Abstract
Objective
Hemorrhagic shock may contribute to acute kidney injury by profoundly altering renal mitochondrial function. Resveratrol (RSV), a naturally occurring sirtuin-1 (SIRT1) activator, has been shown to promote mitochondrial function and reduce oxidative damage in a variety of aging-related disease states. We hypothesized that RSV treatment during resuscitation would ameliorate kidney mitochondrial dysfunction and decrease oxidative damage following hemorrhagic shock.
Method
Using a decompensated hemorrhagic shock model, male Long-Evans rats (n=6 per group) were sacrificed prior to hemorrhage (Sham), at severe shock, and following either lactated Ringer’s (LR) Resuscitation or LR+RSV Resuscitation (RSV: 30mg/kg). At each time point, blood samples were assayed for arterial blood gases, lactate, blood urea nitrogen (BUN) and serum creatinine. Mitochondria were also isolated from kidney samples in order to assess individual electron transport complexes (CI, CII, and CIV) using high-resolution respirometry. Total mitochondria reactive oxygen species (ROS) were measured using fluorometry and lipid peroxidation was assessed by measuring 4-hydroxynonenal by Western blot. qPCR was used quantify mRNA from PGC1-α, SIRT1, and proteins known to mitigate oxidative damage and promote mitochondrial biogenesis.
Results
RSV supplementation during resuscitation restored mitochondrial respiratory capacity, decreased mitochondrial ROS and lipid peroxidation. Compared to standard LR resuscitation, RSV treatment significantly increased SIRT1 and PGC1-α expression and significantly increased both SOD2 and catalase expression. Although RSV was associated with decreased lactate production, pH, BUN and serum creatinine values did not differ between resuscitation strategies.
Conclusions
Resuscitation with RSV significantly restored renal mitochondrial function and decreased oxidative damage following hemorrhagic shock.
Keywords: resveratrol, hemorrhagic shock, kidney, resuscitation, mitochondria, oxidative stress
Introduction
Although hemorrhagic shock remains a common cause of early mortality in severely injured patients, a significant number of deaths occur indirectly as the result of multi-organ failure days to weeks after the initial injury (1). The kidney is especially vulnerable to acute blood loss due to its complex microvascular structure and high oxygen dependency (2). Moreover, acute renal damage is independently associated with higher mortality following trauma. In a review of nearly 400,000 trauma patients, those who developed renal failure were over 5 times more likely to die than similarly injured patients (3). Additionally, the development of acute kidney injury results in the need for longer hospital stays and significantly higher healthcare costs (4). Resuscitative strategies that mitigate acute renal failure could improve trauma patient outcomes and decrease overall healthcare costs.
In severely injured patients, persistent mitochondrial dysfunction is associated with the development of multi-organ failure and higher mortality (5). Because mitochondria utilize oxygen to synthesize nearly 95% of the adenosine triphosphate (ATP) required to meet the body’s energy needs, impaired perfusion during hemorrhagic shock results in mitochondrial and energetic stress (6); Although resuscitation can restore adequate tissue oxygen levels, reperfusion may exacerbate mitochondrial dysfunction by increasing the generation of reactive oxygen species (ROS) (5). ROS not only directly damage mitochondrial proteins, but they can also promote the formation of the mitochondrial permeability transition pore, increase release of cytochrome c, and enhance apoptosis (7). Despite adequate resuscitation following hemorrhagic shock, persistent mitochondrial dysfunction may contribute to ongoing cellular injury and organ failure (8). Improving mitochondrial function and decreasing oxidative damage, therefore, are important steps in mitigating shock-induced kidney injury.
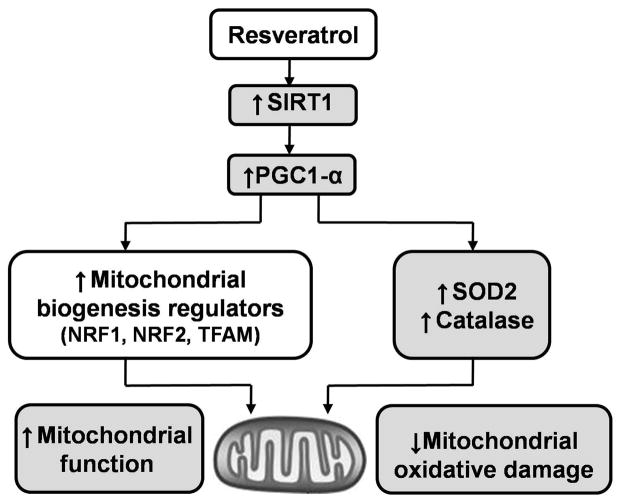
Resveratrol (RSV), a natural polyphenol produced by several plants, has been shown to promote mitochondrial function and reduce oxidative damage in a variety of aging-related diseases (9, 10). Many of the biological effects of RSV are thought to be secondary to its activation of sirtuin-1 (SIRT1) – a highly conserved NAD+-dependent enzyme deacetylase that plays a critical role in aging and stress resistance (11). SIRT1 promotes mitochondrial function by deacetylating and activating peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC1-α), a master regulator of mitochondrial biogenesis, and by inducing the transcription of antioxidant genes (Figure 1) (12, 13). Resveratrol may also have antioxidant effects through a variety of complementary mechanisms, including the activation of nuclear factor erythroid 2-related factor 2 (Nrf2) and the aryl hydrocarbon receptor, which induce antioxidant and drug-metabolizing enzymes. When used in animal models of hemorrhagic shock, RSV has been shown to reduce inflammation and mitigate organ dysfunction (14, 15). There is little information, however, on the impact of RSV on mitochondrial function following acute blood loss.
Figure 1. Potential mechanisms of beneficial effects of resveratrol in mitochondrial protection and anti-oxidative properties.
SIRT1, sirtuin 1; PGC1-α, peroxisome proliferator-activated receptor gamma coactivator 1-α; NRF, nuclear respiratory factor; TFAM, mitochondrial transcription factor A; SOD2, superoxide dismutase 2.
Here, we investigated decompensated hemorrhagic shock in a rodent model and hypothesized that resuscitation with RSV would ameliorate shock-induced kidney mitochondrial dysfunction and decrease oxidative damage by activating SIRT1 dependent pathways.
Materials and Methods
Experimental Protocol
All animal procedures were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania and in accordance with the guidelines established by the National Institutes of Health. Male Long-Evans rats (250–300 g) were housed in a facility with constant temperature and humidity with a 12-hour light/dark cycle. Animals were allowed to acclimate at least 2 days before surgery and given access to food and water ad lib. Using a well-validated decompensated hemorrhagic shock model (16), rats were anesthetized using vaporized isoflurane (2–4%) and underwent placement of femoral vascular catheters (PE50, Braintree Scientific, Inc., Brain-tree, MA). A 5-cm midline laparotomy was performed to simulate soft tissue trauma. All surgical sites were bathed in 1% lidocaine and closed in layers. Animals received 0.25% buprenorphine (0.05mg/kg) and were allowed to emerge from anesthesia. Mean arterial pressure (MAP) and heart rate (HR) were continuously monitored and recorded throughout the experimental protocol (Digi-Med Signal Analyzers, Louisville, KY, USA). After full reversal of anesthesia and 30 minutes of hemodynamic stability, animals were randomized to 1 of 4 groups: Sham, Severe Shock, and Resuscitation with either LR or LR+ RSV. Animals in the Severe Shock or either Resuscitation group were passively bled via the femoral artery and maintained at a MAP of 40 mmHg. When the blood pressure could no longer be maintained without fluid infusion, a MAP of 40 mmHg was sustained by incrementally infusing 0.2 cc boluses of lactated Ringer’s solution (LR). Animals were considered to be in Severe Shock when 40% of the total shed volume had been returned in the form of LR boluses. Animals were then Resuscitated with four times the shed volume in LR with or without RSV (30 mg/kg, Orchid Pharmaceuticals, Lalilab, Durham NC) over 60 minutes (17). RSV was suspended in 50% DMSO (50mg/ml) and an equivalent dose of DMSO was given in animals resuscitated with only LR. Animals (n=6 per group) were euthanized prior to hemorrhage (Sham), at Severe Shock, and following resuscitation with LR or LR+RSV using intravenous pentobarbitol (150mg/kg) and the kidneys were removed immediately. Before sacrifice, blood samples were taken and assayed for arterial blood gases, lactate, hemoglobin, glucose, blood urea nitrogen (BUN) and electrolytes (i-STAT, Abbot Point of Care Inc., Princeton, NJ). Serum creatinine was determined using a Vitros 350 blood biochemistry analyser (Ortho Clinical Diagnostics, Rochester, NY). Serum Neutrophil gelatinase-associated lipocalin (NGAL) levels were measured by ELISA (Bioporto Diagnostics, Denmark). Animals were then euthanized (intravenous Pentobarbitol 150mg/kg) and kidneys were removed immediately.
Isolation of mitochondria
Whole kidneys (4–6 g) were excised immediately each time point following pentobarbital injection. After quickly dissecting away connective tissue and fat, samples were weighed and immersed in ice-cold mitochondrial isolation buffer (MIB) (210 mMmannitol, 70 mM sucrose, 10 mM HEPES, 1 mM EDTA with final pH adjusted to 7.2 using KOH and supplemented with 0.5% fatty acid-free BSA). Tissue was minced and then homogenized with an additional 10 volumes (wt/vol) of MIB with 5% BSA using Potter Elvehjem homogenizer with a loose-fitting Teflon pestle. Mitochondrial isolation was performed using differential centrifugation (18). The homogenate was centrifuged for 10 minutes at 1000 × g (4°C). The supernatant was collected and recentrifuged for 10 minutes at 9,600 × g. The pellet was then resuspended in 15 ml MIB without BSA and centrifuged for an additional 10 minutes at 9,600 × g for further mitochondrial purification. The final mitochondrial pellet was resuspended in MIB and protein concentration was determined spectrophotometrically using the Biuret method with BSA as standard.
Mitochondrial respiratory capacity using high resolution respirometry
A standard substrate/inhibitor titration protocol was used for functional analysis of mitochondrial respiratory chain complexes (19). Individual complex activity directly impacts overall mitochondrial function and contributes to both basal and maximal respiration. Isolated mitochondria (0.15 mg) were resuspended in respiration medium (110mM mannitol, 0.5mM EGTA, 3mM MgCl2, 20mM taurine 10mM KH2PO4, 60mM K lactobionate, 0.3mM DTT, and 0.1% BSA (fatty acid free), adjusted to pH of 7.1 with KOH) (19). Oxygen consumption was measured using high-resolution respirometry (Oxygraph-2k Oroboros Instruments, Innsbruck, Austria). Following stabilization (3–5 minutes), real-time oxygen concentration and flux data were collected continuously (DatLab software 4.3, Oroboros Instruments, Innsbruck, Austria). After the basal respiration rate was recorded, complex I (CI)-dependent mitochondrial respiration was induced by adding 10 mM glutamate, 5 mM malate and 1 mM ADP. In order to determine complex II (CII)-dependent respiration, rotenone (0.5 μM), a selective inhibitor of CI, was added to the medium followed by 10 mM of succinate. Antimycin A (5 μM) was then added to inhibit complex III (CIII) in order to measure non-mitochondrial respiration; followed by addition of TMPD (0.5 mM) and ascorbate (2 mM) as artificial substrates for complex IV. In order to ensure that the respiratory capacity of complex IV (CIV) was not limited by cytochrome c depletion, respiration was measured after the addition cytochrome c (10 μM) (19). This protocol was completed within 60 to 70 min.
Mitochondrial protein 4-hydroxynonenal and 3-nitrotyrosine analysis
Overall tissue damage by reactive oxygen and nitrogen species was assessed by measuring 4-hydroxynonenal and 3-nitrotyrosine by Western blot (Abcam, Cambridge, MA) (20, 21). Briefly, mitochondrial protein (20 μg) was loaded in a 4–12% polyacrylamide gel and separated by electrophoresis (Invitrogen, San Diego, CA). Proteins were transferred onto a nitrocellulose membrane (Bio-Rad, Richmond, CA). After the membranes were blocked for 1 hour at room temperature (10 mmol/L Tris, 150 mmol/L NaCl, and 0.05% Tween-20 supplemented with 5% dry milk), they were incubated with the respective primary antibodies at 1:1000 dilution overnight at 4°C. After washing, membranes were incubated with peroxidase-linked donkey anti-rabbit or sheep anti-mouse IgG secondary antibodies (Amersham, Buckinghamshire, UK) at 1:5,000 dilution for 1 hour at room temperature. Signals were developed by enhanced chemiluminescence (PerkinElmer Life Sciences, Boston, MA). Bands were scanned and quantified by densitometry using ImageJ software (National Institutes of Health, Bethesda, MD).
Total Production of Mitochondrial-derived ROS
To analyze the total production of ROS, isolated mitochondria (10 μg) were suspended in 1 ml of buffer (250 mM sucrose, 20 mM 3-[N-morpholino] butane sulfonic acid, 10 mMTris-base, 100 μMPi [K], 0.5 mM Mg2+, pH 7.0; 30°C) containing CI substrates (malate/glutamate, 2.5/2.5 mM) with 10 μM H2DCFDA. Antimycin A (an inhibitor of CIII; 0.5 μM) was added to allow the production of ROS (22). After incubation at 30°C for 1 hour, the fluorescent signal from dichlorofluorescein (DCF; excitation 488 nm, emission 525 nm) was detected and quantified using a Modulus Microplate Reader (Turner Biosystems, Sunnyvale, CA).
Citrate synthase activity
Citrate synthase activity is commonly used as a quantitative enzyme marker for the presence of intact mitochondria [19]. Citrate synthase activity was determined according to the method described by Srere and Matsuoka (23), which couples coenzyme A to 5, 5′-dithiobis-2-nitrobenzoic acid (DTNB). Kidney tissue (100 μg) was suspended in an assay buffer that included 0.1 mM DTNB (in 1M Tris buffer, pH 8.0), 0.3 mM acetyl coenzyme A and 0.05% Triton X-100. Following the addition of 1 mM oxaloacetate, citrate synthase activity was determined spectrophotometrically by measuring the absorbance of thio-nitrobenzoic acid at 412 nm at 37°C.
NAD+-to-NADH ratio measurements
In order to determine NAD+ and NADH levels, flash-frozen kidney samples (30–60 mg) were treated with HClO4 (0.6 M) or KOH (0.25N) respectively. Treated samples were lysed for 1 minute (20Hz) using a Qiagen Tissue Lyser (Qiagen, West Sussex, UK). After centrifugation (12,000 rpm for 10 min at 4°C), the supernatant was removed and diluted in ice- cold 100mM phosphate buffer (pH 8). Extracts for NADH analysis were treated at 55°C for 10 minutes to hydrolyze free NAD prior to analysis. Diluted extracts were mixed with 0.1M phosphate buffer (pH8), 0.1mg/ml BSA, 10mM nicotinamide, 10uM flavin mononucleotide, 20uM resazurin, and 2% ethanol. A fluorescent signal (excitation at 540/535nm, emission at 590 nm) was detected and quantified in Modulus Microplate Reader (Turner Biosystems, Sunnyvale, CA) (24). Increasing concentrations of NAD (Roche Diagnostics, Indianapolis, IN) were also loaded to generate a standard curve.
Quantitative Polymerase Chain Reaction (qPCR)
Total RNA was isolated by using the TRIzol reagent extraction kit (Invitrogen, Carlsbad, CA), according to the manufacturer’s instructions. Total RNA (1μg) was subsequently reverse-transcribed to cDNA by using the high-capacity cDNA RT kit (Applied Biosystems, Foster City, CA). Diluted cDNA samples were subjected to qPCR using SYBR Selected Master Mix kit (Applied Biosystems, Foster City, CA) and specific primers for individual genes (ABI Prism TM 7900HT Sequence Detection System, Applied Biosystems, Foster City, CA). Relative mRNA expression levels were calculated using the ΔΔCt method and normalized to a housekeeping gene RPLP0 (60S acidic ribosomal protein P0). Sequences of all primers used for amplification reaction assays are summarized in Table 1.
Table 1.
Primers used for qPCR
| Transcript | Forward Primer | Reverse Primer |
|---|---|---|
| RPLP0 | TGGGAATTTTGGTGTTTCAGACT | ACCGCATCGTTAGAACCAGAC |
| SIRT1 | CAGGTTGCAGGAATCCAAA | CAAATCAGGCAAGATGCTGT |
| PGC1-α | CCAGTCTACGGCTGTTTGGT | TGGAAGAACAGATGTGCCCC |
| NRF1 | ACAGATAGTCCTGTCTGGGGAAA | TGGTACATGCTCACAGGGATCT |
| NRF2 | TGAAGTTCGCATTTTGATGGC | CTTTGGTCCTGGCATCTCTAC |
| TFAM | GTTTCGTGCGGGTTTGTGAA | GAAACTGCAATGGCTCTGCC |
| SOD2 | GCCTGCACTGAAGTTCAATG | ATCTGTAAGCGACCTTGCTC |
| CAT | ACCCTCTTATACCAGTTGGC | GCATGCACATGGGGCCATCA |
| COX-2 | ATTCTTTGCCCAGCACTTCA | ATCATCAGACCAGGCACCA |
Primers used for quantitative polymerase chain reaction (qPCR): RPLP0 - 60S acidic ribosomal protein P0; SIRT1 - sirtuin 1; PGC1-α - peroxisome proliferator-activated receptor gamma coactivator 1-alpha; NRF1 - nuclear respiratory factor 1; NRF2 - nuclear respiratory factor 2; TFAM - mitochondrial transcription factor A; SOD2 - superoxide dismutase 2; CAT - catalase; COX-2 - cyclooxygenase 2.
Statistics
Analyses were performed using SPSS 16.0 (SPSS Inc., Armonk, NY) and GraphPad Prism 4 (Graphpad Software Inc., San Diego, CA). Data were analyzed using one-way ANOVA with a post hoc Tukey’s test. Results are presented as mean ± SEM. A p value of less than 0.05 was considered statistically significant.
RESULTS
Physiologic and Laboratory Parameters
Blood pressure was maintained at a fixed MAP of 40 mmHg during shock. As previously described, this decompensated hemorrhagic shock model resulted in severe lactic acidosis as well as hyperkalemia, uremia, and an increase in creatinine. A significant anemia also developed given the crystalloid-only resuscitation (16). Compared to LR alone, LR+RSV resuscitation was associated with significantly less lactate production (10.2 ± 1.2 vs. 6.9 ± 1.3mmol/L, p< 0.05), improved HCO3- levels (11.3 ± 1.1 vs.17.2 ± 1.4mmol/L, p< 0.05), less hyponatremia (130 ± 3 vs. 138 ± 1mmol/L, p< 0.05), and decreased NGAL levels (53.6 ± 1.0 vs. 107.8 ± 28.5 ng/ml). RSV supplementation, however, did not significantly impact the MAP, arterial pH, BUN or creatinine values (Table 2).
Table 2.
Physiologic and laboratory parameters
| Characteristics | Sham (n=6) | Severe Shock (n=6) | LR Resuscitation (n=6) | LR+RSV Resuscitation (n=6) |
|---|---|---|---|---|
| MAP (mmHg) | 111 ± 2 | 41 ± 1a | 78 ± 5a,b | 86 ± 2a,b |
| HR (bpm) | 440 ± 12 | 419 ± 8a | 438 ± 23 | 456 ± 15 |
| Lactate (mmol/L) | 1.3 ± 0.3 | 17.2 ± 1.3a | 10.2 ± 1.2a,b | 6.9 ± 1.3b,c |
| pH | 7.40 ± 0.01 | 7.05 ± 0.09a | 7.20 ± 0.04a,b | 7.32 ± 0.03b |
| PCO2 (mmHg) | 47 ± 4 | 17 ± 2a | 28 ± 1a,b | 33 ± 1b |
| PO2 (mmHg) | 85 ± 4 | 128 ± 4a | 113 ± 5a | 105 ± 2b |
| HCO3− (mmol/L) | 29.1 ± 2.0 | 5.4 ± 1.6a | 11.3 ± 1.1a,b | 17.2 ± 1.4b,c |
| BUN (mg/dl) | 23 ± 2 | 29 ± 2a | 25 ± 1 | 30 ± 3 |
| Creatinine (mg/dl) | 0.28 ± 0.03 | 0.63 ± 0.03a | 0.53 ± 0.05a | 0.57 ± 0.06a |
| Na+ (mmol/L) | 135 ± 1 | 128 ± 1a | 130 ± 3 | 138 ± 1b,c |
| K+ (mmol/L) | 4.5 ± 0.3 | 6.4 ± 0.6a | 5.0 ± 0.4b | 5.4 ± 0.4b |
| Cl− (mmol/L) | 103 ± 1 | 101 ± 1a | 100 ± 2 | 112 ± 3b,c |
| Hemoglobin (g/L) | 12.3 ± 0.4 | 5.0 ± 0.5a | 3.8 ± 0.1b | 4.1 ± 0.3 |
| NGAL (ng/ml) | 60.5 ± 8.2 | 73 ± 23.2 | 107.8 ± 28.5 a,b | 53.6 ± 1.0 b,c |
LR = Lactated Ringer’s solution; RSV = Resveratrol; MAP = Mean Blood Pressure; HR = Heart Rate; NGAL = Neutrophil gelatinase-associated lipocalin; BUN = Blood Urea Nitrogen. Values are mean ± SEM. n = 6.
p<0.05 versus Sham.
p<0.05 versus Severe Shock.
p<0.05 versus LR Resuscitation.
RSV supplementation during resuscitation restored renal mitochondrial function following HS and resuscitation
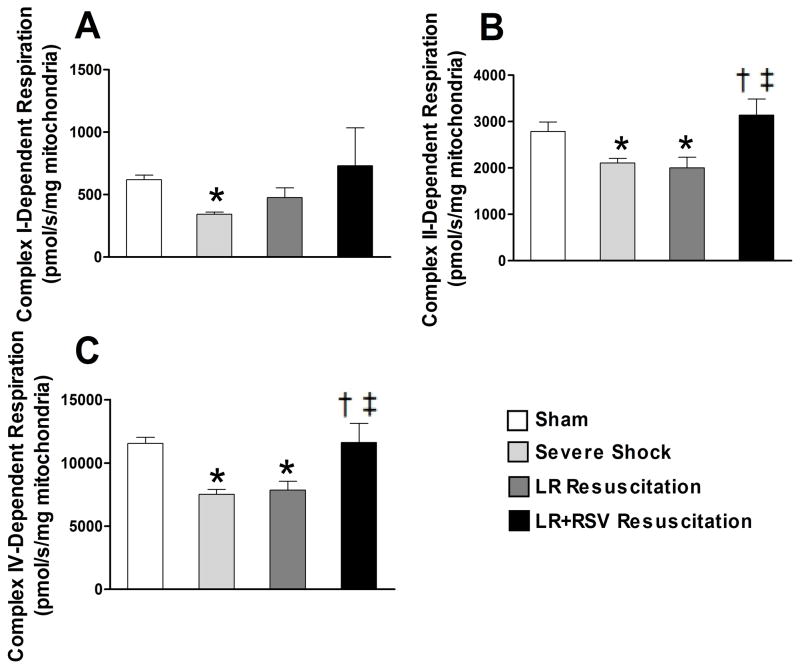
Using high-resolution respirometry, we observed that hemorrhagic shock resulted in significantly decreased respiratory capacity of all mitochondrial complexes (Fig. 2). Compared to LR alone, RSV supplementation during resuscitation significantly restored CII and CIV-dependent respiration (2000.0 ± 234.9 vs. 3137.0 ± 349.5 pmol/s/mg mitochondria and 7857.1 ± 695.8 vs. 11615.4 ± 1518.9 pmol/s/mg mitochondria, respectively; all p< 0.05, Fig. 2B and C).
Figure 2. Resveratrol treatment during resuscitation restored renal mitochondrial function following hemorrhagic shock and resuscitation.
Mitochondrial respiratory capacities in isolated intact mitochondria were assessed by high resolution respirometry. Hemorrhagic shock decreased the respiratory capacity of all complexes in this model of decompensated hemorrhagic shock. RSV supplementation significantly restored Complexes II and IV-dependent respiratory capacity. LR = Lactated Ringer’s solution; RSV = Resveratrol. Values are mean ± SEM. n = 6,*p<0.05 versus Sham; †p<0.05 versus Severe Shock; ‡p<0.05 versus LR Resuscitation.
RSV treatment during resuscitation ameliorated renal mitochondrial oxidative stress following HS and resuscitation
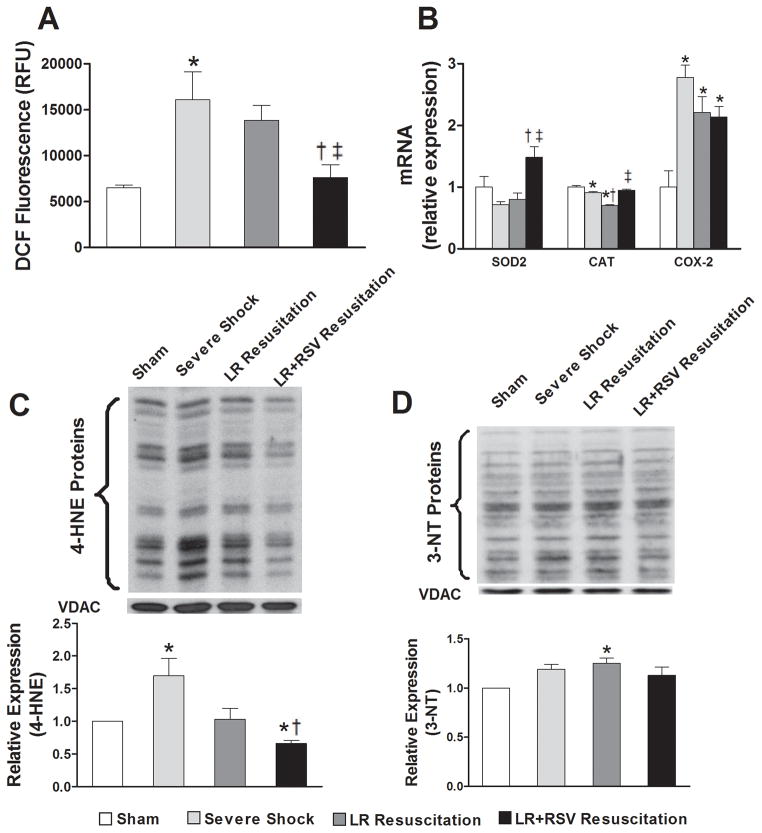
RSV supplementation during resuscitation significantly reduced mitochondrial ROS production following hemorrhagic shock (p< 0.05, Fig. 3A). Furthermore, resuscitation with RSV resulted in a significant increase in the mRNA expression of superoxide dismutase 2 (SOD2) and catalase (CAT) in kidney tissue when compared to resuscitation with LR alone (p< 0.05, Fig. 3B). In contrast, severe hemorrhagic shock was associated with a marked increase in the expression of cyclooxygenase 2 (COX-2) mRNA that was not influenced by either resuscitative strategy (Fig. 3B).
Figure 3. Resveratrol treatment during resuscitation ameliorated renal mitochondrial oxidative stress following hemorrhagic shock and resuscitation.
A, Mitochondrial-derived reactive oxygen species (ROS) production was detected by measuring the fluorescent signal from dichlorofluorescein. RSV supplementation significantly reduced the production of ROS following hemorrhagic shock and resuscitation. B, LR+RSV resuscitation significantly increased the mRNA expression of dismutase 2 (SOD2) and catalase (CAT) in kidney tissue when compared to LR resuscitation. C, 4-hydroxynonenal (4-HNE) was measured by western blotas a marker of mitochondrial lipid peroxidation. 4-HNE levels robustly increased following Severe Shock and were significantly reduced with LR+RSV resuscitation. D, Expression levels of 3-nitrotyrosine (3-NT) in mitochondria were determined by western blot. 3-NT levels increased with LR resuscitation, but did not increase with LR+RSV resuscitation. LR = Lactated Ringer’s solution; RSV = Resveratrol; COX = cyclooxygenase. Values are mean ± SEM. n = 6, *p<0.05 versus Sham; †p<0.05 versus Severe Shock; ‡p<0.05 versus LR Resuscitation.
Oxidative damage to mitochondrial proteins increased dramatically following severe hemorrhagic shock and improved with RSV treatment during resuscitation. 4-hydroxynonenal, a measure of lipid peroxidation from reactive oxygen species, increased robustly following severe shock (Fig. 3C). RSV supplementation significantly ameliorated the degree of lipid peroxidation observed. We also measured the degree of nitrosative stress. While 3-nitrotyrosine levels increased with LR resuscitation, resuscitation with RSV was not associated with enhanced nitrosative stress following hemorrhagic shock (Fig. 3D).
RSV supplement increased the mRNA expression of SIRT1 and the NAD+-NADH ratio in kidney
Severe shock resulted in a dramatic decline in the mRNA expression of SIRT1. Resuscitation with RSV, however, rescued expression of SIRT1 to levels seen in the sham group (p< 0.05, Fig. 4A). Although hemorrhagic shock and subsequent resuscitation with LR alone did not significantly alter NAD+ or NADH concentrations, resuscitation with RSV significantly decreased tissue NADH levels (160.6 ± 21.3 vs. 265.7 ± 24.5 nmol/mg protein; p< 0.05) and nearly doubled the NAD+-to-NADH ratio (Fig. 4B).
Figure 4. Resveratrol supplementation enhanced the mRNA expression of Sirtuin 1(SIRT1) and increased the nicotinamide adenine dinucleotide (NAD+) -nicotinamide adenine dinucleotide dehydrogenase (NADH) ratio in kidney.
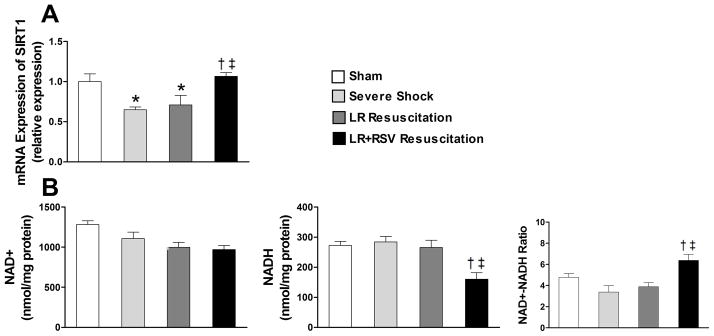
A, The decline in the mRNA expression level of SIRT1 after severe shock and LR resuscitation was reversed with RSV administration during resuscitation. B, NAD+, NADH concentration and NAD+-NADH ratio in kidney tissue were obtainedby detecting the fluorescent signal of resazurin. LR+RSV resuscitation significantly decreased the NADH concentration and nearly doubled the NAD+-to-NADH ratio when compared to LR resuscitation. LR = Lactated Ringer’s solution; RSV = Resveratrol. Values are mean ± SEM. n = 6, *p<0.05 versus Sham; †p<0.05 versus Severe Shock; ‡p<0.05 versus LR Resuscitation.
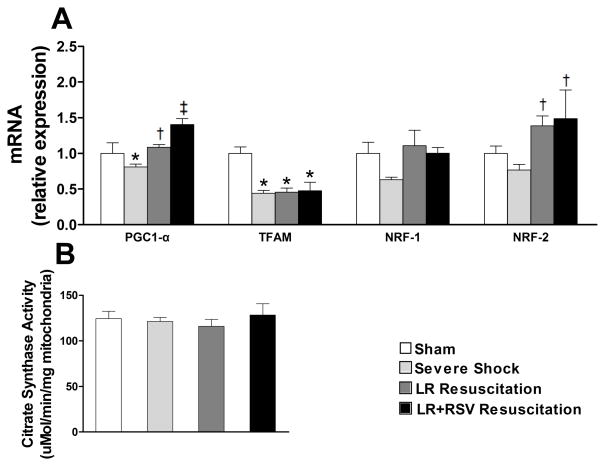
RSV increased mRNA expression of PGC1-α in kidney, but did not promote mitochondrial biogenesis
To gain further insight into the potential impact of RSV on mitochondrial function following hemorrhagic shock, we evaluated the expression of mitochondrial transcriptional regulators including PGC1-α in kidney tissues. Notably, while the mRNA expression of PGC1-α was significantly elevated in the RSV resuscitation group, RSV did not restore the expression of mitochondrial transcription factor A (TFAM) (Fig. 5A). Interestingly, the expression of nuclear respiratory factor (NRF) -1 was not significantly altered by either hemorrhagic shock or by resuscitation, whereas both resuscitation with LR alone and LR+RSV were associated with an increased expression of NRF-2 (Fig. 5A). Lastly, we did not observe a significant change in mitochondrial abundance as measured by citrate synthase activity at any time point (Fig.5B).
Figure 5. mRNA expression of mitochondrial biogenesis factors and mitochondria abundance in kidney.
A, mRNA expression of peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC1-α) was significantly elevated in LR+RSV resuscitation group compared to the LR resuscitation only group. However, the mRNA expressions of mitochondrial transcription factor A (TFAM), nuclear respiratory factor (NRF) 1 and 2 were not influenced by RSV supplementation. B, Citrate synthase activity was determined spectrophotometrically based on coenzyme A coupled to 5, 5′-dithiobis-2-nitrobenzoic acid. Citrate synthase activity did not significantly change with Severe Shock or with resuscitative strategy. LR = Lactated Ringer’s solution; RSV = Resveratrol. Values are mean ± SEM. n = 6, *p<0.05 versus Sham; †p<0.05 versus Severe Shock; ‡p<0.05 versus LR Resuscitation.
DISCUSSION
Resveratrol is a naturally occurring compound with known antioxidant and anti-inflammatory properties (9, 10). Because ischemia-reperfusion events are frequently complicated by mitochondrial dysfunction and exuberant ROS production, we hypothesized that adding resveratrol to standard fluid resuscitation would improve mitochondrial function following acute blood loss. In our model of decompensated hemorrhagic shock, resveratrol significantly ameliorated renal mitochondrial dysfunction and robustly restored Complex II and IV dependent respiratory capacity. Resveratrol also significantly decreased the production of mitochondrial ROS and minimized the subsequent damage from lipid peroxidation. Furthermore, our data suggests that resveratrol benefits mitochondrial function in the acute setting, not by promoting mitochondrial biogenesis, but by activating SIRT1 and PGC1-α-mediated antioxidant pathways.
We elected to investigate novel resuscitative strategies that target renal function because the kidney is frequently one of the first organs damaged by profound blood loss. Although it accounts for only 2% of total body weight, the kidney receives approximately 25% of the cardiac output (2). With acute blood loss, however, cardiac output drops dramatically and the kidney is subjected to profound vasoconstriction in an attempt to preserve blood flow to more vital organs such as the heart and brain (25). As such, hemorrhagic shock significantly decreases renal perfusion. With resuscitation and subsequent tissue reoxygenation, a cascade of intracellular events is triggered that can result in oxidative damage, tissue dysfunction, and multi-organ failure (26). In our study, severe hemorrhagic shock was associated with increased reactive oxygen species and enhanced lipid peroxidation. We also observed that following hemorrhagic shock the kidney suffered severe mitochondrial dysfunction as reflected by a decrease in the respiratory capacity of all mitochondrial complexes. Moreover, hemorrhagic shock was associated with a mild, but statistically significant, increase in both the BUN, creatinine and NGAL suggesting a possible correlation between mitochondrial dysfunction and renal impairment.
Currently, the standard approach to treating hemorrhagic shock is volume replacement with crystalloid fluids in order to increase the circulating intravascular volume, restore blood pressure, and maintain organ perfusion (27). Crystalloid resuscitation, however, does not prevent systemic inflammation or oxidative stress (28). Moreover, we have previously reported that resuscitation with lactated Ringer’s does not restore mitochondrial respiratory capacity in vital organs such as the heart, liver and kidney (6). Similarly, in this study, resuscitation with lactated Ringer’s did not restore mitochondrial respiratory capacity or mitigate mitochondrial oxidative stress in the kidney. Because mitochondrial dysfunction can further exacerbate cellular damage by reducing aerobic ATP production and increasing the generation of reactive oxygen species (ROS) (5), resuscitative fluids that preserve mitochondrial function or augment antioxidant capacity could prove beneficial.
Resveratrol has previously been shown to be protective in several models of critical illness. In septic animals, treatment with resveratrol preserved tissue morphology in the lung and kidney (29), mitigated acute lung injury and prevented myocardial depression (17, 30). Resveratrol has also been shown to be beneficial in several trauma-hemorrhage models. Resuscitation with resveratrol not only improves cardiac output (14), it has been shown to decrease hepatic injury and inflammation following hemorrhagic shock (15). As such, resveratrol may provide a useful therapeutic adjunct to standard fluid resuscitation following traumatic injury.
In our hemorrhagic shock model, resveratrol significantly alleviated acute kidney injury (AKI). Although resveratrol did not improve post-resuscitation serum creatinine levels, its use was associated with a significant decrease in serum NGAL. NGAL, a 25 kDa protein covalently bound to matrix metalloproteinase-9 in neutrophils, is markedly induced following epithelial injury and appears to be more sensitive biomarker for AKI than serum creatinine (31, 32). Elevations in NGAL precede changes in serum creatinine and can be used to diagnose AKI up to 48 hours prior to a clinical change in creatinine or urine output. Given this sensitivity, changes in NGAL are thought to reflect AKI in real-time and following NGAL levels may allow for the institution of earlier, and more effective, renoprotective therapies (31).
Although the mechanism underlying resveratrol’s benefit remains controversial, resveratrol appears to attenuate injury by activating SIRT1 dependent pathways. SIRT1, a NAD+ dependent “survival” enzyme, deacetylates and activates PGC1-α; a key regulator of mitochondrial function and metabolism(12). In turn, PGC1-α impacts two different pathways that may be critical to cell survival following ischemia-reperfusion (Figure 1). Firstly, PGC-1α is required for the induction of many ROS-detoxifying enzymes, including SOD2 and catalase (13). Secondly, PGC1-α induces mitochondrial biogenesis by binding to NRFs and enhancing their activity (33). NRFs subsequently increase the expression of TFAM, an enzyme directly responsible for the transcription of nuclear-encoded mitochondrial proteins (13). As an upstream regulator of PGC-1α, strategies that enhance SIRT1 expression or activity may also play an important role in regulating oxidative stress and mitochondrial biogenesis in vitro. Importantly, the antioxidant and mitochondrial effects of resveratrol also appear to be SIRT1 mediated. In cell culture models, knocking down SIRT1 not only blocked the protective effects of resveratrol on mitochondrial oxidative stress (34), it also prevented resveratrol-induced up-regulation of mitochondrial biogenesis factors (35).
In this study, adding resveratrol to standard resuscitation with lactated ringer’s restored mitochondrial function while mitigating oxidative damage. While resveratrol has been shown to enhance the enzymatic activity of succinate dehydrogenase, a protein that constitutes complex II on the inner mitochondrial membrane (9), in our study, resveratrol also appears to restore CIV activity. Although it is possible that resveratrol could directly impact CIV activity, it is more likely that the benefit observed stems from resveratrol’s known antioxidant effects (33). Both ROS and lipid peroxidation products can effectively inhibit CIV activity following ischemia-reperfusion (36, 37). In our study, resuscitation with resveratrol resulted in decreased ROS production and 4-hydroxynonenal damage following hemorrhagic shock. Thus, the salutary effect of resveratrol on mitochondrial function could be in part secondary to decreased CIV inhibition by oxidant byproducts.
Resveratrol also appears to enhance antioxidant defenses following hemorrhagic shock. With ischemia-reperfusion, the expression of two critical antioxidants, SOD2 and catalase, were significantly decreased. In this study, resveratrol significantly increased the expression of both SOD2 and catalase following hemorrhagic shock, which could have contributed to the observed decrease in mitochondrial oxidative stress. Alternatively, resveratrol supplementation in our study effectively prevented the reduction in CII and CIV activity following hemorrhagic shock, which may have also contributed to the observed decrease in ROS production. Thus, the improvement in oxidative damage observed with resveratrol may be multifactorial – resuscitation with resveratrol may either increase antioxidant defenses, decrease mitochondrial ROS production, or both.
In contrast to previous studies demonstrating augmented mitochondrial biogenesis (9, 33, 35), resveratrol does not seem to promote mitochondrial biogenesis in our acute hemorrhagic shock model. Although the expression of PGC1-α was enhanced, resveratrol had no effect on the other transcriptional factors regulating mitochondrial biogenesis (e.g. NRF-1, NRF-2 and TFAM) and did not change mitochondrial content as measured by citrate synthase activity. Since NRF-1, NRF-2 and TFAM are downstream targets of PGC1-α, further studies with longer observation are needed to determine if resveratrol has any long-term effects on mitochondrial biogenesis. Finally, because resuscitation with resveratrol increased the transcript levels of SIRT1, PGC1-α, and the downstream targets, SOD2 and catalase, we conclude that the acute benefits of resveratrol appear to be mediated via an antioxidant, rather than mitochondrial biogenesis pathway.
Resveratrol may also improve mitochondrial function by favorable impacting cellular redox potential (38). In this study, resveratrol supplementation significantly decreased the NADH concentration and nearly doubled the NAD+ to NADH ratio. As an NAD+-dependent deacetylase, SIRT1 activity may be directly affected by fluctuations in the concentration of NAD+ or changes in the NAD+ to NADH ratio. As such, using resveratrol during resuscitation potentially promoted the entry of NADH into electron transport, thus decreasing the overall concentration of NADH and increasing the NAD+-NADH ratio. It is possible that this favorable change in the NAD+ to NADH ratio may have influenced SIRT1 activity in our model (39).
While intriguing, this study has several limitations. First, we only explored the acute impact of resveratrol following acute blood loss. Further studies are needed to determine if treating hemorrhagic shock with resveratrol improves mitochondrial function and prevents acute kidney injury in the long-term. Second, we only tested one dose of resveratrol. It is possible that alternative dosing regimens may preferentially influence downstream targets of SIRT1 or PGC1α. Third, we only explored the impact of resveratrol on one tissue type. Because activating SIRT1 has been reported to both increase and decrease inflammation in different tissues, it is possible that resveratrol may also have tissue-specific effects. Finally, our studies do not definitively determine if resveratol’s benefit in hemorrhagic shock is PGC1-α dependent. In order to fully explore the role of PGC1-α, it would be necessary to evaluate our model using a PGC1-α antagonist or knock out strain. Unfortunately, an agent that specifically targets PGC1-α is not currently available commercially. Additionally, although a PGC1-α knock out mouse is available, our current experiments were done in a rat model. While we anticipate repeating our experiments using transgenic strains, this endeavor is beyond the scope of the current report.
In this work we explored the effects of resveratrol on kidney mitochondrial injury following hemorrhagic shock and resuscitation. Resveratrol supplementation led to a restoration in kidney mitochondrial function and alleviated oxidative stress injury following hemorrhagic shock. These mitochondrial-protective effects appear to be mediated via stimulation of a SIRT1-PGC1-α-antioxidant pathway rather than via mitochondrial biogenesis. Further work is needed to determine if the restoration of CII and CIV activity observed following resveratrol treatment is secondary to decreased oxidative damage or the result of resveratrol-induced post-translational modifications. Nonetheless, resveratrol may represent a novel therapeutic agent that may limit renal damage following hemorrhagic shock.
Acknowledgments
Source of Funding
Supported, in part, by grant 1K08GM097614-01 from the National Institute of Health and the Clowes ACS/AAST NIGMS Mentored Clinical Scientist Development Award
We thank James G. Davis for technical assistance, David W. Frederick and Anthony Davila for their help with qPCR.
Footnotes
The address for reprints: The Trauma Center at Penn, 3400 Spruce Street, 5 Maloney, Philadelphia, PA 19104
Conflicts of Interest
The authors have no conflicts of interest or financial disclosures.
References
- 1.Minei JP, Cuschieri J, Sperry J, Moore EE, West MA, Harbrecht BG, O’Keefe GE, Cohen MJ, Moldawer LL, Tompkins RG, Maier RV. The changing pattern and implications of multiple organ failure after blunt injury with hemorrhagic shock. Crit Care Med. 2012;40(4):1129–35. doi: 10.1097/CCM.0b013e3182376e9f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Evans RG, Gardiner BS, Smith DW, O’Connor PM. Intrarenal oxygenation: unique challenges and the biophysical basis of homeostasis. Am J Physiol Renal Physiol. 2008;295(5):F1259–70. doi: 10.1152/ajprenal.90230.2008. [DOI] [PubMed] [Google Scholar]
- 3.Benns M, Carr B, Kallan MJ, Sims CA. Benchmarking the incidence of organ failure after injury at trauma centers and nontrauma centers in the United States. J Trauma Acute Care Surg. 2013;75(3):426–31. doi: 10.1097/TA.0b013e31829cfa19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16(11):3365–70. doi: 10.1681/ASN.2004090740. [DOI] [PubMed] [Google Scholar]
- 5.Kozlov AV, Bahrami S, Calzia E, Dungel P, Gille L, Kuznetsov AV, Troppmair J. Mitochondrial dysfunction and biogenesis: do ICU patients die from mitochondrial failure? Ann Intensive Care. 2011;1(1):41. doi: 10.1186/2110-5820-1-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karamercan MA, Weiss SL, Villarroel JP, Guan Y, Werlin E, Figueredo R, Becker LB, Sims C. Can Peripheral Blood Mononuclear Cells be Used as a Proxy for Mitochondrial Dysfunction in Vital Organs During Hemorrhagic Shock and Resuscitation? Shock. 2013;40(6):476–84. doi: 10.1097/SHK.0000000000000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Skripchenko A, Myrup A, Thompson-Montgomery D, Awatefe H, Moroff G, Wagner SJ. Periods without agitation diminish platelet mitochondrial function during storage. Transfusion. 2010;50(2):390–9. doi: 10.1111/j.1537-2995.2009.02450.x. [DOI] [PubMed] [Google Scholar]
- 8.Cairns CB. Rude unhinging of the machinery of life: metabolic approaches to hemorrhagic shock. Curr Opin Crit Care. 2001;7(6):437–43. doi: 10.1097/00075198-200112000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, Geny B, Laakso M, Puigserver P, Auwerx J. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127(6):1109–22. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 10.Lopez-Lluch G, Irusta PM, Navas P, de Cabo R. Mitochondrial biogenesis and healthy aging. Exp Gerontol. 2008;43(9):813–9. doi: 10.1016/j.exger.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5(6):493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 12.Scarpulla RC. Metabolic control of mitochondrial biogenesis through the PGC-1 family regulatory network. Biochim Biophys Acta. 2011;1813(7):1269–78. doi: 10.1016/j.bbamcr.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.St-Pierre J, Drori S, Uldry M, Silvaggi JM, Rhee J, Jager S, Handschin C, Zheng K, Lin J, Yang W, Simon DK, Bachoo R, Spiegelman BM. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell. 2006;127(2):397–408. doi: 10.1016/j.cell.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 14.Tsai YF, Liu FC, Lau YT, Yu HP. Role of Akt-dependent pathway in resveratrol-mediated cardioprotection after trauma-hemorrhage. J Surg Res. 2012;176(1):171–7. doi: 10.1016/j.jss.2011.05.039. [DOI] [PubMed] [Google Scholar]
- 15.Yu HP, Yang SC, Lau YT, Hwang TL. Role of Akt-dependent up-regulation of hemeoxygenase-1 in resveratrol-mediated attenuation of hepatic injury after trauma hemorrhage. Surgery. 2010;148(1):103–9. doi: 10.1016/j.surg.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 16.Souba W, Wilmore D. In: Surgical Research. Ayala A, Wang P, Chaudry I, editors. San Diego, CA: Academic Press; 2001. pp. 325–327. [Google Scholar]
- 17.Smeding L, Leong-Poi H, Hu P, Shan Y, Haitsma JJ, Horvath E, Furmli S, Masoom H, Kuiper JW, Slutsky AS, Parker TG, Plotz FB, dos Santos CC. Salutary effect of resveratrol on sepsis-induced myocardial depression. Crit Care Med. 2012;40(6):1896–907. doi: 10.1097/CCM.0b013e31824e1370. [DOI] [PubMed] [Google Scholar]
- 18.Palmeira CM, Moreno AJ. In: Mitochondrial bioenergetics: methods and protocols. Pesta D, Gnaiger E, editors. New York, NY: Humana Press; 2012. pp. 25–58. [Google Scholar]
- 19.Kuznetsov AV, Veksler V, Gellerich FN, Saks V, Margreiter R, Kunz WS. Analysis of mitochondrial function in situ in permeabilized muscle fibers, tissues and cells. Nat Protoc. 2008;3(6):965–76. doi: 10.1038/nprot.2008.61. [DOI] [PubMed] [Google Scholar]
- 20.Supale S, Thorel F, Merkwirth C, Gjinovci A, Herrera PL, Scorrano L, Meda P, Langer T, Maechler P. Loss of prohibitin induces mitochondrial damages altering beta-cell function and survival and is responsible for gradual diabetes development. Diabetes. 2013;62(10):3488–99. doi: 10.2337/db13-0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu H, Yu S, Xu W, Xu J. Enhancement of 26S proteasome functionality connects oxidative stress and vascular endothelial inflammatory response in diabetes mellitus. Arteriosclerosis Thrombosis and Vascular Biology. 2012;32(9):2131–40. doi: 10.1161/ATVBAHA.112.253385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mattiasson G. Analysis of mitochondrial generation and release of reactive oxygen species. Cytometry A. 2004;62(2):89–96. doi: 10.1002/cyto.a.20089. [DOI] [PubMed] [Google Scholar]
- 23.Srere PA, Matsuoka Y. Inhibition of rat citrate synthase by acetoacetyl CoA and NADH. Biochem Med. 1972;6(3):262–6. doi: 10.1016/0006-2944(72)90047-6. [DOI] [PubMed] [Google Scholar]
- 24.Lee JS, Park AH, Lee SH, Kim JH, Yang SJ, Yeom YI, Kwak TH, Lee D, Lee SJ, Lee CH, Kim JM, Kim D. Beta-lapachone, a modulator of NAD metabolism, prevents health declines in aged mice. PLoS One. 2012;7(10):e47122. doi: 10.1371/journal.pone.0047122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dyess DL, Powell RW, Swafford AN, Jr, Schmacht DC, Roberts WS, Ferrara JJ, Ardell JL. Redistribution of organ blood flow after hemorrhage and resuscitation in full-term piglets. J Pediatr Surg. 1994;29(8):1097–102. doi: 10.1016/0022-3468(94)90287-9. [DOI] [PubMed] [Google Scholar]
- 26.Parke AL, Liu PT, Parke DV. Multiple organ dysfunction syndrome. Inflammopharmacology. 2003;11(1):87–95. doi: 10.1163/156856003321547130. [DOI] [PubMed] [Google Scholar]
- 27.Spahn DR, Cerny V, Coats TJ, Duranteau J, Fernandez-Mondejar E, Gordini G, Stahel PF, Hunt BJ, Komadina R, Neugebauer E, Ozier Y, Riddez L, Schultz A, Vincent JL, Rossaint R. Management of bleeding following major trauma: a European guideline. Crit Care. 2007;11(1):R17. doi: 10.1186/cc5686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aksu U, Bezemer R, Yavuz B, Kandil A, Demirci C, Ince C. Balanced vs unbalanced crystalloid resuscitation in a near-fatal model of hemorrhagic shock and the effects on renal oxygenation, oxidative stress, and inflammation. Resuscitation. 2012;83(6):767–73. doi: 10.1016/j.resuscitation.2011.11.022. [DOI] [PubMed] [Google Scholar]
- 29.Kolgazi M, Sener G, Cetinel S, Gedik N, Alican I. Resveratrol reduces renal and lung injury caused by sepsis in rats. J Surg Res. 2006;134(2):315–21. doi: 10.1016/j.jss.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 30.Li T, Zhang J, Feng J, Li Q, Wu L, Ye Q, Sun J, Lin Y, Zhang M, Huang R, Cheng J, Cao Y, Xiang G, Wu Q. Resveratrol reduces acute lung injury in a LPS induced sepsis mouse model via activation of Sirt1. Mol Med Rep. 2013;7(6):1889–95. doi: 10.3892/mmr.2013.1444. [DOI] [PubMed] [Google Scholar]
- 31.Hjortrup PB, Haase N, Wetterslev M, Perner A. Clinical review: Predictive value of neutrophil gelatinase-associated lipocalin for acute kidney injury in intensive care patients. Crit Care. 2013;17(2):211. doi: 10.1186/cc11855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cruz DN, de Cal M, Garzotto F, Perazella MA, Lentini P, Corradi V, Piccinni P, Ronco C. Plasma neutrophil gelatinase-associated lipocalin is an early biomarker for acute kidney injury in an adult ICU population. Intensive Care Med. 2010;36(3):444–51. doi: 10.1007/s00134-009-1711-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ungvari Z, Sonntag WE, de Cabo R, Baur JA, Csiszar A. Mitochondrial protection by resveratrol. Exerc Sport Sci Rev. 2011;39(3):128–32. doi: 10.1097/JES.0b013e3182141f80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu Y, Nie L, Yin YG, Tang JL, Zhou JY, Li DD, Zhou SW. Resveratrol protects against hyperglycemia-induced oxidative damage to mitochondria by activating SIRT1 in rat mesangial cells. Toxicol Appl Pharmacol. 2012;259(3):395–401. doi: 10.1016/j.taap.2011.09.028. [DOI] [PubMed] [Google Scholar]
- 35.Csiszar A, Labinskyy N, Pinto JT, Ballabh P, Zhang H, Losonczy G, Pearson K, de Cabo R, Pacher P, Zhang C, Ungvari Z. Resveratrol induces mitochondrial biogenesis in endothelial cells. Am J Physiol Heart Circ Physiol. 2009;297(1):H13–20. doi: 10.1152/ajpheart.00368.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Musatov A, Carroll CA, Liu YC, Henderson GI, Weintraub ST, Robinson NC. Identification of bovine heart cytochrome c oxidase subunits modified by the lipid peroxidation product 4-hydroxy-2-nonenal. Biochemistry. 2002;41(25):8212–20. doi: 10.1021/bi025896u. [DOI] [PubMed] [Google Scholar]
- 37.Chen J, Henderson GI, Freeman GL. Role of 4-hydroxynonenal in modification of cytochrome c oxidase in ischemia/reperfused rat heart. J Mol Cell Cardiol. 2001;33(11):1919–27. doi: 10.1006/jmcc.2001.1454. [DOI] [PubMed] [Google Scholar]
- 38.Rodgers JT, Lerin C, Gerhart-Hines Z, Puigserver P. Metabolic adaptations through the PGC-1 alpha and SIRT1 pathways. FEBS Lett. 2008;582(1):46–53. doi: 10.1016/j.febslet.2007.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sack MN, Finkel T. Mitochondrial metabolism, sirtuins, and aging. Cold Spring Harb Perspect Biol. 2012;4(12) doi: 10.1101/cshperspect.a013102. [DOI] [PMC free article] [PubMed] [Google Scholar]