Abstract
Body Mass Index is a commonly used but likely inexact measure of body composition for patients with end-stage liver disease. For this reason, we examined whether body composition measurements from direct visualization on computerized tomography (CT) scans provide new insights both into the degree of malnutrition and also discordant combinations such as obesity with muscle mass loss. This technology is widely used in other medically ill populations but not yet in liver transplantation.
Methods
We examined actual body composition using abdominal CT scan data and software designed to measure fat and muscle compartments.
Results
In 234 liver transplant candidates we found BMI was highly and significantly correlated to subcutaneous and visceral fat. However we additionally found that even among obese patients, cachexia, as defined by muscle mass, was common with 56% of those with BMIs over 30 being cachexic. We also found that patients with non-alcoholic steatohepatitis, compared to other types of liver diseases, were significantly more likely to have larger amounts of visceral fat while also having less muscle. In an exploratory analysis muscle mass corrected for height was a significant predictor of post-transplant survival.
Conclusions
Body composition by CT scan data provides a specific method to identify obesity and muscle wasting for end-stage liver disease patients. Whether these data can aid in the prognostication of outcomes and survival requires further investigation.
Keywords: radiologic assessment, assessment liver transplant candidates, body composition, cachexia
Body composition analyses from radiographic imaging, including the determination of fat and muscle compartments, is widely employed in studies of medical illness such as cancer, obesity, and diabetes (1, 2, 3, 4,) as well as healthy individuals (e.g. exercise physiology) (5). As radiological imaging provides direct visualization of body and tissue compartments, its use in body composition and nutritional assessment is specifically valuable for medical conditions in which traditional measures of nutrition (biochemical markers, weight, or anthropomorphic measurements) have proven less accurate (6). For example, in medically ill populations computerized tomography (CT) scanning provides an exact measure of muscle mass and is proven more valid than externally measured muscle circumference (7). These indications for radiological assessment may be especially applicable to patients with end stage liver disease for whom protein energy malnutrition and muscle wasting is common but whose body mass index (BMI), body weight, or physical body measurements may be inflated due to fluid retention.
Concurrently in the population of patients with end-stage liver disease we have observed the rising epidemic of obesity leading to more patients with high BMIs as well as more patients with non-alcoholic steatohepatitis (NASH or fatty liver disease) being evaluated for liver transplantation (LTX). For example, over the past 10 years the number of adult LTX recipients with NASH has risen 26 fold in the U.S. making it one of the more common single diagnoses for LTX (8). These seemingly incongruous conditions, malnutrition and obesity, create the need for greater accuracy in determining body composition in patients with end-stage liver disease. Additionally it is conceivable that a patient with obesity may not be identified as being nutritionally depleted or as having low muscle mass unless their internal anatomy was examined. Given these issues we decided to examine the application of CT technology in body composition analyses to a cohort of patient with end-stage liver disease who were being evaluated for LTX. Using a standardized cross section of the body commonly examined in body composition studies we compared the readings of specific body compartments of interest to the patients’ computed BMIs and biochemical nutritional markers to determine the degree to which these measures were associated. Using this technique we additionally examined whether a diagnosis of NASH compared to other liver diseases was significantly associated with differences in body fat and muscle composition. In an exploratory analysis we examine the predictive value of these measurements on post-transplant survival. Finally because this is a newer application of an emerging technology we suggest ways in which such detailed data may provide essential information about medical co-morbidity and a potential means to prognosticate about a variety of post-transplant outcomes.
Results
Cohort demographics
Table 1 shows the demographic characteristics of our total cohort. Patients were predominantly Caucasian me, with hepatitis B or C cirrhosis (HCV/HBV) (25%), alcoholic liver disease (ALD) (24%) or both (12%). Based on the patients BMI, 31% were obese or morbidly obese (BMI ≥ 30) regardless of their primary liver diagnosis. Only 1% were underweight (BMI < 18.5).
Table 1.
Demographic, Medical Characteristics, and Body Composition with Gender Comparisons
| Total | Male (n=157) |
Female (n=77) |
Test statistic, p value |
|
|---|---|---|---|---|
| Demographics | ||||
| Age, M (SD) | 55 (9.6) | 55 (10) | 56 (10) | ns |
| Race, % white | 93 | 91 | 100 | 0.003 |
| Medical Variables | ||||
| Hepatitis C infection, % yes | 35 | 43 | 21 | 0.001 |
| MELD score, M (SD) | 21 (8.7) | 21 (8.6) | 21 (8.6) | ns |
| Albumin, M (SD) | 3.0 (0.6) | 3.1 (0.6) | 2.9 (0.5) | ns |
| Creatinine, M (SD) | 1.5 (1.3) | 1.5 (1.4) | 1.4 (1.2) | ns |
| Liver Disease Diagnosis % | ||||
| HCV/HBV | 25 | 29 | 17 | |
| Alcohol | 24 | 28 | 14 | |
| Alcohol and HCV/HBV | 12 | 17 | 1 | |
| NASH | 12 | 8 | 20 | |
| Autoimmune/PSC/PBC | 11 | 6 | 21 | |
| Fulminant failure | 5 | 1 | 13 | |
| All others | 11 | 10 | 14 | |
| Body Composition - mean (SD) | ||||
| BMI | 28.1 (5.5) | 28 (5) | 28 (7) | ns |
| % Obese BMI ≥30 | 32 | 64 | 37 | ns |
| Height cm | 173 (10) | 178 (7) | 163 (7) | 214, <.001 |
| Weight kg | 84 (18) | 89 (16) | 76 (20) | 26, <.001 |
| Visceral Fat cm2 | 152 (104) | 160 (100) | 136 (111) | 4, .048 |
| Subcutaneous Fat cm2 | 217 (128) | 208 (116) | 236 (149) | ns |
| Total Fat cm2 | 369 (198) | 368 (189) | 372 (214) | ns |
| Total Muscle cm2 | 130 (34) | 146 (28) | 99 (19) | 161, <.001 |
| Muscle cm2/height m2 | 43 (10) | 46 (9) | 38(8) | 46, <.001 |
Body Compartment Assessments and Gender Comparisons
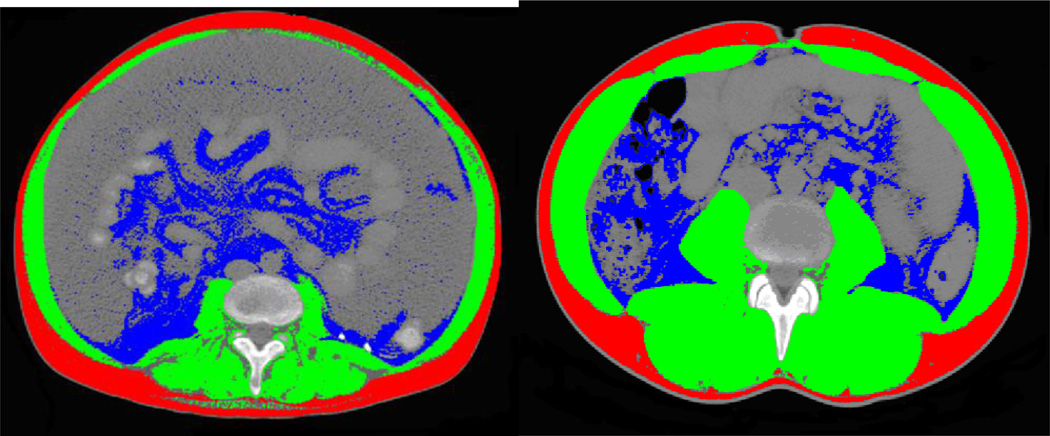
Figure 1 shows examples of how the radiological scans appear for the body composition analyses with the demarcation of the specific body compartments. As expected we found gender differences with respect to body composition (Table 1). While BMI mean scores were not different, men had more visceral fat while women had significantly less muscle.
Figure 1. Examples of Body Composition Analysis at L3–4 Transverse Section.
red is subcutaneous fat, blue is visceral fat and green is muscle.
BMI, Body Composition and Conventional Nutritional Comparisons
Controlling for age, height, gender and race, BMI was moderately to strongly correlated with visceral fat (r = 0.53, p<0.001) and subcutaneous fat (r = 0.77, p<0.001) but only slightly correlated with muscle (r = 0.27, p<0.001). Total muscle was slightly correlated with subcutaneous (r = 0.17, p=0.01) and visceral fat (r = 0.15, p=0.02). Controlling for the amount of ascites (large vs. other) did not improve these correlations. Interestingly, we found that patients with BMIs in the obese range were significantly less likely to have large amounts of ascites (χ2 =9.8, p=0.007).
Other biochemical variables associated with nutritional status were considered with body composition. The serum protein was modestly and significantly associated with total muscle (r = 0.2, p=0.01). Creatinine was mildly negatively correlated to total muscle (r= −0.13, p=0.045). Albumin was not associated with any body compartment.
BMI, Body Composition and Cachexia
By the established definitions of cachexia used for cancer patients which are based on the muscle mass, nearly 70% of the cohort was categorized as cachectic. Interestingly while women were nearly evenly split between cachectic/non-cachectic groups, men were significantly more likely to be cachectic (76% of men, χ2 =11.6, p=0.001). Examining specific thresholds of BMI, we found even in the obese category (BMI ≥30) 56 % were cachectic (Table 2).
Table 2.
BMI by Standardized Categories
| BMI Category | Frequency Total Cohort |
Percent Total Cohort |
% Cachetic | % Large Ascites* |
|---|---|---|---|---|
| Below 18.5 underweight | 2 | 1 | 100 | 0 |
| 18.5 to 24.9 normal weight | 71 | 30 | 79 | 20 |
| 25 to 29.9 overweight | 87 | 37 | 71 | 23 |
| 30 to 39.9 obese | 65 | 28 | 57 | 6 |
| ≥40 morbid obesity | 9 | 4 | 56 | 0 |
p=0.026 Fisher’s Exact test
Liver Disease Comparisons
Table 3 indicates that patients with NASH did not have higher mean BMIs when compared to patients with other types of liver disease. However, compared to the other groups those with NASH had significantly more visceral fat and the lowest mean value for total muscle.
Table 3.
Comparisons Between Different Types of Liver Diseases Controlling for Gender, Age, and Race
| Liver Diseases | |||||||
|---|---|---|---|---|---|---|---|
| Body Composition M (SD) |
NASH | Alcohol | Alcohol and HCV/HBV |
HBV/HCV | Autoimmune | All Others |
Test Statistic, p value |
| BMI | 30 (6) | 27 (5) | 27 (5) | 29 (6.1) | 29 (6) | 29 (5) | ns |
| Visceral Fat* | 183 (100) | 150 (95) | 116 (78) | 148 (100) | 133 (81) | 117 (120) | 5.8, 0.001 |
| Subcutaneous Fat | 236 (116) | 186 (111) | 192 (125) | 235 (145) | 211 (145) | 241 (107) | ns |
| Total Fat | 419 (190) | 339(176) | 309 (178) | 382 (206) | 345 (211) | 418 (190) | ns |
| Total Muscle | 118 (36) | 132 (31) | 147 (26) | 140 (33) | 115 (35) | 125 (30) | 55, 0.001 |
Square root transformed for analyses due to skewness – untransformed means presented for clarity
Exploratory Analyses of Survival
When examining the impact of body composition on outcomes for the total sample (n=234) we found that BMI was not associated with survival nor were any of the fat compartments (subcutaneous or visceral fat). However controlling for age, gender, race, and pre-transplant MELD score the muscle mass corrected for height was significantly associated with survival post-transplantation (full model χ2 = 14.25, p=0.014)(Table 4).
Table 4.
Muscle Predicting Survival Following Transplantation
| Variable | Hazard Ratio | p value | Confidence Interval |
|---|---|---|---|
| Muscle* | 0.97 | 0.04 | 0.94 – 0.99 |
| Gender | 1.30 | 0.34 | 0.75 – 2.25 |
| Age | 1.02 | 0.07 | 0.99 – 1.0 |
| Race | 0.92 | 0.92 | 0.33 – 2.67 |
| MELD score | 1.03 | 0.02 | 1.00–1.06 |
Muscle corrected for height
Conclusions
The determination of protein energy malnutrition can be inaccurate using biologic indicators of nutritional status. For example in moderate to advanced chronic renal disease, urinary creatinine, as a surrogate of muscle mass, has been shown not covary with weight, BMI or anthropomorphic measures of muscle mass (14). Additionally examining actual body composition can reveal discordant combinations that may not be identified by serum markers alone (e.g. large BMI with low muscle mass). Such incongruent combinations can provide new and unexpected insights into outcomes (15). Our data demonstrate how body composition by radiological imaging could bring such insights to light for LTX candidates.
We determined BMI is strongly correlated with visceral and subcutaneous fat visualized on transverse section. This suggests despite concerns of overinflated BMI ratings due to ascites in advanced liver disease, BMI closely reflects the true amount of body fat.. McHugh et al. created an equation to estimate the dry weight of liver disease patients using the grade of ascites and CT body composition analyses. They determined on average those with large ascites had body weights that were inflated only by 8–9 pounds (McHugh 2010). Thus while some individuals may have excessive fluid contributing to their weight and calculated BMI, higher BMIs are strongly associated body compositions that contain substantial subcutaneous and/or visceral fat. Additionally we found those at the highest BMIs were the least likely to have large amounts of ascites.
Although patients with NASH on average had high amounts of total fat, they significantly differed from other types of liver diseases by the location of the fat in the viscera. Additionally that NASH patients tended to have more visceral fat and less muscle could have important implications for overall strength such that post-LTX physical rehabilitation may be slower.
Most importantly we discovered that over 50% of those with BMIs in the obese range were actually cachectic on CT body composition analysis. This percentage is similar to another study of liver transplant candidates which identified 40% of their sample with sarcopenia (Montano-Loza 2012). Additionally nutritional markers commonly used to identify malnutrition (albumin, protein and creatinine) were not associated with BMI or cachexia. Only creatinine was slightly correlated to muscle mass. Thus commonly used nutritional parameters did not reflect the degree of malnutrition and muscle wasting in our patients.
A case-controlled study demonstrated that overweight LTX patients (BMI >27) have longer hospital stays and obese patients (BMI >31) additionally have higher perioperative complication rates (p=0.01), as well as more respiratory (p=0.009) and vascular complications (p=0.04) following transplantation (16). Additionally, in obesity fat can also be distributed within the muscle tissue making it less functional and, as we have identified, obese patients may be deficient in muscle mass. Although we did not examine the infiltration of muscle tissue with fat, body composition technology is advancing to be able to caculate the degree of fat within muscle tissue.
Furthermore there may be combinations of body composition that may be especially disadvantageous, for example those who are both obese and have significant muscle loss. A phenomenon of sarcopenic obesity (decreased muscle mass in obese patients) can be determined through CT scan data (4) and is associated with poorer survival during chemotherapy for pancreatic cancer (4) and increased morbidity/mortality in geriatric populations (18).
Finally, although only exploratory, we found muscle mass was associated with post-transplant survival whereas BMI and the amount of fat were not. One study examined the relationship of BMI with post-LTX patient and graft survival using information on nearly 27,000 LTX recipients from UNOS database and found that those at the extremes of BMIs <19 and ≥40 had an increased likelihood of death even after controlling for comorbidities (17). Contrary to our expectations only 1% of our cohort were underweight (BMI < 18.5), while 5% had BMIs ≥ 40.
Limitations
We did not have methods to directly measure total ascites, peripheral edema or overall fluid retention. However we were able to comment on the relative amount of ascites based on the full abdominal/pelvic CT scan data and controlled for the amount of ascites in our analyses. Other levels of transverse section could be used. However the L3–4 transverse section is the standard section for body composition analyses and will allow future comparisons of our data to a growing literature on body composition in other types of medical illnesses.
Future Directions
The ability to measure compartments of fat and muscle provides a more exact way to assess the specific contribution of fat or malnourishment to outcomes. In one study the cross-sectional measurement of a single muscle group, the psoas muscle, generated from routine abdominal CT scans was used to identify sarcopenia in LTX patients (19). Englesbe et al. found this determination of sarcopenia was highly and significantly correlated with post-LTX survival after controlling for other commonly considered medical covariates (19). They introduced the concept of using CT scan data in LTX patients to measure cachexia and recommended further studies to examine its measurement and impact on LTX outcomes (19). Another study using CT scan data and the same thresholds for cachexia identified that patients with cirhorris, some wait listed for LTX, had significantly poor survival if cachetic (HR 2.2, p=.008) (Montano-Loza 2012). We believe an examination of total body composition, both muscle and fat, provides a more comprehensive assessment of the individuals robustness or conversely frailty. Such detailed information could provide important characteristics to determine the physical robustness for the rigors of LTX surgery and long-term recovery. As abdominal CT scans are routinely performed during the pre-LTX evaluation phase the quantification of body tissues by this technology could be easily determined without additional scanning.
In the future as the field of body composition by CT scan data evolves to examine ratios of body compartments (such as the estimation of sacropenic obesity), and other techniques are developed, these methods could be applied to the scans of LTX candidates. Morbid obesity remains a significant problem in patients with ESLD and this study only begins to elucidate the potential role of body composition analyses as a refined assessment for body composition in patients with high BMIs.
Ultimately if these factors provide useful information in the prognostication of LTX recipient outcomes, then pre-LTX interventions to increase muscle and decrease fat could be developed. A recent review of the literature on exercise capacity in patients with cirrhosis identified two studies where pre-transplant exercise capacity predicted post-transplant survival and two other studies suggesting exercise training was well tolerated in patients with cirrhosis (Jones 2012). Also importantly for outcomes, obese LTX recipients can redevelop fatty liver disease and even cirrhosis < 1.5 years post-LTX (20, 21), and long term interventions to prevent further development or redevelopment of obesity post-LTX are also needed. For these reasons, we believe that body composition analyses by CT scan data can have wide reaching applications to transplant candidate assessment, preparation for, and even long-term survival after transplantation.
Methods
Study Sample
From January 2005 to December 2008 234 adult LTX candidates who were evaluated at the Starzl Transplant Institute underwent abdominal computerized tomography (CT) scans as part of their pre-transplant evaluation and these digital scans were archived in our electronic medical record system. Although we had evaluated nearly 550 adult patients during that time frame the other candidates’ data were not available for analyses because a digital scan was not available, patients underwent MRI instead of CT, or the abdominal scan did not reach the lumbar section required for these analyses.
Measures
Medical Records
During LTX evaluation patients have height/weight measurements and routine laboratory testing. Following an IRB approved protocol we retrieved the CT scan data and additional medical variables of interest from our electronic medical records (including demographic data, height/weight, creatinine, total protein, albumin, type of liver disease and Model for End-Stage Liver Disease - MELD -score). For the exploratory survival analyses we chose CT scan, laboratory data, and BMI calculations that were the closest to the date of LTX (median time of 74 days). From the radiologists determination of the amount of ascites observed in the abdomen or pelvis for each patient’s CT scan we rated them as one of three categories; none/trace/small, medium/moderate or massive/severe/large. For the analyses we examined large ascites vs. others. Dates of death post-LTX were also retrieved.
CT Scans
Radiographic CT scans are converted into digital images produced by the Stentor™ Picture Archive and Communications System that creates high quality, reliable data even better than film as CT technology is inherently digital. We chose a section of measurement commonly used to study medical illnesses, the L3–4 transverse section. A radiology technician retrieved the CT scan data and noted the transverse section closest to the L3–4 disc space based on a spinal scout film.
Body Composition Analyses
To perform the body composition analyses we used the SliceOmatic® software (by TomoVision Magog, Quebec, Canada) software developed for and used extensively by researchers in body composition who need accurate analysis of body composition. SliceOmatic®converts CT scan data into specific body compartments of interest. SliceOmatic® has powerful edge and line tracking tools to quickly allow outlining of tissue planes and uses mathematical morphology to perform tissue segmentation. In particular, specific tissues are demarcated using Hounsfield unit (HU) thresholds. We chose three compartments of interest; visceral fat, subcutaneous fat and total muscle. Adipose tissues were identified and quantified by using HU thresholds of −190 to −30 for both subcutaneous and visceral adipose (4, 9). Skeletal muscle was identified by HU thresholds of 0 to +100 (4, 10). These measurements were computed at the L3–4 transverse section by summing the tissue pixels and multiplying by pixels surface area. At the L3–4 transverse section muscle groups include rectus abdominis, pyramidalis, transversus abdominis, internal and external obliques, lattissimus dorsi, quadratus lumborum, psoas major and minor, and erector spinae (see Figure 1 for example of scan data and colorization demonstrating different body compartments). Cross sectional areas are reported in cm2. Additionally we computed the total fat as the sum of the visceral and subcutaneous fat. A research assistant trained to reliability in body composition analysis with the SliceOmatic® software using a training program designed by our body compartment analysis expert (BG) measured all of the body compartment data. A random sample of scans was re-computed by another expert and comparisons of body compartments achieved intraclass correlations of 0.94–0.97. Using the SliceOmatic® software the approximate time to generate the muscle, visceral and subcutaneous fat measurements at the L3–4 section is about 8 minutes.
Statistical Analyses
We examined descriptive data using estimates of central tendency (means, medians) and spread (standard deviation, range) for continuous data and frequencies and percentages for categorical data. BMI was computed from weight divided by height squared. Due to the differences between males and females on body size we examined gender differences. Comparisons between BMI and the variables of interest were made using bivariate partial correlations. In computing these correlations we controlled for age, height, gender and race because lean body mass declines with age and differs based on height, gender and race. We did separate analyses controlling for the categories of ascites determined on the abdominal CT scan to examine correlations between body compartments and BMI without the influence of the amount of ascites. Muscle mass determined on the CT scan L3–4 transverse section is linearly related to whole body muscle mass (11) and, as is conventional for BMI and other body composition analyses, was normalized for height using the ratio L3–4 skeletal muscle in cm2 divided by height in m2 and reported in units of cm2/m2. The height correction is necessary to determine relative muscle mass as muscle is highly correlated with height (Baumgarter 1988). In studies of cancer patients using L3–4 tranverse CT scan data established cutoffs for cachexia are ≤38.5 cm2/m2 for women and ≤52.4 cm2/m2 for men (12, 13). In these studies sarcopenia was defined using optimal stratification with gender specific cutoffs associated with mortality. To examine associations of interest we used cross tabulation procedures with the appropriate test statistic (Pearson Chi Square or Fisher’s Exact) for categorical variables or analysis of variance for continuous variables. We also examined mean differences between BMI and body compartments based on type of liver disease as we were specifically interested in those patients with NASH compared to other liver diseases. For the comparisons of body compartments we excluded participants who were transplanted for fulminant liver failure (n=12) (83% from acetaminophen overdoses) because these patients were typically not chronically ill from liver disease at the point of LTX and are not representative of LTX candidates with end-stage liver disease. In the analyses between liver diseases we also controlled for age, gender and race. The measurements of visceral fat were square root transformed prior to analyses due to skewness. Cox regression was used for univariate analysis of survival.
Acknowledgements
This research is funded by grant R01 DK066266 from the National Institute of Digestive Disorders and Kidney Diseases Rockville, MD and by grant 5UL1 RR024153-04 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIDDK, NCRR or NIH. Information on NCRR is available at http://www.ncrr.nih.gov/.
List of Abbreviations
- BMI
Body Mass Index
- CT
Computerized tomography
- ESLD
End-stage liver disease
- HBV
Hepatitis B cirrhosis
- HCV
Hepatitis C cirrhosis
- HU
Hounsfield unit
- LTX
liver transplant
- MELD
Model for End-Stage Liver Disease
- NASH
Non-alcoholic steatohepatitis
- PSC
Primary Sclerosing Cholangitis
- ALD
Alcoholic Liver Disease
Footnotes
The contents do not reflect the views of the Department of Veterans Affairs or the United States Government.
The authors of this manuscript have no conflicts of interest to disclose
Participated in research design
Participated in the performance of the research
Participated in data analysis
Participated in the writing of the paper
References
- 1.Prado Carla MM, Birdsell Laura A, Baracos Vickie E. The emerging role of computerized tomography in assessing cancer cachexia. Current Opinion in Supportive & Palliative Care. 2009;3(4):269–275. doi: 10.1097/SPC.0b013e328331124a. [DOI] [PubMed] [Google Scholar]
- 2.Goodpaster BH, Thaete FL, Simoneau JA, Kelley DE. Subcutaneous abdominal fat and thigh muscle composition predict insulin sensitivity independently of visceral fat. Diabetes. 1997;46(10):1579–1585. doi: 10.2337/diacare.46.10.1579. [DOI] [PubMed] [Google Scholar]
- 3.Goodpaster BH, Kelley DE, Wing RR, Meier A, Thaete FL. Effects of weight loss on regional fat distribution and insulin sensitivity in obesity. Diabetes. 1999;48(4):839–847. doi: 10.2337/diabetes.48.4.839. [DOI] [PubMed] [Google Scholar]
- 4.Tan B, Birdsell LA, Martin L, Baracos VE, Fearon KCH. Sarcopenia in an Overweight or Obese Patient Is an Adverse Prognostic Factor in Pancreatic Cancer. Clin Cancer Res. 2009;15:6973–6979. doi: 10.1158/1078-0432.CCR-09-1525. Published Online First November 3, 2009. [DOI] [PubMed] [Google Scholar]
- 5.Manini TM, Clark BC, Nalls MA, Goodpaster BH, Ploutz-Snyder LL, Harris TB. Reduced physical activity increases intermuscular adipose tissue in healthy young adults. Am J Clin Nutr. 2007;85(2):377–384. doi: 10.1093/ajcn/85.2.377. [DOI] [PubMed] [Google Scholar]
- 6.Marquis K, Debigare R, Lacasse Y, et al. Midthigh muscle crosssectional area is a better predictor of mortality than body mass index in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;166:809–813. doi: 10.1164/rccm.2107031. [DOI] [PubMed] [Google Scholar]
- 7.Ohkawa S, Odamaki M, Yoneyama T, Hibi I, Miyaji K, Kumagai H. Standardized thigh muscle area measured by computed axial tomography as an alternate muscle mass index for nutritional assessment of hemodialysis patients. Am J Clin Nutr. 2000;71:485–490. doi: 10.1093/ajcn/71.2.485. [DOI] [PubMed] [Google Scholar]
- 8.UNOS data on liver transplant recipients by diagnoses and year of transplant. [Accessed June 30, 2011]; Available at: http://optn.transplant.hrsa.gov.
- 9.Vehmas T, Kairemo KJ, Taavitsainen MJ. Measuring visceral adipose tissue content from contrast enhanced computed tomography. Int J Obes Relat Metab Disord. 1996;20:570–573. [PubMed] [Google Scholar]
- 10.Mitsiopoulos N, Baumgartner RN, Heymsfield SB, Lyons W, Gallagher D, Ross R. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol. 1998;85:115–122. doi: 10.1152/jappl.1998.85.1.115. [DOI] [PubMed] [Google Scholar]
- 11.Mourtzakis M, Prado CM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab. 2008;33:997–1006. doi: 10.1139/H08-075. [DOI] [PubMed] [Google Scholar]
- 12.Prado CM, Lieffers JR, McCargar LJ, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 2008;9:629–635. doi: 10.1016/S1470-2045(08)70153-0. [DOI] [PubMed] [Google Scholar]
- 13.Baracos VE, Reiman T, Mourtzakis M, Gioulbasanis I, Antoun S. Body composition in patients with non–small cell lung cancer: a contemporary view of cancer cachexia with the use of computed tomography image analysis. Am J Clin Nutr. 2010;91(suppl):1133S–1137S. doi: 10.3945/ajcn.2010.28608C. [DOI] [PubMed] [Google Scholar]
- 14.Kopple JD, Greene T, Chumlea WC, et al. Relationship between nutritional status and the glomerular filtration rate: results from the MDRD study. Kidney Int. 2000;57:1688–1703. doi: 10.1046/j.1523-1755.2000.00014.x. [DOI] [PubMed] [Google Scholar]
- 15.Kalantar-Zadeh K, Streja E, Kovesdy C, Oreopoulos A, Noori N, Jing J, et al. The Obesity Paradox and Mortality Associated With Surrogates of Body Size and Muscle Mass in Patients Receiving Hemodialysis. Mayo Clin Proc. 2010 Nov;85(11):991–1001. doi: 10.4065/mcp.2010.0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nair S, Cohen DB, Cohen MP, Tan H, Maley W, Thuluvath PJ. Postoperative morbidity, mortality, costs, and long-term survival in severely obese patients undergoing orthotopic liver transplantation. Am J Gastroenterology. 2001;96(3):842–845. doi: 10.1111/j.1572-0241.2001.03629.x. [DOI] [PubMed] [Google Scholar]
- 17.Rustgi VK, Marino G, Rustgi S, et al. Impact of body mass index on graft failure and overall survival following liver transplant. Clinical Transplantation. 2004;18(6):634–637. doi: 10.1111/j.1399-0012.2004.00141.x. [DOI] [PubMed] [Google Scholar]
- 18.Stenholm S, Harris TB, Rantanen T, Visser M, Kritchevsky SB, Ferrucci L. Sarcopenic obesity - definition, etiology and consequences. Curr Opin Clin Nutr Metab Care. 2008;11(6):693–700. doi: 10.1097/MCO.0b013e328312c37d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Englesbe MJ, Patel SP, He K, et al. Sarcopenia and Mortality after Liver Transplantation. J Am Coll Surg. 2010;211:271–278. doi: 10.1016/j.jamcollsurg.2010.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carson K, Washington MK, Treem WR, Clavien PA, Hunt CM. Recurrence of nonalcoholic steatohepatitis in a liver transplant recipient. Liver Transpl Surg. 1997;3(2):174–176. doi: 10.1002/lt.500030211. [DOI] [PubMed] [Google Scholar]
- 21.Molloy RM, Komorowski R, Varma RV. Recurrent nonalcoholic steatohepatitis and cirrhosis after liver transplantation. Liver Transpl Surg. 1997;3:177–178. doi: 10.1002/lt.500030212. [DOI] [PubMed] [Google Scholar]
- McHugh PP, Shah SH, Johnston TD, Gedaly R, Ranjan D. Predicting dry weigh in patient with ascites and liver cirrhosis using computed tomography imaging. Hepato-gastroenterology. 2010;57:591–597. [PubMed] [Google Scholar]
- Montano-Loza AJ. Muscle wasting is associated with mortality in patients with cirrhosis. Clin Gastroenterol Hepatol. 2012;10(2):166–173. doi: 10.1016/j.cgh.2011.08.028. [DOI] [PubMed] [Google Scholar]
- Jones JC, et al. Exercise capacity and muscle strength in patients with cirrhosis. Liver Transpl. 2012;18(2):146–151. doi: 10.1002/lt.22472. [DOI] [PubMed] [Google Scholar]
- Baumgartner RN, Koehler KM, Gallagher D, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147:755–763. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]