Abstract
Oligonucleotides manifest much promise as potential therapeutic agents. However, understanding of how oligonucleotides function within living organisms is still rather limited. A major concern in this regard is the mechanisms of cellular uptake and intracellular trafficking of both ‘free’ oligonucleotides and oligonucleotides associated with various polymeric or nanocarrier delivery systems. Here we review basic aspects of the mechanisms of endocytosis and intracellular trafficking and how insights from these processes can be used to understand oligonucleotide delivery. In particular we discuss opportunities for escape of oligonucleotides from endomembrane compartments and describe recent studies using small molecules to enhance oligonucleotide effects.
Graphical Abstract

1. Introduction
Realization of the potential oligonucleotides as therapeutic agents began more than three decades ago with the discovery of antisense molecules [1]. The discovery of RNA interference [2, 3] increased enthusiasm and was further reinforced by insights into the complex roles of non-coding RNAs in regulating genome function [4]. Many aspects of oligonucleotide therapeutics are covered in this theme issue. Here we will stress a key basic aspect of oligonucleotide behavior that underlies all potential therapeutic utilization, namely the cellular uptake and intracellular trafficking of these molecules.
Despite FDA approval of the first antisense drug [5] and the advent of multiple clinical trials in cancer and other diseases [6–10], oligonucleotide therapeutics has progressed slowly. A major issue has been the poor efficacy of oligonucleotides. In large part this because effective delivery of such large, polar molecules to their sites of action within tissues is a very challenging problem [11–13]. This review will primarily emphasize the behavior of ‘free’ oligonucleotides and of molecular-scale oligonucleotide conjugates. Oligonucleotides associated with nanocarriers are more fully discussed elsewhere in this theme issue. The emphasis here will be on processes at the cellular and subcellular level rather than on classical pharmacokinetics and biodistribution. A key theme for this article is the observation that the processes that govern intracellular traffic of internalized molecules are enormously complex and that understanding these processes is vital for the future of oligonucleotide therapeutics.
2. Endocytotic and Trafficking Pathways
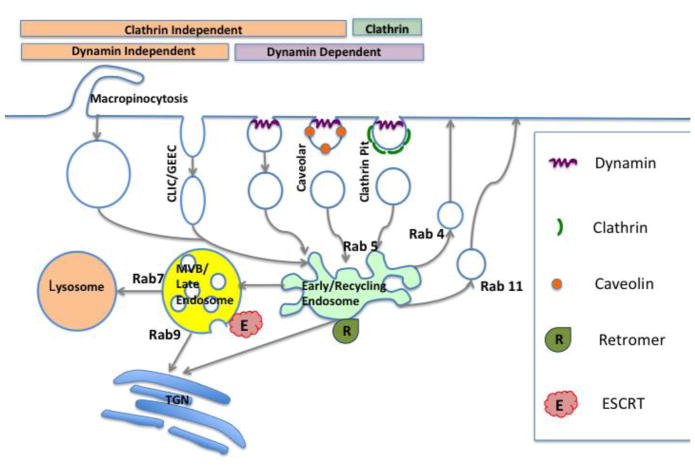
Oligonucleotides usually enter cells via endocytosis; however, it is important to realize the complexities associated with the multiple pathways of internalization and subsequent trafficking. These pathways are regulated by a plethora of unique proteins and lipids that underlie all aspects of internalization and trafficking. An illustration of some of the pathways is given in Figure 1.
Figure 1. Pathways of Endocytosis and Trafficking.
The figure illustrates several of the major internalization and trafficking pathways discussed in the text. Phagocytosis takes places only in specialized cells such as macrophages and granulocytes while the uptake pathways illustrated here are found in many cell types. A few of the key proteins or protein complexes involved in some of the pathways are indicated; however many other essential proteins are not depicted. Specific Rab GTPases play key roles controlling the flow of vesicles between individual compartments. The role of the Retromer complex in endosome to Golgi traffic and the role of the ESCRT complex in the formation of MVBs are discussed in the text. (figures adapted with modification from reference 89).
2.1. Pathways and mechanisms of endocytosis
2.1.1 Coated pits
During classical clathrin mediated endocytosis ligand-bound cell surface receptors associate with AP-2 and other adaptor proteins, and with various accessory proteins, that cluster the receptors into specialized membrane areas subtended by a network of clathrin triskelions [14–16]. The clathrin network, functioning with specialized BAR domain proteins, including SNX9 and amphiphysin, that sense and alter membrane curvature, then produces an invagination of the coated pit. Following invagination, there is a pinching off of a clathrin-coated vesicle mediated by the dynamin GTPase [17]. The coated endosomal vesicle is then quickly uncoated via several proteins including auxilin and hsc70. The uncoated vesicle then begins its intracellular journey. Important receptors and ligands internalized by this pathway include LDL, transferrin, and many activated G Protein Coupled Receptors [18, 19].
2.1.2 Caveolae
Many cells display small, cholesterol-rich membrane invaginations that contain caveolin1. This is a 21kD protein that inserts a hydrophobic hairpin into the lipid bilayer while both N- and C-termini are cytosolic [20, 21]. Stabilization of cavelolar structures also involves coat proteins called cavins that act with caveolin. There is some controversy as to whether caveolae can mature into independent intracellular vesicles or whether they remain associated with the plasma membrane as tubular structures. However, evidence now suggests that caveolae can contribute vesicular structures to intracellular membrane traffic. For example, caveolae have been implicated in the turnover of β1 integrins and the ENaC sodium channel, but the precise mechanisms are unclear. Caveolar vesicles are usually smaller (<100 nanometers) than other types of endosomes, which may reach diameters of several hundred nanometers. Previously it had been suggested that internalized caveloae contributed to unique endomembrane structures termed caveosomes. However, more recently this view has been discredited and caveolar-derived vesicles likely fuse with typical early endosomes. Dynamin seems to be involved in the disjunction of caveolae from the plasma membrane, but the evidence is not as abundant as in the case of clathrin coated pits. Many other proteins are associated with caveolae, particularly molecules involved in signal transduction [22, 23].
2.1.3 Other pathways
Several clathrin- and caveolin- independent pathways have been documented [16, 18, 24]. These pathways are often described in terms of the morphologies of the vesicles they generate or based on the cargo that is preferentially internalized. For example, the flotillins are membrane-inserted proteins that may organize lipid domains and promote subsequent endocytosis, functioning similarly to caveolin. Interestingly some studies indicate that dynamin is not required for internalization of cargo via the flotillin vesicle pathway [25]. Thus the flotillin pathway is an example of a clathrin and caveolin (and possibly dynamin) independent endocytotic pathway.
The CLIC/GEEC pathway is another important mechanism that seems to make a major contribution to fluid phase endocytosis [18, 24]. The acronym is for Clathrin and Dynamin Independent Carriers (CLIC)/GPI-AP Enriched Early Endosomal Compartments (GEEC). This pathway generates tubular endosomes of high volume that are enriched in GPI-proteins and that typically contain markers of fluid phase endocytosis (e.g. dextrans). As implied by their name, dynamin is not required for the disjunction of these vesicles from the plasma membrane. Rather membrane scission probably involves GRAF1, a GTPase activating protein containing a BAR domain [26]. Additionally the small GTPase CDC42 has been implicated in the CLIC/GEEC pathway.
There are additional clathrin and caveolin independent pathways [18, 24, 26]. One is a pathway for the internalization of a type of IL2-Receptor, certain potassium channels, and the FCεR1 immunoglobulin receptor. This pathway utilizes dynamin for scission of vesicles from the plasma membrane, while the pathway is also regulated by protein kinases of the PAK family and by the Rho GTPase. Another pathway that generates both vesicular and tubular structures is involved in the internalization of MHC class I histocompatibility proteins; it utilizes the Arf 6 GTPase, but the involvement of dynamin is unclear.
Macropinocytosis involves cell protrusions that engulf large volumes of extracellular fluid thus substantially contributing to fluid phase endocytosis [27]. Formation of these relatively large structures requires actinomyosin and its characteristic regulators such as the Rac GTPase and PAK family kinases, but probably not dynamin. Additional internalization mechanisms such as phagocytosis and entosis are characteristic of specialized cells but do not play a role in trafficking of oligonucleotides in most cell types [18]. The actinomyosin contractile machinery is involved in most of the processes described above; however, not all endocytotic events require actin. For example, certain arenaviruses utilize a pathway that is independent of clathrin, caveolin, dynamin and actin to enter cells [28]. Interestingly, our studies suggest that phosphorothioate antisense oligonucleotides may enter cells by a pathway similar to that of arenaviruses [29].
Thus multiple pathways for endocytosis are known while additional ones probably remain to be discovered. This provides daunting challenges but also important possibilities for oligonucleotide pharmacology. It should be possible to influence the initial route of endocytosis by targeting oligonucleotides to specific cell surface receptors. Subsequently this may have important consequences for downstream trafficking and for the ultimate biological effect of the oligonucleotide.
2.2. Trafficking downstream of initial internalization
2.2.1 Overview
Irrespective of whether it is a ‘free’ molecule, a molecular scale conjugate or associated with a form of nanoparticle, an oligonucleotide that enters a cell via endocytosis must traverse a complex maze of intracellular pathways leading to many destinations and regulated by intricate protein machinery [30–32]. Major subcellular membrane-bound compartments include early and recycling endosomes, late endosomes/multi-vesicular bodies, lysosomes, the Golgi apparatus and the endoplasmic reticulum (see Figure 1). There are two broad fates for materials in endomembrane compartments; they can be trafficked to lysosomes for degradation or they can be recycled to the plasma membrane and cell exterior. A relatively minor but interesting alternative trafficking route leads from endosomes to the trans-Golgi apparatus and is termed the Retrograde pathway [33]. There are two somewhat inconsistent models of the intracellular trafficking process. One model posits that early and late endosomes are stable compartments that serve as docking stations for smaller vesicles that carry membranes and intraluminal contents between compartments. Another model suggests a gradual maturation of early/recycling endosomes to late endosomes and lysosomes. In actuality it is likely that elements of both models come into play [34, 35]. The intricate ballet of intracellular trafficking is regulated by a host of proteins or protein complexes. Some key examples include the multitudinous Rab family of small GTPases [36, 37], SNARE complexes [38], tethers [39, 40], the ESCRT complex [41] and the Retromer complex [42]. These various proteins play important functional roles but as well they also serve as easily recognizable markers for specific endomembrane compartments.
2.2.2 Basic Mechanisms of Trafficking
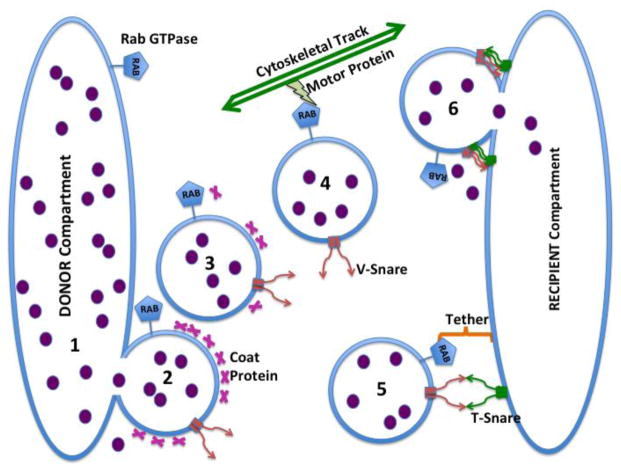
There has been great progress in understanding the mechanisms of intracellular trafficking, acknowledged by a recent Nobel Prize in this area [43]. A basic conserved process underlies all membrane traffic: (a) disjunction of a coated vesicle from a donor membrane compartment; (b) uncoating of the vesicle allowing the display or binding of tethering and fusion proteins; (c) migration of the vesicle to its destination along ’tracks’ provided by actin- or tubulin- based cytoskeletal structures; (d) recognition by the vesicle of its target membrane compartment using tethering proteins and then completion the fusion process using SNARE proteins and delivery of membrane and contents to the target compartment [30, 44, 45]. Figure 2 shows a simple diagram of this process. There are numerous variations of this theme and a plethora of proteins are implicated in different situations, but the general picture is quite clear.
Figure 2. Proposed Mechanism of Vesicular Trafficking of Oligonucleotides.
Oligonucleotides (depicted as small filled circles) are initially accumulated in an endomembrane compartment (the DONOR compartment, for example, early endosomes) and are then trafficked by means of shuttle vesicles to various other endomembrane compartments (the RECIPIENT compartment, for example, the trans-Golgi). The first step (1) involves disjunction (’pinching off’) of a shuttle vesicle under the influence of a coat protein as well as other accessory proteins. At this stage there are non-bilayer regions at the junction between the membranes of the DONOR compartment and the shuttle vesicle. This provides an opportunity for some oligonucleotide to escape to the cytosol. Step 2 involves uncoating of the coated vesicle; Rab proteins can contribute to this step. Step 3 comprises movement of the shuttle vesicle toward its destination along cytoskeletal tracks. Motor proteins such as various myosins (for the actin system) or dyneins or kinesins (for the microtubular system) propel the vesicle. Rab proteins are involved in forming the appropriate linkages to the cytoskeleton. Step 4 entails recognition of the RECIPIENT (‘target’) compartment by the shuttle vesicle. Tether proteins work with Rab proteins to provide interaction specificity while v-SNARE proteins in the vesicle membrane interact with t-SNARE proteins in the RECIPIENT compartment membrane to provide firm bridging, as well as contributing to specificity. In step 5 the SNARE proteins undergo major conformational changes, and with the assistance of accessory proteins, trigger fusion of the shuttle vesicle membrane with the membrane of the RECIPIENT compartment. At this stage non-bilayer regions exist at the junction between shuttle and RECIPIENT membranes potentially allowing escape of oligonucleotide.
The Rab proteins comprise a large (>60) family of small GTPases that act as molecular switches to modulate many aspects of intracellular trafficking [37, 46, 47]. Activated GTP-loaded Rabs bind to and affect the functions of downstream effector proteins, usually acting at the cytoplasmic face of endomembrane compartments. The inactive GDP-loaded form of Rabs associate with cytosolic Rab-GDI (Rab GDP Dissociation Inhibitor) proteins that stabilize the inactive form and regulate the ratio between cytosolic and membrane bound Rab. The balance between active and inactive forms is also regulated by Rab-specific GEFs (Guanine Nucleotide Exchange Factors) and GAPs (GTPase Activating Proteins). Rabs play many roles in modulating intracellular trafficking; this includes vesicle uncoating, movement of vesicles along cytoskeletal tracks, and the ultimate fusion events involving tethers and SNARES. Rab proteins also serve as excellent markers of individual membrane compartments and trafficking pathways. For example, as we will discuss in more detail below, Rab5 is a marker for early endosomes while Rab7 serves for late endosomes, and Rab 9 for endosome-Golgi trafficking. However, a complicated aspect of Rab function involves the presence of ‘Rab domains’ within particular endosomal compartments. Using low levels of expressed green fluorescent protein-Rab chimeras, it has been demonstrated that different Rab proteins localized on the same endomembrane compartment can occupy distinct membrane microdomains [46]. This micro-segregation of Rabs likely contributes to the endosomal sorting processes discussed above [26].
As mentioned, the initial event in intracellular trafficking is the disjunction of a coated vesicle from a donor membrane. The formation of clathrin coated vesicles at the plasma membrane is one example, but there are other examples including the COPI and COPII coats of the Golgi and ER. The initial budding of the coated vesicle is often accomplished by dynamin, but other mechanisms exist as well. For example, vesicle disjunction from late endosomes to the trans-Golgi is accomplished by the Retromer complex [33] utlizing SNX proteins that sense and affect membrane curvature via BAR domains [48].
Tethering proteins create preferential interactions between vesicles and their ultimate target membrane compartments. There are two broad types of tethering proteins; the coiled-coil tethers such as the Golgins and the multi-subunit tethers [38–40]. Tethers are thought to bridge membranes and promote fusion by binding to both Rab proteins and SNARES. However, the multisubunit tethers clearly have multiple functions including possibly ‘proof-reading’ SNARE complexes to assure fusion of the correct vesicular partners. For example, this seems to be true of the late endosomal HOPS tethering complex [40]. There is little clarity about when and how tethering proteins become associated with trafficking vesicles. For example, it is unclear whether tethers bind vesicle coat proteins, or if tethering takes place after uncoating [30].
The ultimate transfer of both membrane material and contents from the shuttle vesicle to the target compartment is achieved through a fusion process mediated by SNAREs (soluble N-methylmaleimide sensitive factor attachment protein receptors) [38, 49]. After initial recognition via tethering proteins, vesicle SNARES (v-SNARES) interact with target compartment SNARES (t-SNARES) to form a four-helix bundle that undergoes a dramatic conformational change to induce membrane fusion by driving close apposition of the lipid bilayer membranes. There is substantial specificity in these events since only particular cognate pairs of v- and t-SNARES will support fusion. Further specificity may be provided by tethering complexes. Resegregation of the v-SNARES and t-SNARES into their original compartments is achieved by the ATP-dependent NSF/SNAP protein complex.
2.2.3 To Lysosomes-or Not
All the manifold pathways of endocytosis seem to initially lead to the early/recycling endosome compartment. These relatively large structures with a central lumen and multiple tubular extensions play a key role in sorting. Vesicles derived from the clathrin, caveolar or other pathways merge with the early endosomes. Material destined for lysosomal degradation accumulates in the central lumen while that destined to return to the plasma membrane migrates to the tubules where disjunction or ‘pinching off’ of smaller shuttle vesicles occurs [32]. Despite the common nexus of the early endosome, it is clear that receptors, ligands and cargo that have been internalized by different initial endocytotic mechanisms can traffic to distinct subcellular compartments. However, we only partially understand this process. There is evidence suggesting that that membrane domains originating from different internalization pathways maintain their identity within early endosomes. This sets the stage for individualized sorting and trafficking to unique downstream destinations [26].
The Rab5 GTPase plays a key role in maintaining the identity and function of early endosomes (EEs) [50]. Rabex-5, a RabGEF, acts with a cofactor Rabaptin-5 to activate Rab5 on the early endosome surface. This results in the recruitment of Rab5 effectors, including the tethering factor EEA1 that can interact with SNARE proteins, and the PI 3-OH kinase Vps34 that helps to enrich the EE membrane in PI3P. Initially this process is self-sustaining allowing the EE to interact with other EEs and to contribute to recycling of membrane constituents to the cell surface via Rab4 and Rab11 regulated vesicular trafficking pathways [51]. However, eventually other proteins are recruited to the EE that drive its maturation to a late endosome (LE) accompanied by displacement of Rab5 and association with Rab7. Thus two sets of effectors are recruited that seem to work in tandem in the EE to LE conversion. The SAND-1/Mon complex binds PI3P, binds Rab7, displaces Rabex 5 and interacts with the HOPs complex [32]. The HOPS tethering complex includes a GEF for Rab 7 [37] that drives Rab7 activation. Paralleling the Rab5 to Rab7 conversion, the endosome loses ability to interact with EE partners and instead acquires the ability to associate with LE partners.
One of the key aspects of the EE to LE conversion is the formation of intraluminal vesicles (ILVs) to create the late endosome/multivesicular body (LE/MVB) compartment [41, 52]. This serves to further concentrate certain proteins and lipids in the endosome lumen thus directing them to lysosomal degradation. The key agents in this process are the five multi-protein complexes of the ESCRT machinery, ESCRTs 0-III and Vps4-Vta1, that recognize ubiquitinated membrane proteins and drive them into invaginations that ultimately vesiculate into the interior of the LE/MVB. Thus, like the clathrin/dynamin system, the ESCRT assembly is a membrane deforming nanomachine; however, topologically speaking, the ESCRT process is the inverse of clathrin mediated endocytosis. An interesting aspect of the formation of ILVs is their unusual lipid composition that is distinct from the endosomal membrane and is highly enriched in lysobisphosphatidic acid (LBPA)[32]. It should be noted that other lipid changes take place in the progression from the plasma membrane to various endosomal membranes, particularly in terms of phosphoinositides that play a role in the binding of various proteins to endomembrane surfaces [52].
Late endosomes are distinctly different in appearance from EEs, lacking the highly tubular structures and being filled with ILVs. They also migrate from a peripheral to a perinuclear location where they can interact/fuse with other LEs and with lysosomes; the migration is thought to involve dynein-dependent minus end directed microtubular transport [32]. However, in addition to their traditional role as a delivery vehicle to lysosomes, two new roles for LE/MVBs have recently emerged. One is the formation of exosomes during which ILVs are disgorged to the cell exterior where they can be taken up by other cells and thus play a role in cell-cell communication [53, 54]. Since exosomes entrap a sampling of the cytosol, they can be used to convey endogenous miRNAs, or exogenous siRNA (after pre-loading the donor cells), from one cell to another [54, 55]. Another surprising finding, very relevant to the oligonucleotide area, is that elements of the miRNA processing machinery, including the RISC complex, are found associated with endomembranes. While some studies implicate LE/MVBs as the key site [56], others indicate that the RISC complex primarily functions in association with rough endoplasmic reticulum [57]. A excellent recent publication reviews the evidence on this topic [58].
Lysosomes are often disparaged as the being the cell’s trash can but these are actually complex and versatile organelles [59]. Like other trafficking events, fusion between late endosomes and lysosomes is very precisely controlled. An important new role for lysosomes has emerged recently in the context of the complex phenomenon of autophagy [60]. However, not all trafficking pathways lead to lysosomes.
In addition to the recycling events that that take place at the EE level, other alternatives to lysosomal trafficking exist. An important one is the retrograde trafficking pathway that links endosomes to the trans-Golgi [61, 62]. The classic example of retrograde transfer is the recapture of mannose-6 phosphate receptors from endosomes to the Golgi, while their hydrolase ligands journey to lysosomes. However, many pathogens have ‘hijacked’ this pathway for their own use. For example, several bacterial and plant toxins reach the cytosol by following a retrograde pathway to the trans-Golgi and ultimately the endoplasmic reticulum [63]. A large number of proteins are involved in various facets of retrograde transport. For example, EE to trans-Golgi trafficking involves both clathrin and the retromer complex. The retromer includes a Vps26-Vps35-Vps29 trimer that serves as a recognition complex that binds to the cytoplasmic tails of potential cargo proteins. It also includes SNX proteins that have PX domains that recognize phosphoinositides and BAR domains that can sense and alter membrane curvature. This results in the tubulation of the EE membrane and eventual formation of vesicles. The LE to trans-Golgi pathway seems to be distinct and is regulated by the Rab9 GTPase. Retrograde trafficking from EEs and LEs also involves tethering proteins, particularly members of the golgin group, as well as SNARES.
In summary, there are multiple trafficking pathways for membrane-delimited vesicles within cells. The physiological role of these pathways is to transport various cellular macromolecules to the sites where they are needed. However, these pathways can also transport exogenous materials, as pathogens long ago discovered. The intracellular trafficking machinery can potentially also be an efficient means to deliver therapeutic agents such as oligonucleotides. However, we need to attain better understanding and better control of the interactions between therapeutic molecules and the intracellular trafficking machinery.
3. Endocytosis and Trafficking of Oligonucleotides
3.1 Overview
There are thousands of publications on the pharmacokinetics, biodistribution, cellular uptake and biological effects of oligonucleotides as individual molecules or associated with various carriers. To provide an overview of this complex literature, this theme issue contains timely reviews on oligonucleotide pharmacokinetics and biodistribution by Geary and colleagues, on the behavior of oligonucleotide conjugates by Ming and colleagues, and on delivery using lipoplexes or cell-penetrating peptides by Huang and co-workers and by Lebleu and colleagues respectively.
The overall picture of the in vivo behavior of oligonucleotides has been well described [64, 65]. Uncharged oligonucleotides including morpholino and peptide nucleic acid derivatives, as well as most forms of siRNA, are rapidly excreted via the kidney. However, phosphorothioate (PS) oligonucleotides display stronger binding to plasma proteins and cells allowing retention in the body for longer periods. Preferential in vivo uptake by certain cell types including kidney proximal tubule cells and liver Kupffer cells has been observed for PS oligonucleotides and for siRNA [66, 67].
The most widespread approach to delivery of antisense, siRNA or other types of oligonucleotides is to include the nucleic acid into some type of nanoparticle, with the goal of increasing both cell uptake and release from membrane compartments [11, 12, 68–70]. Lipid based carriers have been shown to be efficacious for the delivery of siRNA to the liver [71]. A wide variety of polymers [72] and other types of nanocarriers [73] have also been developed for siRNA delivery. Effective delivery of siRNA to tumors has been challenging, but recently targeted lipid based nanoparticles have provided substantial activity in this context [74]. Importantly, however, the biodistribution of most nanocarriers is limited by the permeability properties of the endothelial barrier that prevents access of nanoparticles to the parenchyma of many tissues [11, 75].
In terms of ‘free’, molecular scale oligonucleotides, cellular uptake mechanisms are strongly influenced by the chemical characteristics of the molecule, but in many cases precise mechanistic insights are lacking. Phosphorothioate-based antisense or splice switching oligonucleotides tend to bind strongly to proteins, including those on the cell surface, and thus can enter cells through non-specific adsorptive endocytosis. Additionally PS-oligonucleotides bind certain serum proteins (e.g. alpha-2 macroglobulin) that in turn bind to specific receptors on some cells [76]. Thus uptake mechanisms for PS-oligonucleotides are complex. Uncharged morpholino or peptide-nucleic acid oligomers are taken up by cells or tissues much less effectively than PS-oligonucleotides, likely by fluid phase endocytosis, and are thus often used in conjunction with cationic cell penetrating peptides [77] as is described in detail in the review by Lebleu and colleagues in this theme issue. Similarly unmodified siRNA is poorly taken up by cells and is thus usually used in association with a nanocarrier or conjugated to a ligand that interacts with a cellular receptor. Interesting studies in cell culture and in mouse models have involved the so-called ‘gymnotic’ uptake of antisense oligonucleotides modified with LNA (locked nucleic acid) moieties. Subcellular distribution studies surprisingly indicated that deoxy LNA oligomers became associated with P-bodies that are usually thought to be sites of siRNA action [78].
Attempts to identify endogenous receptors for antisense or siRNA molecules have proven problematic. Integrins of the beta2 subclass [79] and scavenger receptors [66] have been suggested as candidates, but this is controversial [80]. A putative oligonucleotide transporter has been described [81, 82] but there has been little confirmatory work on this finding by other groups. Another candidate is the mammalian homolog of the double-stranded RNA (dsRNA) transport protein SID-1 found in Caenorhabditis elegans [83]. However, initial reports of a role for SID-1 in uptake of siRNA by mammalian cells [84–86] have not been followed up.
An important consideration concerns the binding of oligonucleotides to proteins both on the cell surface and within the cell. Recently there has been some progress regarding interactions of PS-oligonucleotides with intracellular proteins. For example, the chaperonin T-complex 1 and paraspeckle proteins have shown associations with PS-oligonucleotides [87, 88].
Oligonucleotides conjugated with various ligands have drawn increasing interest in recent years [89, 90]. While initial emphasis was on cholesterol conjugates that would affiliate with plasma lipoproteins [91], more recently ligands that can target specific receptors including aptamers [92, 93], small organic molecules [94], peptides [95, 96], and carbohydrates [97, 98] have all been explored. The characteristics of cell surface receptors that may permit successful oligonucleotide targeting have also been discussed [99]. Presumably ligand-oligonucleotide conjugates will internalize by the same pathway as the ligand receptor itself. For example, we have found that conjugates of antisense or siRNA with RGD (arg-gly-asp) peptides known to bind the αvβ3 integrin internalize via a caveolar pathway, as does the integrin itself [95, 100]. However, backbone chemistry does come into play. Thus there is a much greater differential cell uptake of conjugated versus non-conjugated oligonucleotide for siRNA than for PS-antisense; this is presumably due to the fact that there is considerable non-specific uptake due to the PS-backbone. Recently excellent progress has been made with use of ligand-oligonucleotide conjugates in vivo including those targeting the asialoglyoprotein receptor of liver [98] and integrins of tumor cells [101]. A very novel and interesting recent study involved developing a chemically modified, non-charged form of siRNA followed by conjugation to a carbohydrate moiety that binds the asialoglycoprotein receptor [102]. This entity displayed strong RNAi effects in a mouse model.
3.2. Mechanistic Studies of Oligonucleotide Endocytosis and Trafficking
Despite the extensive literature on the biology of oligonucleotides relatively few studies have addressed the mechanisms of oligonucleotide uptake and intracellular trafficking using state-of-the-art molecular or imaging tools. Several recent reviews, in addition to the current effort, have provided good accounts of some of the issues involved and of the limited progress to date [13, 103–105]. Here we will engage in a highly selective discussion of a few key reports that use effective contemporary techniques to address oligonucleotide uptake and trafficking. We will discuss both molecular scale oligonucleotides and conjugates and some nanocarrier systems. Although not focused on oligonucleotides, a recent report on mass transfer in cells serves as a model for how a combination of molecular biological techniques, sophisticated imaging, and mathematical modeling can provide powerful insights into intracellular trafficking processes [106].
In our own laboratory our initial insights into the functional importance of uptake and trafficking mechanisms came from studies comparing the effects of splice switching oligonucleotides administered either with or without conjugation to a targeting ligand [29, 100, 107]. We found that an oligonucleotide taken up by a receptor-mediated process had greater pharmacological effect than an oligonucleotide taken up by a non-specific mechanism, even when the same level of total oligonucleotide accumulated in the cells. This suggested that the route of internalization could affect the ultimate action of the oligonucleotide. These studies also prompted us to begin to deploy more precise techniques for understanding oligonucleotide trafficking including use of dominant negative mutants of key proteins involved in uptake and trafficking, and Green Fluorescent Protein chimeras of proteins that are well-understood markers of different endomembrane compartments [108, 109].
Other investigators have described similar results regarding trafficking pathways. A report using phosphorothioate antisense oligonucleotides in a transformed liver cell line and in murine livers indicated the co-existence of productive and non-productive paths of uptake [110]. The non-productive pathway apparently involved trafficking to lysosomes, while the pathway that resulted in antisense effects involved trafficking that led to interaction with cellular pre-mRNA. Another interesting report involved delivery of siRNA by targeting Toll-Like Receptors [111]. An un-methylated CpG oligonucleotide able to interact with TLR9 was chemically conjugated to a siRNA. This produced enhanced uptake by TLR-9 expressing dendritic cells, macrophages and B-cells, as well as ‘knockdown’ of endogenous and reporter genes. Interestingly, presence of TLR9 was essential for effective ‘knockdown’ although cells lacking the TLR could still take up the conjugate, thus once again implicating the uptake and trafficking pathway in pharmacological effectiveness.
An interesting report deviated from the standard view that cationic lipid carriers function by delivering siRNA via endocytosis followed by escape from endosomes [112]. It was found that although much of the lipid and siRNA entered cells by some type of endocytosis, only a minor component of the cell-associated siRNA contributed to ‘knock down’ and this component likely came from fusion between the siRNA lipoplexes and the plasma membrane. This study employed molecular tools such as dominant negative forms of dynamin and caveolin to probe lipoplex uptake pathways. Another interesting study examined uptake and trafficking of siRNA within perfluorocarbon nanoparticles [113], and found that delivery was via generation of cell-nanoparticle hemifusion complexes followed by lipid raft mediated internalization. This study effectively used the strategy of co-localization with markers that are known to be internalized through particular pathways.
Spherical nucleic acids are entities comprised of siRNA (or other oligonucleotide) tightly adsorbed to gold nanoparticles. A recent report investigated the intracellular fate of such structures in some detail [114]. Fairly sophisticated imaging, as well as GFP chimeras of specific endomembrane proteins were used to demonstrate trafficking from early to late endosomes but not to lysosomes, followed by gradual degradation and export of the oligonucleotide but not the carrier. A recent study using splice switching peptide nucleic acids conjugated to cell penetrating peptides found striking differences in endocytotic mechanisms between different cell types [115]. Thus skeletal muscle cells took up the conjugate by a caveolin-mediated process while cardiac muscle cells utilized clathrin-mediated endocytosis. In addition, the conjugate seemed to exit from endosomes more easily in differentiated as opposed to undifferentiated skeletal muscle cells.
Two articles appearing simultaneously in 2013 have provided unprecedented insights into the intracellular fates of siRNA lipoplexes. Both articles used advanced imaging techniques as well as powerful molecular tools. Thus Gilleron et al [116] found that lipoplexes were initially taken up by clathrin-mediated endocytosis but that event provoked further accumulation by macropinocytosis. The lipoplexes accumulated in an early-late endosome hybrid compartment but only 1–2% of the siRNA reached the cytosol. Sahay et al [117] also emphasized a role for macropinocytosis, but found that much of the siRNA was re-exported from late endosomes/lysosomes utilizing a process that involved the NPC1 lipid transporter protein.
Perhaps the most important recent study of oligonucleotide trafficking is one that definitively links the endosomal machinery to the pharmacological effects of oligonucleotides [118]. ESCRT-1 (the endosomal sorting complex required for transport) plays a key role in the formation of late endosomes/multi-vesicular bodies. Using an shRNA screen, the investigators found that ‘knock down’ of a component of ESCRT-1 machinery dramatically improved the effects of an oligonucleotide antagomir that targets miR-21. This is an unequivocal demonstration that the trafficking machinery influences the actions of oligonucleotides.
There have been a number of other recent studies examining the uptake and trafficking of various forms of oligonucleotides. Selected examples are summarized in Table 1.
Table 1.
| Oligonucleotide | Experimental System | Delivery Method | Technique Used | Conclusion | Reference |
|---|---|---|---|---|---|
| SSO and siRNA | HeLa cervical cancer cell culture | Cell penetrating peptide | Synthesized large unilamellar vesicles | Cargo type affects CPP ability to permeate membranes | [119] |
| Antisense | Tumor cells | Quantum dots | Transmission electron microscopy | Dual targeting system enhances uptake | [120] |
| Antisense | Primary rat myocardial cell culture and rat myocardial infarction model | Antibody modified liposomes | Time lapse live cell imaging and live animal imaging | Antibody complexed liposomes improved targeted delivery to damaged cells | [121] |
| PMO | A375 melanoma three dimensional cell culture | RGD conjugated albumin | Three dimensional spheroid cultures | RGD enhances particle penetration into spheroids | [122] |
| SSO | A375 melanoma and HeLa cervical cancer cell culture | RGD conjugation, small molecules | Time lapse live cell imaging and colocalization quantification with endosomal compartments | Small molecule Retro-1 can enhance oligonucleotide release from cellular compartments | [123] |
| Double strand ODN | bEnd5 mouse endothelial cell culture and Balb/c mouse perfusion | Antibody modified PEI | Double labeled (Oligo & PEI) for tracking complex dissociation in vivo | Antibody modified PEI improves penetration of the blood brain barrier | [124] |
| Antisense | KASUMI-1, MV4-11, and K562 AML cell culture | Cenersen | Hybridization based fluorescence ELISA | ELISA can measure concentration of oligonucleotide in plasma and cells | [125] |
In summary, there are diverse routes for the initial uptake of oligonucleotides presented in various forms including clathrin-dependent, caveolin-dependent and non-clathrin/caveolin dependent pathways. There is also substantial cell type dependent variation in the uptake processes. After initial uptake oligonucleotides can traffic within cells by routes that are more or less productive in terms of ultimate pharmacological effect. Finally there is an indication that the late endosome compartment may play a key role in functional delivery of oligonucleotides to the cytosol and nucleus.
3.3. Escape From Endosomes During Intracellular Trafficking
The complex pathways of endocytosis and trafficking are intended to move endogenous molecules to their appropriate cellular destinations. While that machinery is usually quite efficient, there are nonetheless opportunities for molecules to be released from endomembrane compartments to the cytosol. Intracellular trafficking involves a dynamic flux of membrane vesicles that participate in a plethora of fusion and fission events. Fusion mechanisms in both natural and artificial lipid membranes have been studied extensively [126, 127]. Although this area is largely beyond the scope of this review, it is important to note a few key points. First, fusion creates localized stress that can result in the formation of non-bilayer lipid domains in the fusion partners [126, 128]. Second, it has been observed that non-bilayer regions can be much more permeable to solutes than bilayer regions [129, 130]. Third, enveloped viruses that fuse with cell membranes often utilize specialized proteins that act in a manner similar to cellular SNARE proteins; the influenza virus fusion protein is a good example [131]. These proteins can also induce membrane permeability increases [132]. Thus there is an inherent relationship between the fusion events involved in intracellular trafficking and the opportunity for leakage of vesicular contents (see Figure 2). Therefor the activity of oligonucleotides in cells may be attributable to a low level of continuous leakage from endomembrane vesicles that takes place during intracellular trafficking.
There are several loci in the intracellular trafficking network that may be particularly susceptible to increases in permeability that may allow egress of oligonucleotides to the cytosol [31]. One is at the stage of early/sorting endosomes where there is extensive tubulation and formation of vesicles for return of receptors to the plasma membrane via Rab 4 or Rab 11 dependent mechanisms. However, most studies suggest that materials transit rather rapidly through the sorting endosome compartment. A second locus is at the stage of LE/MVBs where the ESCRT complex directs the formation of inward protrusions of the endosome membrane that ultimately become intraluminal vesicles Third, retrograde traffic from early or late endosomes utilizing the retromer complex or Rab9 mechanisms offers another possibility for membrane instability during tubulation and vesicle formation. Finally, SNARE driven membrane fusions clearly afford opportunities for partial leakage of vesicular contents. Several publications have observed such leakage using in vitro systems designed to mimic fusions driven by SNAREs or viral fusion proteins [132, 133]. It seems feasible to explore some of these potential mechanisms for oligonucleotide delivery to the cytosol. For example, one could use siRNAs to reduce expression of key proteins involved in a particular mechanism, or alternatively use dominant negative versions of those proteins, and determine if that influences the pharmacological effect of an antisense or siRNA that addresses a different target. Another approach would be to use cell lines with genetic defects in intracellular transport processes. There are now several examples of studies using such approaches in the oligonucleotide literature [109, 110, 117, 118].
Once oligonucleotides reach the cytosol they have good access to their ultimate sites of action. Thus for siRNAs the RISC machinery is cytosolic. Antisense and SSOs that primarily act within the nucleus, particularly those with phosphorothioate backbones, are able to rapidly shuttle between the nucleus and the cytoplasm. This process is mediated by nuclear pore structures but does not involve classical nuclear localization signals [134]. For conventional phosphodiester oligonucleotides both passive diffusion and active transport have been described as nuclear entry mechanisms.
3.4 Small Molecules that Affect Intracellular Trafficking
The plethora of unique proteins and lipids involved in intracellular trafficking present an opportunity to develop a chemical biology approach to the elucidation of underlying mechanisms, as well as the possibility of manipulating trafficking for therapeutic purposes. However this approach is not very well developed currently. A recent review article provides a good overview of the limited number of small molecules that affect trafficking that have been developed to date [135].
Most effort has focused on inhibitors of endocytosis. This has been of interest for a long time and a number of drugs have traditionally been used as supposedly selective blockers of various endocytotic routes. This includes chloropromazine for clathrin-mediated endocytosis, amiloride for macropinocytosis, cholesterol depletion by cyclodextrins for caveolar uptake, as well as others. However, these approaches rest on very shaky ground as these agents are known to have multiple effects on cells in addition to the intended one [135]. More recently several agents have emerged that are more precise in their actions on endocytotic mechanisms. Dynasore [136] is a highly selective inhibitor of the dynamin GTPase and thus affects all routes that involve this protein. Improved versions of dynasore are under development [137]. The dynoles are another group of compounds that are excellent inhibitors of dynamin [138]. The pitstops [139] are small molecules that bind to clathrin and are putatively selective inhibitors of clathrin-mediated endocytosis. However, one report suggests that pitstops may also affect clathrin-independent endocytosis [140] thus emphasizing the difficulty in designing truly selective inhibitors. Various inhibitors of endocytosis will be useful in defining pathways of oligonucleotide trafficking. However, they obviously will not enhance effects of oligonucleotides that require endocytosis for uptake. For that we need to turn to intracellular trafficking processes.
A number of agents have been developed that affect the organization of intracellular organelles or the dynamics of trafficking processes. One of the best known is brefeldin A a fungal metabolite that disrupts the organization of the Golgi apparatus by inhibiting several guanine nucleotide exchange factors (GEFs) for the Arf1 GTPase [135]. Golgicide is a more recently developed compound that selectively inhibits the GBF1 Arf-GEF thus more precisely defining its role [141]; like brefeldin, golgicide disrupts the organization of the Golgi apparatus and trans-Golgi network.
Some of the most interesting compounds have come from screens designed to find molecules that block the pathogenic effects of bacterial or plant toxins [142–144]. Screening for agents that block anthrax toxin led to a molecule, EGA, that seemed to act at the early endosome to late endosome transfer stage and that affected other proteins that traffic via acidified compartments [144]. However, EGA did not affect intra-lysosomal pH. Interestingly, EGA did not affect toxins such as ricin that traffic via the retrograde pathway. In contrast, a high throughput screen for molecules that block ricin toxicity led to a series of molecules termed ‘Retro compounds’ that affect retrograde transport to the trans-Golgi [143]. These molecules very selectively inhibited the trafficking and actions of those toxins such as shiga and ricin that utilize this pathway. However, the Retro compound had little effect on other trafficking pathways including recycling of transferrin receptor and biosynthesis and export of viral proteins. In contrast to agents such as Golgicide (which can also block toxin effects) there was no disruption of the morphology of intracellular organelles; the only biochemical correlate of Retro action observed thus far was the re-localization of the SNARE protein syntaxin 5.
One of the compounds that emerged from the ricin screen has been tested for actions on oligonucleotides. Thus the agent Retro-1 was shown to strongly enhance the effects of antisense, splice-switching, and siRNA oligonucleotides in cell culture models [123, 145]. This compound acts by selective partial release of oligonucleotides from late endosomes, but not from lysosomes or other intracellular compartments. These effects take place at concentrations that are minimally toxic. Retro-1 also displayed modest but clear-cut oligonucleotide enhancing effects in a xenograft model. It is surprising that a compound that blocks endosome to trans-Golgi retrograde transfer would also cause release of contents from late endosomes. However, while Retro-1 effectively blocks toxin trafficking at approximately 20 uM it requires a higher concentration of 80–100 uM to significantly enhance oligonucleotide effects. Thus it is possible that the actions of Retro-1 on oligonucleotides represents a ‘side-effect’ with a different mechanism than its effects on toxins.
The observations with Retro-1 opened the path to using small molecules to improve the pharmacological effects of oligonucleotides. While Retro-1 is less than ideal because of its relatively low potency, we felt that it should be possible to identify small molecules with similar actions but of greater potency and efficacy. We pursued this by high throughput screening of >150,000 compounds for their ability to enhance the effects of a splice switching oligonucleotide on a luciferase reporter. We identified several compound series, one of which has been recently published [146]. When used at ~10 uM levels, these 3-deazapteridine analogs strongly enhance the effect of antisense, siRNA and splice switching oligonucleotides in cell culture; often they are as effective as cationic lipid delivery agents. They act in a manner similar to Retro-1 in that they trigger release of oligonucleotide from late endosomes; however the molecular targets of the 3-deazapteridines and the Retro-1 compounds are likely distinct since there is no structural similarity between these molecules. The 3-deazapteridines were also effective in a transgenic mouse model that involves induction of a reporter gene by splice switching oligonucleotides.
There have also been a few other studies that connect small molecules to intracellular delivery of nucleic acids. For example, small molecule drugs that inhibit protein kinase A activity can prevent trafficking of polyplexes and lipoplexes into the late endosomal/lysosomal compartments thus improving the transfection efficiency [147]. Another interesting study, primarily germane to muscle tissue, found that the clinically utilized drug dantrolene improved the effects of SSOs that correct dystrophin expression in a model of Duchennne muscular dystrophy [148]; however the precise mechanism of action is unclear. Yet another study demonstrated increased delivery of siRNA using guanidine-like small molecules that complex with the siRNA and that may also bind to cell surface proteoglycans to improve cell uptake [149]. A recent conference proceeding [150] described a small molecule, Guanabenz, that also complexed with siRNA and enhanced uptake and effects in cell culture. Thus there is increasing interest in the use of small molecule/chemical biology approaches to enhance pharmacological effects of oligonucleotides.
4. Conclusions
The therapeutic development of antisense, siRNA, SSOs and other types of oligonucleotides has, to some degree, outstripped our fundamental knowledge of how these molecules behave in cells and in the body. Thus although there is much information regarding the overall pharmacokinetics and biodistribution of oligonucleotides, there is not an equivalent depth of knowledge about their behavior at the cellular and intracellular levels. Recent work has uncovered some interesting and surprising observations regarding cellular uptake and intracellular trafficking of oligonucleotides. For example, conventional phosphorothioate antisense molecules seem to have an unusual uptake mechanism involving both a productive and a less productive path to nuclear sites of action. Our studies and those of others have shown that the pharmacological effectiveness of an oligonucleotide can strongly depend on its route of uptake and trafficking. In particular, certain receptor-mediated processes seem to support productive delivery. Detailed studies of the uptake and trafficking of siRNA lipoplexes have identified both escape pathways from endosomes and recycling to the cell exterior. Emerging studies are beginning to identify small molecules that can enhance oligonucleotide effects by modulating their intracellular trafficking; this could be an important new approach for oligonucleotide delivery. As the field of oligonucleotide therapeutics matures, and particularly with increasing emphasis on enhancing specificity through targeted delivery, it will be important to take account of fundamental cell biological principles. Thus additional insights into the intracellular trafficking of oligonucleotides will greatly facilitate the design of improved delivery systems for the therapeutic use of these molecules.
Acknowledgments
This work was supported by NIH grant RO1 CA151964 to RLJ.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- 1.Zamecnik PC, Stephenson ML. Inhibition of Rous sarcoma virus replication and cell transformation by a specific oligodeoxynucleotide. Proc Natl Acad Sci U S A. 1978;75:280–284. doi: 10.1073/pnas.75.1.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mello CC, Conte D., Jr Revealing the world of RNA interference. Nature. 2004;431:338–342. doi: 10.1038/nature02872. [DOI] [PubMed] [Google Scholar]
- 3.Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- 4.Moazed D. Small RNAs in transcriptional gene silencing and genome defence. Nature. 2009;457:413–420. doi: 10.1038/nature07756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tse MT. Regulatory watch: Antisense approval provides boost to field. Nature Reviews Drug Discovery. 2013;12:179. [Google Scholar]
- 6.Coelho T, Adams D, Silva A, Lozeron P, Hawkins PN, Mant T, Perez J, Chiesa J, Warrington S, Tranter E, Munisamy M, Falzone R, Harrop J, Cehelsky J, Bettencourt BR, Geissler M, Butler JS, Sehgal A, Meyers RE, Chen Q, Borland T, Hutabarat RM, Clausen VA, Alvarez R, Fitzgerald K, Gamba-Vitalo C, Nochur SV, Vaishnaw AK, Sah DW, Gollob JA, Suhr OB. Safety and efficacy of RNAi therapy for transthyretin amyloidosis. The New England journal of medicine. 2013;369:819–829. doi: 10.1056/NEJMoa1208760. [DOI] [PubMed] [Google Scholar]
- 7.Miller TM, Pestronk A, David W, Rothstein J, Simpson E, Appel SH, Andres PL, Mahoney K, Allred P, Alexander K, Ostrow LW, Schoenfeld D, Macklin EA, Norris DA, Manousakis G, Crisp M, Smith R, Bennett CF, Bishop KM, Cudkowicz ME. An antisense oligonucleotide against SOD1 delivered intrathecally for patients with SOD1 familial amyotrophic lateral sclerosis: a phase 1, randomised, first-in-man study. Lancet neurology. 2013;12:435–442. doi: 10.1016/S1474-4422(13)70061-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koo T, Wood MJ. Clinical trials using antisense oligonucleotides in duchenne muscular dystrophy. Human gene therapy. 2013;24:479–488. doi: 10.1089/hum.2012.234. [DOI] [PubMed] [Google Scholar]
- 9.Raal FJ, Santos RD, Blom DJ, Marais AD, Charng MJ, Cromwell WC, Lachmann RH, Gaudet D, Tan JL, Chasan-Taber S, Tribble DL, Flaim JD, Crooke ST. Mipomersen, an apolipoprotein B synthesis inhibitor, for lowering of LDL cholesterol concentrations in patients with homozygous familial hypercholesterolaemia: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;375:998–1006. doi: 10.1016/S0140-6736(10)60284-X. [DOI] [PubMed] [Google Scholar]
- 10.Bianchini D, Omlin A, Pezaro C, Lorente D, Ferraldeschi R, Mukherji D, Crespo M, Figueiredo I, Miranda S, Riisnaes R, Zivi A, Buchbinder A, Rathkopf DE, Attard G, Scher HI, de Bono J, Danila DC. First-in-human Phase I study of EZN-4176, a locked nucleic acid antisense oligonucleotide to exon 4 of the androgen receptor mRNA in patients with castration-resistant prostate cancer. British journal of cancer. 2013;109:2579–2586. doi: 10.1038/bjc.2013.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Juliano R, Bauman J, Kang H, Ming X. Biological barriers to therapy with antisense and siRNA oligonucleotides. Mol Pharm. 2009;6:686–695. doi: 10.1021/mp900093r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Whitehead KA, Langer R, Anderson DG. Knocking down barriers: advances in siRNA delivery. Nat Rev Drug Discov. 2009;8:129–138. doi: 10.1038/nrd2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Varkouhi AK, Scholte M, Storm G, Haisma HJ. Endosomal escape pathways for delivery of biologicals. J Control Release. 2011;151:220–228. doi: 10.1016/j.jconrel.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 14.Kirchhausen T, Owen D, Harrison SC. Molecular structure, function, and dynamics of clathrin-mediated membrane traffic. Cold Spring Harbor perspectives in biology. 2014;6:a016725. doi: 10.1101/cshperspect.a016725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doherty GJ, McMahon HT. Mechanisms of endocytosis. Annu Rev Biochem. 2009;78:857–902. doi: 10.1146/annurev.biochem.78.081307.110540. [DOI] [PubMed] [Google Scholar]
- 16.Mayor S, Parton RG, Donaldson JG. Clathrin-independent pathways of endocytosis. Cold Spring Harbor perspectives in biology. 2014;6 doi: 10.1101/cshperspect.a016758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mettlen M, Pucadyil T, Ramachandran R, Schmid SL. Dissecting dynamin’s role in clathrin-mediated endocytosis. Biochem Soc Trans. 2009;37:1022–1026. doi: 10.1042/BST0371022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doherty GJ, McMahon HT. Mechanisms of endocytosis. Annual review of biochemistry. 2009;78:857–902. doi: 10.1146/annurev.biochem.78.081307.110540. [DOI] [PubMed] [Google Scholar]
- 19.Wolfe BL, Trejo J. Clathrin-dependent mechanisms of G protein-coupled receptor endocytosis. Traffic. 2007;8:462–470. doi: 10.1111/j.1600-0854.2007.00551.x. [DOI] [PubMed] [Google Scholar]
- 20.Lajoie P, Nabi IR. Lipid rafts, caveolae, and their endocytosis. Int Rev Cell Mol Biol. 2010;282:135–163. doi: 10.1016/S1937-6448(10)82003-9. [DOI] [PubMed] [Google Scholar]
- 21.Parton RG, del Pozo MA. Caveolae as plasma membrane sensors, protectors and organizers. Nat Rev Mol Cell Biol. 2013;14:98–112. doi: 10.1038/nrm3512. [DOI] [PubMed] [Google Scholar]
- 22.Lingwood D, Simons K. Lipid rafts as a membrane-organizing principle. Science. 2010;327:46–50. doi: 10.1126/science.1174621. [DOI] [PubMed] [Google Scholar]
- 23.Sorkin A, von Zastrow M. Endocytosis and signalling: intertwining molecular networks. Nat Rev Mol Cell Biol. 2009;10:609–622. doi: 10.1038/nrm2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sandvig K, Pust S, Skotland T, van Deurs B. Clathrin-independent endocytosis: mechanisms and function. Curr Opin Cell Biol. 2011;23:413–420. doi: 10.1016/j.ceb.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 25.Glebov OO, Bright NA, Nichols BJ. Flotillin-1 defines a clathrin-independent endocytic pathway in mammalian cells. Nat Cell Biol. 2006;8:46–54. doi: 10.1038/ncb1342. [DOI] [PubMed] [Google Scholar]
- 26.Howes MT, Mayor S, Parton RG. Molecules, mechanisms, and cellular roles of clathrin-independent endocytosis. Curr Opin Cell Biol. 2010;22:519–527. doi: 10.1016/j.ceb.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 27.Kerr MC, Teasdale RD. Defining macropinocytosis. Traffic. 2009;10:364–371. doi: 10.1111/j.1600-0854.2009.00878.x. [DOI] [PubMed] [Google Scholar]
- 28.Kunz S. Receptor binding and cell entry of Old World arenaviruses reveal novel aspects of virus-host interaction. Virology. 2009;387:245–249. doi: 10.1016/j.virol.2009.02.042. [DOI] [PubMed] [Google Scholar]
- 29.Alam MR, Ming X, Dixit V, Fisher M, Chen X, Juliano RL. The biological effect of an antisense oligonucleotide depends on its route of endocytosis and trafficking. Oligonucleotides. 2010;20:103–109. doi: 10.1089/oli.2009.0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Angers CG, Merz AJ. New links between vesicle coats and Rab-mediated vesicle targeting. Semin Cell Dev Biol. 2011;22:18–26. doi: 10.1016/j.semcdb.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scott CC, Vacca F, Gruenberg J. Endosome maturation, transport and functions. Semin Cell Dev Biol. 2014;31:2–10. doi: 10.1016/j.semcdb.2014.03.034. [DOI] [PubMed] [Google Scholar]
- 32.Huotari J, Helenius A. Endosome maturation. The EMBO journal. 2011;30:3481–3500. doi: 10.1038/emboj.2011.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johannes L, Wunder C. Retrograde transport: two (or more) roads diverged in an endosomal tree? Traffic. 2011;12:956–962. doi: 10.1111/j.1600-0854.2011.01200.x. [DOI] [PubMed] [Google Scholar]
- 34.Kerr M, Teasdale RD. Live imaging of endosome dynamics. Semin Cell Dev Biol. 2014;31:11–19. doi: 10.1016/j.semcdb.2014.03.027. [DOI] [PubMed] [Google Scholar]
- 35.Pfeffer SR. Rab GTPase regulation of membrane identity. Curr Opin Cell Biol. 2013;25:414–419. doi: 10.1016/j.ceb.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barr FA. Review series: Rab GTPases and membrane identity: causal or inconsequential? J Cell Biol. 2013;202:191–199. doi: 10.1083/jcb.201306010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hutagalung AH, Novick PJ. Role of Rab GTPases in membrane traffic and cell physiology. Physiol Rev. 2011;91:119–149. doi: 10.1152/physrev.00059.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hong W, Lev S. Tethering the assembly of SNARE complexes. Trends in cell biology. 2014;24:35–43. doi: 10.1016/j.tcb.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 39.Brocker C, Engelbrecht-Vandre S, Ungermann C. Multisubunit tethering complexes and their role in membrane fusion. Curr Biol. 2010;20:R943–952. doi: 10.1016/j.cub.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 40.Solinger JA, Spang A. Tethering complexes in the endocytic pathway: CORVET and HOPS. The FEBS journal. 2013;280:2743–2757. doi: 10.1111/febs.12151. [DOI] [PubMed] [Google Scholar]
- 41.Henne WM, Buchkovich NJ, Emr SD. The ESCRT pathway. Dev Cell. 2011;21:77–91. doi: 10.1016/j.devcel.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 42.Attar N, Cullen PJ. The retromer complex. Adv Enzyme Regul. 2010;50:216–236. doi: 10.1016/j.advenzreg.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 43.Mellman I, Emr SD. A Nobel Prize for membrane traffic: vesicles find their journey’s end. J Cell Biol. 2013;203:559–561. doi: 10.1083/jcb.201310134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cai H, Reinisch K, Ferro-Novick S. Coats, tethers, Rabs, and SNAREs work together to mediate the intracellular destination of a transport vesicle. Dev Cell. 2007;12:671–682. doi: 10.1016/j.devcel.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 45.Barlan K, Rossow MJ, Gelfand VI. The journey of the organelle: teamwork and regulation in intracellular transport. Curr Opin Cell Biol. 2013;25:483–488. doi: 10.1016/j.ceb.2013.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nature reviews Molecular cell biology. 2009;10:513–525. doi: 10.1038/nrm2728. [DOI] [PubMed] [Google Scholar]
- 47.Pfeffer SR. Rab GTPase regulation of membrane identity. Curr Opin Cell Biol. 2013 doi: 10.1016/j.ceb.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bonifacino JS, Hurley JH. Retromer. Curr Opin Cell Biol. 2008;20:427–436. doi: 10.1016/j.ceb.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Malsam J, Kreye S, Sollner TH. Membrane fusion: SNAREs and regulation. Cellular and molecular life sciences : CMLS. 2008;65:2814–2832. doi: 10.1007/s00018-008-8352-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zerial M, McBride H. Rab proteins as membrane organizers. Nat Rev Mol Cell Biol. 2001;2:107–117. doi: 10.1038/35052055. [DOI] [PubMed] [Google Scholar]
- 51.Hsu VW, Prekeris R. Transport at the recycling endosome. Curr Opin Cell Biol. 2010;22:528–534. doi: 10.1016/j.ceb.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bissig C, Gruenberg J. Lipid sorting and multivesicular endosome biogenesis. Cold Spring Harbor perspectives in biology. 2013;5:a016816. doi: 10.1101/cshperspect.a016816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kowal J, Tkach M, Thery C. Biogenesis and secretion of exosomes. Curr Opin Cell Biol. 2014;29C:116–125. doi: 10.1016/j.ceb.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 54.El Andaloussi S, Lakhal S, Mager I, Wood MJ. Exosomes for targeted siRNA delivery across biological barriers. Adv Drug Deliv Rev. 2013;65:391–397. doi: 10.1016/j.addr.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 55.Penfornis P, Vallabhaneni KC, Whitt J, Pochampally R. Extracellular vesicles as carriers of microRNA, proteins and lipids in tumor microenvironment. Int J Cancer. 2015 doi: 10.1002/ijc.29417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gibbings DJ, Ciaudo C, Erhardt M, Voinnet O. Multivesicular bodies associate with components of miRNA effector complexes and modulate miRNA activity. Nat Cell Biol. 2009;11:1143–1149. doi: 10.1038/ncb1929. [DOI] [PubMed] [Google Scholar]
- 57.Stalder L, Heusermann W, Sokol L, Trojer D, Wirz J, Hean J, Fritzsche A, Aeschimann F, Pfanzagl V, Basselet P, Weiler J, Hintersteiner M, Morrissey DV, Meisner-Kober NC. The rough endoplasmatic reticulum is a central nucleation site of siRNA-mediated RNA silencing. The EMBO journal. 2013;32:1115–1127. doi: 10.1038/emboj.2013.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim YJ, Maizel A, Chen X. Traffic into silence: endomembranes and post-transcriptional RNA silencing. The EMBO journal. 2014;33:968–980. doi: 10.1002/embj.201387262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Luzio JP, Pryor PR, Bright NA. Lysosomes: fusion and function. Nat Rev Mol Cell Biol. 2007;8:622–632. doi: 10.1038/nrm2217. [DOI] [PubMed] [Google Scholar]
- 60.Marino G, Madeo F, Kroemer G. Autophagy for tissue homeostasis and neuroprotection. Curr Opin Cell Biol. 2011;23:198–206. doi: 10.1016/j.ceb.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 61.Johannes L, Popoff V. Tracing the retrograde route in protein trafficking. Cell. 2008;135:1175–1187. doi: 10.1016/j.cell.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 62.Chia PZ, Gunn P, Gleeson PA. Cargo trafficking between endosomes and the trans-Golgi network. Histochemistry and cell biology. 2013;140:307–315. doi: 10.1007/s00418-013-1125-6. [DOI] [PubMed] [Google Scholar]
- 63.Sandvig K, Skotland T, van Deurs B, Klokk TI. Retrograde transport of protein toxins through the Golgi apparatus. Histochemistry and cell biology. 2013;140:317–326. doi: 10.1007/s00418-013-1111-z. [DOI] [PubMed] [Google Scholar]
- 64.Geary RS. Antisense oligonucleotide pharmacokinetics and metabolism. Expert Opin Drug Metab Toxicol. 2009;5:381–391. doi: 10.1517/17425250902877680. [DOI] [PubMed] [Google Scholar]
- 65.Bennett CF, Swayze EE. RNA targeting therapeutics: molecular mechanisms of antisense oligonucleotides as a therapeutic platform. Annu Rev Pharmacol Toxicol. 2010;50:259–293. doi: 10.1146/annurev.pharmtox.010909.105654. [DOI] [PubMed] [Google Scholar]
- 66.Butler M, Stecker K, Bennett CF. Cellular distribution of phosphorothioate oligodeoxynucleotides in normal rodent tissues. Lab Invest. 1997;77:379–388. [PubMed] [Google Scholar]
- 67.Molitoris BA, Dagher PC, Sandoval RM, Campos SB, Ashush H, Fridman E, Brafman A, Faerman A, Atkinson SJ, Thompson JD, Kalinski H, Skaliter R, Erlich S, Feinstein E. siRNA targeted to p53 attenuates ischemic and cisplatin-induced acute kidney injury. J Am Soc Nephrol. 2009;20:1754–1764. doi: 10.1681/ASN.2008111204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Akhtar S, Benter IF. Nonviral delivery of synthetic siRNAs in vivo. J Clin Invest. 2007;117:3623–3632. doi: 10.1172/JCI33494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tamura A, Nagasaki Y. Smart siRNA delivery systems based on polymeric nanoassemblies and nanoparticles. Nanomedicine (Lond) 2010;5:1089–1102. doi: 10.2217/nnm.10.76. [DOI] [PubMed] [Google Scholar]
- 70.Szoka F. Molecular biology. The art of assembly. Science. 2008;319:578–579. doi: 10.1126/science.1154253. [DOI] [PubMed] [Google Scholar]
- 71.Love KT, Mahon KP, Levins CG, Whitehead KA, Querbes W, Dorkin JR, Qin J, Cantley W, Qin LL, Racie T, Frank-Kamenetsky M, Yip KN, Alvarez R, Sah DW, de Fougerolles A, Fitzgerald K, Koteliansky V, Akinc A, Langer R, Anderson DG. Lipid-like materials for low-dose, in vivo gene silencing. Proc Natl Acad Sci U S A. 2010;107:1864–1869. doi: 10.1073/pnas.0910603106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kabanov AV, Vinogradov SV. Nanogels as pharmaceutical carriers: finite networks of infinite capabilities. Angew Chem Int Ed Engl. 2009;48:5418–5429. doi: 10.1002/anie.200900441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Petros RA, DeSimone JM. Strategies in the design of nanoparticles for therapeutic applications. Nat Rev Drug Discov. 2010;9:615–627. doi: 10.1038/nrd2591. [DOI] [PubMed] [Google Scholar]
- 74.Tseng YC, Mozumdar S, Huang L. Lipid-based systemic delivery of siRNA. Adv Drug Deliv Rev. 2009;61:721–731. doi: 10.1016/j.addr.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Maeda H, Nakamura H, Fang J. The EPR effect for macromolecular drug delivery to solid tumors: Improvement of tumor uptake, lowering of systemic toxicity, and distinct tumor imaging in vivo. Adv Drug Deliv Rev. 2013;65:71–79. doi: 10.1016/j.addr.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 76.Bennett CF, Swayze EE. RNA targeting therapeutics: molecular mechanisms of antisense oligonucleotides as a therapeutic platform. Annu Rev Pharmacol Toxicol. 50:259–293. doi: 10.1146/annurev.pharmtox.010909.105654. [DOI] [PubMed] [Google Scholar]
- 77.Betts CA, Wood MJ. Cell penetrating peptide delivery of splice directing oligonucleotides as a treatment for Duchenne muscular dystrophy. Current pharmaceutical design. 2013;19:2948–2962. doi: 10.2174/1381612811319160009. [DOI] [PubMed] [Google Scholar]
- 78.Stein CA, Hansen JB, Lai J, Wu S, Voskresenskiy A, Hog A, Worm J, Hedtjarn M, Souleimanian N, Miller P, Soifer HS, Castanotto D, Benimetskaya L, Orum H, Koch T. Efficient gene silencing by delivery of locked nucleic acid antisense oligonucleotides, unassisted by transfection reagents. Nucleic Acids Res. 2010;38:e3. doi: 10.1093/nar/gkp841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Benimetskaya L, Loike JD, Khaled Z, Loike G, Silverstein SC, Cao L, el Khoury J, Cai TQ, Stein CA. Mac-1 (CD11b/CD18) is an oligodeoxynucleotide-binding protein. Nat Med. 1997;3:414–420. doi: 10.1038/nm0497-414. [DOI] [PubMed] [Google Scholar]
- 80.Butler M, Crooke RM, Graham MJ, Lemonidis KM, Lougheed M, Murray SF, Witchell D, Steinbrecher U, Bennett CF. Phosphorothioate oligodeoxynucleotides distribute similarly in class A scavenger receptor knockout and wild-type mice. J Pharmacol Exp Ther. 2000;292:489–496. [PubMed] [Google Scholar]
- 81.Hanss B, Leal-Pinto E, Bruggeman LA, Copeland TD, Klotman PE. Identification and characterization of a cell membrane nucleic acid channel. Proc Natl Acad Sci U S A. 1998;95:1921–1926. doi: 10.1073/pnas.95.4.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hanss B, Leal-Pinto E, Teixeira A, Christian RE, Shabanowitz J, Hunt DF, Klotman PE. Cytosolic malate dehydrogenase confers selectivity of the nucleic acid-conducting channel. Proc Natl Acad Sci U S A. 2002;99:1707–1712. doi: 10.1073/pnas.022355499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Winston WM, Sutherlin M, Wright AJ, Feinberg EH, Hunter CP. Caenorhabditis elegans SID-2 is required for environmental RNA interference. Proc Natl Acad Sci U S A. 2007;104:10565–10570. doi: 10.1073/pnas.0611282104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Duxbury MS, Ashley SW, Whang EE. RNA interference: a mammalian SID-1 homologue enhances siRNA uptake and gene silencing efficacy in human cells. Biochem Biophys Res Commun. 2005;331:459–463. doi: 10.1016/j.bbrc.2005.03.199. [DOI] [PubMed] [Google Scholar]
- 85.Tsang SY, Moore JC, Huizen RV, Chan CW, Li RA. Ectopic expression of systemic RNA interference defective protein in embryonic stem cells. Biochem Biophys Res Commun. 2007;357:480–486. doi: 10.1016/j.bbrc.2007.03.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wolfrum C, Shi S, Jayaprakash KN, Jayaraman M, Wang G, Pandey RK, Rajeev KG, Nakayama T, Charrise K, Ndungo EM, Zimmermann T, Koteliansky V, Manoharan M, Stoffel M. Mechanisms and optimization of in vivo delivery of lipophilic siRNAs. Nat Biotechnol. 2007;25:1149–1157. doi: 10.1038/nbt1339. [DOI] [PubMed] [Google Scholar]
- 87.Liang XH, Shen W, Sun H, Prakash TP, Crooke ST. TCP1 complex proteins interact with phosphorothioate oligonucleotides and can co-localize in oligonucleotide-induced nuclear bodies in mammalian cells. Nucleic Acids Res. 2014;42:7819–7832. doi: 10.1093/nar/gku484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shen W, Liang XH, Crooke ST. Phosphorothioate oligonucleotides can displace NEAT1 RNA and form nuclear paraspeckle-like structures. Nucleic Acids Res. 2014;42:8648–8662. doi: 10.1093/nar/gku579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Juliano RL, Ming X, Nakagawa O. The chemistry and biology of oligonucleotide conjugates. Accounts of chemical research. 2012;45:1067–1076. doi: 10.1021/ar2002123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nielsen C, Kjems J, Sorensen KR, Engelholm LH, Behrendt N. Advances in targeted delivery of small interfering RNA using simple bioconjugates. Expert Opin Drug Deliv. 2014;11:791–822. doi: 10.1517/17425247.2014.896898. [DOI] [PubMed] [Google Scholar]
- 91.Zimmermann TS, Lee AC, Akinc A, Bramlage B, Bumcrot D, Fedoruk MN, Harborth J, Heyes JA, Jeffs LB, John M, Judge AD, Lam K, McClintock K, Nechev LV, Palmer LR, Racie T, Rohl I, Seiffert S, Shanmugam S, Sood V, Soutschek J, Toudjarska I, Wheat AJ, Yaworski E, Zedalis W, Koteliansky V, Manoharan M, Vornlocher HP, MacLachlan I. RNAi-mediated gene silencing in non-human primates. Nature. 2006;441:111–114. doi: 10.1038/nature04688. [DOI] [PubMed] [Google Scholar]
- 92.Dassie JP, Liu XY, Thomas GS, Whitaker RM, Thiel KW, Stockdale KR, Meyerholz DK, McCaffrey AP, McNamara JO, 2nd, Giangrande PH. Systemic administration of optimized aptamer-siRNA chimeras promotes regression of PSMA-expressing tumors. Nat Biotechnol. 2009;27:839–849. doi: 10.1038/nbt.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kotula JW, Pratico ED, Ming X, Nakagawa O, Juliano RL, Sullenger BA. Aptamer-mediated delivery of splice-switching oligonucleotides to the nuclei of cancer cells. Nucleic acid therapeutics. 2012;22:187–195. doi: 10.1089/nat.2012.0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nakagawa O, Ming X, Huang L, Juliano RL. Targeted intracellular delivery of antisense oligonucleotides via conjugation with small-molecule ligands. J Am Chem Soc. 2010;132:8848–8849. doi: 10.1021/ja102635c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Alam MR, Ming X, Fisher M, Lackey JG, Rajeev KG, Manoharan M, Juliano RL. Multivalent Cyclic RGD Conjugates for Targeted Delivery of Small Interfering RNA. Bioconjug Chem. 2011;22:1673–1681. doi: 10.1021/bc200235q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sethi D, Chen CP, Jing RY, Thakur ML, Wickstrom E. Fluorescent peptide-PNA chimeras for imaging monoamine oxidase A mRNA in neuronal cells. Bioconjug Chem. 2012;23:158–163. doi: 10.1021/bc2004507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Akinc A, Querbes W, De S, Qin J, Frank-Kamenetsky M, Jayaprakash KN, Jayaraman M, Rajeev KG, Cantley WL, Dorkin JR, Butler JS, Qin L, Racie T, Sprague A, Fava E, Zeigerer A, Hope MJ, Zerial M, Sah DW, Fitzgerald K, Tracy MA, Manoharan M, Koteliansky V, Fougerolles A, Maier MA. Targeted delivery of RNAi therapeutics with endogenous and exogenous ligand-based mechanisms. Mol Ther. 2010;18:1357–1364. doi: 10.1038/mt.2010.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nair JK, Willoughby JL, Chan A, Charisse K, Alam MR, Wang Q, Hoekstra M, Kandasamy P, Kel’in AV, Milstein S, Taneja N, O’Shea J, Shaikh S, Zhang L, van der Sluis RJ, Jung ME, Akinc A, Hutabarat R, Kuchimanchi S, Fitzgerald K, Zimmermann T, van Berkel TJ, Maier MA, Rajeev KG, Manoharan M. Multivalent N-acetylgalactosamine-conjugated siRNA localizes in hepatocytes and elicits robust RNAi-mediated gene silencing. J Am Chem Soc. 2014;136:16958–16961. doi: 10.1021/ja505986a. [DOI] [PubMed] [Google Scholar]
- 99.Juliano RL, Carver K, Cao C, Ming X. Receptors, endocytosis, and trafficking: the biological basis of targeted delivery of antisense and siRNA oligonucleotides. Journal of drug targeting. 2013;21:27–43. doi: 10.3109/1061186X.2012.740674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Alam MR, Dixit V, Kang H, Li ZB, Chen X, Trejo J, Fisher M, Juliano RL. Intracellular delivery of an anionic antisense oligonucleotide via receptor-mediated endocytosis. Nucleic Acids Res. 2008;36:2764–2776. doi: 10.1093/nar/gkn115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liu X, Wang W, Samarsky D, Liu L, Xu Q, Zhang W, Zhu G, Wu P, Zuo X, Deng H, Zhang J, Wu Z, Chen X, Zhao L, Qiu Z, Zhang Z, Zeng Q, Yang W, Zhang B, Ji A. Tumor-targeted in vivo gene silencing via systemic delivery of cRGD-conjugated siRNA. Nucleic Acids Res. 2014;42:11805–11817. doi: 10.1093/nar/gku831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Meade BR, Gogoi K, Hamil AS, Palm-Apergi C, van den Berg A, Hagopian JC, Springer AD, Eguchi A, Kacsinta AD, Dowdy CF, Presente A, Lonn P, Kaulich M, Yoshioka N, Gros E, Cui XS, Dowdy SF. Efficient delivery of RNAi prodrugs containing reversible charge-neutralizing phosphotriester backbone modifications. Nat Biotechnol. 2014;32:1256–1261. doi: 10.1038/nbt.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Juliano RL, Ming X, Nakagawa O. Cellular uptake and intracellular trafficking of antisense and siRNA oligonucleotides. Bioconjug Chem. 2012;23:147–157. doi: 10.1021/bc200377d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Duncan R, Richardson SC. Endocytosis and intracellular trafficking as gateways for nanomedicine delivery: opportunities and challenges. Mol Pharm. 2012;9:2380–2402. doi: 10.1021/mp300293n. [DOI] [PubMed] [Google Scholar]
- 105.Xu S, Olenyuk BZ, Okamoto CT, Hamm-Alvarez SF. Targeting receptor-mediated endocytotic pathways with nanoparticles: rationale and advances. Adv Drug Deliv Rev. 2013;65:121–138. doi: 10.1016/j.addr.2012.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pangarkar C, Dinh AT, Mitragotri S. Endocytic pathway rapidly delivers internalized molecules to lysosomes: an analysis of vesicle trafficking, clustering and mass transfer. J Control Release. 2012;162:76–83. doi: 10.1016/j.jconrel.2012.05.022. [DOI] [PubMed] [Google Scholar]
- 107.Ming X, Alam MR, Fisher M, Yan Y, Chen X, Juliano RL. Intracellular delivery of an antisense oligonucleotide via endocytosis of a G protein-coupled receptor. Nucleic Acids Res. 2010;38:6567–6576. doi: 10.1093/nar/gkq534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ming X, Carver K, Wu L. Albumin-based nanoconjugates for targeted delivery of therapeutic oligonucleotides. Biomaterials. 2013;34:7939–7949. doi: 10.1016/j.biomaterials.2013.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ming X, Sato K, Juliano RL. Unconventional internalization mechanisms underlying functional delivery of antisense oligonucleotides via cationic lipoplexes and polyplexes. J Control Release. 2011;153:83–92. doi: 10.1016/j.jconrel.2011.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Koller E, Vincent TM, Chappell A, De S, Manoharan M, Bennett CF. Mechanisms of single-stranded phosphorothioate modified antisense oligonucleotide accumulation in hepatocytes. Nucleic Acids Res. 2011 doi: 10.1093/nar/gkr089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kortylewski M, Swiderski P, Herrmann A, Wang L, Kowolik C, Kujawski M, Lee H, Scuto A, Liu Y, Yang C, Deng J, Soifer HS, Raubitschek A, Forman S, Rossi JJ, Pardoll DM, Jove R, Yu H. In vivo delivery of siRNA to immune cells by conjugation to a TLR9 agonist enhances antitumor immune responses. Nat Biotechnol. 2009;27:925–932. doi: 10.1038/nbt.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lu JJ, Langer R, Chen J. A novel mechanism is involved in cationic lipid-mediated functional siRNA delivery. Mol Pharm. 2009;6:763–771. doi: 10.1021/mp900023v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kaneda MM, Sasaki Y, Lanza GM, Milbrandt J, Wickline SA. Mechanisms of nucleotide trafficking during siRNA delivery to endothelial cells using perfluorocarbon nanoemulsions. Biomaterials. 2010;31:3079–3086. doi: 10.1016/j.biomaterials.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wu XA, Choi CH, Zhang C, Hao L, Mirkin CA. Intracellular fate of spherical nucleic acid nanoparticle conjugates. J Am Chem Soc. 2014;136:7726–7733. doi: 10.1021/ja503010a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lehto T, Castillo Alvarez A, Gauck S, Gait MJ, Coursindel T, Wood MJ, Lebleu B, Boisguerin P. Cellular trafficking determines the exon skipping activity of Pip6a-PMO in mdx skeletal and cardiac muscle cells. Nucleic Acids Res. 2014;42:3207–3217. doi: 10.1093/nar/gkt1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gilleron J, Querbes W, Zeigerer A, Borodovsky A, Marsico G, Schubert U, Manygoats K, Seifert S, Andree C, Stoter M, Epstein-Barash H, Zhang L, Koteliansky V, Fitzgerald K, Fava E, Bickle M, Kalaidzidis Y, Akinc A, Maier M, Zerial M. Image-based analysis of lipid nanoparticle-mediated siRNA delivery, intracellular trafficking and endosomal escape. Nat Biotechnol. 2013;31:638–646. doi: 10.1038/nbt.2612. [DOI] [PubMed] [Google Scholar]
- 117.Sahay G, Querbes W, Alabi C, Eltoukhy A, Sarkar S, Zurenko C, Karagiannis E, Love K, Chen D, Zoncu R, Buganim Y, Schroeder A, Langer R, Anderson DG. Efficiency of siRNA delivery by lipid nanoparticles is limited by endocytic recycling. Nat Biotechnol. 2013;31:653–658. doi: 10.1038/nbt.2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wagenaar TR, Tolstykh T, Shi C, Jiang L, Zhang J, Li Z, Yu Q, Qu H, Sun F, Cao H, Pollard J, Dai S, Gao Q, Zhang B, Arlt H, Cindhuchao M, Hoffmann D, Light M, Jensen K, Hopke J, Newcombe R, Garcia-Echeverria C, Winter C, Zabludoff S, Wiederschain D. Identification of the endosomal sorting complex required for transport-I (ESCRT-I) as an important modulator of anti-miR uptake by cancer cells. Nucleic Acids Res. 2015;43:1204–1215. doi: 10.1093/nar/gku1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Vasconcelos LD, Madani F, Arukuusk P, Parnaste L, Graslund A, Langel U. Effects of cargo molecules on membrane perturbation caused by transportan10 based cell-penetrating peptides. Biochim Biophys Acta. 2014 doi: 10.1016/j.bbamem.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 120.Zhang MZ, Li C, Fang BY, Yao MH, Ren QQ, Zhang L, Zhao YD. High transfection efficiency of quantum dot-antisense oligonucleotide nanoparticles in cancer cells through dual-receptor synergistic targeting. Nanotechnology. 2014;25:255102. doi: 10.1088/0957-4484/25/25/255102. [DOI] [PubMed] [Google Scholar]
- 121.Liu M, Li M, Sun S, Li B, Du D, Sun J, Cao F, Li H, Jia F, Wang T, Chang N, Yu H, Wang Q, Peng H. The use of antibody modified liposomes loaded with AMO-1 to deliver oligonucleotides to ischemic myocardium for arrhythmia therapy. Biomaterials. 2014;35:3697–3707. doi: 10.1016/j.biomaterials.2013.12.099. [DOI] [PubMed] [Google Scholar]
- 122.Carver K, Ming X, Juliano RL. Multicellular tumor spheroids as a model for assessing delivery of oligonucleotides in three dimensions. Molecular therapy Nucleic acids. 2014;3:e153. doi: 10.1038/mtna.2014.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ming X, Carver K, Fisher M, Noel R, Cintrat JC, Gillet D, Barbier J, Cao C, Bauman J, Juliano RL. The small molecule Retro-1 enhances the pharmacological actions of antisense and splice switching oligonucleotides. Nucleic Acids Res. 2013;41:3673–3687. doi: 10.1093/nar/gkt066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ko YT. Nanoparticle-mediated delivery of oligonucleotides to the blood-brain barrier: in vitro and in situ brain perfusion studies on the uptake mechanisms. Journal of drug targeting. 2013;21:866–873. doi: 10.3109/1061186X.2013.829077. [DOI] [PubMed] [Google Scholar]
- 125.Alachkar H, Xie Z, Marcucci G, Chan KK. Determination of cellular uptake and intracellular levels of Cenersen (Aezea((R)), EL625), a p53 antisense oligonucleotide in acute myeloid leukemia cells. Journal of pharmaceutical and biomedical analysis. 2012;71:228–232. doi: 10.1016/j.jpba.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Blumenthal R, Clague MJ, Durell SR, Epand RM. Membrane fusion. Chem Rev. 2003;103:53–69. doi: 10.1021/cr000036+. [DOI] [PubMed] [Google Scholar]
- 127.Wickner W, Schekman R. Membrane fusion. Nat Struct Mol Biol. 2008;15:658–664. doi: 10.1038/nsmb.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Weinreb G, Lentz BR. Analysis of membrane fusion as a two-state sequential process: evaluation of the stalk model. Biophys J. 2007;92:4012–4029. doi: 10.1529/biophysj.106.090043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Allen TM, Hong K, Papahadjopoulos D. Membrane contact, fusion, and hexagonal (HII) transitions in phosphatidylethanolamine liposomes. Biochemistry. 1990;29:2976–2985. doi: 10.1021/bi00464a013. [DOI] [PubMed] [Google Scholar]
- 130.Ortiz A, Killian JA, Verkleij AJ, Wilschut J. Membrane fusion and the lamellar-to-inverted-hexagonal phase transition in cardiolipin vesicle systems induced by divalent cations. Biophys J. 1999;77:2003–2014. doi: 10.1016/S0006-3495(99)77041-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Martens S, McMahon HT. Mechanisms of membrane fusion: disparate players and common principles. Nat Rev Mol Cell Biol. 2008;9:543–556. doi: 10.1038/nrm2417. [DOI] [PubMed] [Google Scholar]
- 132.Frolov VA, Dunina-Barkovskaya AY, Samsonov AV, Zimmerberg J. Membrane permeability changes at early stages of influenza hemagglutinin-mediated fusion. Biophys J. 2003;85:1725–1733. doi: 10.1016/S0006-3495(03)74602-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wang T, Smith EA, Chapman ER, Weisshaar JC. Lipid mixing and content release in single-vesicle, SNARE-driven fusion assay with 1-5 ms resolution. Biophys J. 2009;96:4122–4131. doi: 10.1016/j.bpj.2009.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lorenz P, Misteli T, Baker BF, Bennett CF, Spector DL. Nucleocytoplasmic shuttling: a novel in vivo property of antisense phosphorothioate oligodeoxynucleotides. Nucleic Acids Res. 2000;28:582–592. doi: 10.1093/nar/28.2.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.von Kleist L, Haucke V. At the crossroads of chemistry and cell biology: inhibiting membrane traffic by small molecules. Traffic. 2012;13:495–504. doi: 10.1111/j.1600-0854.2011.01292.x. [DOI] [PubMed] [Google Scholar]
- 136.Macia E, Ehrlich M, Massol R, Boucrot E, Brunner C, Kirchhausen T. Dynasore, a cell-permeable inhibitor of dynamin. Dev Cell. 2006;10:839–850. doi: 10.1016/j.devcel.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 137.McCluskey A, Daniel JA, Hadzic G, Chau N, Clayton EL, Mariana A, Whiting A, Gorgani NN, Lloyd J, Quan A, Moshkanbaryans L, Krishnan S, Perera S, Chircop M, von Kleist L, McGeachie AB, Howes MT, Parton RG, Campbell M, Sakoff JA, Wang X, Sun JY, Robertson MJ, Deane FM, Nguyen TH, Meunier FA, Cousin MA, Robinson PJ. Building a better dynasore: the dyngo compounds potently inhibit dynamin and endocytosis. Traffic. 2013;14:1272–1289. doi: 10.1111/tra.12119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hill TA, Gordon CP, McGeachie AB, Venn-Brown B, Odell LR, Chau N, Quan A, Mariana A, Sakoff JA, Chircop M, Robinson PJ, McCluskey A. Inhibition of dynamin mediated endocytosis by the dynoles--synthesis and functional activity of a family of indoles. J Med Chem. 2009;52:3762–3773. doi: 10.1021/jm900036m. [DOI] [PubMed] [Google Scholar]
- 139.von Kleist L, Stahlschmidt W, Bulut H, Gromova K, Puchkov D, Robertson MJ, MacGregor KA, Tomilin N, Pechstein A, Chau N, Chircop M, Sakoff J, von Kries JP, Saenger W, Krausslich HG, Shupliakov O, Robinson PJ, McCluskey A, Haucke V. Role of the clathrin terminal domain in regulating coated pit dynamics revealed by small molecule inhibition. Cell. 2011;146:471–484. doi: 10.1016/j.cell.2011.06.025. [DOI] [PubMed] [Google Scholar]
- 140.Dutta D, Williamson CD, Cole NB, Donaldson JG. Pitstop 2 is a potent inhibitor of clathrin-independent endocytosis. PLoS One. 2012;7:e45799. doi: 10.1371/journal.pone.0045799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Saenz JB, Sun WJ, Chang JW, Li J, Bursulaya B, Gray NS, Haslam DB. Golgicide A reveals essential roles for GBF1 in Golgi assembly and function. Nat Chem Biol. 2009;5:157–165. doi: 10.1038/nchembio.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Saenz JB, Doggett TA, Haslam DB. Identification and characterization of small molecules that inhibit intracellular toxin transport. Infection and immunity. 2007;75:4552–4561. doi: 10.1128/IAI.00442-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Stechmann B, Bai SK, Gobbo E, Lopez R, Merer G, Pinchard S, Panigai L, Tenza D, Raposo G, Beaumelle B, Sauvaire D, Gillet D, Johannes L, Barbier J. Inhibition of retrograde transport protects mice from lethal ricin challenge. Cell. 2010;141:231–242. doi: 10.1016/j.cell.2010.01.043. [DOI] [PubMed] [Google Scholar]
- 144.Gillespie EJ, Ho CL, Balaji K, Clemens DL, Deng G, Wang YE, Elsaesser HJ, Tamilselvam B, Gargi A, Dixon SD, France B, Chamberlain BT, Blanke SR, Cheng G, de la Torre JC, Brooks DG, Jung ME, Colicelli J, Damoiseaux R, Bradley KA. Selective inhibitor of endosomal trafficking pathways exploited by multiple toxins and viruses. Proc Natl Acad Sci U S A. 2013;110:E4904–4912. doi: 10.1073/pnas.1302334110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Juliano RL, Ming X, Carver K, Laing B. Cellular uptake and intracellular trafficking of oligonucleotides: implications for oligonucleotide pharmacology. Nucleic acid therapeutics. 2014;24:101–113. doi: 10.1089/nat.2013.0463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Yang B, Ming X, Cao C, Laing B, Yuan A, Porter MA, Hull-Ryde EA, Maddry J, Suto M, Janzen WP, Juliano RL. High-throughput screening identifies small molecules that enhance the pharmacological effects of oligonucleotides. Nucleic Acids Res. 2015;43:1987–1996. doi: 10.1093/nar/gkv060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Rehman ZU, Hoekstra D, Zuhorn IS. Protein kinase A inhibition modulates the intracellular routing of gene delivery vehicles in HeLa cells, leading to productive transfection. J Control Release. 2011 doi: 10.1016/j.jconrel.2011.07.015. [DOI] [PubMed] [Google Scholar]
- 148.Kendall GC, Mokhonova EI, Moran M, Sejbuk NE, Wang DW, Silva O, Wang RT, Martinez L, Lu QL, Damoiseaux R, Spencer MJ, Nelson SF, Miceli MC. Dantrolene enhances antisense-mediated exon skipping in human and mouse models of Duchenne muscular dystrophy. Science translational medicine. 2012;4:164ra160. doi: 10.1126/scitranslmed.3005054. [DOI] [PubMed] [Google Scholar]
- 149.Gooding M, Adigbli D, Edith Chan AW, Melander RJ, MacRobert AJ, Selwood DL. A bifurcated proteoglycan binding small molecule carrier for siRNA delivery. Chemical biology & drug design. 2014;84:24–35. doi: 10.1111/cbdd.12295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.OM, et al. Oligonucleotide Therapeutics. San Diego: 2014. Small molecule enhanced efficacy of therapeutic oligonucleotides. [Google Scholar]