The assessment critically ill patients’ cardiovascular state through hemodynamic monitoring is essential to define both stability and change. But monitoring can be improved by maneuvers designed to stress the cardiovascular state. For example, gallop rhythms heard increasing with spontaneous inspiration or associated paradoxical septal shift by echocardiography connote right heart failure. Similarly, tachycardia and near syncope upon sitting up from a supine position connotes hypovolemia. These maneuvers reflect functional bedside tests of the patient’s physiologic reserve. Functional Hemodynamic Monitoring (FHM) is the process of assessing the dynamic response of a measured hemodynamic variable to a defined, reproducible and readily reversible extrinsic stress (1). FHM parameters is commonly used to predict cardiac output responses to volume loading (2, 3), although its applications are broader.
Since a primary cardiovascular management decision in shock is whether or not to give intravascular fluids to increase blood flow (3), knowing if a patient is volume responsive before giving fluids will both prevent excess over hydration of non-responsive patients and aid in monitoring the response to fluid resuscitation in responsive ones. Unfortunately, static hemodynamic measures of ventricular preload poorly predict volume responsiveness (3). The reasons why are due to inherit cardiac responses to changes in loading. Beat-to-beat changes in ventricular end-diastolic volume induce proportional changes in contractility owing to dynamic end-diastolic sarcomere length changes altering intracellular calcium sensitivity (4). Such matching of dynamic changes in ventricular end-diastolic volume and contractility are essential to match the varying outputs of the two ventricles to each other over short time transients. This is referred to as heterometric regulation or Starling’s Law of the Heart. However, over minutes intrinsic myocardial contractility also changes to meet these changing demands causing this relationship to dissolve because under increased loads, steady state cardiac muscle calcium transients up-regulate (5). This steady state change in contractility is referred as intrinsic autoregulation or the Anrep effect. Thus, steady state measures of preload poorly predict volume responsiveness, whereas dynamic ones predict it very well.
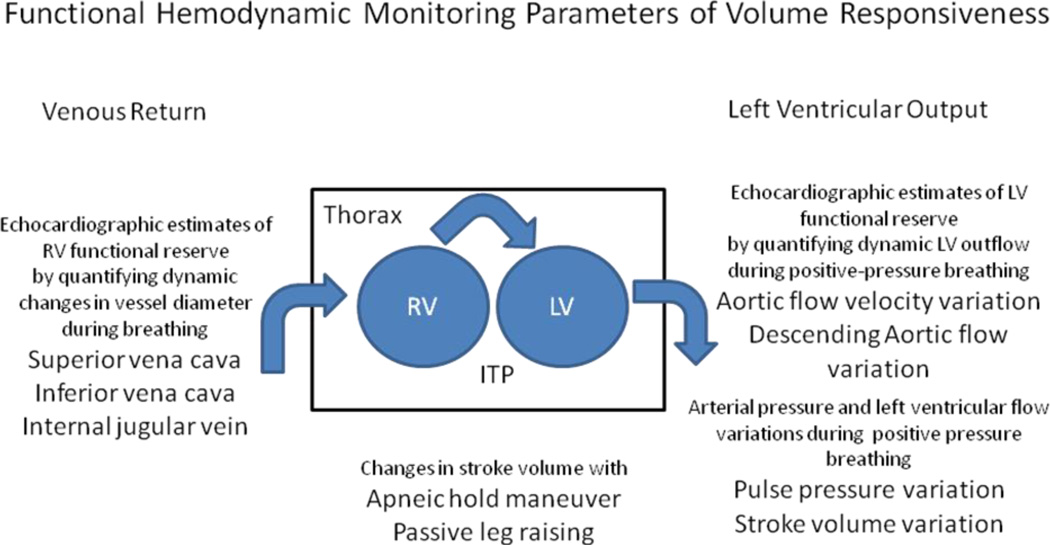
Many FHM approaches take advantage of these dynamic transients to measure either the capacity of the ventricles to fill as the pressure gradient for ventricular filling changes or for the ventricles to proportionally eject this varying amount of volume (6). Since the circulation has two pumps that work both in parallel (ventricular interdependence) and in series, one can assess either right or left-sided function to assess overall cardiovascular reserve. Both spontaneous and positive-pressure breathing, by altering the pressure gradients for venous return to the right ventricle can be used to assess both right and left ventricular preload reserve (7)(Fig. 1). Both right and left ventricular preload reserve need to be present for these dynamic hemodynamic changes to exist. If either ventricle is in failure the dynamic response to venous pressure changes will not alter flow out of either ventricle. Dynamic venous flow changes during spontaneous and positive-pressure ventilation track the right ventricle’s ability to handle the changing volume loads induced by these transient increases in the driving pressure for venous return (8). Thus, dynamic changes in inferior vena caval (9), superior vena caval (10) and internal jugular venous diameters (11), as surrogate for the ability of the right ventricle to accept changing inflows without over-distending measures the adaptability of the right side of the circulation. Threshold values above 10–15% change in diameter exist in volume responsiveness subjects. These analyses can be easily taught and performed but cannot be assessed continuously.
Figure 1.
Schematic readouts of functional measures that purport to assess volume responsiveness
Although interest in left ventricular stroke volume variation (SVV) and arterial pulse pressure variation (PPV), as continuous markers of volume responsiveness, have emerged as functional hemodynamic parameters (2), they are limited in their application to those subjects on positive-pressure ventilation and without severe cor pulmonale or intra-abdominal hypertension (Table 1). They remain valuable if high variability if observed in low tidal volume ventilation (12). Still, during the early phases of resuscitation from severe circulatory shock and in most intra-operative surgical patients, these measures remain important continuous parameters of volume responsiveness. Furthermore, PPV and SVV can also be estimated using many techniques, including ultrasound measures of aortic outflow or descending aortic flow (13) and pulse oximter pleth variability (14). Still, if these dynamic parameters are at the lower threshold of prediction, the so-called “grey zone,” other maneuvers, like small volume fluid challenges or passive leg raising (PLR) maneuvers (infra vide), may need to define volume responsiveness.
Table 1.
Limitations to the Use of Functional Hemodynamic Monitoring Parameters to Predict Volume Responsiveness
|
Because both SVV and PPV sensitivity degrade during spontaneous ventilation, cor pulmonale, high levels of positive end-expiratory airway pressure and low tidal volume ventilation (12), alterative tests have been proposed. Specifically, performing PLR and monitoring transient changes in cardiac output is very sensitive and specific predictor of volume responsiveness under most conditions (15). It becomes inaccurate when intra-abdominal hypertension exists (16). Still, these parameters reflect discrete discontinuous measures.
At the end of the day, we are left with certain clinical realities. First, no monitoring device, no matter how insightful its data or displays will improve patient outcome unless coupled to a treatment, which itself improves outcome (1,3). Resuscitation efforts will only be beneficial if viable tissue is at risk for ischemic dysfunction or post-insult inflammation are salvaged by that resuscitation effort. Second, not all patients who are volume responsive need fluid resuscitation and those that do need fluid resuscitation may not need it up until they are no longer volume responsive. The goals of resuscitation need to be defined based on quantifiable targets of tissue perfusion, organ function and overall host viability, not on fixed values of oxygen delivery or arterial pressure. Third, we rarely know the right combination of therapies needed in most complex patients presenting with cardiovascular insufficiency, so to treat all patients the same volume/pressor/inotrope approach without regard to their individual responses and initial functional status and co-morbidities is to do many of our patients a disservice.
Acknowledgments
This work supported in part by NIH grants HL007820, HL067181 and HL074316
Footnotes
Potential conflicts of interest for Michael R. Pinsky, MD:
Inventor of a University of Pittsburgh US patent No. 6,776,764 “Use of aortic pulse pressure and flow in bedside hemodynamic management.”
Is or was a consultant to Cheetah Medical, Edwards LifeSciences, LiDCO Ltd, Masimo Inc, and Pulsion Ltd.
Has stock options with Cheetah Medicinal Inc. and LiDCO Ltd.
Has received honoraria for lectures from Masimo Inc.
Is the recipient of a grant funded by Edwards LifeSciences, Inc. to the University of Pittsburgh
References
- 1.Pinsky MR, Payen D. Functional hemodynamic monitoring. Crit Care. 2005;9:566–572. doi: 10.1186/cc3927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Michard F, Boussat S, Chemla D, Anguel N, Mercat A, Lecarpentier Y, Richard C, Pinsky MR, Teboul J-L. Relation between respiratory changes in arterial pulse pressure and fluid responsiveness in septic patients with acute circulatory failure. Am J Respir Crit Care Med. 2000;162:134–138. doi: 10.1164/ajrccm.162.1.9903035. [DOI] [PubMed] [Google Scholar]
- 3.Cecconi M, De Backer D, Antonelli M, Beale R, Bakker J, Hofer C, Jaesche R, Mebazaa A, Pinsky MR, Teboul JL, Vincent JL, Rhodes A. Consensus on Circulatory Shock and Hemodynamic Monitoring, Task Force of the European Society of Intensive Care Medicine. Intensive Care Med. 2014;49:1795–1815. doi: 10.1007/s00134-014-3525-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fuchs F, Wang Y. Sarcomere length versus interfilament spacing as determinants of cardiac myofilament Ca2+ sensitivity and Ca2+ binding. J Mol Cell Cardiol. 1996;28:1375–1383. doi: 10.1006/jmcc.1996.0129. [DOI] [PubMed] [Google Scholar]
- 5.Alverez BV, Perez NG, Ennis IL, Camilion de Hurtado MC, Cingolani HE. Mechanisms underlying the increase in force and Ca2+ transient that follow stretch of cardiac muscle: a possible explanation of the Anrep effect. Circ Res. 1999;85:716–722. doi: 10.1161/01.res.85.8.716. [DOI] [PubMed] [Google Scholar]
- 6.Perner A, De Backer D. Understanding hypovolaemia. Intensive care med. 2014;40:613–615. doi: 10.1007/s00134-014-3223-x. [DOI] [PubMed] [Google Scholar]
- 7.Pinsky MR. The hemodynamic consequences of mechanical ventilation: An evolving story. Intensive Care Med. 1997;23:493–503. doi: 10.1007/s001340050364. [DOI] [PubMed] [Google Scholar]
- 8.Pinsky MR. Determinants of pulmonary artery flow variation during respiration. J Appl Physiol. 1984;56:1237–1245. doi: 10.1152/jappl.1984.56.5.1237. [DOI] [PubMed] [Google Scholar]
- 9.Feissel M, Michard F, Faller J, Teboul JL. The respiratory variation in inferior vena cava diameter as a guide to fluid therapy. Intensive Care Med. 2004;30:1834–1837. doi: 10.1007/s00134-004-2233-5. [DOI] [PubMed] [Google Scholar]
- 10.Vieillard-Baron A, Chergui K, Rabiller A, Peyrouset O, Page B, Beauchet A, Jardin Superior vena cava collapsibility as a gauge of volume status in ventilated septic patients. Intensive Care Med. 2004;30:1734–1739. doi: 10.1007/s00134-004-2361-y. [DOI] [PubMed] [Google Scholar]
- 11.Guarracino F, Ferro B, Forfori F, Bertini P, Magliacane L, Pinsky MR. Jugular Vein Distensibility predicts fluid responsiveness in septic patients. Crit Care. 0214;18:647. doi: 10.1186/s13054-014-0647-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Backer D, Heenen S, Piagnerelli M, Koch M, Vincent JL. Pulse pressure variations to predict fluid responsiveness: influence of tidal volume. Intensive Care Med. 2005;31:517–523. doi: 10.1007/s00134-005-2586-4. [DOI] [PubMed] [Google Scholar]
- 13.Monnet X, Rienzo M, Osman D, Anguel N, Richard C, Pinsky MR, Teboul JL. Esophageal Doppler monitoring predicts fluid responsiveness in critically ill ventilated patients. Intensive Care Med. 2005;31:1195–1201. doi: 10.1007/s00134-005-2731-0. [DOI] [PubMed] [Google Scholar]
- 14.Sandroni C, Cavallaro F, Marano C, Falcone C, De Santis P, Antonelli M. Accuracy of plethysmographic indices as predictors of fluid responsiveness in mechanically ventilated adults: a systematic review and meta-analysis. Intensive Care Med. 2012;38:1429–1437. doi: 10.1007/s00134-012-2621-1. [DOI] [PubMed] [Google Scholar]
- 15.Monnet X, Rienzo M, Osman D, Anguel N, Richard C, Pinsky MR, Teboul JL. Passive leg raising predicts fluid responsiveness in the critically ill. Crit Care Med. 2006;34:1402–1407. doi: 10.1097/01.CCM.0000215453.11735.06. [DOI] [PubMed] [Google Scholar]
- 16.Mahjoub Y, Touzeau J, Airapetian N, Lorne E, Hijazi M, Zogheib E, Tinturer F, Slama M, Dupont H. The passive leg-raising maneuver cannot accurately predict fluid responsiveness in patients with intra-abdominal hypertension. Crit Care Med. 2010;38:1824–1829. doi: 10.1097/CCM.0b013e3181eb3c21. [DOI] [PubMed] [Google Scholar]