Abstract
Non-Hodgkin lymphoma (NHL) and Hodgkin lymphoma (HL) represent a heterogeneous group of malignant lymphoid tumors, which have distinct histological and/or biological characteristics with preferential nodal involvement. However, none of the previous studies have assessed the prevalence of common NHL and HL subtypes at each nodal site of involvement.
The aim of our study was to determine the prevalence of HL and NHL subtypes depending on their nodal sites of involvement.
We conducted a single-center retrospective study of 938 lymphoma cases diagnosed in the Pathology Department of Toulouse Purpan Hospital in France between 2001 and 2008, taking into account the site that corresponded to the diagnostic biopsy.
The most frequent sites were cervical lymph nodes (36.8% of all cases), inguinal lymph nodes (16.4%), axillary lymph nodes (11.9%), and supraclavicular lymph nodes (11%). We found an unexpected association between intraparotid nodes and nodular lymphocyte predominant Hodgkin lymphoma (NLPHL) and between inguinal nodes and follicular lymphoma. The risk of having classical Hodgkin lymphoma (CHL) was 15 times greater in patients with mediastinal lymphoma compared to those with other sites of involvement. Regarding HL, nodal and extranodal mediastinal sites and supraclavicular nodes were more likely to be involved by nodular sclerosis Hodgkin lymphoma (NSCHL). In addition, intra-abdominal lymph nodes were more frequently involved by lymphocyte depleted Hodgkin lymphoma compared to inguinal nodes where NLPHL predominated.
Our study shows that some lymph node sites have a disproportionate prevalence of specific subtypes of lymphoma. Identifying these sites may aid to diagnose and better elucidate the pathogenesis of these tumors.
INTRODUCTION
Non-Hodgkin lymphoma (NHL) and Hodgkin lymphoma (HL) represent a heterogeneous group of malignant lymphoid proliferations of extra medullary origin. This heterogeneity is associated with various clinical, pathological, immunological, and cytogenetic presentations, and thus the prognoses are quite variable.1 The primary site of involvement of NHL and HL, however, is essentially nodal, and some NHLs and subtypes of HL may have preferential nodal sites of involvement. The anatomic distribution of HL has been previously studied and usually located in the lymph nodes of the neck and the mediastinum.2 Furthermore, the commonest site of involvement was cervical region followed by axillary region.3–5 In a study including 500 malignant lymphomas,6 the authors found that the primary site of involvement for HL was in lymph nodes or the spleen in 99.5% of cases with prominent involvement in cervical and mediastinal areas, whereas so-called “lymphosarcomas” occurred mainly in axillary, iliac, and inguinal regions. These observations were confirmed in another study by Mauch et al.7 Interestingly, the authors found different patterns of presentation depending on the subtype of HL. Patients with the HL lymphocyte predominant (nodular lymphocyte predominant Hodgkin lymphoma [NLPHL]) subtype were less likely than patients with nodular sclerosis (NS) to have mediastinal involvement, and their main sites of involvement were cervical and inguinal. Few studies have analyzed the distribution of distinct NHL types. The time to diagnosis could be shortened by focusing on the most frequent lymphomas based on nodal site and patient characteristics. Moreover, because the most frequent sites of involvement are most likely the sites of origin for each lymphoma, this information may provide indirect clues for the pathogenesis and etiology of each type of lymphoma. The aim of this study was to assess the prevalence of common NHLs and HLs subtypes at each nodal site of involvement. We selected lymph nodes or other sites used to make the diagnosis, because these are clinically relevant (revelation of the tumor) and are generally the largest found and thus likely to be located in one of the primary sites of the disease.
MATERIALS AND METHODS
Study Design
We conducted an observational, retrospective case study based on a single hospital.
Data Collection
We reviewed the medical records of all cases of primary NHL and HL diagnosed in the Pathology Department of Purpan Hospital, Toulouse, France, from January 2001 to June 2008. Classification was made according to the 4th edition of the World Health Organisation (WHO) classification of tumors of hematopoietic and lymphoid tissues.1 In total, 1949 cases were reviewed using the following inclusion criteria: histologically documented primary diagnosis of any subtype of HL or of a common NHL (follicular lymphoma [FL], diffuse large B-cell lymphoma (DLBCL), T-cell rich DLBCL, Burkitt lymphoma (BL), mantle cell lymphoma, nodal marginal zone B-cell lymphoma, B-cell prolymphocytic lymphoma, anaplastic large B-cell lymphoma, chronic lymphocytic leukemia, lymphoplasmocytic lymphoma, angioimmunoblastic T-cell lymphoma (TCL), T-lymphoblastic lymphoma, and anaplastic large TCL; and the presence of at least 1 histologically documented nodal or mediastinal involvement. Cases with nodal involvement at an unspecified location were excluded. For cases with mediastinal involvement, we included the mediastinum as a site of involvement even when nodal involvement was not histologically certain because it is often difficult or impossible to distinguish between a nodal or adjacent tissue involvement (such as the thymus). There were 1105 cases with at least 1 nodal (1059) or mediastinal (56) site of involvement, and 167 cases were excluded from the study. Institutional ethical approval was obtained in compliance with the Helsinki protocol.
Sample Size
Because the aim of our study was primarily descriptive, we did not estimate a required sample size. However, based on the prevalence of NHL and HL in the general population, an a priori computation of the statistical power of our study was performed. The precision was estimated to be ±5% to 10% for the prevalence of common NHLs and HL subtypes in the most frequent nodal sites of involvement.
Documented Variables
For each case, demographic data (sex and age), diagnosis, Epstein-Barr virus (EBV) status (based on LMP1 immunostaining and/or EBER probe for HL cases), nodal involvement sites, number of nodal involvement sites, and presence of extranodal involvement sites were recorded. The nodal site of involvement was defined as the nodal site at the time of diagnosis that allowed the histological diagnosis. For the analysis, age was grouped into 3 classes: under 14-years old, between 15 and 59-years old, and above 60-years old. Indeed, NHL can affect any age group but mostly occurs in older patients. However, some NHLs (such as mediastinal B-cell lymphoma) and HL also primarily affect younger adults and children.1
Statistical Analysis
In our descriptive analysis, frequencies are presented as counts (n) and percentages (%), whereas quantitative variables are presented as arithmetic means (m) and standard deviations (SD). For each parameter, the 95% confidence interval (95% CI) was calculated. Prevalences were analyzed according to 2 perspectives: first, the prevalence of common NHLs and subtypes of HL by nodal involvement; second, the prevalence of nodal involvement sites by lymphoma. Univariate analyses were conducted to compare the prevalence of common NHLs and subtypes of HL by nodal involvement site using the chi-square test or Fisher exact test (when required). This association between nodal site and lymphoma subtypes was estimated using logistic regression, where the odds ratio (OR) was adjusted odds ratio (aOR) for sex and age. The uncorrected P-values are presented. Additionally, due to the large number of tested null hypotheses, P-value correction for multiple tests was performed using the Benjamini and Hochberg8–10 false discovery rate under general dependence for each site of involvement, where the false discovery rate was kept at 5%.
To assess the potential bias due to excluding data with unspecified locations, we compared the age, sex, and the prevalence of NHL and HL subtypes in the excluded group to the prevalence in our dataset.
Statistical significance was assessed at a P-value of 0.05 (2-tailed), and analyses were performed using STATA v9.1 (10).
RESULTS
Clinical Presentation
We reviewed 938 cases of NHL and HL. The patients were 60.1% male (95% CI: 56.9%–63.2%) and 39.9% female (95% CI: 36.8%–43.1%). The average age was 53.5 years (SD:±21.1) for men and 57.5 years (±21.8) for women. Among all patients, 44% (40.1%–47.3%) had B-cell lymphoma, 46.4% (95% CI: 43%–49.5%) had HL, and 7.8% (95% CI: 6.1%–9.7%) had TCL. The most common lymphomas were DLBCL, FL, and nodular sclerosis classical Hodgkin lymphoma (NSCHL) with a respective prevalence of 17.2% (95% CI: 14.8%–19.7%), 14.1% (95% CI: 11.9%–16.5%), and 21.8% (95% CI: 19.1%–24.5%), respectively (Table 1). Among HL cases, 32% (95% CI: 37.3%–27.6%) were positive for EBV, and there was an increased association with mixed cellularity Hodgkin lymphoma and a decreased association with NLPHL (64.7% and 3.2%, respectively, P < 0.0001).
TABLE 1.
Distribution of NHL and HL
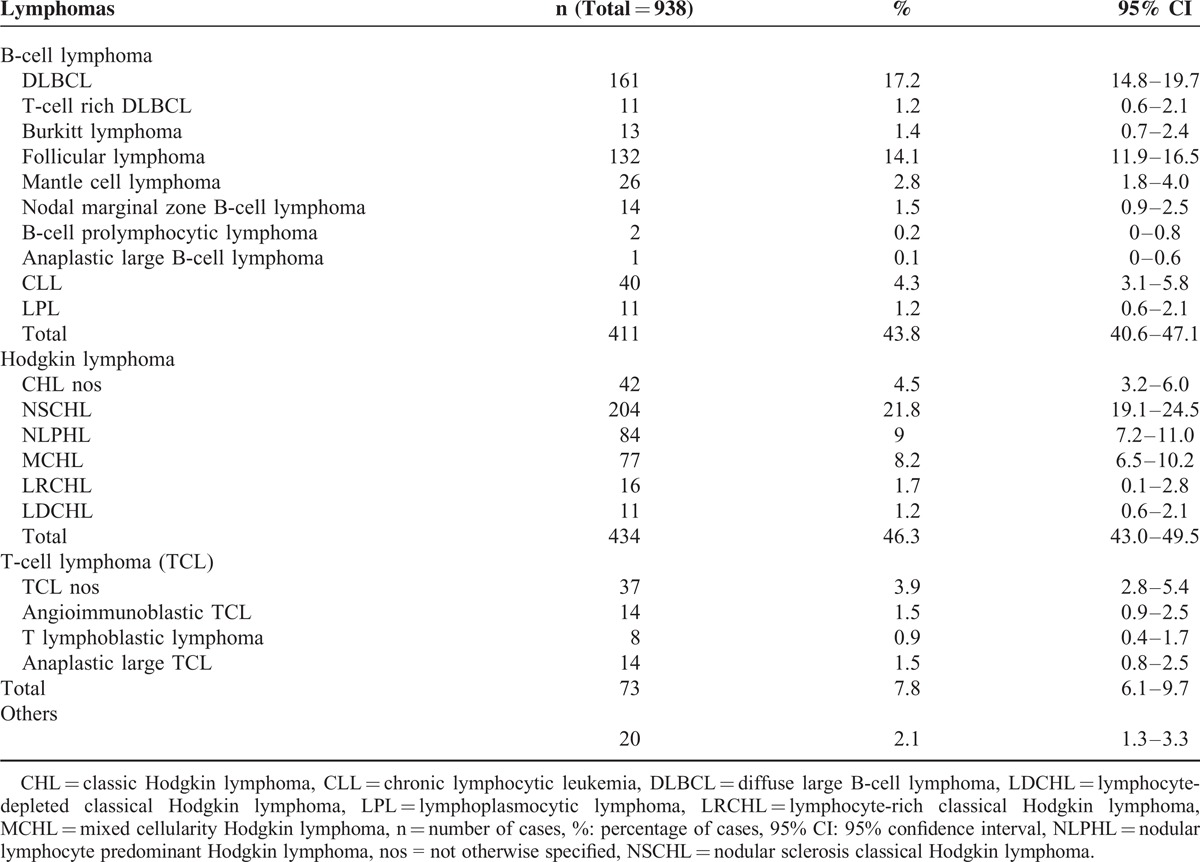
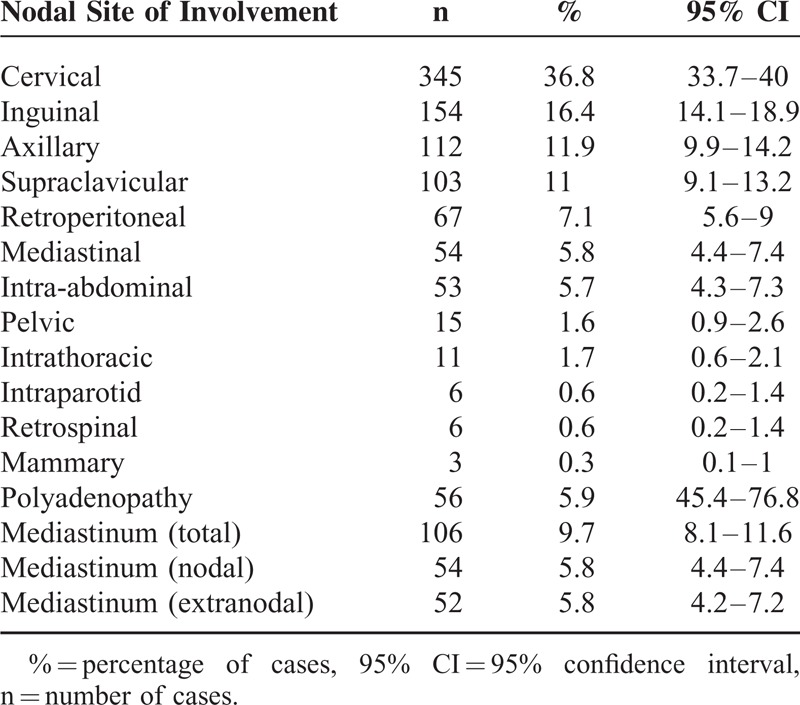
Table 2 summarizes NHL and HL sites of involvement. The most frequently involved sites were cervical lymph nodes (36.8% of all cases, 95% CI: 33.7%–40%), inguinal lymph nodes (16.4%, 95% CI: 14.1%–18.9%), axillary lymph nodes (11.9%, 95% CI: 9.9%–13.2%), and supraclavicular lymph nodes (11%, 95% CI: 9.1%–13.2%).
TABLE 2.
Distribution of Nodal and Mediastinal Sites of Involvement in NHL and HL
NHL and HL Prevalence by Nodal and Mediastinal Site of Involvement
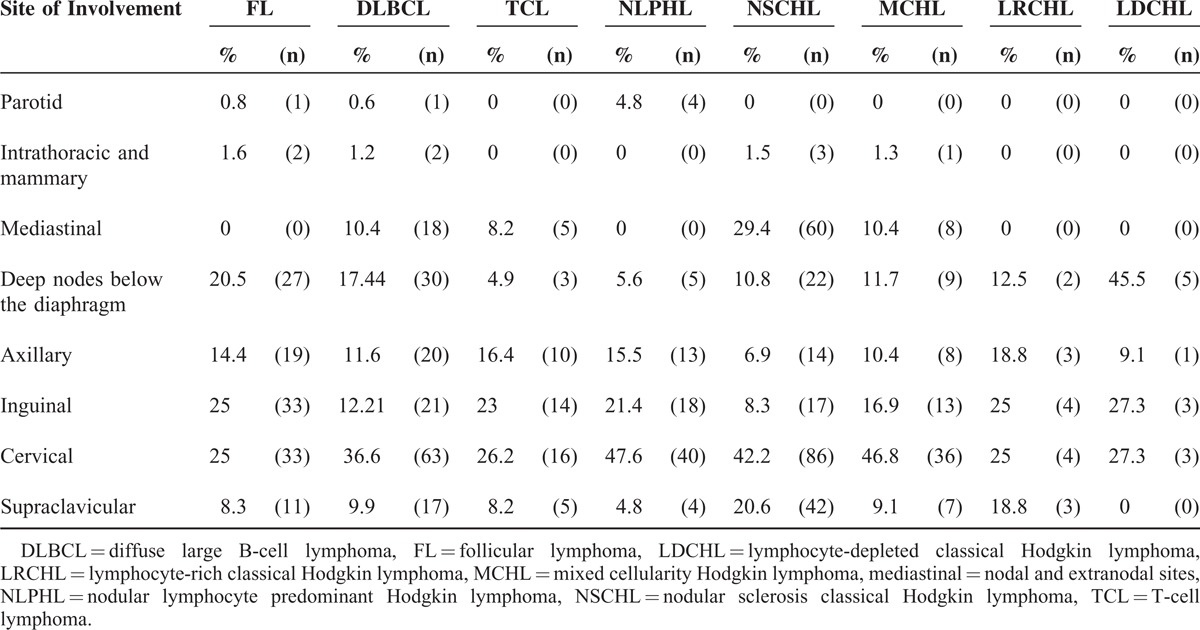
For each nodal site and the extranodal mediastinal site, we examined the distribution of NHL and HL and reported the most frequent lymphomas in Table 3. Some nodal sites had a high prevalence of certain subtypes of lymphoma. For example, mediastinal lymph nodes were 15 times more likely to be classical Hodgkin lymphoma (CHL) compared to other sites (OR: 15, 95% CI: 6.3–37.6, multiple test corrected P-value [c-P] = 0.0001). Similarly, supraclavicular nodes were more frequently involved in CHL (OR = 2.1, 95% CI: 1.4–3.2, c-P = 0.004) and TCL most often displayed polyadenopathy (OR = 4.6, 95% CI: 2.1–9.6, c-P = 0.0001).
TABLE 3.
Distribution of the Most Frequent NHL and HL by Nodal and Extranodal Sites of Involvement
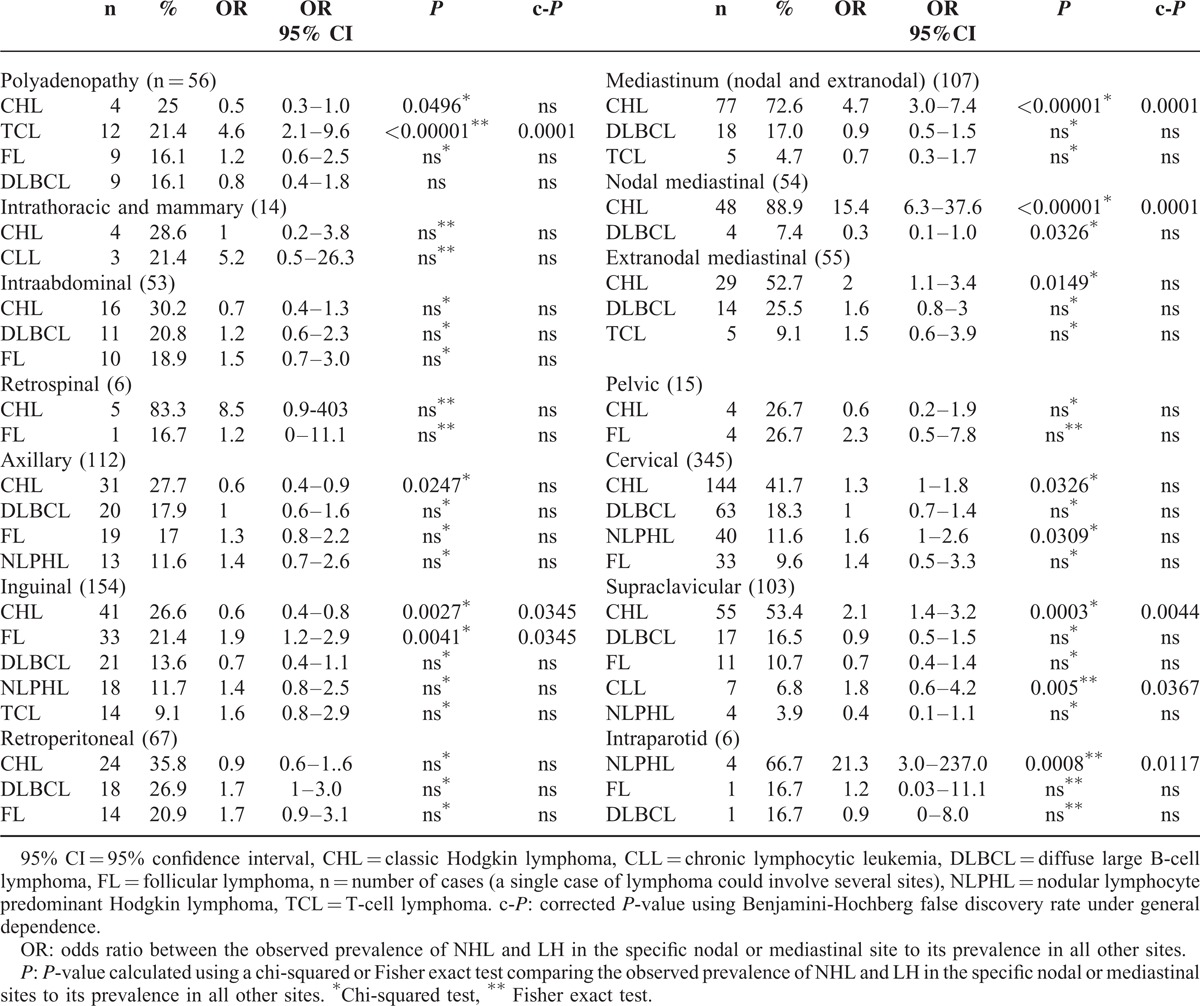
On the other hand, CHL was uncommon in inguinal nodes (OR = 0.6, 95% CI: 0.4–0.8, c-P = 0.034) (Table 3). These results are consistent with known associations, especially between CHL and cervical nodes and between supraclavicular nodes and nodal or extranodal mediastinal sites. However, we also found an unexpected but interesting and significant association of intraparotid nodes with NLPHL (OR = 21.3, 95% CI: 3–237, c-P = 0.011) and inguinal nodes with FL (OR = 1.9, 95% CI: 1.2–2.9, c-P = 0.0345).
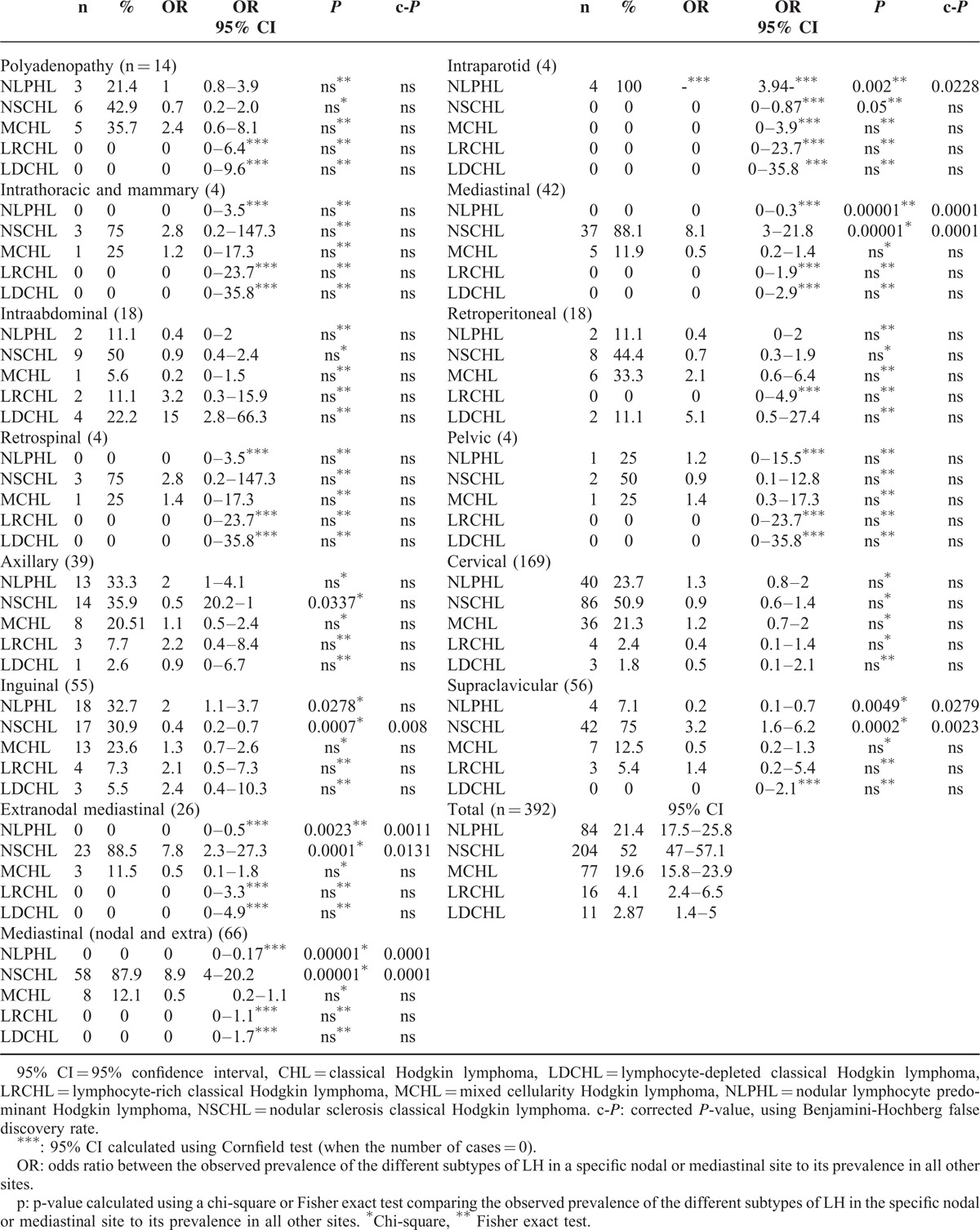
We also studied the distribution of HL subtypes among the 392 HL cases in which it was documented. We observed that mediastinal sites (nodal or extranodal) and supraclavicular nodes were more likely to be associated with NSCHL (OR = 8.9, 95% CI: 4–20.2, c-P = 0.0001 and OR = 3.2, 95% CI: 1.6–6.2, c-P = 0.002, respectively). Inguinal sites were associated with NLPHL (OR = 2, 95% CI: 1.1–3.7); however, this result was not significant after adjustments for multiple testing. NSCHL was found less frequently in inguinal nodes (OR = 0.4, 95% CI: 0.2–0.7, c-P = 0.008), whereas NLPHL was less frequent in mediastinal and supraclavicular sites (OR = 0, 95% CI: 0–0.17, c-P = 0.0001; OR = 0.2, 95% CI: 0.1–0.7, c-P = 0.03, respectively) (Table 4). Additionally, we did not find any association between EBV infection and the site of involvement (data not shown).
TABLE 4.
Distribution of Different Subtypes of HL by Site of Involvement
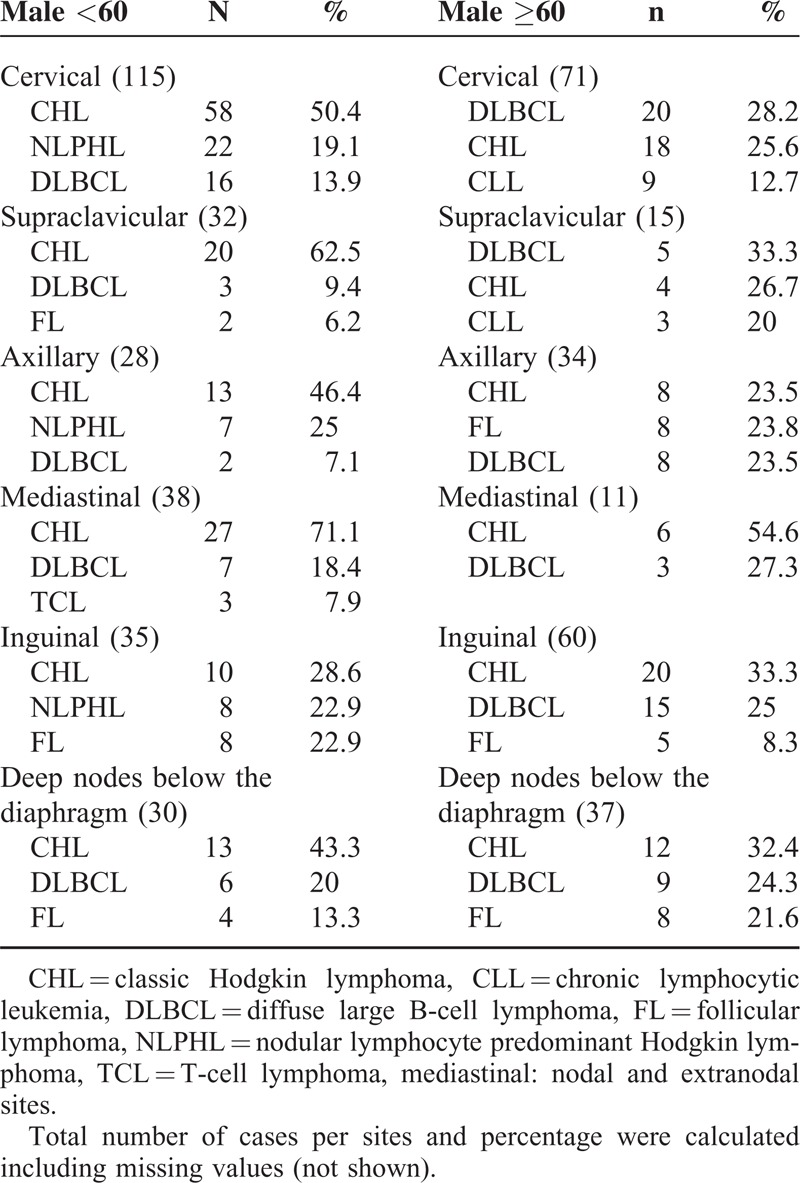
Some lymphomas occurred at different frequencies in males and females, and some were linked to age. Younger patients of both sexes with cervical or supraclavicular node involvement were most likely to have HL, whereas in older patients, the most common diagnosis was DLBCL. Furthermore, for male patients, especially younger ones, NLPHL was also common at these sites. Similarly, FL and NLPHL with inguinal node involvement were common diagnoses in younger patients irrespective of sex (Tables 5 and 6).
TABLE 5.
Distribution of Different Subtypes of HL by Site of Involvement
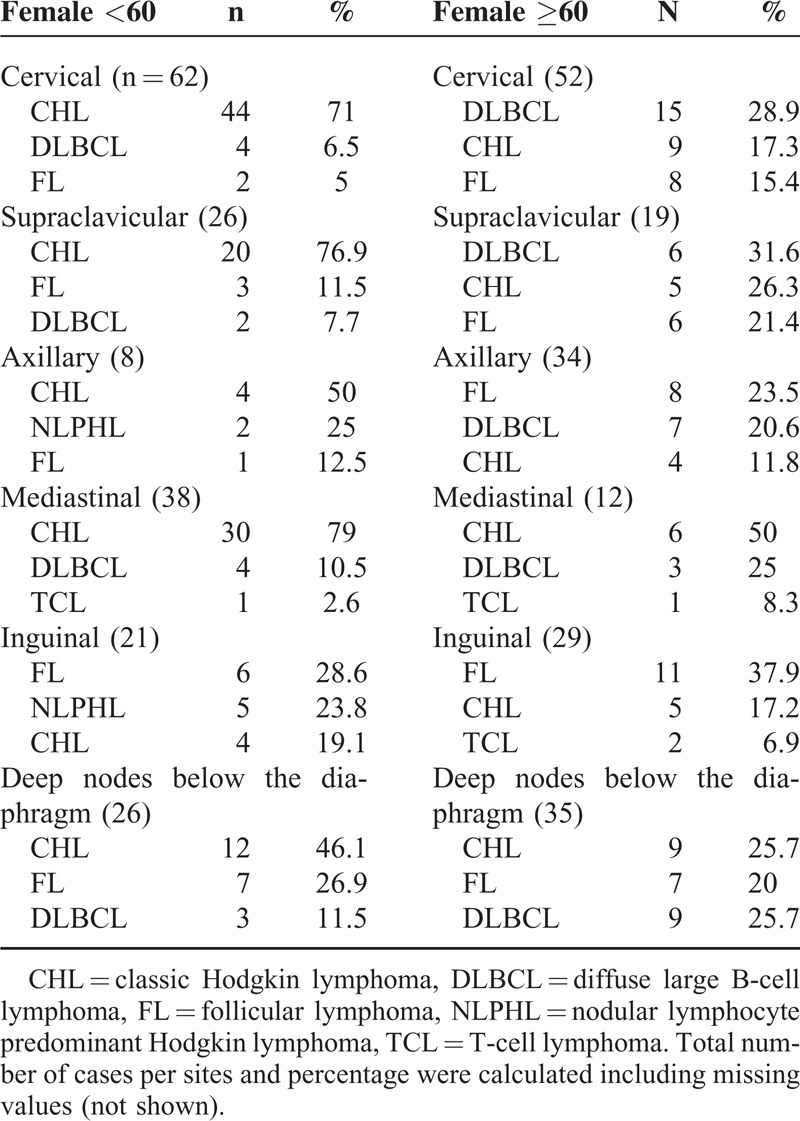
TABLE 6.
Adult Male (15 to 60-years old) Distribution of the Most Frequent NHL and HL by Site of Involvement and Age
Because age and sex might be confounding factors, we adjusted ORs for age and sex. After adjustment, the association between the following sites and diagnoses remained significant (Table 7): polyadenopathy and TCL (aOR: 3.8, [1.8–8.2]); inguinal nodes and FL (aOR: 1.7 [1.1–2.7]); inguinal nodes and CHL (aOR: 0.6, 95% CI: 0.4–0.96); mediastinal site and CHL (a-OR: 4, 95% CI: 2.5–6.4); supraclavicular nodes and CHL (aOR: 1.9, 95% CI: 1.2–3); intraparotid nodes and NLPHL (aOR: 19.1, 95% CI: 3.1–117.2). However, the aOR for the association between chronic lymphocytic leukemia (chronic lymphoid leukemia) and supraclavicular nodes was no more significant. With respect to HL, the aOR for the association between mediastinal site and NSCHL was 7.5 [3.4–16.3]; the aOR for supraclavicular node and NSCHL was 3.1 (95% CI: 1.6–6); and the aOR for supraclavicular node and NLPHL was 0.3 (95% CI: 0.09–0.7).
TABLE 7.
Nodal Sites of Involvement by Lymphoma Type
DISCUSSION
Our study shows that the distribution of various NHLs and subtypes of HL strongly depends on the specific nodal site of involvement. We reviewed 938 consecutive cases of NHL and HL and reported the nodal sites of involvement for each case. Previous studies have looked at sites of involvement in lymphoma,6,7,11–13 but may be limited by focusing on a specific lymphoma subtype or on a specific outcome. To our knowledge, none has systematically correlated these sites with common NHL and HL subtypes. Our descriptive study characterizes the distribution of lymphomas at each site and the preferential localization of each lymphoma. A limitation of this study is that not all lymph nodes may have been tested at diagnosis and no data on the disease stage was available. Consequently, we could not determine the site of origin of the tumor with certainty. However, we believe that the lymph nodes used to make the diagnosis are generally the largest found and are thus likely to be located in one of the primary sites of the disease. To assess the potential bias from excluding diagnoses with an unspecified location, we compared our sample with the excluded sample for age, sex, and lymphoma subtypes and did not find any significant differences (data not shown). Additionally, due to the retrospective design of our study, we were unable to adjust our results for other potentially confounding factors (other than age and sex), such as history of viral infection, that could influence the site of involvement. Despite these limitations, our results provide interesting clues for further investigation.
As lymphomas are heterogeneous, data on the preferential localization of each type will aid in early clinical diagnosis. For example, a lymphoma primarily associated with inguinal lymph nodes in a male under 60 is more frequently CHL or NLPHL compared with older males where it is more likely CHL. As the prognosis of these 2 subtypes of HL is quite different, rapid differential diagnosis is essential. Among females that are 60 years or older, the most frequent diagnosis associated with an inguinal primary site is FL, comprising about 37% of the cases, compared to 21% in the general population. Interestingly, FL is more frequent in inguinal sites among females (regardless of age) than among males. Likewise, an intraparotid lymphoma was diagnosed as NLPHL in 66% of cases, while the overall prevalence of this subtype was only 9%. These findings suggest that some lymphomas may have preferential primary sites of involvement.
As the causes of most lymphoid neoplasms remain unknown, analyses of these preferential nodal sites may provide clues to their etiology. Among potential environmental etiologies, the well-studied is infection.14–16 Infection may increase proliferation and decrease apoptosis of lymphoid cells and thus contribute to lymphocyte transformation. The role of EBV in HL pathogenesis is now well established.17–22 Furthermore, O’Grady et al23 showed that the prevalence of EBV in HL is related to the site of origin of the tumor and was higher in nodes draining tonsillar and oropharyngeal lymphoepithelial tissue. These findings are not surprizing because the oropharyngeal epithelium is the main site of EBV proliferation after infection, although latently infected B-cells in circulation also carry the virus and account for EBV-positive tumors in nonneck sites. Another study24 indicated that EBV-associated TCLs were highly site restricted, nasal TCLs were EBV-associated, whereas pulmonary and gastrointestinal TCLs were less frequently associated with EBV. Hence, TCLs arising from different sites but with similar morphology may show differences in lymphomagenesis.
Extending this concept, the predilection of lymphomas for specific sites of involvement might be consistent with certain tissue-specific infections. Two mechanisms are often proposed to explain how infection can induce lymphoma. First, some viral agents, such as EBV and human herpes virus 8 (HHV8),25,26 are able to directly infect and transform lymphocytes due to the expression of oncogenes. Second, other infections may induce chronic immune stimulation, including the persistent activation of lymphocytes, which may increase the risk of lymphoma. This model of antigen-driven lymphoproliferation has been described particularly in infection-associated lymphomas derived from marginal zone B cells27 the best example being gastric mucosa-associated lymphatic tissue lymphoma associated with helicobacter pylori. The presence of preferential primary nodal sites of involvement is consistent with a chronic site-specific infection, a persisting antigen or an unknown lymphotropic-transforming pathogen that might be at play in the process of lymphomagenesis.
In conclusion, our study shows that some lymph node sites have a disproportionate prevalence of specific subtypes of lymphoma. Identifying these sites may aid to diagnose and better elucidate the pathogenesis of these tumors. In particular, these nodal areas may represent specific entry sites for putative triggering antigens.
Acknowledgements
The authors thank Institut Universitaire de France (PB) and Labex TOUCAN and the CAPTOR consortium for the support.
Footnotes
Abbreviations: aOR = adjusted odds ratio, CHL = classical Hodgkin lymphoma, CLL = chronic lymphocytic leukemia, DLBCL = diffuse large B-cell lymphoma, EBV = Epstein-Barr virus, FL = follicular lymphoma, HL = Hodgkin lymphoma, IC = confidence interval, NHL = non-Hodgkin lymphoma, NLPHL = nodular lymphocyte predominant Hodgkin lymphoma, NSCHL = nodular sclerosis Hodgkin lymphoma, TCL = T-cell lymphoma.
CL and CD contributed equally to this work.
CL, GRP, SV, and PB collected and reviewed the cases. CL, CD, and PAG analyzed data. CD, CL, and PB designed and supervised the study. CL, CD, SV, and PB wrote the manuscript.
This work was supported by Institut Universitaire de France (PB), and also supported by grants from the Labex TOUCAN and the CAPTOR consortium (CL and PB).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Swerdlow SH, Harris NL, Jaffe ES, et al. Tumors of Hematopoeitic and Lymphoid Tissues. Lyon: IARC Press; 2008. [Google Scholar]
- 2.Weber AL, Rahemtullah A, Ferry JA. Hodgkin and non-Hodgkin lymphoma of the head and neck: clinical, pathologic, and imaging evaluation. Neuroimaging Clin North Am 2003; 13:371–392. [DOI] [PubMed] [Google Scholar]
- 3.Olu-Eddo AN, Omoti CE. Hodgkin lymphoma: clinicopathologic features in Benin City, Nigeria and update on its biology and classification. Nigerian J Clin Pract 2011; 14:440–444. [DOI] [PubMed] [Google Scholar]
- 4.Oliapuram Jose B, Koerner P, Bertolone S, et al. Pediatric Hodgkin's disease. J Kentucky Med Assoc 2004; 102:104–106. [PubMed] [Google Scholar]
- 5.Horning SJ. Hodgkin's lymphoma. In: Lichtman MA, Kipps TJ, Kaushansky, et al. Williams Hematology. New York: Mc Graw Hill; 2006:1461–1482. [Google Scholar]
- 6.Banfi A, Bonadonna G, Carnevali G, et al. Malignant lymphomas: further studies on their preferential sites of involvement and possible mode of spread. Lymphology 1969; 2:130–138. [PubMed] [Google Scholar]
- 7.Mauch PM, Kalish LA, Kadin M, et al. Patterns of presentation of Hodgkin disease. Implications for etiology and pathogenesis. Cancer 1993; 71:2062–2071. [DOI] [PubMed] [Google Scholar]
- 8.Benjamini Y, Drai D, Elmer G, et al. Controlling the false discovery rate in behavior genetics research. Behav Brain Res 2001; 125:279–284. [DOI] [PubMed] [Google Scholar]
- 9.Hochberg Y, Benjamini Y. More powerful procedures for multiple significance testing. Stat Med 1990; 9:811–818. [DOI] [PubMed] [Google Scholar]
- 10.Binquet C, Verret C, Chene G, et al. Major statistical software usable in epidemiology. Rev Epidemiol Sante publique 1998; 46:329–336. [PubMed] [Google Scholar]
- 11.Banfi A, Bonadonna G, Carnevali G, et al. Lymphoreticular sarcomas with primary involvement of Waldeyer's ring. Clinical evaluation of 225 cases. Cancer 1970; 26:341–351. [DOI] [PubMed] [Google Scholar]
- 12.Banfi A, Bonadonna G, Carnevali G, et al. Preferential sites of involvement and spread in maignant lymphomas. Eur J Cancer 1968; 4:319–324. [DOI] [PubMed] [Google Scholar]
- 13.Bonadonna G, Banfi A, Carnevali G, et al. Preferential sites and methods of diffusion of malignant lymph nodes studied on the basis of 500 cases. Tumori 1967; 53:551–564. [DOI] [PubMed] [Google Scholar]
- 14.Engels EA. Infectious agents as causes of non-Hodgkin lymphoma. Cancer Epidemiol Biomarkers Prevent 2007; 16:401–404. [DOI] [PubMed] [Google Scholar]
- 15.Hennessy BT, Hanrahan EO, Daly PA. Non-Hodgkin lymphoma: an update. Lancet Oncol 2004; 5:341–353. [DOI] [PubMed] [Google Scholar]
- 16.Hjalgrim H, Engels EA. Infectious aetiology of Hodgkin and non-Hodgkin lymphomas: a review of the epidemiological evidence. J Intern Med 2008; 264:537–548. [DOI] [PubMed] [Google Scholar]
- 17.Armstrong AA, Weiss LM, Gallagher A, et al. Criteria for the definition of Epstein-Barr virus association in Hodgkin's disease. Leukemia 1992; 6:869–874. [PubMed] [Google Scholar]
- 18.Brousset P, Chittal S, Schlaifer D, et al. Detection of Epstein-Barr virus messenger RNA in Reed-Sternberg cells of Hodgkin's disease by in situ hybridization with biotinylated probes on specially processed modified acetone methyl benzoate xylene (ModAMeX) sections. Blood 1991; 77:1781–1786. [PubMed] [Google Scholar]
- 19.Jarrett RF, Gallagher A, Jones DB, et al. Detection of Epstein-Barr virus genomes in Hodgkin's disease: relation to age. J Clin Pathol 1991; 44:844–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.MacMahon B. Epidemiology of Hodgkin's disease. Cancer Res 1966; 26:1189–1201. [PubMed] [Google Scholar]
- 21.Macmahon B. Epidemiological evidence of the nature of Hodgkin's disease. Cancer 1957; 10:1045–1054. [DOI] [PubMed] [Google Scholar]
- 22.Pallesen G, Hamilton-Dutoit SJ, Rowe M, et al. Expression of Epstein-Barr virus latent gene products in tumour cells of Hodgkin's disease. Lancet 1991; 337:320–322. [DOI] [PubMed] [Google Scholar]
- 23.O’Grady J, Stewart S, Elton RA, et al. Epstein-Barr virus in Hodgkin's disease and site of origin of tumour. Lancet 1994; 343:265–266. [DOI] [PubMed] [Google Scholar]
- 24.de Bruin PC, Jiwa M, Oudejans JJ, et al. Presence of Epstein-Barr virus in extranodal T-cell lymphomas: differences in relation to site. Blood 1994; 83:1612–1618. [PubMed] [Google Scholar]
- 25.Cesarman E, Chang Y, Moore PS, et al. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. New Engl J Med 1995; 332:1186–1191. [DOI] [PubMed] [Google Scholar]
- 26.Oksenhendler E, Boulanger E, Galicier L, et al. High incidence of Kaposi sarcoma-associated herpesvirus-related non-Hodgkin lymphoma in patients with HIV infection and multicentric Castleman disease. Blood 2002; 99:2331–2336. [DOI] [PubMed] [Google Scholar]
- 27.Suarez F, Lortholary O, Hermine O, et al. Infection-associated lymphomas derived from marginal zone B cells: a model of antigen-driven lymphoproliferation. Blood 2006; 107:3034–3044. [DOI] [PubMed] [Google Scholar]


