Abstract
Objective
Using a large sample of colorectal cancer (CRC) survivors we 1) describe pain interference (PI) prevalence across the cancer continuum; 2) identify demographic and clinical factors associated with PI and changes in PI; and 3) examine PI’s relationship with survivors’ job changes.
Methods
CRC participants of the Cancer Care Outcomes Research and Surveillance Consortium completed surveys during the initial phase of care (baseline, <1 year, n=2,961) and follow-up (about 1-year post-diagnosis, n=2,303). PI was measured using the SF-12 item. Multiple logistic regression was used to identify predictors of PI. Model 1 evaluated moderate/high PI at baseline, Model 2 evaluated new/continued/increasing PI post-diagnosis follow-up, and Model 3 restricted to participants with baseline PI (N=603) and evaluated predictors of equivalent/increasing PI. Multivariable logistic regression was also used to examine whether PI predicted job change.
Results
At baseline and follow-up, 24.7% and 23.7% of participants reported moderate/high PI, respectively. Among those with baseline PI, 46% had equivalent/increasing PI at follow-up. Near diagnosis and at follow-up, female gender, comorbidities, depression, chemotherapy and radiation were associated with moderate/high PI while older age was protective of PI. Pulmonary disease and heart failure comorbidities were associated with equivalent/increasing PI. PI was significantly associated with no longer having a job at follow-up among employed survivors.
Conclusion
Almost half of survivors with PI during the initial phase of care had continued PI into post-treatment. Comorbidities, especially cardiovascular and pulmonary conditions, contributed to continued PI. PI may be related to continuing normal activities, i.e., work, after completed treatment.
Introduction
The prevalence of pain among cancer survivors ranges from 20% to more than 60%, making cancer a priority area in the Institute of Medicine’s (IOM) call for addressing pain in the United States. (1) The prevalence, duration, and intensity of pain can vary depending on several factors, including cancer type. (2-4) For example, gastrointestinal cancer survivors, including colorectal cancer (CRC) survivors, often report less pain intensity than head and neck, lung, and breast cancer survivors. (5-7)
While pain intensity is an informative metric, the IOM recommends that we improve data collection efforts to better document both the prevalence of pain how pain interferes with outcomes such as disability, work, and activities of daily living. (1) For example, mild or moderate intensity pain may still result in considerable interference with physical functioning and disruption in daily activities, social engagement and normal work, (8) and ultimately lead to increased depressive symptoms, lower quality of life, and perceived disability. (9) Moreover, in one study, 55% of survivors of different cancer types with comorbid pain and depression reported health-related unemployment. (10) The inability to either continue or resume normal paid work is a significant concern given that survivors not only have financial constraints due to medical costs, but work provides a sense of normalcy that benefits quality of life. (11) Understanding pain interference’s impact is fundamental to improving normal function and activities, including work, of the rapidly growing population of CRC survivors: over 1 million Americans are currently living with a history of CRC and, given the decreasing mortality trend, this number continues to grow. (12)
Despite the serious consequences of such pain interference, there is little guidance on the factors that are associated with interference and its persistence from the point of care into survivorship. Factors predictive of pain intensity, such as age, gender, race, treatment and comorbidities, are likely also associated with interference, but given the conceptual difference between severity and interference, these relationships should be evaluated with pain interference. (13-15) Comorbidities are of significant importance because not only are comorbidities one of the top contributors to pain in cancer survivors, (16) but compared to the non-cancer population, cancer survivors are less likely to adequately care for and manage comorbid conditions such as diabetes, leading to additional pain-related complications. (17) This is particularly a problem among CRC survivors: compared to breast and prostate cancer survivors, CRC survivors are the least likely to manage comorbid conditions. (17)
The purpose of this study was to address some of the research gaps on CRC-related pain interference. We aimed to: 1) describe the prevalence of pain interference among a racially/ethnically diverse group of CRC patients during the initial phase of care and at post treatment follow-up; 2) identify sociodemographic and clinical variables associated with pain interference according to a biopsychosocial model (biological, psychological, and social factors); (18-20) and 3) examine the relationship of pain interference with changes in job status. To our knowledge, this is the first study to examine the prevalence of pain interference over time in a population-based sample of CRC patients. Overall, few cancer studies assess pain interference at more than one time point, (21, 22) in racially/ethnically diverse populations (13) and/or from more than one health care site. Moreover, most studies focus on pain intensity in CRC patients grouped with other gastrointestinal cancers. (6, 7, 15) Recent studies have called for larger, population-based studies to obtain more reliable estimates of pain interference in CRC survivors. (14)
METHODS
Data
The Cancer Care Outcomes Research and Surveillance (CanCORS) Consortium was established by the National Cancer Institute in 2001.(23) It is comprised of five geographically distinct sites, five Cancer Research Network integrated health systems, and 15 Veterans Health Administration hospitals. Between 2003 and 2005, newly diagnosed (majority within 4 to 7 months) CRC and lung cancer patients, recruited through state cancer registries and health care administrative data. Minorities (African American, Asian/Pacific Islander, and Hispanic) were oversampled to ensure a diverse population. Survivors completed a survey during the initial phases of care (i.e., baseline survey). The survey collected information about initial treatment, care, and symptoms (CanCORS I). Surveys were conducted in English, Spanish, or Chinese. Clinical information was abstracted from medical records. Approximately one year after baseline (beginning in August 2004), participants were contacted for a follow-up survey on symptoms and overall health in the previous 12 months. At both baseline and follow-up, abbreviated and surrogate surveys were available for patients who were too ill to complete the full survey or deceased at the time of survey, respectively. Surveys were pilot tested prior to implementation and comprised of validated questionnaires as well as new items developed for CanCORS.(24)
Sample selection criteria
Baseline data included 2,961 CRC patients who completed the full version of the baseline survey, reported a race of White, African American, Hispanic, or Asian, and had complete data on the primary outcome (<2% had missing values). For the follow-up survey, attrition among the 2,961 participants was due to: death (n=154), refusal (n=197), unable to contact (n=98), not contacted because follow-up enrollment was reached (n=25) and other reasons (e.g., patient unable to communicate, language problem) (n=187). This resulted in 2,303 survivors at follow-up.
Pain interference
Pain interference was measured at baseline and at follow-up by an item from the Medical Outcomes Study Short Form-12 (MOS SF-12): “During the past four weeks, how much did pain interfere with your normal work? (including both work outside the home and housework).”(25) Response categories were on a 5-point Likert-type scale with 1 corresponding to ‘not at all’ and 5 corresponding to ‘extremely.’ We defined one dichotomous outcome variable for the initial phase of care, “baseline pain interference:” this was coded as 0 if the participant reported minimal interference (“not at all/a little bit”) and 1 if the participant reported moderate/high interference (“moderately/quite a bit/extremely”). (26, 27) A similar dichotomous outcome variable was defined for the post-treatment follow-up, “follow-up pain interference. ” Moreover, we defined “continued pain interference ” only for those CanCORS respondents who had moderate/high interference at baseline. This was equal to 0 if, at follow-up, pain interference was minimal (“not at all/a little bit”) and 1 if it was moderate/high (“moderately/quite a bit/extremely”). The latter group was comprised of those who at follow up reported equivalent pain interference (e.g., “moderately” at baseline and follow-up) or increasing pain interference (e.g. report “moderately” at baseline and “quite a bit” at follow-up).
Pain severity
To describe overall pain severity of the sample, three items from the Brief Pain Inventory (BPI) were evaluated: “pain at its least”, “pain on average”, and “pain at its worst”. (28) Each item is scored on 0 to 10 scale with higher scores indicating greater pain severity. Scores of 1-4 indicate mild pain, 5-6 for moderate pain, and 7-10 for severe pain. (29)
Job change
Job change was defined as a dichotomous variable using self-reported information from the baseline and follow-up surveys. This was equal to 0 if a survivors reported having a paid job at baseline and at post-treatment follow-up (no job change), and equal to 1 if a survivor reported having a job at baseline and not at post-treatment follow-up (job change).
Covariates
The choice of covariates was guided by the biopsychosocial model of pain (20). Biological and social factors included sociodemographic variables self-reported at baseline such as race/ethnicity, age, sex, income, insurance, and education. (7, 13, 30-32). Other clinical factors included variables abstracted from medical records such as cancer stage (33) time since treatment, (7) and treatment received. (34) Self-reported clinical variables included comorbidity, (35) and stoma. (36) We also collected information on any pain medication use but did not include as a covariate. (37) Survivors were asked whether they were taking any type of medication (prescription or non-prescription) for pain for any reason. For comorbidities, at the baseline survey, survivors responded to “Have you ever had…” the following conditions: diabetes, kidney disease, Crohn’s disease , pulmonary disease (emphysema, chronic obstructive pulmonary disease), heart failure, heart attack, and stroke. Psychological factors included a self-reported history of depression or emotional problems prior to diagnosis. (18)
Statistical methods
Pain interference
Descriptive analyses of the demographic and clinical variables were conducted separately for baseline and follow-up. Frequencies are presented for categorical variables and mean and standard deviations (SD) are presented for continuous variables. Associations between sociodemographic and clinical variables with baseline pain interference and follow-up pain interference were examined using chi-square tests for categorical variables and analysis of variance for continuous variables with adjustment for multiple comparisons (Tukey-Kramer).
Logistic regression was applied for three models to obtain odds ratios (ORs) with 95% confidence intervals (CI). Model 1 evaluated “baseline pain interference”, Model 2 evaluated “follow-up pain interference” and Model 3 evaluated “continued pain interference” among those with baseline pain interference. We included covariates in two sequential blocks: 1) sociodemographic variables only and 2) sociodemographic and clinical variables. Pain medication use was not included due to its strong correlation with pain interference. Logistic regression analysis utilized those CanCORS data sets that included imputation (5 imputed data sets) adjusting for non-response (38) for all items except pain interference and race/ethnicity, where missing observations were excluded. Multi-collinearity checks indicated no significant collinearity issues. Findings were consistent between imputed and non-imputed data sets.
Pain interference and pain severity
Mean scores and standard deviations were calculated for the three BPI items. (29) Due to non-normal distribution, Spearman correlation coefficients were calculated to describe the association between the SF-12 pain interference item (using the 1 to 5 scale) and the pain severity items (using the 0 to 10 scale).
Job change
Job change analyses were restricted to survivors at follow-up who reported a paid job at baseline (n=1,007). Among survivors who did not have a job at baseline, there was no significant change in job status: 97% also reported no job at follow-up. Frequencies of job change across pain interference outcomes were calculated and chi-square tests were used to test for significant differences. Three multivariable logistic regression analyses were used to evaluate the association of pain interference and job change, where job change was the dependent variable and each pain interference outcome served as the primary independent variable. The analysis controlled for age, sex, race, income, education, stage at diagnosis, and treatment.
SAS 9.3 was used for all analyses. (39) This study was approved by human subjects’ review boards at all participating institutions.
RESULTS
Population characteristics
Over half of patients were male (54.1%) and White (69.6%) (Table 1). Age was evenly distributed between age categories, with 22% to 26% of the sample in each age category. About 29% reported a college degree or higher. Approximately 45% of participants had at least one comorbid condition and 19% reported a depression or emotional problem prior to cancer. About 45% were diagnosed at Stage III or IV, 92% received surgery as treatment, and 26% reported taking medication for pain.
Table 1.
Baseline study characteristics of colorectal cancer patients
| Baseline*
(n=2961) |
Follow-up*,†
(n=2303) |
||||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Race | |||||
| White | 2060 | 69.57 | 1671 | 72.56 | |
| African American | 459 | 15.50 | 345 | 14.98 | |
| Hispanic | 265 | 8.95 | 184 | 7.99 | |
| Asian | 177 | 5.98 | 103 | 4.47 | |
| Age‡ | |||||
| ≤54 years | 774 | 26.14 | 580 | 25.18 | |
| 55 to 64 years | 745 | 25.16 | 591 | 25.66 | |
| 65 to 74 years | 787 | 26.58 | 627 | 27.23 | |
| ≥75 years | 655 | 22.12 | 505 | 21.93 | |
| Sex‡ | |||||
| Male | 1602 | 54.10 | 1259 | 54.67 | |
| Female | 1359 | 45.90 | 1044 | 45.33 | |
| Income‡ | |||||
| <$20,000 | 718 | 24.25 | 527 | 22.88 | |
| $20,000 to <$40,000 | 740 | 24.99 | 579 | 25.14 | |
| $40,000 to <$60,000 | 470 | 15.87 | 387 | 16.80 | |
| >$60,000 | 762 | 25.73 | 626 | 27.18 | |
| Education‡ | |||||
| Less than high school | 469 | 15.84 | 336 | 14.59 | |
| High school graduate/GED | 838 | 28.30 | 640 | 27.79 | |
| Some college/ vocational school | 784 | 26.48 | 625 | 27.14 | |
| College degree or higher | 857 | 28.94 | 692 | 30.05 | |
| Covered by insurance‡ | |||||
| No | 84 | 2.84 | 57 | 2.48 | |
| Yes | 2869 | 97.89 | 2241 | 97.31 | |
| Comorbid conditions§ | |||||
| No | 1620 | 54.82 | 1412 | 61.31 | |
| Yes | 1335 | 45.18 | 887 | 38.51 | |
| Depression | |||||
| No | 2387 | 80.61 | 1848 | 80.24 | |
| Yes | 562 | 18.98 | 447 | 19.41 | |
| Stage at diagnosis | |||||
| Stage I | 674 | 22.76 | 554 | 24.06 | |
| Stage II | 806 | 27.22 | 647 | 28.09 | |
| Stage III | 832 | 28.10 | 674 | 29.27 | |
| Stage IV | 421 | 14.22 | 270 | 11.72 | |
| Time since diagnosis (days) | |||||
| Mean (SD) | 144 | (53) | 440 | (121) | |
| (Range) | (41-364) | (269-1170) | |||
| Surgery | |||||
| No | 209 | 7.06 | 87 | 3.78 | |
| Yes | 2749 | 92.84 | 2214 | 96.14 | |
| Treatment | |||||
| Neither | 1380 | 46.61 | 1031 | 44.77 | |
| Radiation | 19 | 0.64 | 16 | 0.69 | |
| Chemotherapy | 1146 | 38.70 | 887 | 38.51 | |
| Both | 409 | 13.81 | 365 | 15.85 | |
| Stoma | |||||
| No | 2605 | 87.98 | 2043 | 88.71 | |
| Yes | 352 | 11.89 | 258 | 11.20 | |
| Brief Pain Inventory: In the last 4 weeks.. | |||||
| Pain severity at its least | |||||
| Mean (SD) | 0.76 | (1.63) | 1.98 | (2.06) | |
| Range | (0-10) | ||||
| Pain severity on average | |||||
| Mean (SD) | 1.45 | (2.29) | 3.80 | (2.08) | |
| Range | (0-10) | (0-10) | |||
| Pain severity at its worst | |||||
| Mean (SD) | 2.29 | (3.37) | 5.95 | (2.45) | |
| Range | (0-10) | (0-10) | |||
| Pain medication use | |||||
| Yes | 778 | 26.27 | 602 | 26.14 | |
| No | 2176 | 73.49 | 1699 | 73.77 | |
Percentages may not total 100% due to missing values. Missing was less than 10% on all variables;
Participants’ demographic and clinic characteristic were not statistically significantly different at baseline and follow-up (p>0.05); except for time since diagnosis (p<0.0001) and pain severity at its least (p=0.03);
Chi-square test results show that these sociodemographic factors are all significantly different by race/ethnicity, age, sex, income and education (all p-value <0.001) and insurance (p=0.02).
Comorbid conditions assessed included heart attack, heart failure, stroke, diabetes, pulmonary function problems (emphysema), kidney disease, diabetes, Crohn’s disease
Pain interference: frequency and bivariate associations
Overall, 27.4% and 23.8% of survivors reported moderate/high pain interference at baseline and follow-up, respectively. All sociodemographic and clinical variables (with the exception of time since diagnosis and stage) were significantly associated with baseline and follow-up pain interference (Table 2). Age, income, comorbidities, depression and pain medication were significantly associated with both follow-up pain interference and continued pain interference among the sub-group with interference at baseline (Table 2).
Table 2.
Bivariate analysis of factors associated with pain interference during initial phase of care and pain interference at follow-up
| Baseline (n=2961) | Follow-up (n=2303) | Sub-group at follow-up*
(n=603) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Moderate/ high N |
Row %† | p-value | New/Continued /Increasing N |
Row %† | p-value | Equivalent /increasing N |
Row %† | p-value‡ | ||
| Overall | 812 | (27.4) | 548 (23.8) | (23.8) | 276 | (45.8) | ||||
| Race | ||||||||||
| White | 523 | (25.4) | <0.001 | 380 | (22.7) | 0.021 | 189 | (46.7) | 0.243 | |
| African-American | 158 | (34.4) | 103 | (29.9) | 55 | (49.7) | ||||
| Hispanic | 86 | (32.5) | 46 | (25.0) | 23 | (39.7) | ||||
| Asian | 45 | (25.4) | 19 | (18.5) | 9 | (31.0) | ||||
| Age | ||||||||||
| ≤54 years | 272 | (35.1) | <0.001 | 162 | (27.9) | <0.001 | 77 | (39.3) | 0.010 | |
| 55 to 64 years | 244 | (32.8) | 159 | (26.9) | 97 | (54.5) | ||||
| 65 to 74 years | 153 | (19.4) | 113 | (18.0) | 49 | (39.8) | ||||
| ≥75 years | 143 | (21.8) | 114 | (22.6) | 53 | (50.0) | ||||
| Sex | ||||||||||
| Male | 395 | (24.7) | <0.001 | 270 | (21.5) | 0.004 | 128 | (44.9) | 0.689 | |
| Female | 417 | (30.7) | 278 | (26.6) | 148 | (46.5) | ||||
| Income | ||||||||||
| <$20,000 | 229 | (31.9) | <0.001 | 169 | (32.1) | <0.001 | 91 | (56.2) | 0.011 | |
| $20,000 to <$40,000 | 212 | (28.7) | 152 | (26.3) | 78 | (49.4) | ||||
| $40,000 to <$60,000 | 117 | (24.9) | 72 | (18.6) | 32 | (35.2) | ||||
| ≥$60,000 | 171 | (22.4) | 118 | (18.9) | 53 | (37.3) | ||||
| Education | ||||||||||
| Less than high school | 146 | (31.1) | <0.001 | 90 | (26.8) | <0.001 | 46 | (48.4) | 0.147 | |
| High school | 253 | (30.2) | 188 | (29.4) | 93 | (52.0) | ||||
| graduate/GED | ||||||||||
| Some college/ | 217 | (27.7) | 140 | (22.4) | 74 | (43.0) | ||||
| vocational school | ||||||||||
| College degree or | 195 | (22.8) | 130 | (18.8) | 63 | (40.4) | ||||
| higher | ||||||||||
| Stage at diagnosis | ||||||||||
| Stage I | 166 | (24.6) | 0.070 | 123 | (22.2) | 0.078 | 64 | (50.8) | 0.405 | |
| Stage II | 217 | (26.9) | 149 | (23.0) | 77 | (46.4) | ||||
| Stage III | 255 | (30.7) | 157 | (23.3) | 87 | (42.2) | ||||
| Stage IV | 115 | (27.3) | 81 | (30.0) | 33 | (50.8) | ||||
| Time since diagnosis (days)|| | 0.489 | 0.242 | 0.541 | |||||||
| Mean | 143 | 143 | 435 | 431 | ||||||
| Treatment | ||||||||||
| Neither | 319 | (23.1) | <0.001 | 221 | (21.4) | <0.001 | 106 | (49.1) | 0.242 | |
| Chemotherapy or radiation only |
304 | (26.0) | 206 | (22.8) | 96 | (41.6) | ||||
| Chemotherapy and radiation |
188 | (46.0) | 121 | (33.2) | 74 | (47.7) | ||||
| Stoma | ||||||||||
| Yes | 696 | (26.7) | 0.033 | 469 | (23.0) | 0.007 | 236 | (45.1) | 0.360 | |
| No | 113 | (32.1) | 79 | (30.6) | 40 | (50.6) | ||||
| Comorbid conditions § | ||||||||||
| No | 391 | (24.1) | <0.001 | 293 | (20.8) | <0.001 | 139 | (41.1) | 0.007 | |
| Yes, any | 417 | (31.2) | 254 | (28.6) | 137 | (52.1) | ||||
| Diabetes | ||||||||||
| No | 623 | (26.2) | 0.007 | 406 | (21.9) | <0.001 | 196 | (42.7) | 0.004 | |
| Yes | 183 | (31.8) | 141 | (31.6) | 80 | (56.3) | ||||
| Kidney disease | ||||||||||
| No | 710 | (27.0) | 0.221 | 474 | (23.1) | 0.039 | 238 | (45.2) | 0.317 | |
| Yes | 95 | (30.3) | 71 | (29.1) | 38 | (51.4) | ||||
| Crohn’s disease | ||||||||||
| No | 759 | (26.7) | <0.001 | 520 | (23.5) | 0.083 | 257 | (45.4) | 0.397 | |
| Yes | 37 | (43.5) | 21 | (32.8) | 15 | (53.6) | ||||
| Pulmonary disease | ||||||||||
| No | 660 | (25.4) | <0.001 | 443 | (22.0) | <0.001 | 211 | (43.3) | 0.021 | |
| Yes | 142 | (41.2) | 100 | (36.6) | 61 | (55.5) | ||||
| Heart failure | ||||||||||
| No | 757 | (26.9) | 0.035 | 503 | (22.9) | <0.001 | 251 | (44.4) | 0.001 | |
| Yes | 45 | (35.4) | 43 | (45.7) | 24 | (75.0) | ||||
| Heart attack | ||||||||||
| No | 713 | (26.7) | 0.014 | 479 | (23.1) | 0.014 | 242 | (45.2) | 0.278 | |
| Yes | 90 | (33.7) | 64 | (30.6) | 32 | (52.5) | ||||
| Stroke | ||||||||||
| No | 731 | (26.8) | 0.010 | 489 | (23.0) | 0.003 | 239 | (44.4) | 0.023 | |
| Yes | 76 | (34.9) | 33.1 | (33.1) | 37 | (59.7) | ||||
| Depression | ||||||||||
| No | 582 | (24.4) | <0.001 | 398 | (21.5) | <0.001 | 183 | (42.9) | 0.018 | |
| Yes | 222 | (39.5) | 148 | (33.1) | 92 | (53.5) | ||||
| Pain medication use | ||||||||||
| Yes | 451 | (58.0) | <0.001 | 336 | (55.8) | <0.001 | 194 | (71.4) | <0.001 | |
| No | 361 | (16.6) | 212 | (12.5) | 81 | (24.6) | ||||
Percentages reflect the percentage of participants within each row reporting moderate/high PI, new,equivalent/increasing pain. For example, during initial phase of care 25% of White patients reported moderate/high pain interference and 34% of African American patients reported moderate/high pain interference.
p-value for X2 difference test between minimal pain interference at diagnosis vs. moderate/high interference
p-value for X2 difference test between decreased pain interference at follow-up vs. equivalent/increasing pain interference at follow-up
Analysis of variance with adjustment for multiple comparisons (Tukey-Kramer)
Pain interference and pain severity
The mean pain severity score was less than5, which indicates mild pain, (29) for all BPI items except at follow-up, where the mean severity score of “pain at its worst” was 5.95, (SD=2.45). Demographic and clinical characteristics were similar at baseline and follow-up (p-values >0.05) with the exception of pain severity at its least (p=0.03). Spearman correlation coefficients (r) indicate that pain interference, using the 1 to 5 scale, was significantly associated with the three BPI items on the 0 to 10 scale: “pain at its least” (r=0.48, p<0.001), “pain on average” (r=0.57, p<0.001), and “pain at its worst” (r=0.58, p<0.001).
Logistic Regression
Model 1: Baseline pain interference: moderate/high
African American survivors were more likely to report pain interference than White cancer survivors (Table 3). Older survivors (65 to 75 and >75 years) had lower odds of pain interference than younger survivors (<55 years) (ORrange 0.38-0.51). Females were significantly more likely to report pain interference than males. Low income was significantly associated with moderate/high pain interference, but after adjusting for clinical variables, income was no longer significant. CRC survivors with a history of diabetes, Crohn’s disease, heart attack and/or stroke, and depression/emotional concerns were more likely to report moderate/high pain interference than those who did not report ever having those conditions. Receiving chemotherapy and radiation (OR=2.97, 95% CI, 2.20-4.02) was also significantly associated with reporting pain interference at baseline..
Table 3.
Predictors of moderate/high pain interference during initial phase of care (ref= minimal pain interference: “not at all/a little bit”) N=2961
| Demographics | Demographics and Clinical | ||
|---|---|---|---|
|
| |||
| Demographics | Moderate/high PI OR (95% CI) |
Moderate/high PI OR (95% CI) |
|
| Race (ref*=White) | |||
| African American | 1.17 (0.93-1.47) | 1.33 (1.03-1.72) | |
| Hispanic | 1.06 (0.79-1.42) | 1.19 (0.87-1.64) | |
| Asian | 0.89 (0.62-1.28) | 1.11 (0.75-1.65) | |
| Age (ref=<55 years) | |||
| 55 to 64 years | 0.90 (0.72-1.12) | 0.85 (0.67-1.09) | |
| 65 to 74 years | 0.40 (0.31-0.51) | 0.38 (0.29-0.51) | |
| ≥75 | 0.44 (0.34-0.57) | 0.51 (0.38-0.68) | |
| Sex (ref=male) | |||
| Female | 1.29 (1.09-1.52) | 1.36 (1.13-1.63) | |
| Income (ref=≥$60,000) | |||
| <$20,000 | 1.73 (1.31-2.29) | 1.31 (0.96-1.79) | |
| $20,000 to <$40,000 | 1.60 (1.22-2.10) | 1.33 (0.99-1.80) | |
| $40,000 to <$60,000 | 1.27 (0.96-1.69) | 1.18 (0.86-1.63) | |
| Education (ref=College degree or higher) | |||
| Less than high school | 1.30 (0.97-1.74) | 1.28 (0.94-1.75) | |
| High school graduate/GED | 1.21 (0.95-1.54) | 1.18 (0.93-1.52) | |
| Some college/ vocational school | 1.14 (0.95-1.44) | 1.06 (0.82-1.36) | |
| Clinical characteristics | |||
| Stage at diagnosis (ref=Stage I) | |||
| Stage II | 0.98 (0.76-1.28) | ||
| Stage III | 1.13 (0.84-1.52) | ||
| Stage IV | 0.95 (0.67-1.34) | ||
| Time since diagnosis (days) | 0.99 (0.99-1.00) | ||
| Treatment† (ref=neither) | |||
| Chemotherapy or radiation only | 1.04 (0.81-1.35) | ||
| Chemotherapy and radiation | 2.97 (2.20-4.02) | ||
| Stoma (ref=no) | |||
| Yes | 0.89 (0.67-1.18) | ||
| Diabetes (ref=no) | |||
| Yes | 1.37 (1.09-1.73) | ||
| Kidney disease (ref=no) | |||
| Yes | 1.14 (0.85-1.53) | ||
| Crohn’s disease (ref=no) | |||
| Yes | 2.20 (1.34-3.60) | ||
| Pulmonary disease (ref=no) | |||
| Yes | 2.12 (1.63-2.76) | ||
| Heart failure (ref=no) | |||
| Yes | 1.19 (0.77-1.83) | ||
| Heart attack (ref=no) | |||
| Yes | 1.54 (1.11-2.13) | ||
| Stroke (ref=no) | |||
| Yes | 1.44 (1.03-2.02) | ||
| Depression (ref=no) | |||
| Yes | 1.77 (1.43-2.21) | ||
|
| |||
| Goodness-of-fit (Hosmer-Lemeshow) | |||
| X2 (df) p-value | 8.68 (8) p=0.370 | 3.35 (8) p=0.910 | |
| C-statistic | 0.64 | 0.70 | |
Ref = Reference category;
Treatment status reflects the treatment received at the time of the first survey
Model 2: Follow-up pain interference: new, increasing or continued
Similar to baseline pain interference, older survivors were less likely to report pain interference at follow-up (ORrange 0.46-0.66). Female survivors and those with lower income were more likely to report pain interference than their counterparts, even after adjusting for clinical variables. Survivors who self-reported diabetes, pulmonary disease, heart failure, or depression were more likely to report new, continued, or increased pain interference at follow-up. Additionally, those who had chemotherapy and radiation were more likely to report new, continued, or increased pain interference at follow-up (OR 1.78 95% CI: 1.24-2.55).
Model 3: Continued pain interference
Among the survivors who had moderate/high pain interference at baseline, age and income were significant predictors of continued pain interference (Model 3 with sociodemographic variables only, Table 4), but after including clinical variables, these factors became non-significant. Survivors who reported pulmonary disease or having had heart failure were significantly more likely to report equivalent or increasing pain interference at follow-up.
Table 4.
Predictors of follow-up pain interference (ref: no pain/decreased pain interference) n=2303
| Demographics | Demographics and Clinical | ||
|---|---|---|---|
|
| |||
| New/Continued/Highe r Pain interference † |
New/Continued/Higher Pain interference † |
||
| Demographics | OR (95% CI) | OR (95% CI) | |
| Race (ref*=White) | |||
| African American | 1.09 (0.83-1.43) | 1.15 (0.85-1.55) | |
| Hispanic | 0.92 (0.63-1.33) | 0.91 (0.61-1.35) | |
| Asian | 0.72 (0.43-1.22) | 0.86 (0.50-1.48) | |
| Age (ref=<54 years) | |||
| 55 to 64 | 0.90 (0.69-1.18) | 0.82 (0.61-1.09) | |
| 65 to 74 | 0.47 (0.35-0.63) | 0.46 (0.33-0.63) | |
| ≥75 | 0.61 (0.45-0.82) | 0.66 (0.47-0.93) | |
| Sex (ref=male) | |||
| Female | 1.23 (1.01-1.50) | 1.33 (1.07-1.65) | |
| Income (ref=≥$60,000) | |||
| <$20,000 | 2.06 (1.51-2.82) | 1.57 (1.22-2.20) | |
| $20,000 to <$40,000 | 1.58 (1.17-2.12) | 1.30 (0.97-1.78) | |
| $40,000 to <$60,000 | 0.99 (0.71-1.39) | 0.93 (0.66-1.31) | |
| Education (ref= College degree or higher) | |||
| Less than high school | 1.19 (0.84-1.68) | 1.23 (0.85-1.78) | |
| High school graduate/GED | 1.41 (1.06-1.86) | 1.32 (0.98-1.78) | |
| Some college/ vocational school | 1.05 (0.79-1.39) | 1.00 (0.75-1.34) | |
| Clinical characteristics | |||
| Time since diagnosis (days) | 0.99 (0.99-1.00) | ||
| Stage at diagnosis (ref=Stage I) | |||
| Stage II | 0.93 (0.69-1.26) | ||
| Stage III | 0.92 (0.65-1.32) | ||
| Stage IV | 1.42 (0.94-2.17) | ||
| Treatment† (ref=neither) | |||
| Chemotherapy or radiation only | 1.00 (0.73-1.38) | ||
| Chemotherapy and Radiation | 1.78 (1.24-2.55) | ||
| Stoma (ref=no) | |||
| Yes | 1.21 (0.87-1.69) | ||
| Diabetes (ref=no) | |||
| Yes | 1.52 (1.17-1.98) | ||
| Kidney disease (ref=no) | |||
| Yes | 1.21 (0.86-1.69) | ||
| Crohn’s disease (ref=no) | |||
| Yes | 1.33 (0.73-2.42) | ||
| Pulmonary disease (ref=no) | |||
| Yes | 2.01 (1.49-2.71) | ||
| Heart failure (ref=no) | |||
| Yes | 2.23 (1.38-3.60) | ||
| Heart attack (ref=no) | |||
| Yes | 1.27 (0.87-1.86) | ||
| Stroke (ref=no) | |||
| Yes | 1.45 (0.99-2.12) | ||
| Depression (ref=no) | |||
| Yes | 1.55 (1.21-2.00) | ||
|
| |||
| Goodness-of-fit (Hosmer-Lemeshow) | |||
| X2 (df) p-value | 9.87 (8) p=0.275 | 4.06 (8) p=0.851 | |
| C-statistic | 0.62 | 0.69 | |
Ref = Reference category;
Treatments that had been received by the time of the follow-up survey.
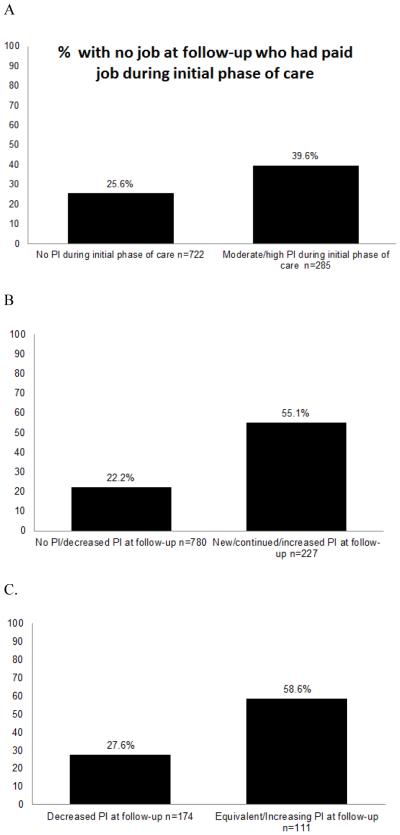
Job change and pain interference
Approximately 40% of employed survivors with pain interference at baseline reported no job at follow-up, vs. 26% of those with minimal pain interference (Figure 1, p<0.002; Adjusted OR 1.77 95% CI:1.24-2.54). Over half of survivors with follow-up pain interference reported a job change compared to less than a quarter of survivors with no follow-up pain interference (Figure 1, p<0.001; Ajusted OR 3.35 95% CI: 2.31-4.88). Among the sub-group of survivors who had pain interference at baseline, 58.6% of those with continued pain interference reported a job change compared to only 28% of those with no continued pain interference (Figure 1, p<0.001; Adjusted OR 2.94 95% CI: 1.50-5.77).
Figure 1.
Survivors reporting no job at follow-up by pain interference (PI) among those with a paid job near diagnosis (n=1,007). A) proportion of survivors with no job at follow-up by PI level near diagnosis; B) proportion of survivors with no job at follow-up by level of PI at follow-up; C) proportion of survivors with PI near diagnosis with no job at follow-up by change in PI. Chi-square tests for all comparisons were statistically significant at p<0.001.
DISCUSSION
One in four CRC survivors reported moderate/high pain interference during the initial phase of care and approximately one year later, with about half of those reporting at least moderate interference near diagnosis having equivalent or increased pain interference at follow-up. Consistent with the biopsychosocial model of pain, multiple factors contributed to a higher likelihood of pain interference during the initial phase of care, including being female and younger, having comorbidities and depression problems, and having received chemotherapy and radiation. These same factors were associated with reporting pain interference at post-treatment follow-up. However, among those survivors who reported pain interference during the initial phase of care, a sub-set of comorbidities, including pulmonary disease and heart failure, predicted equivalent or increased pain interference one year post-diagnosis. Understanding the prevalence and factors associated with experiencing pain interference is critical to delivering quality cancer care and optimizing survivor outcomes. The impact of unresolved pain interference is substantial: survivors employed during the initial phase of care were more likely to report no job post-treatment if they continued to experience pain interference.
Prevalence of pain interference
Our pain interference prevalence estimate at the initial phase of care was similar to another study’s estimate of interference in a heterogeneous cancer patient sample (27% vs 32%, respectively). (21) Our finding that one in two CRC survivors with at least moderate pain interference reported the same or higher pain interference in the follow-up period also supports the aforementioned study’s finding of no significant decrease in pain interference over time. (21) A recent study of racially/ethnically diverse veterans with CRC found that one in three patients reported not getting the help they needed regarding pain.(40) Reported barriers to effective pain management that may contribute to poor pain interference outcomes include lack of physician knowledge to effectively treat cancer-related pain and physician concerns for some pain medication side-effects (i.e., opioid addiction). Moreover, discrepancies in priorities related to pain treatment between patients and physicians exist. (41) For example, while cancer survivors are mainly concerned about etiology of pain, duration, and intensity with respect to its interference in function,(42) physicians identified intensity, breakthrough pain, and treatment as the primary concerns.(43) Such discrepancies may lead to differences in how pain is assessed and managed.
Predictors of pain interference
Understanding what contributes to pain interference entails considering a number of factors that include clinical and non-clinical characteristics of survivors. Previous studies have shown that sources of cancer-related pain for CRC survivors include gastrointestinal symptoms, including general stomachache (more common in survivors with chemotherapy, 11% vs 3%) and cramping (more common in those with ostomies, 17% vs. 5%). (44) Bowel symptoms, including pain symptoms, are prominent especially among rectal cancer survivors.(45) Consistent with previous studies, we found that younger age, female gender, race/ethnicity, treatment (chemotherapy and radiation), and comorbidities, specifically Crohn’s disease, diabetes, pulmonary disease, previous heart attack or stroke, and depression/emotional problems, were associated with higher likelihood of reporting pain interference during the initial phase of care. (18, 21, 22) The contribution of comorbid conditions to pain interference is of particular importance. Comorbidities such as Crohn’s disease likely exacerbate any cancer-related general stomachache and bowel discomfort(46) experienced by CRC survivors during the initial phase of treatment. Comorbidities such as diabetes contribute in other ways, for example because of poor diabetes management during cancer treatment: this may contribute to pain interference through painful lower limb neuropathy or other complications. (47)
At follow-up, in addition to the age, gender, treatment, and depression/emotional problems, low income and other comorbidities were associated with pain interference that continued or was newly reported since the initial phase of care. Heart failure, diabetes, and pulmonary disease were significant predictors, while Crohn’s disease, heart attack, and stroke were no longer associated with pain interference. Cardiovascular conditions, including heart failure, diabetes requiring insulin, and chronic pulmonary disease have been identified as “high severity” comorbidities for cancer patients.(48) These findings are in line with reports from a non-cancer population where patients with multiple health conditions and those with psychological conditions are more likely to report pain. (49) However, only heart failure and pulmonary disease were associated with continued pain interference among the survivors who had moderate/high pain interference during the initial phase of care. This is in contrast to previous studies of breast cancer patients in which multiple demographic and clinical factors were associated with increased pain interference, not just comorbid conditions.(21, 22) Overall these findings suggest that the cancer-related information (i.e., treatment or stage at diagnosis) reviewed by physicians may not provide enough information to predict which survivors may be at risk for persistent pain interference. Assessments of current comorbidity or a history of conditions, such as heart failure, and their management may provide critical information to help patients reduce pain-related problems. Factors not present in our study, including those related psychological or social factors (e.g., coping strategies, social support, or perceived control over pain)(50) likely also contribute to the change in pain interference at follow-up.
Job change and pain interference
The importance of considering pain interference in studies of survivors is demonstrated by its association with job change from the treatment period to post-treatment follow-up.(51) About one-third of CRC survivors in our sample were employed during their initial phase of care, a lower proportion compared to other studies of younger populations with multiple types of cancer.(52) Pain interference, particularly when it continued or increased post-treatment, was strongly associated with no longer having a job post-treatment. Available interventions for work-related issues in cancer survivors may not target pain specifically, but focus on other aspects, for example exercise rehabilitation and psychological counseling.(53) Much of the available research on work outcomes in survivors focuses on reintegration into the workplace or legal aspects in the workplace: it does not address work retention after diagnosis. (51, 52) In addition to traditional pain management strategies, occupational health strategies from a therapist may assist the survivor/employee to improve the ability to continue working. (54)
The limitations of this study include the CanCORS study population, which has a higher level of education than the average population and the majority of survivors (>97%) have health insurance, limiting generalizability to other populations. However, this is a multi-site study incorporating several health care delivery systems and geographically distinct sites. Second, survivors unable to complete the full version of the survey were not included in our analysis. Pain interference may be underestimated due to the inability to include those in worse health states. Third, our single-item measure of pain interference may not be sensitive to all aspects of interference (i.e., social and cognitive activities), underestimating the level of pain interference. Instruments such as the PROMIS scale (55) or the pain interference scale of the BPI (56) are able to measure areas such as social engagement interference or mood/affective interference. However, single-item measures of pain interference do have good criterion-validity with quality of life(57) and the single question provides a realistic representation of what could be asked by physicians in time-constrained clinic visits. Finally, we are not able to determine whether the pain interference is directly related to cancer or whether the job change is specifically related to the pain interference. However, our multivariate models for job change support the strong association between pain and job change.
Despite limitations, the clinical implications are important given our findings of the large proportion of unresolved pain interference as well as the data from a previous study that about 30% of CRC survivors are not asked about pain and only 55% report a discussion with their doctor regarding pain. (40) In light of the evidence that CRC survivors are less likely to care for comorbidities, (17) and our finding that specific comorbidities were strongly associated with continued pain interference are particularly important. Practitioners may need to comprehensively evaluate survivors’ management of their conditions to determine whether additional treatment or modification is needed. The 2014 National Comprehensive Cancer Network guidelines, while focusing on pain intensity, emphasize the goals of pain management are to address comfort, function, and safety. (58) Some discussion of whether pain (limited or severe) affects normal function is needed to identify or modify strategies to manage pain, such as treating comorbidities, pain medication (i.e., dosage, medication type), other therapy (e.g., physical therapy and exercise).
A problem exists with pain interference past the initial treatment phase among CRC survivors and this is associated to survivors remaining employed. Given that pain brings high national costs in terms of disability and health care burden, (59, 60) and high personal costs in terms of physical functioning, quality of life, and return to work, (10, 33, 51, 61) it is imperative that we find ways to address the problem. Addressing chronic comorbidities that are likely contributing to the source of pain, managing expectations for recovery, discussion of pain medication use, and identifying therapies for treating pain-related functional problems and/or psychological conditions are important considerations for taking a comprehensive approach reducing pain interference.
Table 5.
Predictors of continued pain interference (same or higher) at follow-up among those who reported moderate/high pain interference at baseline (ref: decreased pain interference at follow-up) n=603
| Demographics | Demographics and Clinical | ||
|---|---|---|---|
|
| |||
| Equivalent/increasing PI | Equivalent/increasing PI | ||
| Demographics | OR (95% CI) | OR (95% CI) | |
| Race (ref*=White) | |||
| African American | 0.96 (0.62-1.50) | 1.11 (0.68-1.83) | |
| Hispanic | 0.74 (0.41-1.34) | 0.72 (0.38-1.35) | |
| Asian | 0.59 (0.26-1.30) | 0.54 (0.22-1.34) | |
| Age (ref=<54 years) | |||
| 55 to 64 | 1.58 (1.03-2.42) | 1.43 (0.90-2.27) | |
| 65 to 74 | 0.84 (0.52-1.37) | 0.85 (0.49 -1.46) | |
| ≥75 | 1.21 (0.73-2.01) | 1.09 (0.60-1.99) | |
| Sex (ref=male) | |||
| Female | 1.03 (0.78-1.53) | 1.19 (0.83-1.71) | |
| Income (ref=≥$60,000) | |||
| <$20,000 | 1.91 (1.12-3.25) | 1.54 (0.87-2.71) | |
| $20,000 to <$40,000 | 1.42 (0.87-2.33) | 1.09 (0.91-1.86) | |
| $40,000 to <$60,000 | 0.91 (0.52-1.58) | 0.74 (0.41-1.33) | |
| Education (ref= College degree or higher) | |||
| Less than high school | 1.02 (0.57-1.82) | 1.01 (0.59-2.06) | |
| High school graduate/GED | 1.23 (0.76-1.99) | 1.13 (0.68-1.89) | |
| Some college/ vocational school | 0.95 (0.60-1.52) | 0.94 (0.57-1.54) | |
| Clinical characteristics | |||
| Time since diagnosis (days) | 0.99 (0.99-1.00) | ||
| Stage at diagnosis (ref=Stage I) | |||
| Stage II | 0.91 (0.54-1.55) | ||
| Stage III | 0.87 (0.49-1.56) | ||
| Stage IV | 1.39 (0.66-2.94) | ||
| Treatment† (ref=neither) | |||
| Chemotherapy or radiation only | 0.74 (0.42-1.28) | ||
| Chemotherapy and Radiation | 1.19 (0.66-2.13) | ||
| Stoma (ref=no) | |||
| Yes | 1.08 (0.60-1.92) | ||
| Diabetes (ref=no) | |||
| Yes | 1.54 (0.98-2.42) | ||
| Kidney disease (ref=no) | |||
| Yes | 1.62 (0.70-3.74) | ||
| Crohn’s disease (ref=no) | |||
| Yes | 1.62 (0.70-3.74) | ||
| Pulmonary disease (ref=no) | |||
| Yes | 1.71 (1.07-2.76) | ||
| Heart failure (ref=no) | |||
| Yes | 4.10 (1.51-11.10) | ||
| Heart attack (ref=no) | |||
| Yes | 0.72 (0.35-1.47) | ||
| Stroke (ref=no) | |||
| Yes | 1.63 (0.88-3.03) | ||
| Depression (ref=no) | |||
| Yes | 1.24 (0.82-1.87) | ||
|
| |||
| Goodness-of-fit (Hosmer-Lemeshow) | |||
| X2 (df) p-value | 5.41 (8) p=0.713 | 12.11 (8) p=0.146 | |
| C-statistic | 0.58 | 0.67 | |
Ref = Reference category;
Treatments that had been received by the time of the follow-up survey.
Acknowledgements
None
Funding: CanCORS consortium was supported by grants from the National Cancer Institute (NCI) to the Statistical Coordinating Center (U01 CA093344) and the NCI-supported Primary Data Collection and Research Centers (Dana-Farber Cancer Institute/Cancer Research Network [U01 CA093332], Harvard Medical School/Northern California Cancer Center [U01 CA093324], RAND/UCLA [U01 CA093348[, University of Alabama at Birmingham [U01CA093329], University of Iowa [U01CA093339], University of North Carolina [U01 CA 093326] and by a Department of Veterans Affairs grant to the Durham VA Medical Center [CRS 02-164]; and grant 2 T32 HS013852 from the Agency for Healthcare Research and Quality, Rockville, MD (KK)
Footnotes
Conflict of interest: All authors confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome. There are no disclosures to report.
References
- 1.National Research Council . Relieving pain in America: A blueprint for transforming prevention, care, education, and research. The National Academies Press; Washington, DC: 2011. [PubMed] [Google Scholar]
- 2.Goudas LC, Bloch R, Gialeli-Goudas M, Lau J, Carr DB. The epidemiology of cancer pain. Cancer Invest. 2005;23(2):182–90. [PubMed] [Google Scholar]
- 3.Chang VT, Hwang SS, Feuerman M, Kasimis BS. Symptom and quality of life survey of medical oncology patients at a Veterans Affairs medical center: A role for symptom assessment. Cancer. 2000;88(5):1175–83. doi: 10.1002/(sici)1097-0142(20000301)88:5<1175::aid-cncr30>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 4.van den Beuken-van Everdingen M, de Rijke J, Kessels A, Schouten H, van Kleef M, Patijn J. Prevalence of pain in patients with cancer: A systematic review of the past 40 years. Ann Oncol. 2007;18(9):1437–49. doi: 10.1093/annonc/mdm056. [DOI] [PubMed] [Google Scholar]
- 5.Breivik H, Cherny N, Collett B, et al. Cancer-related pain: A pan-European survey of prevalence, treatment, and patient attitudes. Ann of Onco. 2009;20(8):1420–33. doi: 10.1093/annonc/mdp001. [DOI] [PubMed] [Google Scholar]
- 6.Rustoen T, Fossa SD, Skarstein J, Moum T. The impact of demographic and disease-specific variables on pain in cancer patients. J Pain Symptom Manage. 2003;26(2):696–704. doi: 10.1016/s0885-3924(03)00239-2. [DOI] [PubMed] [Google Scholar]
- 7.Stuver SO, Isaac T, Weeks JC, et al. Factors associated with pain among ambulatory patients with cancer with advanced disease at a comprehensive cancer center. J Oncol Pract. 2012;8(4):e17–23. doi: 10.1200/JOP.2011.000388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu JSY, Beaton D, Smith PM, Hagen NA. Patterns of pain and interference in patients with painful bone metastases: A Brief Pain Inventory validation study. J Pain Symptom Manage. 2010;39(2):230–40. doi: 10.1016/j.jpainsymman.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 9.Day MA, Thorn BE. The relationship of demographic and psychosocial variables to pain-related outcomes in a rural chronic pain population. Pain. 2010;151(2):467–74. doi: 10.1016/j.pain.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kroenke K, Theobald D, Wu J, Loza JK, Carpenter JS, Tu W. The association of depression and pain with health-related quality of life, disability, and health care use in cancer patients. J Pain Symptom Manage. 2010;40(3):327–41. doi: 10.1016/j.jpainsymman.2009.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wells M, Williams B, Firnigl D, et al. Supporting 'work-related goals' rather than 'return to work' after cancer? A systematic review and meta-synthesis of 25 qualitative studies. Psychooncology. 2013;22(6):1208–19. doi: 10.1002/pon.3148. [DOI] [PubMed] [Google Scholar]
- 12.American Cancer Society Colorectal cancer facts & figures 2014-1016. American Cancer Society. 2014 [cited 04/04/2014] [Google Scholar]
- 13.Green CR, Hart-Johnson T, Loeffler DR. Cancer-related chronic pain: Examining quality of life in diverse cancer survivors. Cancer. 2011;117(9):1994–2003. doi: 10.1002/cncr.25761. [DOI] [PubMed] [Google Scholar]
- 14.Lowery AE, Starr T, Dhingra LK, et al. Frequency, characteristics, and correlates of pain in a pilot study of colorectal cancer survivors 1-10 years post-treatment. Pain Med. 2013;14(11):1673–80. doi: 10.1111/pme.12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van den Beuken-van Everdingen, Marieke HJ, de Rijke JM, Kessels AG, Schouten HC, van Kleef M, Patijn J. High prevalence of pain in patients with cancer in a large population-based study in the Netherlands. Pain. 2007;132(3):312–20. doi: 10.1016/j.pain.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 16.Moryl N, Coyle N, Essandoh S, Glare P. Chronic pain management in cancer survivors. J Natl Compr Canc Netw. 2010;8(9):1104–10. doi: 10.6004/jnccn.2010.0079. [DOI] [PubMed] [Google Scholar]
- 17.Snyder CF, Frick KD, Herbert RJ, et al. Quality of care for comorbid conditions during the transition to survivorship: Differences between cancer survivors and noncancer controls. J Clin Oncol. 2013;31(9):1140–8. doi: 10.1200/JCO.2012.43.0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang HL, Kroenke K, Wu J, Tu W, Theobald D, Rawl SM. Predictors of cancer-related pain improvement over time. Psychosom Med. 2012;74(6):642–7. doi: 10.1097/PSY.0b013e3182590904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fisch MJ, Lee J, Weiss M, et al. Prospective, observational study of pain and analgesic prescribing in medical oncology outpatients with breast, colorectal, lung, or prostate cancer. J Clin Oncol. 2012;30(16):1980–8. doi: 10.1200/JCO.2011.39.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gatchel RJ, Peng YB, Peters ML, Fuchs PN, Turk DC. The biopsychosocial approach to chronic pain: Scientific advances and future directions. Psychol Bull. 2007;133(4):581–624. doi: 10.1037/0033-2909.133.4.581. [DOI] [PubMed] [Google Scholar]
- 21.Moye J, June A, Martin LA, Gosian J, Herman LI, Naik AD. Pain is prevalent and persisting in cancer survivors: Differential factors across age groups. J Geriatr Oncol. 2014;5(2):190–6. doi: 10.1016/j.jgo.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Castel LD, Saville BR, DePuy V, Godley PA, Hartmann KE, Abernethy AP. Racial differences in pain during 1 year among women with metastatic breast cancer. Cancer. 2008;112(1):162–70. doi: 10.1002/cncr.23133. [DOI] [PubMed] [Google Scholar]
- 23.Ayanian JZ, Chrischilles EA, Fletcher RH, et al. Understanding cancer treatment and outcomes: The Cancer Care Outcomes Research and Surveillance Consortium. J Clin Oncol. 2004;22(15):2992–6. doi: 10.1200/JCO.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 24.Malin JL, Ko C, Ayanian JZ, et al. Understanding cancer patients' experience and outcomes: Development and pilot study of the Cancer Care Outcomes Research and Surveillance patient survey. Support Care Cancer. 2006;14(8):837–48. doi: 10.1007/s00520-005-0902-8. [DOI] [PubMed] [Google Scholar]
- 25.Ware J, Jr, Kosinski M, Keller SD. A 12-item short-form health survey: Construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–33. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Lacey RJ, Belcher J, Croft PR. Does life course socio-economic position influence chronic disabling pain in older adults? A general population study. Eur J Public Health. 2013;23(4):534–40. doi: 10.1093/eurpub/cks056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thomas E, Peat G, Harris L, Wilkie R, Croft PR. The prevalence of pain and pain interference in a general population of older adults: Cross-sectional findings from the North Staffordshire Osteoarthritis Project (NorStOP) Pain. 2004;110(1–2):361–8. doi: 10.1016/j.pain.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 28.Cleeland CS, Gonin R, Hatfield AK, et al. Pain and its treatment in outpatients with metastatic cancer. N Engl J Med. 1994;330(9):592–6. doi: 10.1056/NEJM199403033300902. [DOI] [PubMed] [Google Scholar]
- 29.Cleeland C. Brief Pain Inventory user guide. University of Texas MD Anderson; Houston: 2009. [Google Scholar]
- 30.Im EO, Chee W, Guevara E, et al. Gender and ethnic differences in cancer pain experience: A multiethnic survey in the United States. Nurs Res. 2007;56(5):296–306. doi: 10.1097/01.NNR.0000289502.45284.b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Green CR, Hart-Johnson T. Cancer pain: An age-based analysis. Pain Med. 2010;11(10):1525–36. doi: 10.1111/j.1526-4637.2010.00957.x. [DOI] [PubMed] [Google Scholar]
- 32.Cano A, Mayo A, Ventimiglia M. Coping, pain severity, interference, and disability: The potential mediating and moderating roles of race and education. J Pain. 2006;7(7):459–68. doi: 10.1016/j.jpain.2006.01.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mantyh PW. Cancer pain and its impact on diagnosis, survival and quality of life. Nat Rev Neurosci. 2006;7(10):797–809. doi: 10.1038/nrn1914. [DOI] [PubMed] [Google Scholar]
- 34.Polomano RC, Farrar JT. Pain and neuropathy in cancer survivors. surgery, radiation, and chemotherapy can cause pain; research could improve its detection and treatment. Am J Nurs. 2006;106(3 Suppl):39–47. doi: 10.1097/00000446-200603003-00015. [DOI] [PubMed] [Google Scholar]
- 35.Das SC, Khurana H, Gupta D, Mishra S, Bhatnagar S. Comorbidities in a cancer patient: Problems in pain management and palliation. Am J Hosp Palliat Care. 2009;26:60–3. doi: 10.1177/1049909108322297. [DOI] [PubMed] [Google Scholar]
- 36.Wilson TR, Alexander DJ, Kind P. Measurement of health-related quality of life in the early follow-up of colon and rectal cancer. Dis Colon Rectum. 2006;49(11):1692–702. doi: 10.1007/s10350-006-0709-9. [DOI] [PubMed] [Google Scholar]
- 37.Nersesyan H, Slavin KV. Current aproach to cancer pain management: Availability and implications of different treatment options. Ther Clin Risk Manag. 2007;3(3):381–400. [PMC free article] [PubMed] [Google Scholar]
- 38.He Y, Zaslavsky AM, Landrum MB, Harrington DP, Catalano P. Multiple imputation in a large-scale complex survey: A practical guide. Stat Methods Med Res. 2010;19(6):653–70. doi: 10.1177/0962280208101273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.SAS Institute SAS 9.3. 2012 9.3. [Google Scholar]
- 40.van Ryn M, Phelan SM, Arora NK, et al. Patient-reported quality of supportive care among patients with colorectal cancer in the veterans affairs health care system. J Clin Oncol. 2014 doi: 10.1200/JCO.2013.49.4302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schreiber J. Understanding the cancer pain experience. Curr Pain Headache Rep. 2014;18(8):1–6. doi: 10.1007/s11916-014-0440-5. [DOI] [PubMed] [Google Scholar]
- 42.Knudsen AK, Aass N, Heitzer E, et al. Interviews with patients with advanced cancer--another step towards an international cancer pain classification system. Support Care Cancer. 2012;20(10):2491–500. doi: 10.1007/s00520-011-1361-z. [DOI] [PubMed] [Google Scholar]
- 43.Hjermstad MJ, Fainsinger R, Kaasa S. European Palliative Care Research Collaborative (EPCRC) Assessment and classification of cancer pain. Curr Opin Support Palliat Care. 2009;3(1):24–30. doi: 10.1097/SPC.0b013e3283260644. [DOI] [PubMed] [Google Scholar]
- 44.Schneider EC, Malin JL, Kahn KL, Ko CY, Adams J, Epstein AM. Surviving colorectal cancer. Cancer. 2007;110(9):2075–82. doi: 10.1002/cncr.23021. [DOI] [PubMed] [Google Scholar]
- 45.Denlinger CS, Barsevick AM. The challenges of colorectal cancer survivorship. J Natl Compr Cancr Netw. 2009;7(8):883–94. doi: 10.6004/jnccn.2009.0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Crohn's disease: MedlinePlus medical encyclopedia 2013Available at: http://www.nlm.nih.gov/medlineplus/ency/article/000249.htm. Accessed 12/18, 2014.
- 47.Said G. Diabetic neuropathy. Handb Clin Neurol. 2013;115:579–89. doi: 10.1016/B978-0-444-52902-2.00033-3. [DOI] [PubMed] [Google Scholar]
- 48.Yancik R, Ganz PA, Varricchio CG, Conley B. Perspectives on comorbidity and cancer in older patients: Approaches to expand the knowledge base. J Clin Oncol. 2001;19(4):1147–51. doi: 10.1200/JCO.2001.19.4.1147. [DOI] [PubMed] [Google Scholar]
- 49.Mailis-Gagnon A, Nicholson K, Yegneswaran B, Zurowski M. Pain characteristics of adults 65 years of age and older referred to a tertiary care pain clinic. Pain Res Manag. 2008;13(5):389–94. doi: 10.1155/2008/541963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hanley MA, Raichle K, Jensen M, Cardenas DD. Pain catastrophizing and beliefs predict changes in pain interference and psychological functioning in persons with spinal cord injury. J Pain. 2008;9(9):863–71. doi: 10.1016/j.jpain.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Feuerstein M, Todd BL, Moskowitz MC, et al. Work in cancer survivors: A model for practice and research. J Cancer Surviv. 2010;4(4):415–37. doi: 10.1007/s11764-010-0154-6. [DOI] [PubMed] [Google Scholar]
- 52.Short PF, Vasey JJ, Tunceli K. Employment pathways in a large cohort of adult cancer survivors. Cancer. 2005;103(6):1292–301. doi: 10.1002/cncr.20912. [DOI] [PubMed] [Google Scholar]
- 53.Thijs KM, de Boer AG, Vreugdenhil G, van de Wouw AJ, Houterman S, Schep G. Rehabilitation using high-intensity physical training and long-term return-to-work in cancer survivors. J Occup Rehabil. 2012;22(2):220–9. doi: 10.1007/s10926-011-9341-1. [DOI] [PubMed] [Google Scholar]
- 54.Macmillan Cancer Support/ICM Returning to work: Cancer and vocational rehabilitation. report of a scoping study for Macmillan Cancer Support. 2008 [Google Scholar]
- 55.Amtmann D, Cook KF, Jensen MP, et al. Development of a PROMIS item bank to measure pain interference. Pain. 2010;150(1):173–82. doi: 10.1016/j.pain.2010.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cleeland CS, Ryan KM. Pain assessment: Global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23(2):129–38. [PubMed] [Google Scholar]
- 57.Jensen MP. The validity and reliability of pain measures in adults with cancer. J Pain. 2003;4(1):2–21. doi: 10.1054/jpai.2003.1. [DOI] [PubMed] [Google Scholar]
- 58.National Comprehensive Cancer Network Adult cancer pain. 2014 In: NCCN Guidelines. [Google Scholar]
- 59.Stewart WF, Ricci JA, Chee E, Morganstein D, Lipton R. Lost productive time and cost due to common pain conditions in the US workforce. JAMA. 2003;290(18):2443–54. doi: 10.1001/jama.290.18.2443. [DOI] [PubMed] [Google Scholar]
- 60.Gaskin DJ, Richard P. The economic costs of pain in the United States. J of Pain. 2012;13(8):715–24. doi: 10.1016/j.jpain.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 61.Black B, Hartford JA, Herr K, et al. The relationships among pain, non-pain symptoms, and quality of life measures in older adults with cancer receiving hospice care. Pain Med. 2011;12(6):880–9. doi: 10.1111/j.1526-4637.2011.01113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]