Abstract
To evaluate effectiveness of 23-valent pneumococcal polysaccharide vaccine (PPSV23) inoculated during defined “vaccination period,” first 6 months post cancer diagnosis (ie, an anti-cancer treatment period), in elderly lung cancer patients on community-acquired pneumonia (CAP) hospitalization incidence.
This was a nationwide population-based cohort study of 157 newly diagnosed elderly lung cancer patients receiving PPSV23 during “vaccination period”, and 628 age and sex one-to-one matched controls enrolled in the National Health Insurance Research Database (NHIRD) of Taiwan between 2007 and 2010. All patients were ≥75 years old and still survival post “vaccination period.” Incidence density (ID) of all-cause inpatient CAP and cumulative survival risk were analyzed by multivariate Poisson regression and Kaplan–Meier method, respectively.
After a 4-year follow-up, IDs of all-cause inpatient CAP for vaccination and control cohorts were 297 and 444 per 1000 PYs, respectively. Less vaccinated patients had CAP incidence density >1 time per PY (12.7% vs 21.2%) than non-vaccinated patients. After adjusting for potential confounding variables, like influenza vaccination, comorbidities, cancer treatment modalities, and socioeconomic status, adjusted inpatient CAP incidence rate in PPSV23 vaccination cohort was 0.74 times lower than control cohort (incidence rate ratio [IRR] = 0.740, P = 0.0339). Two-year cumulative CAP hospitalization rates and overall survival rates were 37.1% vs. 55.4%, and 46.6% vs. 26.2%, respectively, for lung cancer patients with and without PPSV23 (both P < 0.001). Subgroup analysis showed that for elderly lung cancer patients not ever receiving influenza vaccine, PPSV23 still had trend to reduce all-cause inpatient CAP.
For elderly lung cancer patients aged ≥75 years, PPSV23 inoculated during anti-cancer treatment period could reduce CAP hospitalizations and improve survival.
INTRODUCTION
Among adults in United States, Streptococcus pneumoniae (pneumococcus) remains a leading cause of serious illness along with bacteremia, meningitis, and pneumonia. An estimated 4000 deaths occur in United States each year because of S pneumoniae, primarily among adults.1 Incidence rate of invasive disease ranges from 3.8 per 100,000 among persons aged 18 to 34 years to 36.4 per 100,000 among those aged ≥65 years.2 Adults with certain medical conditions, such as lung cancer3,4 or human immunodeficiency virus,5 are also at increased risk for invasive pneumococcal disease (IPD) or community-acquired pneumonia (CAP). The disease rates for adults in these groups can be >20 times than those for adults without high-risk medical conditions. S pneumoniae is also the main etiology for CAP (about 60%–75% prevalence) according to data from the British Thoracic Society.6 Several studies have showed between 69% and 72.4% of cancer patient IPD cases were caused by serotypes covered in 23-valent pneumococcal polysaccharide vaccine (PPSV23).4,7 In Taiwanese studies, PPSV23 coverage was 92.2%.8
In United States, PPSV23 is recommended for all adults aged ≥65 years, adults aged 19 to 64 years who have serious long-term health issues, smokers, and children >2 years of age with serious long-term health problems; the vaccine provides protection of ≥5 years.9,10 However, for immunocompromised adults, for example, HIV patients, PPSV23 efficacy still remain undetermined.5,11 In a HIV patient cohort, PPSV23 decreased IPD incidence from 342/100,000 person-years (PYs) to 187/100,000 PYs within 3 years of PPSV23 immunization.5 But another double-blind, randomized, and placebo-controlled trial showed PPSV23 was ineffective in HIV-1-infected Ugandan adults.11 However, for patients with cancer, PPSV23 efficacy has only been investigated in Hodgkin's disease in a few studies12,13 whereas solid cancer diseases have not yet been investigated, including lung cancer.
Lung cancer is the leading cause of death among all cancer types as it accounts for 13% of cancer mortality worldwide.14 It is expected to be 26% to 28% of all American cancer deaths in 2013,15 and was 19.7% of all Taiwanese cancer deaths in 2012.16 A substantial body of evidence suggests that either chronic obstructive pulmonary disease (COPD) or impaired lung function is associated with lung cancer.17 Besides, lung cancer patients often require anti-cancer therapy, such as surgery, chemotherapy, or radiotherapy, which might affect immune status and result in lung damage.18 Significantly increased rates of IPD in those with lung cancer have been reported.4 However, in clinical, physicians often focus on cancer treatments for elderly lung cancer patients and ignore necessity and benefit of pneumococcal vaccine vaccination, even though PPSV23 is recommended for all adults aged ≧ 65 years by the Centers for Disease Control and Prevention (CDC) of United States.10
Whether lung cancer patient receiving pneumonia vaccination largely depends on attitude of oncology doctor to cancer treatment and pneumonia vaccination. Therefore, in this study, we plan to clarify effectiveness of PPSV inoculated during anti-cancer treatment course in elderly lung cancer patients.
MATERIALS AND METHODS
Ethics Statement
This study has been submitted to the Institutional Review Board of our institution and has been approved with approval number B10001019.
Database
Patients were drawn from the National Health Insurance Research Database (NHIRD), which was released for research purposes by the National Health Research Institutes, Taiwan. The NHIRD provides all medical claims for approximately 99% of Taiwanese people.19 To ensure accuracy of the claims, NHIRD performs quarterly expert reviews on a sample taken from every 50 to 100 ambulatory and inpatient claims.20 False diagnostic reports yield severe penalties from NHIRD.21 Information obtained from NHIRD is considered both complete and accurate.22 We used several NHIRD datasets in this study. These included ambulatory care visit claims, inpatient hospitalization expenditures, a registry for contracted medical facilities, a registry for beneficiaries, and also a registry for catastrophic illness patients.
Patient Allocation and Study Groups
There were 91,075 patients who were initially identified with a lung cancer diagnosis between 1997 and 2010 according to the International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM) code 162. These patients were validated using cross-linked data from the catastrophic illness registry. Since 2007, PPSV23 was funded in Taiwan by the Wang Jhan-Yang Social Welfare Foundation for general population ≥75 years of age. Hence, we excluded lung cancer patients diagnosed before 2007. A total of 33,297 incident lung cancer cases were diagnosed between 2007 and 2010.
Among them, 10,859 patients were older than 75 years. Besides, vaccination effect might be affected by cancer treatment modalities and the relative short survival time of lung cancer patients. Therefore, for exploration of cancer treatment modalities effect on PPSV23 and fair comparison of these two cohort groups, we defined the “vaccination period” as the first six months post cancer diagnosis when most cancer treatment modalities took place and observation time was calculated from 6 months post cancer diagnosis in both cohort groups (Figure 1). Therefore, we excluded 964 patients who died within the “vaccination period,” 732 patients who had been vaccinated before cancer diagnosis, and 160 patients who received vaccination after the “vaccination period.” Finally, there were total 157 vaccinated persons aged ≥75 years old receiving PPSV23 during the “vaccination period” and survived after “vaccination period” (Figure 2).
FIGURE 1.
The cohort study timeline of vaccination period and observation time in elderly lung cancer patients ≥75 years of age. The primary endpoint is the frequency of all-cause inpatient CAP (ie, the number of CAP per person-year observed).
FIGURE 2.
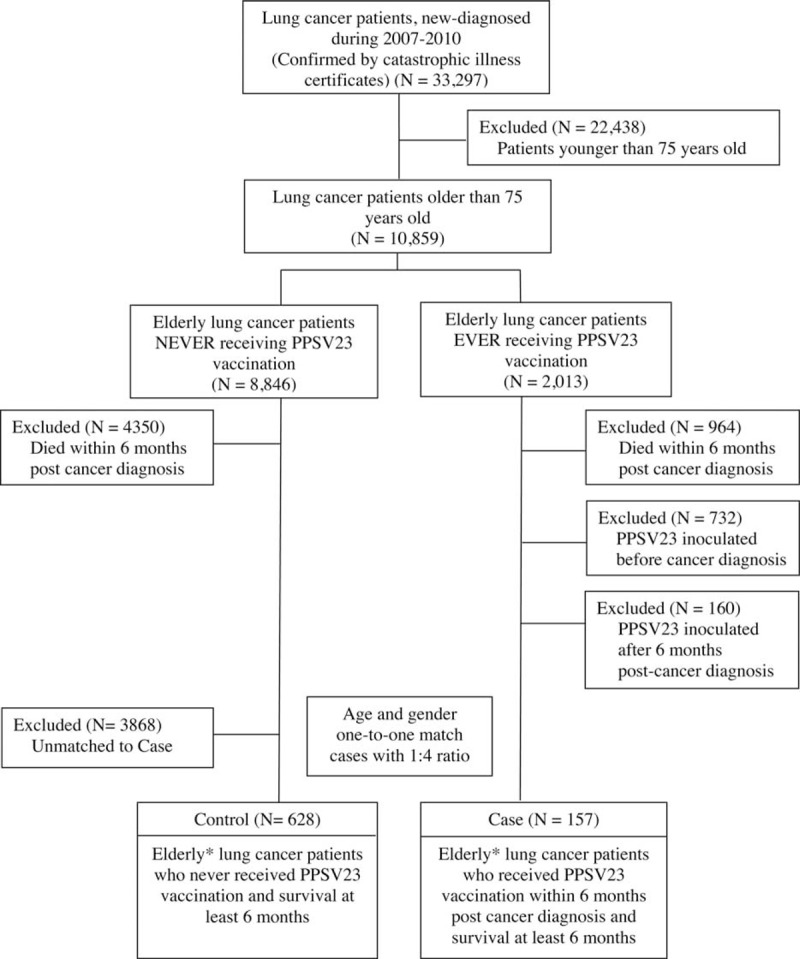
Study design flowchart. ICD-9 = International Classification of Diseases, Ninth Revision, NHI = National Health Insurance, PPSV23 = 23-valent Pneumococcal polysaccharide vaccine. ∗All cases and controls were 75 years of age or older. (PPSV was funded by Wang Jhan-Yang Social Welfare Foundation in Taiwan for general population aged ≥ 75 years).
After one-to-one matching for age and sex with a 1:4 ratio, 628 elderly lung cancer patients aged ≥75 years who never received PPSV23 vaccinations were then recruited into control cohort. All patients of 2 cohorts survived over half year after cancer diagnosis.
Measurements of Endpoints and Covariates
The primary outcome in the study was incidence density (ID), the number of CAP per person-year (PYs) observed, of inpatient all-cause CAP (ICD-9-CM codes 481–482 and 485–486), rather than inpatient pneumococcal CAP. Frequency of inpatient pneumococcal CAP is severely under-estimated in clinical practice and would result in wrong conclusion because definite pathogen culture result is not necessary during treatment process of pneumonia in clinical.
Observation time was calculated from the last day of vaccination period to end date of follow-up; this was either the withdrawal date from the NHI program (including death) or study termination date (the last day of 2010).
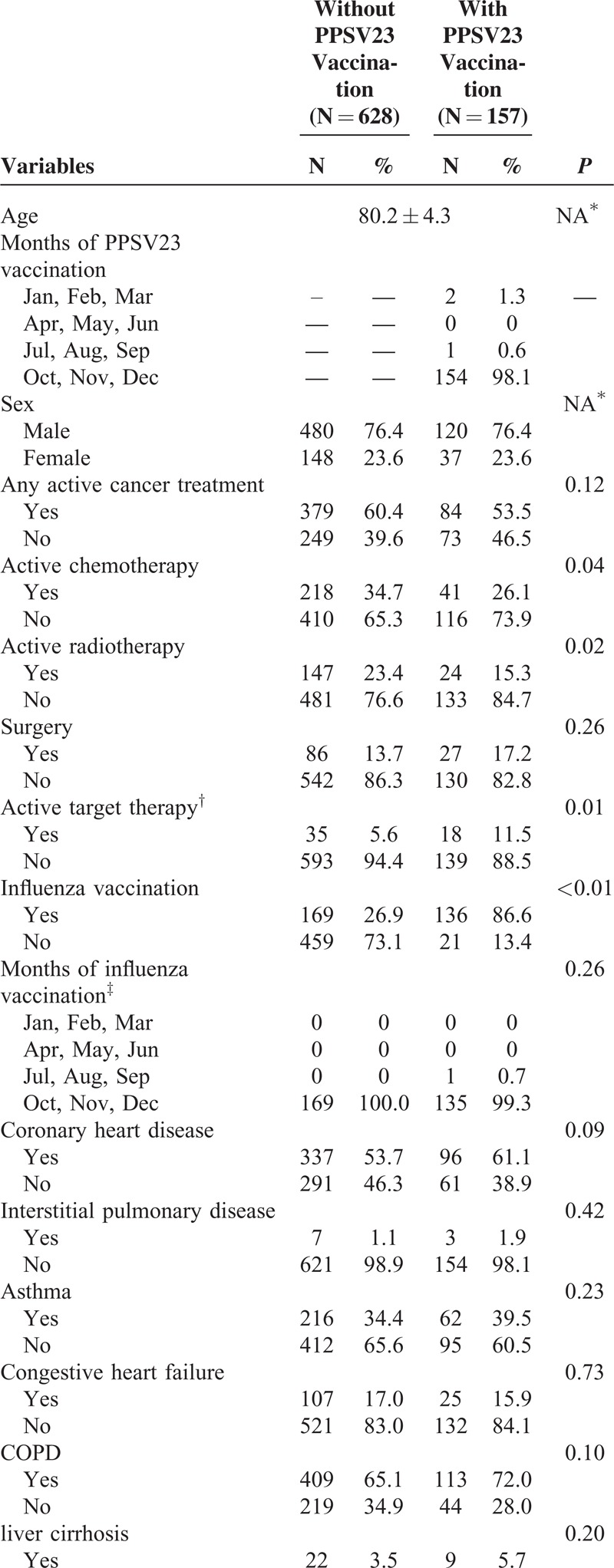
Independent variables were age, sex, cancer treatment modalities, influenza vaccination, comorbidities, and sociodemographic variables (Table 1 ). Sociodemographic variables included geographic region, urbanization level, and socioeconomic status. Influenza vaccination status was identified 1 year prior to “vaccination period” to the end date of follow-up from either outpatient or inpatient claims database. The cancer treatment modalities possibly affecting immune system or susceptibility to pneumonia (surgery, radiotherapy, chemotherapy, and target therapy) were adjusted accordingly.23–25 Comorbidities considered in our analysis included a number of major illnesses, such as coronary heart disease, interstitial pulmonary disease, asthma, congestive heart failure (CHF), COPD, liver cirrhosis, diabetes mellitus (DM), chronic renal failure, systemic lupus erythematosus (SLE), stroke, and dementia; these were found to significantly impact CAP cause and outcome.26 Insurance premium for each NHI beneficiary was proportional to his or her wages; a higher premium indicated higher wages. For those not actively employed, their insurance premium was zero. We grouped patient residential areas into 3 urbanization levels (ie, metropolis, satellite city, and rural area) according to the proposed classification scheme of Liu et al.27 We adjusted for regional variables because Taiwan demonstrates a distinct urban–rural difference in medical care accessibility.28 Information related to insurance premiums and residential areas was obtained from the beneficiary registry.
TABLE 1.
Demographic Characteristics and Comorbidities of Elderly Lung Cancer Patients With and Without 23-valent Pneumococcal Polysaccharide Vaccine Vaccination
Statistical Analysis
Patient characteristic comparisons between the 2 study groups were conducted using a chi-square test. Our endpoint is frequency of CAP (ie, the number of CAP per PY observed), which follows the Poison distribution, not whether a study subject encountered an episode of CAP, considering the relative short survival time of lung cancer patients to prevent observation time bias between study and control groups. We analyzed frequency distribution of inpatient CAP and performed a log-linear Poisson regression model to calculate both incidence rate ratio (IRR) and 95% confidence interval (CI), with adjustment for potential confounders by multivariate Poisson regression analysis. Kaplan-Meier method was used to estimate cumulative risk of CAP hospitalization and overall survival time. The SAS software (version 9.2, SAS Institute, Cary, NC, USA) was used for statistical analyses. A 2-sided P value of <0.05 was considered statistically significant.
RESULTS
Distribution of demographic characteristics and comorbidities seen for these 2 cohorts is shown in Table 1 . The mean age was 80.2 years for these 2 groups. PPSV23 and influenza vaccination were both initiated from October every year in Taiwan, without significant difference of vaccination time distribution (Table 1 ). Most patients received active anti-lung cancer therapy, 60.4% and 53.5 %, respectively. During a 4-year follow-up period, a total of 281 study subjects encountered 446 CAP hospitalization episodes in 1091 observed PYs, which represented an ID of 408 per 1000 PYs. ID of study and control cohort was respectively 297 and 444 per 1000 PYs (Table 2).
TABLE 1 (Continued).
Demographic Characteristics and Comorbidities of Elderly Lung Cancer Patients With and Without 23-valent Pneumococcal Polysaccharide Vaccine Vaccination
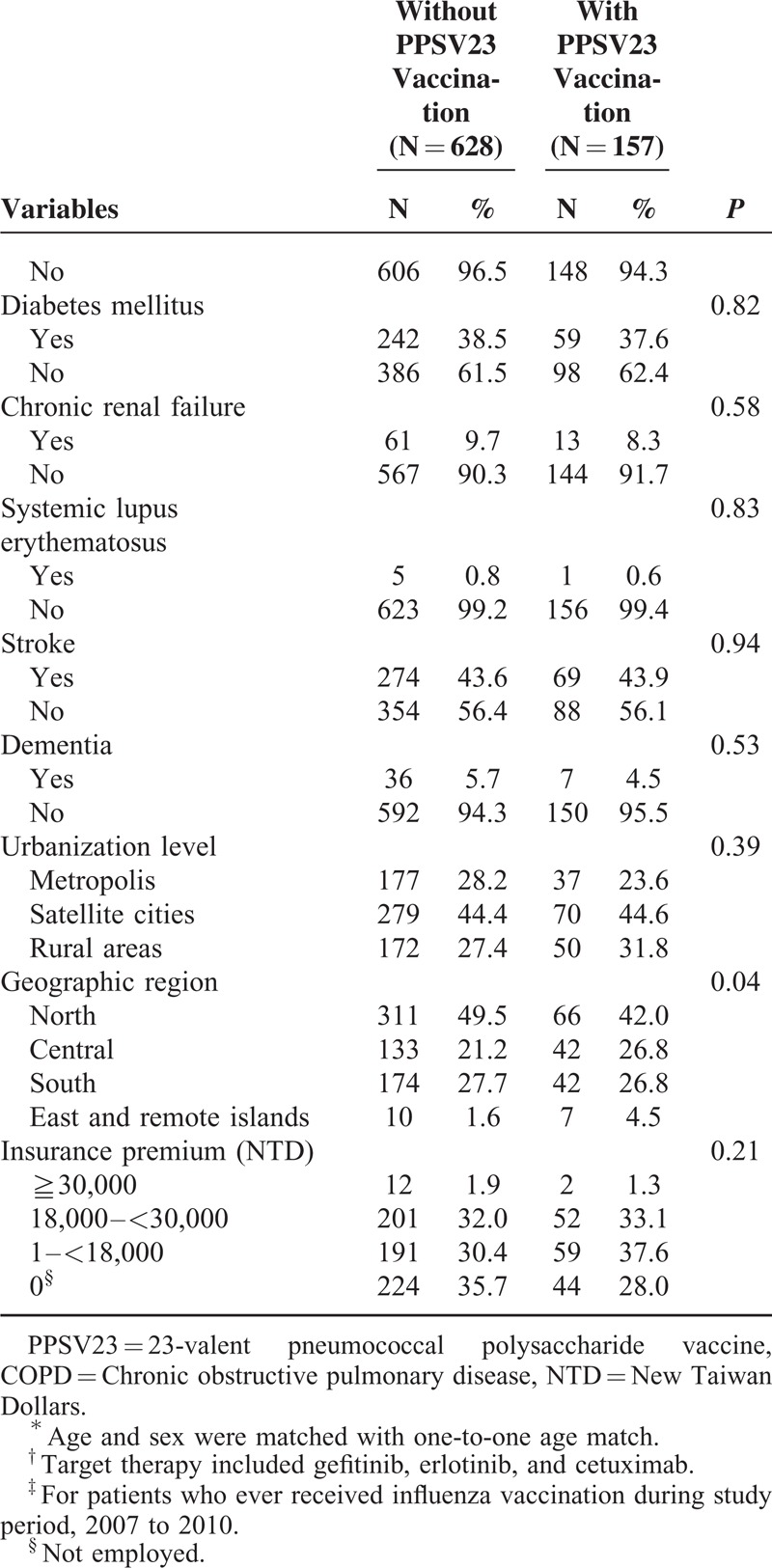
Table 3 shows frequency distribution of CAP hospitalization according to PPSV23 vaccination status. More patients in vaccination cohort group never had CAP hospitalization (69.4% vs. 62.9%) or had a CAP hospitalization frequency between 0 and 1 times per PY (17.8% vs. 15.9%) than non-vaccination cohort group. However, for individuals with a CAP hospitalization frequency from 1 to 2, 2 to 3, and >3 times per PY, non-vaccination cohort had nearly twice as many subjects (15.4% vs. 9.6%, 4.5% vs. 2.5%, and 1.3% vs. 0.6%, respectively) than did vaccination group cohort (Table 3). It meant that less vaccinated patients had CAP incidence density over 1 time per PY (12.7% vs. 21.2%) than non-vaccinated patients.
TABLE 2.
Incidence Density of Hospitalization for Inpatient Pneumococcal CAP and All-cause CAP in Elderly Lung Cancer Patients With and Without PPSV23
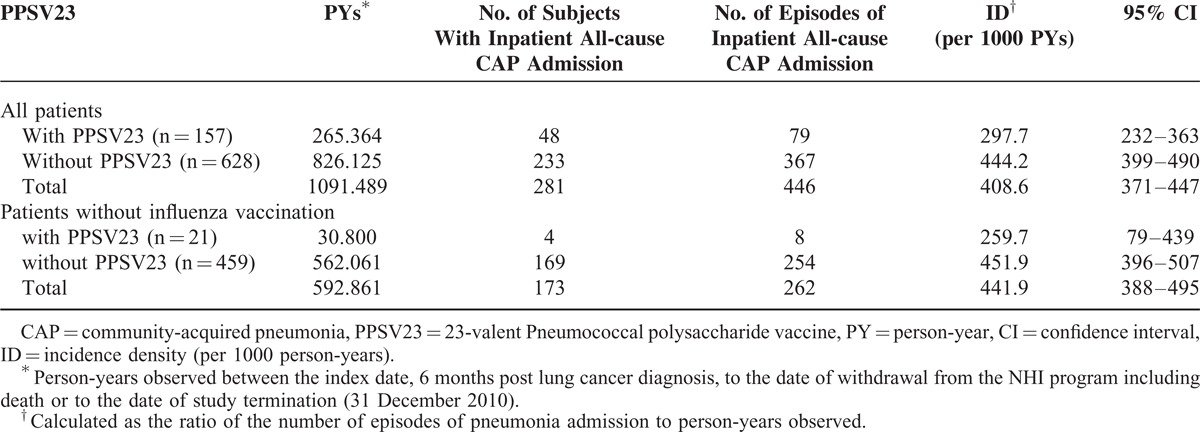
Two-year cumulative CAP hospitalization rates were 37.1% vs 55.4%, respectively, for lung cancer patients with and without PPSV23 vaccination (P < 0.001, Figure 3). Two-year overall survival rates with and without PPSV23 vaccination were 46.6% and 26.2%, respectively (P < 0.001, Figure 4).
FIGURE 3.
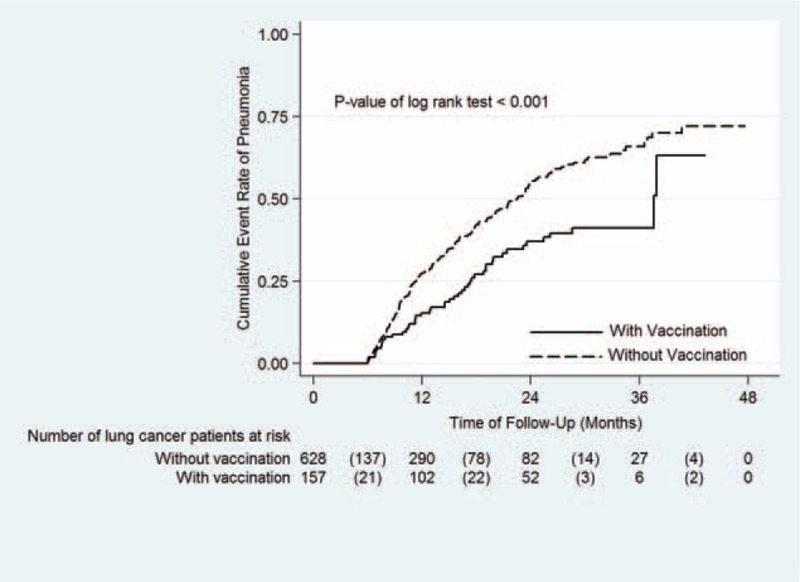
Comparison of Kaplan–Meier failure estimates of community-acquired pneumonia (CAP) hospitalization between lung cancer patients with and without PPSV23 vaccination. The 2-year cumulative CAP hospitalization rates were 37.1% vs 55.4%, respectively, for lung cancer patients with and without PPSV23 vaccination (P < 0.001). Continuous line indicates lung cancer patients with PPSV23 vaccination, Dash line indicates lung cancer patients without PPSV23 vaccination.
FIGURE 4.
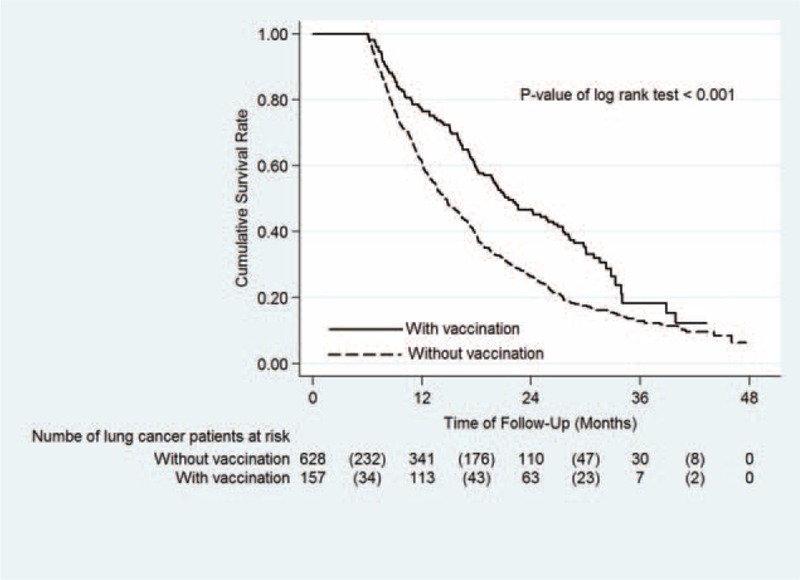
Kaplan–Meier survival curve for lung cancer patients with and without PPSV23 vaccination. The 2-year overall survival rates of lung cancer patients with and without PPSV23 vaccination were 46.6% and 26.2%, respectively (P < 0.001). Continuous line indicates lung cancer patients with PPSV23 vaccination, Dash line indicates lung cancer patients without PPSV23 vaccination.
Table 4 shows both unadjusted and adjusted IRR of CAP hospitalization. After adjustment for potential confounders, PPSV23 vaccination still significantly and substantially reduced CAP hospitalization risk, with an IRR of 0.74 (P = 0.0339). The regression coefficient (β) was −0.3006, which indicated a negative association.
TABLE 3.
Frequency Distribution of CAP Admission Numbers per PY in Elderly Lung Cancer Patients With and Without 23-valent Pneumococcal Polysaccharide Vaccine Vaccination
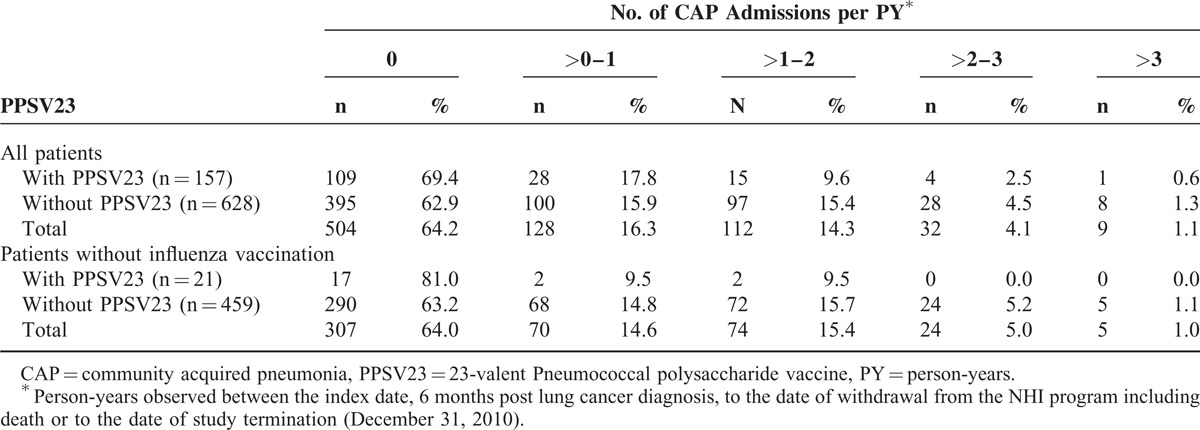
TABLE 4.
Crude and Adjusted IRR of Community-acquired Pneumonia Admission in Elderly Lung Cancer Patients With and Without 23-valent Pneumococcal Polysaccharide Vaccine Vaccination
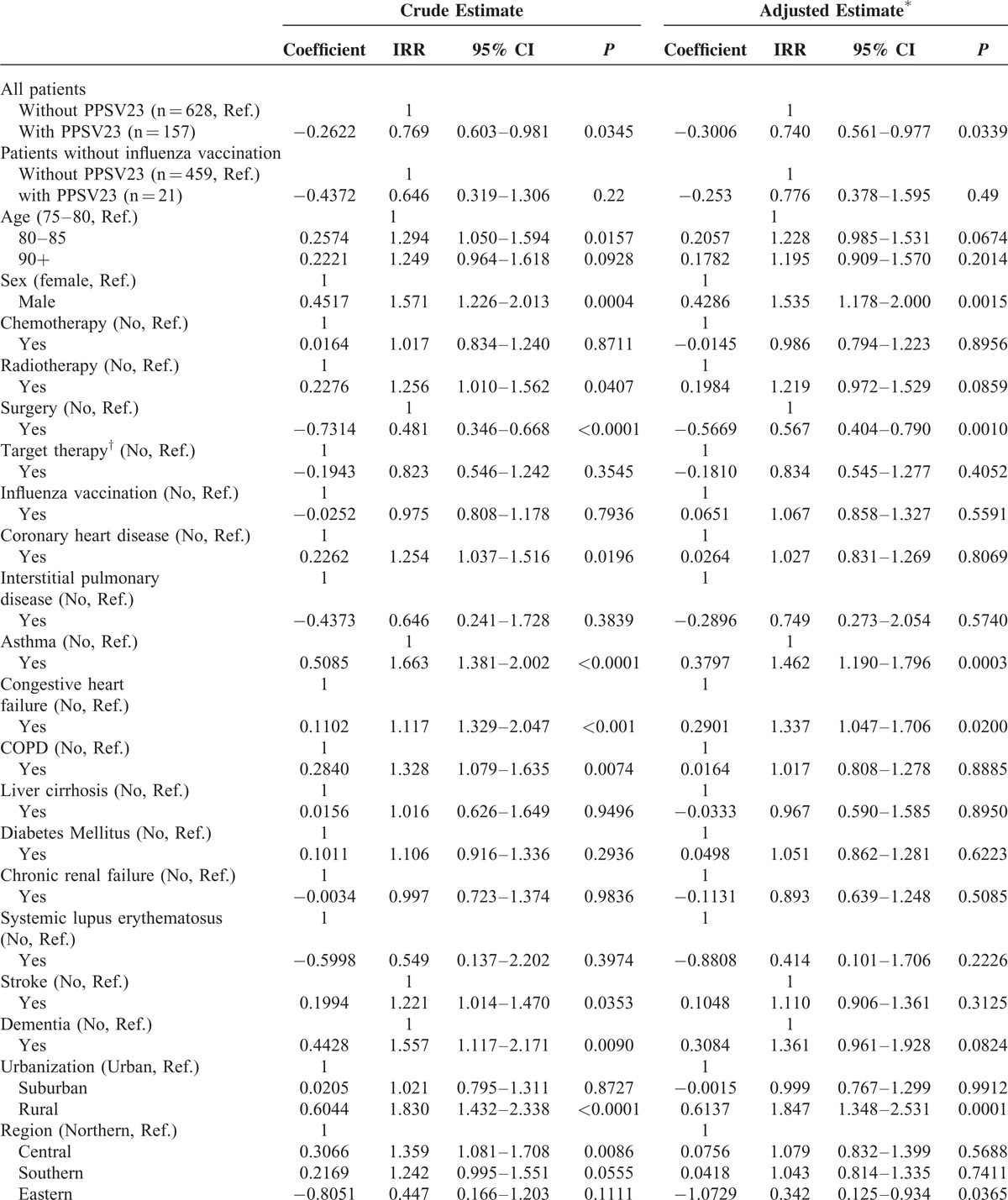
TABLE 4 (Continued).
Crude and Adjusted IRR of Community-acquired Pneumonia Admission in Elderly Lung Cancer Patients With and Without 23-valent Pneumococcal Polysaccharide Vaccine Vaccination
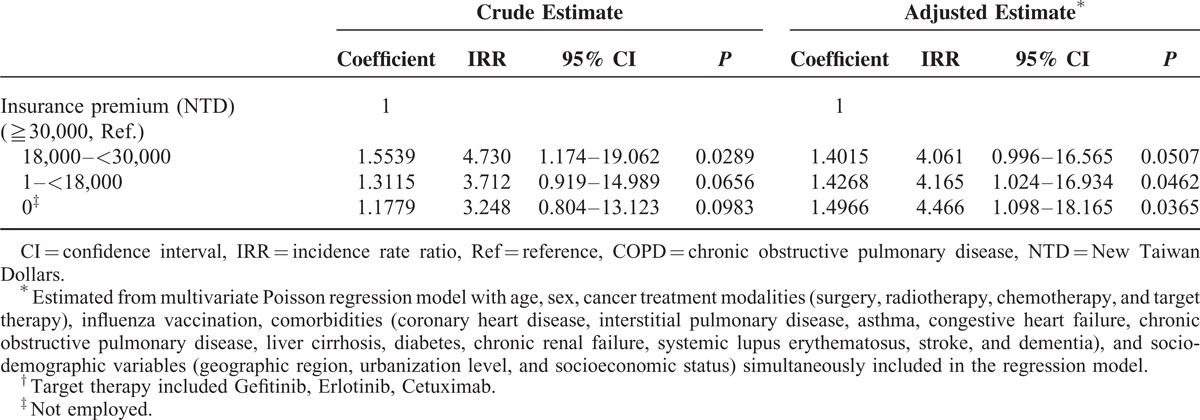
Table 4 also shows effect of other potential confounding variables. Male patients had a significantly higher adjusted IRR (IRR = 1.535, P = 0.0015) than female patients. Patients who received lung surgery had a significantly reduced IRR (adjusted IRR = 0.567, P = 0.0010). Although radiotherapy had a significant increase in unadjusted IRR (unadjusted IRR = 1.256, P = 0.0407), after adjustment for confounders, the effect did not reach significance. Both chemotherapy and target therapy did not have any significant effect. Our data showed that influenza vaccination did not have any significant effect on CAP hospitalization. For further excluding potential confounding effect of influenza vaccination, we further exclude patients who ever received influenza vaccine during study period, 2007 to 2010, in subgroup analysis (Tables 2–4). Subgroup analysis showed that for elderly lung cancer patients not ever receiving influenza vaccine, PPSV23 still has trend to reduce all-cause inpatient CAP, although not statistically significant mainly due to not enough vaccinated patient number.
For comorbidities, asthma (adjusted IRR = 1.462, P = 0.0003) and congestive heart failure (adjusted IRR = 1.337, P = 0.0200) were both significantly and positively associated with an increase in adjusted IRR. Although coronary heart disease, COPD, stroke, and dementia had a significant increase in unadjusted IRR, they had no significant effect after adjusting for confounders. In comparison with urban areas, patients living in rural areas had higher IRR of CAP hospitalization which may be due to more elderly patients living alone, poorer environmental sanitation and less medical resources in rural areas. Patients living in Northern regions with more available medical resources tended to have a lower incidence rate than the Central and Southern regions; however, this difference was not statistically significant. Lung cancer patients who lived in the Eastern regions whose characteristics were low pollution and low population density had the lowest IRR as compared with other regions; however, only 2% of all study subjects lived in the Eastern regions. Patients with the highest insurance premium (≥30,000 New Taiwan Dollars) had the lowest IRR of CAP hospitalization.
DISCUSSION
The levels of information and evidence related to PPV23 in cancer patients are limited and controversial.29 There have been only a few reports about effectiveness of PPSV23 in cancer patients, mainly focusing on Hodgkin disease. A study ever reported significant antibody response to PPSV in children with untreated Hodgkin disease with vaccine inoculated before or after splenectomy.12 The same significant antibody response also has been reported in splenectomized patients due to autoimmune disease.13 However, after reviewing the literature, this was the first study that examined effect of PPSV in lung cancer patients.
Elderly patients are at risk of CAP. A previous study found the likelihood of CAP increased with age (adjusted hazard ratio 1.03, 95% CI: 1.02–1.04).3 Several studies reported an incidence rate of 7.51 per 1000 PYs for all-cause CAP in general population aged ≥60 years,30 and 30 per 1000 PYs for those aged ≥70 years.31 The later study also showed a protective effect related to recent PPV23 vaccination (within the previous five years) against both pneumococcal and all-cause CAP for elderly people aged ≥60 years.30 In our 2 elderly lung cancer cohorts aged ≥75 years, patients had higher incidences of CAP hospitalization, 298 and 444 per 1000 PYs, respectively, which may result from older age, occasional immunocompromised status during cancer treatment, and poor pulmonary function accompanied by lung cancer. This also highlighted the importance of preventing pneumonia in such a high-risk group.
The newest result of CAPITA trial in 2015 shows that PCV13 has efficacy in reducing CAP and IPD of any pneumococcal strain, included both vaccine-type and non-vaccine-type pneumococcal strain, though not any cause pneumonia, in healthy elderly population aged > 65 years, with study endpoint being first episode of CAP and IPD.32 Contrast to that study, our study shows PPSV23 has efficacy in all-cause pneumonia in elderly lung cancer patients. There are several differences between these 2 studies. First, our population 75 years or older were weaker and much more susceptible to pneumonia than population older than 65 years in CAPITA trial. Besides, our study subjects were all newly diagnosed lung cancer patients under anti-cancer treatments who were very susceptible to pneumonia. Second, we included only inpatient CAP which was generally more severe than outpatient CAP of CAPITA trial collected at 101 temporary community-based sites throughout the Netherlands. Third, our endpoint is not whether a study subject encountered an episode of CAP, it is frequency of CAP (ie, the number of CAP per PY observed), considering the relative short survival time of lung cancer patients to prevent observation time bias between study and control groups.
In a population-based study of people aged ≥65 years, risk factors for CAP included age, male sex, COPD, asthma, DM, CHF, stroke, dementia, lung cancer, and smoking by multivariate analysis.33 Another population-based case–control study that included 96 patients with clinical chronic pulmonary diseases (ie chronic bronchitis, emphysema and/or asthma) showed PPSV23 was associated with a non-statistically significant reduction in all pneumococcal pneumonia risk among persons aged ≥75 years (adjusted odds ratio: 0.45; 95% CI: 0.16–1.27) but there was no apparent protective effect among people aged 50 to 74 years.34 As compared with previous studies, our cohorts focusing on elderly lung cancer patients aged ≥75 years showed that male sex, asthma, and CHF were independent significant risk factors for CAP hospitalization.
As compared with patients not receiving surgery, patients who had received lung surgery were found to have a lower risk of subsequent CAP hospitalization. This result may be because patients who had surgery, in general, would have fewer comorbidities and less advanced cancer, or because their primary lung lesions have been removed. Our results did not show an elevated risk of all-cause CAP hospitalization associated with chemotherapy or radiotherapy, although radiotherapy may result in radiation pneumonitis.35 In our study, influenza vaccine did not have a significant effect on CAP hospitalization. Different virus strains circulating at different years might be one of the reasons.
Although lung cancer patients of these cohorts received vaccine inoculation during anti-cancer treatment period, our study results still showed benefit of PPSV23. One of the reasons may be that not all patients in vaccination cohort had ever received chemotherapy but only 26% patients because of their old age. Another reason maybe because chemocytotoxic agents used for treating lung cancer included both immunosuppressant and non-immunosuppressant agents and oncology doctors may choose less toxic agents for these elderly patients aged ≥75 years. For example, interleukin-6-blocking therapy with target therapy tocilizumab used in rheumatoid arthritis patients would not affect the humoral immune response to either influenza or pneumococcal vaccines.36 The other possibility maybe that immune status of these patients was closely monitored during cancer treatments period and inoculation time was precisely determined by well-trained clinical physician in Taiwan.
Indirect vaccine effect could be offered by other vaccine, like pneumococcal conjugate vaccine (PCV), for example, introduction of routine infant 7-valent PCV immunization program post year 2000 in United States reduce pneumococcal infections among unvaccinated persons of all ages, including those aged ≥65 years.37 PCV7 was provided free of charge in Taiwan since 2009. However, serotype 19A was the major serotype in Taiwan and was not covered by PCV7. PCV13 was introduced into Taiwan since 2011 and was provided to children aged between 2 and 5 years of age for free since 2013.38 This study period was from 2007 to 2010 and the indirect vaccine effects would not be expected in this study.
Study Strengths
This study had several strengths. First, it was a nationwide population-based study, which included all lung cancer patients and all hospitals in Taiwan; therefore, this study had little room for selection bias and attrition bias. Compared with other country where less elderly people ever received PPSV23 vaccination because of vaccine payment at their own expense, elderly people in Taiwan age over 75 years were offered free PPSV23 vaccination since 2007. Less lung patients would receive vaccination because physicians often focus on treatments of cancer and ignore potential possible benefit of pneumococcal vaccine, for example, only 15% elderly lung cancer patients ever received pneumococcal vaccine before or after cancer diagnosis in Taiwan. This nationwide population-based study has included a relative large sample of vaccinated elderly lung cancer patients. Second, effect of vaccine was evaluated by incidence rate ratio and Poisson regression, eliminating bias due to different survival time and different cancer stages. Third, it utilized insurance claim datasets to provide access to longitudinal records of demographically diverse patients.39 Fourth, this lung cancer cohort was collected from the NHI database, entailing little likelihood of cohort member nonresponse or loss to follow-up. At last, several potential confounding factors have been evaluated in this study by multivariate Poisson regression, including socioeconomic levels, influenza vaccination, cancer treatment modalities, and comorbidities.
Study Limitations
Our study also featured several limitations. First, this is an observational nationwide population-based cohort study rather than a randomized controlled trial, though these 2 cohorts patients were one-to-one matched by sex and age and analyzed by multivariate analysis. Second, because only patients surviving post “vaccination period” were recruited to evaluate effect of cancer treatment modalities and make sure these 2 cohorts were properly compared, it is still unknown about PPSV23 efficacy on elderly lung cancer patients who died within 6 months post cancer diagnosis. However, considering their short survival time, they seem unlikely to benefit from any vaccination. Third, vaccinated people have high healthy awareness and take care for aspiration pneumonia or other infections. This bias may result in overestimate efficacy of PPSV23. However, we have adjusted personal socioeconomic status to decrease that effect as much as possible.
The United States has recommended the use of PCV13 followed ≥8 weeks thereafter with PPSV23 in all immunocompromised persons since October 2012 and in persons 65 years or older in 2014. This study showed PPSV23 alone without PCV13 boost had efficacy on inpatients CAPs in elderly lung cancer patients 75 years or older. Further investigation of PPSV23 efficacy in cancer patients with short survival time is needed.
CONCLUSIONS
For elderly lung cancer patients aged ≥75 years, PPSV23 inoculated during anti-cancer treatment period could reduce CAP hospitalizations and improve survival.
Acknowledgments
None.
Footnotes
Abbreviations: aHR = adjusted hazard ratio, aOR = adjusted odds ratio, CAP = community-acquired pneumonia, CHF = congestive heart failure, CI = confidence interval, COPD = chronic obstructive pulmonary disease, DM = diabetes mellitus, ICD-9-CM = International Classification of Disease, Ninth Revision, Clinical Modification, ID = incidence density, IRR = incidence rate ratio, NHIRD = National Health Insurance Research Database, NTD = New Taiwan Dollars, PCV = pneumococcal conjugate vaccine, PPSV23 = 23-valent pneumococcal polysacch/aride vccine, PYs = person-years, SLE = systemic lupus erythematosus.
This study is partly based on data from the National Health Insurance Research Database provided by the Bureau of National Health Insurance, Department of Health in Taiwan and managed by National Health Research Institutes (registry number 99029). However, the interpretation and conclusions contained herein are not those of the Bureau of National Health Insurance, Department of Health, or National Health Research Institutes.
The authors declare no conflicts of interests.
REFERENCES
- 1.CDC. Active Bacterial Core surveillance report, Emerging Infections Program Network, Streptococcus pneumoniae, 2010. Atlanta, GA: US Department of Health and Human Services, CDC; 2011. Available at http://www.cdc.gov/abcs/reports-findings/survreports/spneu10-orig.html Accessed October 1, 2012. [Google Scholar]
- 2.CDC. Active Bacterial Core surveillance report, Emerging Infections Program Network, Streptococcus pneumoniae, 2010. Atlanta, GA: US Department of Health and Human Services, CDC; 2011. Available at http://www.cdc.gov/abcs/reports-findings/survreports/spneu10-orig.html Accessed February 16, 2015. [Google Scholar]
- 3.Lin SH, Ji BC, Shih YM, et al. Comorbid pulmonary disease and risk of community-acquired pneumonia in COPD patients. Int J Tuberc Lung Dis 2013; 17:1638–1644. [DOI] [PubMed] [Google Scholar]
- 4.Wong A, Marrie TJ, Garg S, et al. Increased risk of invasive pneumococcal disease in haematological and solid-organ malignancies. Epidemiol Infect 2010; 138:1804–1810. [DOI] [PubMed] [Google Scholar]
- 5.Siemieniuk RA, Gregson DB, Gill MJ. The persisting burden of invasive pneumococcal disease in HIV patients: an observational cohort study. BMC Infect Dis 2011; 11:314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartlett JG, Mundy LM. Community-acquired pneumonia. N Engl J Med 1995; 333:1618–1624. [DOI] [PubMed] [Google Scholar]
- 7.Garcia-Vidal C, Ardanuy C, Gudiol C, et al. Clinical and microbiological epidemiology of Streptococcus pneumoniae bacteremia in cancer patients. J Infect 2012; 65:521–527. [DOI] [PubMed] [Google Scholar]
- 8.Hsueh PR, Chen HM, Lu YC, et al. Antimicrobial resistance and serotype distribution of Streptococcus pneumoniae strains isolated in southern Taiwan. J Formos Med Assoc 1996; 95:29–36. [PubMed] [Google Scholar]
- 9.Centers for Disease C, Prevention. Pneumococcal Polysaccharide Vaccine Information Statement. 2009. [Google Scholar]
- 10.CDC. Updated recommendations for prevention of invasive pneumococcal disease among adults using the 23-valent pneumococcal polysaccharide vaccine (PPSV23). MMWR 2010; 59:1102–1106. [PubMed] [Google Scholar]
- 11.French N, Nakiyingi J, Carpenter LM, et al. 23-valent pneumococcal polysaccharide vaccine in HIV-1-infected Ugandan adults: double-blind, randomised and placebo controlled trial. Lancet 2000; 355:2106–2111. [DOI] [PubMed] [Google Scholar]
- 12.Addiego JE, Jr, Ammann AJ, Schiffman G, et al. Response to pneumococcal polysaccharide vaccine in patients with untreated Hodgkin's disease. Children's Cancer Study Group Report. Lancet 1980; 2:450–452. [DOI] [PubMed] [Google Scholar]
- 13.Landgren O, Bjorkholm M, Konradsen HB, et al. A prospective study on antibody response to repeated vaccinations with pneumococcal capsular polysaccharide in splenectomized individuals with special reference to Hodgkin's lymphoma. J Int Med 2004; 255:664–673. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization (2008) Cancer mortality and morbidity. Available: http://www.who.int/gho/ncd/mortality_morbidity/cancer/en/. Accessed February 16, 2015. [Google Scholar]
- 15.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013; 63:11–30. [DOI] [PubMed] [Google Scholar]
- 16.Taiwan National Health Insurance Administration MoHW (2013) Statistics & Surveys. Available: http://www.nhi.gov.tw/English/webdata/webdata.aspx?menu=11&menu_id=296&webdata_id=1942&WD_ID=296. Accessed February 16, 2015. [Google Scholar]
- 17.Tockman MS. Samet JM. Other host factors and lung cancer susceptibility. Epidemiology of Lung Cancer. New York, NY: Marcel Dekker; 1994. 397–412. [Google Scholar]
- 18.Crestani B, Valeyre D, Roden S, et al. Bronchiolitis obliterans organizing pneumonia syndrome primed by radiation therapy to the breast. The Groupe d’Etudes et de Recherche sur les Maladies Orphelines Pulmonaires (GERM“O”P). Am J Respir Crit Care Med 1998; 158:1929–1935. [DOI] [PubMed] [Google Scholar]
- 19.Lu JF, Hsiao WC. Does universal health insurance make health care unaffordable? Lessons from Taiwan. Health affairs 2003; 22:77–88. [DOI] [PubMed] [Google Scholar]
- 20.Cheng TM. Taiwan's new national health insurance program: genesis and experience so far. Health Affairs 2003; 22:61–76. [DOI] [PubMed] [Google Scholar]
- 21.Bureau of National Health Insurance Website (2000) Methods for estimating false claims. Available from: Available at: http://www.nhi.gov.tw/information/bulletin_file/421-0890036465-19.doc (last accessed 1 October 2009). [Google Scholar]
- 22.Sun Y, Chang YH, Chen HF, et al. Risk of Parkinson disease onset in patients with diabetes: a 9-year population-based cohort study with age and sex stratifications. Diabetes Care 2012; 35:1047–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yen TT, Lin CH, Jiang RS, et al. Incidence of Late-onset Pneumonia in Patients after Treatment with Radiotherapy for Nasopharyngeal Carcinoma: a nationwide population-based study. Head Neck 2014; Jul 2. doi: 10.1002/hed.23827. [DOI] [PubMed] [Google Scholar]
- 24.Lee JY, Jin SM, Lee CH, et al. Risk factors of postoperative pneumonia after lung cancer surgery. J Korean Med Sci 2011; 26:979–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee JO, Kim DY, Lim JH, et al. Risk factors for bacterial pneumonia after cytotoxic chemotherapy in advanced lung cancer patients. Lung Cancer 2008; 62:381–384. [DOI] [PubMed] [Google Scholar]
- 26.Cilloniz C, Polverino E, Ewig S, et al. Impact of age and comorbidity on cause and outcome in community-acquired pneumonia. Chest 2013; 144:999–1007. [DOI] [PubMed] [Google Scholar]
- 27.Liu CY, Hung YT, Chuang YL, et al. Incorporating development stratification of Taiwan townships into sampling design of large scale health interview survey. J Health Manage 2006; 4:1–22. [Google Scholar]
- 28.Chen HF, Ho CA, Li CY. Age and sex may significantly interact with diabetes on the risks of lower-extremity amputation and peripheral revascularization procedures: evidence from a cohort of a half-million diabetic patients. Diabetes Care 2006; 29:2409–2414. [DOI] [PubMed] [Google Scholar]
- 29.CDC. Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) for Pneumococcal Vaccines for Immunocompromised Adults. Available at http://www.cdc.gov/vaccines/acip/recs/GRADE/pneumo-immuno-adults.html. Page last updated: October 10, 2012. [Google Scholar]
- 30.Ochoa-Gondar O, Vila-Corcoles A, Rodriguez-Blanco T, et al. Effectiveness of the 23-valent pneumococcal polysaccharide vaccine against community-acquired pneumonia in the general population aged >/= 60 years: 3 years of follow-up in the CAPAMIS study. Clin Infect Dis 2014; 58:909–917. [DOI] [PubMed] [Google Scholar]
- 31.Garcia-Vidal C, Calbo E, Pascual V, et al. Effects of systemic steroids in patients with severe community-acquired pneumonia. Eur Respir J 2007; 30:951–956. [DOI] [PubMed] [Google Scholar]
- 32.Bonten MJ, Huijts SM, Bolkenbaas M, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med 2015; 372:1114–1125. [DOI] [PubMed] [Google Scholar]
- 33.Jackson ML, Neuzil KM, Thompson WW, et al. The burden of community-acquired pneumonia in seniors: results of a population-based study. Clin Infect Dis 2004; 39:1642–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vila-Corcoles A, Ochoa-Gondar O, Rodriguez-Blanco T, et al. Clinical effectiveness of 23-valent pneumococcal polysaccharide vaccine against pneumonia in patients with chronic pulmonary diseases: a matched case-control study. Human Vaccin Immunother 2012; 8:639–644. [DOI] [PubMed] [Google Scholar]
- 35.Leprieur EG, Fernandez D, Chatellier G, et al. Acute radiation pneumonitis after conformational radiotherapy for nonsmall cell lung cancer: clinical, dosimetric, and associated-treatment risk factors. J Cancer Res Ther 2013; 9:447–451. [DOI] [PubMed] [Google Scholar]
- 36.Tsuru T, Terao K, Murakami M, et al. Immune response to influenza vaccine and pneumococcal polysaccharide vaccine under IL-6 signal inhibition therapy with tocilizumab. Mod Rheumatol 2014; 24:511–516. [DOI] [PubMed] [Google Scholar]
- 37.Pilishvili T, Lexau C, Farley MM, et al. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis 2010; 201:32–41. [DOI] [PubMed] [Google Scholar]
- 38.(Taiwan) C. Press Releases: Taiwan CDC announces this year's first pneumococcal death in infant and urges parents to get children meeting eligibility criteria for government-funded pneumococcal conjugate vaccine vaccinated. http://www.cdc.gov.tw/english/info.aspx?treeid=bc2d4e89b154059b&nowtreeid=ee0a2987cfba3222&tid=520647D35E5023F4. Accessed February 16, 2015. in press. [Google Scholar]
- 39.Jollis JG, Ancukiewicz M, DeLong ER, et al. Discordance of databases designed for claims payment versus clinical information systems. Implications for outcomes research. Ann Intern Med 1993; 119:844–850. [DOI] [PubMed] [Google Scholar]


