Abstract
Objective
To evaluate the role of computed tomographic texture analysis (CTTA) in assessing tumor angiogenesis and survival of soft tissue sarcoma (STS).
Methods
In twenty patients with STSs, tumor texture parameters, which were measured on pre-therapeutic CT using CTTA software with the spatial scale filter (SSF) extracting fine to coarse texture, were compared with microvessel density (MVD), plasma VEGF, soluble VEGF receptor-1 (sVEGFR-1), and overall survival (OS).
Results
Mean of positive pixels (MPP) showed a positive correlation with MVD (P=0.02). Entropy at medium texture scales (SSF=3,4,5) showed positive correlations with VEGF (P=0.03, P=0.009, P=0.02, respectively), and entropy without filtration showed a positive correlation with sVEGFR-1 (P=0.02). In univariate analysis, kurtosis at a medium texture scale and MPP showed significant correlations with OS (P=0.04, P=0.007), and multivariate analysis demonstrated that MPP was an independent prognostic factor (P=0.01).
Conclusion
Texture parameters are associated with tumor angiogenesis and OS in STS.
Keywords: computed tomography, soft tissue sarcoma, texture analysis, heterogeneity, angiogenesis
INTRODUCTION
Heterogeneity in the structure or blood supply is a well-recognized feature of malignancy.1,2 One way for noninvasive assessment of tumor heterogeneity is computed tomographic (CT) texture analysis (CTTA). CTTA is an image processing algorithm that can be used to quantify the heterogeneity within the tissue by assessing the distribution of texture coarseness and irregularity within a lesion. In previous studies, CTTA of tumors on contrast–enhanced (CE) CT or non-CECT images have been reported to have a correlation with survival in non–small cell lung cancer, esophageal cancer, hepatocellular carcinoma, colon cancer, and metastatic renal cell carcinoma.1,3–6 Furthermore, CTTA has successfully demonstrated biological associations with glucose metabolism, hypoxia, and angiogenesis.3,4,7
Soft tissue sarcomas (STSs) are a heterogeneous group of rare tumors that arise from mesenchymal cells at all body sites, and neoadjuvant therapy provides several advantages in the treatment of STSs.8 Treatment-induced cytoreduction potentially facilitates a less radical surgical resection, thus decreasing the operative and postoperative morbidity. Moreover, the addition of radiotherapy (RT) has been prospectively demonstrated to decrease the incidence of local recurrence.9–11 and recently, neoadjuvant therapy with the combination of bevacizumab (BV) and RT showed that BV increased the efficacy of RT against STS and might reduce the incidence of local recurrence.12
Given the emerging benefit of neoadjuvant therapy in STS, predicting treatment response prior to the therapy is of great importance. Thus, we hypothesize that CTTA, which reflects tumor biology, can predict clinical outcome in STS treated with neoadjuvant BV and RT. In this preliminary study, our aim was to evaluate the heterogeneity of STS by means of CTTA in patients treated with neoadjuvant BV and RT. We particularly assessed correlations of the texture parameters with angiogenesis and survival.
MATERIALS AND METHODS
Patient population
This preliminary study was part of the phase II clinical trial on STS12, which was in compliance with Health Insurance Portability and Accountability Act regulations, and was approved by the institutional review board at Dana-Farber/Harvard Cancer Center (Boston, MA). All patients were required to provide written informed consent before study participation according to institutional and federal guidelines. The eligibility and treatment schedule have been detailed previously.12 Briefly, the patient eligibility criteria included the following; (i) patients had histopathologically proven measurable primary STS or an isolated local recurrence of STS after previous surgery; (ii) they had no metastatic disease; (iii) they had no surgery, chemotherapy, immunotherapy, experimental therapy, or radiotherapy within 4 weeks of first day of study drug dosing; and (iv) they had adequate renal function (serum creatinine level ≤ 1.4 mg/dl). Exclusion criteria included the following: (i) significant medical comorbidities; (ii) clinically significant cardiovascular disease including uncontrolled hypertension, myocardial infarction, and unstable angina; (iii) pregnancy or lactation; (iv) known history of deep vein thrombus or pulmonary embolus; and (v) an inability to give written informed consent. 20 patients with STSs were enrolled in this study from August 2006 to June 2009. The median follow-up time was 53.11 months.
Treatment
The treatment schedule and the dose modification schema have been detailed previously.12 Briefly, patients received four doses of BV (5 mg/kg) every 2 weeks. RT was started with the second dose of BV to a total dose of 50.4 Gy in 28 fractions within 5.5 weeks. Surgical resection of the tumor was performed with curative intent at 6–7 weeks after RT completion and 8–9 weeks after the last dose of BV.
Pathological analysis
Tumor samples were obtained by image guided core needle biopsy before the therapy. Tumor grade was assessed according to French Federation of Cancer Centers Sarcoma Group (FNCLCC) guideline. Microvessel density (MVD) was also assessed by immnohistochemical analysis using an antibody against CD31 as previously described.12 After the surgery, all surgical specimens were evaluated in a standard fashion. The extent of necrosis was assessed relative to the percentage of residual viable tumor in each case and in an identical manner to that established for bone tumors.8,13 The percentage of necrosis ranged from 0% to 100%.
Plasma markers of angiogenesis
The plasma marker analysis has been detailed previously.12 All blood samples were collected in ethylenediaminetetraacetic acid (EDTA)-containing Vacutainer tubes and spun at 1000g for 15 min, and plasma was aliquoted and frozen immediately before the. The plasma samples were analyzed using multiplex array plates from Meso-Scale Discovery (Gaithersburg, MD) for serial measurements of the VEGF, soluble VEGF receptor-1 (sVEGFR-1).
Imaging acquisition and analysis
All patients were examined on a 16/64-section multi-detector row CT (MDCT) scanner (Light Speed/Discovery; GE Medical Systems, Milwaukee, WI) within 2 weeks before the therapy. The following CT parameters were used for acquisition of volume data: 100 kVp; 150–200 mA; 0.5-second rotation time; field of view, 220–360 mm; matrix, 512 mm; 5 mm reconstructed slice thickness. Tumor size was measured in the longest cross-sectional dimension for each lesion based on RECIST 1.1 guidelines.14
CT Texture analysis (CTTA)
CTTA was performed on the non-CECT patient images using TexRAD (TexRAD Ltd, Somerset, UK, www.texrad.org), a novel commercially available research software for image heterogeneity assessment. This CTTA algorithm has been previously reported in other tumor applications.1,3–5,7,15,16 The single axial CT slice with the largest cross-sectional area of the tumor was used for CTTA. A freehand region of interest (ROI) was carefully drawn contouring the periphery of the tumor (Figure 1A) by a single reader (K.H., with 10 years of experience in CT interpretation), who was blinded to the clinical and survival data. Mean ROI size was 9988 pixels (range, 2875–34944 pixels). The CTTA algorithm had an additional thresholding procedure that excluded any pixels with attenuation values below −50HU. CTTA comprised an image filtration technique using a Laplacian of Gaussian spatial band-pass filter to produce a series of derived images extracting and enhancing features of different sizes and intensity variations at different spatial scale filter (SSF) values highlighting fine (SSF=2, features of 2mm in radius, Fig. 1B), medium (SSF=3, 4, 5, features of 3mm, 4mm – Fig. 1C, 5mm in radius) and coarse (SSF=6, features of 6mm in radius, Fig. 1D) texture scales, and was followed by texture (heterogeneity) quantification using entropy (a measure of irregularity), mean value of positive pixels (MPP; the average value of all the pixels with positive values), skewness (a measure of asymmetry of the histogram) and kurtosis (a measure of peakedness and tailedness). In addition these histogram parameters were also quantified from the conventional CT image without filtration (i.e. SSF=0). A recent article further described what these texture parameters mean in terms of image features.17 To assess interobserver variability, the other reader (F.T., with 14 years of experience in CT interpretation), who was blinded to the clinical and survival data, independently evaluated CTTA of STS in the same manner as first reader.
Fig. 1.
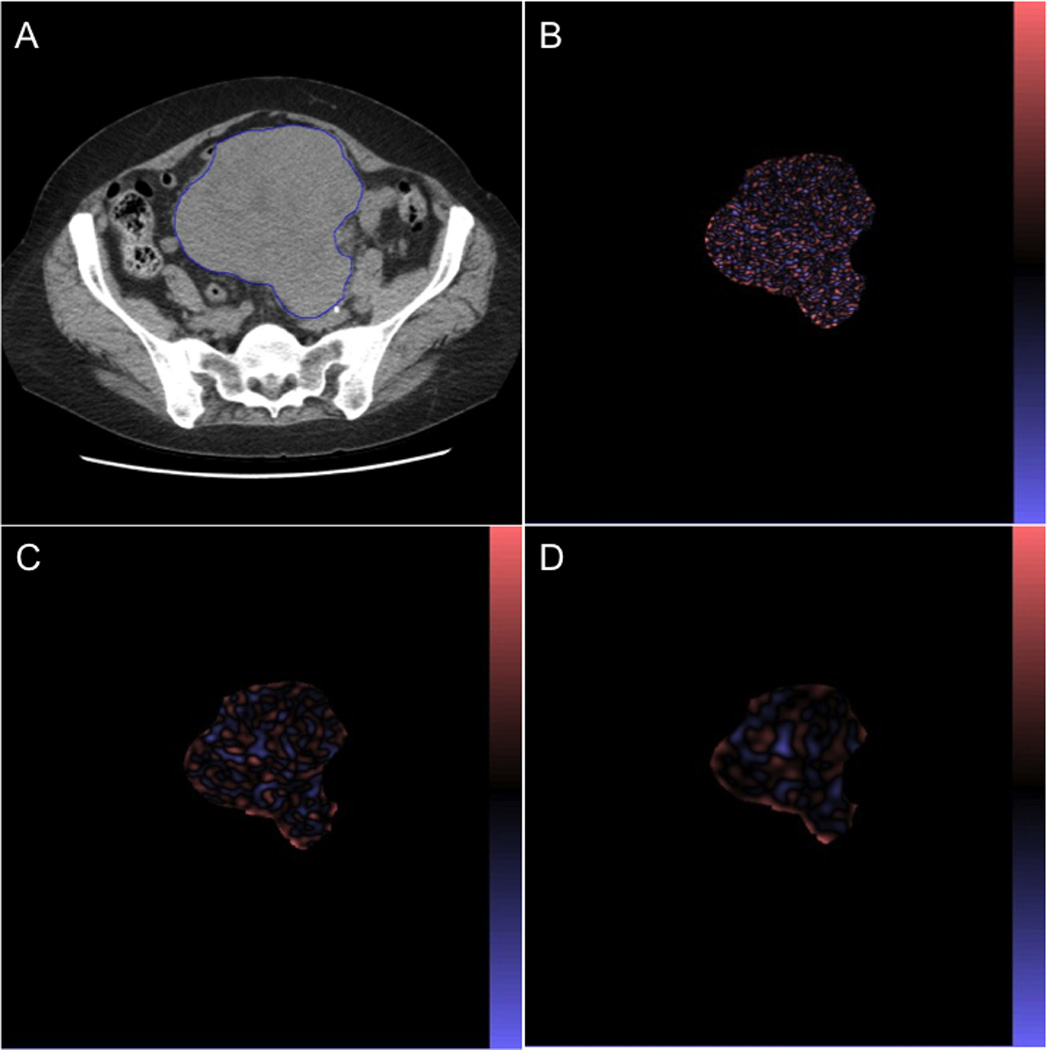
(A) Non-CECT image of 66-year-old woman with STS. Corresponding images in the same patient selectively display (B) fine, (C) medium, (D) coarse texture and were obtained by using SSF of 2mm, 4mm, and 6mm in radius.
Statistical analysis
Statistical analyses were carried out using the JMP 10.0 (SAS Institute, Inc., Cary, NC, USA), and for all comparisons, P < 0.05 was considered to indicate a statistically significant difference. Interobserver agreement of CTTA analysis was assessed by means of spearman’s rank correlation coefficient. Correlations between texture parameters and markers of angiogenesis were also analyzed using spearman’s rank correlation coefficients. The association of the continuous variables with overall survival (OS) was evaluated using the Cox proportional hazards regression model. In the univariate analyses, each variable was included in a Cox regression model alone. In the multivariable analyses, each continuous variable was added to a Cox regression model, and the influence of each variable was assessed. Kaplan-Meier analysis was also performed for OS analysis, and the log-rank test was employed. Kaplan-Meyer survival analysis identified the respective optimal threshold based on an iterative method for each parametric threshold that can best separate the patients into poor and good survival groups (indicated by the best P-value from log-rank test).
RESULTS
Patient Characteristics
A total of twenty patients were eligible in the current study. The subjects included sixteen men and four women, with a median age of 58.5 years (range 26–75 years). The histologic subtypes of tumors were, fibroblastic sarcoma (n=8), liposarcoma (n=6), leiomyosarcoma (n=4), fibroblastic osteosarcoma (n=1) and undifferentiated sarcoma (n=1). Tumors of fourteen patients were located in the extremity, and six in the retroperitoneum. Patients’ characteristics were summarized in Table 1.
Table 1.
Patient characteristics
| Patient Demographics | Variables | Value |
|---|---|---|
| Sex | Male / Female | 16 / 4 |
| Age | Median / range | 54.8 / 26–75 |
| Tumor size | Median / range | 5.85 / 3.1–18.9 |
| Tumor grade | 1 / 2 / 3 | 2 / 8 / 10 |
| Necrosis after the therapy (%) | Median / range | 65.0 / 20–100 |
Interobserver agreement of CTTA
Interobserver agreement was assessed by comparing the two sets of CTTA parameters of STS tumors measured by two independent readers. Interobserver agreement for CTTA parameters was summarized in Table 2. CTTA parameters showed good correlations between two readers (Spearman's correlation coefficient, 0.698–0.990).
Table 2.
Interobserver agreement (Spearman’s rank correlation)
| Entropy | MPP | Skewness | Kurtosis | |||||
|---|---|---|---|---|---|---|---|---|
| SSF | R | P | R | P | R | P | R | P |
| 0 | 0.964 | < 0.0001 | 0.980 | < 0.0001 | 0.853 | < 0.0001 | 0.799 | < 0.0001 |
| 2 | 0.978 | < 0.0001 | 0.990 | < 0.0001 | 0.909 | < 0.0001 | 0.971 | < 0.0001 |
| 3 | 0.928 | < 0.0001 | 0.974 | < 0.0001 | 0.912 | < 0.0001 | 0.858 | < 0.0001 |
| 4 | 0.859 | < 0.0001 | 0.952 | < 0.0001 | 0.898 | < 0.0001 | 0.698 | 0.0006 |
| 5 | 0.867 | < 0.0001 | 0.984 | < 0.0001 | 0.938 | < 0.0001 | 0.843 | < 0.0001 |
| 6 | 0.942 | < 0.0001 | 0.990 | < 0.0001 | 0.917 | < 0.0001 | 0.841 | < 0.0001 |
Texture parameters and biological markers of angiogenesis
Correlations of texture parameters with MVD, plasma VEGF, sVEGFR-1 were shown in Table 3. MVD showed a positive correlation with MPP with no filtration (R=0.516, P=0.02). Entropy at medium texture scales (SSF=3,4,5) showed positive correlations with VEGF (R=0.507, P=0.03; R=0.595, P=0.009; R=0.521, P=0.02; respectively), and entropy without filtration showed positive correlations with sVEGFR-1 (R=0.541, P=0.02). Other texture parameters showed no significant correlations with the biological markers.
Table 3.
Correlations of texture parameters with MVD, plasma VEGF, and sVEGFR-1
| Entropy | MPP | Skewness | Kurtosis | |||||
|---|---|---|---|---|---|---|---|---|
| SSF | R | P | R | P | R | P | R | P |
| MVD | ||||||||
| 0 | −0.269 | 0.2 | 0.516 | 0.02 | 0.160 | 0.5 | 0.213 | 0.3 |
| 2 | −0.376 | 0.1 | −0.340 | 0.1 | 0.213 | 0.3 | 0.217 | 0.3 |
| 3 | −0.373 | 0.1 | −0.329 | 0.1 | 0.008 | 0.9 | 0.233 | 0.3 |
| 4 | −0.233 | 0.3 | −0.302 | 0.2 | −0.139 | 0.5 | 0.147 | 0.5 |
| 5 | −0.081 | 0.7 | −0.245 | 0.3 | −0.117 | 0.6 | 0.022 | 0.9 |
| 6 | 0.044 | 0.8 | −0.202 | 0.4 | −0.126 | 0.6 | 0.047 | 0.8 |
| Plasma VEGF | ||||||||
| 0 | 0.330 | 0.1 | 0.119 | 0.6 | −0.302 | 0.2 | −0.250 | 0.3 |
| 2 | 0.416 | 0.08 | 0.328 | 0.1 | −0.326 | 0.1 | −0.126 | 0.6 |
| 3 | 0.507 | 0.03 | 0.361 | 0.1 | −0.322 | 0.1 | −0.057 | 0.8 |
| 4 | 0.595 | 0.009 | 0.440 | 0.06 | −0.074 | 0.7 | −0.137 | 0.5 |
| 5 | 0.521 | 0.02 | 0.402 | 0.09 | −0.052 | 0.8 | −0.265 | 0.2 |
| 6 | 0.273 | 0.2 | 0.277 | 0.26 | −0.036 | 0.8 | 0.034 | 0.8 |
| sVEGFR-1 | ||||||||
| 0 | 0.541 | 0.02 | −0.010 | 0.9 | −0.394 | 0.1 | −0.241 | 0.3 |
| 2 | 0.435 | 0.07 | 0.457 | 0.05 | −0.264 | 0.2 | −0.108 | 0.6 |
| 3 | 0.384 | 0.1 | 0.435 | 0.07 | −0.135 | 0.5 | 0.034 | 0.8 |
| 4 | 0.242 | 0.3 | 0.398 | 0.1 | 0.122 | 0.6 | 0.038 | 0.8 |
| 5 | −0.023 | 0.9 | 0.347 | 0.1 | 0.157 | 0.5 | 0.177 | 0.4 |
| 6 | −0.278 | 0.2 | 0.324 | 0.1 | −0.267 | 0.2 | 0.133 | 0.5 |
Univariate analysis of correlations between texture parameters and OS
In univariate analysis, MPP with no filtration (SSF=0), kurtosis at a medium texture scale (SSF=5) showed significant associations with OS (P=0.007, P=0.04, respectively) (Table 4). For these two texture parameters, survival curves were analyzed with use of Kaplan-Meyer analysis (Fig. 2). Patients with tumor showing lower MPP (≤ 34.65) with no filtration (SSF=0) showed better survival (P = 0.009). Patients with higher kurtosis (> 0.58) at medium texture scale (SSF=5) also showed a good correlation with OS, but this tendency was not significant (P = 0.05).
Table 4.
Univariate Cox regression analysis of correlations between texture parameters and OS
| Filter value / parameters | HR | 95%CI | P |
|---|---|---|---|
| SSF = 0 | |||
| Entropy | 2.36 | 0.30–25.37 | 0.4 |
| MPP | 1.24 | 1.04–1.62 | 0.007 |
| Skewness | 1.58 | 0.35–4.34 | 0.4 |
| Kurtosis | 1.12 | 0.61–1.67 | 0.6 |
| SSF = 2 | |||
| Entropy | 2.83 | 0.53–16.51 | 0.2 |
| MPP | 1.01 | 0.97–1.05 | 0.3 |
| Skewness | 1.78 | 0.41–6.23 | 0.4 |
| Kurtosis | 1.06 | 0.78–1.30 | 0.6 |
| SSF = 3 | |||
| Entropy | 4.36 | 0.71–29.06 | 0.1 |
| MPP | 1.02 | 0.96–1.06 | 0.4 |
| Skewness | 1.05 | 0.38–4.39 | 0.9 |
| Kurtosis | 0.98 | 0.65–1.28 | 0.9 |
| SSF = 4 | |||
| Entropy | 4.21 | 0.64–26.15 | 0.1 |
| MPP | 1.02 | 0.95–1.09 | 0.4 |
| Skewness | 1.19 | 0.57–3.03 | 0.6 |
| Kurtosis | 0.86 | 0.49–1.13 | 0.3 |
| SSF = 5 | |||
| Entropy | 3.07 | 0.55–21.56 | 0.2 |
| MPP | 1.03 | 0.95–1.09 | 0.3 |
| Skewness | 1.07 | 0.56–1.91 | 0.8 |
| Kurtosis | 0.47 | 0.12–0.98 | 0.04 |
| SSF = 6 | |||
| Entropy | 1.47 | 0.61–7.15 | 0.4 |
| MPP | 1.02 | 0.96–1.06 | 0.4 |
| Skewness | 1.50 | 0.68–4.51 | 0.3 |
| Kurtosis | 0.72 | 0.40–1.04 | 0.1 |
Fig. 2.
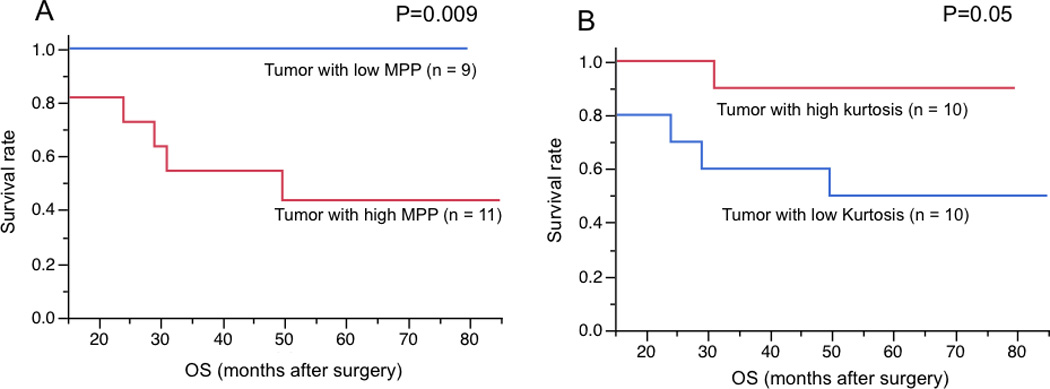
Kaplan-Meier analysis of MPP without filtration (A) and kurtosis at medium texture scale (SSF=5) (B) in OS. Patients with tumor showing lower MPP (≤ 34.65) and higher kurtosis (> 0.58) showed better survival.
The influence of MPP on OS in multivariate analysis
MPP with no filtration showed a best correlation with OS, and therefore, we assessed the influence of MPP on OS using the Cox proportional hazards regression model in comparison with clinicopathologic features (age, size, necrosis, tumor grade) that were reported as prognostic markers of STS in previous studies.18–24 In the multivariate analyses, MPP was identified an independent factor for OS (P=0.01; hazards ratio, 1.27; 95% CI, 1.03–1.77) (Table 5).
Table 5.
Multivariate analysis of MPP and clinicopathological features for OS using Cox regression model
| Variables | HR | 95%CI | P |
|---|---|---|---|
| MPP without filtration | 1.27 | 1.03, 1.77 | 0.01 |
| Age | 1.00 | 0.91, 1.11 | 0.9 |
| Tumor size | 0.97 | 0.63, 1.46 | 0.8 |
| Grade | 2.25 | 0.23, 53.94 | 0.5 |
| Necrosis | 0.97 | 0.92, 1.01 | 0.3 |
DISCUSSION
Human tumors arise from single cells that have accumulated the necessary number and type of heritable alterations. Each such cell leads to dysregulated growth and eventually the formation of a tumor. Despite their monoclonal origin, at the time of diagnosis, most tumors show a striking amount of intratumor heterogeneity, which has implications for diagnosis, treatment efficacy, and the identification of drug targets.25 Thus, assessment of tumor heterogeneity is relevant to everyday clinical practice, and CTTA is emerging as a method to quantify heterogeneity within the tumor. In the validation of this approach, establishment of histologic and prognostic correlates is an important step. Recently several papers about CTTA have been published, and texture parameters on CECT or non-CECT have been demonstrated their correlations with survival in non–small cell lung cancer, esophageal cancer, colon cancer, and metastatic renal cell carcinoma.1,3–5 Furthermore, CTTA has successfully demonstrated its histological correlations with glucose metabolism, hypoxia, and tumor angiogenesis in non–small cell lung cancer and esophageal cancer.3,4,7 However, to date, no studies have directly addressed the intratumoral heterogeneity of STS.
In this study, texture parameters were compared with biologic markers of angiogenesis, and MPP showed a positive correlation with MVD. Basically, fresh blood, including intravascular blood is typically of higher attenuation.17 And MPP is the average brightness of positive values of the image. Based on these, the tumor with higher MVD may have more bright objects. In addition, entropy showed positive correlations with plasma VEGF and sVEGFR-1, which have been previously proposed as biomarkers for antiangiogenic therapy.26,27 One possible reason for these correlations may be the development of hypoxia in the tumor. Entropy, which is a measure of irregularity, represents heterogeneity in tumor. Structural heterogeneity leads to a heterogeneous blood supply in the tumor, which may result in a hypoxic tumor environment. In turn, hypoxia may lead to increased plasma VEGF and sVEGFR-1.28
In survival analysis, patients with low MPP showed better OS, and MPP was demonstrated to be an independent prognostic factor associated with OS. Several previous studies reported a correlation between MVD and survival in patients with STS, and demonstrated that high MVD in STS correlated with poor OS.29,30 Therefore, we assume that high MPP tumor, which tends to show high MVD, may be associated with poor OS in STS. In a previous study of non-small cell lung carcinoma, MPP on non-CECT showed a negative correlation with MVD.7 But, in that study, MPP value was calculated with a filtration, and therefore, the association between MPP and histology may change according to the filter value. Or it may just reflect biological differences of angiogenesis between STS and non-small cell lung carcinoma.
We also observed that low kurtosis in STS associated with a poor OS. Kurtosis is a measure of the "peakedness" and “tailedness” of the histogram, and high kurtosis tends to have a distinct peak near the mean.17 In colon cancer, Ng et al. reported that lower kurtosis of tumor on CECT associated with poorer 5-year OS.5 They hypothesized that these might be tumors with greater cell packing and more uniform distribution in tumor structure.5 Another assumption is that intratumoral fibrosis may affect tumor kurtosis. In lung, kurtosis has been used for the evaluation of interstitial pulmonary fibrosis, and lung with higher fibrosis has been reported to show lower kurtosis.31,32 Given the importance of fibrotic tumor stroma in cancer progression33–35, STS with an abundant of fibrotic tumor stroma, which leads low kurtosis in tumor, may have a poor prognosis. The influence of texture parameters on survival may change according to the tumor biology such as angiogenesis and stromal fibrosis.
Our study has several limitations. First, our findings are based on single-center data, and the sample size was very small. Our findings need to be confirmed in multicenter investigations, and a larger patient population should be studied. Second, acquisition parameters, such as tube voltage or tube current, may affect texture parameters. A previous water phantom study in which researchers used varying tube current (100–250 mAs) at 80 and 120 kV and then varying tube voltage at a fixed tube current of 150 mAs suggests this has less of an effect on a texture parameter.16 Third, the definition of the tumor ROIs was subjective. A computerized tumor segmentation method with high reproducibility and reliability should be employed in the further studies.
In conclusion, our results suggested that the tumor texture on non-CECT image might be associated with angiogenesis and survival of STS patients treated with BV + RT. Because CT is commonly used in the staging process of STS, image post-processing techniques such as CT texture analysis can serve as a widely applicable and beneficial biomarker for predicting OS of patients with STSs. We believe that our results will provide an important insight into selecting the optimal therapeutic strategy for patients with STSs.
Acknowledgments
Source of Funding
Supported by NIH grants 1 R21 CA117128 and 1R01 CA158301 (S.S.Y). One of the authors (B.G.) is the scientific director of TexRAD, and provided technical advices for the software used for this analysis.
Footnotes
Conflicts of Interest
All other authors have no conflict of interest.
REFERENCES
- 1.Goh V, Ganeshan B, Nathan P, et al. Assessment of response to tyrosine kinase inhibitors in metastatic renal cell cancer: CT texture as a predictive biomarker. Radiology. 2011;261:165–171. doi: 10.1148/radiol.11110264. [DOI] [PubMed] [Google Scholar]
- 2.Pries AR, Cornelissen AJ, Sloot AA, et al. Structural adaptation and heterogeneity of normal and tumor microvascular networks. PLoS Comput Biol. 2009;5:e1000394. doi: 10.1371/journal.pcbi.1000394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ganeshan B, Panayiotou E, Burnand K, et al. Tumour heterogeneity in non-small cell lung carcinoma assessed by CT texture analysis: a potential marker of survival. Eur Radiol. 2012;22:796–802. doi: 10.1007/s00330-011-2319-8. [DOI] [PubMed] [Google Scholar]
- 4.Ganeshan B, Skogen K, Pressney I, et al. Tumour heterogeneity in oesophageal cancer assessed by CT texture analysis: preliminary evidence of an association with tumour metabolism, stage, and survival. Clin Radiol. 2012;67:157–164. doi: 10.1016/j.crad.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 5.Ng F, Ganeshan B, Kozarski R, et al. Assessment of primary colorectal cancer heterogeneity by using whole-tumor texture analysis: contrast-enhanced CT texture as a biomarker of 5-year survival. Radiology. 2013;266:177–184. doi: 10.1148/radiol.12120254. [DOI] [PubMed] [Google Scholar]
- 6.Hayano K, Yoshida H, Zhu AX, et al. Fractal Analysis of Contrast-Enhanced CT Images to Predict Survival of Patients with Hepatocellular Carcinoma Treated with Sunitinib. Dig Dis Sci. 2014;59:1996–2003. doi: 10.1007/s10620-014-3064-z. [DOI] [PubMed] [Google Scholar]
- 7.Ganeshan B, Goh V, Mandeville HC, et al. Non-small cell lung cancer: histopathologic correlates for texture parameters at CT. Radiology. 2013;266:326–336. doi: 10.1148/radiol.12112428. [DOI] [PubMed] [Google Scholar]
- 8.Eilber FC, Rosen G, Eckardt J, et al. Treatment-induced pathologic necrosis: a predictor of local recurrence and survival in patients receiving neoadjuvant therapy for high-grade extremity soft tissue sarcomas. J Clin Oncol. 2001;19:3203–3209. doi: 10.1200/JCO.2001.19.13.3203. [DOI] [PubMed] [Google Scholar]
- 9.Yoon SS, Chen YL, Kirsch DG, et al. Proton-beam, intensity-modulated, and/or intraoperative electron radiation therapy combined with aggressive anterior surgical resection for retroperitoneal sarcomas. Ann Surg Oncol. 2010;17:1515–1529. doi: 10.1245/s10434-010-0935-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pawlik TM, Pisters PW, Mikula L, et al. Long-term results of two prospective trials of preoperative external beam radiotherapy for localized intermediate- or high-grade retroperitoneal soft tissue sarcoma. Ann Surg Oncol. 2006;13:508–517. doi: 10.1245/ASO.2006.05.035. [DOI] [PubMed] [Google Scholar]
- 11.Yang JC, Chang AE, Baker AR, et al. Randomized prospective study of the benefit of adjuvant radiation therapy in the treatment of soft tissue sarcomas of the extremity. J Clin Oncol. 1998;16:197–203. doi: 10.1200/JCO.1998.16.1.197. [DOI] [PubMed] [Google Scholar]
- 12.Yoon SS, Duda DG, Karl DL, et al. Phase II study of neoadjuvant bevacizumab and radiotherapy for resectable soft tissue sarcomas. Int J Radiat Oncol Biol Phys. 2011;81:1081–1090. doi: 10.1016/j.ijrobp.2010.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wunder JS, Paulian G, Huvos AG, et al. The histological response to chemotherapy as a predictor of the oncological outcome of operative treatment of Ewing sarcoma. J Bone Joint Surg Am. 1998;80:1020–1033. doi: 10.2106/00004623-199807000-00011. [DOI] [PubMed] [Google Scholar]
- 14.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 15.Davnall F, Yip CS, Ljungqvist G, et al. Assessment of tumor heterogeneity: an emerging imaging tool for clinical practice? Insights Imaging. 2012;3:573–589. doi: 10.1007/s13244-012-0196-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miles KA, Ganeshan B, Griffiths MR, et al. Colorectal cancer: texture analysis of portal phase hepatic CT images as a potential marker of survival. Radiology. 2009;250:444–452. doi: 10.1148/radiol.2502071879. [DOI] [PubMed] [Google Scholar]
- 17.Miles KA, Ganeshan B, Hayball MP. CT texture analysis using the filtration-histogram method: what do the measurements mean? Cancer Imaging. 2013;13:400–406. doi: 10.1102/1470-7330.2013.9045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coindre JM, Terrier P, Guillou L, et al. Predictive value of grade for metastasis development in the main histologic types of adult soft tissue sarcomas: a study of 1240 patients from the French Federation of Cancer Centers Sarcoma Group. Cancer. 2001;91:1914–1926. doi: 10.1002/1097-0142(20010515)91:10<1914::aid-cncr1214>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 19.Singer S, Corson JM, Gonin R, et al. Prognostic factors predictive of survival and local recurrence for extremity soft tissue sarcoma. Ann Surg. 1994;219:165–173. doi: 10.1097/00000658-199402000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weitz J, Antonescu CR, Brennan MF. Localized extremity soft tissue sarcoma: improved knowledge with unchanged survival over time. J Clin Oncol. 2003;21:2719–2725. doi: 10.1200/JCO.2003.02.026. [DOI] [PubMed] [Google Scholar]
- 21.Kattan MW, Leung DH, Brennan MF. Postoperative nomogram for 12-year sarcoma-specific death. J Clin Oncol. 2002;20:791–796. doi: 10.1200/JCO.2002.20.3.791. [DOI] [PubMed] [Google Scholar]
- 22.Pisters PW, Leung DH, Woodruff J, et al. Analysis of prognostic factors in 1,041 patients with localized soft tissue sarcomas of the extremities. J Clin Oncol. 1996;14:1679–1689. doi: 10.1200/JCO.1996.14.5.1679. [DOI] [PubMed] [Google Scholar]
- 23.Coindre JM, Terrier P, Bui NB, et al. Prognostic factors in adult patients with locally controlled soft tissue sarcoma. A study of 546 patients from the French Federation of Cancer Centers Sarcoma Group. J Clin Oncol. 1996;14:869–877. doi: 10.1200/JCO.1996.14.3.869. [DOI] [PubMed] [Google Scholar]
- 24.Ueda T, Yoshikawa H, Mori S, et al. Influence of local recurrence on the prognosis of soft-tissue sarcomas. J Bone Joint Surg Br. 1997;79:553–557. doi: 10.1302/0301-620x.79b4.7487. [DOI] [PubMed] [Google Scholar]
- 25.Durrett R, Foo J, Leder K, et al. Intratumor heterogeneity in evolutionary models of tumor progression. Genetics. 2011;188:461–477. doi: 10.1534/genetics.110.125724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jain RK, Duda DG, Willett CG, et al. Biomarkers of response and resistance to antiangiogenic therapy. Nat Rev Clin Oncol. 2009;6:327–338. doi: 10.1038/nrclinonc.2009.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duda DG, Willett CG, Ancukiewicz M, et al. Plasma soluble VEGFR-1 is a potential dual biomarker of response and toxicity for bevacizumab with chemoradiation in locally advanced rectal cancer. Oncologist. 2010;15:577–583. doi: 10.1634/theoncologist.2010-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilson WR, Hay MP. Targeting hypoxia in cancer therapy. Nat Rev Cancer. 2011;11:393–410. doi: 10.1038/nrc3064. [DOI] [PubMed] [Google Scholar]
- 29.Comandone A, Foglione A, Berardengo E, et al. Neoangiogenesis in adult soft tissue sarcomas (STS): prognostic significance of microvessel density (MVD) correlated to grading and stage. A perspective study. Journal of Bone & Joint Surgery, British Volume. 2009;91:269-269. [Google Scholar]
- 30.Kubo T, Shimose S, Fujimori J, et al. Diversity of angiogenesis among malignant bone tumors. Molecular and Clinical Oncology. 2013;1:131–136. doi: 10.3892/mco.2012.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Best AC, Meng J, Lynch AM, et al. Idiopathic pulmonary fibrosis: physiologic tests, quantitative CT indexes, and CT visual scores as predictors of mortality. Radiology. 2008;246:935–940. doi: 10.1148/radiol.2463062200. [DOI] [PubMed] [Google Scholar]
- 32.Kamiya A, Murayama S, Kamiya H, et al. Kurtosis and skewness assessments of solid lung nodule density histograms: differentiating malignant from benign nodules on CT. Jpn J Radiol. 2014;32:14–21. doi: 10.1007/s11604-013-0264-y. [DOI] [PubMed] [Google Scholar]
- 33.Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression. Nature. 2004;432:332–337. doi: 10.1038/nature03096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 35.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]


