Abstract
Severe combined immunodeficiency (SCID) is most frequently caused by mutations in the cytokine receptor common gamma chain, CD132, encoded by the X-linked gene, IL2RG. Most patients present in the first year of life with failure to thrive, severe, opportunistic infections and absence of CD3+ T cells. We present a patient with pediatric illness and a diagnosis of combined variable immune deficiency (CVID) who was diagnosed at age 23 with an inherited IL2RG mutation causing loss of signal transduction through CD132. His peripheral blood included CD3/CD4 and CD3/CD8 positive cells as well as low levels of CD19+ B cells containing a reversion to the wildtype IL2RG allele. The reversion, which was not present at birth, may account for his mild phenotype and late diagnosis.
Keywords: X-SCID, IL2RG, Reversion, Common gamma chain
Introduction
Severe combined immunodeficiency (SCID) typically presents in infancy with poor survival beyond 2 years without definitive immune reconstitution therapy. Increasingly, though, less severe presentations have been associated with mutations in SCID genes. Most of these “leaky” SCID cases are due to hypomorphic mutations. However, there are a few cases of reversion mutations leading to prolonged survival and a less severe phenotype. We present an individual diagnosed with CVID who was identified with a mutation in IL2RG at 25 years old. Samples from the patient and his mother were obtained and records were reviewed with informed consent under approved protocols at the National Institutes of Health and the University of California San Francisco.
Case
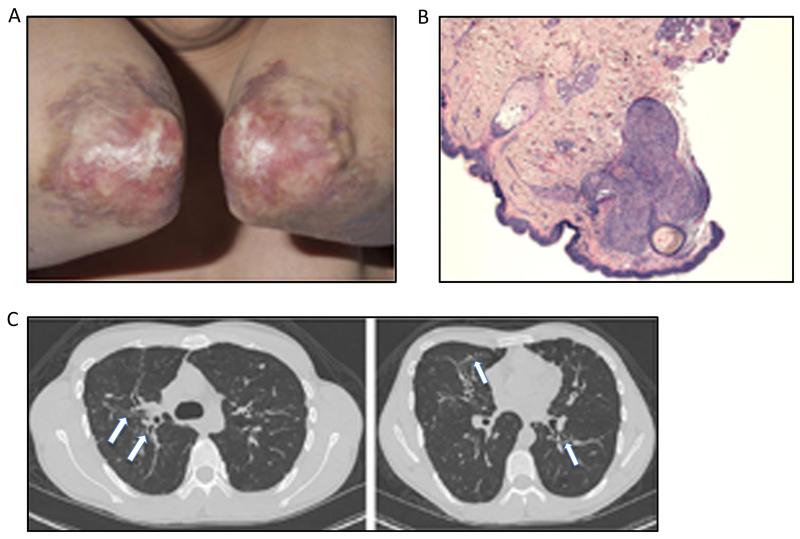
The patient developed recurrent ear, sinus and lung infections in early childhood. At the age of 5 years, he was diagnosed with common variable immunodeficiency (CVID) and was started on intravenous immunoglobulin (IVIG) therapy with decrease in the number of infections. Skin lesions developed around age 6 years, affecting primarily the elbow (Fig. 1a), nose and right cheek and neck, with atrophy and some soft tissue loss of the right nasal alae. Biopsy of the lesions showed granulomatous inflammation (Fig. 1b) with presence of non-clonal CD3+CD4+ and CD3+CD8+CD57-lymphocytes. Stains and cultures were negative for bacteria, fungi and mycobacteria. These lesions improved with intermittent topical tacrolimus, but several secondary bacterial infections of the elbow lesions occurred, associated twice with osteomyelitis of the underlying bone. Now at 27 years old, he requires antibiotic courses for respiratory exacerbations about once yearly and has stable bronchiectasis on chest CT (Fig. 1c). He has not had viral skin infections such as warts, molluscum contagiosum or herpes zoster. He denies gastrointestinal symptoms and has no evidence of malabsorption; however has vitamin B12 deficiency with negative intrinsic factor autoantibodies.
Fig. 1.
Clinical presentation a. Dermatitis on elbows of patient; note central atrophy, hypopigmentation and scaling with areas of erythema and induration at the perimeter. b. Histological stain demonstrating granuloma with intense lymphocytic infiltration in the superficial dermis. c. Chest CT at 26 years with features of bronchiectasis, irregular scarring, distention and thickening of the airways (arrows), throughout the lung parenchema
Lymphocyte phenotyping showed normal numbers of CD3+ T cells, but low CD4 cells, high CD8 cells and inverted CD4/CD8 ratio, with high numbers and percentages of CD3+CD8+CD57+ cytotoxic T cells. B cells were within the normal range, but without isotype switching. NK cells were reduced while the NK-T population was expanded compared to normal controls. Total lymphocyte counts evaluated three times over a 4-year period have declined from 1520/uL (89.8 % CD3+) at age 23 to 1150/uL (95.7 % CD3+) at age 25 and 890/uL (95.2 % CD3+) at age 27. The CD4/CD8 ratio has remained at 0.3. Surprisingly, the NK-T cell numbers have remained elevated in the mid 400′s/uL despite declining numbers of total lymphocytes. B cell numbers have also declined (117/uL, 40/uL and 30/uL at ages 23, 25 and 27 respectively). Consistent with a T-cell immunodeficiency, flow cytometric analysis of TCR Vb usage showed a limited T cell repertoire [1] with over-represented Vb2, Vb13.1 and Vb20 in CD4+ cells and Vb 3, Vb13.1 and Vb 16 in CD8+ cells. (data not shown). DNA from multiple peripheral blood samples was amplified for detection of immunoglobulin and T-cell receptor gene rearrangements. In all samples, there was polyclonal rearrangement of the immunoglobulin locus and clonal or oligoclonal rearrangement of the TCR locus, replicating the limited T cell repertoire seen by Vb staining. DNA from the skin biopsy demonstrated polyclonal TCR rearrangement in the tissues. The patient has normal levels of plasma IgA and IgM (447 and 130 mg/dL respectively) however IgG levels are not reliable due to the administration of IVIg.
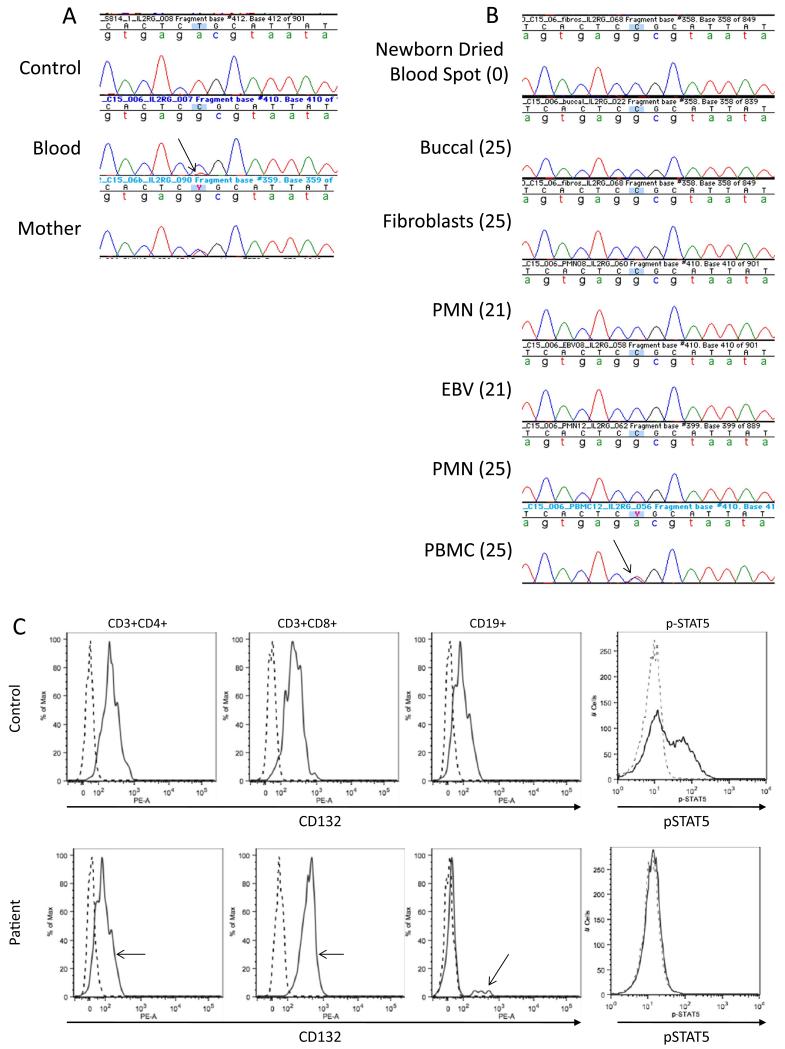
The clinical phenotype of granulomatous skin lesions and bronchiectasis led to consideration of RAG1, RAG2 and TAP defects, the first two ruled out by sequencing and the latter by showing normal MHC class 1 expression. The combined T and B cell defect suggested a leaky form of SCID, so we sequenced IL2RG, encoding the common gamma chain for multiple cytokine receptors (CD132), using DNA isolated from whole blood. We found a hemizygous T>C transition in IL2RG at c.260 resulting in p.L87P, with a minor wild-type peak detected under the mutant peak (Fig. 2a). To distinguish whether this double peak was due to somatic mosaicism or reversion, DNA from maternal buccal swabs was sequenced and demonstrated heterozygosity of c.260 T>C. Therefore, the patient inherited the mutant, maternal allele and the wild-type allele is due to a reversion event. As further support for reversion, DNA isolated from the newborn dried blood spot card (DBS) obtained from the California Department of Public Health Genetic Disease Laboratory had undetectable TREC amplification (normal >25 copies/uL) despite 31,800 copies of the control amplicon (normal >10,000 copies/uL), indicative of severe T cell lymphopenia at birth [2]. Furthermore, IL2RG sequence of this DNA showed only the mutant c.260C allele (Fig. 2b).
Fig. 2.
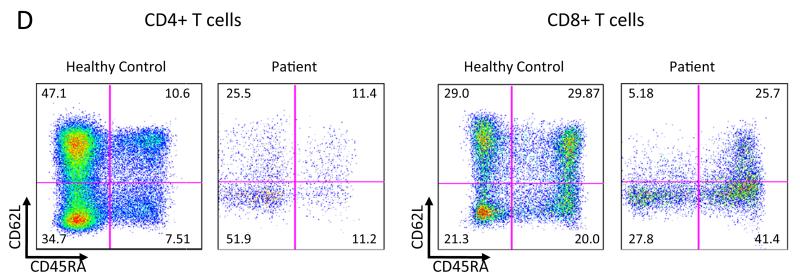
Genetic and functional demonstration of reversion mutation a IL2RG genomic sequencing. DNA from a healthy control, whole blood from the patient demonstrating the c.260 T>C mutation and a minority contribution of the wild-type revertant peak (arrow), and buccal swab of the patient’s mother. b IL2RG sequence from patient cells and age at collection (parentheses). c Surface staining for CD132 (solid line) on CD3+CD4+, CD3+CD8+ and CD20+ PBMC. Revertant CD132+ population indicated by arrows. IL-2 induced pSTAT5 in EBV-B cells. Solid line IL-2 stimulated, dotted line unstimulated. d Subtyping of CD4+ and CD8+ T cells. The numbers indicate percentage of gated cells in each quadrant. The patient is notable for the decreased central memory (CD62L+ CD45RA−) population in both CD4+ and CD8+ cells
To evaluate different lineages for the reversion, DNA isolated from archived polymorphonuclear cells (PMNs) and Epstein-Barr virus (EBV) transformed B cells, both collected at age 21, as well as peripheral blood mononuclear cells (PBMCs), PMNs, buccal swab cells and fibroblasts collected at age 25 were sequenced for IL2RG exon 2. (Fig. 2b). Only the mutant allele was present in DNA from buccal cells, fibroblasts, PMNs, and the EBV transformed B cell line from age 21 as well as PMNs from age 25. In contrast, the first available PBMC sample, from age 25, had equal amounts of mutant and wild-type DNA, demonstrating the prominence of the reversion within the lymphoid lineage.
To examine the effect of the above p.L87P mutation, we stained the EBV transformed B cells, demonstrating loss of anti-CD132 antibody recognition of the mutant protein. We then isolated PBMCs from whole blood and stained for CD3, CD4, CD8, CD19 and CD132 to determine which cells had detectable surface expression of CD132 indicating revertant cells. As expected, all CD3+CD4+ and CD3+CD8+ cells expressed CD132 (Fig. 2c). We next examined CD62L versus CD45RA in CD4+ and CD8+ cells for T cell subsets (Fig. 2d). While naïve (CD45RA+ CD62L+) T cells were present in percentages comparable to normal (10.6 % vs 11.4 in CD4+ and 29.87 vs 25.7 % in CD8+), the increased T cell effector memory and transitional effector memory subsets are expanded indicating repopulation towards terminally differentiated and exhausted subsets. Surprisingly, there was a small population (1.1 %) of CD19+ cells that were CD132 positive as well. Given the low percentage of CD132+ B cells, we repeated the staining on frozen PBMCs from a different visit and again detected a CD132+ B cell population. We further subtyped the CD132+ B cells using CD10 versus CD27 staining; the patient had increased percentages of naïve and transitional CD132+ B cells (38, 31 % respectively compared to 28, 10 % in the normal control) however he had only 18 % memory B cells (56 % in the normal control). IL-2 was unable to induce STAT5 phosphorylation (pSTAT5) in the patient EBV cells compared to EBV cells from a healthy control (Fig. 2c) indicating the missense change affects signal transduction through CD132.
Discussion
X-linked severe combined immune deficiency (X-SCID) comprises both cellular and humoral immune defects. Unless detected by a positive family history or newborn screening, patients typically present in the first year due to opportunistic infections and failure to thrive, and mortality is high in the first 2 years without immune reconstitution from bone marrow transplantation. X-SCID is caused by mutations occurring in IL2RG encoding the cytokine common gamma chain (γc) [3, 4] which supports signaling by multiple interleukins (IL) including IL-2, IL-4 [5], IL-7 [6], IL-9 [7], IL-15 [8] and IL-21 [9]. Signaling through IL-7 is essential for differentiation of T cells from the common lymphoid progenitor, while IL-21 signaling induces proliferation, immunoglobulin (Ig) isotype switching and antibody secretion in B cells [10]. IL-15 is required for maturation of natural killer (NK) cells [11]. The lymphocyte profile is therefore a lack of T and NK cells, while B cells are present but unable to make specific antibody responses.
In four previously reported cases of spontaneous reversion in IL2RG [12-15], only CD3+ T cells demonstrated reversion to wild-type while all other lineages examined remained mutant. This is the first identified case of X-SCID in which the reversion is found in CD19+ B cells as well as T-cells, indicating a reversion event at the level of common lymphoid progenitor. This early reversion is detectable within circulating B cells but does not appear to be sufficient to correct the patient’s specific immunoglobulin defect.
Milder phenotypes in patients have been associated with mosaicism due to somatic mutation [16] while improvement of severe phenotype has been demonstrated in cases of somatic reversion occurring in the appropriate cell population [14, 15, 17]. We describe a patient whose early childhood infections may have improved due to selective expansion of wild type T cells during early life as well as IVIG therapy. He nevertheless experienced cumulative pulmonary injury and extensive skin lesions. Similar to “leaky” SCID due to RAG defects, granulomatous skin disease was a predominant clinical feature. Newborn TREC screening, which is becoming more widely available, would have detected SCID for this patient and others with leaky SCID allowing early institution of optimal therapy [18].
Acknowledgments
This research was supported in part by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health, USA. This project has been funded in part with federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. HHSN261200800001E.
Footnotes
Publication Disclaimer The views expressed in this article are those of the authors and do not reflect the official policy of the U.S. Government.
Conflict Interest The authors declare that they have no conflict of interest.
References
- 1.Hsieh MY, Hong WH, Lin JJ, Lee WI, Lin KL, Wang HS, et al. T-cell receptor excision circles and repertoire diversity in children with profound T-cell immunodeficiency. J Microbiol Immunol Infect. 2013;46(5):374–81. doi: 10.1016/j.jmii.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 2.Puck JM. Laboratory technology for population-based screening for severe combined immunodeficiency in neonates: the winner is T-cell receptor excision circles. J Allergy Clin Immunol. 2012;129:607–16. doi: 10.1016/j.jaci.2012.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Puck JM, Deschenes SM, Porter JC, Dutra AS, Brown CJ, Willard HF, et al. The interleukin-2 receptor g chain maps to Xq13.1 and is mutated in X-linked severe combined immunodeficiency, SCIDX1. Hum Mol Genet. 1993;2(8):1099–104. doi: 10.1093/hmg/2.8.1099. [DOI] [PubMed] [Google Scholar]
- 4.Noguchi M, Yi Huafang Y, Rosenblatt HM, Filipovich AH, Adelstein S, Modi WS, et al. Interleukin-2 receptor g chain mutation results in X-linked severe combined immunodeficiency in humans. Cell. 1993;73(1):147–57. doi: 10.1016/0092-8674(93)90167-o. [DOI] [PubMed] [Google Scholar]
- 5.Russell SM, Keegan AD, Harada N, Nakamura Y, Noguchi M, Leland P, et al. Interleukin-2 receptor gamma chain: a functional component of the interleukin-4 receptor. Science. 1993;262(5141):1880–3. doi: 10.1126/science.8266078. [DOI] [PubMed] [Google Scholar]
- 6.Noguchi M, Nakamura Y, Russell SM, Ziegler SF, Tsang M, Cao X, et al. Interleukin-2 receptor gamma chain: a functional component of the interleukin-7 receptor. Science. 1993;262(5141):1877–80. doi: 10.1126/science.8266077. [DOI] [PubMed] [Google Scholar]
- 7.Kimura Y, Takeshita T, Kondo M, Ishii N, Nakamura M, Van Snick J, et al. Sharing of the IL-2 receptor gama chain with the functional IL-9 receptor complex. Int Immunol. 1995;7(1):115–20. doi: 10.1093/intimm/7.1.115. [DOI] [PubMed] [Google Scholar]
- 8.Giri JG, Anderson DM, Kumaki S, Park LS, Grabstein KH, Cosman D. IL-15, a novel T cell growth factor that shares activities and receptor components with IL-2. J Leukoc Biol. 1995;57(5):763–6. doi: 10.1002/jlb.57.5.763. [DOI] [PubMed] [Google Scholar]
- 9.Habib T, Senadheera S, Weinberg K, Kaushansky K. The common gamma chain (gamma c) is a required signaling component of the IL-21 receptor and supports IL-21-induced cell proliferation via JAK3. Biochemistry. 2002;41(27):8725–31. doi: 10.1021/bi0202023. [DOI] [PubMed] [Google Scholar]
- 10.Recher M, Berglund LJ, Avery DT, Cowan MJ, Gennery AR, Smart J, et al. IL-21 is the primary common g chain-binding cytokine required for human B-cell differentiation in vivo. Blood. 2011;118(26):6824–35. doi: 10.1182/blood-2011-06-362533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vosshenrich CA, Ranson T, Samson SI, Corcuff E, Colucci F, Rosmaraki EE, et al. Roles for common cytokine receptor gamma-chain-dependent cytokines in the generation, differentiation, and maturation nof NK cell precursors and peripheral NK cells in vivo. J Immunol. 2005;174(3):1213–21. doi: 10.4049/jimmunol.174.3.1213. [DOI] [PubMed] [Google Scholar]
- 12.Volker S, Volker W, Le Deist F, Dirksen U, Broker B, Muller-Fleckenstein I, et al. Atypical X-linked severe combined immunodeficiency due to possible spontaneous reversion of the genetic defect in T cells. N Engl J Med. 1996;335:1563–7. doi: 10.1056/NEJM199611213352104. [DOI] [PubMed] [Google Scholar]
- 13.Speckmann C, Pannicke U, Wiech E, Schwarz K, Fisch P, Friedrich W, et al. Clinical and immunologic consequences of a somatic reversion in a patient with X-linked severe combined immunodeficiency. Blood. 2008;112(10):4090–7. doi: 10.1182/blood-2008-04-153361. [DOI] [PubMed] [Google Scholar]
- 14.Kawai T, Saito M, Nishikomori R, Yasumi T, Izawa K, Murakami T, et al. Multiple reversions of an IL2RG mutation restore T cell function in an X-linked severe combined immunodeficiency patient. J Clin Immunol. 2012;32(4):690–7. doi: 10.1007/s10875-012-9684-1. [DOI] [PubMed] [Google Scholar]
- 15.Kuijpers TW, van Leeuwen EM, Barendregt BH, Klarenbeek P, Aan de Kerk DJ, Baars PA, et al. A reversion of an IL2RG mutation in combined immunodeficiency providing competititive advantage to the majority of CD8+ T cells. Haematologica. 2013;98(7):1030–8. doi: 10.3324/haematol.2012.077511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsu AP, Sowerwine KJ, Lawrence MG, Davis J, Henderson CJ, Zarember KA, et al. Intermediate phenotypes in patients with auto-somal dominant hyper-IgE syndrome caused by somatic mosai-cism. J Allergy Clin Immunol. 2013;131(6):1586–93. doi: 10.1016/j.jaci.2013.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uzel G, Tng E, Rosenzweig SD, Hsu AP, Shaw JM, Horwitz ME, et al. Reversion mutations in patients with leukocyte adhesion deficiency type-1 (LAD-1) Blood. 2008;111(1):209–18. doi: 10.1182/blood-2007-04-082552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kwan A, Abraham RS, Currier R, Brower A, Andruszewski K, Abbott JK, et al. Newborn screening for SCID in the United States: experience from 11 screening programs. JAMA. 2014;312(7):729–38. doi: 10.1001/jama.2014.9132. [DOI] [PMC free article] [PubMed] [Google Scholar]