Supplemental digital content is available in the text
Abstract
Pharmacists record data on all drugs claimed and may build a personal relationship with their clients. We hypothesized that loyalty to a single pharmacy could be associated with a better quality of drug use.
To assess the association between pharmacy loyalty and quality of drug use among individuals treated with oral antidiabetes drugs (OADs).
This is a cohort study using Quebec Health Insurance Board data. Associations were assessed using multivariable logistic regression.
New OAD users, aged ≥18 years.
Individuals who filled all their prescription drugs in the same pharmacy during the first year of treatment were considered loyal. During year 2 of treatment we assessed 4 quality indicators of drug use: persistence with antidiabetes treatment, compliance with antidiabetes treatment among those considered persistent, use of an angiotensin-converting enzyme inhibitor or of an angiotensin II receptor blocker (ACEi/ARB), and use of a lipid-lowering drug.
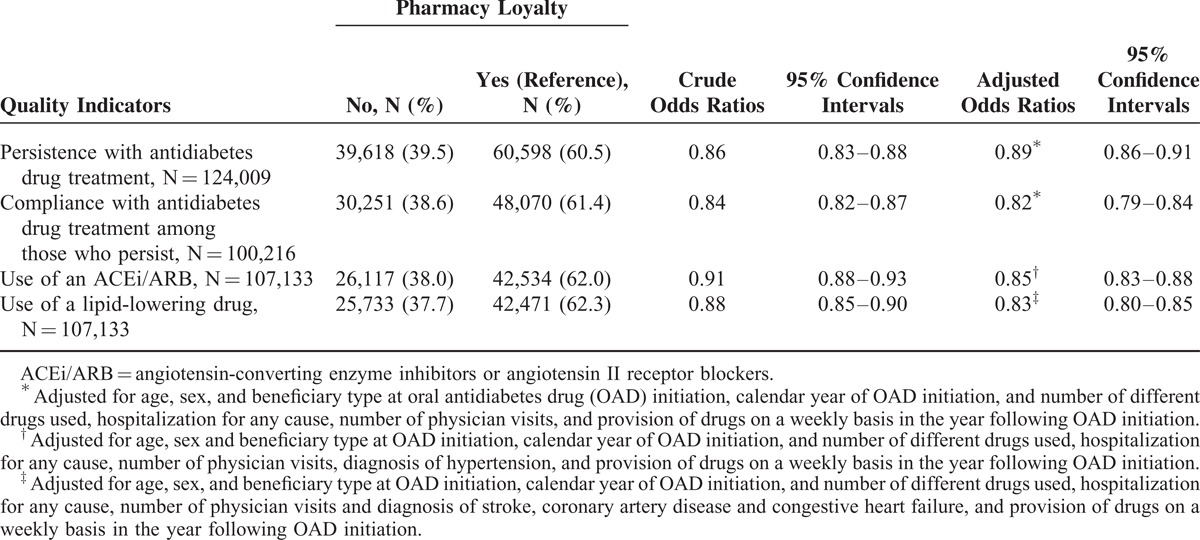
Of 124,009 individuals, 59.75% were identified as loyal. Nonloyal individuals were less likely to persist with their antidiabetes treatment (adjusted odds ratio = 0.89; 95% CI: 0.86–0.91), to comply with their antidiabetes treatment (0.82; 0.79–0.84), to use an ACEi/ARB (0.85; 0.83–0.88) and to use a lipid-lowering drug (0.83; 0.80–0.85). Quality of drug use decreased as the number of different pharmacies increased (linear contrast tests <0.001).
Results underscore the important role pharmacists could play in helping their clients with chronic diseases to better manage their drug treatments. Further research is needed to determine to what extent the positive effects associated with pharmacy loyalty are specifically due to pharmacists.
INTRODUCTION
Community pharmacists play a crucial role in optimizing their clients’ drug therapy. However, to properly assess and deal with any drug therapy-related problem they might detect, pharmacists need reliable and complete information on the prescribed drugs purchased. In Canada's Quebec province, pharmacists must maintain a pharmacy record for each client dispensed prescription drugs. These records include client information (name, address, birth date, known allergies, disease, etc.), plus the prescribed drugs purchased (drug name, dosage, number of authorized renewals, date of purchase, number of pills dispensed, number of days’ supply, etc.). By putting this information to use, pharmacists can manage their clients’ drug therapy. However, since these records are largely pharmacy-based, unless clients purchase all their drugs at the same pharmacy (loyal to 1 pharmacy only) pharmacists cannot detect drug therapy-related problems and take action to optimally manage the whole drug therapy.
Instances of drug therapy-related problems include nonpersistence (not continuously refilling prescriptions for the prescribed length of time) and noncompliance (not taking the drug in accordance with the prescribed dosage and schedule) with treatment, and the absence of guideline-recommended treatments. To our knowledge, only 3 studies1–3 have evaluated the association between pharmacy loyalty and quality of drug use. In the first study, the use of a single dispensing pharmacy was associated with a lowered likelihood of a potential inappropriate drug combination.1 Marcum et al2 observed that those using multiple pharmacies had higher likelihood of noncompliance (defined as the proportion of days covered [PDC] lesser than 0.80) and of potential drug–drug interaction. While Choudhry et al3 observed, using that same definition of noncompliance, that it decreased as the number of pharmacy visits increased among statins and angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers (ACEi/ARB) users. However, the study populations were limited to seniors and did not examine important aspects of quality use of drugs, such as persistence with treatment and the use of guidelines-recommended drugs.
Type 2 diabetes is a chronic condition necessitating the long-term use of many drugs, notably an oral antidiabetes drug (OAD) or insulin and cardioprotective agents: ACEi/ARB and lipid-lowering drugs.4 Nonpersistence and noncompliance with OAD treatment in Quebec province have been well described.5,6 For example, researchers reported a 65% and 56% likelihood of persisting with the initial OAD over a 12-month period for patients initiated on metformin and sulfonylurea, respectively.5 Among new users of OAD, 38% would not persist or not comply (ie, obtain drug supplies for at least 80% of the days) with their antidiabetes drug treatment during the first year of treatment.6 Furthermore, drug management of cardiovascular risks among patients taking an antidiabetes drug treatment is suboptimal.7,8
We hypothesized that pharmacy loyalty could be associated with a better quality of drug use (ie, persistence and compliance with treatment and exposure to guideline-recommended drugs), and that quality could decrease as the number of different pharmacies visited increases. Consequently, we conducted a study among new OAD users to assess the association between pharmacy loyalty and 4 quality indicators of drug use: persistence with antidiabetes drugs, compliance with antidiabetes drugs among those considered persistent, use of an ACEi/ARB and use of a lipid-lowering drug. In addition, we assessed the association between the number of different pharmacies visited and the same 4 quality indicators.
METHODS
Study Design
We conducted an inception cohort study. We assessed pharmacy loyalty and the number of different pharmacies visited during the first year after OAD initiation, and then we measured quality indicators of drug use in the second year. Figure 1 shows the study design and timeline representation of the variables measured.
FIGURE 1.
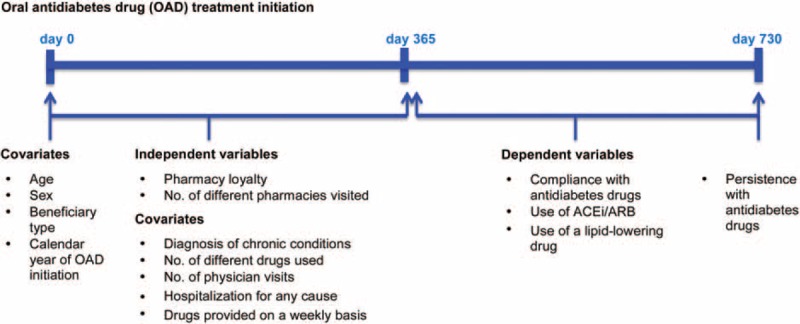
Study design and timeline representation of variables measured. ACEi/ARB = angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers, OAD = oral antidiabetes drug.
Data Sources
We used administrative data from Quebec's provincial health insurance agency (Régie de l’assurance maladie du Québec (RAMQ)). The provincial public health insurance plan covers all permanent residents of Quebec province for both medical services and hospitalizations. In addition, the public drug plan covers prescribed drugs for all residents aged 65 or more, residents without a private drug insurance group plan and all welfare recipients. Beneficiaries of the public drug plan represent 43% of the province's population.9 The RAMQ databases contain specifics on all beneficiaries and billing data from physicians and pharmacists. RAMQ also manages the Quebec hospitalization registry. In short, for each insured individual the RAMQ beneficiary database contains the beginning and end dates of eligibility for the public drug plan, age, sex, beneficiary type, and date of death, if applicable. The physicians’ billing database contains the dates of medical visits and the main reason (International Classification of Diseases (ICD) codes) for those visits. For each drug dispensed the pharmaceutical services database includes: prescription fill date, International Non-proprietary Name (INN) codes, plus dosage form, number of days’ supply, and the anonymous identification number of the dispensing pharmacy. The RAMQ drug plan database is considered accurate for prescription drug claims.10 The hospitalization registry contains information on hospitalization: dates, length of stay, primary and secondary diagnoses (ICD-9 or ICD-10 codes).
Study Population
We asked RAMQ to identify all individuals insured with the Quebec public drug plan, aged 18 or more, with at least one claim for an OAD recorded in the prescription claim database between January 1, 2000 and December 31, 2008 and who had been continuously eligible for the public drug plan throughout the entire year before the first claim registered on or after January 1, 2000. RAMQ provided the data recorded in its databases pertaining to each of these individuals for the entire period beginning 1 year before the first OAD claim date (OAD initiation) on or after January 1, 2000, and continuing to December 31, 2008, or the date of loss of drug plan eligibility or death, whichever came first.
Using this information, we assembled a population that included only new OAD users with a minimum 2-year follow-up (Figure 2). This enabled us to measure pharmacy loyalty and quality indicators of drug use for every individual in the first and second year of follow-up, respectively. Hence, we excluded all individuals with 1 or more claims of an OAD in the 1-year period before the OAD initiation date or after January 1, 2000; those whose first OAD claim was after December 31, 2006; those not eligible for the Quebec drug plan for the entire 2-year period after OAD initiation; and those with no drug claim recorded in the database (irrespective of the pharmacological class) during the year after OAD initiation. The RAMQ database contains no records of drugs taken in the hospital, so, to ensure a valid measurement of compliance with drugs, we then excluded any individuals hospitalized for 275 or more days in the first or second year after OAD initiation. We did this because it was suggested that researchers use the expected number of days’ supply covered by at least 3 claims to estimate drug use compliance.11 Since Quebec pharmacists supply most patients with their OADs for a 30-day period,5 this corresponds to approximately 90 days’ supply. Finally, for the assessment of the association between pharmacy loyalty and use of an ACEi/ARB and use of a lipid-lowering drug, we also excluded individuals aged less than 50 years at OAD initiation. We did this because Canadian clinical guidelines available during the study period, consistently recommended that individuals with diabetes, aged 50 years or more, take those drugs.4,12,13
FIGURE 2.
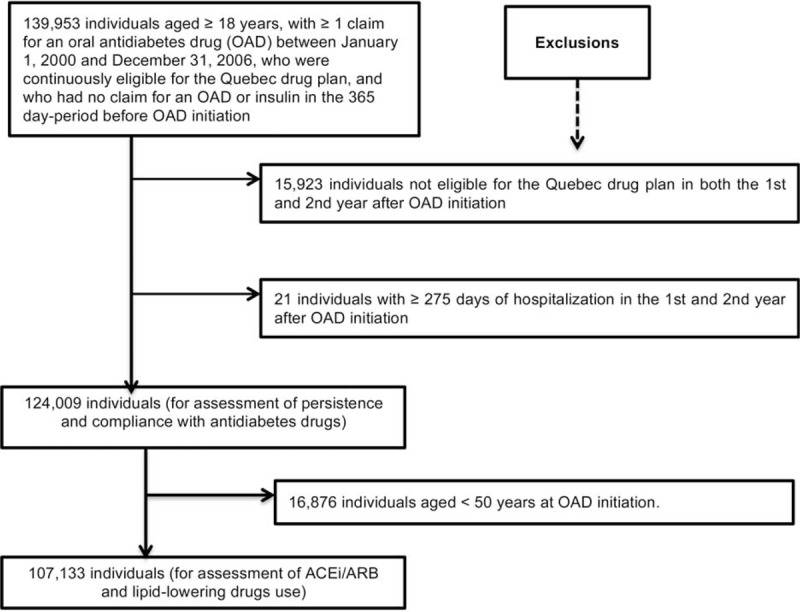
Selection of the study population. ACEi/ARB = angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers, OAD = oral antidiabetes drug.
Independent Variables
Pharmacy loyalty during the first year after OAD initiation was assessed using 2 independent variables. First, individuals who filled all their prescriptions, irrespective of the drug class, at the same pharmacy were considered loyal. Second, we classed individuals in 4 categories (1, 2, 3, 4–23 pharmacies) according to the number of different pharmacies where they filled drugs.
Quality Indicators
During the second year after OAD initiation we assessed the 4 quality indicators: persistence and compliance (among those who persisted) with antidiabetes drug treatment, use of an ACEi/ARB and use of a lipid-lowering drug.
Patients were deemed persistent with their antidiabetes drug treatment 2 years after initiation, if they had filled any OAD or insulin in the OAD initiation second-year anniversary period. For any OAD, we defined this period as equal to the number of days supplied at the most recent dispensing before the second anniversary date (day 730) plus a permissible gap of 0.5 times the days’ supply. For insulin, the second-year anniversary refill period was the 90-day period before the second anniversary date.6,14 Patients hospitalized at day 730 were considered persistent if they had filled any OAD or insulin in the period before the date of their most recent hospital entry, similar to those defined for the second-year anniversary period.
We used PDC to assess compliance with antidiabetes drugs among the individuals persisting with their treatment. The PDC is the total number of days covered by either any OAD or insulin, divided by the number of days in the second year after OAD initiation, that is, 365. For OADs, the number of days’ supply was derived directly from the RAMQ database. Since insulin use may vary on a daily basis, the number of days’ supply was defined beforehand as 90 for all insulin prescriptions dispensed.6,14 Drugs taken in hospital are not recorded in the RAMQ database, so we removed the number of days spent in hospital from both the PDC numerator and denominator. Individuals with a PDC of 80% or more were deemed compliant. Previously published studies have used the 80% threshold to measure compliance with OADs.6,15,16
Individuals supplied an ACEi/ARB or a lipid-lowering drug for at least 1 day in the second year after OAD initiation, were regarded as taking these drugs. Table—Supplemental Digital Content 1, http://links.lww.com/MD/A322 shows the INN codes used to classify the drugs.
Potential Confounding Variables
Besides calendar year of OAD initiation, potential confounders also included sociodemographic, health-related, and healthcare use variables. We assessed sociodemographic characteristics at OAD initiation date (age (continuous), sex, and beneficiary type (no/partial/maximum guaranteed income supplement) as an indicator of socioeconomic status). In the first year after OAD initiation, we looked for the presence of certain health-related variables and healthcare use variables: history of hypertension, stroke, coronary artery disease, and congestive heart failure (see Table—Supplemental Digital Content 2, http://links.lww.com/MD/A322 for the corresponding ICD-9 and ICD-10 codes), number of different drugs used as an indicator of comorbidity,17 number of physician visits, hospitalization for any cause, and the provision of antidiabetes drugs or ACEi/ARB or lipid-lowering drugs on a weekly basis. In Quebec province, several pharmacies offer their clientele the possibility of having drugs dispensed for 7 days in pill organizers, instead of the usual 30 days provision. This practice may affect both pharmacy loyalty and the quality of drugs use. In our study, if individuals claimed ≥80% of their antidiabetes drugs or ACEi/ARB or lipid-lowering drugs for 7-day periods in the year following OAD initiation, they were considered to be supplied their drugs on a weekly basis.
Statistical Analysis
Frequency distributions described the study population's characteristics at OAD initiation and throughout the following year. We also calculated the proportion of loyal individuals according to each quality indicator. We conducted 4 multivariable logistic regression analyses for the association between pharmacy loyalty and persistence with antidiabetes drug treatment, compliance with antidiabetes drugs, use of an ACEi/ARB, and use of a lipid-lowering drug. In the first and second logistic regression analyses, potential confounders included: calendar year of OAD initiation, age (continuous variable), sex, beneficiary type, number of different drugs used, number of physician visits, hospitalization for any cause and provision of drugs on a weekly basis. In the third analysis, besides these variables we also included history of hypertension, and in the fourth analysis, history of stroke, coronary artery disease, and congestive heart failure. We did so because hypertension is a condition for which the use of ACEi/ARB is recommended, while lipid-lowering drugs are recommended to patients suffering from stroke, coronary artery disease, and congestive heart failure. Multicollinearity was assessed using the procedure described by Belsley et al.18 We obtained adjusted odds ratios (AOR) with their 95% confidence intervals (CI).
Similar analyses took place to assess the association between the number of different pharmacies visited and the 4 quality indicators. We also did a linear contrast test for all quality indicators to determine whether there was a significant trend effect with the increase in the number of different pharmacies visited.
We tested the sensitivity of our results to the 80% PDC threshold for compliance by repeating the analysis using 70% and 90% as cut-off points. Analyses were performed using SAS, version 9.3 (SAS Institute, Inc., Cary, NC).
Ethical Considerations
To guarantee anonymity, RAMQ assigned each individual a unique encrypted number. The Commission d’accès à l’information du Québec gave approval for RAMQ to send us the data. The research ethics committee of the CHU de Québec approved the study.
RESULTS
Of the 124,009 individuals comprising our study population (Figure 2), 74,098 (59.8%) were deemed loyal to a single pharmacy. Of those remaining, 33,754 (27.2%), 10,860 (8.8%) and 5297 (4.3%) filled drugs in 2, 3 and 4 to 23 pharmacies, respectively. Of the individuals who filled drugs in 2 pharmacies, 11,398 (33.8%) filled only one prescription in the second pharmacy, 9231 (27.4%) switched definitely to the second and 9648 (28.6%) went back and forth between the 2. Since 3477 (10.3%) of the 33,754 individuals filled their last prescription of the first year in the second pharmacy we could reach no conclusion concerning their pharmacy-visiting pattern. Table 1 gives the study population characteristics according to pharmacy loyalty.
TABLE 1.
Characteristics of Patients at Oral Antidiabetes Drug Initiation or in the Year After, According to Pharmacy Loyalty (N = 124,009)
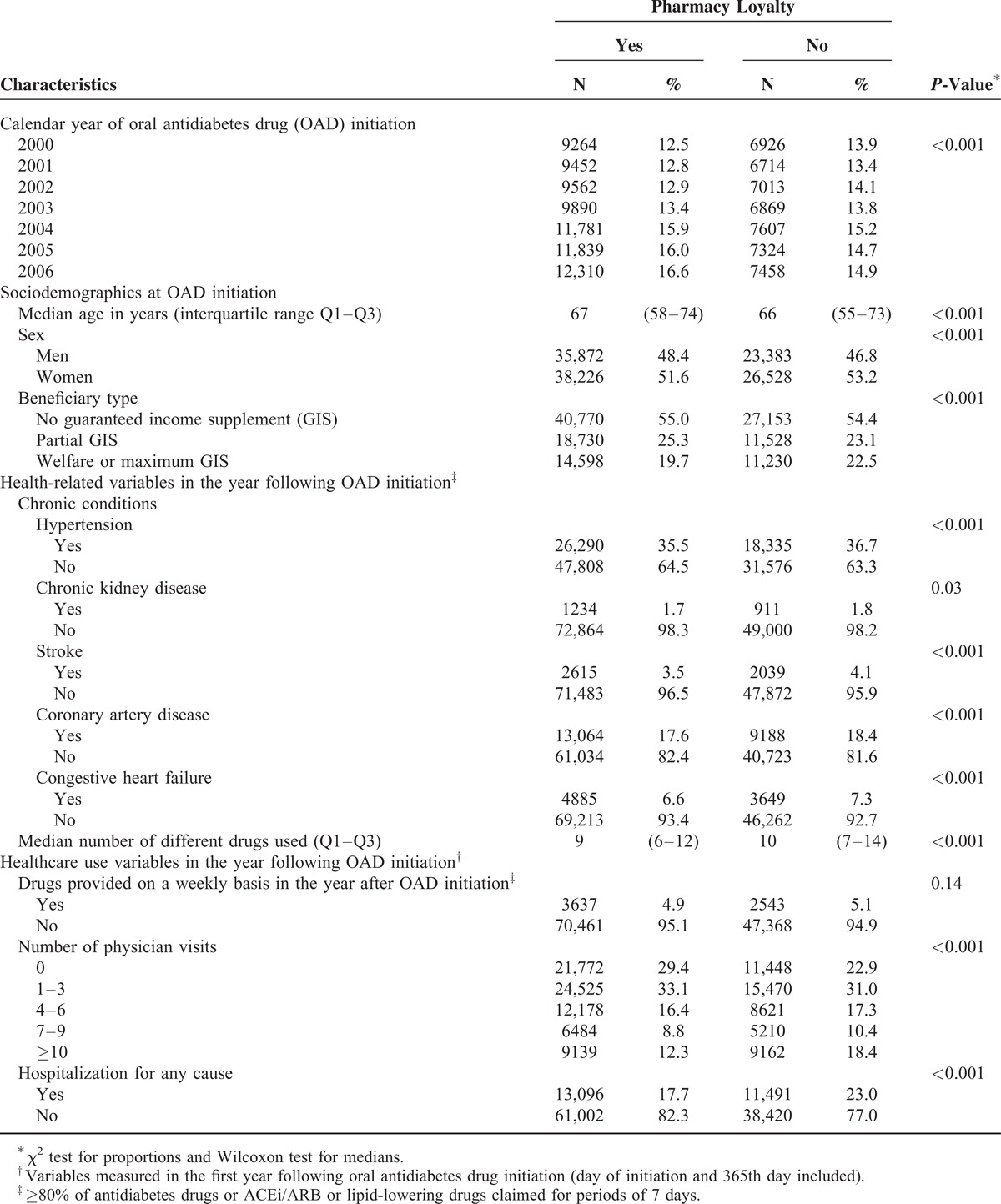
In total, 100,216 (80.8%) individuals were persistent with their antidiabetes drug treatment 2 years after OAD initiation (Table 2). Individuals not loyal to a single pharmacy were 11% less likely to be persistent. Among the persistent individuals, 78,321 (78.2%) were compliant with antidiabetes drugs during their second year of treatment. Individuals not loyal to a single pharmacy were 18% less likely to be compliant. Of the 107,133 individuals included in the ACEi/ARB and lipid-lowering drug use analyses, 64.1% and 63.7% used an ACEi/ARB and a lipid-lowering drug, respectively. Individuals not loyal to a single pharmacy were 15% less likely to use an ACEi/ARB and 17% less likely to use a lipid-lowering drug in the second year after OAD treatment initiation.
TABLE 2.
Quality Indicators of Drug Use in the Second Year Following Antidiabetes Drug Treatment Initiation According to Pharmacy Loyalty in the First Year Following This Initiation
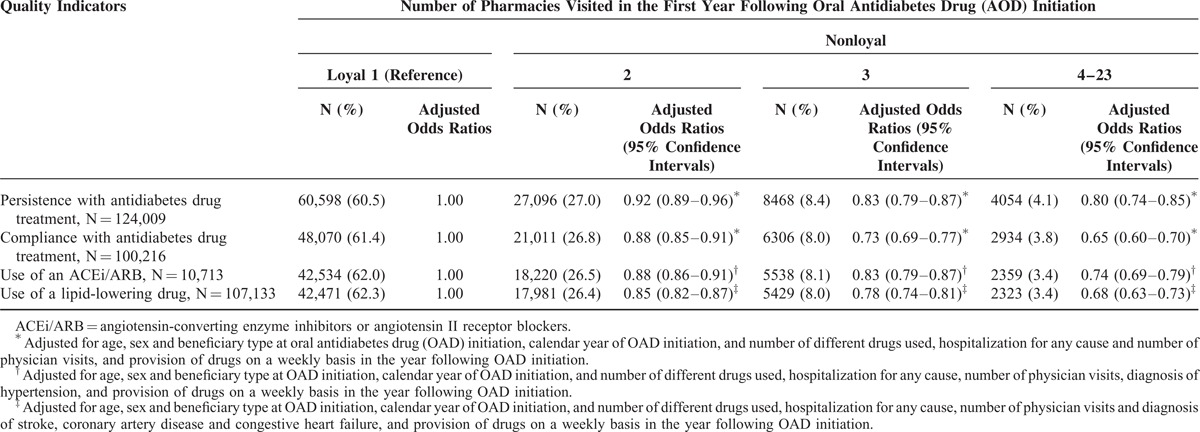
Compared to loyal individuals, those who visited 2, 3, and 4 to 23 different pharmacies were 8%, 17%, and 20% less likely to be persistent with their antidiabetes drug treatment 2 years after OAD initiation, respectively (Table 3). Of those who persisted, individuals who visited 2, 3, and 4 to 23 different pharmacies were 12%, 27%, and 35% less likely, respectively, than loyal individuals to be compliant with their antidiabetes drugs. Compared to loyal individuals, those who visited 2, 3, and 4 to 23 different pharmacies were also 12%, 17%, and 26% less likely, respectively, to use an ACEi/ARB and 15%, 22%, and 32% less likely to use a lipid-lowering drug. The trend of a decrease in quality with an increase in the number of pharmacies was statistically significant (linear contrast test for all quality indicators was <0.001).
TABLE 3.
Quality Indicators of Drug Use in the Second Year Following Antidiabetes Drug Treatment Initiation According to the Number of Different Pharmacies Visited in the First Year Following This Initiation
Since the prevalence of the quality indicators was high, we carried out 4 log-binomial regression analyses,19 in addition to the 4 logistic regression analyses. The associations observed between pharmacy loyalty and the 4 quality indicators remained unchanged in effect, size, and statistical significance (data not shown). Similarly, associations were not sensitive to the cut-off point's variation for PDC (data not shown).
DISCUSSION
Two major findings emerged from this study: individuals loyal to a single pharmacy were more likely to have better quality indicators of drug use and, for all indicators, the quality decreased with the increase in number of pharmacies where drugs were filled.
We found that nonloyal individuals were less likely to be persistent with antidiabetes drugs and nonloyal persistent individuals were less likely to be compliant with antidiabetes drugs than loyal individuals. As far as we knew, the association between pharmacy loyalty and persistence with treatment had never been assessed. Our findings are however, consistent with a recent study in which elderly beneficiaries of Medicare Part D who used a single pharmacy had 7% to 24% higher odds of being covered for ≥80% of the days in a year with biguanides, sulfonylureas, thiazolidinediones, and dipeptidyl peptidase-IV inhibitors.2 Our findings are also consistent with results from a study using data from a United States Pharmacy Benefit Manager, in which each additional pharmacy visited by patients was associated with a 1.6% and 2.0% decrease in the PDC in a year by a statin and an ACEi/ARB, respectively.3 In a recent systematic review of pharmacy services interventions on adherence, Jalal et al20 concluded that patient education by pharmacists and telephone contact had a statistically significant positive effect on medication adherence. Our results suggest this could be facilitated when patients fill their drugs in the same pharmacy.
We observed that nonloyal individuals were less likely to use an ACEi/ARB and a lipid-lowering drug; a finding consistent with findings of 2 previous studies in which individuals who obtained all their drugs in a single pharmacy had a lower risk of receiving a potential inappropriate drug combination1 and of a potential drug–drug interaction.2 We believe our study is the first to investigate the effect of loyalty to a pharmacy on the use of guidelines-recommended drugs. This result is also in line with our hypothesis that with comprehensive information available, pharmacists might also detect drug-related problems of nonexposure to guidelines-recommended drugs, and suggests that pharmacists might intervene to resolve said problems, for example, by discussion with prescribers.
This study has some limitations. First, we assumed that all drugs claimed were taken, which may have led to overestimation of persistence and compliance. This nondifferential misclassification could have biased the effect estimates toward the null. Second, we assessed pharmacy loyalty in the first year of treatment and quality indicators in the year after. Therefore, we cannot assume that the association we have observed between pharmacy loyalty and drug use quality would remain the same if quality indicators were assessed in subsequent years. We also assumed there was no information sharing between pharmacies. However, this is not always the case in the province of Quebec. For example, at the client's request, pharmacists may transfer the pharmacy record to another pharmacy. Moreover, in pharmacies affiliated with one of Quebec's pharmacy chains, client records are chain based rather than pharmacy based. We had no way to identify individuals whose record was transferred to another pharmacy, nor those individuals who filled their drugs in more than 1 pharmacy of a pharmacy chain with shared records. Nonetheless, despite the fact that some pharmacists had the information available on drugs that their “nonloyal” clients had filled elsewhere, we still observed an association between pharmacy loyalty and better quality of drug use. This tends to suggest that better quality of drug use might to some extent be due to the quality of the personal relationships that pharmacists were able to develop with the clients who filled all their drugs in their pharmacy. Next, our results apply only to the population covered by the RAMQ public drug plan. However, our results are likely to be generalizable to jurisdictions sharing, on one hand, similar guidelines for the treatment of type 2 diabetes and, on the other hand, a similar organization of pharmacy practice and similar public drug program.
Our study also has strengths. First, it involved a great number of patients insured under the provincial drug benefit plan and who initiated an OAD between 2000 and 2006. Furthermore, the group includes all seniors in the general population, providing population-based data for this age group. Second, ours is a real-life study that took into account that some individuals change medication patterns (OAD to another OAD or insulin). Moreover, we took the use of insulin into account when we assessed persistence and compliance. Insulin remains an important component of the treatment for individuals with diabetes and failure to consider its use would have led us to underestimate persistence and compliance with all antidiabetes drugs. Third, contrary to previous studies that were cross-sectional,1,2 loyalty to pharmacy was defined before measuring quality indicators in order to ensure its anteriority.
The positive association between pharmacy loyalty and persistence and compliance with antidiabetes drugs and with guidelines-recommended drugs underscores the important role pharmacists could play in helping their clients with chronic diseases to better manage their drug treatments. However, only further research can determine to what extent the positive effects associated with pharmacy loyalty are specifically due to pharmacists, and not due to other factors such as convenience (eg, people use the same pharmacy simply because the location is convenient).
Acknowledgments
The authors thank Eric Demers for statistical analyses and Joanne Vidal for editing the manuscript. These persons gave permission to be named.
Footnotes
Abbreviations: ACEi/ARB = angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker, AOR = adjusted odds ratio, CI = confidence interval, ICD = International Classification of Diseases, INN = International Non-proprietary Name, OAD = oral antidiabetes drug, PDC = Proportion of days covered, RAMQ = Régie de l’assurance maladie du Québec.
This study was funded by the Laval University Chair on Adherence to Treatments. The Chair was supported by nonrestricted grants from: AstraZeneca Canada, Merck Canada, Sanofi Canada, Pfizer Canada and the Prends soin de toi Program.
The authors have no conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website (www.md-journal.com).
REFERENCES
- 1.Tamblyn RM, McLeod PJ, Abrahamowicz M, et al. Do too many cooks spoil the broth? Multiple physician involvement in medical management of elderly patients and potentially inappropriate drug combinations. CMAJ 1996; 154:1177–1184. [PMC free article] [PubMed] [Google Scholar]
- 2.Marcum ZA, Driessen J, Thorpe CT, et al. Effect of multiple pharmacy use on medication adherence and drug-drug interactions in older adults with Medicare Part D. J Am Geriatr Soc 2014; 62:244–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choudhry NK, Fischer MA, Avorn J, et al. The implications of therapeutic complexity on adherence to cardiovascular medications. Arch Intern Med 2011; 171:814–822. [DOI] [PubMed] [Google Scholar]
- 4.Canadian Diabetes Association Clinical Practice Guidelines Expert Committee Canadian Diabetes Association 2008 Clinical Practice Guidelines for the Prevention and Management of Diabetes in Canada. Can J Diabetes 2008; 32 (Suppl. 2):S1–S225. [DOI] [PubMed] [Google Scholar]
- 5.Gregoire JP, Sirois C, Blanc G, et al. Persistence patterns with oral antidiabetes drug treatment in newly treated patients--a population-based study. Value Health 2010; 13:820–828. [DOI] [PubMed] [Google Scholar]
- 6.Guenette L, Moisan J, Breton MC, et al. Difficulty adhering to antidiabetic treatment: factors associated with persistence and compliance. Diabetes Metab 2013; 39:250–257. [DOI] [PubMed] [Google Scholar]
- 7.Guenette L, Breton MC, Hamdi H, et al. Important treatment gaps in vascular protection for the elderly after type 2 diabetes therapy initiation. Can J Cardiol 2013; 29:1593–1598. [DOI] [PubMed] [Google Scholar]
- 8.Sirois C, Moisan J, Poirier P, et al. Underuse of cardioprotective treatment by the elderly with type 2 diabetes. Diabetes Metab 2008; 34:169–176. [DOI] [PubMed] [Google Scholar]
- 9.Quebec Health Insurance Agency (RAMQ) 2010-2011 Annual Management Report. http://www.ramq.gouv.qc.ca/SiteCollectionDocuments/citoyens/fr/rapports/rappann1112-fr.pdf [Google Scholar]
- 10.Tamblyn R, Lavoie G, Petrella L, et al. The use of prescription claims databases in pharmacoepidemiological research: the accuracy and comprehensiveness of the prescription claims database in Quebec. J Clin Epidemiol 1995; 48:999–1009. [DOI] [PubMed] [Google Scholar]
- 11.Andrade SE, Kahler KH, Frech F, et al. Methods for evaluation of medication adherence and persistence using automated databases. Pharmacoepidemiol Drug Saf 2006; 15:565–574.discussion 567–575. [DOI] [PubMed] [Google Scholar]
- 12.Canadian Diabetes Association Clinical Practice Guidelines Expert Committee Canadian Diabetes Association 1998 Clinical Practice Guidelines for the Prevention and Management of Diabetes in Canada. JAMC 1998; 159 (Suppl. 8):S1–S32. [PMC free article] [PubMed] [Google Scholar]
- 13.Canadian Diabetes Association Clinical Practice Guidelines Expert Committee Canadian Diabetes Association 2003 Clinical Practice Guidelines for the Prevention and Management of Diabetes in Canada. Can J Diabetes 2003; 27 (Suppl. 2):S1–S151. [DOI] [PubMed] [Google Scholar]
- 14.Bonafede MM, Kalsekar A, Pawaskar M, et al. A retrospective database analysis of insulin use patterns in insulin-naive patients with type 2 diabetes initiating basal insulin or mixtures. Patient Prefer Adherence 2010; 4:147–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheong C, Barner JC, Lawson KA, et al. Patient adherence and reimbursement amount for antidiabetic fixed-dose combination products compared with dual therapy among Texas Medicaid recipients. Clin Ther 2008; 30:1893–1907. [DOI] [PubMed] [Google Scholar]
- 16.Colombi AM, Yu-Isenberg K, Priest J. The effects of health plan copayments on adherence to oral diabetes medication and health resource utilization. J Occup Environ Med 2008; 50:535–541. [DOI] [PubMed] [Google Scholar]
- 17.Schneeweiss S, Seeger JD, Maclure M, et al. Performance of comorbidity scores to control for confounding in epidemiologic studies using claims data. Am J Epidemiol 2001; 154:854–864. [DOI] [PubMed] [Google Scholar]
- 18.Belsley DA, Edwin K, Welsch RE. Regression Diagnostics: Identifying Influential Data and Sources of Collinearity. New York: John Wiley and Sons; 1980. [Google Scholar]
- 19.Deddens JA, Petersen MR. Approaches for estimating prevalence ratios. Occup Environ Med 2008; 65:481.486–501. [DOI] [PubMed] [Google Scholar]
- 20.Jalal ZS, Smith F, Taylor D, et al. Pharmacy care and adherence to primary and secondary prevention cardiovascular medication: a systematic review of studies. Eur J Hosp Pharm 2014; 2014: 21:238–244. [Google Scholar]


