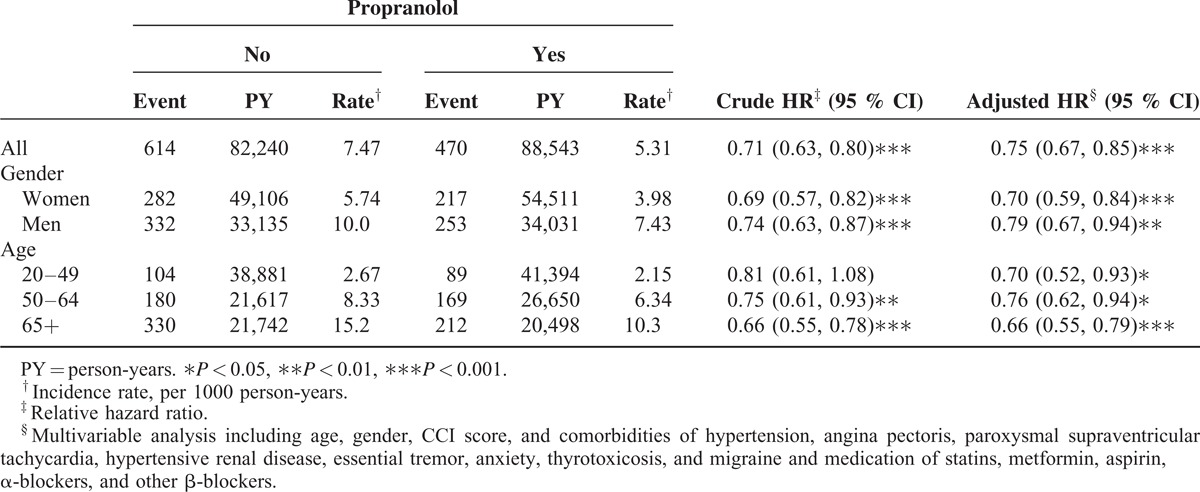
The incidences were higher in men than in women in both cohorts. The HR of cancer was significantly low in both men and women in the propranolol cohort, respectively (HR: 0.79, 95% CI: 0.67–0.94; HR: 0.70, 95% CI: 0.59–0.84). In both cohorts, the age-specific incidence of cancer increased with age. The age-specific propranolol to nonpropranolol-cohort HR of cancer was low in all age groups, and the effect was most significant in the age group ≧65 years (HR: 0.66; 95% CI: 0.55–0.79).
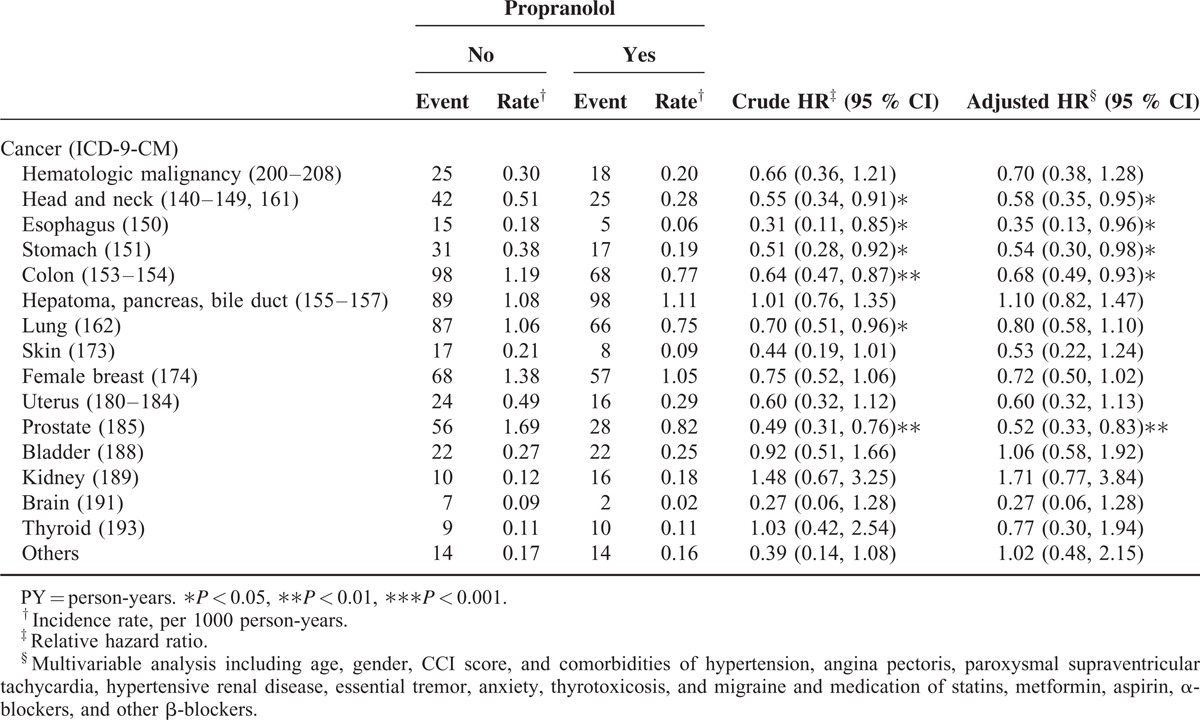
Table 3 shows the specific analyses of cancer types. Compared with the patients who did not take propranolol, the patients who received propranolol treatment exhibited a significantly lower risk of cancer in the head and neck (HR: 0.58; 95% CI: 0.35–0.95), esophagus (HR: 0.35; 95% CI: 0.13–0.96), stomach (HR: 0.54; 95% CI: 0.30–0.98), colon (0.68; 95% CI: 0.49–0.93), and prostate (HR: 0.52; 95% CI: 0.33–0.83).
TABLE 3.
Comparison of Incidence and Hazard Ratio of Cancer Types in the Matched Cohorts with Propranolol Treatment and Without Propranolol Treatment
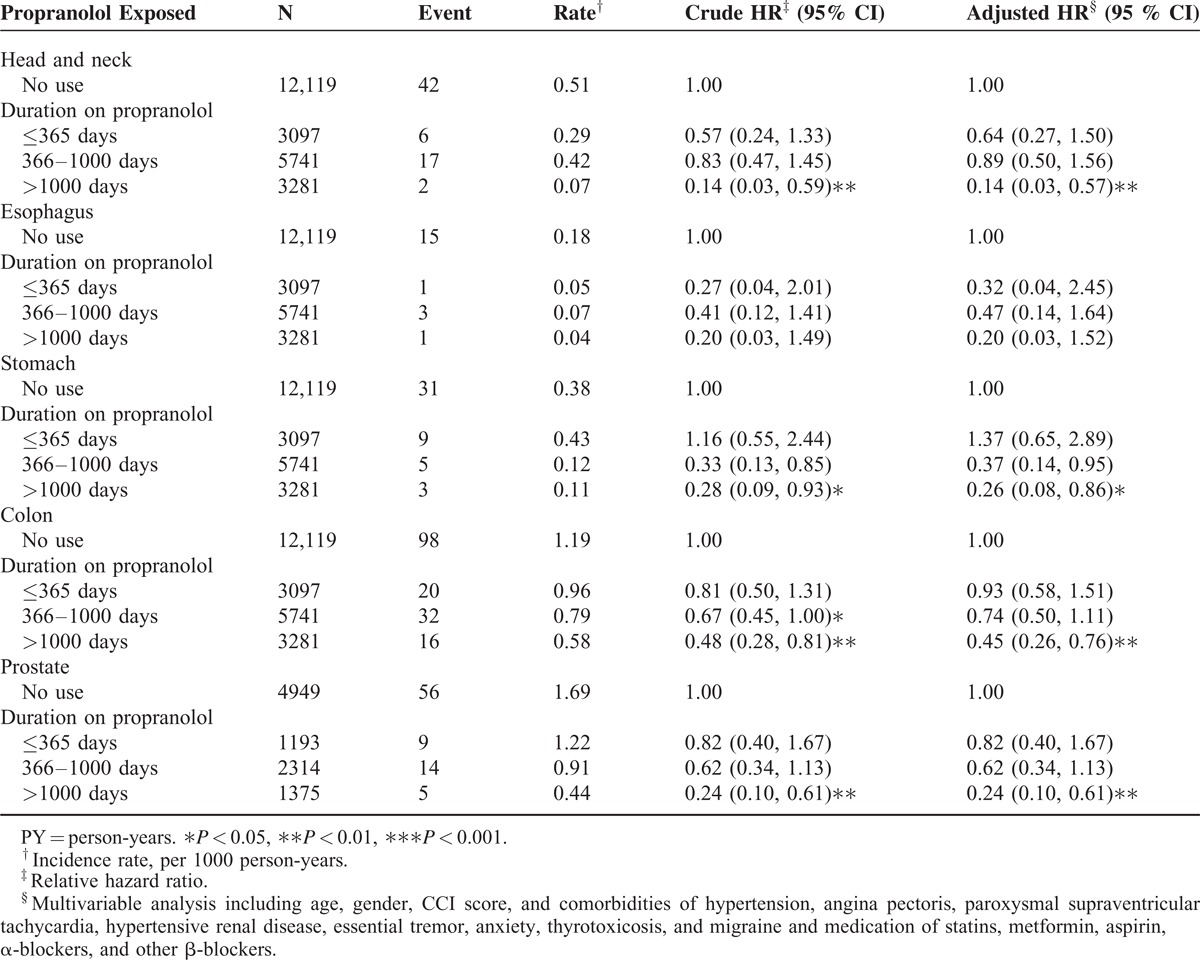
In addition, the duration of propranolol use was associated with the reduced risk of cancer. Table 4 shows the incidences of the 5 cancer types stratified according to the duration of propranolol use. The risk of head and neck, stomach, colon, and prostate cancer decreased markedly when the patients used propranolol for longer than 1000 days.
TABLE 4.
Incidence and Adjusted Hazard Ratio of Subdivision Cancer in the Matched Cohorts Stratified by Duration of Propranolol Use
DISCUSSION
The relevance of the β-AR signaling system in cancer biology has been demonstrated in cancer cell lines and animal studies.2–9 The effects of stress are mediated mainly through activation of the cancer cell β2-AR and its downstream cell cyclic AMP-protein kinase A signaling pathway.1,4 These studies have clarified the relationships between stress and cancer progression.2–9 Thus, β-AR may be a therapeutic target for intervention. The protective roles of β-AR blockers have been reported in several retrospective studies.10–12,16,17,20,21 However, other studies have yielded conflicting results and not supported the proposition that β-AR blockers can improve cancer outcomes.13–15,18,19,2–24 Several studies have not discriminated β1-AR from β2-AR activity and categorized β-AR blockers as a single pharmacologic group.11,12,16–18,20,21,24 In addition, β1-selective agents have replaced shorter-acting and nonselective propranolol in the treatment of common cardiovascular diseases such as hypertension. These retrospective studies have mostly used β1-selective AR blockers for treatment. Although β-AR blockers are labeled according to the selectivity, they exhibit an affinity for both β1-AR and β2-AR because of the similarity between β1-AR and β2-AR.36 This may partly explain the results in the studies and may be consistent with the hypothesis suggested in the preclinical studies.
The association between β-AR blockers and cancer risk is complex. Several studies have supported the notion that β-AR blockers reduce cancer risk,25–28 whereas other studies have revealed that β-AR blockers do not exert an influence on cancer development.29–32 One study used propranolol, but most studies classified β-AR blockers as 1 group.26,27,29–31 Aspirin, metformin, and statins have been reported to reduce cancer risk33–35 and can confound the effects of propranolol. In the present study, we determined that propranolol could lower the risk of cancer development by 25% after adjustment of these medications. Our study demonstrated that propranolol could reduce cancer risk in all age groups, particularly in the age group ≥65 years. Hypertension, purportedly associated with malignancy,37 is a prevalent disorder among aging people.38 Therefore, this may explain why, in our study, propranolol usage reduced cancer risk obviously in patients ≥65 years.
We determined that propranolol reduced the risk of head and neck, esophagus, stomach, colon, and prostate cancers. The protective effect of propranolol for head and neck, stomach, colon, and prostate cancers was most substantial when exposure duration exceeded 1000 days. Our results are similar to those of Assimes et al28 who reported a decreased risk of colorectal cancer and a slightly decreased risk of head and neck cancer as well as hematological and other gastrointestinal cancers in β-AR blockers ever users. In addition, the results of Perron et al26 also support our findings in prostate cancer-risk reduction. Jansen et al32 reported that β-AR blocker use was not associated with colorectal cancer risk, but long-term usage was associated with stage IV disease. The sample size in the stage-specific analyses was small, and the results should be interpreted with caution.
The main strength of our study was the large size of the sample derived from a generally accurate nationwide database with a wide coverage, which facilitated tracing the histories of medical services and comprehensive follow-ups. The sample provided an adequate statistical power to examine the associations between propranolol and cancer risk in our study.
However, our study had several limitations in addition to the limitation related to the retrospective design. First, the cancer incidence rate (per 1000 person-years) in the nonpropranol (7.47) and propranolol cohorts (5.31) were both higher than the general population (3.85). The propranolol group matched with the nonpropranolol group with comorbidity condition in order to decrease diversion. Therefore, both groups have more comorbidities than the general population. Patients with comorbidities might visit physicians more often and may thus be more likely to participate in cancer screening programs, which enable early cancer detection.39 Although matched comorbidities in both propranolol and nonpropranolol cohorts may lead to selection bias, the finding that propranolol can reduce the risk of cancers was true. Second, we could not adjust the established risk factors of cancer, such as smoking, body mass index, and family history. Third, it has been proposed that hypertension is a risk factor for malignancy; therefore, it can be a confounding factor. Finally, investigating the dose–response relations was beyond the scope of the present study.
In conclusion, this study supports the proposition that propranolol can reduce the risk of cancers, particularly head and neck, esophagus, stomach, colon, and prostate cancers. In head and neck, stomach, colon, and prostate cancer, the protective effect is most substantial when propranolol is used for a duration exceeding 1000 days. Further prospective study is necessary to confirm these findings.
Acknowledgments
The authors thank Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW104-TDU-B-212-113002); China Medical University Hospital, Academia Sinica Taiwan Biobank, Stroke Biosignature Project (BM104010092); NRPB Stroke Clinical Trial Consortium (MOST 103-2325-B-039 -006); Tseng-Lien Lin Foundation, Taichung, Taiwan; Taiwan Brain Disease Foundation, Taipei, Taiwan; Katsuzo and Kiyo Aoshima Memorial Funds, Japan; and Health, and welfare surcharge of tobacco products, China Medical University Hospital Cancer Research Center of Excellence (MOHW104-TDU-B-212-124-002, Taiwan) for the support.
Footnotes
Abbreviations: CI = confidence interval, β-AR = β-adrenergic receptor, NHI = the National Health Insurance, HR = hazard ratio, ICD-9-CM = International Classification of Diseases, Ninth Revision, Clinical Modification, LHID2000 = the Longitudinal Health Insurance Database 2000, NHIRD = National Health Insurance Research Database.
All authors have contributed to this manuscript, and all authors agree with the content of the manuscript. Conception/Design: P-YC, W-YH, C-HK; Provision of study materials: C-HK; Data analysis and interpretation: P-YC, W-YH, C-LL, C-HK; Collection and/or assembly of data, Manuscript writing, Final approval of manuscript: All authors.
This study was supported in part by the Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW104-TDU-B-212-113002); China Medical University Hospital, Academia Sinica Taiwan Biobank, Stroke Biosignature Project (BM104010092); NRPB Stroke Clinical Trial Consortium (MOST 103-2325-B-039 -006); Tseng-Lien Lin Foundation, Taichung, Taiwan; Taiwan Brain Disease Foundation, Taipei, Taiwan; Katsuzo and Kiyo Aoshima Memorial Funds, Japan; and Health, and welfare surcharge of tobacco products, China Medical University Hospital Cancer Research Center of Excellence (MOHW104-TDU-B-212-124-002, Taiwan).
The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. No additional external funding was received for this study.
The authors report no conflicts of interest.
REFERENCES
- 1.Cole SW, Sood AK. Molecular pathways: beta-adrenergic signaling in cancer. Clin Cancer Res 2012; 18:1201–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lutgendorf SK, Cole S, Costanzo E, et al. Stress-related mediators stimulate vascular endothelial growth factor secretion by two ovarian cancer cell lines. Clin Cancer Res 2003; 9:4514–4521. [PubMed] [Google Scholar]
- 3.Sood AK, Bhatty R, Kamat AA, et al. Stress hormone-mediated invasion of ovarian cancer cells. Clin Cancer Res 2006; 12:369–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thaker PH, Han LY, Kamat AA, et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med 2006; 12:939–944. [DOI] [PubMed] [Google Scholar]
- 5.Sood AK, Armaiz-Pena GN, Halder J, et al. Adrenergic modulation of focal adhesion kinase protects human ovarian cancer cells from anoikis. J Clin Invest 2010; 120:1515–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo K, Ma Q, Wang L, et al. Norepinephrine-induced invasion by pancreatic cancer cells is inhibited by propranolol. Oncol Rep 2009; 22:825–830. [DOI] [PubMed] [Google Scholar]
- 7.Palm D, Lang K, Niggemann B, et al. The norepinephrine-driven metastasis development of PC-3 human prostate cancer cells in BALB/c nude mice is inhibited by beta-blockers. Int J Cancer 2006; 118:2744–2749. [DOI] [PubMed] [Google Scholar]
- 8.Liao X, Che X, Zhao W, et al. The β-adrenoceptor antagonist, propranolol, induces human gastric cancer cell apoptosis and cell cycle arrest via inhibiting nuclear factor κB signaling. Oncol Rep 2010; 24:1669–1676. [DOI] [PubMed] [Google Scholar]
- 9.Zhang D, Ma Q, Shen S, et al. Inhibition of pancreatic cancer cell proliferation by propranolol occurs through apoptosis induction: the study of beta-adrenoceptor antagonist's anticancer effect in pancreatic cancer cell. Pancreas 2009; 38:94–100. [DOI] [PubMed] [Google Scholar]
- 10.Barron TI, Connolly RM, Sharp L, et al. Beta blockers and breast cancer mortality: a population- based study. J Clin Oncol 2011; 29:2635–2644. [DOI] [PubMed] [Google Scholar]
- 11.Melhem-Bertrandt A, Chavez-Macgregor M, Lei X, et al. Beta-blocker use is associated with improved relapse-free survival in patients with triple-negative breast cancer. J Clin Oncol 2011; 29:2645–2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Botteri E, Munzone E, Rotmensz N, et al. Therapeutic effect of (-blockers in triple-negative breast cancer postmenopausal women. Breast Cancer Res Treat 2013; 140:567–575. [DOI] [PubMed] [Google Scholar]
- 13.Sørensen GV, Ganz PA, Cole SW, et al. Use of β-blockers, angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, and risk of breast cancer recurrence: a Danish nationwide prospective cohort study. J Clin Oncol 2013; 31:2265–2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cardwell CR, Coleman HG, Murray LJ, et al. Beta-blocker usage and breast cancer survival: a nested case-control study within a UK Clinical Practice Research Datalink cohort. Int J Epidemiol 2013; 42:1852–1861. [DOI] [PubMed] [Google Scholar]
- 15.Cardwell CR, Coleman HG, Murray LJ, et al. Beta-blocker usage and prostate cancer survival: a nested case-control study in the UK Clinical Practice Research Datalink cohort. Cancer Epidemiol 2014; 38:279–285. [DOI] [PubMed] [Google Scholar]
- 16.Grytli HH, Fagerland MW, Fosså SD, et al. Association between use of β-blockers and prostate cancer-specific survival: a cohort study of 3561 prostate cancer patients with high-risk or metastatic disease. Eur Urol 2014; 65:635–641. [DOI] [PubMed] [Google Scholar]
- 17.Diaz ES, Karlan BY, Li AJ. Impact of beta blockers on epithelial ovarian cancer survival. Gynecol Oncol 2012; 127:375–378. [DOI] [PubMed] [Google Scholar]
- 18.Johannesdottir SA, Schmidt M, Phillips G, et al. Use of β-blockers and mortality following ovarian cancer diagnosis: a population-based cohort study. BMC Cancer 2013; 13:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCourt C, Coleman HG, Murray LJ, et al. Beta-blocker usage after malignant melanoma diagnosis and survival: a population-based nested case-control study. Br J Dermatol 2014; 170:930–938. [DOI] [PubMed] [Google Scholar]
- 20.De Giorgi V, Grazzini M, Gandini S, et al. Treatment with β-blockers and reduced disease progression in patients with thick melanoma. Arch Intern Med 2011; 171:779–781. [DOI] [PubMed] [Google Scholar]
- 21.Lemeshow S, Sørensen HT, Phillips G, et al. β-Blockers and survival among Danish patients with malignant melanoma: a population-based cohort study. Cancer Epidemiol Biomarkers Prev 2011; 20:2273–2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Livingstone E, Hollestein LM, van Herk-Sukel MP, et al. β-Blocker use and all-cause mortality of melanoma patients: results from a population-based Dutch cohort study. Eur J Cancer 2013; 49:3863–3871. [DOI] [PubMed] [Google Scholar]
- 23.Hicks BM, Murray LJ, Powe DG, et al. β-Blocker usage and colorectal cancer mortality: a nested case-control study in the UK Clinical Practice Research Datalink cohort. Ann Oncol 2013; 24:3100–3106. [DOI] [PubMed] [Google Scholar]
- 24.Shah SM, Carey IM, Owen CG, et al. Does β-adrenoceptor blocker therapy improve cancer survival? Findings from a population-based retrospective cohort study. Br J Clin Pharmacol 2011; 72:157–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nkontchou G, Aout M, Mahmoudi A, et al. Effect of long-term propranolol treatment on hepatocellular carcinoma incidence in patients with HCV-associated cirrhosis. Cancer Prev Res (Phila) 2012; 5:1007–1014. [DOI] [PubMed] [Google Scholar]
- 26.Perron L, Bairati I, Harel F, et al. Antihypertensive drug use and the risk of prostate cancer (Canada). Cancer Causes Control 2004; 15:535–541. [DOI] [PubMed] [Google Scholar]
- 27.Monami M, Filippi L, Ungar A, et al. Further data on beta-blockers and cancer risk: observational study and meta-analysis of randomized clinical trials. Curr Med Res Opin 2013; 29:369–378. [DOI] [PubMed] [Google Scholar]
- 28.Assimes TL, Elstein E, Langleben A, et al. Long-term use of antihypertensive drugs and risk of cancer. Pharmacoepidemiol Drug Saf 2008; 17:1039–1049. [DOI] [PubMed] [Google Scholar]
- 29.Coleman CI, Baker WL, Kluger J, et al. Antihypertensive medication and their impact on cancer incidence: a mixed treatment comparison meta-analysis of randomized controlled trials. J Hypertens 2008; 26:622–629. [DOI] [PubMed] [Google Scholar]
- 30.Fryzek JP, Poulsen AH, Lipworth L, et al. A cohort study of antihypertensive medication use and breast cancer among Danish women. Breast Cancer Res Treat 2006; 97:231–236. [DOI] [PubMed] [Google Scholar]
- 31.Li CI, Daling JR, Tang MT, et al. Use of antihypertensive medications and breast cancer risk among women aged 55 to 74 years. JAMA Intern Med 2013; 173:1629–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jansen L, Below J, Chang-Claude J, et al. Beta blocker use and colorectal cancer risk: population-based case-control study. Cancer 2012; 118:3911–3919. [DOI] [PubMed] [Google Scholar]
- 33.Cuzick J, Otto F, Baron JA, et al. Aspirin and nonsteroidal anti-inflammatory drugs for cancer prevention: an international consensus statement. Lancet Oncol 2009; 10:501–507. [DOI] [PubMed] [Google Scholar]
- 34.Quinn BJ, Kitagawa H, Memmott RM, et al. Repositioning metformin for cancer prevention and treatment. Trends Endocrinol Metab 2013; 24:469–480. [DOI] [PubMed] [Google Scholar]
- 35.Bonovas S. Statins: do they have a potential role in cancer prevention and modifying cancer-related outcomes? Drugs 2014; 74:1841–1848. [DOI] [PubMed] [Google Scholar]
- 36.Smith C, Teitler M. Beta-blocker selectivity at cloned human beta 1- and beta 2-adrenergic receptors. Cardiovasc Drugs Ther 1999; 13:123–126. [DOI] [PubMed] [Google Scholar]
- 37.Goon PK, Stonelake PS, Lip GY. Hypertension, anti-hypertensive therapy and neoplasia. Curr Pharm Des 2007; 13:2539–2544. [DOI] [PubMed] [Google Scholar]
- 38.Leenen FH, Dumais J, McInnis NH, et al. Results of the Ontario survey on the prevalence and control of hypertension. CMAJ 2008; 178:1441–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bostick RM, Sprafka JM, Virnig BA, et al. Predictors of cancer prevention attitudes and participation in cancer screening examinations. Prev Med 1994; 23:816–826. [DOI] [PubMed] [Google Scholar]


