Abstract
This study examines the effects of fetal exposure to a synthetic stress hormone (synthetic glucocorticoids) on children’s susceptibility to postnatal sociodemographic adversity. We recruited two groups of children who were born healthy and at term. Twenty-six had been treated with steroid hormones (glucocorticoids) during the prenatal period (treatment group); eighty-five did not receive any treatment (comparison group). Only children exposed to both prenatal stress hormones and sociodemographic adversity showed impaired performance on standardized tests of memory function. The association was specific to long-term memory. General intellectual functioning and expressive language were not affected by fetal glucocorticoid exposure. Results were independent of maternal intelligence and concurrent maternal depression. These findings are consistent with a vulnerability-stress model. Prenatal exposure to synthetic stress hormones is associated with increased susceptibility to subsequent adversity with consequences for cognitive functioning that persist 6 – 10 years after birth.
Keywords: betamethasone, glucocorticoids, cortisol, prenatal, cognition, memory, stress
Introduction
Human life advances from a single-cell zygote to a fully formed infant capable of living outside of the womb. The period from conception through birth is a time of remarkable development, and one that is especially vulnerable to environmental exposures. The fetus has enormous capacity to adapt to changes in the prenatal environment, however compelling data from both animal (Weinstock, 2008) and human studies (Sandman & Davis, 2010) show that this early plasticity has long-term consequences for health and development. Here we report that human fetal exposure to a stress hormone (synthetic glucocorticoids) increases the vulnerability to subsequent stress (sociodemographic adversity). These exposures have implications for cognitive functioning that persist through middle childhood. The present study is a unique test of prevailing models of the developmental origins of mental health and considers the synergistic roles of the prenatal and postnatal environments in shaping child neurodevelopment.
The influential developmental-origins-of disease model also known as the fetal programming hypothesis is the prevailing framework for understanding the earliest origins of health and disease in human development. The model proposes that exposure to stressors during the fetal period increases subsequent risk for both physical (cardiovascular disease, hypertension, non-insulin-dependent diabetes mellitus, obesity, and asthma) (Barker, 1998) and mental health outcomes (cognitive functioning and psychiatric illness) (Davis & Sandman, 2012). Until recently, empirical support for the fetal programming model came from retrospective research that examined the relation between birth phenotype (e.g., length of gestation, birth weight) and later health outcomes. However, a growing literature based on prospective research has reported links between fetal stress and stress hormone exposures and a range of infant and child outcomes, providing further support for the fetal programming hypothesis (e.g., Davis & Sandman, 2012; Monk, Spicer, & Champagne, 2012; O’Connor et al., 2007; Van den Bergh et al., 2005). With few exceptions (Bergman, Sarkar, Glover, & O’Connor, 2010), studies evaluating fetal programming in human subjects do not consider the joint role of the prenatal and the postnatal environment in determining later outcomes.
According to the vulnerability-stress model suboptimal outcomes derive from the synergistic effect of a vulnerability factor inherent in the individual that interacts with risk factors or stress in the environment (Monroe & Simons, 1991). Although vulnerability-stress models are the dominant paradigm invoked to explain variation in the potency of environmental influences on adaptation, development and health (e.g., Calvete, Orue, & Hankin, 2013; Smith et al., 2012), most studies that have tested these models in humans have not included subjects with a known prenatal exposure to a biologically active stress signal.
Alternative models question the disease/dysfunction emphasis of the programming and vulnerability stress models and suggest that early environmental signals may shape development to increase adaptation to the environment (Gluckman & Hanson, 2004; Nederhof & Schmidt, 2012; Pluess & Belsky, 2011; Sandman, Davis, & Glynn, 2012). The predictive-adaptive-response (PAR) model (Gluckman & Hanson, 2004), also known as the weather-forecasting model (Bateson et al., 2004), predicts that, under certain conditions, organisms that are stressed in utero may have an adaptive advantage if they are confronted with stress later in development but an increased risk for disease if the conditions of their postnatal environment are favorable (Bogin, Silva, & Rios, 2007). The differential susceptibility (Pluess & Belsky, 2011) and adaptive calibration models (ACM; Ellis and Del Giudice, 2013), similarly have featured the patterns or the stability of environmental signals over time and emphasized that responses to early adversity may confer adaptive advantages. These models propose that early experiences may influence developmental plasticity, or the degree to which individuals are susceptible to both positive and negative environments.
The present study provides a unique evaluation in 6 to 10-year-old children, of the relation between known fetal exposure to a biological stress hormone, a synthetic glucocorticoid, and the consequences of exposure to sociodemographic adversity. The synthetic glucocorticoid, betamethasone, is routinely administered to women presenting with preterm labor between 24 and 34 gestational weeks, primarily to promote development of the fetal lungs and to increase survival in the case of preterm birth (National Institutes of Health Consensus Development Conference Statement, August 17–18, 2000). Randomized clinical trials have shown that treatment with glucocorticoids effectively reduces morbidity and mortality among infants who are born preterm (McKinlay, Crowther, Middleton, & Harding, 2012). Unlike cortisol, betamethasone freely crosses the placenta. Further, glucocorticoids, including betamethasone, pass through the blood brain barrier and target receptors throughout the central nervous system (Trenque et al., 1994). Glucocorticoids play a central role in brain development (Harris & Seckl, 2011; Lupien, McEwen, Gunnar, & Heim, 2009). Studies in animal models conclude that prenatal exposure to elevated levels of glucocorticoids permanently modifies the structure and function of the developing central nervous system, especially prefrontal and limbic regions, including the hippocampus (Coe & Lubach, 2005; Uno et al., 1994).
This study evaluates prevailing developmental models by determining whether a known fetal exposure to a biologically active stress signal increases the sensitivity to adversity. Overwhelming evidence documents that children growing up in socieoeconomically-deprived circumstances suffer from pervasive physical and mental health consequences (Bates, Lewis, & Weiss, 2013; Evans & Kim, 2012; Karlamangla et al., 2009). The present study assessed exposure to postnatal adversity in children with a validated index of sociodemographic risk (Poehlmann, Schwichtenberg, Bolt, & Dilworth-Bart, 2009). Specifically in this study we evaluate whether fetal exposure to a synthetic stress hormone (glucocorticoids) sensitizes the child to sociodemographic adversity.
Methods
Participants
Participants were one hundred and eleven mothers and their 6 to 10-year-old children (n=56 boys, n=55 girls; M=8.17 years, SD = 1.34 years) recruited based on delivery records from a major medical center in Southern California. All children were full term at birth (gestational age at birth greater than 37 weeks based on ACOG dating criteria) (“ACOG Practice Bulletin No. 101: Ultrasonography in pregnancy,” 2009). Inclusion criteria were appropriate weight for gestational age at birth and singleton status. Exclusion criteria were chromosomal or other congenital anomalies (e.g., trisomy 21), postnatal steroid administration and major neonatal illness (e.g., sepsis), maternal preeclampsia including HELLP syndrome, maternal drug use, as well as maternal disorders during pregnancy requiring corticosteroid treatment or thyroid medication. Seven percent of glucocorticoid exposed children and 5% from the comparison group failed to meet eligibility criteria described above and were not recruited into in the present investigation. Eligible subjects were recruited into two groups: those with and without prenatal exposure to synthetic glucocorticoids. Of those contacted, 86% of glucocorticoid exposed and 63% non-exposed mother-child pairs consented to participate. The glucocorticoid group comprised 26 children who received prenatal glucocorticoid treatment (betamethasone) because of risk for preterm delivery, but delivered full term. The glucocorticoids were given via 2 intramuscular injections as per standard of care between 24 and 34 gestational weeks (M = 30.0 weeks, SD = 3.0 weeks) and between 29 and 107 days prior to delivery (M = 60.0 days, SD = 21.1 days). The comparison group consisted of 85 children born full term and without prenatal exposure to synthetic glucocorticoids. Power analyses supported that this sample size provides adequate power to test the consequences of early life experiences and prenatal glucocorticoid exposure on child cognitive outcomes and determined recruitment. Sensitivity analysis revealed that our sample was sufficient to detect small-to-moderate effects (f2 = .07).
Measures
Child cognitive development
Standardized and validated measures were used to assess general cognitive ability, expressive language, and verbal recall memory. A delayed verbal recall memory task was included because brain regions involved in delayed recall (e.g., the hippocampus) contain high concentrations of glucocorticoid receptors (Harris & Seckl, 2011; Lupien et al., 2009) and are particularly susceptible to the effects of synthetic glucocorticoids (Uno et al., 1994).
Children’s general intelligence was assessed using the Perceptual Reasoning Index (PRI) of the Wechsler Intelligence Scale for Children (WISC-IV). The PRI is relatively language free and culturally independent (Baron, 2004) and two of the subscales (Matrix Reasoning and Block Design) have been shown to be excellent indicators of general intelligence (Baron, 2004; Wechsler, 2002). The verbal list-learning tests from the Wide Range Assessment of Memory and Learning – Second Edition (WRAML-2) were used to assess three aspects children’s verbal memory; immediate recall, delayed recall, and delayed recognition. The two delay conditions were included because performance on delayed memory tasks has been shown to be indicative of hippocampal functioning (Squire, 1992). The subtests have demonstrated validity, good internal consistency (r = .70 through r = .90) and acceptable stability (r = .59 through r = .78) (Sheslow & Adams, 2003). Expressive language was evaluated using the Expressive Vocabulary Test, Second Edition (EVT-2: Williams, 2007). The EVT-2 uses both labeling and synonym items to assess expressive vocabulary in children and adults ages 2.5 to 90+ without relying on reading or writing. The EVT-2 has been shown to have good validity, internal reliability (α=.94 through α=.97 for children ages 6 to 10 years), and excellent stability (r = .95) (Williams, 2007).
Sociodemographic risk
Sociodemographic and other background data were collected at the time of study entry by standardized maternal interview. A validated index of sociodemographic risk was computed as a measure of adversity based on previously published work (Poehlmann et al., 2009). One point was given for meeting each of the following risk factors; birth of index child before mother turned 18 years, maternal education below high school levels, mother is a single parent, four or more children 18 years or younger living in the home, family income below federal poverty guidelines (adjusted for family size). Scores ranged from 0 to 5, with higher scores reflecting a greater number of sociodemographic risk factors.
Maternal IQ and mood
Maternal intellectual ability and mood were assessed at the time of child cognitive testing to control for potential confounding effects. Intellectual ability was determined from scores on the Perceptual Organization Index (POI) of the Wechsler Adult Intelligence Scale – 3rd Edition; the adult parallel of the PRI given to children (Wechsler, 1997). The POI is a measure of reasoning and problem-solving skills. The POI is a well-validated scale that has excellent internal consistency (α = .94) and test-retest reliability (r = .88).
Maternal concurrent mood was assessed using the Beck Depression Inventory – Second Edition (BDI-II: Beck, Steer, & Brown, 1996). The BDI-II is a 21-item questionnaire that asks respondents to rate their feelings (on a 4-point scale) over the past two weeks. Total scores range from 0–63, with higher scores indicating a higher severity of depression. Analyses indicated an excellent level of internal consistency in the current study (α = .90).
Prenatal course and birth outcome
Maternal and infant medical records were reviewed to assess pregnancy complications and birth outcome. A standard score assessing prenatal obstetric risk was derived (Hobel, 1982).
Data analyses
Preliminary analyses were performed to identify maternal (i.e., intelligence, depressive symptoms), neonatal (i.e., gestational age at birth, birth weight, five-minute Apgar score) or child (i.e., age at assessment, sex, first language, birth order) variables that might influence child cognitive development. The only factor associated with child outcomes with a p-value of .05 or less was maternal intelligence. Maternal intelligence was included as a covariate in all analyses. To address the possibility that group differences could contribute to study findings prenatal obstetric risk and maternal depression additionally were included as covariates.
Regression analyses were used to test the independent and combined (interactive) influences of prenatal glucocorticoid exposure and sociodemographic risk on children’s cognitive functioning. The homogeneity of variance regression assumption was met. A separate model was tested for each of the outcome variables: general cognitive ability (WISC-IV Perceptual Reasoning Index), expressive language (EVT-2) and verbal memory (WRAML2 immediate recall, delayed recall and delayed recognition scales). Prenatal glucocorticoid treatment (1 = yes; 0 = no), sociodemographic risk (risk index), and their product term interaction were entered in each model as independent variables after controlling for the effects of maternal intelligence, maternal depressive symptoms, and the number of obstetric risks. Significant interactions were probed by calculating and plotting simple slopes for children exposed to exogenous prenatal glucocorticoids and comparison group. In addition, we inspected regions of significance (Preacher, Curran, & Bauer, 2006) and identified values of sociodemographic risk at which glucocorticoid exposure had a significant negative effect on child outcomes.
Results
The treatment and comparison group did not differ significantly on the sociodemographic risk index or any of the maternal (intelligence, depressive symptoms, education, marital status), demographic (household income, race/ethnicity), neonatal (gestational age at birth, birth weight, Apgar score) or child (age, sex, birth order) factors (all p’s > 0.10). See Tables 1 and 2.
Table 1.
Descriptive information for children in the study sample
| Prenatal sGC n = 26 | Comparison group n = 85 | |
|---|---|---|
| Race/Ethnicity (%) | ||
| Hispanic | 42 | 54 |
| Non-Hispanic White | 23 | 25 |
| Other | 35 | 21 |
| GA at first dose (weeks) | 29.9 (2.9) | N/A |
| Days between first dose and delivery | 60.0 (21.1) | N/A |
| Gestational age at birth (weeks) | 38.5 (1.0) | 39.3 (1.4) |
| Birth order (% firstborn) | 31% | 32% |
| Birth weight (grams) | 3405.5 (430.1) | 3413.9 (470.9) |
| Sex (% female) | 50% | 49% |
| Apgar score at 5-minutes | 8.9 (0.4) | 8.9 (0.4) |
| Child age at assessment(years) | 8.2 (1.2) | 8.2 (1.4) |
Table 2.
Descriptive information for mothers in the study sample
| Prenatal sGC n = 26 | Comparison group n = 85 | |
|---|---|---|
| Maternal age at delivery (years) | 29.4 (5.7) | 28.3 (6.8) |
| Married or cohabitating (%) | 85 | 82 |
| Highest level of education (%) | ||
| Primary, elementary, or middle school | 8 | 21 |
| High school or equivalent | 15 | 18 |
| Associates or vocational | 54 | 40 |
| Bachelor degree | 23 | 13 |
| Graduate degree | 0 | 8 |
| Annual household income (%) | ||
| $0 – $30,000 | 15 | 34 |
| $30,001 – $60,000 | 42 | 24 |
| $60,001 – $100,000 | 23 | 18 |
| Over $100,000 | 20 | 24 |
| WAIS: POI score | 94.4 (13.8) | 93.32 (17.5) |
| BDI-II total score | 5.5 (5.7) | 7.5 (8.0) |
Consistent with prior research, even after covarying maternal intelligence, maternal depressive mood, and the number of obstetric risks, elevated postnatal sociodemographic risk was associated with impairments in general cognitive ability [WISC-IV PRI; β = −0.19, t(103) = −1.92, p = 0.05] and marginally associated with expressive language [EVT scaled score; β = −0.17, t(103) = −1.81, p = 0.074]. Elevated sociodemographic risk was not associated with memory deficits (all p’s >.05). A significant main effect of glucocorticoid treatment was not present for general cognitive ability, expressive language or memory (See Table 3; ps >0.10).
Table 3.
Children’s cognitive functioning
| Prenatal sGC n = 26 | Comparison group n = 85 | |
|---|---|---|
| General cognitive ability (WISC-IV) | ||
| Perceptual Reasoning Index (PRI) | 101.2 (13.5) | 98.1 (13.6) |
| Expressive Language (EVT-2) | 101.5 (17.5) | 98.3 (12 |
| Verbal memory (WRAML2) | ||
| Immediate recall | 9.1 (3.1) | 9.4 (2.4) |
| Delayed recall | 9.1 (2.2) | 9.5 (2.1) |
| Recognition | 9.7 (3.6) | 9.5 (2.8) |
Note: Mean and Standard Deviation for the glucocorticoid treatment and comparison groups are presented here.
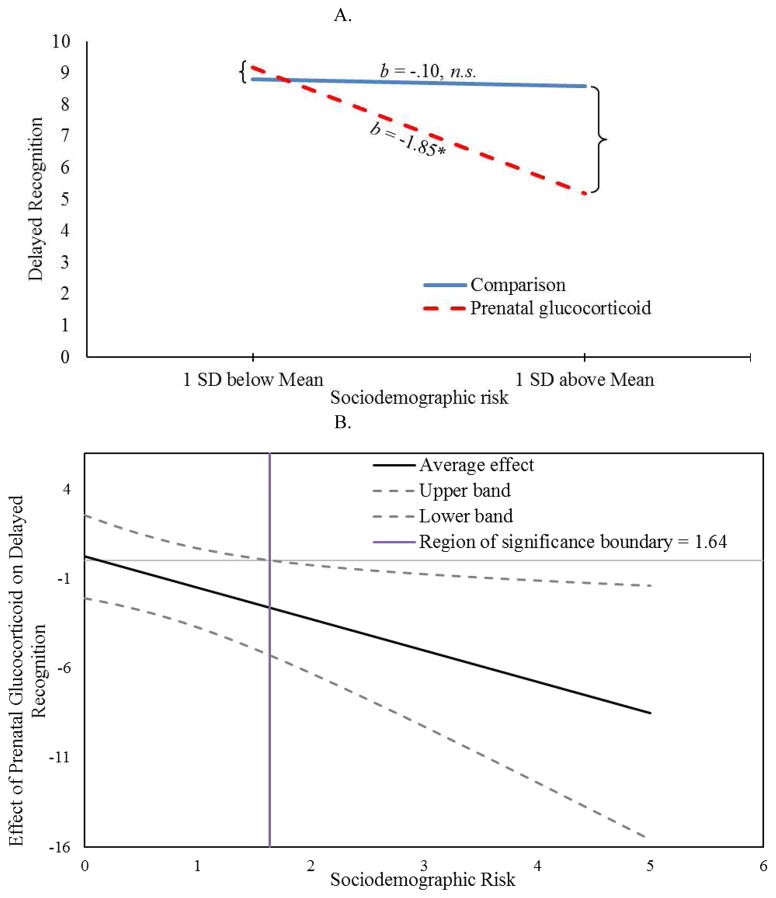
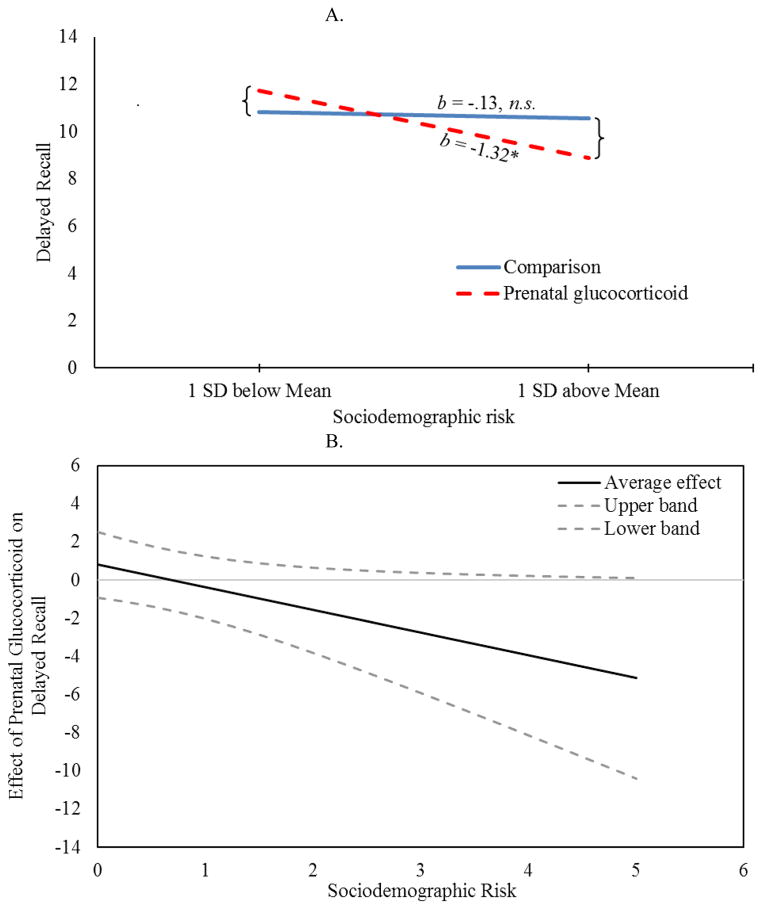
Notably, a significant interaction was observed for the two delayed memory tasks. Significant interactions confirmed that only children exposed to both fetal glucocorticoids and sociodemographic adversity had impaired performance on delayed recognition and delayed recall tasks (p’s <.05). See Figures 1A and 2A and Table 4. Importantly, these associations remained after statistically covarying obstetric risk, maternal depressive symptoms and maternal or child general intellectual functioning. Inspection of the regions of significance (Figure 1B) revealed that prenatal glucocorticoid treatment was associated with significantly impaired delayed recognition for children exposed to sociodemographic risk above the value of 1.64. For delayed recall, inspection of regions of significance (Figure 2B) revealed that prenatal glucocorticoids did not confer statistically significant risk for any values of sociodemographic risk observed in our study (ranging from 0 to 5). No significant interactions between prenatal glucocorticoids and sociodemographic risk were observed for WISC, EVT or immediate recall (all p’s >0.05). Further, no significant associations with sex were observed nor was there significant interactions with sex and either risk factor (all p’s >0.05).
Figure 1.
Effects of Sociodemographic Risks on Delayed Recognition by Prenatal Glucocorticoid Treatment (A) Children exposed to prenatal glucocorticoids are vulnerable to sociodemographic risk whereas comparison children are not; (B) Region of significance analysis indicate that the effect of prenatal glucocorticoids on delayed recognition is significant for children exposed to sociodemographic risk greater than 1.64.
Figure 2.
Effects of Sociodemographic Risks on Delayed Recall by Prenatal Glucocorticoid Treatment (A) Children exposed to prenatal glucocorticoids are vulnerable to sociodemographic risk whereas comparison children are not; (B) Although the effect of prenatal glucocorticoids on delayed recall becomes more negative with increasing sociodemographic risk, region of significance analysis indicate that children with prenatal glucocorticoid exposure are not significantly different from the comparison group across the observed values of sociodemographic risk.
Table 4.
Hierarchical Regression model examining whether the interaction between sociodemographic risk and prenatal steroid exposure accounts for unique variance in child delayed recognition memory (Table 4a) or delayed recall memory (Table 4b).
| A. Delayed recognition
| |||
|---|---|---|---|
| R2 | ΔR2 | β | |
| Model 1 | .02 | ||
| Maternal intelligence | .10 | ||
| Maternal depressive symptoms | −.06 | ||
| Number of obstetric risks | .07 | ||
|
| |||
| Model 2 | .04 | .02 | |
| Maternal intelligence | .05 | ||
| Maternal depressive symptoms | −.06 | ||
| Number of obstetric risks | .15 | ||
| Prenatal steroid exposure | −.13 | ||
| Sociodemographic risk | −.13 | ||
|
| |||
| Model 3 | .08 | .05* | |
| Maternal intelligence | .06 | ||
| Maternal depressive symptoms | −.09 | ||
| Number of obstetric risks | .18 | ||
| Prenatal steroid exposure | .03 | ||
| Sociodemographic risk | −.04 | ||
| Prenatal Steroids x Sociodemographic risk | −.29* | ||
| B. Delayed recall
| |||
|---|---|---|---|
| R2 | ΔR2 | β | |
| Model 1 | .02 | ||
| Maternal intelligence | −.01 | ||
| Maternal depressive symptoms | −.11 | ||
| Number of obstetric risks | −.11 | ||
|
| |||
| Model 2 | .04 | .02 | |
| Maternal intelligence | −.08 | ||
| Maternal depressive symptoms | −.11 | ||
| Number of obstetric risks | −.15 | ||
| Prenatal steroid exposure | .01 | ||
| Sociodemographic risk | −.15 | ||
|
| |||
| Model 3 | .08 | .04* | |
| Maternal intelligence | −.07 | ||
| Maternal depressive symptoms | −.14 | ||
| Number of obstetric risks | −.12 | ||
| Prenatal steroid exposure | .16 | ||
| Sociodemographic risk | −.07 | ||
| Prenatal Steroids x Sociodemographic risk | −.27* | ||
Note: Because of multicollinearity between child and maternal general intelligence, the model is presented with only maternal intelligence included. Note that the steroid by sociodemographic risk interaction also remains significant when child general intelligence is included in the model (data not shown).
Discussion
The current study takes advantage of a common treatment given to women at risk for preterm delivery to perform a direct test of the role of fetal exposure to stress hormones in the development of susceptibility to later stress. These novel findings show that known human fetal exposure to synthetic glucocorticoids alters the sensitivity to environmental signals. Only those children who experienced both fetal glucocorticoid exposure and sociodemographic risk expressed memory impairments. These data are consistent with the hypothesis that prenatal experiences, especially those that influence stress physiology, shape developmental trajectories in ways that influence adaptation to the environment (Pluess & Belsky, 2011; Robinson, Lichtenstein, Anckarsater, Happe, & Ronald, 2013).
The present study provides a novel test of several prevailing models characterizing the developmental consequences of early life stress by assessing outcomes among children with a known exposure to synthetic glucocorticoids during the prenatal period. Study findings support the vulnerability-stress hypothesis and illustrate that, for memory, sociodemographic adversity only is associated with impairments if a prior vulnerability (prenatal glucocorticoid treatment) is present. Thus, the children are faced with double jeopardy--danger from two different sources. Although the main effect of glucocorticoid administration on memory predicted by the fetal programming model was not found, these data suggest that the underlying vulnerability may be “programmed” during the fetal period. Prenatal exposure to high levels of a biologically active stress hormone may influence fetal adaptation with implications for responses to the environment. As proposed by the differential susceptibility and adaptive calibration models, it is plausible that glucocorticoid treatment increases lability of responses to subsequent environments and this may account for the memory impairments in the context of adversity. In contrast to predictions by differential susceptibility and adaptive calibration models, glucocorticoid treatment was not associated with enhanced memory in the context of low sociodemographic risk. However, we argue that the present findings do not fully test the differential susceptibility or the adaptive calibration models because the sociodemographic risk measure characterizes level of adversity and does not capture variability in positive environmental influences.
Fetal exposure to excess stress hormones may alter fetal neurodevelopmental trajectories (Davis, Sandman, Buss, Wing, & Head, 2013) resulting in memory impairment among the children who are exposed to subsequent risk. The consequences of fetal exposure to glucocorticoids are specific to memory. One possible alternative explanation for the lack of effects of synthetic glucocorticoids on general intellectual functioning and expressive language (either directly or in concert with sociodemographic risk) may be due to low sample size. However, sensitivity analysis revealed that our sample was sufficient to detect small-to-moderate effects (f2 = .07). Our finding that sociodemographic risk is associated with general measures of intellectual functioning and language, regardless of glucocorticoid exposure, is consistent with prior research (Bates et al., 2013). Fetal exposure to glucocorticoids may only increase susceptibility to subsequent adversity for functions such as memory that are dependent on brain regions that are rich in glucocorticoid receptors. The hippocampus, integral to learning and memory (Squire, 1992), may be specifically influenced by prenatal treatment with synthetic glucocorticoids (Waffarn & Davis, 2012) because this region has a high concentration of glucocorticoid receptors (Conrad, 2008; Harris & Seckl, 2011; Lupien et al., 2009; Rodriguez et al.) and these receptors are present in the human fetal hippocampus during the gestational window when glucocorticoid treatment is administered (24–34 gestational weeks) (Noorlander, De Graan, Middeldorp, Van Beers, & Visser, 2006).
Numerous animal studies illustrate that early stress experiences shape the animals’ responses to subsequent environments. This may occur through sensitization of the HPA axis. Fetal glucocorticoid exposure may result in epigenetic modifications in the HPA axis thus leading to long lasting changes that influence responses to subsequent environments (Daskalakis, Bagot, Parker, Vinkers, & de Kloet, 2013; Karsten & Baram, 2013). Few human studies have evaluated epigenetic mechanisms. However, fetal exposure to adversity, including maternal depression and smoking, have been associated with methylation of the glucocorticoid receptor gene and altered patterns of HPA axis reactivity (Oberlander et al., 2008; Stroud et al., 2014). These findings in conjunction with animal research suggest that an epigenetic mechanism may underlie the persisting influence of fetal glucocorticoid exposure on responses to the environment.
There are several novel contributions of our study. Previous human studies evaluating the impact of fetal exposure to glucocorticoids often are limited by i) the inclusion of preterm infants which means that the consequences of glucocorticoids cannot be dissociated from the well-known effects of preterm birth, ii) a focus on intelligence or developmental delays rather than processes, such as memory, that rely on brain regions that are particularly susceptible to glucocorticoids, and iii) a failure to consider the influence of the postnatal environment (Waffarn & Davis, 2012). The present study addresses these limitations and illustrates that prenatal glucocorticoid treatment may be associated with specific vulnerabilities in the context of postnatal adversity in a healthy and low-risk sample of children who were full term at birth. A primary limitation of the present investigation is that prenatal glucocorticoid treatment was not randomly assigned and thus, we cannot rule out the possibility of a confounding factor that accounts for the study findings. Further, because women and children were not prospectively evaluated during the prenatal period, we were not able to characterize other aspects of the prenatal environment (e.g., maternal stress) that may influence fetal development. It is the case that the treatment and comparison groups do not differ significantly on any of the sociodemographic factors assessed or on maternal factors such as intelligence or depression and that considering these potential confounding factors did not account for study findings.
Acknowledgments
This research was supported by NIH awards HD-50662 and HD-6582 to EPD and Conte Center Award MH-96889. The authors wish to thank the families who participated in this project. The assistance of Megan Faulkner, Natalie Hernandez, and Kendra Leak of the Women and Children’s Health and Well-Being Project, Department of Psychiatry & Human Behavior, University of California is gratefully acknowledged.
References
- ACOG Practice Bulletin No. 101: Ultrasonography in pregnancy. Obstet Gynecol. 2009;113(2 Pt 1):451–461. doi: 10.1097/AOG.0b013e31819930b0. [DOI] [PubMed] [Google Scholar]
- Barker DJP. In utero programming of chronic disease. Clinical Sciences. 1998;95:115–128. [PubMed] [Google Scholar]
- Baron IS. Neuropsychological Evaluation of the Child. New York: Oxford University Press Inc; 2004. [Google Scholar]
- Bates TC, Lewis GJ, Weiss A. Childhood socioeconomic status amplifies genetic effects on adult intelligence. Psychol Sci. 2013;24(10):2111–2116. doi: 10.1177/0956797613488394. [DOI] [PubMed] [Google Scholar]
- Bateson P, Barker D, Clutton-Brock T, Deb D, D’Udine B, Foley RA, et al. Developmental plasticity and human health. Nature. 2004;430(6998):419–421. doi: 10.1038/nature02725. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Beck Depression Inventory–II (BDI–II) San Antonio, TX: The Psychological Corporation; 1996. [Google Scholar]
- Bergman K, Sarkar P, Glover V, O’Connor TG. Maternal prenatal cortisol and infant cognitive development: moderation by infant-mother attachment. Biol Psychiatry. 2010 doi: 10.1016/j.biopsych.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogin B, Silva MI, Rios L. Life history trade-offs in human growth: adaptation or pathology? American Journal of Human Biology. 2007;19(5):631–642. doi: 10.1002/ajhb.20666. [DOI] [PubMed] [Google Scholar]
- Calvete E, Orue I, Hankin BL. Transactional relationships among cognitive vulnerabilities, stressors, and depressive symptoms in adolescence. J Abnorm Child Psychol. 2013;41(3):399–410. doi: 10.1007/s10802-012-9691-y. [DOI] [PubMed] [Google Scholar]
- Coe CL, Lubach GR. Developmental consequences of antenatal dexamethasone treatment in nonhuman primates. Neurosci Biobehav Rev. 2005;29(2):227–235. doi: 10.1016/j.neubiorev.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Conrad CD. Chronic stress-induced hippocampal vulnerability: the glucocorticoid vulnerability hypothesis. Rev Neurosci. 2008;19(6):395–411. doi: 10.1515/revneuro.2008.19.6.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daskalakis NP, Bagot RC, Parker KJ, Vinkers CH, de Kloet ER. The three-hit concept of vulnerability and resilience: toward understanding adaptation to early-life adversity outcome. Psychoneuroendocrinology. 2013;38(9):1858–1873. doi: 10.1016/j.psyneuen.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EP, Sandman CA. Prenatal psychobiological predictors of anxiety risk in preadolescent children. Psychoneuroendocrinology. 2012;37(8):1224–1233. doi: 10.1016/j.psyneuen.2011.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EP, Sandman CA, Buss C, Wing DA, Head K. Fetal glucocorticoid exposure is associated with preadolescent brain development. Biol Psychiatry. 2013;74(9):647–655. doi: 10.1016/j.biopsych.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans GW, Kim P. Childhood poverty and young adults’ allostatic load: the mediating role of childhood cumulative risk exposure. Psychol Sci. 2012;23(9):979–983. doi: 10.1177/0956797612441218. [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA. Living with the past: evolution, development, and patterns of disease. Science. 2004;305(5691):1733–1736. doi: 10.1126/science.1095292. [DOI] [PubMed] [Google Scholar]
- Harris A, Seckl J. Glucocorticoids, prenatal stress and the programming of disease. Hormones and Behavior. 2011;59(3):279–289. doi: 10.1016/j.yhbeh.2010.06.007. [DOI] [PubMed] [Google Scholar]
- Karlamangla AS, Miller-Martinez D, Aneshensel CS, Seeman TE, Wight RG, Chodosh J. Trajectories of cognitive function in late life in the United States: demographic and socioeconomic predictors. Am J Epidemiol. 2009;170(3):331–342. doi: 10.1093/aje/kwp154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsten CA, Baram TZ. How Does a Neuron “know” to Modulate Its Epigenetic Machinery in Response to Early-Life Environment/Experience? Front Psychiatry. 2013;4:89. doi: 10.3389/fpsyt.2013.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10(6):434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- McKinlay CJD, Crowther CA, Middleton P, Harding JE. Repeat antenatal glucocorticoids for women at risk of preterm birth: A Cochrane Systematic Review. American Journal of Obstetrics and Gynecology. 2012;206(3):187–194. doi: 10.1016/j.ajog.2011.07.042. [DOI] [PubMed] [Google Scholar]
- Monk C, Spicer J, Champagne FA. Linking prenatal maternal adversity to developmental outcomes in infants: The role of epigenetic pathways. Dev Psychopathol. 2012;24(4):1361–1376. doi: 10.1017/S0954579412000764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe SM, Simons AD. Diathesis-stress theories in the context of life stress research: implications for the depressive disorders. Psychological Bulletin. 1991;110(3):406–425. doi: 10.1037/0033-2909.110.3.406. [DOI] [PubMed] [Google Scholar]
- Nederhof E, Schmidt MV. Mismatch or cumulative stress: toward an integrated hypothesis of programming effects. Physiol Behav. 2012;106(5):691–700. doi: 10.1016/j.physbeh.2011.12.008. [DOI] [PubMed] [Google Scholar]
- Noorlander CW, De Graan PN, Middeldorp J, Van Beers JJ, Visser GH. Ontogeny of hippocampal corticosteroid receptors: effects of antenatal glucocorticoids in human and mouse. J Comp Neurol. 2006;499(6):924–932. doi: 10.1002/cne.21162. [DOI] [PubMed] [Google Scholar]
- O’Connor TG, Caprariello P, Blackmore ER, Gregory AM, Glover V, Fleming P. Prenatal mood disturbance predicts sleep problems in infancy and toddlerhood. Early Human Development. 2007;83(7):451–458. doi: 10.1016/j.earlhumdev.2006.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlander TF, Weinberg J, Papsdorf M, Grunau R, Misri S, Devlin AM. Prenatal exposure to maternal depression, neonatal methylation of human glucocorticoid receptor gene (NR3C1) and infant cortisol stress responses. Epigenetics. 2008;3(2):97–106. doi: 10.4161/epi.3.2.6034. [DOI] [PubMed] [Google Scholar]
- Pluess M, Belsky J. Prenatal programming of postnatal plasticity? Dev Psychopathol. 2011;23(1):29–38. doi: 10.1017/S0954579410000623. [DOI] [PubMed] [Google Scholar]
- Poehlmann J, Schwichtenberg AJM, Bolt D, Dilworth-Bart J. Predictors of depressive symptom trajectories in mothers of preterm or low birth weight infants. Journal of Family Psychology. 2009;23(5):690–704. doi: 10.1037/a0016117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher KJ, Curran PJ, Bauer DJ. Computational Tools for Probing Interactions in Multiple Linear Regression, Multilevel Modeling, and Latent Curve Analysis. Journal of Educational and Behavioral Statistics. 2006;31(4):437–448. [Google Scholar]
- Robinson EB, Lichtenstein P, Anckarsater H, Happe F, Ronald A. Examining and interpreting the female protective effect against autistic behavior. Proc Natl Acad Sci U S A. 2013;110(13):5258–5262. doi: 10.1073/pnas.1211070110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez JS, Zurcher NR, Keenan KE, Bartlett TQ, Nathanielsz PW, Nijland MJ. Prenatal betamethasone exposure has sex specific effects in reversal learning and attention in juvenile baboons. Am J Obstet Gynecol. doi: 10.1016/j.ajog.2011.01.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandman CA, Davis EP. Gestational stress influences cognition and behavior. Future Neurology. 2010;5(5):675–690. [Google Scholar]
- Sandman CA, Davis EP, Glynn LM. Prescient human fetuses thrive. Psychol Sci. 2012;23(1):93–100. doi: 10.1177/0956797611422073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheslow D, Adams W. Wide Range Assessment of Memory and Learlning Administration and Technical Manual. 2. Lutz, FL: Psychological Assessment Resources; 2003. [Google Scholar]
- Smith HJ, Sheikh HI, Dyson MW, Olino TM, Laptook RS, Durbin CE, et al. Parenting and Child DRD4 Genotype Interact to Predict Children’s Early Emerging Effortful Control. Child Dev. 2012;83(6):1932–1944. doi: 10.1111/j.1467-8624.2012.01818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR. Memory and the hippocampus: A synthesis from findings with rats, monkeys, and humans. Psychological Review. 1992;99(2):195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- Stroud LR, Papandonatos GD, Rodriguez D, McCallum M, Salisbury AL, Phipps MG, et al. Maternal smoking during pregnancy and infant stress response: test of a prenatal programming hypothesis. Psychoneuroendocrinology. 2014;48:29–40. doi: 10.1016/j.psyneuen.2014.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trenque T, Lamiable D, Vistelle R, Millart H, Leperre A, Choisy H. Comparative pharmacokinetics of two diastereoisomers dexamethasone and betamethasone in plasma and cerebrospinal fluid in rabbits. Fundam Clin Pharmacol. 1994;8(5):430–436. doi: 10.1111/j.1472-8206.1994.tb00822.x. [DOI] [PubMed] [Google Scholar]
- Uno H, Eisele S, Sakai A, Shelton S, Baker E, DeJesus O, et al. Neurotoxicity of glucocorticoids in the primate brain. Hormone and Behavior. 1994;28:336–348. doi: 10.1006/hbeh.1994.1030. [DOI] [PubMed] [Google Scholar]
- Van den Bergh BRH, Mennes M, Oosterlaan J, Stevens V, Stiers P, Marcoen A, et al. High antenatal maternal anxiety is related to impulsivity during performance on cognitive tasks in 14- and 15-year-olds. Neuroscience and Biobehavioral Reviews. 2005;29(2):259–269. doi: 10.1016/j.neubiorev.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Waffarn F, Davis EP. Effects of antenatal corticosteroids on the hypothalamic-pituitary-adrenocortical axis of the fetus and newborn: experimental findings and clinical considerations. Am J Obstet Gynecol. 2012;207(6):446–454. doi: 10.1016/j.ajog.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. WAIS-III administration and scoring manual. San Antonio, TX: Psychological Corporation; 1997. [Google Scholar]
- Wechsler D. Wechsler Preschool and Primary Scale of Intelligence. San Antonio, TX: The Psychological Corporation; 2002. [Google Scholar]
- Weinstock M. The long-term behavioural consequences of prenatal stress. Neuroscience & Biobehavioral Reviews. 2008;32(6):1073–1086. doi: 10.1016/j.neubiorev.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Williams KT. Expressive Vocabulary Test. 2. Minneapolis, MN: NCS Pearson, Inc; 2007. [Google Scholar]