Abstract
Purpose
Extracorporeal photopheresis (ECP) alone or in combination therapy is effective for treatment of leukemic cutaneous T-cell lymphoma (L-CTCL), but its mechanism(s) of action remain unclear. This study was designed to investigate the effect of ECP on regulatory T-cell and CD8+ T-cells in L-CTCL patients.
Experimental Design
Peripheral blood from 18 L-CTCL patients at baseline, Day 2, 1-month, 3-month, and 6-month post-ECP therapy were analyzed by flow cytometry for CD4+CD25+/high, CD4+Foxp3+CD25+/-, CD3+CD8+, CD3+CD8+CD69+, and CD3+CD8+IFN-γ+ T-cells. Clinical responses were assessed and correlated with changes in these T-cell subsets.
Results
Twelve of 18 patients achieved clinical responses. The average baseline number of CD4+CD25+/high T-cells of PBMCs in L-CTCL patients was normal (2.2%), but increased at 6-month post-therapy (4.3%, p<0.01). The average baseline number of CD4+Foxp3+ T-cells out of CD4+ T-cells in 9 evaluable patients was high (66.8±13.7%), mostly CD25 negative. The levels of CD4+Foxp3+ T cells in responders were higher (n=6, 93.1±5.7%) than non-responders (n=3, 14.2±16.0%, p<0.01), and they declined in parallel with malignant T-cells. The numbers of CD3+CD8+CD69+ and CD3+CD8+ IFN-γ+ T-cells increased at 3-month post-therapy in 5 of 6 patients studied.
Conclusions
ECP alone or in combination therapy might be effective in L-CTCL patients whose malignant T-cells have a CD4+Foxp3+CD25- phenotype.
Keywords: Sézary Syndrome, Mycosis fungoides, regulatory T-cells, CD25, foxp3, CD8+ T-cells, extracorporeal photopheresis
Introduction
Cutaneous T-cell Lymphomas (CTCL) are extra-nodal non-Hodgkin T-cell lymphomas that first present as cutaneous lesions with clonal expansion of malignant helper T-cells (1). The leukemic variants (L-CTCL) include Sézary Syndrome (SS) and a portion of mycosis fungoides (MF) harboring blood CD4+CD26- or/and CD4+CD7- malignant T-cells with diffuse erythroderma. The degree of blood involvement in patients with L-CTCL is associated with worse overall survival (2-4). To date, L-CTCL/SS are rarely curable except long-term complete response after allogeneic stem cell transplantation(5, 6).
Extracorporeal photopheresis (ECP) is used commonly as frontline therapy for L-CTCL, with which leukocytes are exposed ex vivo to 8-methoxypsoralen (8-MOP) and UVA radiation, and then reinfused into the patient circulation. The overall response rate of ECP in CTCL patients is between 54% and 74% with a 14%-33.3% complete response rate (7-9). It is well-tolerated with minimal side effects and increased overall survival (9-11). To achieve more complete responses, biological response modifiers (BRM), especially interferons and retinoids, are often administered together with ECP and is known as combined immunomodulatory therapy. However, many question about how the therapy works remain unclear.
Regulatory T-cells (Treg cells) are “professional” regulatory/suppressor T-cells critical for maintenance of immune homeostasis and prevention of autoimmunity (12). Treg cells are characterized by constitutive expression of the transcription factor forkhead box P3 (Foxp3) essential for Treg cell development and suppressive activity. The expression of CD25, the α-chain of IL-2 receptor, is also a feature of Treg cells, but its expression is less specific, because CD25 is also expresses by conventional activated T-cells. However, Treg cells express higher levels of CD25 compared to conventional T-cells (12). Therefore, the expression of Foxp3 and the high level of CD25 are widely-used as phenotypic markers for Treg cells.
Interestingly, malignant T-cells in L-CTCL, especially in SS, share many features with Treg cells. SS cells derive from CD4+ helper T-cells, and a portion of them are positive for CD25(13), are anergic to activation stimuli, and are also immunosuppressive (14). Berger et al reported that after being co-cultured with dendritic cells loaded with apoptotic tumor cells ex vivo, CD4+CTCL cells adopted a Treg cell phenotype expressing CD25 and Foxp3 and secreting IL-10 and TGF-β (15). They, therefore, proposed that CTCL might be a malignancy of Treg cells. Heid et al also found that a subset of SS patients had malignant CD4+Foxp3+CD25- T-cells with regulatory function (16). However, discordant findings have simultaneously been reported, especially in MF patients (17-20). How Treg cells are modulated during therapy with ECP has not been established. Addressing the controversy of Treg cells in CTCL and understanding the effects of ECP on Treg cells may be helpful to develop more effective and less immunosuppressive therapies.
Although the immune tolerance mediated by Treg cells may explain the effects of ECP in graft-versus-host disease (GVHD), the anti-tumor immunity mediated by CD8+ cytotoxic T lymphocytes may underlie the efficacy of ECP in L-CTCL(21). Higher numbers of blood CD8+ T-cells are associated with better clinical response to ECP(22). Clinical improvement after ECP in CTCL patients is associated with a shift from a Th2 phenotype to a IL-12/Th1 phenotype (23). We recently reported that in patients with L-CTCL, ECP augments blood myeloid dendritic cells (mDC), a subset of DCs producing IL-12 that polarizes naïve T-cells toward a Th1 phenotype (24).
This translational pilot study was designed to further investigate the effect of ECP treatment on Treg cells and CD8+ T-cell function. By flow cytometry, we analyzed CD4+CD25+/high, CD4+Foxp3+CD25+/-, CD3+CD8+, CD3+CD8+CD69+, and CD3+CD8+IFN-γ+T-cell subsets in peripheral blood from L-CTCL patients at baseline, Day 2, and 1, 3, and 6 months during ECP therapy. Clinical responses over six months of therapy were correlated with changes in these T-cell subsets.
Materials and Methods
Study Design and patients
Patients with L-CTCL starting ECP treatment during 04/2007 -11/2010 signed informed consents to enroll in this study. The study was approved by the University of Texas MD Anderson Cancer Center Institutional Review Board, and conducted according to the Declaration of Helsinki Principles. The revised diagnosis criteria for staging of MF and SS by ISCL/EORTC was used (25). All patients were treated with the UVAR XTS photopheresis system (Therakos, Inc. Raritan, NJ) over 2 consecutive days every 2-4 weeks per cycle. Fresh peripheral blood was collected at baseline (BL) and after ECP on Day 2 (D2), 1 month (1M), 3-4 months (3-4M), and 6-7 months (6-7M). Peripheral blood samples from normal donors (ND) were obtained from the Department of Transfusion Medicine at our institution. Peripheral mononuclear cells (PBMC) were isolated by Ficoll density gradient centrifugation.
Clinical response analysis
Evaluation of skin involvement by modified severity-weighted assessment tool (mSWAT) was performed (MD) at baseline (BL) and after treatment at 1 month, 3-4 months, and 6-7 months. In this study, we defined circulating malignant T-cells as CD3+CD4+CD26- and/or CD7- T-cells by flow cytometry (26), and assessed them before and at 1 month, 3-4 months, and 6-7 months over a treatment course (10, 27-30). Patients with complete responses (CR) had complete disease disappearance in skin or blood. Patients with partial responses (PR) had greater than 50% improvement in skin or blood involvement. Minor response (MR) patients had 25%-50% improvement of skin or blood. Patients with CR, PR and MR were considered as responders. Patients with stable disease (SD) whose changes in skin or blood were within 25%, and patients with progressive disease (PD) whose changes in skin or blood were 25% worse from baseline, were grouped as non-responders.
Flow cytometry analysis of regulatory T-cell subsets
Immunofluorescence surface staining for CD3+CD4+CD25+/high T-cells
The standard three-color flow cytometry was used to analyze CD3+CD4+CD25+/high T-cells with fresh PBMCs. Antibodies used were APC/Cy7 anti-human CD3 (BioLegend, San Diego, CA), FITC anti-human CD4, and PE anti-human CD25 (BD Biosciences). APC/Cy7-, FITC- or PE-conjugated IgG1κ, IgG2α were isotype controls.
Immunofluorescence intracellular staining for Foxp3+ T-cells
Frozen PBMCs from all time points for each patient were examined by flow cytometry at the same time. PBMCs were stained with CD3, CD4, and CD25 surface markers first. After a fixation and permeabilization step (Fixation/Permeabilization Solution, BD Bioscience), PE anti-human Foxp3 antibody (clone PCH101, eBioscience, Inc. San Diego, CA) was then added. Counting beads (Spherotech, Inc. Lake Forest, IL) were added afterward. Samples were analyzed on a Gallios flow cytometer using Kaluza software (Beckman Coulter, Inc. Brea CA), and absolute numbers were calculated (31).
Flow cytometry analysis of CD8+ T-cell subsets
Cell activation
Frozen PBMCs from all time points for each patient were incubated with the leukocyte activation cocktail (BD Biosciences) at 2μl/106 cells for 4 hours at 37°C before staining.
Immunofluorescence intracellular staining for CD3+CD8+IFNγ+ T-cells
FITC anti-human CD3 (BD Biosciences) and APC anti-human CD8- (Biolegend) were added into activated cells. After a fixation and permeabilization step, cells were then incubated with PerCyPCy 5.5 anti-human CD69 (Biolegend), followed with PE anti-human IFN-γ (R&D Systems). Isotype controls were also run in parallel.
Quantitative Real-Time PCR
Total RNA extraction from PBMCs, 1st strand cDNA synthesis, quantitative RT-PCR for Foxp3 (Hs00203958), and fold change calculation were done as previously described (32-34).
Statistical analysis
Differences in each parameter [mean (SD) and median (range)] among groups at baseline were examined by Kruskal-Wallis tests. Linear mixed models were used to assess the changes from baseline as a function of time point and clinical response (PR or MR vs. PD or SD). P-values <0.05 were considered as significantly different. A correlation was determined by Pearson product-moment correlation coefficient R ≥0.5-1.0 based on the Cohen scale. Statistical analyses were done in SPSS Statistics 17.0 and Microsoft Office Excel 2007.
Results
Response of L-CTCL patients to ECP alone or in combination therapy
Eighteen L-CTCL patients completed the 6-month treatment course and were evaluable for clinical response in skin and blood (35). Their demographics were summarized in Table 1. All patients had generalized erythroderma except Patient #17 who had high numbers of circulating tumor cells but no visible skin lesions as previously reported (33). All patients had ≥ 50% circulating malignant T-cells exception Patient #4 (Table 2). The circulating tumor cells in 17 patients had a CD4+CD26- phenotype except for Patient #12 who had a CD4+CD7- phenotype. All L-CTCL patients had previously received skin directed therapies, but none had received radiation, chemotherapy, or immunosuppressive agents. Six of 18 L-CTCL patients were treated with ECP only. Twelve patients who had no improvement on ECP alone at 3 months had addition of biological response modifiers, bexarotene or/and IFN-α.
Table 1. Clinical characteristics and responses of L-CTCL patients.
| Characteristics | Patients (n=18) |
|---|---|
| Age - Years old | |
| Median | 67.0 |
| Range | 54.0 – 79.0 |
|
| |
| Gender - no. of patients (%) | |
| Female | 5 (27.8%) |
| Male | 13 (72.2%) |
|
| |
| Stage - no. of patients (%) | |
| III | 2 (11.1%) |
| IV | 16 (88.9%) |
|
| |
| Modified skin-weighted assessment tool (mSWAT) | |
| ≤ 5.0 | 1(5.5%) |
| 39.0– 80.0 | 10 (55.6%) |
| ≥ 80.0 | 7 (38.9%) |
|
| |
| Blood involvement * | |
| B1: low blood tumor burden | 4 (22.2%) |
| B2: high blood tumor burden | 14 (77.8%) |
|
| |
| TCR vβ - no. of patients (%) | |
| Positive | 16 (88.9%) |
| Negative | 2 (11.1%) |
|
| |
| ECP - no. of cycles** | |
| Median | 9.5 |
| Range | 6 - 13 |
|
| |
| Combination immunotherapy - no. of patients (%) | |
| ECP alone | 6 (33.3%) |
| + Bexarotene | 5 (27.8%) |
| + IFN-α | 2 (11.1%) |
| + Bexarotene and IFN-α | 5 (27.8%) |
|
| |
| Response - no. of patients (%) | |
| Partial response (PR) | 8 (44.4%) |
| Minor response (MR) | 4 (22.2%) |
| Stable disease (SD) | 3 (16.7%) |
| Progressive disease (PD) | 3 (16.7%) |
: circulating CD4+CD26- or CD4+CD7- T-cells;
: excluding Patient#8 who had L-CTCL plus GVHD
Table 2. Flow cytometry analysis of T-cell subsets in L-CTCL patients at baseline.
| Patient # | TCR vβ (%) | Circulating CD4+CD26- malignant T-cells | CD4+CD25+/high T-cells (%) | CD4+Foxp3+ T-cells (%) ** | CD8+ T-cells (/μl) | Clinical response | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| % | /μl | Total | CD25- | CD25+ | Total | CD69+ | IFN-γ+ | ||||
| 1 | 70.0 | 64.2 | 1751.0 | 0.3 | - | - | - | 364.0 | 8.3 | 0.6 | MR |
|
| |||||||||||
| 2 | 91.0 | 82.2 | 413.0 | 0.31 | - | - | - | 3.0 | - | - | PR |
|
| |||||||||||
| 5 | 85.0 | 89.9 | 3928.0 | 0.25 | 91.5 | 74.6 | 14.3 | 426.0 | - | - | PR |
|
| |||||||||||
| 6 | 73.7 | 73.7 | 2218.0 | 1.02 | 95.5 | 67.8 | 28.3 | 322.0 | - | - | PR |
|
| |||||||||||
| 7 | 83.0 | 79.1 | 1671.0 | 1.67 | 97.7 | 38.7 | 59.0 | 175.0 | - | - | PR |
|
| |||||||||||
| 8 | 76.0 | 64.4 | 227.0 | 6.51 | 83.4 | 61.4 | 19.9 | 13.0 | - | - | MR |
|
| |||||||||||
| 10 | 93.0 | 85.9 | 2752.0 | 0.66 | - | - | - | 80.0 | - | - | PR |
|
| |||||||||||
| 11 | 97.1 | 87.5 | 11581.0 | 1.96 | - | - | - | 106.0 | 17.1 | 15.8 | PR |
|
| |||||||||||
| 14 | 94.0 | 94.7 | 22751.0 | 0.78 | 99.1 | 83.8 | 15.0 | 240.0 | - | - | PR |
|
| |||||||||||
| 16 | n/a | 91.9 | 4300.0 | 0.86 | - | - | - | ---- | - | - | PR |
|
| |||||||||||
| 17 | 95.0 | 91.7 | 5139.0 | 1.19 | 91.3 | 78.5 | 12.7 | 184.0 | - | - | MR |
|
| |||||||||||
| 18 | 97.0 | 91.7 | 5500.0 | 0.01 | - | - | - | 138.0 | 9.2 | 6.6 | MR |
|
| |||||||||||
| N=12 | 86.8±9.97‡ | 83.1±10.6 | 5185.9±6311.3 | 1.3±1.7 | 93.1±5.7 | 67.5±16.2 | 24.9±17.7 | 186.5±139.4 | 11.5±4.8 | 7.7±7.7 | |
|
| |||||||||||
| 3 | n/a | 55.5 | 525.0 | 0.7 | 39.8 | 29.9 | 5.5 | 192 | - | - | PD |
|
| |||||||||||
| 4 | 54.0 | 20.6 | 69.0 | 1.4 | - | - | - | 55 | - | - | SD |
|
| |||||||||||
| 9 | 97.0 | 93.8 | 17981.0 | 0.9 | - | - | - | 192 | - | - | SD |
|
| |||||||||||
| 12* | 94.0 | 56.0 | 1284.6 | 3.4 | - | - | - | 179 | 0.2 | 0.2 | PD |
|
| |||||||||||
| 13 | 95.0 | 94.5 | 9977.0 | 0.2 | 2.1 | 1.5 | 0.3 | 252 | 1.8 | 0.7 | PD |
|
| |||||||||||
| 15 | 95.0 | 77.1 | 2600.0 | 16.7 | 0.6 | 0.5 | 0.1 | 47 | 1.5 | 0.1 | SD |
|
| |||||||||||
| N=6 | 87.0±18.5 | 66.3±28.2 | 5406.1±7158.1 | 3.91±6.4 | 14.2±16.0 | 10.6±12.1 | 1.9±2.2 | 152.8±82.9 | 1.2±0.8 | 0.3±0.3 | |
|
| |||||||||||
| p value | NS | 0.0811 | NS | NS | 0.0001 | 0.0017 | 0.0679 | NS | 0.0217 | 0.0035 | |
n/a: not available; PR: partial response, MR: minimal response, SD: stable disease, PD: progressive disease;
: Patient#12 with CD4+CD7- phenotype;
: % of CD4+ T-cells;
: Mean ±SD
After 6 months of treatment, there was an average 54.1 % reduction of circulating malignant T-cells and a 34.6 % reduction in mSWAT. Twelve of 18 patients (66.7%) achieved an overall response (Figure 1, Table 1): 11 had responses in skin and 3 had responses in blood. Of interest, four of six L-CTCL patients who received ECP alone and 8 of 12 who received ECP combination immunotherapy achieved the same overall clinical response rate of 66.7%. There were no significant differences in baseline mSWAT scores between responders and non-responders, but marginally higher percentages of CD4+CD26- or CD7- malignant T-cells were present in the responders compared to non-responders (Table 2).
Figure 1.
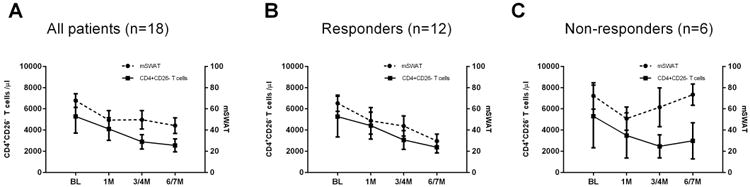
CD4+CD25+/high T-cell subsets in L-CTCL patients increased after ECP
The first analysis by flow cytometry was to measure CD4+CD25+/high T-cells in peripheral blood from 18 L-CTCL patients at baseline. The average numbers of CD4+CD25+/high T-cells in PBMCs were 2.2±3.8% for all 18 L-CTCL patients, and 0.8±0.6% for 15 patients if excluding three outliers (Patient# 8, #12, and #15), which were comparable to those in healthy donors (0.6±0.5%, n=8, Figure 2A, B).
Figure 2.
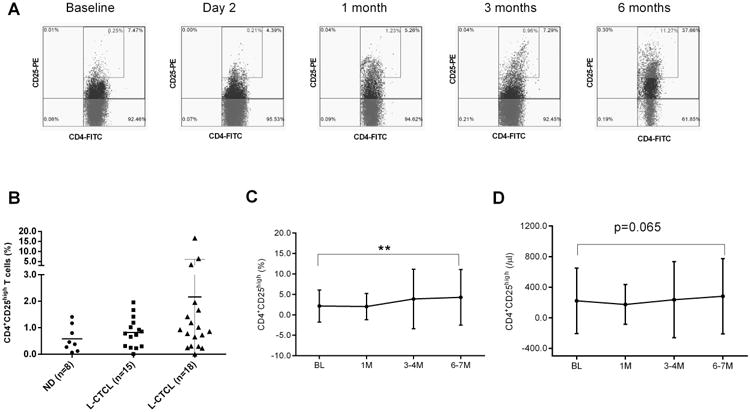
After treatment, the average numbers of CD4+CD25+/high T-cells increased, and average percentages of CD4+CD25High T-cells in PBMCs of 18 patients were 2.2% at baseline, 2.0% at 1 month, 3.9% at 3-4 months, and 4.3% at 6-7 months post-ECP (p<0.01, Figure 2A,C, Table S1). The average absolute numbers of CD4+CD25High T-cells were 221.1 cells/μl at baseline, 174.2 cells/μl at 1 month, 236.6 cells/μl at 3-4months, and 281.3 cells/μl at 6-7months post-ECP, respectively (Figure 2D, Table S1). In the 12 responders, the numbers of CD4+CD25+/high T-cells increased from 1.3% or 114.6 cells/μl at baseline to 4.5% or 224.33 cells/μl at 6-7 months post-ECP. However, in the 6 non-responders, the numbers of CD4+CD25+/high T-cells were unchanged from 3.9% or 416.4 cells/μl at baseline to 3.9% or 395.4 cells/μl at 6 months post-ECP (Table S1).
CD4+Foxp3+ T-cells in L-CTCL patients decreased after ECP
Malignant T-cells in L-CTCL have been reported to have a regulatory T-cell phenotype (CD4+Foxp3+) with either CD25 positive or CD25 negative (15, 16). In this study, we found increased Foxp3 mRNA levels in PBMCs of L-CTCL patients (n=17, 13.4±8.5 fold) compared to normal donors (n=5, 0.9±0.2 fold). Two patients (Patient#6 and Patient #17) had extremely high levels (80.3 and 126.8 fold). Patient #17, who had 1.19% CD4+CD25+/high T-cells in PBMCs, was previously reported as an invisible leukemic cutaneous T-cell lymphoma with a regulatory T-cell clone (33).
Next, we examined the intracellular expression of Foxp3 in CD4+ T-cells by flow cytometry. Due to limited cell numbers, we were only able to examine CD4+Foxp3+ T-cells in 9 L-CTCL patients. There were higher numbers of CD4+Foxp3+ T-cells out of total CD4+ T-cells in L-CTCL patients (n=9, 66.8±13.7%, Figure 3A) compared to normal donors (n=5, 5.8±2.2 %, t-test, p<0.05). About two thirds of CD4+Foxp3+ T-cells were CD25 negative (48.5±30.4%, Figure 3B). Interestingly, of 9 L-CTCL patients tested, 6 responders had much higher percentages of CD4+Foxp3+ T-cells (93.1±5.7%) which were similar to the levels of CD4+CD26- malignant T-cells (82.3%) and TCRvβ clonal T-cells (84.5%). In contrast, 3 non-responders had only low numbers of CD4+Foxp3+ T-cells (14.2±16.0%, p<0.01) although of high levels of CD4+CD26- malignant T-cells (75.7%) and TCRvβ clonal T-cells (95.0%) (Figure 3C, Table 2). CD4+Foxp3+ T-cells were predominantly CD25 negative in both responders (67.5±16.2 %) and non-responders (10.6±12.1%) compared to CD25 positive portions in responders (24.9±17.7 %) and non-responders (1.9±2.2 %), respectively.
Figure 3.
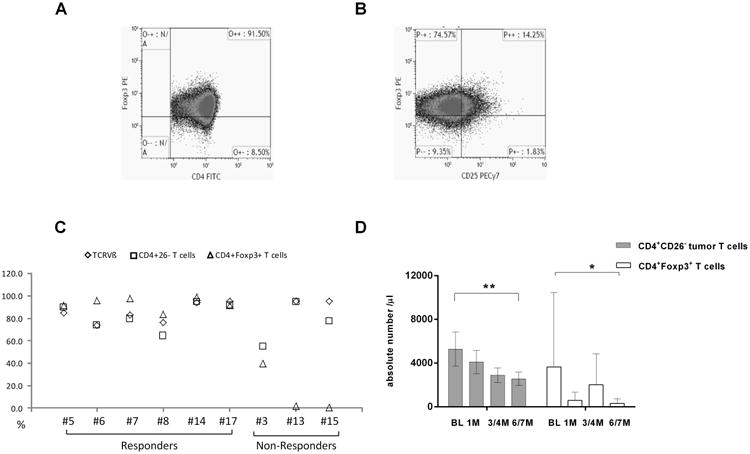
After ECP, the average absolute numbers of malignant T-cells in 18 patients decreased from 5903.9 cells cells/μl at baseline to 4326.5 cells/μl at 1 month, 3070.3 cells/μl at 3-4 months, and 2711.8 cells/μl at 6-7 months post-ECP. Meanwhile, the average numbers of CD4+Foxp3+ T-cells were 3640.7 cells/μl at baseline (n=9), 595.1 cells/μl at 1 month (n=5), 2010.9 cells/μl at 3-4 months (n=3), and 314.9 cells /μl at 6-7 months (n=5) post treatment (Figure 3D, Table S1).
Activated and functional CD3+CD8+ T-cells in L-CTCL patients increased after ECP
Compared to CD3+CD8+ T-cells in normal donors (596.6±412.8 cells/μl, n=3, Figure 4A), the average numbers in L-CTCL patients were lower (174.6±124.1 cells/μl, n=6, p<0.01, Figure 4B). Using CD69, a human transmembrane C-Type lectin protein as an activation marker, we found that activated CD8+CD69+ T-cells were much lower in L-CTCL patients (6.3±6.5 cells/μl, n=6, Figure 4B) than in normal donors (288.5 ± 185.6 cells /μl, n=3, p<0.01, Figure 4A). In addition, functional CD8+ T-cells were highly suppressed as indicated by their low expression of intracellular IFN-γ after activation. The CD3+CD8+IFN-γ+ T-cells in L-CTCL patients were rare (4.0 ±6.3 cells/μl, n=6, Figure 4B) versus normal donors (160.1±111.3 cells /μl, n=3, p<0.01, Figure 4A).
Figure 4.
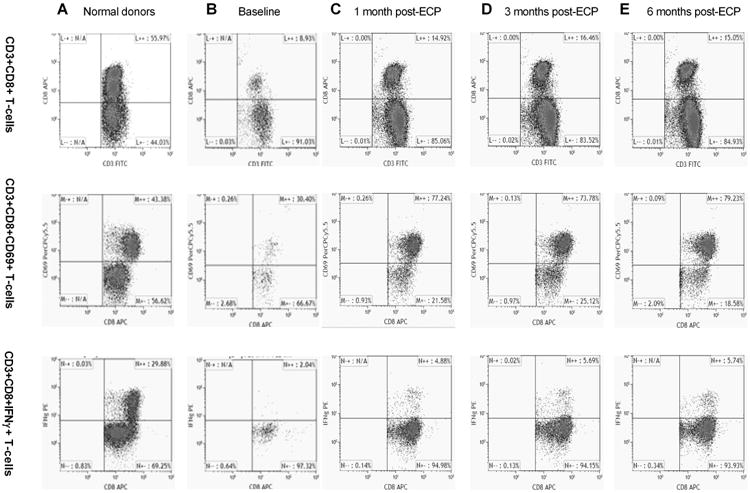
As shown in Table 2, responders had only slightly higher baseline CD8+ T-cell numbers (186.5±139.4 cells/μl, n=11) versus non-responders (152.8±82.9 cells/μl, n=6). Responders had higher baseline activated CD8+CD69+ T-cells (11.5±4.8 cells/μl, n=3) than non-responders (1.2±0.8 cells/μl, p<0.05, n=3). Functional CD3+CD8+INF-γ+ T-cells were also higher in responders (7.7±7.7 cells/μl, n=3) than in non-responders at baseline (0.3±0.3 cells/μl, n=3, p<0.01).
After treatment, the absolute numbers of CD8+ T-cells decreased in most patients (Table S1). In contrast, the percentages of CD8+ T-cells were unchanged or slightly increased during ECP (Table S1, Figure 4B-E). Interestingly, of 6 L-CTCL patients tested, activated CD3+CD8+CD69+ T-cells were increased in 5 patients until 3-4 months after ECP but their numbers were not sustained at 6 months. On average, CD3+CD8+CD69+ numbers increased from 6.3±2.7 cells/μl at baseline to 73.4 ±65.2 cells/μl at 1 month, and to 111.3±111.3 cells /μl at 3 months post-ECP (Figure 4C-E). Moreover, functional CD8+ T-cells also increased in 5 of 6 patients over ECP therapy. On average, CD3+CD8+ IFN-γ+ T-cell numbers increased from 4.0±2.6 cells/μl at baseline to 9.6±5.1 cells/μl at 1 month, and 8.1±8.0 cells/μl at 3-4 months after therapy.
Discussion
In this study, 12 of 18 L-CTCL patients (66.7%) achieved overall clinical responses to ECP alone or in combination therapy. Interestingly, the decrease in the numbers of CD4+Foxp3+ T-cells mirrors the blood improvement over 6 month treatment in some of L-CTCL patients. Indeed, 6 of 9 patients who had high numbers of circulating CD4+Foxp3+CD25- cells were responders compared to patients who had low CD4+Foxp3+ T-cells at baseline and did not respond. Meanwhile, we also observed an increase in activated and functional CD8+ T-cells during the first three months of therapy. To our knowledge, there is no previous study on the clinical response to ECP or ECP combination therapy in L-CTCL patients stratified by levels of blood CD4+Foxp3+ T-cells.
There are previous studies supporting the idea that malignant T-cells in L-CTCL patients are malignant Treg cells (15, 16). In this study, we showed that average Foxp3 mRNA expression was high in PBMCs from L-CTCL patients, which was consistent with our previous study (33, 34). CD4+Foxp3+T-cell populations were high at baseline in 6 of 9 L-CTCL patients studied, which were similar to the numbers of CD4+CD26- malignant T-cells and clonal T-cells in the peripheral blood. Over 6 months of ECP therapy, the numbers of circulating CD4+CD26- malignant T-cells declined, as did the numbers of CD4+Foxp3+ cells. Our results suggest that the majority of circulating malignant T-cells in some L-CTCL patients might have a CD4+Foxp3+ phenotype which appears to be more sensitive to ECP therapy. The future study with a bigger sample size needs to test this hypothesis. Recently, a distinct epigenetic modification in a region of the Foxp3 locus called the Treg-cell-specific demethylated region (TSDR) was found to be fully demethylated in Treg cells, and it was also found in SS patients with high numbers of Foxp3+ malignant T-cells (16).
Human Treg cells are functionally and phenotypically diverse. They can display molecular and functional heterogeneity, can sometimes be naïve and effector- or memory-like cells, and can produce effector cytokines (36-38). CD4+Foxp3+Treg cells might also become unstable. Under certain physiological states and inflammatory conditions, they might adopt a phenotype that is more characteristic of effector CD4+T-cells (36, 39). CD25 expression on Treg cells can be down-regulated in a lymphopenic environment (36, 40). In this study, we found that the heterogeneity of Treg cells with respect to expression of CD25 was observed in peripheral blood of L-CTCL patients. Two thirds of L-CTCL patients had high numbers of CD4+Foxp3+ T-cells. At baseline, two thirds of CD4+Foxp3+ T-cells were CD25 negative, and one-third were CD25 positive. Although there were high numbers of CD4+Foxp3+CD25- T-cells, the numbers of CD4+Foxp3+CD25+ T cells and CD4+CD25+/high T-cells were relatively normal in most of L-CTCL patients. It is possible that malignant T-cells in these L-CTCL patients may be Treg cells, but multiple phenotypes may co-exist. CD4+Foxp3+CD25- T-cells may be present in the setting of chronic inflammatory environments in CTCL. In fact, two separate Treg makers in our study, Foxp3 and CD25, showed discordant changes in ECP responders: decreasing in CD4+Foxp3+ T-cells but increasing in CD4+CD25+/high T-cells. Further study to understand the discrepancy and diversity of Treg cells in CTCL may help us uncover the mystery of ECP therapy in CTCL.
Studies have also shown that activation-induced Foxp3 expression in activated T-cells could be one component of homeostasis initiated by these cells to exert negative feedback during an immune response (12). Long et al found that low-dose antigen stimulation can promote Foxp3 expression in human CD4+T-cells (41). Chronic antigen or superantigen stimulation, perhaps from staphylococci or viruses, has been suggested to initiate T-cell proliferation and accumulation in CTCL lesions (42-44). As an example, human T-lymphotropic virus type 1 (HTLV-1), first isolated from an MF patient, is associated with the development of MF lesions (45). In support of this concept, PBMCs from HTLV-1+ patients have been found to have high levels of Foxp3 expression. CD4+CD25- T-cells from normal healthy donors were shown to acquire both CD25 and Foxp3 expression after being infected with HTLV-1(46). Krejsgaard et al even proposed a scheme describing the plasticity model where the phenotype of malignant T-cells in CTCL is modulated by the local microenvironments in skin lesions and blood(47).
Decreased numbers of CD8+ T-cells are thought to contribute to the impaired cell immunity seen in L-CTCL (22, 48, 49). As expected, we observed a low number of CD3+CD8+ T-cells in the blood of L-CTCL patients. Depressed activity and function of CD3+CD8+ T-cells were indicated by low CD69 expression and IFN-γ secretion. Activated CD8+CD69+ T-cells and IFN-γ secreting CD8+T-cells were increased at 1 or 3 months after ECP treatment in L-CTCL patients. The increase in CD8+T-cells was more profound in L-CTCL patients who responded to ECP than in unresponsive patients. The enhanced activity and function of CD8+ T-cells after ECP could be partially attributed to the removal of suppression from malignant T-cells and/or by dendritic cells stimulation as we have previously reported (35). We conclude that ECP alone or in combination therapy exerts multiple effects on immune cells in L-CTCL patients: 1) reduce immunosuppressive malignant T-cells; 2) augment immunostimulatory dendritic cells; and 3) enhance the activity and function of CD3+CD8+ T-cells.
The limitation of this study is that two thirds of patients were given ECP therapy combined with biological response modifiers, thus it is not known whether effects on T-cell subsets in these patients are from ECP or from other biological modifiers. The small sample size is another weakness of this study. We only examined 9 patients for CD4+Foxp3+ T-cells in our cohort, so our results have low statistical power and may be due to the chance, and must be confirmed in a larger study.
Nevertheless, our results from this study allow us to propose a new working hypothesis that ECP therapy alone or in combination therapy might be more effective in in L-CTCL patients whose malignant T-cells have a CD4+Foxp3+CD25- phenotype. Analysis of CD4+Foxp3+ T-cell subsets might be useful to better tailor ECP therapy for CTCL patients.
Supplementary Material
Summary statement.
Extracorporeal photopheresis (ECP) is effective for treatment of leukemic cutaneous T-cell lymphoma (L-CTCL), but its mechanism(s) of action remain unclear. Regulatory T-cells (Treg) express Foxp3 and CD25. Although malignant T-cells of CTCL gain the Treg cell phenotype in vitro; it is unclear whether this also occurs in vivo. Our results indicate that ECP therapy alone or in combination therapy might be more effective in patients with high baseline percentages of CD4+Foxp3+ T-cells, and this T-cell subset declines in parallel with malignant T cells during treatment. Analysis of CD4+Foxp3+ T-cell subsets might be useful to better tailor ECP therapy for CTCL patients.
Acknowledgments
Funding / Grants: This study was supported by following research funds: investigator-initiated research grant from Therakos, Inc. to XN and MD (LS2006-00018652AB); Mr. and Mrs. R. John Stanton, Jr. CTCL Research Fund to MD; CCTS T32 Pre-doctoral Training Program (TL1RR024147 and UL1RR024148) to LHS; and the BCM/UT CFAR AI36211to DEL. FACS was funded by National Cancer Institute Core grant, CA-16672.
We thank the nurse team from the apheresis unit, the Department of Stem Cell Transplantation, for sample collection. Thanks for clinical fellows and residents of UT-Houston Dermatology Residency Program for assisting with clinic visits. Many thanks to all patients participated in this study. Authors want to thank Dennis Parenti MD and Jillian Davis PhD for their valuable discussion during the manuscript preparation.
References
- 1.Willemze R, Jaffe ES, Burg G, Cerroni L, Berti E, Swerdlow SH, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768–3785. doi: 10.1182/blood-2004-09-3502. [DOI] [PubMed] [Google Scholar]
- 2.Scarisbrick JJ, Whittaker S, Evans AV, Fraser-Andrews EA, Child FJ, Dean A, et al. Prognostic significance of tumor burden in the blood of patients with erythrodermic primary cutaneous T-cell lymphoma. Blood. 2001;97:624–630. doi: 10.1182/blood.v97.3.624. [DOI] [PubMed] [Google Scholar]
- 3.Vidulich KA, Talpur R, Bassett RL, Duvic M. Overall survival in erythrodermic cutaneous T-cell lymphoma: an analysis of prognostic factors in a cohort of patients with erythrodermic cutaneous T-cell lymphoma. Int J Dermatol. 2009;48:243–252. doi: 10.1111/j.1365-4632.2009.03771.x. [DOI] [PubMed] [Google Scholar]
- 4.Talpur R, D S, Seyfer S, Liu P, Duvic M. 1st World Congress of Cutaneous Lymphoma. Chicago, IL: 2010. Long-term outcomes of 1263 patients with mycosis fungoides and Sezary syndrome from 1982-2009. [Google Scholar]
- 5.Duvic M, Donato M, Dabaja B, Richmond H, Singh L, Wei W, et al. Total skin electron beam and non-myeloablative allogeneic hematopoietic stem-cell transplantation in advanced mycosis fungoides and Sezary syndrome. J Clin Oncol. 2010;28:2365–2372. doi: 10.1200/JCO.2009.25.8301. [DOI] [PubMed] [Google Scholar]
- 6.Polansky M, Talpur R, Daulat S, Hosing C, Dabaja B, Duvic M. Long-Term Complete Responses to Combination Therapies and Allogeneic Stem Cell Transplants in Patients With Sezary Syndrome. Clin Lymphoma Myeloma Leuk. 2014;5:00423–00426. doi: 10.1016/j.clml.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 7.Zic JA. The treatment of cutaneous T-cell lymphoma with photopheresis. Dermatol Ther. 2003;16:337–346. doi: 10.1111/j.1396-0296.2003.01646.x. [DOI] [PubMed] [Google Scholar]
- 8.Crovetti G, Carabelli A, Berti E, Guizzardi M, Fossati S, De Filippo C, et al. Photopheresis in cutaneous T-cell lymphoma: five-year experience. Int J Artif Organs. 2000;23:55–62. [PubMed] [Google Scholar]
- 9.Knobler R, Duvic M, Querfeld C, Straus D, Horwitz S, Zain J, et al. Long-term follow-up and survival of cutaneous T-cell lymphoma patients treated with extracorporeal photopheresis. Photodermatol Photoimmunol Photomed. 2012;28:250–257. doi: 10.1111/j.1600-0781.2012.00689.x. [DOI] [PubMed] [Google Scholar]
- 10.Gottlieb SL, Wolfe JT, Fox FE, DeNardo BJ, Macey WH, Bromley PG, et al. Treatment of cutaneous T-cell lymphoma with extracorporeal photopheresis monotherapy and in combination with recombinant interferon alfa: a 10-year experience at a single institution. J Am Acad Dermatol. 1996;35:946–957. doi: 10.1016/s0190-9622(96)90119-x. [DOI] [PubMed] [Google Scholar]
- 11.Edelson R, Berger C, Gasparro F, Jegasothy B, Heald P, Wintroub B, et al. Treatment of cutaneous T-cell lymphoma by extracorporeal photochemotherapy. Preliminary results. N Engl J Med. 1987;316:297–303. doi: 10.1056/NEJM198702053160603. [DOI] [PubMed] [Google Scholar]
- 12.Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol. 2010;10:490–500. doi: 10.1038/nri2785. [DOI] [PubMed] [Google Scholar]
- 13.Talpur R, Jones DM, Alencar AJ, Apisarnthanarax N, Herne KL, Yang Y, et al. CD25 expression is correlated with histological grade and response to denileukin diftitox in cutaneous T-cell lymphoma. J Invest Dermatol. 2006;126:575–583. doi: 10.1038/sj.jid.5700122. [DOI] [PubMed] [Google Scholar]
- 14.Ni X, Zhang C, Talpur R, Duvic M. Resistance to Activation-Induced Cell Death and Bystander Cytotoxicity Via the Fas//Fas Ligand Pathway Are Implicated in the Pathogenesis of Cutaneous T Cell Lymphomas. J Investig Dermatol. 2005;124:741–750. doi: 10.1111/j.0022-202X.2005.23657.x. [DOI] [PubMed] [Google Scholar]
- 15.Berger CL, Tigelaar R, Cohen J, Mariwalla K, Trinh J, Wang N, et al. Cutaneous T-cell lymphoma: malignant proliferation of T-regulatory cells. Blood. 2005;105:1640–1647. doi: 10.1182/blood-2004-06-2181. [DOI] [PubMed] [Google Scholar]
- 16.Heid JB, Schmidt A, Oberle N, Goerdt S, Krammer PH, Suri-Payer E, et al. FOXP3(+)CD25(-) Tumor Cells with Regulatory Function in Sezary Syndrome. J Invest Dermatol. 2009;129:2875–2885. doi: 10.1038/jid.2009.175. [DOI] [PubMed] [Google Scholar]
- 17.Gjerdrum LM, Woetmann A, Odum N, Burton CM, Rossen K, Skovgaard GL, et al. FOXP3+ regulatory T cells in cutaneous T-cell lymphomas: association with stage and survival. Leukemia. 2007;21:2512–2518. doi: 10.1038/sj.leu.2404913. [DOI] [PubMed] [Google Scholar]
- 18.Klemke CD, Fritzsching B, Franz B, Kleinmann EV, Oberle N, Poenitz N, et al. Paucity of FOXP3+ cells in skin and peripheral blood distinguishes Sezary syndrome from other cutaneous T-cell lymphomas. Leukemia. 2006;20:1123–1129. doi: 10.1038/sj.leu.2404182. [DOI] [PubMed] [Google Scholar]
- 19.Tiemessen MM, Mitchell TJ, Hendry L, Whittaker SJ, Taams LS, John S. Lack of suppressive CD4+CD25+FOXP3+ T cells in advanced stages of primary cutaneous T-cell lymphoma. J Invest Dermatol. 2006;126:2217–2223. doi: 10.1038/sj.jid.5700371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krejsgaard T, Gjerdrum LM, Ralfkiaer E, Lauenborg B, Eriksen KW, Mathiesen AM, et al. Malignant Tregs express low molecular splice forms of FOXP3 in Sezary syndrome. Leukemia. 2008;22:2230–2239. doi: 10.1038/leu.2008.224. [DOI] [PubMed] [Google Scholar]
- 21.Edelson RL. Cutaneous T cell lymphoma: the helping hand of dendritic cells. Ann N Y Acad Sci. 2001;941:1–11. [PubMed] [Google Scholar]
- 22.Heald P, Rook A, Perez M, Wintroub B, Knobler R, Jegasothy B, et al. Treatment of erythrodermic cutaneous T-cell lymphoma with extracorporeal photochemotherapy. J Am Acad Dermatol. 1992;27:427–433. doi: 10.1016/0190-9622(92)70212-x. [DOI] [PubMed] [Google Scholar]
- 23.Di Renzo M, Rubegni P, De Aloe G, Paulesu L, Pasqui AL, Andreassi L, et al. Extracorporeal photochemotherapy restores Th1/Th2 imbalance in patients with early stage cutaneous T-cell lymphoma. Immunology. 1997;92:99–103. doi: 10.1046/j.1365-2567.1997.00325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ni X, Duvic M. Dendritic cells and cutaneous T-cell lymphomas. G Ital Dermatol Venereol. 2011;146:103–113. [PubMed] [Google Scholar]
- 25.Olsen E, Vonderheid E, Pimpinelli N, Willemze R, Kim Y, Knobler R, et al. Revisions to the staging and classification of mycosis fungoides and Sezary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European Organization of Research and Treatment of Cancer (EORTC) Blood. 2007;110:1713–1722. doi: 10.1182/blood-2007-03-055749. [DOI] [PubMed] [Google Scholar]
- 26.Jones D, Dang NH, Duvic M, Washington LT, Huh YO. Absence of CD26 expression is a useful marker for diagnosis of T-cell lymphoma in peripheral blood. Am J Clin Pathol. 2001;115:885–892. doi: 10.1309/U1Y6-J4AG-5M4M-7AYV. [DOI] [PubMed] [Google Scholar]
- 27.Pierson DM, Jones D, Muzzafar T, Kersh MJ, Challagundla P, Medeiros LJ, et al. Utility of CD26 in flow cytometric immunophenotyping of T-cell lymphomas in tissue and body fluid specimens. Cytometry B Clin Cytom. 2008;74:341–348. doi: 10.1002/cyto.b.20431. [DOI] [PubMed] [Google Scholar]
- 28.Duvic M, Talpur R, Ni X, Zhang C, Hazarika P, Kelly C, et al. Phase 2 trial of oral vorinostat (suberoylanilide hydroxamic acid, SAHA) for refractory cutaneous T-cell lymphoma (CTCL) Blood. 2007;109:31–39. doi: 10.1182/blood-2006-06-025999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duvic M, Hymes K, Heald P, Breneman D, Martin AG, Myskowski P, et al. Bexarotene is effective and safe for treatment of refractory advanced-stage cutaneous T-cell lymphoma: multinational phase II-III trial results. J Clin Oncol. 2001;19:2456–2471. doi: 10.1200/JCO.2001.19.9.2456. [DOI] [PubMed] [Google Scholar]
- 30.Olsen EA, Kim YH, Kuzel TM, Pacheco TR, Foss FM, Parker S, et al. Phase IIb multicenter trial of vorinostat in patients with persistent, progressive, or treatment refractory cutaneous T-cell lymphoma. J Clin Oncol. 2007;25:3109–3115. doi: 10.1200/JCO.2006.10.2434. [DOI] [PubMed] [Google Scholar]
- 31.Montes M, Jaensson EA, Orozco AF, Lewis DE, Corry DB. A general method for bead-enhanced quantitation by flow cytometry. J Immunol Methods. 2006;317:45–55. doi: 10.1016/j.jim.2006.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ni X, Richmond HM, Liao XM, Decker WK, Shiue LH, Shpall EJ, et al. Induction of T-cell responses against cutaneous T-cell lymphomas ex vivo by autologous dendritic cells transfected with amplified tumor mRNA. J Invest Dermatol. 2008;128:2631–2639. doi: 10.1038/jid.2008.125. [DOI] [PubMed] [Google Scholar]
- 33.Shiue LH, Ni X, Prieto VG, Jorgensen JL, Curry JL, Goswami M, et al. A case of invisible leukemic cutaneous T cell lymphoma with a regulatory T cell clone. Int J Dermatol. 2012:1365–4632. doi: 10.1111/j.1365-4632.2011.05351.x. [DOI] [PubMed] [Google Scholar]
- 34.Ni X, Jorgensen JL, Goswami M, Challagundla P, Decker WK, Kim YH, et al. Reduction of Regulatory T cells by Mogamulizumab, a Defucosylated Anti-CC Chemokine Receptor 4 Antibody, in Patients with Aggressive/Refractory Mycosis Fungoides and Sezary Syndrome. Clin Cancer Res. 2014;5 doi: 10.1158/1078-0432.CCR-14-0830. [DOI] [PubMed] [Google Scholar]
- 35.Shiue LH, Alousi AM, Wei C, Hosing CM, Duvic M, Ni X. Augmentation of blood dendritic cells by extracorporeal photopheresis in patients with leukemic cutaneous T-cell lymphoma and graft-versus-host disease. J Invest Dermatol. 2013;133:2098–2100. doi: 10.1038/jid.2013.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sakaguchi S, Vignali DA, Rudensky AY, Niec RE, Waldmann H. The plasticity and stability of regulatory T cells. Nat Rev Immunol. 2013;13:461–467. doi: 10.1038/nri3464. [DOI] [PubMed] [Google Scholar]
- 37.Li L, Boussiotis VA. Molecular and functional heterogeneity of T regulatory cells. Clin Immunol. 2011;141:244–252. doi: 10.1016/j.clim.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Singh M, Basu S, Camell C, Couturier J, Nudelman RJ, Medina MA, et al. Selective expansion of memory CD4(+) T cells by mitogenic human CD28 generates inflammatory cytokines and regulatory T cells. Eur J Immunol. 2008;38:1522–1532. doi: 10.1002/eji.200737929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nie H, Zheng Y, Li R, Guo TB, He D, Fang L, et al. Phosphorylation of FOXP3 controls regulatory T cell function and is inhibited by TNF-alpha in rheumatoid arthritis. Nat Med. 2013;19:322–328. doi: 10.1038/nm.3085. [DOI] [PubMed] [Google Scholar]
- 40.Duarte JH, Zelenay S, Bergman ML, Martins AC, Demengeot J. Natural Treg cells spontaneously differentiate into pathogenic helper cells in lymphopenic conditions. Eur J Immunol. 2009;39:948–955. doi: 10.1002/eji.200839196. [DOI] [PubMed] [Google Scholar]
- 41.Long SA, Rieck M, Tatum M, Bollyky PL, Wu RP, Muller I, et al. Low-dose antigen promotes induction of FOXP3 in human CD4+ T cells. J Immunol. 2011;187:3511–3520. doi: 10.4049/jimmunol.1003880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tokura Y, Heald PW, Yan SL, Edelson RL. Stimulation of cutaneous T-cell lymphoma cells with superantigenic staphylococcal toxins. J Invest Dermatol. 1992;98:33–37. doi: 10.1111/1523-1747.ep12494184. [DOI] [PubMed] [Google Scholar]
- 43.Lee PY, Charley M, Tharp M, Jegasothy BV, Deng JS. Possible role of Epstein-Barr virus infection in cutaneous T-cell lymphomas. J Invest Dermatol. 1990;95:309–312. doi: 10.1111/1523-1747.ep12485017. [DOI] [PubMed] [Google Scholar]
- 44.Herne KL, Talpur R, Breuer-McHam J, Champlin R, Duvic M. Cytomegalovirus seropositivity is significantly associated with mycosis fungoides and Sezary syndrome. Blood. 2003;101:2132–2136. doi: 10.1182/blood-2002-07-2247. [DOI] [PubMed] [Google Scholar]
- 45.Poiesz BJ, Ruscetti FW, Gazdar AF, Bunn PA, Minna JD, Gallo RC. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci U S A. 1980;77:7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Walsh PT, Benoit BM, Wysocka M, Dalton NM, Turka LA, Rook AH. A role for regulatory T cells in cutaneous T-Cell lymphoma; induction of a CD4 + CD25 + Foxp3+ T-cell phenotype associated with HTLV-1 infection. J Invest Dermatol. 2006 Mar;126(3):690–2. doi: 10.1038/sj.jid.5700121. [DOI] [PubMed] [Google Scholar]
- 47.Krejsgaard T, Odum N, Geisler C, Wasik MA, Woetmann A. Regulatory T cells and immunodeficiency in mycosis fungoides and Sézary syndrome. Leukemia. 2012;26:424–432. doi: 10.1038/leu.2011.237. [DOI] [PubMed] [Google Scholar]
- 48.Rook AH, Vowels BR, Jaworsky C, Singh A, Lessin SR. The immunopathogenesis of cutaneous T-cell lymphoma. Abnormal cytokine production by Sezary T cells. Arch Dermatol. 1993;129:486–489. [PubMed] [Google Scholar]
- 49.Hoppe RT, Medeiros LJ, Warnke RA, Wood GS. CD8-positive tumor-infiltrating lymphocytes influence the long-term survival of patients with mycosis fungoides. J Am Acad Dermatol. 1995;32:448–453. doi: 10.1016/0190-9622(95)90067-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


