Abstract
Peromyscus leucopus , the white-footed mouse, is one of the more abundant mammals of North America and is a major reservoir host for at least five tickborne diseases of humans, including Lyme disease and a newly-recognized form of relapsing fever. In comparison to Mus musculus, which is not a natural reservoir for any of these infections, there has been little research on experimental infections in P. leucopus. With the aim of further characterizing the diversity of phenotypes of host responses, we studied a selection of quantitative traits in colony-bred and –reared outbred P. leucopus adults that were uninfected, infected with the relapsing fever agent Borrelia hermsii alone, or infected after immunization with Lyme disease vaccine antigen OspA and keyhole limpet hemocyanin (KLH). The methods included measurements of organ weights, hematocrits, and bleeding times, quantitative PCR for bacterial burdens, and enzyme immunoassays for serum antibodies against both the immunization proteins and cellular antigens of the infecting organism. The results included the following: (i) Uninfected animals displayed wide variation in relative sizes of their spleens and in their bleeding times. (ii) In an experiment with matched littermates, no differences were observed between females and males at 7 days of infection in bacterial burdens in blood and spleen, relative spleen size, or antibody responses to the B. hermsii specific-antigen, FbpC. (iii) In studies of larger groups of males or females, the wide variations between bacterial burdens and in relative spleen sizes between individuals was confirmed. (iv) In these separate groups of males and females, all animals showed moderate-to-high levels of antibodies to KLH but wide variation in antibody levels to OspA and to FbpC. The study demonstrated the diversity of host responses to infection and immunization in this species and identified quantitative traits that may be suitable for forward genetics approaches to reservoir-pathogen interactions.
Keywords: tickborne, Lyme disease, relapsing fever, reservoir, vaccine, wildlife
Introduction
The genus Peromsyscus is one of the most widely distributed, ecologically variable, and speciose among rodent taxa of North America (Baker, 1968; Kirkland and Layne, 1989). As E.R. Hall describes peromyscines in Mammals of North America, “In most places within the geographic range of the genus, these mice are the most abundant mammal” (Hall, 1979). The deer mouse P. maniculatus and the white-footed mouse P. leucopus have broad and overlapping distributions and are particularly important as major reservoirs for several human pathogens. In the case of P. maniculatus, which exists in variety of habitats mainly in central and western United States, these pathogens include hantaviruses (Amman et al., 2013). P. leucopus, which is distributed across much of the central and eastern United States, serves as a reservoir host for at least five tick-borne human pathogens: the Lyme disease agent Borrelia burgdorferi (Levine et al., 1985), the cause of human granulocytic anaplasmosis Anaplasma phagocytophilum (Telford et al., 1996), the babesiosis agent Babesia microti (Telford and Spielman, 1993), the flavirus that causes deer tick virus encephalitis (Ebel et al., 2000), and the newly-recognized disease agent Borrelia miyamotoi (Barbour et al., 2009; Scoles et al., 2001). For all these infections the disease vector for humans in the northeastern and north-central United States and adjoining areas of Canada is the northern form of the blacklegged tick Ixodes scapularis. The larval and nymphal stages of this tick heavily exploit P. leucopus as a source of their blood meals in many if not all of the areas where the aforementioned pathogens are endemic (Piesman and Schwan, 2010). P. leucopus is the first candidate for transmission-blocking vaccines targeting wildlife with the aim of reducing the prevalence of B. burgdorferi in ticks (Bhattacharya et al., 2011; Meirelles Richer et al., 2011; Tsao et al., 2004).
Given the key role of P. leucopus in the life cycle of several different human pathogens, including the cause of Lyme disease, the most frequent arthropod-borne disease in the United States (Centers for Disease Control, 2014), one might expect that it would be the subject of much attention as a laboratory model for these infections. But that has not been the case. The number of reports of experimental infections of the laboratory mouse Mus musculus greatly outnumber those of P. leucopus, even though the house mouse is not a reservoir. Among the possible explanations for this neglect of P. leucopus is the assumption that because rodent is familiarly called a “mouse”, it is not only expedient but also sufficient to substitute the laboratory mouse.
Doubtless much has been learned from experimental infections with B. burgdorferi of M. musculus, especially in its highly defined inbred varieties (Barthold et al., 2010). But it is unclear to what extent the findings from this model animal can be extrapolated to P. leucopus, let alone other natural reservoirs, like shrews (Brisson et al., 2008). A presumption of close-relatedness between these two “mice”, which superficially resemble each other, does not match current understanding of the evolution of rodents. The genus Peromyscus is in the family Cricetidae, along with hamsters and voles, while M. musculus, as well as that other laboratory standby, Rattus rattus, is in Muridae, a different family, (Steppan et al., 2004). The Peromsycus lineage is estimated to have diverged from the common ancestor of Mus and Rattus 25 million years ago (Dewey and Dawson, 2001). One Cricetidae member with a long history of utility for studies of infectious diseases in the laboratory is the golden hamster, Mesocricetus auratus. And, indeed, the first published report of infection of a rodent with B. burgdorferi in the laboratory was on hamsters (Johnson et al., 1984). But citations to experimental B. burgdorferi infections of M. auratus are dwarfed by those to M. musculus.
Cognizant of the lengthening list of infectious agents carried by Peromyscus species and the evidence of increasing distribution and incidence of these zoonoses in North America (Centers for Disease Control, 2014), we set out to further develop P. leucopus as an experimental model for those tick-borne pathogens for which it serves as a major reservoir. A P. leucopus experimental system is feasible in part because there are at least two institutional sources for colony-bred and –reared outbred P. leucopus in the United States: in South Carolina (Crossland et al., 2014) and in Massachusetts (Bhattacharya et al., 2011). Advances in the physical mapping and sequencing of the genomes of different Peromyscus species (Kenney-Hunt et al., 2014; Worley, 2015), including P. leucopus, also mean that investigators likely will not be dependent on using inbred strains for the identification of loci for either Mendelian or quantitative traits. Such forward genetics approaches as high-density SNP microarrays and genome-wide association studies are most informative when there are well-characterized phenotypes of biological relevance and with no or limited co-variation. To this end we examined several distinct parameters of host response of P. leucopus in experiments with either Borrelia hermsii infection alone or infection in combination with immunization with antigens that were anticipated to be irrelevant to the course of the infection. The soft tick-borne relapsing fever agent B. hermsii was chosen for the experiment because of the expectation of higher pathogen densities in the blood with this organism than with B. burgdorferi (Barbour et al., 2009). We had found that P. leucopus could be infected with B. hermsii by needle inoculation and for there to be a detectable and specific antibody response to the infection (Baum et al., 2012).
Our primary question for the present study was, To what extent do these colony-bred animals differ in selected phenotypes of responses to infection and immunization? In other words, how much would they individually vary if placed under the same experimental conditions?
Materials and Methods
Animals
All animal studies were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of California, Irvine. Severe-combined immunodeficient mice (SCID) C.B-17/Icr-Prkdcscid/IcrIcoCrl were obtained from Charles River Laboratories and were housed in sterile barrier cages (Tecniplast USA Inc., Exton, PA). Adult male and female colony-bred and –reared Peromyscus leucopus (LL stock) were obtained from the Peromyscus Genetic Stock Center (PGSC) colony at the University of South Carolina, Columbia, SC (Peromyscus Genetic Stock Center, 2015). The colony was derived from 38 wild ancestors captured at a single site near Linville, NC between 1982 and 1985. Pedigrees are kept on all mice, and siblings are excluded from breeding pairs (Crossland et al., 2014). The mice were routinely monitored for common rodent viruses, intestinal parasites, and mites (Wiedmeyer et al., 2014). At U.C. Irvine, animals were housed under BSL2 conditions in groups of 5 in Tecniplast cages (Tecniplast USA Inc., Exton, PA). They were accomodated to the new environment for at least 3 days before experiments. Rodent feed (Teklad 8604 Rodent Diet, Harlan Laboratories, Indianapolis, IN) and water were supplied ad libitum. Animals were on a 12 hour light and 12 hour dark schedule and at an ambient temperature of 22 °C. Total body masses and dissected organ masses were determined to 0.01 g with a PL3002 laboratory balance scale (Mettler-Toledo, Columbus, OH) before, during, or at termination of experimental infections. Body lengths (i.e. from nose to base of tail) were measured with Adobe Photoshop v. 7 from digital photographs of the animals on a calibrated grid.
Bacterial strains
B. hermsii strain CC1 (Dai et al., 2006) was propagated in SCID mice from frozen stocks of infected mouse plasma as described (Lewis et al., 2014). The concentrations of B. hermsii in the blood were determined by phase-contrast microscopy of wet mounts in a Petroff-Hausser counting chamber as described (Barbour and Bundoc, 2001). The plasma was diluted with Barbour-Stoenner-Kelly II (BSK II) culture medium (Barbour, 1984) to provide the chosen inoculum in a volume of 50 μl.
Infection and immunization
On day 0, P. leucopus animals were each injected subcutaneously with B. burgdorferi strain B31 recombinant lipidated OspA (lot # 42537 from Aventis Pasteur, Swiftwater, PA) (Pal et al., 2003; Tsao et al., 2004) at a dose of 0.45 μg and keyhole limpet hemocyanin (Sigma–Aldrich, St. Louis, MO) (Martin et al., 2007a) at 1.4 μg per gram of animal mass and in a volume of 100 μl of the following buffer: 50 mM Tris, pH 7.5–10 mM NaCl-0.3% Triton X-100. The purity and the absence of proteolysis of the recombinant OspA was confirmed by sodium dodecyl sulfate-polyacrylamide gel electrophoreis. Protein concentrations were determined with a Microplate BCA Protein Assay Kit (Pierce, Rockford, IL). On day 21, P. leucopus were each intraperitoneally injected with 104 cells of B. hermsii in infected plasma. Animals were euthanized on day 28 by CO2 overdose, followed immediately by cardiocentesis and cervical dislocation. Blood was collected into 1.7 ml tubes coated with lithium heparin (Becton-Dickinson, Franklin Lakes, NJ). Plasma was recovered by serial 5 s centrifugations at 3000 × g in a microcentrifuge and then pooled. The liver, spleen, and heart were removed intact by dissection and weighed. Whole blood specimens and spleens were kept frozen at −80° C until DNA extraction. The dissected livers were fixed in 10% neutral-buffered formalin solution.
Enzyme immunoassay (EIA)
Antigens were purified recombinant Fibronectin Binding Protein C (FbpC; also known as BHA007) of B. hermsii (Lewis et al., 2014), recombinant Borrelia miyamotoi GlpQ protein (Krause et al., 2014), and the recombinant OspA and KLH preparations used for the immunization. To the wells of 96-well Microtest™ U-bottomed polystyrene microtiter plates (Becton Dickinson Co., Franklin Lakes, NJ) were added 100 μl protein solution at a concentration of 1 μg per milliliter of carbonate buffer (0.35 M Na2CO3- 0.036 M NaHCO3, pH 9.6), and then the plates were incubated for 16 h at 22°C. The wells were washed 3 times with 300 μl phosphate-buffered saline (PBS) with 0.05% Tween 20 (Bio-Rad, Irvine, CA) (PBS-T) and then blocked for 1 h with 200 μl 10% bovine serum albumin (EMD Milipore, Billerica, MA) in PBS (PBS-A) at 22°C. This was followed by 3 washes with 300 μl of PBS-T each. To each well was then added 100 μl of serum diluted 1:100 in PBS-A. Each sample was assayed in triplicate. When replicates of the assays were carried out on different days the coefficients of determination (R2) were ≥0.95. Sera from uninfected P. leucopus and wells with buffer only were used as negative controls. The plates were incubated for 3 h at 22°C, and then the plates were washed 3 times with 300 μl PBS-T. Bound antibody was detected with a 100 μl volume of alkaline phosphatase-conjugated goat anti-Peromyscus leucopus IgG heavy and light chain antibody (Kirkegaard and Perry Laboratories, Gaithersburg, MD) diluted 1:2000 in PBS-A. The plates were incubated for 16 h at 22°C. The wells were washed 3 times with 300 μl PBS-T, and the colorimetric reaction was performed with 100 μl per well of p-nitrophenyl phosphate (Sigma-Aldrich, St. Louis, MO). The optical density was determined at 405 nm with a Synergy II plate reader (BioTek, Winooski, VT).
Quantitative polymerase chain reaction (qPCR)
DNA was isolated from blood and spleens using the Qiagen DNeasy Blood and Tissue Kit (Qiagen, Valencia, CA). In brief, dissected and cut-up tissues or whole blood were lysed 12 to 16 hours at 56°C in 180 μl ATL buffer and 20 μl Proteinase K (20 mg/ml). Sample processing was automated in a QiaCube apparatus (Qiagen, Valencia, CA). Eluted samples were then cleaned and concentrated using ZymoResearch Clean and Concentrator kit (Zymo Research, Irvine, CA) following the manufacturer’s protocol. The elution volume was 30 μl. DNA concentrations were determined with a Nanodrop 1000 Spectrophotometer (Thermo Scientific, Waltham, MA) or a Qubit® 2.0 Fluorometer (Life Technologies, Carlsbad, CA). qPCR for 16S ribosomal DNA of B. hermsii was carried out as described (Barbour and Travinsky, 2010). The probe for B. burgdorferi 16S ribosomal DNA in the assay served as a control for the specificity of the B. hermsii probe in quantitating genome copies of B. hermsii in the extracted DNA. Assays were carried out in duplicate; R2 values between replicates were ≥ 0.95.
Other blood studies
For the hematocrit measurements, cardiac blood was collected with heparinized capillary tubes (Fisher Scientific, Waltham, MA), and these were centrifuged in a ZIPocrit Microhematocrit Centrifuge (LW Scientific, Lawrenceville, GA) for 5 minutes. The bleeding time was the time in 30 second intervals for bleeding to cease after 1 mm of the tip of the tail was snipped with sterile scissors and blood intermittently drawn off with a gauze.
Histopathology
Livers from P. leucopus were fixed in 10% neutral-buffered formalin and then processed at University of California Davis’ Comparative Pathology Laboratory, where they were paraffin-embedded, sectioned, and stained with hematoxylin and eosin. The sections were examined blindly as to infection state. The histology of the liver was evaluated and graded with respect to five pathologic features: hemophagocytosis by Kupfer cells, portal and periportal infiltration by mononuclear cells, lymphofollicular hyperplasia, centrilobular lipoidosis, and presence and extent of ground-glass appearance. The absence of a feature was assigned a score of 0, and assessments of mild, moderate, or severe were assigned scores of 1, 2, or 3, respectively. The composite pathology score was the sum of the individual feature scores with a maximum score of 13. Livers from two uninfected mice had scores of 0 by blind reading.
Statistics
Descriptive statistics of means, medians, standard deviations (SD), coefficients of variation (CV), 95% confidence intervals (CI), coefficients of determination (R2), linear and power regressions, and general linear models were calculated by Stata/MP v. 10.1 (Stata Corp., College Station, TX) or SYSTAT v. 13 (Systat Software, Chicago, IL). Parametric hypothesis testing was by 2-tailed paired or unpaired t test or ANOVA. The Shapiro-Wilk W test for normal data was used.
Results
Overview of the design and conditions
The subjects for this study were colony-bred and –reared P. leucopus of both sexes and aged 13 to 81 weeks, with the majority in the range of 20 to 50 weeks. P. leucopus have life spans of up to 8 years (416 weeks) in captivity (Joyner et al., 1998), so these were mature but not aged animals. Four different experiments were carried out: 1, a group of 28 uninfected males; 2, a group with littermate-matched males and females; 3, a group of 30 males; and 4, a group of 30 females, for an overall total of 102 animals. In experiment 2 the mixed sex set of animals were infected with Borrelia hermsii and examined with respect to the differences in pathogen burden and host responses. The two antigens, GlpQ and FbpC, used in the immunoassays were known to be immunogenic during B. hermsii infections of M. musculus (Lewis et al., 2014; Schwan et al., 1996).
In experiments 3 and 4, the two larger sets with single sex, the animals were also infected with B. hermsii but after first being immunized 3 weeks before with single unadjuvanted doses of keyhole limpet hemocyanin (KLH), a commonly used antigen in experimental studies, including of P. leucopus (Martin et al., 2007a), and B. burgdorferi’s OspA lipoprotein, which is the basis of a recombinant dog vaccine against Lyme disease (Conlon et al., 2000) and the principal candidate antigen for a wildlife vaccine targeting P. leucopus and possibly other reservoirs in the field (Bhattacharya et al., 2011; Meirelles Richer et al., 2011; Tsao et al., 2004).
Diversity of selected traits in uninfected animals
In experiment 1, the 28 males were the offspring of 25 different mating pairs and were aged 119 to 525 days (mean 258 d; median 175 d). Their body masses ranged from 14.0 to 24.9 g (mean 18.4 g; median 18.7 g), and their lengths from nose to the base of tail were from 8.0 to 9.3 cm (8.7 cm mean; 8.8 cm median). Neither body mass (R2 = 0.008) nor length (R2 = 0.015) correlated with age among these animals. The mean (CI) change in body mass between two weighings 21 d apart was −0.19 (−0.40 to +0.02) g.
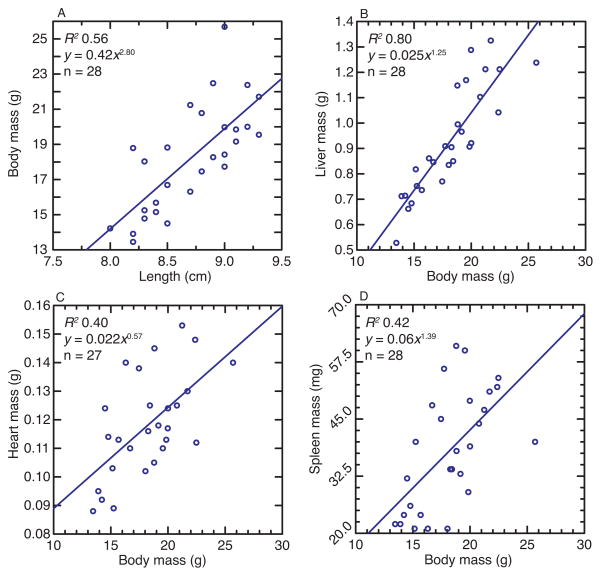
Body mass (M) scaled to body length (L) from nose to the base of the tail with an exponent of 2.8 (i.e., M ∝ L2.8), close to the theoretical expectation of 3 (Thompson, 1961) (Figure 1). The allometric scaling of liver mass to body mass with an exponent of 1.25 was similar to the value of 1.23 that one obtains with the combined data from 6 different strains of M. musculus of Konarzewski and Diamond (Konarzewski and Diamond, 1995). For 16 week old males of 7 strains of M. musculus (B6D2F1, BALB/c, C3H/HeJ, C56BL/6, CBA, DBA/2, and FVB/N) the exponent for the power trend line of liver mass on body mass was 1.21 (The Jackson Laboratory, 2015). Within each of the 6 inbred strains in Konarzewski and Diamond study, the CV ranged from 0.07 to 0.10 for wet body mass and 0.08 to 0.16 for liver mass. In contrast, the corresponding CV values for the 28 uninfected P. leucopus were 0.17 for body mass and 0.23 for liver mass, evidence of broader variation in these traits among the outbred P. leucopus.
Figure 1.
Four scatter plots of the relationships between body mass and either body length (panel A), liver mass (B), heart mass (C), or spleen mass (D) of 28 uninfected male P. leucopus (experiment 1). The least-squares linear regression line, the number (n) of animals in the dataset, the coefficient of determination (R2), and the power equation for the trend line (where x is the x-axis value and y is the y-axis value) are shown.
The ratio of spleen mass to whole body mass was more variable than that observed for the liver, but the allometric exponent at 1.39 was similar (Figure 1). The ratio of heart to whole body also was more variable between animals than the liver to body ratio. Unlike the liver and spleen, which tended to be proportionately greater as body mass increased, the heart mass (Ht) of larger animals was proportionately smaller (Ht ∝ M0.57). (Another quantitative trait, bleeding time, that varied between uninfected animals is described below.)
Comparison of infected male and female animals
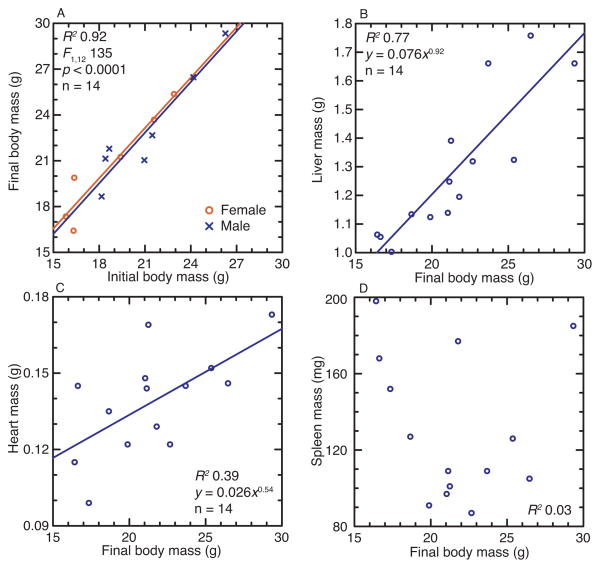
In experiment 2, fourteen animals (7 females and 7 males) were injected with 104 B. hermsii on day 0 and then euthanized on day 7. Male and female littermates were paired, and, accordingly, the mean (CI) ages in days were 211 (192–229) were the same for the two sub-groups. Although males were heavier than matched female littermates, by a mean (CI) of 18% (7–25%), before the infection, there was no discernible difference between sexes in the amount of change in mass between days 0 and 21 (Figure 2 and Table 1). Females and males were comparable in their relative spleen, liver, and heart sizes at the peak of infection. For the combined group of 14 animals, the heart mass scaled to body mass similarly to uninfected animals (Ht ∝ M0.54), but the liver masses (Lv) were proportionately smaller in the infected animals (Lv ∝ M0.92) and more variable (Figures 1 and 2). There was no association between age in days and relative spleen size (R2 = 0.03) for the 14 animals in the sample.
Figure 2.
Four scatter plots of the relationships between final (day 7) body mass and either initial (day 0) body mass (panel A), liver mass (B), heart mass (C), or spleen mass (D) of 7 female and 7 littermate-matched P. leucopus infected with B. hermsii on day 0 (experiment 2). The least-squares linear regression line, the number (n) of animals in the dataset, coefficient of determination (R2), and the power equation for the trend line (where x is the x-axis value and y is the y-axis value) are shown. In panel A the female and male animals are distinguished by symbols and colors, as shown, and by separate regression lines. In the other panels females and males are not distinguished.
Table 1.
Characteristics of Borrelia hermsii (Bh) infection of female and male Peromyscus leucopus
| Sex | Change in mass (g) (95% CI) a | % spleen mass b | % liver mass b | % heart mass b | Blood Bh genomes × 105 c | Spleen Bh genomes × 103 | FbpC EIA (OD) d |
|---|---|---|---|---|---|---|---|
| Female (n=7) | 1.91 (1.14–2.69) | 0.71 (0.47–0.95) | 6.1 (5.7–6.6) | 0.68 (0.59–0.77) | 1.10 (0.06–18.5) | 4.32 (2.26–8.26) | 0.72 (0.53–0.91) |
| Male (n=7) | 1.86 (0.91–2.81) | 0.55 (0.43–0.67) | 5.9 (5.5–6.2) | 0.63 (0.57–0.68) | 1.52 (0.11–21.0) | 7.35 (1.42–38.1) | 0.52 (0.27–0.76) |
| Paired t test p value | 0.91 | 0.14 | 0.30 | 0.39 | 0.86 | 0.60 | 0.10 |
95% confidence interval
Percentage (%) of spleen, liver, or heart mass to total body mass
Quantitative PCR for 16S rRNA gene; per μg total DNA of the extract
Enzyme immunoassay optical density (OD) at 450 nm for antibodies to FbpC protein; mean of duplicates
Littermate-matched females and males were also similar in their spirochete burdens in the blood and spleen and in their antibody responses to the FbpC protein after 7 days of infection (Table 1). By linear regression there was no discernible association at the 0.05 level of confidence of age with spirochete burdens in blood or spleen or with anti-FbpC antibody levels in this sample. The variation in spleen masses in the 14 infected animals (Figure 2) was not accounted for by spirochete burdens in the spleen, as estimated by qPCR (R2 = 0.08).
Combined infection and immunization
Two groups of 30 animals of the same sex were studied separately, but the protocol was the same: immunization with KLH and OspA on day 0, inoculation with B. hermsii on day 21, and euthanasia with blood and tissue collection on day 28. The most comprehensive studies were done on a group of 30 males that were offspring of 29 different mating pairs (experiment 3). They ranged in age from 90 to 566 d with a median of 200 and a mean of 270 d. Table 2 summarizes the values of several of the parameters representing the animals’ bodies, the burdens of spirochetes in the blood and spleen, pathology of the liver, and antibodies to proteins in response to a single immunization 28 d previously (KLH and OspA) and to the infecting pathogen after 7 d (GlpQ and FbpC). The CV of the preinfection body masses was 0.19, similar to the 0.17 observed with 28 males in the uninfected group. After 7 d of the infection there was little change in body mass for the animals, but there were greater variances in relative spleen size. The R2 for spleen mass regressed on body mass was only 0.11, similar to what was observed with 14 females and males (Figure 2).
Table 2.
Selected quantitative traits of 30 male P. leucopus in combined infection/immunization experiment
| Parameter | Median | Mean | SD a | CV b |
|---|---|---|---|---|
| Day 21 mass (g) | 22.82 | 23.32 | 4.46 | 0.19 |
| Δ mass by day 28 c | −0.03 | −0.08 | 1.11 | na d |
| Spleen mass (mg) | 86.5 | 93.7 | 36.0 | 0.38 |
| Spleen % | 0.37 | 0.41 | 0.15 | 0.38 |
| Hematocrit (%) | 44.0 | 43.7 | 4.3 | 0.10 |
| Bleeding time (min) | 3.0 | 4.2 | 3.0 | 0.71 |
| Pathology score e | 2.5 | 2.9 | 1.7 | 0.60 |
| Blood genomes f | 0.91 × 107 | 13.0 × 107 | 39.6 × 107 | 2.27 |
| Spleen genomes f | 0.22 × 105 | 4.1 × 105 | 10.2 × 105 | 2.48 |
| KLH EIA (OD450) g | 1.022 | 1.036 | 0.158 | 0.15 |
| FbpC EIA | 0.190 | 0.387 | 0.517 | 1.34 |
| GlpQ EIA | 0.242 | 0.283 | 0.115 | 0.41 |
| OspA EIA | 0.224 | 0.484 | 0.616 | 1.27 |
SD, standard deviation
CV, coefficient of variation
Change (Δ) in mass in gram (g) between day 21 and day 28
na, not applicable
Composite score of histological findings (see text); maximum score of 13
Number of B. hermsii genomes by qPCR per μg of total DNA in extracts of whole blood or spleen
Enzyme immunoassay optical density (OD) at 450 nm; mean of duplicate determinations
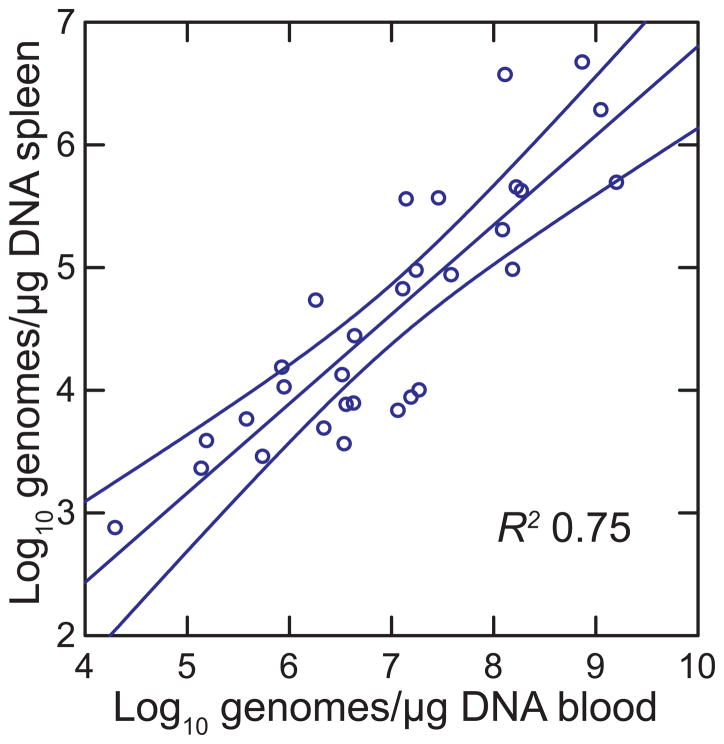
The spirochete burdens in the blood and spleen 7 days into the infection varied over several orders of magnitude between individual animals. The log-transformed values for both blood and spleen approximated normal distributions (Shapiro-Wilk W test p > 0.3). In this experiment with a larger sample size (n = 30) we noted a positive association between the log10 of the genome copies per μg of extracted spleen DNA and the relative spleen size (R2 = 0.21; p = 0.01). The burden of spirochetes in the spleen in turn plausibly was accounted for by the density of spirochetes in the blood at the time of euthanasia, according to log-log regression (p < 0.0001) (Figure 3). Although older males tended to have lower spirochete burdens than younger animals in the blood (n = 27; R2 = 0.18; p = 0.03), the variances for untransformed values were similar for the 19 animals aged 90–246 days (CV = 2.2) and the 8 animals aged 379–566 days (CV = 1.8) (F1,25 = 0.84; p = 0.36). There was no discernible association of spirochete burdens in the blood or spleen with composite liver pathology scores (R2 = 0.01), which ranged from 1 to 7, or with the most commonly noted feature, hemophagocytosis by Kupfer cells, which ranged from 1 to 3 in score.
Figure 3.
Scatter plot of log-transformed normalized B. hermsii genome copies in spleen on whole blood copies in 30 male P. leucopus at day 7 of infection (experiment 3). Spirochete burdens were estimated by species-specific quantitative PCR (qPCR). The least-squares linear regression with 95% confidence limits and coefficient of determination (R2) are shown.
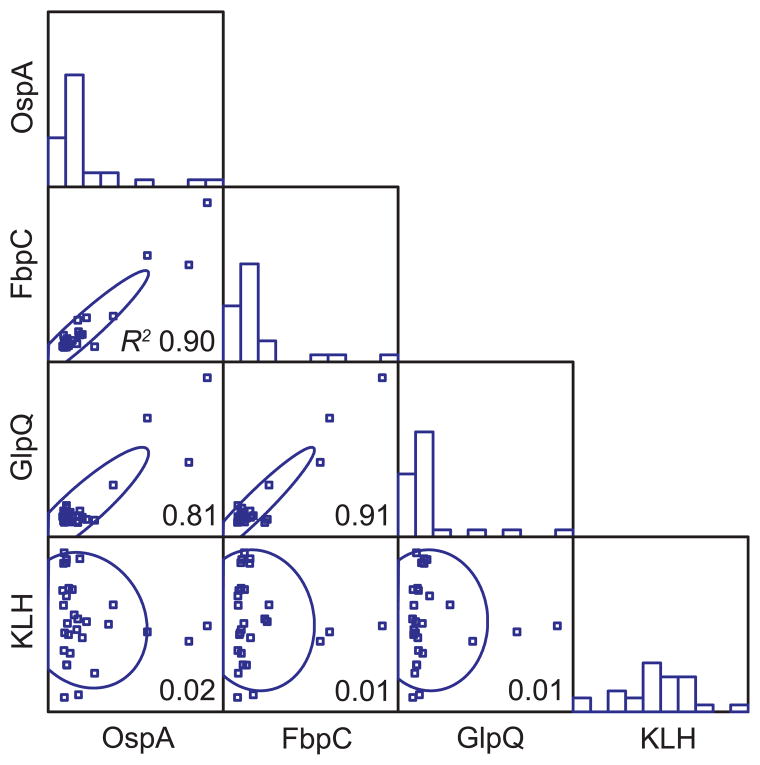
There was also variation between individual animals in their antibody responses to the infection and the immunization (Table 2 and Figure 4). While all 30 animals responded to the immunization with KLH with EIA values of 0.724 or higher and little variation between individuals, only a minority of the animals had more than moderately elevated antibodies to FbpC, GlpQ, and/or OspA. Those animals that responded to one of these three borrelial antigens tended to respond to the others as well (Figure 4). There was no discernible association between age and antibody levels to KLH, FbpC, GlpQ, or OspA (R2 ≤ 0.03). The distinctions between animals in their antibodies to FbpC and GlpQ may be accountable by differences in the dynamics of antibody formation rather than the specificity; this was only 7 days into the infection. But that would not explain a similarly wide spread of values among the mice immunized with OspA along with KLH four weeks prior.
Figure 4.
Combined frequency histograms and scatter plots of the binding of serum antibodies of 30 male P. leucopus (experiment 3) to OspA, FbpC, GlpQ, and keyhole limpet hemocyanin (KLH) proteins. The animals were immunized with OspA and KLH on day 0, infected with B. hermsii on day 21, and euthanized for blood and tissue collection on day 28. Antibody concentrations of the different specificities were determined by enzyme immunoassay (EIA) as described in text. In the histograms the x axes indicate relative EIA values of optical density at 450 nm and the y axes indicate relative counts. In the scatter plots both the x and y axes indicate the relative EIA values in a pairwise fashion. For x axes values rise from left to right, and for y axes values rise from bottom to top of the graphs.
In experiment 4, the group of 30 females were the offspring of 21 different mating pairs. They ranged in age from 143 to 378 d with a median of 273 and a mean of 265 d The 0.08 value for the R2 for spleen mass regressed on body mass was again lower in the infected animals than observed in the uninfected rodents (Figure 1). We noted once more a weak positive association between the relative spleen size and the number of B. hermsii genomes in the spleen after 7 d of infection (R2 = 0.11; p = 0.09), as well as a weak negative association between age and spirochete burdens in the blood (R2 = 0.15; p = 0.07) in the female group.
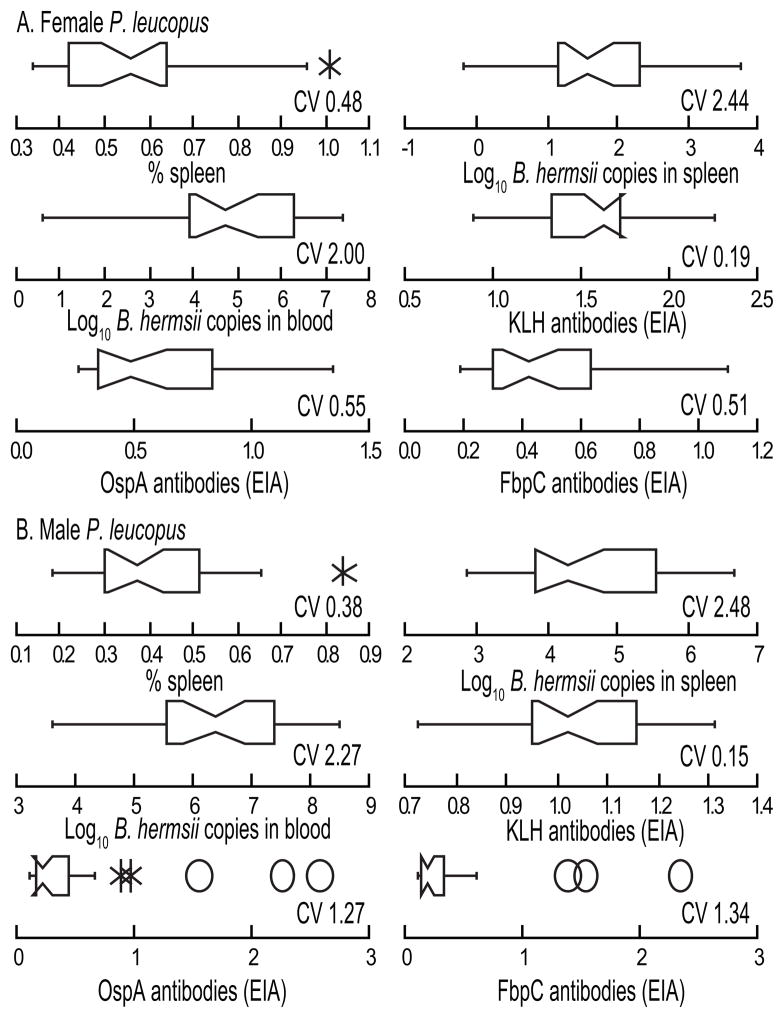
Figure 5 summarizes for the infection of 30 females (experiment 4) the findings of relative spleen size, B. hermsii genomes in spleen and blood, and EIA values for antibodies to KLH, OspA, and FbpC, and compares the values with those of the group of males in experiment 3. Overall, the females were similar to the males in the diversity of the infection phenotypes, in particular, the log normal distributions of B. hermsii in the blood and spleen. Like the males, all the females produced antibodies to KLH within a comparatively narrow range but were more varied in their antibody responses to OspA and FbpC. The distributions of antibody concentrations for these two borrelial antigens among the individuals in this group were skewed, if not to the extent of the males’ values (Figure 5).
Figure 5.
Selective characteristics of 30 female (panel A; experiment 4) and 30 male (panel B; experiment 3) P. leucopus immunized with OspA and keyhole limpet hemocyanin (KLH) and then infected with B. hermsii. The box-whisker plots are of the relative spleen size as percentage (%) of total body mass, the burden of spirochetes in the spleen and blood as determined by qPCR of genome copies of B. hermsii, and antibody levels to KLH and OspA as determined by enzyme immunoassay with readings in optical density at 450 nm. The coefficient of variation (CV) is shown for each determination. The CV for genome copies was calculated from the original and not the log-transformed values. Each horizontal box indicates the first and third quartiles, and the indentation inside the box is the median. The 1.5× interquartile range is indicated by the horizontal line (whiskers) bisecting the box, and values outside this range are indicated by asterisks and ovals.
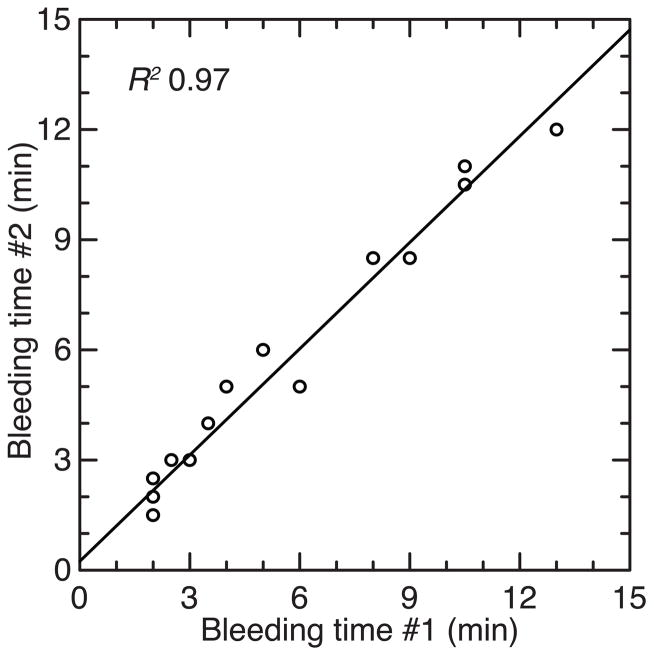
Bleeding time
In the course of taking low-volume blood samples from the animals, we noted variation between the animals in the length of time for bleeding from the clipped tail to stop. This was investigated in more depth with a standardized bleeding time assay that was repeated 4 d later for 30 male mice before infection (experiment 3). The results were reproducible (Figure 6). The averaged replicates for each of the 30 animals ranged from 1.5 to 12 min, and the CV was 0.71 (Table 2). There was little or no association (R2) between mean bleeding time and age (0.00), pre-infection body mass (0.01), hematocrit (0.00), relative spleen size (0.04), the log-transformed number of B. hermsii genomes in the blood (0.06), or the antibody response to OspA immunization (0.00).
Figure 6.
Paired bleeding times of 30 uninfected male P. leucopus (experiment 3) taken 4 days apart. The least-squares linear regression and coefficient of determination (R2) are shown.
Discussion
The experimental animals in this study were outbred rather than inbred, and presumably the sample more broadly represented the phenotypic diversity of species populations undergoing infections in nature. Though outbred rodents have long taken a back seat to inbred varieties of a species in biomedical research, the increasing application of forward genetics to non-model organisms means that experimental systems need not be limited to species that are second or third choice for an investigation. The laboratory mouse, M. musculus, in either inbred or outbred versions, has been elucidative for pathogenesis studies of Borrelia infections but is less relevant for the ecology of these infectious diseases, for modeling pathogen-reservoir-vector interactions and dynamics, and for measures to control these diseases at their sources, such as by targeting vaccines or other interventions to the reservoirs themselves. Needless to say, the outputs from forward genetics or mathematical modeling are only as good as the informativeness and replicability of the phenotypes to be scored in screens or incorporated for simulations.
Further assessment of the diversity of responses to infection and immunity of P. leucopus was the study’s principal aim. There have been previous studies of experimental infections of Peromyscus species, including hantaviruses in P. maniculatus (Botten et al., 2000) and several of B. burgdorferi in P. leucopus (reviewed in Barthold et al., 2010). However, in these studies either the data on individual animals were largely qualitative or the sample sizes were small. One exception was our earlier P. leucopus study, in which we noted a wide range of host responses among individuals animals infected with B. burgdorferi, but the emphasis in that study was on differences between strains of B. burgdorferi in the infections they caused in the animals (Baum et al., 2012). For the descriptive study presented here, the primary end-points instead were quantitative assessments of diversity within groups of up to 30 animals under the same experimental conditions, and not a comparison of treated with untreated animals per se.
To a large degree the study succeeded, as discussed below. Nevertheless, there were limitations to the study. The subjects differed from animals captured in the field in being detectably free of other infectious diseases and parasites, in the predictability and adequateness of their nutrition (Martin et al., 2007a), and their habitat’s controlled, unvarying conditions (Martin et al., 2008). The animals were infected by needle inoculation and not by tick bite, although this may not be an important distinction for experimental B. hermsii infections (Policastro et al., 2013). Though more than a hundred animals were studied in total, these were divided for the experiments in groups of 30 or less. Consequently, the study may have been underpowered for detecting some true differences between uninfected and infected or between males and females. Though we preferred to exclude siblings in an experimental group, the exigencies of breeding schedules and availability contingencies meant that a small number of siblings (but not clones) were represented in some experiments (and were specifically requested for the mixed sex experiment). Finally, although P. leucopus can be infected with B. hermsii by needle inoculation (Baum et al., 2012), it is not the usual reservoir for this species. B. burgdorferi would be suitable, but we reasoned that for a first investigation a pathogen that more predictably produced higher blood and organ burdens in this species could provide a greater potential range of host responses for assessments of phenotype diversity. P. leucopus can be infected with B. miyamotoi (Scoles et al., 2001), and thus this species would be another choice.
The principal findings of the four experiments were the following:
Values for burdens of spirochetes in the blood or spleen, as measured by qPCR with normalization for total DNA in reactions, fit a log-normal distribution with a wide divergence among individual animals (Figure 5), similarly to what was observed in infected wild animals that were captured (Barbour et al., 2009). Spirochete DNA in the spleen closely tracked burdens in the blood at the same time. Residual blood accounts for some or much of the genome target that was measured in the spleen, while additional DNA may represent live or dead sequestered bacterial cells or their breakdown in the spleen. This was a narrow time window for sampling an infection, and delaying the collection a day or two later may have yielded different results, especially if immune responses are building. Notwithstanding this limitation, the employed protocol yielded similar results upon replication. The broad range of burdens in blood and spleen among individuals, first observed in the group of males, was also noted in the study of the female group. While the apparent effect of age on spirochete burdens was modest, it is a parameter to be controlled for in future studies. Among the several testable hypotheses to account for the variances of pathogen burdens are individual differences in a rate-limiting step in the adaptive immune response or innate immunity signaling pathways.
Among uninfected animals, spleen mass scaled allometrically to body mass and similarly to liver mass scaling, but there was comparatively greater variation in relative size of spleen than of the liver. The variation of relative spleen size was greater than what had been observed with M. musculus across a collection of inbred strains. After one week of infection there was further divergence in this characteristic between individual P. leucopus to the point of little or no discernible association of spleen mass with terminal body mass. Pre-infection spleen mass plausibly was an independent determinant of mid-infection spleen mass. But the remaining variance appears to be only partially attributable to the burden of live (or dead) spirochetes in the spleen. Change in relative spleen size during infection may then be an informative quantitative trait that is not solely dependent on pathogen burden. Given the important role of the spleen in local and systemic immunity (Bronte and Pittet, 2013), including for Borrelia infections (Meleney, 1928; Smith et al., 1985), this phenomenon may not be trivial.
All animals in two different experiments of 30 subjects had moderate to high levels of antibodies to the reference antigen KLH four weeks after single dose immunization. The antibody responses were age-independent and were similar in magnitude to what Martin et al. (2007b) observed in P. leucopus. In contrast, only a minority of animals in either of the two groups had comparable EIA values for antibodies to the OspA protein, which had been administered with the KLH. The doses of OspA were equivalent on weight basis to what had elicited strong antibody responses in two inbred strains of M. musculus injected with the same preparation of OspA (Pal et al., 2003). Higher variances for anti-OspA antibody levels than for anti-KLH antibodies across a panel were also noted among 30 females in a separate experiment. The same animals that had high antibody levels to OspA also had higher than the mean levels of antibodies to two other borrelial proteins, FbpC and, to a lesser extent, GlpQ. These antibodies developed in response to an acute infection and not immunization. Like OspA, FbpC is a lipoprotein, so an explanation for co-variation in antibody levels to these proteins might lie in signalling pathways for innate responses to bacterial lipoproteins in general (Salazar et al., 2009). Whatever the explanation, this heterogeneity of response in P. leucopus, the main candidate for OspA-based, transmission-blocking vaccines (Meirelles Richer et al., 2011; Tsao et al., 2004), may have implications for further development and implementation of this reservoir-targeting immunization project.
A serendipitous finding among uninfected animals was reproducible differences over a 10-fold range in the time for hemostasis after the tail was clipped (Figure 6 and Table 2). The technique that was used corresponds to the Duke method for the bleeding time test for defects in hemostasis of humans (Harker and Slichter, 1972). The test is mainly used for detecting evidence of platelet dysfunction, but the time can also be prolonged when platelet concentrations in the blood are abnormally low. Platelet counts in 40 P. leucopus from the PGSC had a mean of 394,000 per μl with a SD of 139,000 and CV of 0.35 (Wiedmeyer et al., 2014). While this is within the range of normal platelet counts for humans, it is about half of what is reported for M. musculus (Sun et al., 2014). P. leucopus and P. maniculatus have also been noted to have unusual timing of the coagulation of their blood, which is a phenomenon that is distinguished from the bleeding time assay used here (Folk et al., 2010). The cause of the varied bleeding times in P. leucopus remains to be determined. But the observed variation conceivably has consequences for tick feeding and the duration of blood meals, given the importance of hemostasis for the embedded tick (Sonenshine and Anderson, 2014).
Conclusions
P. leucopus, as represented by these samplings of an outbred but colony-bred population, manifested considerable diversity in phenotype among uninfected animals and to the same if not greater extent among animals in their responses to infection and immunization. The natural variation observed in animals in a stable and protected environment would be the foundation upon which several different environmental effects, such as other infectious diseases, predators, and climate, would build under natural conditions. Attempts to model the ecology of tick-borne zoonoses should incorporate estimates of variance among these important reservoir hosts for inferences to hold true under field validation. Reservoir-targeted vaccination programs in their planning cannot assume that individual animals will respond in an “average” way. Finally, the findings encourage further development of Peromyscus as a model organism that is particularly relevant for ecological and population genetics studies in North America. Of particular interest as quantitative traits of this reservoir host are relative spleen size, spirochete burdens, and antibody responses to certain lipoproteins.
Acknowledgments
The research was supported by National Institutes of Health grant AI-065359. We thank Janet Crossland, Michael Felder, and Paul Vrana of the Peromyscus Genetic Stock Center for advice and discussions, and Arash Ghalyanchi Langeroudi, Fong Hue, and Azadeh Shojaee Estabragh for their early contributions to the study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amman BR, Manangan AP, Flietstra TD, Calisher CH, Carroll DS, Wagoner KD, Mills JN. Association between movement and Sin Nombre virus (Bunyaviridae: Hantavirus) infection in North American deermice (Peromyscus maniculatus) in Colorado. J Wildl Dis. 2013;49:132–142. doi: 10.7589/2012-02-041. [DOI] [PubMed] [Google Scholar]
- Baker RH. Habitats and distribution. In: King JA, editor. Biology of Peromyscus (Rodentia) American Society of Mammalogists; Stillwater, OK: 1968. [Google Scholar]
- Barbour AG. Isolation and cultivation of Lyme disease spirochetes. Yale J Biol Med. 1984;57:521–525. [PMC free article] [PubMed] [Google Scholar]
- Barbour AG, Bundoc V. In vitro and in vivo neutralization of the relapsing fever agent Borrelia hermsii with serotype-specific immunoglobulin M antibodies. Infect Immun. 2001;69:1009–1015. doi: 10.1128/IAI.69.2.1009-1015.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour AG, Bunikis J, Travinsky B, Hoen AG, Diuk-Wasser MA, Fish D, Tsao JI. Niche partitioning of Borrelia burgdorferi and Borrelia miyamotoi in the same tick vector and mammalian reservoir species. Am J Trop Med Hyg. 2009;81:1120–1131. doi: 10.4269/ajtmh.2009.09-0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour AG, Travinsky B. Evolution and distribution of the ospC gene, a transferable serotype determinant of Borrelia burgdorferi. MBio. 2010;1:e00153–10. doi: 10.1128/mBio.00153-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthold SW, Cadavid D, Phillip MT. Animal models of borreliosis. In: Radolf JD, Samuels DS, editors. Borrelia: Molecular Biology, Host Interaction, and Pathogenesis. Caister Academic Press; Norfolk, UK: 2010. pp. 359–412. [Google Scholar]
- Baum E, Hue F, Barbour AG. Experimental infections of the reservoir species Peromyscus leucopus with diverse strains of Borrelia burgdorferi, a Lyme disease agent. MBio. 2012;3:e00434–00412. doi: 10.1128/mBio.00434-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya D, Bensaci M, Luker KE, Luker G, Wisdom S, Telford SR, Hu LT. Development of a baited oral vaccine for use in reservoir-targeted strategies against Lyme disease. Vaccine. 2011;29:7818–7825. doi: 10.1016/j.vaccine.2011.07.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botten J, Mirowsky K, Kusewitt D, Bharadwaj M, Yee J, Ricci R, Feddersen RM, Hjelle B. Experimental infection model for Sin Nombre hantavirus in the deer mouse (Peromyscus maniculatus) Proc Natl Acad Sci U S A. 2000;97:10578–10583. doi: 10.1073/pnas.180197197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisson D, Dykhuizen DE, Ostfeld RS. Conspicuous impacts of inconspicuous hosts on the Lyme disease epidemic. Proc Biol Sci. 2008;275:227–235. doi: 10.1098/rspb.2007.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronte V, Pittet MJ. The spleen in local and systemic regulation of immunity. Immunity. 2013;39:806–818. doi: 10.1016/j.immuni.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control. Tickborne Diseases of the United States: A Reference Manual for Health Care Professionals. 2. U.S. Department of Health and Human Services; 2014. http://www.cdc.gov/lyme/resources/TickborneDiseases.pdf. [Google Scholar]
- Conlon JA, Mather TN, Tanner P, Gallo G, Jacobson RH. Efficacy of a nonadjuvanted, outer surface protein A, recombinant vaccine in dogs after challenge by ticks naturally infected with Borrelia burgdorferi. Vet Therapeutics. 2000;1:96–107. [PubMed] [Google Scholar]
- Crossland JP, Dewey MJ, Barlow SC, Vrana PB, Felder MR, Szalai GJ. Caring for Peromyscus spp. in research environments. Lab Animal. 2014;43:162–166. doi: 10.1038/laban.504. [DOI] [PubMed] [Google Scholar]
- Dai Q, Restrepo BI, Porcella SF, Raffel SJ, Schwan TG, Barbour AG. Antigenic variation by Borrelia hermsii occurs through recombination between extragenic repetitive elements on linear plasmids. Mol Microbiol. 2006;60:1329–1343. doi: 10.1111/j.1365-2958.2006.05177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey MJ, Dawson WD. Deer mice: “The Drosophila of North American mammalogy”. Genesis. 2001;29:105–109. doi: 10.1002/gene.1011. [DOI] [PubMed] [Google Scholar]
- Ebel GD, Campbell EN, Goethert HK, Spielman A, Telford SR., 3rd Enzootic transmission of deer tick virus in New England and Wisconsin sites. Am J Trop Med Hyg. 2000;63:36–42. doi: 10.4269/ajtmh.2000.63.36. [DOI] [PubMed] [Google Scholar]
- Folk GE, Dickson EW, Lynch RG, Thrift DL. An unusual timing of blood coagulation in deer mice (Peromyscus leucopus) Biological Rhythm Res. 2010;41:1–6. [Google Scholar]
- Hall ER. Mammals of North America. John Wiley and Sons; New York, NY: 1979. [Google Scholar]
- Harker LA, Slichter SJ. The bleeding time as a screening test for evaluation of platelet function. New Engl J Med. 1972;287:155–159. doi: 10.1056/NEJM197207272870401. [DOI] [PubMed] [Google Scholar]
- Johnson RC, Marek N, Kodner C. Infection of Syrian hamsters with Lyme disease spirochetes. J Clin Microbiol. 1984;20:1099–1101. doi: 10.1128/jcm.20.6.1099-1101.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyner CP, Myrick LC, Crossland JP, Dawson WD. Deer mice as laboratory animals. ILAR. 1998;39:322–330. doi: 10.1093/ilar.39.4.322. [DOI] [PubMed] [Google Scholar]
- Kenney-Hunt J, Lewandowski A, Glenn TC, Glenn JL, Tsyusko OV, O’Neill RJ, Brown J, Ramsdell CM, Nguyen Q, Phan T, Shorter KR, Dewey MJ, Szalai G, Vrana PB, Felder MR. A genetic map of Peromyscus with chromosomal assignment of linkage groups (a Peromyscus genetic map) Mamm Genome. 2014;25:160–179. doi: 10.1007/s00335-014-9500-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkland GL, Layne JN. Advances in the Study of Peromyscus (Rodentia) Texas Tech University Press; Lubbock, TX: 1989. [Google Scholar]
- Konarzewski M, Diamond J. Evolution of basal metabolic rate and organ masses in laboratory mice. Evolution. 1995:1239–1248. doi: 10.1111/j.1558-5646.1995.tb04450.x. [DOI] [PubMed] [Google Scholar]
- Krause PJ, Narasimhan S, Wormser GP, Barbour AG, Platonov AE, Brancato J, Lepore T, Dardick K, Mamula M, Rollend L, Steeves TK, Diuk-Wasser M, Usmani-Brown S, Williamson P, Sarksyan DS, Fikrig E, Fish D. Borrelia miyamotoi sensu lato seroreactivity and seroprevalence in the northeastern United States. Emerg Infect Dis. 2014;20:1183–1190. doi: 10.3201/eid2007.131587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine JF, Wilson ML, Spielman A. Mice as reservoirs of the Lyme disease spirochete. Am J Trop Med Hyg. 1985;34:355–360. doi: 10.4269/ajtmh.1985.34.355. [DOI] [PubMed] [Google Scholar]
- Lewis ER, Marcsisin RA, Campeau Miller SA, Hue F, Phillips A, Aucoin DP, Barbour AG. Fibronectin-binding protein of Borrelia hermsii expressed in the blood of mice with relapsing fever. Infect Immun. 2014;82:2520–2531. doi: 10.1128/IAI.01582-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LB, 2nd, Navara KJ, Weil ZM, Nelson RJ. Immunological memory is compromised by food restriction in deer mice Peromyscus maniculatus. Am J Physiol Regulatory, integrative and comparative physiology. 2007a;292:R316–320. doi: 10.1152/ajpregu.00386.2006. [DOI] [PubMed] [Google Scholar]
- Martin LB, 2nd, Weil ZM, Nelson RJ. Immune defense and reproductive pace of life in Peromyscus mice. Ecology. 2007b;88:2516–2528. doi: 10.1890/07-0060.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LB, Weil ZF, Nelson RJ. Seasonal changes in vertebrate immune activity: mediation by physiological trade-offs. Philos Trans R Soc Lond B Biol Sci. 2008;363:321–329. doi: 10.1098/rstb.2007.2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meirelles Richer L, Aroso M, Contente-Cuomo T, Ivanova L, Gomes-Solecki M. Reservoir targeted vaccine for Lyme borreliosis induces a yearlong, neutralizing antibody response to OspA in white-footed mice. Clin Vaccine Immunol. 2011;18:1809–1816. doi: 10.1128/CVI.05226-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meleney HE. Relapse phenomena of Spironema recurrentis. J Exp Med. 1928;48:65–82. doi: 10.1084/jem.48.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal S, Luke CJ, Barbour AG, Peterson E, de la Maza LM. Immunization with the Chlamydia trachomatis major outer membrane protein, using the outer surface protein A of Borrelia burgdorferi as an adjuvant, can induce protection against a chlamydial genital challenge. Vaccine. 2003;21:1455–1465. doi: 10.1016/s0264-410x(02)00680-1. [DOI] [PubMed] [Google Scholar]
- Peromyscus Genetic Stock Center. Peromyscus Genetic Stock Center. University of South Carolina; Coumbia, SC: 2015. http://stkctr.biol.sc.edu. [Google Scholar]
- Piesman J, Schwan TG. Ecology of borreliae and their arthropod vectors. In: Samuels DS, Radolf JD, editors. Borrelia. Molecular Biology, Host Interaction, and Pathogensis. Caister Academic Press; Norfolk, UK: 2010. pp. 251–278. [Google Scholar]
- Policastro PF, Raffel SJ, Schwan TG. Borrelia hermsii acquisition order in superinfected ticks determines transmission efficiency. Infect Immun. 2013;81:2899–2908. doi: 10.1128/IAI.00542-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar JC, Duhnam-Ems S, La Vake C, Cruz AR, Moore MW, Caimano MJ, Velez-Climent L, Shupe J, Krueger W, Radolf JD. Activation of human monocytes by live Borrelia burgdorferi generates TLR2-dependent and -independent responses which include induction of IFN-beta. PLoS Pathogens. 2009;5:e1000444. doi: 10.1371/journal.ppat.1000444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwan TG, Schrumpf ME, Hinnebusch BJ, Anderson DE, Jr, Konkel ME. GlpQ: an antigen for serological discrimination between relapsing fever and Lyme borreliosis. J Clin Microbiol. 1996;34:2483–2492. doi: 10.1128/jcm.34.10.2483-2492.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scoles GA, Papero M, Beati L, Fish D. A relapsing fever group spirochete transmitted by Ixodes scapularis ticks. Vector Borne Zoonotic Dis. 2001;1:21–34. doi: 10.1089/153036601750137624. [DOI] [PubMed] [Google Scholar]
- Smith RD, Miranpuri GS, Adams JH, Ahrens EH. Borrelia theileri: isolation from ticks (Boophilus microplus) and tick-borne transmission between splenectomized calves. Am J Vet Res. 1985;46:1396–1398. [PubMed] [Google Scholar]
- Sonenshine DE, Anderson JM. Mouthparts and digestive system: anatomy and molecular biology of feeding and digestion. In: Sonenshine DE, Roe RM, editors. Biology of Ticks. Oxford University Press; New York, N.Y: 2014. [Google Scholar]
- Steppan S, Adkins R, Anderson J. Phylogeny and divergence-date estimates of rapid radiations in muroid rodents based on multiple nuclear genes. Syst Biol. 2004;53:533–553. doi: 10.1080/10635150490468701. [DOI] [PubMed] [Google Scholar]
- Sun Y, Desierto MJ, Ueda Y, Kajigaya S, Chen J, Young NS. Peromyscus leucopus mice: a potential animal model for haematological studies. International J Exp Pathol. 2014;95:342–350. doi: 10.1111/iep.12091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telford SR, 3rd, Dawson JE, Katavolos P, Warner CK, Kolbert CP, Persing DH. Perpetuation of the agent of human granulocytic ehrlichiosis in a deer tick-rodent cycle. Proc Natl Acad Sci U S A. 1996;93:6209–6214. doi: 10.1073/pnas.93.12.6209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telford SR, 3rd, Spielman A. Reservoir competence of white-footed mice for Babesia microti. J Med Entomol. 1993;30:223–227. doi: 10.1093/jmedent/30.1.223. [DOI] [PubMed] [Google Scholar]
- The Jackson Laboratory. Morphometric (organ weight) survey of 11 inbred strains of mice. The Jackson Laboratory; 2015. http://phenome.jax.org. [Google Scholar]
- Thompson D. On Growth and Form. Cambridge University Press; London: 1961. Abridged ed. [Google Scholar]
- Tsao JI, Wootton JT, Bunikis J, Luna MG, Fish D, Barbour AG. An ecological approach to preventing human infection: vaccinating wild mouse reservoirs intervenes in the Lyme disease cycle. Proc Natl Acad Sci U S A. 2004;101:18159–18164. doi: 10.1073/pnas.0405763102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedmeyer CE, Crossland JP, Veres M, Dewey MJ, Felder MR, Barlow SC, Vrana PB, Szalai G. Hematologic and serum biochemical values of 4 species of Peromyscus mice and their hybrids. J Am Assoc Lab Animal Sci. 2014;53:336–343. [PMC free article] [PubMed] [Google Scholar]
- Worley KC. Peromyscus Genome Project of the Human Genome Sequencing Center. Baylor College of Medicine; 2015. http://www.hgsc.bcm.edu/other-mammals/peromyscus-genome-project. [Google Scholar]