Abstract
Perioperative chemotherapy given to increase the chance of cure for localized disease, and maintenance therapy for metastatic disease, represent distinct aspects of the urothelial cancer disease treatment spectrum. The ability to access both pre and post chemotherapy tissue in the neoadjuvant setting provides important opportunities for translational research to test novel therapies and identify predictors of response to therapy. The maintenance setting may be more complex and study design and endpoints need to be determined based on the candidate drugs mechanism of action and toxicity.
Neoadjvuant Chemotherapy
The use of cisplatin based neoadjuvant chemotherapy for patients with muscle invasive urothelial cancer (UC) of the bladder (MIBC) is supported by level 1 evidence. However the optimal regimen, duration of therapy and patient population has not been defined.
MVAC
In SWOG 8710, 317 patients with stage T2N0M0 to T4aN0M0 bladder cancer were randomized to receive MVAC (methotrexate 30 mg/m2 days 1, 15, 22, vinblastine 3 mg/m2 days 2, 15, 22, doxorubicin 30 mg/m2 day 2 and cisplatin 70 mg/m2 day 2) for three cycles every 28 days followed by cystectomy compared to cystectomy alone1. Grade 3 and 4 toxicities were predominantly hematologic and gastrointestinal, as this study completed accrual prior to the routine use of modern antiemetic and granulocyte growth factor support. At a median follow up of 8.7 years, the median survival for patients receiving chemotherapy and cystectomy was 77 months compared to 46 month for those undergoing cystectomy alone (p=0.05). Patients with pathologic complete response (pCR) in either group experienced improved OS than those with any residual tumor at cystectomy. The rate of pCR in the chemotherapy arm was 38%, compared to 15% in the cystectomy alone arm, which was presumably achieved by vigorous preoperative TURBT. Down staging and survival outcomes in this trial set the benchmark for subsequent neoadjuvant chemotherapy studies in MIBC.
Gemcitabine and Cisplatin
A study of patients with advanced or metastatic bladder cancer randomized to treatment with cisplatin and gemcitabine (GC) versus MVAC showed similar five year outcomes and improved toxicity2. Many have extrapolated this benefit of GC to the perioperative setting, making this the most commonly utilized neoadjuvant regimen in the United States3. An international multicenter retrospective analysis of 212 patients found that the 146 patients with GC had similar pCR rates to the 66 patients treated with MVAC (of which 51 received AMVAC)4.
Future neoadjuvant trials should prospectively evaluate the benefit of cisplatin and gemcitabine compared to other regimens.
Accelerated MVAC
Accelerated MVAC (aMVAC) was found to have improved chemotherapy tolerance, drug delivery, and a trend toward relative reduction in the risk of progression and death in patients in patients with locally advanced unresectable and metastatic bladder cancer when compared to traditional MVAC5. AMVAC consists of the same agents as the traditional MVAC regimen, but given all over day 1 or 2 only with growth factor support. In two separate studies patients with cT2-cT4a N0-N1 MIBC were treated with three (Plimack6) or four cycles (Choueiri7) of neoadjuvant aMVAC. Both studies met their primary endpoint of pathologic response. Of the 40 evaluable patients in the Plimack et al trial, 38% achieved pCR, with another 14% down staged to non-MIBC. Of the 39 patients in the Choueiri study, 49% were down staged to non-MIBC, 10 of whom had pCR. Both groups reported responses in patients with clinical N1 disease, suggesting that tumor biology, and not clinical stage may predict response to neoadjuvant therapy. Both groups cited excellent tolerance to the aMVAC regimen, with significantly fewer grade 3 or 4 toxicities than previously reported with MVAC. Chemotherapy was completed efficiently, with both groups reporting median time to surgery significantly shorter than that described in prior neoadjuvant trials.
In combination with bevacizumab, Seifker –Radtke and colleagues reported a pCR rate of 39% in 44 patients with high risk bladder cancer (lymphovascular invasion cT3b, hydronephrosis, micropapillary features, or tumor in a diverticulum) and 2 year overall survival of 75%8.
CMV
In a trial by the, National Cancer Research Institute Bladder Cancer Clinical Studies Group, patients with T2 –T4a N0/x UCB were randomized to CMV (methotrexate 30 mg/m2 days 1 and 8, vinblastine 4 mg/m2 day 1 and 9, cisplatin 100 mg/m2 day 2 with folinic acid) every 21 days for 3 cycles prior to cystectomy or radiotherapy. At a median follow up of eight years, patients treated with neoadjuvant chemotherapy had a 16% reduction in the risk of death compared to patients in the no chemotherapy arm (CI 0.72 to 0.99, p=0.037). The study was not powered to determine differences between outcomes in patients who underwent surgery, radiotherapy or both9.
Ongoing study
SWOG 1314 - A Randomized Phase II Study of Co-Expression Extrapolation (COXEN) with Neoadjuvant Chemotherapy for Localized, Muscle-Invasive Bladder Cancer (NCT02177695) was activated in 2014. It randomizes patients with newly diagnosed stage cT2-T4a N0 MIBC to either GC or AMVAC. The primary objective of the study is to determine if gene expression profiling (COXEN score) obtained from transurethral biopsy specimens is prognostic of response to neoadjuvant chemotherapy.
Pathologic Response Following Chemotherapy
pCR has been associated with survival in the neoadjuvant setting in urothelial cancer. In randomized controlled trials of neoadjuvant chemotherapy with pCR as an endpoint, the rate of pCR is consistently higher with chemotherapy than in cystectomy alone arms10. However there is some debate in the literature as to whether giving neoadjvuant chemotherapy represents overtreatment, since some patients will achieve pT0 based on TUR alone. In SWOG 8710 trial, OS for patients who achieved pCR in the MVAC arm vs those who had cystectomy alone arm was similar, though the study was not powered to detect a difference in this subgroup1. In a retrospective analysis of patients who received MVAC followed by cystectomy with negative surgical margins, achievement of pCR correlated with improved survival outcomes compared to those who had any residual non-invasive tumors, (pa, pT1, CIS). Patients with residual p>T2 tumors or positive lymph nodes had significantly worse survival outcomes compared to those with complete pathologic response11. In a meta-analysis of 13 studies of 886 patients who underwent neoadjuvant chemotherapy and cystectomy, achievement of pCR despite the treatment arm was associated with a 55% lower risk of death compared to patients with residual invasive or noninvasive cancer (HR 0.45, 95%CI 0.36–0.56)12.
In a retrospective cohort of 1104 cystectomy patients, 120 (11%), achieved pathologic CR. Of those 120 patients, 77 received neoadjuvant chemotherapy, the majority of which was platinum based, and had “high risk” features, (cT3b on exam under anesthesia, cT4a, LVI on TURBT, cN+ on imaging, hydronephrosis). The authors reported no significant difference in overall, disease specific, or relapse free survival in the patients with pCR at cystectomy treated with or without chemotherapy (OS 80% vs 90%, p=0.31, DSS 86% vs 92%, p=0.65, RFS 80% vs 90%, p=0.18)13. They conclude similar outcomes can be obtained based on pCR status with or without chemotherapy. However, since only “high risk” patients received neoadjuvant chemotherapy in this series, one might also conclude that the addition of chemotherapy can improve the prognosis of “high risk” patients in this series to be similar to that of “low risk” who were not offered chemotherapy.
A retrospective review of the Nordic 1 and 2 randomized platinum based neoadjuvant studies showed that patients who had pCR following chemotherapy and cystectomy had a 5 year overall survival rate of 88.2% compared to 57.1% for those with pCR following cystectomy alone (p=0.001)14. These data suggest an additional benefit above increased rate of pathologic down staging with chemotherapy. In a review of 4430 patients at 12 international cancer centers who underwent RC without chemotherapy, found that survival rates were similar for those patients with pCR and pTa or CIS at cystectomy, but statistically superior to those with pT>1 or node positive disease. These authors cited their 10% rate of recurrence, and 7% rate of cancer specific death in patients with pT0 at cystectomy as a caution that all patients require long term surveillance15.
Rate of pCR following multimodality therapy is an efficient and reproducible endpoint in clinical trials of neoadjuvant therapy for UC, and has been associated with survival. However pCR has not been prospectively validated as a surrogate endpoint for OS in urothelial cancer, and thus caution is advised when interpreting clinical trials.
The neoadjuvant paradigm for translational research
Standard acquisition of pre-therapy biopsy tissue with subsequent organ and lymph node resection following neoadjuvant chemotherapy makes UC an ideal disease for translational studies. These studies are important because in the 20 years since the original MVAC study, no studies have shown a significant improvement in the pCR rate of approximately 38% or improvement in survival. Therefore, many patients with what ultimately proves to be platinum resistant disease are being over treated with ineffective chemotherapy, delaying definite surgery.
In addition, the neoadjvuant paradigm provides an opportunity to test the efficacy of molecularly targeted and biologic agents agents16. The EGFR inhibitor erlotinib was tested in 20 patients with muscle invasive UC with in the neoadjuvant setting. Pathologic downstaging to pT0 in 25% and 35% to <pT1 was demonstrated. All patients with pathologic downstaging had acneform drug rash, a marker of response to erlotinib in other malignancies17.
The tyrosine kinase inhibitor dasatinib was studied in patients undergoing cystectomy who were unfit or unwilling the receive neoadjuvant cisplatin. Downstaging was achieved in 14% of patients, and in those with paired TURBT and cystectomy tissue available, a demonstrable decrease in target SRC family kinases was noted.18,19.
Given the encouraging response data for anti PD-120 and anti PD-L121 antibodies in patients with advanced platinum refractory UC, a phase I/II trial of GC combined with the anti PD-1 antibody pembrolizumab is planned through the Hoosier Cancer Research network (NCT02365766). Cisplatin ineligible patients will receive pembrolizumab and gemcitabine alone.
Potential predictors of response
A comprehensive review of efforts to characterize molecular characteristics of responders and non-responders to chemotherapy is beyond the scope of this review, but selected analyses are outlined to demonstrate important areas of future research. Choi found that tumor response was correlated with pre chemotherapy molecular profile. Based on whole genome mRNA expression, “p53-like” tumors were mostly chemotherapy resistant, and residual tumors at cystectomy were enriched for the p53-like subtype following chemotherapy compared to matched pretreatment specimens22.
In a single institution cohort of 81 patients who underwent cystectomy without neoadjuvant chemotherapy, somatic mutations in one or more of six DNA repair genes (ATM, ERCC2, FANCD2, PALB2, BRCA1 and 2) were associated with improvement in recurrence free survival23. In a review of 38 patients who received neoadjuvant platinum based chemotherapy, low ERCC1 expression was associated with improved disease free and overall survival24. In their prospective aMVAC cohort, Plimack demonstrated by DNA sequencing of pretreatment tissue, 13/13 patients who achieved pCR had variants in ≥ 1of ATM, RB1 or FANCC genes, with roles in maintenance of chromatin structure and DNA repair25.
Adjuvant therapy
One of the potential advantages of adjvuvant therapy is that it patients can be fully surgically staged, limiting the toxicity of chemotherapy to patients most likely to benefit, while not delaying curative therapy among the patients who are unlikely to benefit.
Unfortunately data are not adequate to fully characterize the role of adjuvant chemotherapy in muscle invasive bladder cancer after cystectomy. Completing clinical trials in this disease setting has been challenging. EORTC 30994 randomized patients with T3 or T4 or N+ urothelial cancer after cystectomy to either immediate (within 90 days) chemotherapy with MVAC or GC or chemotherapy at the time of relapse. This study closed after accruing 284 of 660 planned patients. There was no difference in overall survival26.
A study of gemcitabine and cisplatin vs observation after cystectomy in patients with Grade 3 T2 disease or T3 or T4,N0–2 patients was closed to poor accrual after 194 patients were enrolled. There was no difference in OS or DFS between the two arms, and only 62% of patients completed planned chemotherapy27. Another study used p53 status by immunohistory chemistry as a prognostic biomarker for recurrence and to identify candidates for adjuvant MVAC. In this study, 499 patients underwent p53 assessment. Of this, 272 were positive, and 114 were randomized to receive three cycles of adjuvant MVAC or placebo. p53 negative patients were observed. After the first 110 patients of the planned 190 were randomized, the study was closed for futility. There was no difference in recurrence risk based on p53 status and there was no difference in outcome between the randomized patients. In addition, of 58 patients assigned to MVAC, only 46 received any treatment and only 39 began cycle 3.28
Given the small size of many adjuvant studies of bladder cancer, a meta-analysis of nine randomized clinical trials of 945 patients suggested a benefit in OS (HR 0.77 (95% CI 0.59–0.99) p=0.049) and DFS (HR 0.66 (95%CI 0.45–0.91)p=0.014).29 A recent population based study using the National Cancer Database suggested a benefit of adjuvant chemotherapy given within 90 days of cystectomy in patients with ≥pT3 and or node positive disease (HR = 0.78 (95% confidence interval [CI], 0.71–0.86).30
Adjuvant Immunotherapy
A randomized study of adjuvant cellular immunotherapy with DN 2402 vs. surveilliance in patients with high risk her 2 positive urothelial carcinoma has completed accrual (NCT01353222). A study of MAGE-A3 + AS-15 vs. placebo in patients with MAGEA3 positive muscle invasive bladder cancer with no evidence of disease after cystectomy is now underway (NCT01435356).
Maintenance treatment
Although response rates to front line cisplatin based chemotherapy are high, relapses are common and median survival is short (14–15 months)2. One potential mechanism to improve overall survival is to consolidate these responses with maintenance therapy. However there is no effective maintenance therapy in UC. This has been done with some success in other disease sites and those experiences may provide guidance into treatment selection and study design for future studies.
Completed studies in UC Suntinib
A double blind, randomized Phase 2 study examined the role of maintenance sunitinib in patients had to have either stable disease (SD) or a complete or partial response to first line chemotherapy for recurrent or metastatic UC.. The study closed early due to poor accrual after randomizing 54 of 84 planned patients over four years. There was no difference in six month PFS or OS. The toxicity profile of sunitnib was consistent with other reports, including thrombocytopenia, diarrhea, mucositis, fatigue and hypertension. Theses resulted in a high rate of dose reductions, delays and discontinuation31.
Lenalidomide
A phase 1 study of gemcitabine, cisplatin and lenalidomide was designed to continue lenalidomide maintenance in patients who had SD, CR or PR following the treatment of all three drugs. This study closed early due to poor accrual and toxicity of the combination. Lenalidomide could not be dose escalated beyond the first dose level (10 mg) due to cytopenias. No patients reached the lenalidomide maintenance arm32.
Studies awaiting results in UC
Bevacizumab
CALGB 90601 (NCT00942331) was a Phase III study of GC with either bevacizumab or placebo. After six cycles of chemotherapy, patients who do not have disease progression or unacceptable toxicity continued on bevacizumab/placebo every three weeks. The endpoint is overall survival. This study has completed accrual.
Phase II studies examining novel agents
A randomized Phase II open label study of vinflunine vs best supportive care in patients who had disease stabilization or response to 4–6 cycles of cisplatin containing chemotherapy has completed accrual (NCT 01529411). Preliminary safety data have found the treatment to be well tolerated with the most common G3/4 AEs being constipation (12.7%), neutropenia (7.9%), fatigue (4.8%) and myalgia (3.2 %)33. A phase II study of gemcitabine and cisplatin adds ipilumumab to cycles 3–6 (NCT01524991). Patients with stable disease after cycle six can continue on single agent ipilumumab maintenance. Another phase II study evaluates docetaxel with or without the Heat Shock Protein (HSP 27) inhibitor apatoresn (OGX 427) in patients with metastatic UC who have relapsed following, or are refractory to platinum based therapy (NCT01780545). Patients who receive 10 cycles of docetaxel in the combination arm can continue on OGX 427 maintenance until disease progression or death. This study is still enrolling patients.
Lessons learned from selected other disease sites
Other disease sites may provide insight into the study design of future studies of maintenance therapy in UC. This is not meant to be a review of all maintenance studies but to guide investigators as they design studies in UC.
Cytotoxic chemotherapy
Although many patients (and physicians) may be hesitant to stop chemotherapy after a pre-determined number of cycles, they may be forced to due to toxicity. There is debate in the literature across disease sites about the benefits of maintenance therapy and whether the added toxicity such as neuropathy is worth the potential benefits in PFS or OS34–36.
Patients with platinum sensitive ovarian cancer who received three cycles of paclitaxel had a shorter PFS than those who received 12 cycles. However, there was no difference in OS. More patients discontinued due to neuropathy in the 12 cycle arm than the 3 cycles, but there was no formal quality of life analysis37.
In an effort to limit chronic neuropathy in patients with advanced colorectal cancer, OPTIMOX 1 randomized patients to FOLFOX (5FU, leucovorin and oxapliatin) every two weeks until disease progression or FOLFOX for six cycles, followed by 5FU/LV Maintenance for 12 cycles followed by FOLFOX for another six cycles. This showed no difference in PFS or OS between the two arms, but reduced Grade 3 and 4 toxicity during the maintenance 5FU/LV alone arm38.
Targeted therapies
Targeted therapies have a more favorable side effect profile than cytotoxic chemotherapy. However as many new agents have been introduced in recent years, characterizing the benefit of maintenance therapy may be challenging as patients may be treated with sequential therapies. Some lessons learned from ovarian and lung cancer are described.
GOG 218 and ICON 7 both examined the role of upfront chemotherapy given with concurrent and maintenance bevacizumab compared to chemotherapy alone. In both studies, compared to the chemotherapy alone group, the bevacizumab arms had an improved in PFS but no improvement in OS.39,40 One potential explanation for these disparate results is that bevacizumab may reduce per-tumoral edema, but not an actual tumor response36.
Similary in patients without disease progression following surgery for ovarian, fallopian tube or peritoneal cancer and at least five cycles of platinum based chemotherapy, compared to those receiving placebo, those on pazopanib demonstrated an improvement in PFS, but not OS. One third of patients in the pazopanib arm (compared to 5.6% in placebo) discontinued therapy due to side effects. including hypertension (30.3%), neutropenia (9.9%) and liver toxicity (9.4%)41.
ECOG 4599 randomized patients with non-small cell lung cancer to carboplatin, paclitaxel and bevacizumab compared to chemotherapy alone for six cycles. Patients on the bevacizumab arm continued on bevacizumab alone until disease progression. The bevacizumab arm demonstrated both improved PFS and OS42.
AVAiL randomized patients to cisplatin and gemcitabine with or without bevacizumab with bevacizumab maintenance in the bevacizumab arms. While there was an improvement in PFS, there was no difference in OS,43 possibly due to the availability of erlotinib and pemetrexed, which were not available during ECOG 4599.
Lessons learned for maintenance therapy for urothelial and other cancers
Taken together, these studies demonstrated several issues that may inform study design for future studies.
Stratification factors
Some studies required patients to have a PR or CR prior to starting maintenance therapy, while others enrolled patients with stable disease. The patients with the most chemoresponsive disease (CR and PR) may be the best candidates for consolidation therapy, although this may limit study accrual. An analysis of patients who received first line chemotherapy for metastatic UC identified baseline performance status, number of visceral metastatic sites, baseline WBC and response to treatment. These may be potential stratification characteristics studies of maintenance therapy in UC.44
Ability to access pathology
In contrast to the neoadjuvant setting, obtaining specimens for correlative studies in the maintenance setting may be difficult as patients would need to undergo repeat biopsies that are not standard of care.
Candidate drug tolerability
Potential agents for maintenance therapy should be well tolerated over an extended period of time. This is particularly important given the advanced age, high frequency of comorbidities and diminished renal function of many patients with advanced UC. Studies should be designed with HRQOL measures to assess whether a potential benefit is worth any reduction of QOL in patients with advanced disease.
Potential molecular predictors
Identification of molecular targets of response to predict patients most likely to benefit may improve response rates and over treatment of patients who are unlikely to benefit. Studies of maintenance have evaluated the benefit of cetuximab in patients with KRAS WT metastatic colorectal cancer45 and olaparib in BRCA mutated ovarian cancer46 Unfortunately unlike other disease sites, there are no predictors of response to therapy for metastatic UC. Hopefully work done in the neoadjuvant setting will inform maintenance studies. In addition, the TCGA may guide selection of candidate agents. An analysis of 131 urothelial carcinomas identified potential therapeutic targets in over two thirds of tumors. The most frequent targets were in the phosphatidylinositol-3-OH kinase/AKT/mTOR pathway RTK/MAPK pathway47.
Endpoints
OS is the gold standard for clinical trials. Unfortunately the life expectancy for advanced UC is still short (12–15 months)2, so OS is still a practical endpoint. There are no FDA approved salvage regimens in the United States, and regimens currently in use such as taxanes and pemetrexed are associated with a very short PFS and OS48,49.
Design
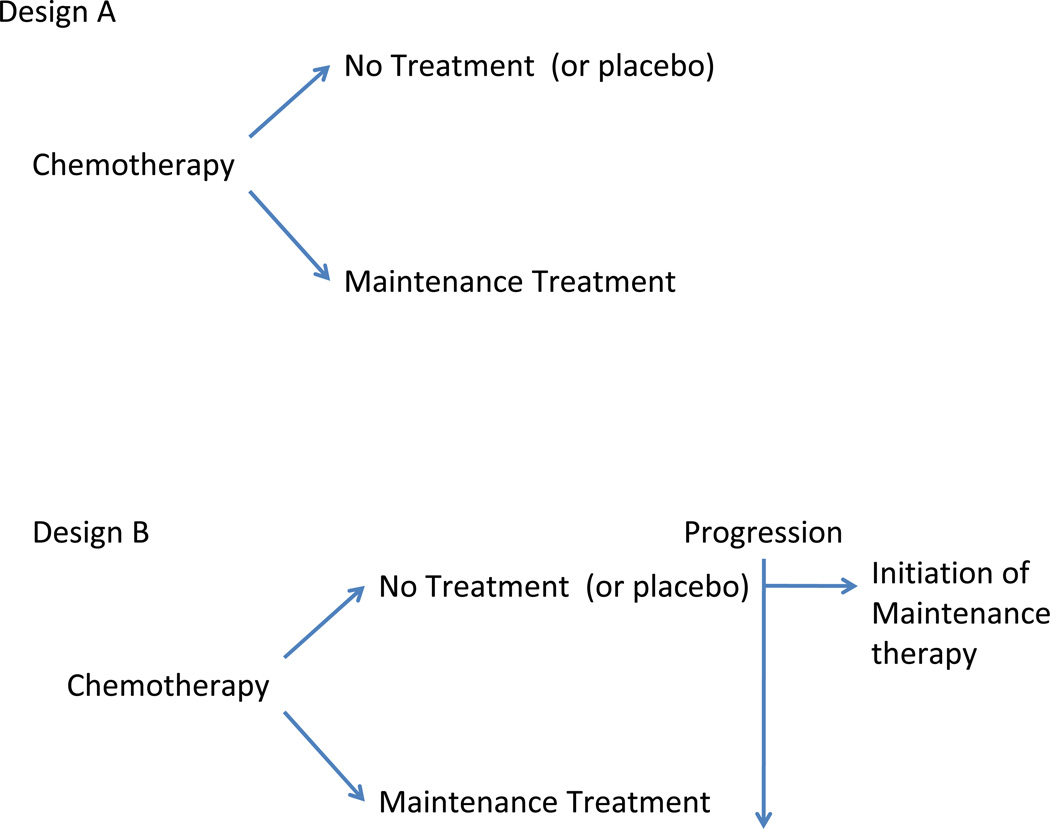
There are several potential study designs which are shown in Figure 1. The choice may depend on the endpoint, sample size, mechanism of action and toxicity of the drug.
Figure 1.
In Design A, patients are randomized to maintenance or no therapy after upfront chemotherapy. In Design B, patients enrolled to the no maintenance therapy are allowed to start the maintenance at time of disease progression. In Design B, OS would be an ideal endpoint as it would clarify if deferred therapy at the time of progression is as effective as continuous therapy, as well as evaluate endpoints such as QOL and resource utilization.
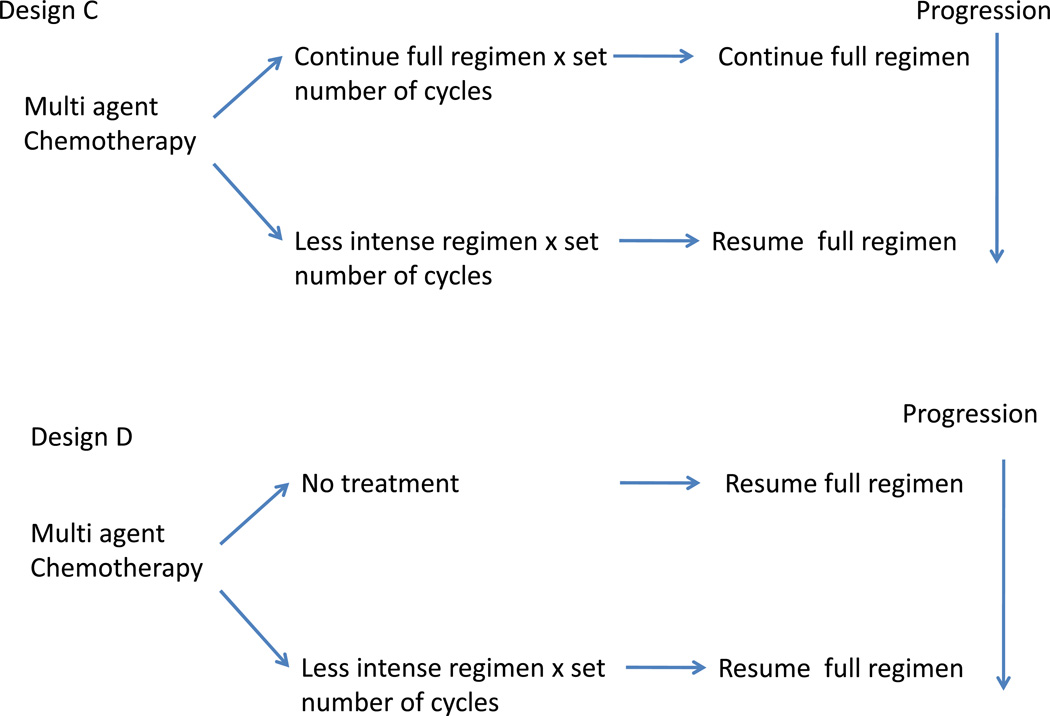
Design C studies the role of less intensive maintenance. This is particularly helpful in determining if discontinuing a component of multi-agent regimen may reduce long term cumulative toxicity. Design D may be a follow up of Design C. This evaluates the role of less intensive treatment (perhaps the regimen identified in Design C), with resumption of intensive treatment at progression.
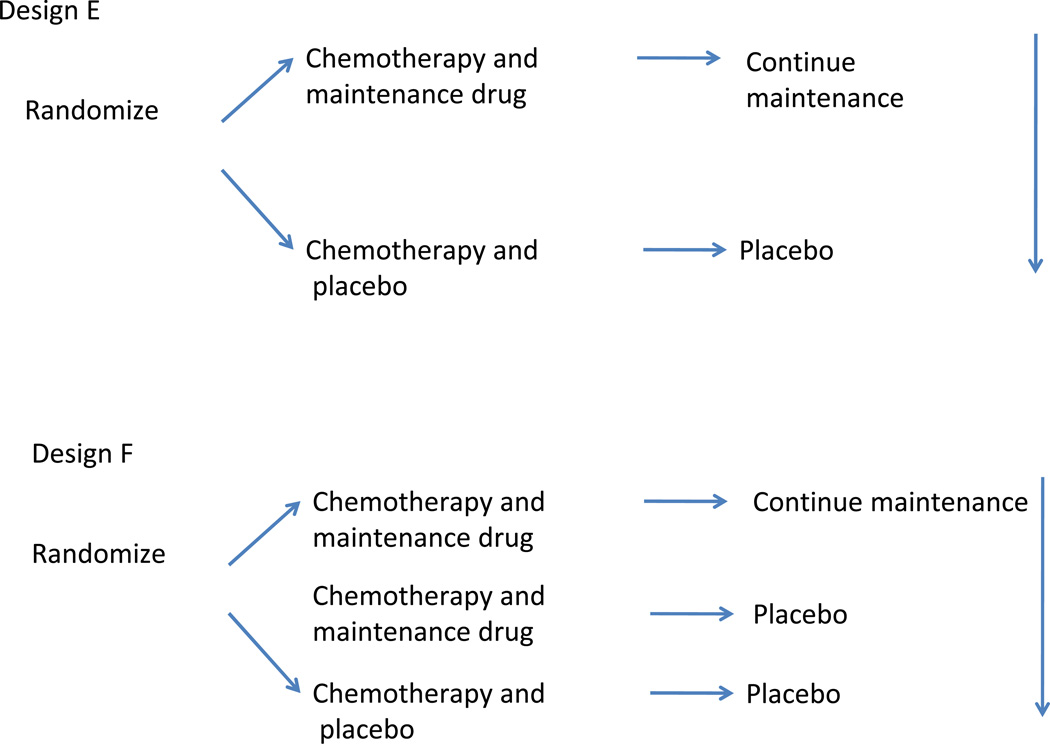
Design E randomizes patients to chemotherapy and a novel agent and continues the novel agent after a set number of cycles in the maintenance setting. Design F is similar to E but the three arms would allow investigators to determine any benefit is due to the upfront treatment, maintenance or both. However, a three arm study would require a larger sample size.
Summary
Although level 1 evidence supports the use of cisplatin-based neoadjvuant chemotherapy for UC, two decades of studies have not improved the pCR and OS rates established in the original Phase 3 study. In addition there remains concern about the toxicity, overtreatment of patients with pCR after TURBT and the need for more effective regimens for patients with cisplatin refractory disease. The neoadjvuant paradigm is an ideal opportunity to test novel agents given the availability of tissue both before and after therapy for translational studies. In addition, the association between pCR and survival allows endpoints to be reached quickly, although both PFS and OS are still important endpoints to evaluate. In contrast, the adjuvant setting has proven to be a difficult space to complete clinical trials, although investigators are optimistic about recent studies of adjuvant immunotherapy.
Unfortunately development of effective agents for metastatic UC has been slow, and as a result there are currently no effective agents approved for second line or maintenance therapy. Studies of maintenance therapy need to be carefully designed to ensure that a investigators can rigorously assess the benefit of a potential maintenance therapy, and balance this against its toxicity.
In addition, given the high prevalence of co-morbidities among many patients with urothelial cancer, identification of better tolerated agents is needed, such as non-cisplatin containing regimens for patients with impaired renal function. Treatments in the adjuvant setting need to be tolerable for patients recovering from cystectomy. For potential maintenance therapies to be effective, they need to be tolerable over a longer period of time. To improve outcomes in patients with urothelial cancer, biomarkers are needed to identify patients most likely to benefit from treatment, and studies should be designed to evaluate treatments that meaningfully improves both quality and length of life.
| Regimen | Phase | Estimated Enrollment |
Status | Primary Endpoint |
Clincialtrials.gov |
|---|---|---|---|---|---|
| Neoadjvuant | |||||
| AMVAC or GC (Testing COXEN) | Phase II | 184 | Open | Prognostic value of Treatment specific COXEN score | NCT02177695 |
| GC and Pembroluzimab or gemcitabine alone and pembroluzimab (cisplatin ineligible) | Phase Ib-II | 81 | Pending | Phase 1b: safety, tolerability Phase II: Pathologic muscle invasive response | NCT02365766 |
| Adjuvant | |||||
| DN 2402 v. observation | Randomized Phase II | 180 | Completed accrual | OS | NCT01353222 |
| MAGE-A3 + AS-15 vs. placebo | Randomized Phase II | 273 | Open | DFS | NCT01435356 |
| Maintenance | |||||
| GC and bevacizumab vs. GC and placebo | Randomized Phase III | 500 | Completed accrual | OS | NCT00942331 |
| Vinflunine vs. best supportive care | Randomized Phase II | 86 | Completed accrual | PFS | NCT 01529411 |
| GC and ipilumumab | Phase II | 36 | Completed accrual | OS | NCT01524991 |
| Docetaxel and OGX 427 vs. docetaxel | Randomized Phase II | 200 | Open | OS | NCT01780545 |
Source: clinicaltrials.gov, accessed March 14, 2015
Acknowledgments
Disclosures
Dr. Wong was supported byP30CA006927 from the National Cancer Institute. Dr. Wong has received research support from Pfizer and Bristol Myers Squibb.
The funding source had no role in the if any, in the writing of the report; and in the decision to submit the paper for publication
Dr. Hoffman-Censits has received research support from Novartis and was a paid consultant to Genentech
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Grossman HB, Natale RB, Tangen CM, Speights VO, Vogelzang NJ, Trump DL, deVere White RW, Sarosdy MF, Wood DP, Jr, Raghavan D, Crawford ED. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349:859–866. doi: 10.1056/NEJMoa022148. [DOI] [PubMed] [Google Scholar]
- 2.von der Maase H, Sengelov L, Roberts JT, Ricci S, Dogliotti L, Oliver T, Moore MJ, Zimmermann A, Arning M. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol. 2005;23:4602–4608. doi: 10.1200/JCO.2005.07.757. [DOI] [PubMed] [Google Scholar]
- 3.Apolo AB, Kim JW, Bochner BH, Steinberg SM, Bajorin DF, Kevin Kelly W, Agarwal PK, Koppie TM, Kaag MG, Quinn DI, Vogelzang NJ, Sridhar SS. Examining the management of muscle-invasive bladder cancer by medical oncologists in the United States. Urologic Oncology: Seminars and Original Investigations. 2014;32:637–644. doi: 10.1016/j.urolonc.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galsky MD, Pal SK, Chowdhury S, Harshman LC, JCS, Wong Y-N, Yu EY, Powles T, Moshier EL, Ladoire S, Hussain SA, Agarwal N, N VU, Recine F, Dominik B, Necchi A, Theodore C, Milowsky MI, Bellmunt J, Rosenberg JE Investigators ftR. Comparative effectiveness of gemcitabine plus cisplatin versus methotrexate, vinblastine, doxorubicin, plus cisplatin as neoadjuvant therapy for muscle-invasive bladder cancer. Cancer. 2015 doi: 10.1002/cncr.29387. In Press. [DOI] [PubMed] [Google Scholar]
- 5.Sternberg CN, de Mulder P, Schornagel JH, Theodore C, Fossa SD, van Oosterom AT, Witjes JA, Spina M, van Groeningen CJ, Duclos B, Roberts JT, de Balincourt C, Collette L, Group EG-UC. Seven year update of an EORTC phase III trial of high-dose intensity M-VAC chemotherapy and G-CSF versus classic M-VAC in advanced urothelial tract tumours. Eur J Cancer. 2006;42:50–54. doi: 10.1016/j.ejca.2005.08.032. [DOI] [PubMed] [Google Scholar]
- 6.Plimack ER, Hoffman-Censits JH, Viterbo R, Trabulsi EJ, Ross EA, Greenberg RE, Chen DY, Lallas CD, Wong YN, Lin J, Kutikov A, Dotan E, Brennan TA, Palma N, Dulaimi E, Mehrazin R, Boorjian SA, Kelly WK, Uzzo RG, Hudes GR. Accelerated methotrexate, vinblastine, doxorubicin, and cisplatin is safe, effective, and efficient neoadjuvant treatment for muscle-invasive bladder cancer: results of a multicenter phase II study with molecular correlates of response and toxicity. J Clin Oncol. 2014;32:1895–1901. doi: 10.1200/JCO.2013.53.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choueiri TK, Jacobus S, Bellmunt J, Qu A, Appleman LJ, Tretter C, Bubley GJ, Stack EC, Signoretti S, Walsh M, Steele G, Hirsch M, Sweeney CJ, Taplin ME, Kibel AS, Krajewski KM, Kantoff PW, Ross RW, Rosenberg JE. Neoadjuvant dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin with pegfilgrastim support in muscle-invasive urothelial cancer: pathologic, radiologic, and biomarker correlates. J Clin Oncol. 2014;32:1889–1894. doi: 10.1200/JCO.2013.52.4785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siefker-Radtke A, Kamat A, Corn P, Matin S, Grossman B, Millikan R, Dinney C. Neoadjuvant chemotherapy with DD-MVAC and bevacizumab in high-risk urothelial cancer: Results from a phase II trial at the University of Texas MD. Anderson Cancer Center. ASCO Meeting Abstracts. 2012;30:4523. [Google Scholar]
- 9.International Collaboration of T, Medical Research Council Advanced Bladder Cancer Working P, European Organisation for R, Treatment of Cancer Genito-Urinary Tract Cancer G, Australian Bladder Cancer Study G, National Cancer Institute of Canada Clinical Trials G, Finnbladder, Norwegian Bladder Cancer Study G, Club Urologico Espanol de Tratamiento Oncologico G. Griffiths G, Hall R, Sylvester R, Raghavan D, Parmar MK. International phase III trial assessing neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: long-term results of the BA06 30894 trial. J Clin Oncol. 2011;29:2171–2177. doi: 10.1200/JCO.2010.32.3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lavery HJ, Stensland KD, Niegisch G, Albers P, Droller MJ. Pathological T0 following radical cystectomy with or without neoadjuvant chemotherapy: a useful surrogate. J Urol. 2014;191:898–906. doi: 10.1016/j.juro.2013.10.142. [DOI] [PubMed] [Google Scholar]
- 11.Sonpavde G, Goldman BH, Speights VO, Lerner SP, Wood DP, Vogelzang NJ, Trump DL, Natale RB, Grossman HB, Crawford ED. Quality of pathologic response and surgery correlate with survival for patients with completely resected bladder cancer after neoadjuvant chemotherapy. Cancer. 2009;115:4104–4109. doi: 10.1002/cncr.24466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petrelli F, Coinu A, Cabiddu M, Ghilardi M, Vavassori I, Barni S. Correlation of pathologic complete response with survival after neoadjuvant chemotherapy in bladder cancer treated with cystectomy: a meta-analysis. Eur Urol. 2014;65:350–357. doi: 10.1016/j.eururo.2013.06.049. [DOI] [PubMed] [Google Scholar]
- 13.Kassouf W, Spiess PE, Brown GA, Munsell MF, Grossman HB, Siefker-Radtke A, Dinney CP, Kamat AM. P0 stage at radical cystectomy for bladder cancer is associated with improved outcome independent of traditional clinical risk factors. Eur Urol. 2007;52:769–774. doi: 10.1016/j.eururo.2007.03.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosenblatt R, Sherif A, Rintala E, Wahlqvist R, Ullen A, Nilsson S, Malmstrom PU, Nordic Urothelial Cancer G. Pathologic downstaging is a surrogate marker for efficacy and increased survival following neoadjuvant chemotherapy and radical cystectomy for muscle-invasive urothelial bladder cancer. Eur Urol. 2012;61:1229–1238. doi: 10.1016/j.eururo.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 15.Tilki D, Svatek RS, Novara G, Seitz M, Godoy G, Karakiewicz PI, Kassouf W, Fradet Y, Fritsche HM, Sonpavde G, Izawa JI, Ficarra V, Lerner SP, Schoenberg M, Stief CG, Dinney CP, Skinner E, Lotan Y, Sagalowsky AI, Reich O, Shariat SF. Stage pT0 at radical cystectomy confers improved survival: an international study of 4,430 patients. J Urol. 2010;184:888–894. doi: 10.1016/j.juro.2010.04.081. [DOI] [PubMed] [Google Scholar]
- 16.Chism DD, Woods ME, Milowsky MI. Neoadjuvant Paradigm for Accelerated Drug Development: An Ideal Model in Bladder Cancer. The Oncologist. 2013;18:933–940. doi: 10.1634/theoncologist.2013-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pruthi RS, Nielsen M, Heathcote S, Wallen EM, Rathmell WK, Godley P, Whang Y, Fielding J, Schultz H, Grigson G, Smith A, Kim W. A phase II trial of neoadjuvant erlotinib in patients with muscle-invasive bladder cancer undergoing radical cystectomy: clinical and pathological results. BJU Int. 2010;106:349–354. doi: 10.1111/j.1464-410X.2009.09101.x. [DOI] [PubMed] [Google Scholar]
- 18.Hahn N, Daneshmand S, Posadas E, Koch M, Bihrle R, Foster R, Masterson T, Cheng L, Liu Z, Breen T, Fleming M, Lance R, Ryan C, Corless C, Galsky M, Alva A, Mitchell C, Shen S, Lerner S, Sonpavde G. A phase II trial of neoadjuvant dasatinib (Neo-D) in muscle-invasive urothelial carcinoma of the bladder (miUCB): Hoosier Oncology Group GU07-122 trial. ASCO Meeting Abstracts. 2012;30:4586. [Google Scholar]
- 19.Knudsen B, Hahn NM, Daneshmand S, Posadas EM, Muhanty S, Lerner SP, Sonpavde G. Biologic activity of dasatinib administered as neoadjuvant therapy preceding radical cystectomy (RC) for muscle-invasive bladder cancer (MIBC) ASCO Meeting Abstracts. 2014;32:324. [Google Scholar]
- 20.Plimack E, Gupta S, Bellmunt J, Berger R, Montgomery B, Gonzalez E, Pulini J, Dolled-Filhart M, Emancipator K, Pathiraja K, Gause C, Perini R, Cheng J, O’Donnell P. A phase 1b study of pembrolizumab (Pembro; MK-3475) in patients (Pts) with advanced urothelial tract cancer. Oral Presentation; ESMO Annual Meeting; Madrid, Spain. 2014. [Google Scholar]
- 21.Powles T, Vogelzang NJ, Fine GD, Eder JP, Braiteh FS, Loriot Y, Cruz Zambrano C, Bellmunt J, Burris HA, Teng S-lM, Shen X, Koeppen H, Hegde PS, Chen DS, Petrylak DP. Inhibition of PD-L1 by MPDL3280A and clinical activity in pts with metastatic urothelial bladder cancer (UBC) ASCO Meeting Abstracts. 2014;32:5011. [Google Scholar]
- 22.Choi W, Porten S, Kim S, Willis D, Plimack ER, Hoffman-Censits J, Roth B, Cheng T, Tran M, Lee IL, Melquist J, Bondaruk J, Majewski T, Zhang S, Pretzsch S, Baggerly K, Siefker-Radtke A, Czerniak B, Dinney CP, McConkey DJ. Identification of distinct basal and luminal subtypes of muscle-invasive bladder cancer with different sensitivities to frontline chemotherapy. Cancer Cell. 2014;25:152–165. doi: 10.1016/j.ccr.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yap KL, Kiyotani K, Tamura K, Antic T, Jang M, Montoya M, Campanile A, Yew PY, Ganshert C, Fujioka T, Steinberg GD, O'Donnell PH, Nakamura Y. Whole exome sequencing of muscle-invasive bladder cancer identifies recurrent mutations of UNC5C and prognostic importance of DNA repair gene mutations on survival. Clinical Cancer Research. 2014 doi: 10.1158/1078-0432.CCR-14-0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ozcan MF, Dizdar O, Dincer N, Balci S, Guler G, Gok B, Pektas G, Seker MM, Aksoy S, Arslan C, Yalcin S, Balbay MD. Low ERCC1 expression is associated with prolonged survival in patients with bladder cancer receiving platinum-based neoadjuvant chemotherapy. Urol Oncol. 2013;31:1709–1715. doi: 10.1016/j.urolonc.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 25.Plimack E, Dunbrack R, Brennan T, Wei Q, Yelensky R, Serebriiskii I, Hoffman-Censits J, Kutikov A, Alpaugh K, Dulaimi E, Viterbo R, Greenberg R, Chen D, Lallas C, Wong Y-N, Trabulsi E, Palma N, Miller V, Golemis E, Ross E. Next-generation sequencing to identify molecular alterations in DNA repair and chromatin maintenance genes associated with pathologic complete response (pT0) to neoadjuvant accelerated methotrexate, vinblastine, doxorubicin, and cisplatin (AMVAC) in muscle-invasive bladder cancer (MIBC) ASCO Meeting Abstracts. 2014;32:4538. [Google Scholar]
- 26.Sternberg CN, Skoneczna I, Kerst JM, Albers P, Fossa SD, Agerbaek M, Dumez H, de Santis M, Théodore C, Leahy MG, Chester JD, Verbaeys A, Daugaard G, Wood L, Witjes JA, de Wit R, Geoffrois L, Sengelov L, Thalmann G, Charpentier D, Rolland F, Mignot L, Sundar S, Symonds P, Graham J, Joly F, Marreaud S, Collette L, Sylvester R. Immediate versus deferred chemotherapy after radical cystectomy in patients with pT3-pT4 or N+ M0 urothelial carcinoma of the bladder (EORTC 30994): an intergroup, open-label, randomised phase 3 trial. The Lancet Oncology. 2015;16:76–86. doi: 10.1016/S1470-2045(14)71160-X. [DOI] [PubMed] [Google Scholar]
- 27.Cognetti F, Ruggeri EM, Felici A, Gallucci M, Muto G, Pollera CF, Massidda B, Rubagotti A, Giannarelli D, Boccardo F. Adjuvant chemotherapy with cisplatin and gemcitabine versus chemotherapy at relapse in patients with muscle-invasive bladder cancer submitted to radical cystectomy: an Italian, multicenter, randomized phase III trial. Annals of Oncology. 2012;23:695–700. doi: 10.1093/annonc/mdr354. [DOI] [PubMed] [Google Scholar]
- 28.Stadler WM, Lerner SP, Groshen S, Stein JP, Shi S-R, Raghavan D, Esrig D, Steinberg G, Wood D, Klotz L, Hall C, Skinner DG, Cote RJ. Phase III Study of Molecularly Targeted Adjuvant Therapy in Locally Advanced Urothelial Cancer of the Bladder Based on p53 Status. Journal of Clinical Oncology. 2011;29:3443–3449. doi: 10.1200/JCO.2010.34.4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leow JJ, Martin-Doyle W, Rajagopal PS, Patel CG, Anderson EM, Rothman AT, Cote RJ, Urun Y, Chang SL, Choueiri TK, Bellmunt J. Adjuvant chemotherapy for invasive bladder cancer: a 2013 updated systematic review and meta-analysis of randomized trials. Eur Urol. 2014;66:42–54. doi: 10.1016/j.eururo.2013.08.033. [DOI] [PubMed] [Google Scholar]
- 30.Galsky M, Stensland K, Moshier E, Sfakianos J, McBride R, Tsao C-K, Casey M, Hall S, Boffetta P, Oh W, Wisnivesky J. Comparative effectiveness of adjuvant chemotherapy (AC) versus observation in patients with >= pT3 and/or pN+ bladder cancer (BCa) ASCO Meeting Abstracts. 33:292. [Google Scholar]
- 31.Grivas PD, Daignault S, Tagawa ST, Nanus DM, Stadler WM, Dreicer R, Kohli M, Petrylak DP, Vaughn DJ, Bylow KA, Wong SG, Sottnik JL, Keller ET, Al-Hawary M, Smith DC, Hussain M. Double-blind, randomized, phase 2 trial of maintenance sunitinib versus placebo after response to chemotherapy in patients with advanced urothelial carcinoma. Cancer. 2014;120:692–701. doi: 10.1002/cncr.28477. [DOI] [PubMed] [Google Scholar]
- 32.Agarwal N, Apolo AB, Tsao C-K, Lee KM, Godbold JH, Soto R, Poole A, Gimpel-Tetra K, Lowe N, Oh WK, Galsky MD. Phase Ib/II Trial of Gemcitabine, Cisplatin, and Lenalidomide as First-Line Therapy in Patients With Metastatic Urothelial Carcinoma. The Oncologist. 2014;19:915–916. doi: 10.1634/theoncologist.2014-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Polo SH, Gonzalez del Alba A, Perez-Valderrama B, Villa Guzman JC, Climent MA, Lainez N, Font A, Duran I, Mellado B, Castellano D, Garcia-Donas J, Virizuela JA, Leon L, LLorente MdM, Domenech M, Morales R, Gallardo E, Puente J, Macia S, Bellmunt J. Vinflunine maintenance therapy versus best supportive care after platinum combination in advanced bladder cancer: A phase II, randomized, open label, study (MAJA study, SOGUG 2011-02)--Interim analysis on safety. ASCO Meeting Abstracts. 2014;32:359. [Google Scholar]
- 34.Seidman AD. The Search for an Elusive Uniform Strategy for a Heterogeneous Disease: Lesson Learned? Journal of Clinical Oncology. 2013;31:1707–1708. doi: 10.1200/JCO.2013.48.6894. [DOI] [PubMed] [Google Scholar]
- 35.Ozols RF. Maintenance Therapy in Advanced Ovarian Cancer: Progression-Free Survival and Clinical Benefit. Journal of Clinical Oncology. 2003;21:2451–2453. doi: 10.1200/JCO.2003.03.039. [DOI] [PubMed] [Google Scholar]
- 36.Oliver KE, McGuire WP. Ovarian Cancer and Antiangiogenic Therapy: Caveat Emptor. Journal of Clinical Oncology. 2014;32:3353–3356. doi: 10.1200/JCO.2014.57.4574. [DOI] [PubMed] [Google Scholar]
- 37.Markman M, Liu PY, Wilczynski S, Monk B, Copeland LJ, Alvarez RD, Jiang C, Alberts D. Phase III Randomized Trial of 12 Versus 3 Months of Maintenance Paclitaxel in Patients With Advanced Ovarian Cancer After Complete Response to Platinum and Paclitaxel-Based Chemotherapy: A Southwest Oncology Group and Gynecologic Oncology Group Trial. Journal of Clinical Oncology. 2003;21:2460–2465. doi: 10.1200/JCO.2003.07.013. [DOI] [PubMed] [Google Scholar]
- 38.Tournigand C, Cervantes A, Figer A, Lledo G, Flesch M, Buyse M, Mineur L, Carola E, Etienne P-L, Rivera F, Chirivella I, Perez-Staub N, Louvet C, Andre T, Tabah-Fisch I, de Gramont A. OPTIMOX1: A Randomized Study of FOLFOX4 or FOLFOX7 With Oxaliplatin in a Stop-and-Go Fashion in Advanced Colorectal Cancer—A GERCOR Study. Journal of Clinical Oncology. 2006;24:394–400. doi: 10.1200/JCO.2005.03.0106. [DOI] [PubMed] [Google Scholar]
- 39.Burger RA, Brady MF, Bookman MA, Fleming GF, Monk BJ, Huang H, Mannel RS, Homesley HD, Fowler J, Greer BE, Boente M, Birrer MJ, Liang SX. Incorporation of Bevacizumab in the Primary Treatment of Ovarian Cancer. New England Journal of Medicine. 2011;365:2473–2483. doi: 10.1056/NEJMoa1104390. [DOI] [PubMed] [Google Scholar]
- 40.Perren TJ, Swart AM, Pfisterer J, Ledermann JA, Pujade-Lauraine E, Kristensen G, Carey MS, Beale P, Cervantes A, Kurzeder C, Bois Ad, Sehouli J, Kimmig R, Stahle A, Collinson F, Essapen S, Gourley C, Lortholary A, Selle F, Mirza MR, Leminen A, Plante M, Stark D, Qian W, Parmar MKB, Oza AM. A Phase 3 Trial of Bevacizumab in Ovarian Cancer. New England Journal of Medicine. 2011;365:2484–2496. doi: 10.1056/NEJMoa1103799. [DOI] [PubMed] [Google Scholar]
- 41.du Bois A, Floquet A, Kim J-W, Rau J, del Campo JM, Friedlander M, Pignata S, Fujiwara K, Vergote I, Colombo N, Mirza MR, Monk BJ, Kimmig R, Ray-Coquard I, Zang R, Diaz-Padilla I, Baumann KH, Mouret-Reynier M-A, Kim J-H, Kurzeder C, Lesoin A, Vasey P, Marth C, Canzler U, Scambia G, Shimada M, Calvert P, Pujade-Lauraine E, Kim B-G, Herzog TJ, Mitrica I, Schade-Brittinger C, Wang Q, Crescenzo R, Harter P. Incorporation of Pazopanib in Maintenance Therapy of Ovarian Cancer. Journal of Clinical Oncology. 2014;32:3374–3382. doi: 10.1200/JCO.2014.55.7348. [DOI] [PubMed] [Google Scholar]
- 42.Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, Lilenbaum R, Johnson DH. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 43.Reck M, von Pawel J, Zatloukal P, Ramlau R, Gorbounova V, Hirsh V, Leighl N, Mezger J, Archer V, Moore N, Manegold C. Overall survival with cisplatin–gemcitabine and bevacizumab or placebo as first-line therapy for nonsquamous non-small-cell lung cancer: results from a randomised phase III trial (AVAiL) Annals of Oncology. 2010 doi: 10.1093/annonc/mdq020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Galsky MD, Moshier E, Krege S, Lin CC, Hahn N, Ecke T, Sonpavde G, Pond G, Godbold J, Oh WK, Bamias A. Posttreatment prognostic nomogram for patients with metastatic urothelial cancer completing first-line cisplatin-based chemotherapy. Urol Oncol. 2014;32:48 e1–48 e8. doi: 10.1016/j.urolonc.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 45.Siu LL, Shapiro JD, Jonker DJ, Karapetis CS, Zalcberg JR, Simes J, Couture F, Moore MJ, Price TJ, Siddiqui J, Nott LM, Charpentier D, Liauw W, Sawyer MB, Jefford M, Magoski NM, Haydon A, Walters I, Ringash J, Tu D, O'Callaghan CJ. Phase III randomized, placebo-controlled study of cetuximab plus brivanib alaninate versus cetuximab plus placebo in patients with metastatic, chemotherapyrefractory, wild-type K-RAS colorectal carcinoma: the NCIC Clinical Trials Group and AGITG CO.20 Trial. J Clin Oncol. 2013;31:2477–2484. doi: 10.1200/JCO.2012.46.0543. [DOI] [PubMed] [Google Scholar]
- 46.Ledermann J, Harter P, Gourley C, Friedlander M, Vergote I, Rustin G, Scott CL, Meier W, Shapira-Frommer R, Safra T, Matei D, Fielding A, Spencer S, Dougherty B, Orr M, Hodgson D, Barrett JC, Matulonis U. Olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer: a preplanned retrospective analysis of outcomes by BRCA status in a randomised phase 2 trial. Lancet Oncol. 2014;15:852–861. doi: 10.1016/S1470-2045(14)70228-1. [DOI] [PubMed] [Google Scholar]
- 47.The Cancer Genome Atlas Research N. Comprehensive molecular characterization of urothelial bladder carcinoma. Nature. 2014;507:315–322. doi: 10.1038/nature12965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vaughn DJ, Broome CM, Hussain M, Gutheil JC, Markowitz AB. Phase II trial of weekly paclitaxel in patients with previously treated advanced urothelial cancer. J Clin Oncol. 2002;20:937–940. doi: 10.1200/JCO.2002.20.4.937. [DOI] [PubMed] [Google Scholar]
- 49.Sweeney CJ, Roth BJ, Kabbinavar FF, Vaughn DJ, Arning M, Curiel RE, Obasaju CK, Wang Y, Nicol SJ, Kaufman DS. Phase II study of pemetrexed for second-line treatment of transitional cell cancer of the urothelium. J Clin Oncol. 2006;24:3451–3457. doi: 10.1200/JCO.2005.03.6699. [DOI] [PubMed] [Google Scholar]