Abstract
Rationale
Previous reports shows rimonabant's inverse properties may be a limiting factor for treating cannabinoid dependence. To overcome this limitation neutral antagonists were developed, to address mechanisms by which an inverse agonist and neutral antagonist elicit withdrawal.
Objective
Introduces an animal model to study cannabinoid dependence by incorporating traditional methodologies and profiling novel cannabinoid ligands with distinct pharmacological properties/modes of action by evaluating their pharmacological effects on CB1-receptor (CB1R) related physiological/behavioral endpoints.
Methods
The cannabinergic AM2389 was acutely characterized in the tetrad (locomotor activity, analgesia, inverted screen/catalepsy bar test and temperature); with some comparisons made to Δ9-tetrahydrocannabinol (THC). Tolerance was measured in mice repeatedly administered AM2389. Antagonist-precipitated withdrawal was characterized in cannabinoid-adapted mice induced by either centrally acting antagonists, rimonabant and AM4113, or an antagonist with limited brain penetration, AM6545.
Results
In the tetrad, AM2389 was more potent and longer acting than THC, suggesting a novel approach for inducing dependence. Repeated administration of AM2389 led to tolerance by attenuating hypothermia that was induced by acute AM2389 administration. Antagonist-precipitated withdrawal signs were induced by rimonabant or AM4113, but not by AM6545. Antagonist-precipitated withdrawal was reversed by reinstating AM2389 or THC.
Conclusions
These findings suggest cannabinoid-precipitated withdrawal may not be ascribed to the inverse properties of rimonabant, but rather to rapid competition with the agonist at the CB1R. This withdrawal syndrome is likely centrally-mediated, since only the centrally acting CB1R antagonists elicited withdrawal, i.e., such responses were absent after the purported peripherally selective CB1R antagonist AM6545.
Keywords: Cannabinoid, THC, AM2389, AM4113, AM6545, Antagonist, Tolerance, Dependence, Withdrawal, Mice
Introduction
Cannabinoid receptor 1 (CB1R) activation by the phytocannabinoid Δ9-tetrahydrocannabinol (THC), the major psychoactive constituent in marijuana, and other synthetic CB1R agonists produces acute effects such as antinociception, hypo-locomotion, hypothermia, and catalepsy, all of which have been observed and quantified in rodent models, as previously reviewed (Maldonado and Rodriguez de Fonseca 2002). THC produces measureable pharmacological signs of tolerance upon repeated administrations in both humans and experimental animals (Carlini 1968; Hart et al. 2002; Jones et al. 1976; Jones et al. 1981). For instance, humans treated with oral THC (70-210 mg/day) for 30 days developed a lessening of the subjective high compared to the initial drug treatment (Jones et al. 1976; Jones et al. 1981).
Abrupt discontinuation of chronic THC treatment does not typically induce spontaneous signs of withdrawal (Jones and Benowitz 1976). Nevertheless, some human subjects have complained of inner unrest, irritability, insomnia, and hot flashes as symptoms of cannabis withdrawal (Budney et al. 1999). Instances where spontaneous THC withdrawal has been detected indicate that the withdrawal symptoms are mild in comparison to antagonist-precipitated withdrawal, making precipitated withdrawal a feasible experimental paradigm for studying robust signs of cannabinoid withdrawal (Lichtman and Martin 2005).
The partial efficacy THC has been the standard cannabinoid agonist for inducing dependence in animal models (prior to precipitated withdrawal); however, commercial preparations of synthetic cannabinoids, such as K2/Spice, mostly contain higher efficacy cannabinergics. Synthetic cannabinoids are currently the second highest abused illicit drug, with cannabis abuse ranked as the highest (Tai and Fantegrossi 2014). The rapid popularity of full efficacy cannabinoids pushes the urgency to develop an animal model for studying cannabinoid dependence. Thus, a CB1R agonist that is capable of producing sustained CB1R activation at full efficacy, such as AM2389, may be more efficient in this regard than THC (Järbe et al. 2012; Nikas et al. 2010). Using drug discrimination with rats suggested a slow onset of effect for AM2389 and a long in vivo functional half-life estimate of around 15 hrs (Järbe et al. 2012). Time course tests with THC in rats suggested a return to vehicle-like responding at about 4.5 hrs post-administration (Järbe et al. 1986; Järbe et al. 1981). At peak effect, there was approximately a 100-fold difference in potency between AM2389 and THC (Järbe et al. 2012).
The CB1R selective antagonist/inverse agonist rimonabant has been a useful experimental tool for inducing signs of withdrawal in cannabinoid-dependent subjects (Hutcheson et al. 1998). Withdrawal in rodents include wet dog shakes, head shakes, facial rubbing, and front paw tremor (Hutcheson et al. 1998; Maldonado and Rodriguez de Fonseca 2002). These behaviors are likely CB1R mediated (Maldonado and Rodriguez de Fonseca 2002). Since THC and rimonabant bind with reasonably high affinity to CB1Rs located both in the CNS and peripheral organs, systemic exposure to these agents in vivo cannot provide insight as to the contribution of central vs. peripheral compartment(s) to the withdrawal effects observed. A CB1R antagonist with limited brain penetration, such as AM6545, could help address this issue. AM6545 has been shown to cause a change in neuronal activity at CB1Rs located in the periphery with no effect on CB1Rs in the brain (Tam et al. 2010). In behavioral tests, AM6545 (10 mg/kg) did not attenuate centrally mediated cannabinergic behaviors, e.g., hypothermia, catalepsy and immobility (Tam et al. 2010). Additionally, in a discriminated drinking aversion procedure using rimonabant as the discriminative stimulus, AM6545 (10 mg/kg) did not suppress drinking whereas the centrally acting neutral antagonist AM4113 did, in a manner similar to but with less potency than rimonabant (Järbe et al. 2011).
By virtue of its inverse agonist property, rimonabant engagement of the CB1R can not only prevent the receptor from binding an agonist, but it can also alter constitutive CB1R-dependent cellular signaling/activity in vitro. For instance, rimonabant increased cAMP levels in the forskolin stimulated cAMP assay (Mato et al. 2002; Sink et al. 2008). To address the above mentioned adverse effects induced by rimonabant, it was suggested that a neutral antagonist would be an alternative approach for treating obesity and related metabolic risk factors (Chambers et al. 2007). A neutral antagonist, such as AM4113, would be devoid of negative efficacy by not affecting constitutive CB1R activity. For instance, AM4113 had no effect on forskolin stimulated cAMP production (Sink et al. 2008). Preclinical studies revealed behavioral differences between an inverse agonist and a neutral antagonist. Briefly, AM4113 did not induce nausea (i.e., conditioned gaping) in rats and vomiting (i.e., emesis) in ferrets as reported with inverse agonists, such as rimonabant and AM251 (Chambers et al. 2007; Salamone et al. 2007); see also (Meye et al. 2013). Our hypothesis is that in vivo signs of cannabinoid withdrawal induced by rimonabant may potentially result not only from CB1R blockade, but also as a negative efficacy effect of rimonabant.
Material and Methods
Animals
Male CD-1 mice (Charles River Breeding Laboratories, Wilmington, MA, USA) weighing 30-35 g (6 weeks old ± 1 SD) were group housed, 5 to a cage, in a temperature controlled (~20°C), animal facility. Mice were habituated to the vivarium for at least 1 week prior to experiments with a light/dark cycle of 12:12 h (lights on at 7 a.m.). Mice were given food and water ad libitum. Experimentally naïve mice were used for all procedures and tested during the light phase. The Animal Care and Use Committee of Northeastern University, Boston, MA, USA approved all procedures. The “Principles of Animal Laboratory Care” (National Institute of Health 1996) was followed.
Drugs
(−)-THC [(−)-delta-9-tetrahydrocannabinol] (Table 1) was delivered as an ethanol (200 mg/ml) solution and stored at −20°C until used. Upon use, appropriate amounts were withdrawn followed by the evaporation of ethanol under a stream of nitrogen. The residue was dissolved (w/v) in an aqueous solution of dimethyl sulfoxide (DMSO, 2%), propylene glycol (PG, 4%) and Tween-80 (T-80, 4%) prior to a final suspension with saline slowly added before animal dosing, the exception being 100 mg/kg THC where the vehicle used was DMSO 2%, PG 10% and T-80 8%. Suspensions were prepared daily shortly before injection. AM2389 [9-Nor-9β-hydroxy-3-(1-hexyl-cyclobut-1-yl)-hexahydrocannabinol] was stored and prepared in the same manner as THC. The ligands AM2389 and AM4113 [5-(4-alkylphenyl)-1-(2,4-dichlorophenyl)-4-methyl-N-(piperidin-1-yl)-1H-pyrazole-3-carboxamide] and AM6545 [5-(4-[4-cyanobut-1-ynyl]phenyl)]-1-(2,4-dichlorophenyl)- 4-methyl-N- (1,1-dioxo-thiomorpholino)-1 H-pyrazole-3-carboxamide] were synthesized at the Center for Drug Discovery (CDD), Northeastern University, Boston, MA. THC and rimonabant were provided by The Research Technology Branch, National Institute of Drug Abuse, Rockville, MD. Doses were administered intraperitoneally (i.p.) in a volume of 10 ml/kg, except for 100 mg/kg THC where the volume was 15 ml/kg. Drugs were administered 20 min (THC) or 90 min (AM2389) prior to testing, unless otherwise indicated.
Table 1.
Structure of cannabinoid ligands with binding affinities. Ki values were provided by the CDD and published data (Thakur et al. 2005).
| Type | Cannabinoid Ligand | Structure | CB1 affinity (Ki in nM) | CB2 affinity (Ki in nM) |
|---|---|---|---|---|
| Agonist | (-)-Δ9-THC |
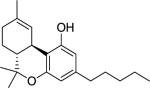
|
39.5 | 40 |
| AM2389 |
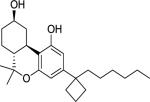
|
0.16 | 4.21 | |
| Antagonist | Rimonabant |
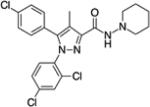
|
10 | 931 |
| AM4113 | N-piperidin-1-yl-2,4-dichlorophenyl-1H-pyrazole-3-carboxamide analog | 0.89 | 92 | |
| AM6545 |
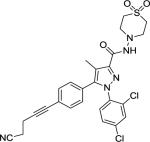
|
1.73 | 523 |
Open Field
Locomotor activity (horizontal exploration) used an open-field photobeam activity monitoring system (San Diego Instruments, San Diego, CA, USA). Activity was recorded by infrared photo-beam sensors in a 16 × 16 in acrylic chamber, which was placed inside a sound attenuating ventilated box to produce a controlled environment. The photo-beam sensors consisted of 4 infrared beam bars placed at 90° angles enclosing the bottom of the chamber, lined with a second row (only for 2 of the bars located parallel to each other) that recorded rearing (vertical exploration). All chambers were wiped with a soap/water solution between tests.
Paw Withdrawal
Paw withdrawal was tested using a method previously described (Hargreaves et al. 1988) and later modified for mice. Mice were placed inside a Plexiglas chamber above a glass plate heated to 30°C. Animals acclimated for ~1 hr prior to testing. The thermal nociceptive stimulus, a focused projection bulb located underneath the glass plate, was aimed at the plantar surface of the hind limb. The heat current was set to 6.0 amperes. Measurements were carried out by an automated motion sensor, which terminated the heat stimulus and recorded the latency to withdrawal. The maximum heat exposure time was 40 sec to prevent tissue damage. The glass plate was cleaned of animal waste prior to taking measurements.
Inverted Screen
The inverted screen was used to measure muscle strength and motor coordination (Coughenour et al. 1977; Lichtman et al. 2004). The inverted screen assay consisted of placing mice in the center of the wire mesh grid (14 × 14 cm2) that was inverted 180° so that the mice were oriented upside down on the bottom of the screen. The latency (sec) to climb onto the reverse (top) side was recorded.
Catalepsy
Catalepsy was assessed with a bar test, i.e., both forelimbs were positioned on a stainless steel rod (0.40 cm in diameter) that was elevated 4.5 cm above the surface (Lichtman et al. 2002). Catalepsy was measured as the length of time each mouse maintained both forelimbs in an elevated position on the bar. Mice that remained immobile were considered cataleptic.
Rectal Temperature
Rectal temperature was measured in mice using a rectal probe of a digital laboratory thermometer, RET-3-ISO, type T thermocouple (Physitemp Instruments Inc, Clifton, NJ). The lubricated probe was inserted ~2.0 cm into the rectum for ~30 sec prior to each recording.
Observation Chambers
Observation chambers were used to score (count/duration) individual mouse behaviors. Chambers consisted of a circular clear glass jar (radius 4.25 cm, height 16 cm) sealed with a ventilated cover, providing clear visibility of movement and ample air circulation. The behaviors scored were tremors, face rubbing and rearing. The behaviors were scored by trained observers blinded to the treatment groups. Definitions for each behavior were: tremors, rapid shaking of the front paw(s); face rubbing, using the front paw(s) to brush their head; rearing, upward movements of the mouse with their entire weight being supported by the hind paw(s). These behaviors were chosen based on previous findings that reported a change in activity levels of tremors, face rubbing and rearing during spontaneous or CB1R antagonist precipitated withdrawal in mice tolerant to CB1R agonists (Aceto et al. 2001; Oliva et al. 2003).
Mice repeatedly treated with AM2389 were given a dose of 0.1 mg/kg on day 1 and 0.3 mg/kg on days 2-5. This dosing regimen was based on preliminary data that determined an appropriate regimen that did not compromise the mice health. Acutely administered 0.3 mg/kg AM2389 produced marked effects such that mice did not eat or drink for at least 24 hrs. Therefore, 0.1 mg/kg AM2389 was used for the initial, day 1 tolerance induction.
Precipitated Withdrawal
For precipitated withdrawal studies, data are presented in 2 phases. Phase I (i.e., pre-antagonist) represents Day 5 data collected from mice that received 5 days of vehicle or AM2389 prior to receiving an antagonist. Phase II (i.e., post-antagonist) represents the same mice that had received an antagonist, to precipitate withdrawal, or vehicle immediately after Phase I data was collected.
In Phase I open-field studies, locomotor and rearing activity were measured as the total number of beam breaks or rearing bouts, respectively, within a 60 min period following 90 min post last vehicle or AM2389 administration in mice pretreated for 5 days with vehicle or AM2389. In Phase II open-field studies, the total number of beam breaks or rearing bouts were recorded for a 75 min period immediately following a single administration of vehicle, rimonabant, AM4113 or AM6545 in mice that were previously exposed to the open-field arena for 60 min (i.e., Phase I).
In Phase I observation studies, tremors, face rubbing and rearing behaviors were scored during a 60 min observation period following 90 min post last vehicle or AM2389 administration in mice pretreated for 5 days with vehicle or AM2389. In Phase II observation studies, behaviors were scored during a 120 min observation period immediately following a single administration of vehicle, rimonabant, AM4113 or AM6545 in mice that were previously exposed to the observation chambers for 60 min (i.e., Phase I).
Data Analysis
Results are presented as means ± SEM. Significant differences (α = 0.05) between group means were calculated with ANOVA [based on the Fisher (F) distribution], followed by Holm-Sidak multiple comparison post-hoc statistical test procedure, except where otherwise indicated.
Results
Acute AM2389 dosing
AM2389-treated mice had a significant dose-dependent decrease in locomotion greater than vehicle-treated mice [F(3,32) = 9.87, P < 0.001] (Table 2). AM2389 at 0.1 and 0.3 mg/kg produced a significant greater paw withdrawal latency than the vehicle group [F(6,65) = 12.00, P < 0.001]. AM2389 at 0.03 mg/kg and THC did not induce any significant change in latency compared to the vehicle group. In the inverted screen test, AM2389 at 0.03, 0.1 and 0.3 mg/kg produced no significant difference [F(3,32) = 2.61; P > 0.05] from vehicle-treated mice, since there was only a trend towards vehicle-treated mice reaching the reverse side of the inverted screen more quickly in comparison to the highest doses of AM2389 (0.1 and 0.3 mg/kg). In the catalepsy bar test, 0.1 mg/kg AM2389 resulted in immobility significantly greater than vehicle-treated mice [F(3,32) = 4.06; P < 0.02]. The dose of 0.3 mg/kg AM2389 yielded a large variation in response; hence, no significant difference in comparison to vehicle. All doses of AM2389 (0.03, 0.1 and 0.3 mg/kg) induced a dose-dependent decrease in temperature in comparison to vehicle [F(6,65) = 60.95; P < 0.001]. In addition, the highest doses of THC (10 and 30 mg/kg) induced a greater decrease in temperature than vehicle.
Table 2.
Data values represent mean ± SEM. 1).
| Dose (mg/kg) | 1) Ambulation (beam breaks) | 2) Withdrawal Latency (sec) | 3) Inverted screen (sec) | 4) Catalepsy (sec) | 5) Temperature (°C) | |
|---|---|---|---|---|---|---|
| Vehicle | --- | 4301 ± 446.7 | 14.4 ± 1.6 | 26.7 ± 5.4 | 1.9 ± 0.4 | 0.3 ± 0.1 |
| AM2389 | 0.03 | 2050.5 ± 633.5* | 24.9 ± 3.5 | 13.5 ± 4.2 | 2.4 ± 0.8 | −1.3 ± 0.3* |
| 0.1 | 1373 ± 421.9* | 41 ± 2.9* | 35.5 ± 9.3 | 17.6 ± 5.4* | −5.4 ± 0.5* | |
| 0.3 | 1088.7 ± 166* | 41 ± 0* | 46.2 ± 9.2 | 12.2 ± 9.6 | −6.6 ± 0.6* | |
| THC | 3 | --- | 22.7 ± 5.1 | --- | --- | 0.4 ± 0.24 |
| 10 | --- | 15.5 ± 1.8 | --- | --- | −2.5 ± 1* | |
| 30 | --- | 18.9 ± 6.5 | --- | --- | −3.4 ± 0.6* | |
Ambulation was measured as the total number of beam breaks within a 60 min period following AM2389 (n = 6 per dose) or vehicle (n = 18). 2). Paw withdrawal was measured as the amount of time utilized to remove the hind paw from a thermal stimulus after injection with AM2389 (n = 6 per dose), THC (n = 6 per dose), or vehicle (n = 36): Cut-off time was 40 sec. 3). Inverted screen test measured the amount of time needed to climb to the reverse side of the screen post administration of AM2389 (n = 6 per dose) or vehicle (n = 18). Mice that failed to climb to the reverse side or fell off were given a score of 60 sec. 4). Catalepsy measured the duration of immobility on the bar until the mouse removed both forepaws from the bar or climbed onto the bar with one or both hind paws: Cut-off time was 60 sec. 5). Rectal temperatures were measured as changes in temperature after treatment with vehicle and AM2389 or THC 90 min or 20 min post-injection, respectively. AM2389 (n = 6 per dose), THC (n = 6 per dose), vehicle (n = 36).
indicates significant difference from the vehicle group at P ≤ 0.05; Holm-Sidak multiple comparisons versus vehicle following one-way ANOVA.
Repeated AM2389 dosing
On day 1 of repeated dosing, mice (n = 4) administered AM2389 (0.1 mg/kg) had maximum hypothermia, a 5.7°C decrease in temperature from baseline (data not shown). This hypothermic effect was significantly attenuated to −3.9, −1.1, 0.9 and −1.4 with repeated AM2389 (0.3 mg/kg) administration at days 2, 3, 4 and 5, respectively [F(4,12) = 36.04; P < 0.001]. Thus, hypothermia was attenuated after repeated dosing, resulting in temperatures approaching the day 1 non-drug baseline temperature (rectal temperatures immediately prior to the first dosing averaged (± SEM) 38.9°C ± 0.1.).
Phase I pre-antagonist open-field
AM2389-pretreated mice had a significant decrease (50%) in locomotion greater than vehicle-pretreated mice [F(1,124) = 100.92, P < 0.001] (data not shown). Similarly, rearing was suppressed in AM2389-pretreated mice by 38% in comparison to vehicle-pretreated mice [F(1,124) = 34.35, P < 0.001].
Phase II post-antagonist open-field
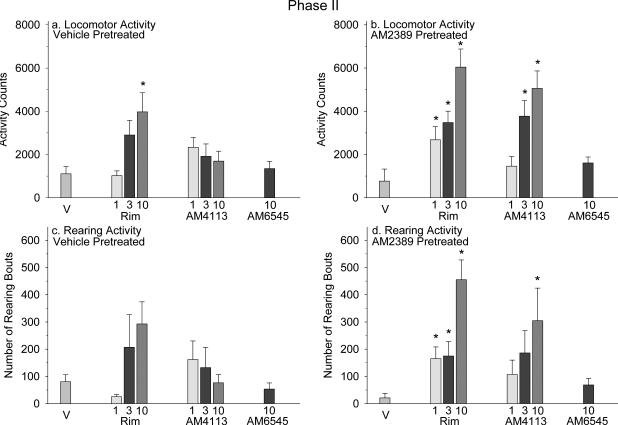
Locomotion and rearing activity measured in vehicle-pretreated mice administered rimonabant, AM4113 or AM6545 were similar to animals treated with vehicle, except for an increase in locomotion at 10 mg/kg rimonabant [F(7,55) = 3.63, P = 0.003] (Figure 1a, c). AM2389-pretreated mice that were administered either rimonabant or AM4113 at 3 and 10 mg/kg had a significant increase in locomotion in comparison to mice that were administered vehicle [F(7,55) = 11.14, P < 0.001] (Figure 1b). AM4113 and AM6545 at 1 and 10 mg/kg, respectively, did not induce a change in locomotion that differed significantly from vehicle. AM2389-pretreated mice administered rimonabant exhibited a significant increase in rearing, which was also observed with 10 mg/kg AM4113 in comparison to vehicle [F(7,55) = 5.39, P < 0.001] (Figure 1d).
Figure 1.
Panels a and b: Phase II locomotor activity was measured following a single administration of vehicle (n = 8), rimonabant (rim; n = 7-8 per dose), AM4113 (n = 8 per dose) or AM6545 (n = 7-8) . Panels c and d: Rearing bouts. Each data plot represents the mean ± SEM for total beam breaks or rearing bouts during 75 min. *, indicates significant difference from the vehicle group in vehicle- or AM2389-pretreated mice at P < 0.05; Holm-Sidak multiple comparisons versus vehicle procedure following one-way ANOVA.
Phase I pre-antagonist observation
AM2389-pretreatment produced no change in tremors or face rubbing in comparison to vehicle-pretreated mice (data not shown). However, there was a significant suppression in rearing by 33% in AM2389-pretreated as compared to control mice [F(1,125) = 65.04, P < 0.001].
Phase II post-antagonist observation
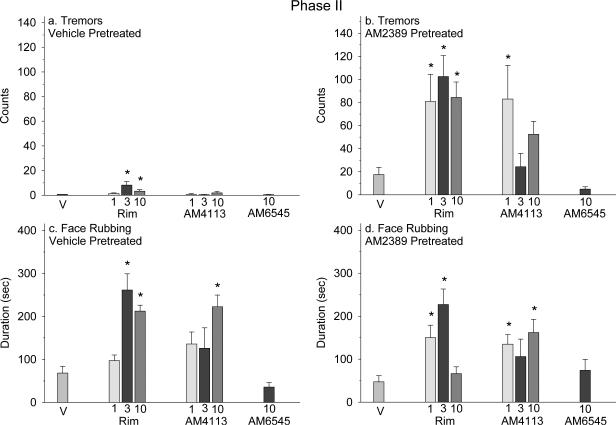
Tremors recorded in vehicle-pretreated mice administered 3 and 10 mg/kg rimonabant occurred frequently in comparison to mice treated with vehicle, which did not exhibit tremor [F(7,56) = 28.39, P < 0.001] (Figure 2a). AM2389-pretreated mice administered rimonabant and 1 mg/kg AM4113 showed an increase in tremors greater than vehicle [F(7,55) = 8.43, P < 0.001] (Figure 2b). Face rubbing in vehicle-pretreated mice was significantly increased after administration of 3 and 10 mg/kg rimonabant and 10 mg/kg AM4113 versus vehicle [F(7,56) = 8.39, P < 0.001] (Figure 2c). AM2389-pretreated mice administered rimonabant at 1 and 3 mg/kg and AM4113 at 1 and 10 mg/kg had a greater duration of face rubbing in comparison to vehicle [F(7,55) = 5.24, P < 0.001] (Figure 2d).
Figure 2.
In Phase II, behaviors were scored immediately following a single administration of vehicle (n = 8), rimonabant (rim; n = 8 per dose), AM4113 (n = 8 per dose) or AM6545 (n = 8). Panels a and b: Tremor counts; and Panels c and d: Face rubbing duration. Each data point represents the mean ± SEM for total tremors or face rubbing sampled for 5 min every 5 min during the 60 min observation session and again for 5 min towards the end of the 120 min observation session.
*, indicates significant difference from the vehicle group in vehicle- or AM2389- pretreated mice at P < 0.05; Holm-Sidak multiple comparisons versus vehicle procedure following one-way ANOVA. Panel a data were analyzed using the Kruskal-Wallis test followed by post-hoc non-parametric method as tremor(s) after vehicle exhibited a mean of zero.
Rearing measured post-administration of all antagonists produced no significant change in comparison to vehicle (data not shown), and an increase in rearing in AM2389-pretreated mice [F(7,55) = 7.88, P < 0.001] at 1 and 10 mg/kg rimonabant (11% and 5%, respectively) and 1 and 3 mg/kg AM4113 (7% and 8%, respectively). AM6545 (10 mg/kg) did not produce a significant change in rearing for either vehicle- or AM2389-pretreated mice.
Cannabinoid reinstatement in the open-field
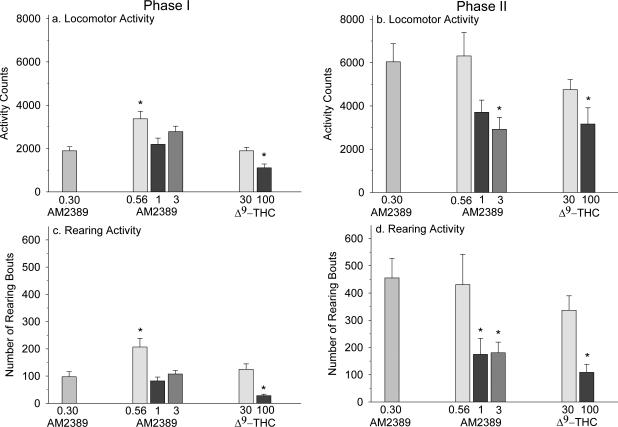
AM2389-pretreated mice administered 0.56 mg/kg had a significant increase in locomotion in comparison to the 0.30 mg/kg AM2389 group F(5,38) = 10.61, P < 0.001], whereas 100 mg/kg THC displayed a decline in locomotion (Figure 3a). These findings corresponded to rearing activity F(5,38) = 11.87, P < 0.001] (Figure 3c).
Figure 3.
Panels a and c: Phase I locomotor and rearing activity was measured in mice pretreated for 5 days with AM2389 (n = 6-7 per dose). On day 5 mice were administered AM2389 at 0.3, 0.56, 1 or 3 mg/kg 90 min or THC at 30 or 100 mg/kg 20 min before being placed into the open-field arena. Each data point represents the mean ± SEM for total beam breaks or rearing bouts during 60 min. Panels b and d: Phase II locomotor and rearing activity were measured immediately following a single administration of 10 mg/kg rimonabant in mice from Phase I. Each data point represents the mean ± SEM for total beam breaks or rearing bouts during 75 min.
*, indicates significant difference from the group administered 0.3 mg/kg AM2389 (Phase I) or 0.3 mg/kg AM2389 plus rimonabant (Phase II) at P < 0.05; Holm-Sidak multiple comparisons versus 0.3 mg/kg AM2389 (Phase I) or 0.3 mg/kg AM2389 plus rimonabant (Phase II) procedure following one-way ANOVA.
After administration of 10 mg/kg rimonabant, in Phase II, there was a trend towards decreased hyperactivity with increasing doses of AM2389 with 3 mg/kg AM2389 producing the greatest suppression in hyperactivity [F(5,38) = 3.56, P = 0.01] (Figure 3b). This suppression in hyperactivity was also displayed by the 100 mg/kg THC group. These finding were similarly reported for rearing activity [F(5,38) = 5.65, P < 0.001], in addition to a suppression in hyperactivity with the 1 mg/kg AM2389 group (Figure 3d).
Discussion
In this study, we characterized physiological and behavioral responses in mice administered AM2389, a high-efficacy and potent agonist with ~26-fold selectivity for the CB1-over the CB2-R (Järbe et al. 2012; Nikas et al. 2010). The psychomotor and temperature alterations observed in the current study support previous findings that suggest these responses were likely due to activation of CB1Rs in the brain (Kishimoto and Kano 2006; Rawls et al. 2002). Furthermore, the behavioral effects of AM2389 measured here share similarities with other CB1R agonists, including THC and WIN55,212-2 (Fan et al. 1994; Lichtman et al. 2001). AM2389 dose-dependently suppressed locomotor activity to a greater degree than vehicle. Furthermore, the results from the inverted screen and catalepsy bar test showed a trend towards increased immobility time in a dose-dependent manner. The inverted screen test outcome may imply that AM2389 induces biphasic effects, i.e., a decrease in latency at low doses and an increase in latency at higher doses. This would follow suit with previous work suggesting that CB1R agonists may induce biphasic effects (Chaperon and Thiebot 1999). However, this finding was not supported by other measures in this study. For instance, in the catalepsy bar test, mice given 0.3 mg/kg AM2389 appeared unable to maintain their posture on the bar and they fell off; this explains the large error bars and thus the lack of significance with respect to the controls.
AM2389 increased the paw withdrawal latency in the presence of a thermal noxious stimulus at lower doses than THC. It is unclear why there was no significant increase in the paw withdrawal latency after THC treatment, even at the highest dose of 30 mg/kg. It is likely that the heat stimulus intensity used to induce a response for low doses of AM2389 surpassed the heat sensitivity threshold for inducing a dose-dependent latency in THC-treated mice. Also, it is possible that other analgesia instruments (e.g., hot-plate and tail-flick) may be more sensitive at detecting THC hypoalgesia than the thermal stimulator used here. However, the sensitivity of this paw stimulator to cannabinoids might be increased during hyperalgesia (e.g., carrageenan induced inflammation), as shown previously (Richardson et al. 1998).
Core body temperature is reduced after administration of AM2389 or THC. The highest dose of AM2389 (0.3 mg/kg) acutely induced a decrease in temperature (−6.63 ± 0.55°C), which was approximately 2-fold greater than the hypothermia elicited by a 100-fold higher dose (30 mg/kg) of THC (−3.36 ± 0.56°C). The rectal temperature changes observed in our CD-1 mice generalizes across mice strains and species, including C57BL/6J mice (Järbe et al. 2012) and Sprague-Dawley rats (Järbe 1978). Furthermore, it was shown that the AM2389 induced hypothermia was attenuated by AM251, a CB1R selective antagonist, suggesting that this physiological change was CB1R mediated (Järbe et al. 2012). It could be argued that the THC doses examined here were not sufficient to induce a greater degree of hypothermia. However, maximal effects on hypothermia with doses 32 and 100 mg/kg THC have been reported in mice (McMahon and Koek 2007). Overall, our data indicate that acute dosing with AM2389 exerts pronounced central effects that were measured in classical behavioral assays associated with CB1R agonist activity, and does so with greater potency than THC. Mice repeatedly administered AM2389 displayed an attenuation of hypothermia by days 3-5, suggesting that tolerance developed to this effect. Collectively, the functional activity of AM2389 administered acutely/chronically is consistent with previously characterized CB1R cannabinergic ligands.
Cannabinoid dependence was studied employing a dose regimen involving mice that were administered AM2389 once daily for 5 days prior to precipitating withdrawal. It was found that during precipitated withdrawal, rimonabant induced profound hyperactivity as well as increased paw tremors in AM2389-tolerant mice. Likewise, AM4113 administered to AM2389-tolerant mice produced increased levels of hyperactivity at the higher doses (3 and 10 mg/kg AM4113) with a slight elevation in paw tremors. In cannabinoid-naïve mice challenged with rimonabant there was a trend towards hyperactivity; however, the level of hyperactivity was not as pronounced as what was observed in AM2389-tolerant mice administered rimonabant. Unlike rimonabant, hyperactivity was not evident in cannabinoid-naïve mice challenged with the neutral CB1R antagonist AM4113. Tremors observed in cannabinoid-naïve mice had a limited elevation after rimonabant treatment, which was even less in AM4113 treated mice. This would indicate that hyperactivity and tremors are plausible signs of cannabinoid dependence. The increase in these behaviors is consistent with previous reports from other groups (Cook et al. 1998; Hutcheson et al. 1998; Lichtman et al. 2001). Furthermore, it was reported that hyperlocomotion and paw tremors were signs of cannabinoid withdrawal (Huang et al. 2009). Huang and colleagues reported a marked elevation in locomotor activity as well as paw tremors post rimonabant (15 mg/kg) administration in C57BL/6 mice tolerant to THC (25 mg/kg twice daily) versus cannabinoid-naïve mice. Similar findings were also reported in mice tolerant to inhaled marijuana (Wilson et al. 2006).
Unlike previous reports, the current study failed to show face rubbing as a marker for cannabinoid withdrawal. However, other reports referred to face rubbing as a precipitated withdrawal sign (Aceto et al. 1996). Aceto et al. (1996) showed that cannabinoid-tolerant rats exhibited a dose-responsive increase in face rubbing during rimonabant induced precipitated withdrawal. This discrepancy in somatic withdrawal behavior may suggest that an increase in face rubbing during precipitated withdrawal is species specific. Conversely, another group reported profound face rubbing in THC-tolerant mice challenged with rimonabant (Hutcheson et al. 1998), though the same mouse strain was also used in the current study. Such findings could cause one to speculate about the challenges in comparing subjective measures across laboratories. For instance, was the absence of profound face rubbing noted in the current work but not by Hutcheson et al. (1998) attributed to the observers interpretation of what this behavior is or are there other variables to account for these differences? One way to overcome different behavioral interpretations would be to provide clear definitions for the behaviors being observed. Additionally, it has been suggested that the endocannabinoid tone during withdrawal may be a determinant and thus, at least partly, explain different outcomes across studies (Huang et al. 2010).
Using the observation chambers test, our study further indicated that during precipitated withdrawal rearing was elevated in AM2389-tolerant mice, whereas the open-field test showed a strong trend towards increased rearing activity. A possible explanation is that the surface area of the open-field arena is larger allowing for more exploratory behavior, while the observation chambers are considerably smaller allowing for measurement of more subtle behaviors. Therefore, it seems possible that having less space to explore increased the habituation rate as reflected by a decrease in rearing. In addition, cannabinoid-naïve mice all had a further decrease in rearing post rimonabant or AM4113 administration in Phase II compared to Phase I, suggesting that centrally acting CB1R antagonists do not increase rearing. Our findings also show that cannabinoid precipitated withdrawal is induced with no measurable influence from a peripheral mechanism. Thus, i.p. administration of AM6545 did not result in hyperactivity when given to AM2389 adapted mice. AM6545 was examined at 10 mg/kg as that has been a commonly used dose in previously published work (Chopda et al. 2013; Tam et al. 2010). For instance, 10 mg/kg AM6545 was unable to attenuate cannabinoid agonist-induced catalepsy, locomotor activity and hypothermia, whereas centrally acting CB1R antagonist rimonabant did attenuate these effects (Tam et al. 2010). Furthermore, rimonabant and AM6545 were equally effective in attenuating obesity-related glucose intolerance and insulin resistance and normalizing the elevated blood glucose and insulin levels in diet-induced obesity mice.
Finally, we attempted to reverse the effects of rimonabant induced precipitated withdrawal in the open-field test. The point of this portion of the study was to ascertain if the rimonabant-precipitated withdrawal response in the open-field was surmountably counteracted by increasing the dose of AM2389 and to determine if this would “generalize” to THC as well. It was shown that increasing doses of AM2389 or THC were able to attenuate the hyperactivity induced by rimonabant in our cannabinoid-tolerant mice. This further supports a report that measured THC induced reversal of paw tremors that were precipitated by rimonabant in THC-tolerant mice (Lichtman et al. 2001). In the latter study, the THC doses used to attenuate paw tremors did not attenuate hyperactivity. An explanation for this differential outcome could be that the THC dose that attenuated paw tremors was not high enough to affect locomotor activity. Thus, it is possible that if a wider dose range of THC had been examined, as in the current study, attenuation of hyperactivity might have occurred. Similar to the drug discrimination results referred to earlier (Järbe et al. 2012), the potency ratio between AM2389 and THC in suppressing cannabinoid antagonist withdrawal was approximately 100-fold.
It was our assumption that CB1R neutral antagonists might precipitate withdrawal to a lesser degree in comparison to CB1R inverse agonists, thereby leading to the development of improved pharmacotherapeutics for managing cannabinoid dependence/withdrawal. The current study did not portray a clear-cut differentiation between a CB1R antagonist with negative efficacy (inverse agonist) versus a CB1R antagonist that does not alter basal signaling (neutral antagonist). Clearly, further investigations are required to develop improved pharmacotherapeutics for cannabinoid dependence. These findings, along with previous studies, should benefit future work aimed at elucidating the underlying mechanism(s) that separate neutral antagonism from inverse agonism.
Acknowledgements
This work was supported by United States Public Health Service Grants DA023142-5 and DA009064-19 from the National Institute on Drug Abuse (NIDA). We thank NIDA for supplies of (−)-THC and rimonabant. We also thank V. Shibad and A. Kulkarni for their assistance in measuring somatic withdrawal signs. Parts of these data were presented at the annual meeting of The International Cannabinoid Research Society Symposium July 6 - 9 at the Pheasant Run Resort, St. Charles, Illinois, USA (Tai et al. 2011).
Footnotes
Disclosure statement
Authors declare that the study sponsors did not have any role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
References
- Aceto MD, Scates SM, Lowe JA, Martin BR. Dependence on delta 9-tetrahydrocannabinol: studies on precipitated and abrupt withdrawal. J Pharmacol Exp Ther. 1996;278:1290–5. [PubMed] [Google Scholar]
- Aceto MD, Scates SM, Martin BB. Spontaneous and precipitated withdrawal with a synthetic cannabinoid, WIN 55212-2. Eur J Pharmacol. 2001;416:75–81. doi: 10.1016/s0014-2999(01)00873-1. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Novy PL, Hughes JR. Marijuana withdrawal among adults seeking treatment for marijuana dependence. Addiction. 1999;94:1311–22. doi: 10.1046/j.1360-0443.1999.94913114.x. [DOI] [PubMed] [Google Scholar]
- Carlini EA. Tolerance to chronic administration of Cannabis sativa (marihuana) in rats. Pharmacology. 1968;1:135–42. doi: 10.1159/000135954. [DOI] [PubMed] [Google Scholar]
- Chambers AP, Vemuri VK, Peng Y, Wood JT, Olszewska T, Pittman QJ, Makriyannis A, Sharkey KA. A neutral CB1 receptor antagonist reduces weight gain in rat. Am J Physiol Regul Integr Comp Physiol. 2007;293:R2185–93. doi: 10.1152/ajpregu.00663.2007. [DOI] [PubMed] [Google Scholar]
- Chaperon F, Thiebot MH. Behavioral effects of cannabinoid agents in animals. Crit Rev Neurobiol. 1999;13:243–81. doi: 10.1615/critrevneurobiol.v13.i3.20. [DOI] [PubMed] [Google Scholar]
- Chopda GR, Vemuri VK, Sharma R, Thakur GA, Makriyannis A, Paronis CA. Diuretic effects of cannabinoid agonists in mice. Eur J Pharmacol. 2013;721:64–9. doi: 10.1016/j.ejphar.2013.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook SA, Lowe JA, Martin BR. CB1 receptor antagonist precipitates withdrawal in mice exposed to Delta9-tetrahydrocannabinol. J Pharmacol Exp Ther. 1998;285:1150–6. [PubMed] [Google Scholar]
- Coughenour LL, McLean JR, Parker RB. A new device for the rapid measurement of impaired motor function in mice. Pharmacol Biochem Behav. 1977;6:351–3. doi: 10.1016/0091-3057(77)90036-3. [DOI] [PubMed] [Google Scholar]
- Fan F, Compton DR, Ward S, Melvin L, Martin BR. Development of cross-tolerance between delta-9-tetrahydrocannabinol, CP 55,940 and WIN 55,212. J Pharmacol Exp Ther. 1994;271:1383–90. [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- Hart CL, Ward AS, Haney M, Comer SD, Foltin RW, Fischman MW. Comparison of smoked marijuana and oral Delta(9)-tetrahydrocannabinol in humans. Psychopharmacology (Berl) 2002;164:407–15. doi: 10.1007/s00213-002-1231-y. [DOI] [PubMed] [Google Scholar]
- Huang P, Liu-Chen LY, Kirby LG. Anxiety-like effects of SR141716-precipitated delta9- tetrahydrocannabinol withdrawal in mice in the elevated plus-maze. Neurosci Lett. 2010;475:165–8. doi: 10.1016/j.neulet.2010.03.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P, Liu-Chen LY, Unterwald EM, Cowan A. Hyperlocomotion and paw tremors are two highly quantifiable signs of SR141716-precipitated withdrawal from delta9-tetrahydrocannabinol in C57BL/6 mice. Neurosci Lett. 2009;465:66–70. doi: 10.1016/j.neulet.2009.08.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutcheson DM, Tzavara ET, Smadja C, Valjent E, Roques BP, Hanoune J, Maldonado R. Behavioural and biochemical evidence for signs of abstinence in mice chronically treated with delta-9-tetrahydrocannabinol. Br J Pharmacol. 1998;125:1567–77. doi: 10.1038/sj.bjp.0702228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Järbe TUC. Delta-9-tetrahydrocannabinol: tolerance after noncontingent exposure in rats. Arch Int Pharmacodyn Ther. 1978;231:49–56. [PubMed] [Google Scholar]
- Järbe TUC, Hiltunen AJ, Lander N, Mechoulam R. Cannabimimetic activity (delta 1-THC cue) of cannabidiol monomethyl ether and two stereoisomeric hexahydrocannabinols in rats and pigeons. Pharmacol Biochem Behav. 1986;25:393–9. doi: 10.1016/0091-3057(86)90015-8. [DOI] [PubMed] [Google Scholar]
- Järbe TUC, LeMay BJ, Vemuri VK, Vadivel SK, Zvonok A, Makriyannis A. Central mediation and differential blockade by cannabinergics of the discriminative stimulus effects of the cannabinoid CB1 receptor antagonist rimonabant in rats. Psychopharmacology (Berl) 2011;216:355–65. doi: 10.1007/s00213-011-2226-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Järbe TUC, Swedberg MD, Mechoulam R. A repeated test procedure to assess onset and duration of the cue properties of (−) delta 9-THC, (−) delta 8-THC-DMH and (+) delta 8-THC. Psychopharmacology (Berl) 1981;75:152–7. doi: 10.1007/BF00432178. [DOI] [PubMed] [Google Scholar]
- Järbe TUC, Tai S, Lemay BJ, Nikas SP, Shukla VG, Zvonok A, Makriyannis A. AM2389, a high-affinity, in vivo potent CB(1)-receptor-selective cannabinergic ligand as evidenced by drug discrimination in rats and hypothermia testing in mice. Psychopharmacology (Berl) 2012;220:417–26. doi: 10.1007/s00213-011-2491-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RT, Benowitz N. The 30-day trip. Clinical studies of cannabis tolerance and dependence. In: Braude MC, Szara S, editors. Pharmacology of Marijuana. Academic Press; New York: 1976. [Google Scholar]
- Jones RT, Benowitz N, Bachman J. Clinical studies of cannabis tolerance and dependence. Ann N Y Acad Sci. 1976;282:221–39. doi: 10.1111/j.1749-6632.1976.tb49901.x. [DOI] [PubMed] [Google Scholar]
- Jones RT, Benowitz NL, Herning RI. Clinical relevance of cannabis tolerance and dependence. J Clin Pharmacol. 1981;21:143S–152S. doi: 10.1002/j.1552-4604.1981.tb02589.x. [DOI] [PubMed] [Google Scholar]
- Kishimoto Y, Kano M. Endogenous cannabinoid signaling through the CB1 receptor is essential for cerebellum-dependent discrete motor learning. J Neurosci. 2006;26:8829–37. doi: 10.1523/JNEUROSCI.1236-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman AH, Fisher J, Martin BR. Precipitated cannabinoid withdrawal is reversed by delta-9-tetrahydrocannabinol or clonidine. Pharmacol Biochem Behav. 2001;69:181–8. doi: 10.1016/s0091-3057(01)00514-7. [DOI] [PubMed] [Google Scholar]
- Lichtman AH, Hawkins EG, Griffin G, Cravatt BF. Pharmacological activity of fatty acid amides is regulated, but not mediated, by fatty acid amide hydrolase in vivo. J Pharmacol Exp Ther. 2002;302:73–9. doi: 10.1124/jpet.302.1.73. [DOI] [PubMed] [Google Scholar]
- Lichtman AH, Leung D, Shelton CC, Saghatelian A, Hardouin C, Boger DL, Cravatt BF. Reversible inhibitors of fatty acid amide hydrolase that promote analgesia: evidence for an unprecedented combination of potency and selectivity. J Pharmacol Exp Ther. 2004;311:441–8. doi: 10.1124/jpet.104.069401. [DOI] [PubMed] [Google Scholar]
- Lichtman AH, Martin BR. Cannabinoid tolerance and dependence. Handb Exp Pharmacol. 2005;168:691–717. doi: 10.1007/3-540-26573-2_24. [DOI] [PubMed] [Google Scholar]
- Maldonado R, Rodriguez de Fonseca F. Cannabinoid addiction: behavioral models and neural correlates. J Neurosci. 2002;22:3326–31. doi: 10.1523/JNEUROSCI.22-09-03326.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mato S, Pazos A, Valdizan EM. Cannabinoid receptor antagonism and inverse agonism in response to SR141716A on cAMP production in human and rat brain. Eur J Pharmacol. 2002;443:43–6. doi: 10.1016/s0014-2999(02)01575-3. [DOI] [PubMed] [Google Scholar]
- McMahon LR, Koek W. Differences in the relative potency of SR 141716A and AM 251 as antagonists of various in vivo effects of cannabinoid agonists in C57BL/6J mice. Eur J Pharmacol. 2007;569:70–6. doi: 10.1016/j.ejphar.2007.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meye FJ, Trezza V, Vanderschuren LJ, Ramakers GM, Adan RA. Neutral antagonism at the cannabinoid 1 receptor: a safer treatment for obesity. Mol Psychiatry. 2013;18:1294–301. doi: 10.1038/mp.2012.145. [DOI] [PubMed] [Google Scholar]
- National Institute of Health . Principles of animal laboratory care. National Academy; Washington, DC: 1996. [Google Scholar]
- Nikas SP, Alapafuja SO, Papanastasiou I, Paronis CA, Shukla VG, Papahatjis DP, Bowman AL, Halikhedkar A, Han X, Makriyannis A. Novel 1′,1′-chain substituted hexahydrocannabinols: 9beta-hydroxy-3-(1-hexyl-cyclobut-1-yl)-hexahydrocannabinol (AM2389) a highly potent cannabinoid receptor 1 (CB1) agonist. J Med Chem. 2010;53:6996–7010. doi: 10.1021/jm100641g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva JM, Ortiz S, Palomo T, Manzanares J. Behavioural and gene transcription alterations induced by spontaneous cannabinoid withdrawal in mice. J Neurochem. 2003;85:94–104. doi: 10.1046/j.1471-4159.2003.01627.x. [DOI] [PubMed] [Google Scholar]
- Rawls SM, Cabassa J, Geller EB, Adler MW. CB1 receptors in the preoptic anterior hypothalamus regulate WIN 55212-2 [(4,5-dihydro-2-methyl-4(4-morpholinylmethyl)-1-(1-naphthalenyl-carbonyl)-6H-pyrrolo[3,2,1ij]quinolin-6-one]-induced hypothermia. J Pharmacol Exp Ther. 2002;301:963–8. doi: 10.1124/jpet.301.3.963. [DOI] [PubMed] [Google Scholar]
- Richardson JD, Kilo S, Hargreaves KM. Cannabinoids reduce hyperalgesia and inflammation via interaction with peripheral CB1 receptors. Pain. 1998;75:111–9. doi: 10.1016/S0304-3959(97)00213-3. [DOI] [PubMed] [Google Scholar]
- Salamone JD, McLaughlin PJ, Sink K, Makriyannis A, Parker LA. Cannabinoid CB1 receptor inverse agonists and neutral antagonists: effects on food intake, food-reinforced behavior and food aversions. Physiol Behav. 2007;91:383–8. doi: 10.1016/j.physbeh.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sink KS, McLaughlin PJ, Wood JA, Brown C, Fan P, Vemuri VK, Peng Y, Olszewska T, Thakur GA, Makriyannis A, Parker LA, Salamone JD. The novel cannabinoid CB1 receptor neutral antagonist AM4113 suppresses food intake and food-reinforced behavior but does not induce signs of nausea in rats. Neuropsychopharmacology. 2008;33:946–55. doi: 10.1038/sj.npp.1301476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai S, Järbe TUC, Nikas SP, Makriyannis A. Characterization of a CB1 receptor agonist (AM2389) with a long duratoin of effect to facilitate the study of CB1 dependence/withdrawal. International Cannabinoid Research Society (ICRS); Research Triangle Park, NC: 2011. [Google Scholar]
- Tam J, Vemuri VK, Liu J, Batkai S, Mukhopadhyay B, Godlewski G, Osei-Hyiaman D, Ohnuma S, Ambudkar SV, Pickel J, Makriyannis A, Kunos G. Peripheral CB1 cannabinoid receptor blockade improves cardiometabolic risk in mouse models of obesity. J Clin Invest. 2010;120:2953–66. doi: 10.1172/JCI42551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur GA, Nikas SP, Makriyannis A. CB1 cannabinoid receptor ligands. Mini Rev Med Chem. 2005;5:631–40. doi: 10.2174/1389557054368772. [DOI] [PubMed] [Google Scholar]
- Wilson DM, Varvel SA, Harloe JP, Martin BR, Lichtman AH. SR 141716 (Rimonabant) precipitates withdrawal in marijuana-dependent mice. Pharmacol Biochem Behav. 2006;85:105–13. doi: 10.1016/j.pbb.2006.07.018. [DOI] [PubMed] [Google Scholar]