Abstract
Rationale
Evidence suggests that the noradrenergic system mediates ethanol-reinforcement. However, preclinical studies suggest that noradrenergic antagonists block other oral reinforcers indicating possible unwanted secondary medication effects.
Methods
This study examined combinations of low-dose prazosin with propranolol or naltrexone using a behavioral paradigm that separately assesses reinforcer-seeking and self-administration. Male alcohol-preferring (P) rats (n=20/experiment) were trained to complete a response requirement (RR) resulting in access to 1% sucrose (n=10) or 10% ethanol (n=10) for 20min. Rats received vehicle, prazosin alone (0.125, 0.25, 0.5, 1.0 mg/kg; intraperitoneally (IP)) or prazosin in combination with propranolol (5 mg/kg (IP); Exp1) or in combination with naltrexone (0.03 mg/kg (subcutaneously (SC); Exp2).
Results
For Exp1, prazosin alone effectively decreased sucrose-seeking more than ethanol-seeking, but decreased ethanol self-administration only. Propranolol alone effectively decreased ethanol-seeking more than sucrose-seeking and decreased ethanol intake only. At some dose combinations, there was a greater attenuation of ethanol and sucrose intake relative to either drug alone. For Exp2, prazosin alone and naltrexone alone were effective in decreasing ethanol-seeking and intake only. Combination treatment was more effective than either drug alone at decreasing ethanol-seeking and consumption and sucrose intake, but not sucrose-seeking.
Conclusions
Propranolol and naltrexone alone were specific to ethanol indicating that low doses of either medication may be beneficial in treating alcohol use disorders. Prazosin in combination with propranolol or naltrexone was more effective than either drug alone, but also reduced sucrose-reinforced behaviors. These data suggest that the noradrenergic system is a viable target for developing treatment approaches for problem drinkers.
Keywords: Noradrenergic, Ethanol, Combination Pharmacotherapy, Prazosin, P Rat, Propranolol, Naltrexone, Alcohol, Self-Administration
Introduction
Recent evidence suggests that the noradrenergic system plays a key role in mediating ethanol-motivated behaviors in both alcohol-dependent and non-dependent humans and rats (Froehlich et al. 2013a,b; Fox et al. 2012; Gilpin and Koob 2010; Rasmussen et al. 2009; Simpson et al. 2009; Verplaetse et al. 2011; Walker et al. 2008). Prazosin, an α1-adrenergic antagonist, has been found to reduce ethanol drinking in home cage, limited access paradigms in P rats (Rasmussen et al. 2009) and block operant responding for ethanol in dependent Wistar rats, with higher doses necessary to be effective in non-dependent animals in a fixed ratio (FR) paradigm (Walker et al. 2008). Prazosin blocks stress-induced reinstatement of ethanol-seeking in Wistar rats (Le et al. 2011), and reduces ethanol drinking throughout prolonged treatment in alcohol-experienced P rats and impedes acquisition of ethanol drinking in naïve P rats (Froehlich et al. 2013b). In the same paradigm used in the present investigation, prazosin attenuated ethanol- and sucrose-seeking and consumption in P rats (Verplaetse et al. 2012). Clinically, prazosin has been shown to decrease relapse, number of drinking days per week, and number of drinks per week in alcoholics (Simpson et al. 2009). Similarly, prazosin also reduces alcohol craving, anxiety, and negative emotions following stress exposure in treatment-seeking, alcohol-dependent individuals (Fox et al. 2012).
Propranolol, a non-selective β-adrenergic antagonist, decreases operant responding for ethanol in dependent Wistar rats, with higher doses necessary to reduce responding in non-dependent Wistar rats in the same FR paradigm (Gilpin and Koob 2010). Higher doses of propranolol also decreases ethanol self-administration during a 2-hour alcohol drinking period in P rats, although the effects of propranolol on ethanol drinking were variable (Rasmussen et al., 2014). Inconsistently, propranolol was not efficacious in reducing ethanol consumption in a two-bottle choice paradigm (ethanol vs water) in Long-Evans rats (Begleiter 1974). Clinically, however, propranolol reduces craving and somatic symptoms associated with alcohol withdrawal (Carlsson and Johansson 1971; Sellers et al. 1977; Tyrer 1972).
One FDA approved medication for the treatment of alcohol use disorders (AUDs) and that has been shown to successfully reduce ethanol intake and responding in multiple behavioral paradigms is naltrexone, a non-selective opioid antagonist (Ciccocioppo et al. 2007; Czachowski and DeLory 2009; Gilpin et al. 2008; Goodwin et al. 2001; Henderson-Redmond and Czachowski 2014; June et al. 1998; Kim et al. 2004; Koistinen et al. 2001; Kuzmin et al. 2007, 2008; Le et al. 1993, 1999; Middaugh et al. 1999; Stromberg et al. 1998a,b, 2004; Walker and Koob 2008; Williams and Broadbridge 2009). Naltrexone has been tested in both alcohol-preferring lines and nonselected lines, including P, Long Evans, (Henderson-Redmond and Czachowski 2014) and Wistar rats (Walker and Koob 2008), and in the same paradigm used in the present study (Henderson-Redmond and Czachowski 2014). Naltrexone also reduces number of drinking days, number of drinks per occasion, and decreases craving and relapse in the clinic (O’Malley et al. 1992; Volpicelli et al. 1992). However, subsequent studies indicate that naltrexone is only modestly effective in reducing alcohol consumption (Kranzler and VanKirk 2001) and does not increase abstinent rates amongst alcoholics (Garbutt et al. 1999).
The emerging literature revealing the efficacy of prazosin and propranolol in reducing the reinforcing effects of ethanol in rodent models suggests a new potential target for the treatment of AUDs. However, the dose range used in experiments to date produces non-specific reductions in sucrose and food consumption in Wistar and P rats (Le et al. 2011; Verplaetse et al. 2012), and naltrexone decreases saccharin and sucrose intake in Wistar, Long Evans, and P rats (Goodwin et al. 2001; Henderson-Redmond and Czachowski 2014), suggesting possible adverse secondary effects of these medications. Subthreshold dosing combinations may be an important next step in providing new treatment strategies for AUDs. This approach is used on the basis that a combination of efficacious medications can target multiple neurotransmitter systems or different receptor subtypes of the same system that may be dysregulated in alcoholism, and thus target different aspects of alcohol-motivated behaviors. Similarly, drugs with different mechanisms of action can be used in combination and in a lower dose range to potentially minimize side effects associated with higher doses of either drug alone (Anton et al. 2006; Mattson and Litten 2005). In fact, recent evidence suggests that prazosin in combination with propranolol suppresses ethanol drinking in P rats during ethanol withdrawal and after prolonged abstinence (Rasmussen et al. 2014), and prazosin in combination with naltrexone decreases ethanol intake in a free-access two-hour, two-bottle choice procedure in P rats (Froehlich et al. 2013a).
Preclinical models of ethanol self-administration have historically focused on an exclusively consummatory response (e.g., home cage drinking) or a combined seeking/drinking response (e.g., lever-press required for access to each 0.1ml of the reinforcer solution). The goal of the present experiments was to extend the current literature and evaluate the effects of subthreshold combinations of efficacious noradrenergic agents and naltrexone on separate measures of ethanol-seeking and drinking at binge-like levels. The paradigm used procedurally separates the motivational or seeking component (i.e., lever-presses) from consummatory behavior (i.e. drinking), and by including a sucrose control reinforcer, determines the specificity of drug treatment for ethanol reinforcement. Finally, this study sought to determine whether combination pharmacotherapy was more effective than either drug alone in decreasing ethanol-seeking or consumption in a rodent line genetically bred for high alcohol drinking.
Methods
Subjects
Subjects were 40 male, alcohol-naïve P rats (n=20/study) from the 76th (Experiment 1) and 77th (Experiment 2) generation of selective breeding (obtained from the Indiana University School of Medicine, Indianapolis, IN). Animals in each of the two studies were randomly assigned to either an ethanol-reinforced group or a sucrose-reinforced group (n=10/group). Ad libitum access to food and water was maintained throughout the experiments except where noted below. Animals were individually housed on a 12-hour light/dark cycle (5:00 AM – 5:00 PM), and all animal care procedures were conducted in accordance with the NIH Guidelines for the Care and Use of Laboratory Animals (2011) and all protocols were approved by the Institutional Animal Care and Use Committee (IACUC).
Apparatus
Daily sessions were conducted in modular chambers (30 × 30 × 24.5 cm; Med-Associates, St. Albans, VT) equipped with a houselight, a retractable lever, and a retractable graduated cylinder tube with rubber stopper and a stainless steel spout with double ball bearings to prevent leakage. The lever was located on the wall opposite to the sipper tube. All chambers were housed in sound-attenuated enclosures equipped with exhaust fans that masked external noise. Electrical inputs and outputs of each chamber were controlled using Med-Associates software.
Drugs
Ethanol solutions were prepared volume/volume in water using 95% ethanol. For sucrose/ethanol solutions and sucrose solutions, the sucrose solution was prepared weight/volume and used as a solute. Prazosin hydrochloride (Santa Cruz Biotechnology, Inc., Santa Cruz, CA; Tocris Bioscience, Ellisville, MO) was prepared by dissolving drug in sterile water [prazosin has limited solubility in saline (Rasmussen et al. 2009)] at 1.0 ml/kg BW. Prazosin (0.0, 0.125, 0.25, 0.5, and 1.0 mg/kg) was injected intraperitoneally (IP) 30 minutes prior to the start of each test session; saline was used as the vehicle control. Propranolol hydrochloride (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) and naltrexone hydrochloride (Sigma-Aldrich, Inc., Milwaukee, WI) were prepared by dissolving drug in saline at 1.0 ml/kg BW. Propranolol (0.0 and 5 mg/kg) was injected IP 30 minutes prior to the start of each test session. Naltrexone (0.0 and 0.03 mg/kg) was injected subcutaneously (SC) 30 minutes prior to the start of each test session.
Training and Ethanol Initiation
For a detailed description of training procedures, see Verplaetse et al., (2012). Briefly, subjects were trained to press the lever on a FR 1 schedule for access to 10% sucrose. During the initial 3 weeks of training, subjects underwent a modified sucrose-fading procedure (Samson, 1986) to initiate ethanol consumption (final solutions: sucrose group, 1% sucrose; ethanol group, 10% ethanol). The final sucrose concentration was chosen as a moderately palatable reinforcer with a similar baseline lever-press responding as compared to the ethanol group, since Verplaetse et al. (2012) found responding for 2% sucrose to be slightly higher than for 10% ethanol in P rats. When subjects in each group were responding appropriately for their reinforcer, the procedural separation between seeking (lever-pressing) and consumption was then instituted. The FR schedule was discontinued, and following the completion of a single RR, access to the sipper tube was provided for 20 uninterrupted minutes. Over 4 weeks, the RR was increased from 4 to 20, and then the RR of 20 was maintained for an additional 2 weeks.
Treatment Schedule and Test Sessions
The behavioral paradigm used in these experiments has both a drinking and seeking component during each daily session, and in addition, separate test sessions focused on one behavior or the other. The consummatory testing phase of each experiment assessed the effects of drug treatments on ethanol and sucrose intake with a minimal seeking requirement (i.e. RR 1). On consummatory testing days the RR was dropped to a 1 so that after 1 lever press the sipper tube extended into the chamber for 20 uninterrupted minutes. A RR was included in consummatory sessions to control for cues and expectancy effects (i.e., all training and test sessions are identical with the exception of the number of responses required) and to enable the assessment of “motivated” (versus “free”) intake, but was reduced to 1 so that subjects were unlikely to fail to obtain access to the reinforcer on a drug testing day. Consummatory testing days were on Tuesdays of each week. On Tuesdays, animals received an IP or SC injection of prazosin alone, prazosin and propranolol (Exp1), or prazosin and naltrexone (Exp2) 30 minutes prior to the start of the operant conditioning session. The appetitive testing phase used the same drug treatments as the consummatory phase and was on Fridays of each week. On Fridays, each injection preceded a single, non-reinforced extinction session in order to remove any possible ceiling effect imposed by the RR. Extinction sessions consisted of 20 minutes of access to the lever, but no reinforcer was provided. Sipper tubes were still placed in the retracted holders to control for possible scent cues. The other 3 days of the week were ‘normal’, non-injection reinforced sessions with a RR of 20.
Blood Ethanol Concentration (BEC) Determination
All subjects reinforced with ethanol underwent one operant conditioning session immediately after all testing was concluded, and immediately following the final 20 minutes of alcohol access during this session, blood samples were collected (100 μl) into heparinized capillary tubes from a nick to the tip of the tail while rats were restrained for a maximum of 2 to 4 minutes. Samples were stored on ice during collection, and then immediately centrifuged and a 5 μl sample of plasma was analyzed using the AM1 Analyzer (Analox Instruments, Lunenburg, MA). Ethanol concentration was determined with an amperometric oxygen electrode that measures oxygen consumption during the enzymatic oxidation of alcohol to acetaldehyde.
Data Analyses and Statistics
Two-way RM ANOVA (with reinforcer and treatment as the main within subjects factors) was used to analyze responding (lever-presses). Intakes were calculated based upon daily body weights and fluid volume consumed, and separate one-way RM ANOVAs (with treatment as the within subjects factor) were performed for each reinforcer. A two-way RM ANOVA was not used on reinforcer intakes because baseline (i.e. vehicle treatment) intake volumes between ethanol and sucrose were significantly different (e.g., Exp 1: ethanol: 1.23 g/kg, sucrose: 0.5 g/kg; p ≤ 0.001, see below). Two-way RM ANOVAs were performed on latency to first lick (consummatory testing phase) and latency to lever press (appetitive testing phase). Latency data were normalized using logarithmic transformation prior to analyses. Post-hoc analyses were conducted when appropriate using either Dunnett’s test or Newman-Keuls. All analyses were conducted using the SigmaStat 3.5 program (Systat Software, Inc., Chicago, IL) with significance accepted at p ≤ 0.05. Data are presented as mean ± SEM.
Results
Experiment 1: Prazosin alone and in combination with propranolol
Consummatory Testing Phase
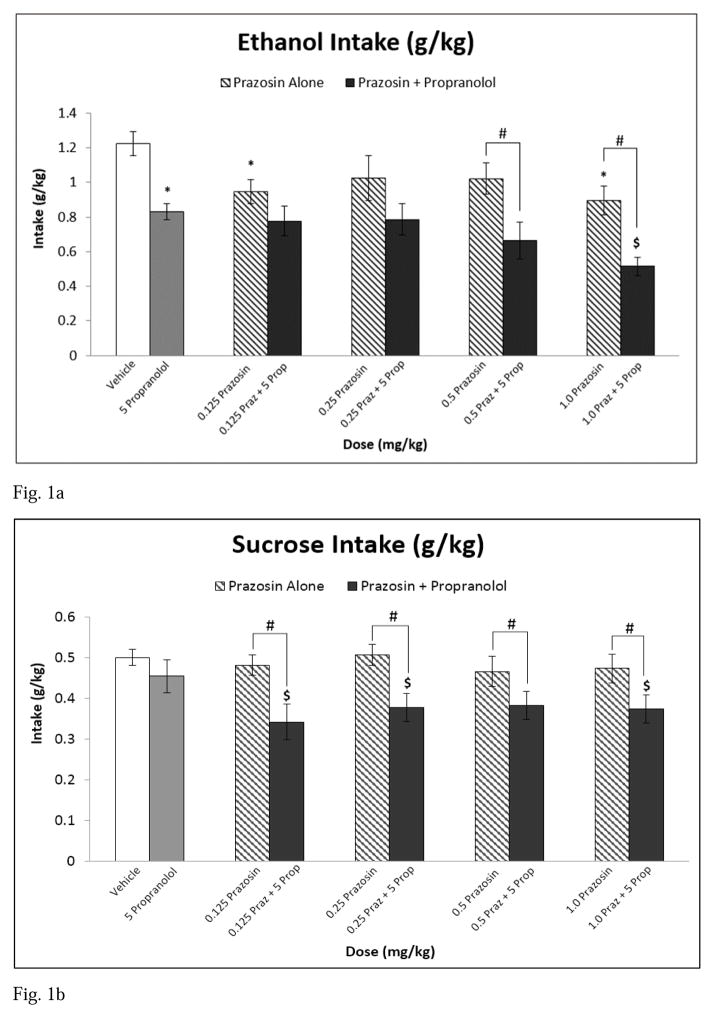
At baseline (vehicle injection), rats consumed on average 1.23 g/kg ethanol and 0.5 g/kg sucrose. Analysis of ethanol intake (g/kg) following treatment showed that there was a main effect of treatment [F(9,81) = 8.993, p ≤ 0.001] (see Fig 1a). Post hoc analyses indicated that 0.125 and 1.0 mg/kg prazosin alone and propranolol alone decreased ethanol consumption relative to vehicle. The highest treatment combination (1.0 mg/kg prazosin + propranolol) reduced ethanol intake compared to propranolol alone. Additionally, the combinations of 0.5 mg/kg prazosin + propranolol and 1.0 mg/kg prazosin + propranolol reduced ethanol consumption relative to each of their corresponding prazosin alone doses. Similarly, all combination treatments decreased ethanol drinking relative to vehicle.
Fig. 1.
Experiment 1: Effect of Prazosin alone, Propranolol alone, or Prazosin + Propranolol on (a) ethanol intake (g/kg), n=10 and (b) sucrose intake (g/kg), n=10. * = Significantly different from Vehicle; $ = Significantly different from Propranolol alone; # = Combination significantly different from corresponding Prazosin alone dose. Note: Significant differences between vehicle and all combination treatments (Fig 1a, b). White bar indicates Vehicle, Gray bar indicates Propranolol alone, striped bars indicate Prazosin alone doses, and black bars indicate Combination Treatments
Analysis of sucrose intake (g/kg) showed that there was a main effect of treatment [F(9, 81) = 9.856, p ≤ 0.001] (see Fig 1b), with post hoc analyses indicating that the combinations of 0.125 mg/kg prazosin + propranolol, 0.25 mg/kg prazosin + propranolol, and 1.0 mg/kg prazosin + propranolol reduced sucrose intake compared to propranolol alone. Similarly, all combination drug treatments attenuated sucrose consumption relative to each of their corresponding prazosin alone doses and all prazosin + propranolol combination treatments decreased sucrose intake relative to vehicle (see Fig 1b).
Appetitive Testing Phase
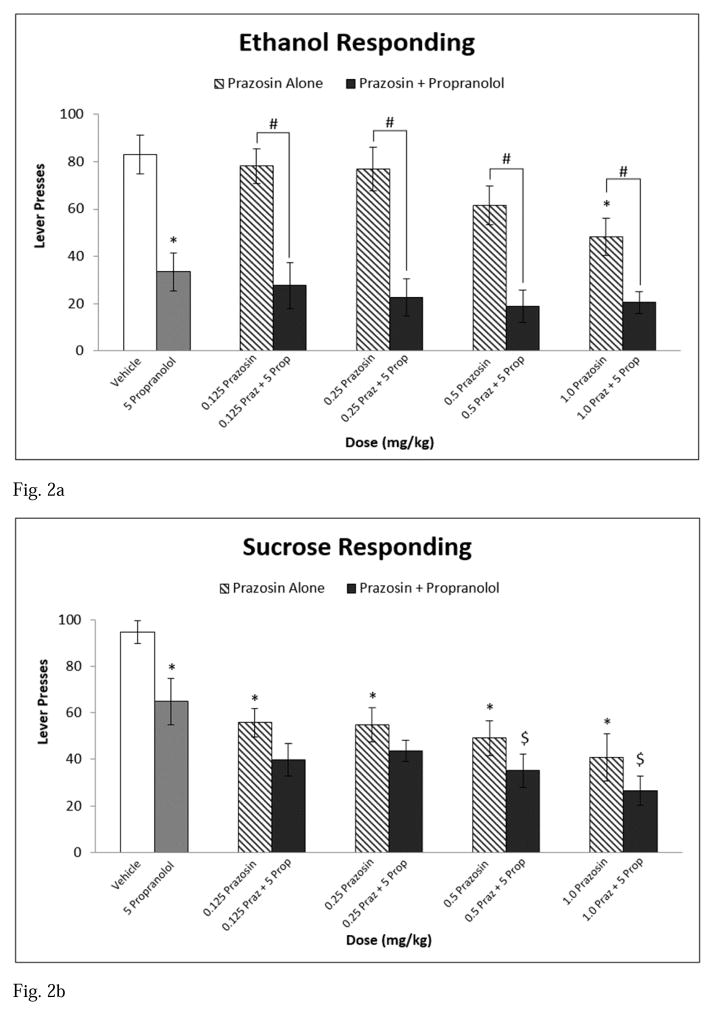
At baseline, rats responded on the lever an average of 83 responses for the ethanol-reinforced group and 94.6 responses for the sucrose-reinforced group. Analysis of appetitive responding revealed a main effect of treatment [F(9,162) = 18.588, p ≤ 0.001] and a significant interaction between reinforcer and treatment [F(9,162) = 3.709, p ≤ 0.001]. In the ethanol-reinforced group, 1.0 mg/kg prazosin alone, propranolol alone, and all combination drug treatments decreased lever pressing relative to vehicle. Further, each combination treatment significantly reduced responding on the lever for ethanol compared to each of their corresponding prazosin alone treatments (see Fig 2a). With regard to responding for the sucrose-reinforced group, 0.125, 0.25, 0.5, and 1.0 mg/kg prazosin alone and propranolol alone decreased lever pressing for sucrose compared to vehicle. Similarly, all combination drug treatments attenuated responding relative to vehicle. Further, the 0.5 mg/kg prazosin + propranolol and 1.0 mg/kg prazosin + propranolol combination treatments significantly lessened responding compared to propranolol alone (see Fig 2b). Subjects significantly dampened lever responding to a greater degree for ethanol relative to sucrose following administration of propranolol alone.
Fig. 2.
Experiment 1: Effect of Prazosin alone, Propranolol alone, or Prazosin + Propranolol on (a) ethanol seeking, n=10 and (b) sucrose seeking, n=10. * = Significantly different from Vehicle; $ = Significantly different from Propranolol alone; # = Combination significantly different from corresponding Prazosin alone dose. Note: Significant differences between vehicle and all combination treatments (Fig 2a, b). White bar indicates Vehicle, Gray bar indicates Propranolol alone, striped bars indicate Prazosin alone doses, and black bars indicate Combination Treatments
Latencies
A two-way RM ANOVA on latency to first lick revealed a main effect of reinforcer [F(1,162) = 7.729, p = 0.012], with ethanol latencies (0.327 ± 0.0436) longer than sucrose latencies (0.155 ± 0.0436), and a main effect of treatment [F(9,162) = 3.920, p < 0.001], with the 0.5 mg/kg prazosin + propranolol treatment prolonging latency to lick for ethanol relative to all other treatments.
In the appetitive testing phase, there was a significant main effect of reinforcer [F(1,162) = 10.158, p = 0.005] (ethanol 1.327 ± 0.0973; sucrose 0.889 ± 0.0973) and a reinforcer by treatment interaction [F(9,162) = 1.912, p = 0.05], such that latency to lever press at baseline was different between ethanol- and sucrose-reinforced groups (p = 0.049).
BEC Determination
Blood ethanol levels were collected following completion of all drug treatments. On the final day of operant conditioning sessions, the average ethanol intake was 1.27 g/kg which yielded BECs between 17.0 and 99.0 mg/dl with an average BEC of 57.2 mg/dl, as measured at the end of the 20 minute self-administration period.
Experiment 2: Prazosin alone and in combination with naltrexone
Consummatory Testing Phase
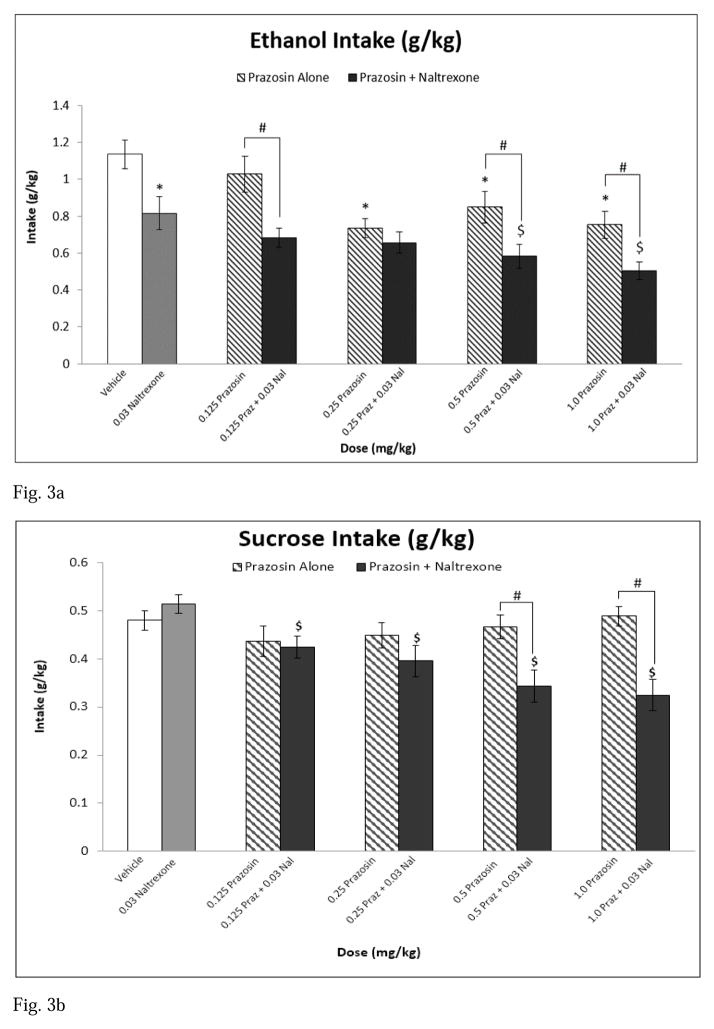
At baseline (vehicle injection), rats consumed on average 1.14 g/kg ethanol and 0.48 g/kg sucrose. Analysis of ethanol intake (g/kg) following treatment revealed a main effect of treatment [F(9,81) = 10.414, p < 0.001] (see Fig 3a). Post hoc analyses indicated that 0.25, 0.5, and 1.0 mg/kg prazosin alone and naltrexone alone decreased ethanol self-administration relative to vehicle. Post hoc analyses revealed that the 0.5 mg/kg prazosin + naltrexone and the 1.0 mg/kg prazosin + naltrexone combination treatments attenuated ethanol intake compared to naltrexone alone. Additionally, the combinations of 0.125 mg/kg prazosin + naltrexone, 0.5 mg/kg prazosin + naltrexone, and 1.0 mg/kg prazosin + naltrexone reduced ethanol self-administration relative to each of their corresponding prazosin alone doses. All combination treatments decreased drinking relative to vehicle.
Fig. 3.
Experiment 2: Effect of Prazosin alone, Naltrexone alone, or Prazosin + Naltrexone on (a) ethanol intake (g/kg), n=10 and (b) sucrose intake (g/kg), n=10. * = Significantly different from Vehicle; $ = Significantly different from Naltrexone alone; # = Combination significantly different from corresponding Prazosin alone dose. Note: Significant differences between Vehicle and all combination treatments (Fig 3a), and significant differences between Vehicle and 0.25 mg/kg Prazosin + 0.03 Naltrexone, 0.5 mg/kg Prazosin + 0.03 Naltrexone, and 1.0 mg/kg Prazosin + 0.03 mg/kg Naltrexone (Fig 3b). White bar indicates Vehicle, Gray bar indicates Propranolol alone, striped bars indicate Prazosin alone doses, and black bars indicate Combination Treatments
Analysis of sucrose intake revealed a main effect of treatment [F(9,81) = 9.883, p < 0.001] (see Fig 3b), with post hoc analyses indicating that all prazosin + naltrexone treatment combinations decreased sucrose consumption relative to naltrexone alone. Similarly, the drug treatment combinations of 0.5 mg/kg prazosin + naltrexone and 1.0 mg/kg prazosin + naltrexone attenuated sucrose intake relative to each of their corresponding prazosin alone doses. Finally, the treatment combinations of 0.25 mg/kg prazosin + naltrexone, 0.5 mg/kg prazosin + naltrexone, and 1.0 mg/kg prazosin + naltrexone reduced sucrose self-administration compared to vehicle.
Appetitive Testing Phase
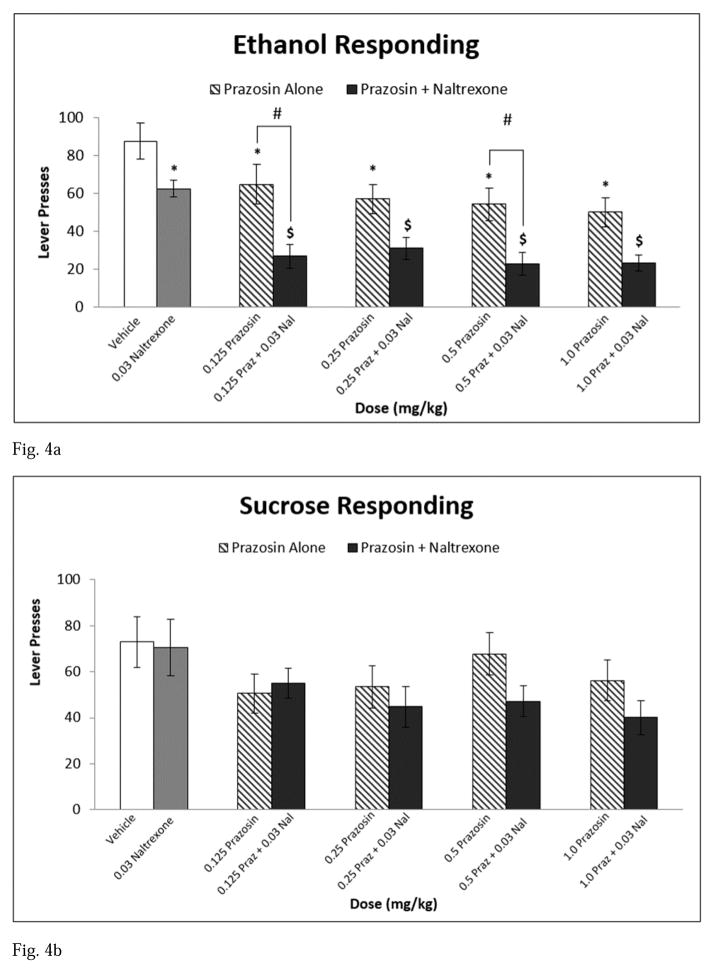
At baseline, rats responded on the lever an average of 87.8 responses for the ethanol-reinforced group and 72.9 responses for the sucrose-reinforced group. Analysis of appetitive responding revealed a main effect of treatment [F(9,162) = 8.493, p < 0.001] and a significant interaction between reinforcer and treatment [F(9,162) = 1.930, p = 0.05]. For ethanol-reinforced responding, all prazosin alone doses, naltrexone alone, and all combination drug treatments reduced responding compared to vehicle. All prazosin + naltrexone combination drug treatments also decreased lever pressing relative to naltrexone alone. Further, 0.125 mg/kg prazosin + naltrexone and 0.5 mg/kg prazosin + naltrexone attenuated appetitive responding for ethanol compared to each of their corresponding prazosin alone doses (see Fig 4a). With regard to the sucrose-reinforced group, there was no effect of prazosin alone, naltrexone alone, or any of the combination drug treatments on lever pressing during non-reinforced extinction sessions in the appetitive testing phase (see Fig 4b).
Fig. 4.
Experiment 2: Effect of Prazosin alone, Naltrexone alone, or Prazosin + Naltrexone on (a) ethanol seeking, n=10 and (b) sucrose seeking, n=10. * = Significantly different from Vehicle; $ = Significantly different from Naltrexone alone; # = Combination significantly different from corresponding Prazosin alone dose. Note: Significant differences between Vehicle and all combination treatments (Fig 4a). White bar indicates Vehicle, Gray bar indicates Propranolol alone, striped bars indicate Prazosin alone doses, and black bars indicate Combination Treatments
Latencies
A two-way RM ANOVA on latency to first lick revealed a main effect of reinforcer [F(1,162) = 7.476, p = 0.014], with ethanol latencies (0.346 ± 0.0351) longer than sucrose latencies (0.210 ± 0.0351), and a main effect of treatment [F(9,162) = 4.585, p < 0.001], such that the 0.5 mg/kg prazosin + naltrexone and the 1.0 mg/kg prazosin + naltrexone prolonged latency to first lick for ethanol compared to vehicle, naltrexone alone, and each of their corresponding prazosin alone doses.
In the appetitive testing phase, a two-way RM ANOVA on latency to first lever press revealed a main effect of reinforcer [F(1,162) = 9.760, p = 0.006], such that the ethanol group (1.305 ± 0.101) took longer to first lever press than the sucrose group (0.860 ± 0.101).
BEC Determination
Blood ethanol levels were collected following completion of all drug treatments. On the final day of operant conditioning sessions, average ethanol intake was 1.31 g/kg which yielded BECS between 13.6 and 82.8 mg/dl with an average BEC of 56.7 mg/dl, as measured at the end of the 20 minute self-administration period.
Discussion
Overall, the results from the present study support a role for the combination of agents within the noradrenergic system and with naltrexone, an antagonist of the opioid system, in modulating ethanol and sucrose self-administration and seeking in a paradigm that separately assesses appetitive (seeking/craving) versus consummatory (intake) behaviors and using animals selectively bred for ethanol preference. In both experiments, rats consumed ethanol at levels considered to be at or above ‘binge-like’ drinking (>1.0 g/kg in under 20 min), and well above the amount shown to be discriminated by rats after oral consumption (e.g. Hodge et al., 2001). BECs were comparable to our previous work where we showed that P rats in this model consume much of the ethanol “up front” and therefore peak BECs are likely higher than we measure at the end of the 20-minute session (Verplaetse et al. 2012). In Experiment 1, animals reduced ethanol intake and seeking following administration of prazosin alone and propranolol alone, and their combination was more effective than monotherapy. However, these effects were not selective for ethanol, in that combination pharmacotherapy attenuated sucrose consumption more effectively than either drug alone, and prazosin and propranolol alone were able to reduce sucrose seeking with no enhanced effects of their combination on responding. In Experiment 2, prazosin alone and naltrexone alone suppressed responding for ethanol and ethanol self-administration, and prazosin in combination with naltrexone reduced this effect more substantially than either drug alone. Naltrexone alone was selective for ethanol-motivated behaviors, however, prazosin in combination with naltrexone decreased sucrose consumption. Sucrose seeking was unaffected by any treatment.
The current findings are consistent with and extend previous research in that prazosin, propranolol, and naltrexone alone all successfully attenuated ethanol self-administration and seeking. With regard to prazosin, the high dose (1.0 mg/kg) consistently decreased ethanol-seeking and drinking in both Experiments, with lower doses showing efficacy varying by experiment. These findings are consistent with and extend that of Le et al. (2011), in that prazosin was found to block stress-induced responding for ethanol at 0.5 and 1.0 mg/kg IP in Wistar rats. An examination of a purely consummatory response (i.e., home cage drinking) in P rats showed that acute prazosin at the 1.0 mg/kg IP dose decreased ethanol intake while 0.5 mg/kg IP prazosin only became effective after 3 consecutive days of drug treatment in that study (1.0 mg/kg remained effective throughout the treatment regimen; Rasmussen et al., 2009). Similarly, Froehlich et al., (2013b) showed that prolonged treatment with 1.0 mg/kg prazosin, administered via an oral gelatin, but not 0.5 mg/kg prazosin, decreased ethanol intake in P rats with a history of alcohol drinking, and this dose was also effective at blocking initiation of ethanol consumption in naïve P rats. However, Walker and colleagues (2008) found that 0.25 to 1.0 mg/kg IP prazosin did not attenuate responding for ethanol in non-dependent, non-selected animals in a combined seeking/drinking response (i.e., FR responding for ‘sips’ of ethanol), indicating that animals selectively bred for excessive drinking may have an increased susceptibility to prazosin’s suppressive effects. Alternatively, the non-dependent Wistar rats in that study had low baseline responding (and therefore intake) which could have masked the effect of this dose range of prazosin. In support of this interpretation, Verplaetse et al. (2012), using P rats in the same paradigm as the present investigation, did not find an effect of prazosin alone at 1.0 mg/kg IP on ethanol intake, but average baseline drinking in that study was 0.86 g/kg ethanol as compared to ~1.2 g/kg ethanol in the present study. Overall, these findings suggest that prazosin may be most effective at decreasing ethanol self-administration in high-drinking animals.
At the 5 mg/kg dose, propranolol was selectively effective in decreasing ethanol intake and reinforcer-seeking. Gilpin and Koob (2010) found that this dose of propranolol (IP) was not effective in decreasing FR responding for ethanol in non-dependent, non-selected Wistar rats, but was effective in dependent animals in the same combined drinking/seeking paradigm mentioned previously. Notably, responding and intake in the dependent animals in that study was higher than in nondependent animals. Other findings using propranolol in alcohol-dependent P rats during early withdrawal and protracted abstinence indicate that the 5 mg/kg IP dose does not reduce alcohol drinking (Rasmussen et al., 2014). This disparity could be due to the use of operant responding for ethanol in the current study versus voluntary drinking or the pattern and extent of alcohol drinking history in P rats used between studies. Together with the present findings, evidence likely suggests that 5 mg/kg propranolol decreases ethanol intake, and ethanol-seeking to a greater degree than sucrose-seeking. Naltrexone has previously been tested at subthreshold doses (0.01, 0.025, and 0.03 mg/kg SC) and has only shown a trend towards a reduction in ethanol consumption and responding in home-cage drinking and FR-responding paradigms in alcohol-preferring rat lines (Koistinen et al. 2001; June et al. 1998). In the present study, 0.03 mg/kg naltrexone alone selectively attenuated ethanol-seeking and intake in P rats. One other study has tested naltrexone in the same paradigm as the present investigation and found that 0.1 mg/kg SC naltrexone (the lowest dose tested) was non-selective in decreasing intake in P rats (Henderson-Redmond and Czachowski 2014). Since naltrexone has been shown to bind preferentially to μ-opioid receptors in doses less than 1.0 mg/kg (Emmerson et al. 1994; Paterson et al. 1984; Wang et al. 2001, 2007), it is likely that the selectivity of naltrexone in reducing ethanol- versus sucrose-motivated behaviors may be attributed to naltrexone’s binding affinity for μ- versus δ- and κ-opioid receptors.
Combination pharmacotherapy in the present study revealed some instances where the combination was more effective than either drug alone at reducing reinforcer intake. Prazosin in combination with propranolol was successful in decreasing ethanol intake at the highest dose combination (1.0 mg/kg prazosin + 5 mg/kg propranolol), while all treatment combinations decreased sucrose self-administration relative to the drugs alone. However, with regard to reinforcer seeking, no combinations of prazosin and propranolol were more effective than either drug alone likely because propranolol alone was so effective in reducing ethanol seeking that it masked any enhanced effect when combined with prazosin. Previous findings had suggested that the 5 mg/kg dose would be subthreshold, however, it was more effective than expected, likely due to the use of the unique paradigm that separately assesses seeking and drinking responses. Similarly, prazosin alone was so effective in reducing sucrose-seeking in Experiment 1 that it made it difficult to detect the any increased effectiveness of treatment combinations. The observed treatment combination effects are consistent with recent findings showing that P rats receiving 1.0 mg/kg IP prazosin in combination with 10 mg/kg IP propranolol or 2 mg/kg IP prazosin in combination with 5 mg/kg IP propranolol suppressed ethanol drinking during withdrawal from chronic ethanol consumption (Rasmussen et al. 2014). Prazosin in combination with naltrexone was more effective than either drug alone at decreasing reinforcer intake and selective effects were observed on ethanol-seeking. Prazosin in combination with naltrexone was able to decrease ethanol and sucrose intake (more so than either drug alone) at the highest two treatment combinations (0.5 mg/kg prazosin + 0.03 mg/kg naltrexone and 1.0 mg/kg prazosin + 0.03 mg/kg naltrexone). Similarly, 0.125 mg/kg prazosin in combination with 0.03 mg/kg naltrexone and 0.5 mg/kg prazosin in combination with 0.03 mg/kg naltrexone were effective in decreasing responding for ethanol only. These two dose combinations of prazosin/naltrexone are the one example of the increased effectiveness of combination treatment on ethanol-reinforced responding that was not also observed with sucrose-reinforced responding. This is also in agreement with Froehlich and colleagues (2013a) in which 2.0 mg/kg prazosin in combination with 10 mg/kg naltrexone, administered via an oral gelatin, reduced ethanol consumption more effectively than either drug alone during one week of consecutive treatment in a free-access 2-hour, 2-bottle choice paradigm in P rats.
Between experiments, prazosin alone consistently decreased ethanol intake with no effects on sucrose intake. Overall, the effects of prazosin on intake were relatively consistent between experiments, with some larger discrepancies in the effects on reinforcer seeking. With regard to ethanol intake, the low dose of prazosin was effective in Experiment 1 while higher doses were required in Experiment 2. However, it should be noted that the prazosin-induced decreases in ethanol intake were relatively small in terms of the pharmacological effects of ethanol. In other words, at baseline, animals were drinking 1.14 – 1.23 g/kg ethanol, and the highest dose of prazosin alone (1.0 mg/kg) decreased ethanol consumption to 0.9 g/kg (Exp 1) and 0.75 g/kg (Exp 2), which are still pharmacologically relevant ethanol intakes, which was expected given that the doses of prazosin were intended to be subthreshold. In Experiment 1, only the high dose of prazosin decreased lever-pressing for ethanol and, in Experiment 2, a decrement in ethanol seeking was observed at all doses tested. More surprisingly, a suppression of sucrose seeking was found at all prazosin doses in Experiment 1, with no effects of prazosin on sucrose seeking in Experiment 2. This disparity could be due to the lower vehicle/vehicle baseline sucrose seeking and slightly higher variance in responding for sucrose seeking overall in Experiment 2, such that a significant reduction in lever-pressing was not detected. Again, at subthreshold doses, some disparity in efficacy is expected and further research on the therapeutic range of prazosin in multiple rat lines with different reinforcers will shed light on the effectiveness and selectivity of this drug.
There were no consistent effects of any treatment on the latency to initiate both lever-press responding and licking across both reinforcers (there were no effects at all on the latency to initiate lever-pressing). All of the instances of increased latency to first lick in the ethanol-reinforced group correspond to a decrease in ethanol intake, suggesting a lack of motivation to initiate ethanol drinking followed by a decreased drinking response once ethanol became available. In other words, there is no evidence for general motor impairing effects of any of these drugs alone or in combination at the doses tested. It should also be noted that all latency measures precede ethanol access, and the seeking response measures were taken during extinction sessions (when no ethanol was available), therefore any impairments of ethanol seeking were not due to an interaction between ethanol and prazosin, propranolol, or naltrexone.
The paradigm used in the present investigation allows for the assessment of selectivity of prospective treatments for ethanol versus other reinforcers. Across experiments, the ability of prazosin alone or in combination with propranolol or naltrexone to decrease seeking and self-administration was not selective for ethanol. These data suggest that the mechanism mediating reinforcement via the noradrenergic and opioid systems may be acting through a pathway that modulates general reinforcement. This notion is consistent with previous research that prazosin, propranolol, and naltrexone alone decrease the self-administration of other drugs of abuse (cocaine, heroin, & nicotine; Bruijnzeel et al. 2010; Forget et al. 2010; Goldberg and Gonzalez 1976; Greenwell et al. 2009) and other palatable, oral reinforcers such as saccharin, sucrose, and food pellets (Czachowski and DeLory 2009; Goodwin et al. 2001; Henderson-Redmond and Czachwoski 2014; June et al. 1998; Le et al. 2011; Verplaetse et al. 2012). However, low “subthreshold” doses of all treatments alone were selective in reducing ethanol self-administration, and prazosin in combination with naltrexone was selective in suppressing ethanol-seeking, indicating that the use of a lower dose range, in already efficacious medications, could bypass unwanted secondary side effects associated with the use of higher doses of the same drugs. In particular, 5 mg/kg propranolol and 0.03 mg/kg naltrexone were selective for decreasing ethanol self-administration and were more effective in reducing ethanol-seeking, and therefore could be viable targets, individually and in combination, for selectively attenuating the motivation to consume alcohol in a heavy drinking population. Prazosin in combination with naltrexone was selective in reducing ethanol-seeking, while prazosin in combination with propranolol or naltrexone reduced both ethanol and sucrose self-administration, suggesting that combination pharmacotherapy may provide an alternative treatment option in a subset of alcoholics who are non-responsive to monotherapy.
Acknowledgments
Funded by T32AA07462 and P60AA007611 from the National Institute on Alcohol Abuse and Alcoholism.
References
- Anton RF, O’Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295(17):2003–17. doi: 10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
- Begleiter H. Propranolol and alcohol consumption in the rat. American Journal of Drug and Alcohol Abuse. 1974;1(1):107–110. doi: 10.3109/00952997409031911. [DOI] [PubMed] [Google Scholar]
- Bruijnzeel AW, Bishnoi M, van Tuijl IA, Keijzers KFM, Yavarovich KR, Pasek TM, Ford J, Alexander JC, Yamada H. Effects of prazosin, clonidine, and propranolol on the elevations in brain reward thresholds and somatic signs associated with nicotine withdrawal in rats. Psychopharmacology. 2010;212(4):485–499. doi: 10.1007/s00213-010-1970-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson C, Johansson T. The psychological effects of propranolol in the abstinence phase of chronic alcoholics. The British journal of psychiatry: the journal of mental science. 1971:119. doi: 10.1192/bjp.119.553.605. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Economidou D, Rimondini R, Sommer W, Massi M, Heilig M. Buprenorphine reduces alcohol drinking through activation of the nociceptin/orphanin FQ-NOP receptor system. Biological Psychiatry. 2007;61:4–12. doi: 10.1016/j.biopsych.2006.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czachowski CL, DeLory MJ. Acamprosate and naltrexone treatment effects on ethanol and sucrose seeking and intake in ethanol-dependent and nondependent rats. Psychopharmacology. 2009;204(2):335–348. doi: 10.1007/s00213-009-1465-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmerson PJ, Liu MR, Woods JH, Medzihradsky F. Binding affinity and selectivity of opioids at mu, delta, and kappa receptors in monkey brains. Journal of Pharmacology and Experimental Therapeutics. 1994;271:1630–1637. [PubMed] [Google Scholar]
- Forget B, Wertheim C, Mascia P, Pushparaj A, Goldberg SR, Le Foll B. Noradrenergic alpha(1) receptors as a novel target for the treatment of nicotine addiction. Neuropsychopharmacology. 2010;35(8):1751–1760. doi: 10.1038/npp.2010.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox HC, Anderson GM, Tuit K, Hansen J, Kimmerling A, Siedlarz KM, Sinha R, et al. Prazosin effects on stress- and cue-induced craving and stress response in alcohol-dependent individuals: preliminary findings. Alcoholism Clin Exp Res. 2012;36(2):351–360. doi: 10.1111/j.1530-0277.2011.01628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froehlich JC, Hausauer BJ, Rasmussen DD. Combining naltrexone and prazosin in a single oral medication decreases alcohol drinking more effectively than does either drug alone. Alcoholism Clin Exp Res. 2013a;37:1763–1770. doi: 10.1111/acer.12148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froehlich JC, Hausauer BJ, Federoff DL, Fischer SM, Rasmussen DD. Prazosin reduces alcohol drinking throughout prolonged treatment and blocks the initiation of drinking in rats selectively bred for high alcohol intake. Alcoholism Clin Exp Res. 2013b;37:1552–1560. doi: 10.1111/acer.12116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbutt JC, West SL, Carey TS, Lohr KN, Crew FT. Pharmacological treatment of alcohol dependence: a review of the evidence. Journal of the American Medical Association. 1999;281:1318–1325. doi: 10.1001/jama.281.14.1318. [DOI] [PubMed] [Google Scholar]
- Gilpin NW, Richardson HN, Koob GF. Effects of CRF(1)-receptor and opioid-receptor antagonists on dependence-induced increases in alcohol drinking by alcohol-preferring (P) rats. Alcoholism Clin Exp Res. 2008;32(9):1535–1542. doi: 10.1111/j.1530-0277.2008.00745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Koob GF. Effects of beta-adrenoceptor antagonists on alcohol drinking by alcohol-dependent rats. Psychopharmacology. 2010;212(3):431–439. doi: 10.1007/s00213-010-1967-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg SR, Gonzalez FA. Effects of propranolol on behavior maintained under fixed ratio schedules of cocaine injection or food presentation in squirrel monkeys. Journal of Pharmacology and Experimental Therapeutics. 1976:198. [PubMed] [Google Scholar]
- Goodwin FLW, Campisi M, Babinska I, Amit Z. Effects of naltrexone on the intake of ethanol and flavored solutions in rats. Alcohol. 2001;25:9–19. doi: 10.1016/s0741-8329(01)00163-x. [DOI] [PubMed] [Google Scholar]
- Greenwell TN, Walker BM, Cottone P, Zorrilla EP, Koob GF. The alpha(1) adrenergic receptor antagonist prazosin reduces heroin self-administration in rats with extended access to heroin administration. Pharmacology Biochemistry and Behavior. 2009;91(3):295–302. doi: 10.1016/j.pbb.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson-Redmond A, Czachowski C. Effects of opioid receptor ligands on ethanol- and sucrose-seeking and drinking in alcohol-preferring (P) and long evans rats. Psychopharmacology (online) 2014 doi: 10.1007/s00213-014-3571-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge CW, Cox AA, Bratt AM, Camarini R, Iller K, Kelley SP, Mehmert KK, Nannini MA, Olive MF. The discriminative stimulus properties of self-administered ethanol are mediated by GABA(A) an NMDA receptors in rats. Psychopharmacology. 2001;154(1):13–22. doi: 10.1007/s002130000619. [DOI] [PubMed] [Google Scholar]
- June HL, Grey C, Warren-Reese C, Durr LF, Ricks-Cord A, Johnson A, McCane S, Williams L, Mason D, Cummings R, Lawrence A. The opioid receptor antagonist nalmefene reduces responding maintained by ethanol presentation: Preclinical studies in ethanol-preferring and outbred Wistar rats. Alcoholism Clin Exp Res. 1998;22:2174–2185. [PubMed] [Google Scholar]
- Kim S-G, Han B-D, Park J-M, Kim M-J, Stromberg MF. Effect of the combination of naltrexone and acamprosate on alcohol intake in mice. Psychiatry and Clinical Neurosciences. 2004;58:30–36. doi: 10.1111/j.1440-1819.2004.01189.x. [DOI] [PubMed] [Google Scholar]
- Koistinen M, Tuomainen P, Hyytia P, Kiianmaa K. Naltrexone suppresses ethanol intake in 6-hydroxydopamine-treated rats. Alcohol Clin Exp Res. 2001;25:1605–1612. [PubMed] [Google Scholar]
- Kranzler HR, Van Kirk J. Efficacy of naltrexone and acamprosate for alcoholism treatment: a meta-analysis. Alcohol Clin Exp Res. 2001;25:1335–1341. [PubMed] [Google Scholar]
- Kuzmin A, Stenback T, Lijequist S. Memantine enhances the inhibitory effects of naltrexone on ethanol consumption. European Journal of Pharmacology. 2008;584(2–3):352–356. doi: 10.1016/j.ejphar.2008.02.015. [DOI] [PubMed] [Google Scholar]
- Le AD, Funk D, Juzytsch W, Coen K, Navarre BM, Cifani C, Shaham Y. Effect of prazosin and guanfacine on stress-induced reinstatement of alcohol and food seeking in rats. Psychopharmacology. 2011;218(1):89–99. doi: 10.1007/s00213-011-2178-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le AD, Poulos CX, Harding S, Watchus J, Juzytsch W, Shaham Y. Effects of naltrexone and fluoxetine on alcohol self-administration and reinstatement of alcohol seeking induced by priming injections of alcohol and exposure to stress. Neuropsychopharmacology. 1999;21:435–444. doi: 10.1016/S0893-133X(99)00024-X. [DOI] [PubMed] [Google Scholar]
- Le AD, Poulos CX, Quan B, Chow S. The effects of selective blockade of delta and mu opiate receptors on ethanol consumption by C57BL/6 mice in a restricted access paradigm. Brain Research. 1993;630:330–332. doi: 10.1016/0006-8993(93)90672-a. [DOI] [PubMed] [Google Scholar]
- Mattson ME, Litten RZ. Combining treatments for alcoholism: why and how? J Stud Alcohol Suppl. 2005;15:8–16. doi: 10.15288/jsas.2005.s15.8. [DOI] [PubMed] [Google Scholar]
- Middaugh LD, Kelley BM, Cuison ER, Groseclose CH. Naltrexone effects on ethanol reward and discrimination in C57BL/6 mice. Alcoholism Clin Exp Res. 1999;23:456–464. [PubMed] [Google Scholar]
- O’Malley SS, Jaffe AJ, Chang G, Schottenfeld RS, Meyer RE, Rounsaville B. Naltrexone and coping skills therapy for alcohol dependence: A controlled study. Archives of General Psychiatry. 1992;49:881–887. doi: 10.1001/archpsyc.1992.01820110045007. [DOI] [PubMed] [Google Scholar]
- Paterson SJ, Corbett AD, Gillan MGC, Kosterlitz HW, McKnight AT, Robson LE. Radioligands for probing opioid receptors. Journal of Receptor Research. 1984;4:143–154. doi: 10.3109/10799898409042545. [DOI] [PubMed] [Google Scholar]
- Rasmussen DD, Beckwith LE, Kincaid CL, Froehlich JC. Combining the α1-adrenregic receptor antagonist, prazosin, with the β-adrenergic receptor antagonist, propranolol, reduces alcohol drinking more effectively than either drug alone. Alcohol Clin Exp Res. 2014;38(6):1532–9. doi: 10.1111/acer.12441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen DD, Alexander LL, Raskind MA, Froehlich JC. The alpha(1)-adrenergic receptor antagonist, prazosin, reduces alcohol drinking in alcohol-preferring (P) rats. Alcoholism Clin Exp Res. 2009;33(2):264–272. doi: 10.1111/j.1530-0277.2008.00829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellers EM, Zilm DH, Degani NC. Comparative efficacy of propranolol and chlordiazepoxide in alcohol withdrawal. Journal of Studies on Alcohol. 1977:38. doi: 10.15288/jsa.1977.38.2096. [DOI] [PubMed] [Google Scholar]
- Simpson TL, Saxon AJ, Meredith CW, Malte CA, McBride B, Ferguson LC, Raskind M. A pilot trial of the alpha-1 adrenergic antagonist, prazosin, for alcohol dependence. Alcohol Clin Exp Res. 2009;33(2):255–263. doi: 10.1111/j.1530-0277.2008.00807.x. [DOI] [PubMed] [Google Scholar]
- Stromberg MF, Volpicelli JR, O’Brien CP. Effects of naltrexone administered repeatedly across 30 or 60 days on ethanol consumption using a limited access procedure in the rat. Alcohol Clin Exp Res. 1998a;22:2186–2191. [PubMed] [Google Scholar]
- Stromberg MF, Casale M, Volpicelli JR, et al. A comparison of the effects of the opioid antagonists naltrexone, naltrindole, and beta-funeltrexamine on ethanol consumption in the rat. Alcohol. 1998b;15(4):281–289. doi: 10.1016/s0741-8329(97)00131-6. [DOI] [PubMed] [Google Scholar]
- Stromberg MF. The effect of baclofen alone and in combination with naltrexone on ethanol consumption in the rat. Pharmacol Biochem Behav. 2004;78:743–750. doi: 10.1016/j.pbb.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Tyrer P. Propranolol in alcohol addiction. Lancet. 1972;2(7779) doi: 10.1016/s0140-6736(72)92110-1. [DOI] [PubMed] [Google Scholar]
- Verplaetse TL, Rasmussen DD, Froehlich JC, Czachowski CL. Effects of prazosin, an α1-adrenergic receptor antagonist, on the seeking and intake of alcohol and sucrose in alcohol-preferring (P) rats. Alcohol Clin Exp Res. 2012;36(5):882–6. doi: 10.1111/j.1530-0277.2011.01653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpicelli JR, Alterman AI, Hayashida M, O’Brien CP. Naltrexone in the treatment of alcohol dependence. Archives of General Psychiatry. 1992;49:876–880. doi: 10.1001/archpsyc.1992.01820110040006. [DOI] [PubMed] [Google Scholar]
- Walker BM, Koob GF. Pharmacological evidence for a motivational role of κ- opioid systems in ethanol dependence. Neuropsychopharmacology. 2008;33:643–652. doi: 10.1038/sj.npp.1301438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Rasmussen DD, Raskind MA, Koob GF. Alpha(1)-noradrenergic receptor antagonism blocks dependence-induced increases in responding for ethanol. Alcohol. 2008;42(2):91–97. doi: 10.1016/j.alcohol.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Raehal KM, Bilsky EJ, Sadee W. Inverse agonist and neutral antagonists at mu opioid receptor (MOR): Possible role of basal receptor signaling in narcotic dependence. Journal of Neurochemistry. 2001;77:1590–1600. doi: 10.1046/j.1471-4159.2001.00362.x. [DOI] [PubMed] [Google Scholar]
- Wang D, Sun X, Sadee W. Different effects of opioid antagonists on mu-, delta-, and kappa-opioid receptors with and without agonist pretreatment. The Journal of Pharmacology and Experimental Therapeutics. 2007;321:544–552. doi: 10.1124/jpet.106.118810. [DOI] [PubMed] [Google Scholar]
- Williams KL, Broadbridge CL. Potency of naltrexone to reduce ethanol self-administration in rats is greater for subcutaneous versus intraperitoneal injection. Alcohol. 2009;43:119–126. doi: 10.1016/j.alcohol.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]