Abstract
Converging data in songbirds support a central role for the medial preoptic nucleus (POM) in motivational aspects of vocal production. Recent data suggest that dopamine in the POM plays a complex modulatory role in the production of sexually-motivated song and that an optimal level of dopamine D1 receptor stimulation is required to facilitate singing behavior. To further explore this possibility, we used quantitative real time PCR to examine relationships between mRNA expression of D1 as well as D2 receptors in the POM (and also the lateral septum and Area X) and sexually-motivated singing behavior in male European starlings. Results showed that both males with the highest and lowest D1 expression in the POM sang significantly less than males with intermediate levels of expression. Furthermore, singing behavior rose linearly in association with increasing levels of D1 expression in POM but dropped abruptly, such that individuals with D1 expression values higher than the mean sang very little. Analysis of birds with low and intermediate levels of D1 expression in POM revealed strong positive correlations between D1 expression and song but negative relationships between D2 receptor expression and song. These findings support prior work suggesting an optimal level of POM D1 receptor stimulation best facilitates sexually-motivated singing behavior. Results also suggest that D2 receptors may work in opposition to D1 receptors in POM to modify vocal production.
Keywords: vocal communication, dopamine, social behavior, songbird, birdsong, motivation
1.0 Introduction
Vocal communication is essential for coordinating many social interactions in vertebrates (Bradbury and Vehrencamp, 2011). Multiple studies in songbirds provide insight into the neural regulation of vocal learning, production, and sensorimotor aspects of vocal behavior (reviewed in (Zeigler and Marler, 2008)). However, less is known about the mechanisms underlying the motivation to communicate. Furthermore, the propensity to communicate varies strongly across individuals, yet little is known about the neural control of individual differences in vocal production. Converging data in songbirds support a central role for the medial preoptic nucleus (often referred to as POM in birds) in motivational aspects of communication (reviewed in (Riters, 2011, 2012)). Recent data indicate that the dopamine system in this region plays a complex modulatory role in the production of sexually-motivated song (Heimovics and Riters, 2008, Heimovics et al., 2009, Riters et al., 2014); however, the mechanisms by which it does so are not well known. The goal of this study is to provide insight into the roles of D1 and D2 receptors in the POM in this form of highly motivated vocal production.
Across vertebrates, the medial preoptic nucleus is centrally involved in male sexually-motivated behaviors (e.g., (Crews, 2005, Hull and Dominguez, 2006, Balthazart and Ball, 2007, Stolzenberg and Numan, 2011)), including the production of courtship song in male songbirds (Riters and Ball, 1999, Alger and Riters, 2006, Alger et al., 2009). Peripheral injections of a D1 receptor agonist facilitate the production of sexually-motivated song in male starlings (Riters et al., 2005, Schroeder and Riters, 2006). The POM contains dopamine, dopamine synthetic enzymes (i.e. tyrosine hydroxylase; TH) and dopamine receptors (Heimovics and Riters, 2008, Kleitz-Nelson et al., 2010b, Kubikova et al., 2010), suggesting it may be one site in which dopamine acts to influence singing behavior. Consistent with this possibility, both measures of D1 dopamine receptors and TH in the POM correlate with male courtship song (Heimovics and Riters, 2008, Heimovics et al., 2009). Furthermore, in rodents and birds several studies show that stimulation of D1 receptors in the medial preoptic area can facilitate other types of sexually-motivated male behavior (Markowski et al., 1994, Stolzenberg and Numan, 2011). However, the relationship between D1 receptor stimulation in the medial preoptic area and sexually-motivated male behaviors (including song) is not straight forward. Although some studies show that stimulation of D1 receptors in the medial preoptic area promotes sexually-motivated behavior (e.g., (Markowski et al., 1994, Stolzenberg and Numan, 2011, Riters et al., 2014)), others demonstrate an inhibitory role (e.g.,(Kleitz-Nelson et al., 2010a)) or no detectable effect (e.g., (Hull et al., 1989)).
The inconsistent effects of D1 receptor manipulations on male sexual behavior have led some researchers to suggest that dopamine in the medial preoptic area is not important for mediating this behavior (Paredes and Agmo, 2004). Another interpretation is that an optimal level of dopamine receptor activity is critical for stimulating sexually-motivated behaviors, including song. In support of this possibility, a dose-response pharmacology experiment in male starlings demonstrated that D1 receptor stimulation in POM influenced song in an inverted-U shaped manner, such that song production was maximally stimulated at intermediate levels of receptor stimulation, but inhibited at both lower and higher levels of stimulation (Riters et al., 2014). If the relationship between singing behavior and D1 receptor activation is an inverted-U shape, conflicting results may be a consequence of studies revealing only a single part of this curve.
The possibility that an optimal level of dopamine D1 receptor stimulation is required to facilitate behavior is well supported by studies of the prefrontal cortex, which show that low and high levels of D1 receptor occupancy can result in low behavioral performance, while intermediate levels of D1 receptor stimulation facilitate performance (reviewed in (Seamans and Yang, 2004, Williams and Castner, 2006, Berridge and Arnsten, 2013)). We propose that if an optimal level of D1 dopamine receptor activity is needed to promote sexually-motivated song, this may be mediated at the level of D1 receptor transcription in the POM. To provide insight into this possibility, here we examined relationships between mRNA expression of D1 receptors in the POM and sexually-motivated song production in male European starlings, Sturnus vulgaris.
In addition to D1 receptors, the POM also contains D2 receptors (Heimovics et al., 2009, Kleitz et al., 2009). To date, D2 receptors in POM have not been implicated in singing behavior (Heimovics et al., 2009); however, peripheral pharmacological manipulations of D1 and D2 receptors indicate that D1 stimulation facilitates whereas D2 stimulation inhibits the production of other forms of sexually-motivated male behavior (Balthazart et al., 1997, Kleitz-Nelson et al., 2010a). In studies of male rats, D1 and D2 receptors in the medial preoptic nucleus have also been shown to influence male sexual behavior in an opposing fashion and it is proposed that the ratio of D2/D1 receptors in this region influences sexual activity (Hull et al., 1989). In contrast, studies in Japanese quail indicate that both D1 and D2 receptors in the POM facilitate sexually-motivated behaviors (Kleitz-Nelson et al., 2010a). Differences in effects of dopamine in the medial preoptic area observed in rats and quail may also relate to differences in the ratios of D1 and D2 receptors found in the POM (Kleitz et al., 2009). The extent to which D1 and D2 receptors in the POM modulate singing behavior in male songbirds is unknown; therefore, we also examined the extent to which D2 expression in POM related to vocal behavior.
2.0 Experimental Procedures
2.1 Capture and housing
Starlings used in this study were captured with baited fly-in traps on a farm in Madison, WI during 2009–2010. Upon capture, individuals were transported to the University of Wisconsin’s Department of Zoology indoor animal facilities and each banded with a unique combination of plastic color bands in addition to a stainless steel identification band. Starlings were then housed in single sex cages (91 cm × 47 cm × 47 cm; 5 birds/cage) and provided food and water ab libitum. To induce a state of photosensitivity (Dawson et al., 2001), individuals were subjected to artificial photoperiods of 18h light (L):6h dark (D) for 6 weeks, followed by 6 weeks of 8L:16D before the onset of the experiment (as in (Heimovics et al., 2009, Kelm et al., 2011)). All procedures and protocols adhered to the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals and a protocol approved by the University of Wisconsin Institutional Animal Care and Use Committee.
2.2 Behavioral measures
In June 2012, 20 male starlings were randomly distributed into 5 outdoor aviaries (2.13 m × 2.4 m × 1.98 m; 5 birds/aviary) 7 days prior to behavioral observations. Aviaries were equipped with nest boxes, perches, nesting material and baths; food and water were provided ad libitum. The birds were exposed to the natural photoperiod which was approximately 13L:11D, which stimulates gonadal recrudescence and spring-typical singing and courtship behaviors. Once males began to demonstrate reproductive behaviors within the aviaries (after 7 days), we exposed each aviary to female stimulus birds for 4 consecutive days and observed the males’ responses. Briefly, a female starling was released into an aviary for 20 minutes and male behavior during her presence was documented. During each 20 min period, a single observer recorded the number of songs produced and the amount of time each male spent singing (secs) as well as bouts of non-specific behaviors, including eating, drinking, and preening (with a bout separated by at least 2 seconds from prior behavior). The stimulus female was removed from the aviary at the end of each behavioral session. A single female was used as a stimulus bird on each day of observations (rotated through all aviaries) and a new female was used each day (4 females total). Stimulus females were housed indoors under similar light cycle length as outdoor aviaries. Starlings were behaviorally tested in social groups because we were interested in examining song behavior as it occurs within a dynamic social system (i.e. a flock). We were specifically interested in the extent to which dominance status (as reflected in whether or not a male acquired a nesting site) related to D1/D2 expression; however, no significant differences were observed between males with and without nesting sites in the present study and these data will not be discussed further.
2.3 Testosterone measures, tissue preparation, and qPCR
Following behavioral observations on day 4, all males were immediately sacrificed by rapid decapitation. Trunk blood samples were collected at this time to assay testosterone (T). Plasma T was measured using a commercial grade competitive enzyme immunoassay (Cayman Chemical, Ann Arbor, MI, USA, Catalog # 582701) as in past studies (e.g., (Cordes et al., 2014b)).
Brains were collected, flash frozen in 2-Methylbutane (M32631, Sigma Aldrich, St. Louis), and stored at −80°C. Using a cryostat, brains were sliced coronally at −15°C into 200μm sections and placed onto gel-coated slides for nucleus-specific punches (Stoelting brain punch set #5740; punch sizes and locations are shown in Figure 1). In addition to POM, Area X and LS were also punched for qPCR analysis because both areas contain dopamine receptors (Casto and Ball, 1994, Heimovics et al., 2009, Kubikova et al., 2010); however, Area X is a song control region not implicated in motivation and D1 receptors in LS have been more tightly linked to agonistically-motivated song as opposed to sexually-motivated song (Heimovics et al., 2009). Tissue punches were transferred to microcentrifuge tubes and stored at −80°C until RNA extraction. Tissue was homogenized with an electric Dremel tool and RNA extracted with a Bio-Rad Aurum Total RNA Fatty and Fibrous Tissue Kit (Catalog No. 732-6830; Bio-Rad, Hercules, CA) following manufacturer’s procedures. RNA concentration was measured with a Nanodrop system (Thermo Scientific, Wilmington, DE) and RNA integrity in a subset of samples was verified with Agilent 2100 BioAnalyzer and Agilent RNA 6000 Pico Kit (Agilent Technologies, Santa Clara, CA). RNA (100ng for POM and Area X and 50ng for LS) was then converted into single stranded cDNA using Invitrogen SuperScript III First-Strand Synthesis System (Catalog No. 18080-051; Life Technologies, Carlsbad, CA). Following cDNA conversion, relative gene expression for D1 and D2 was determined for Area X, POM, and LS using qPCR analysis.
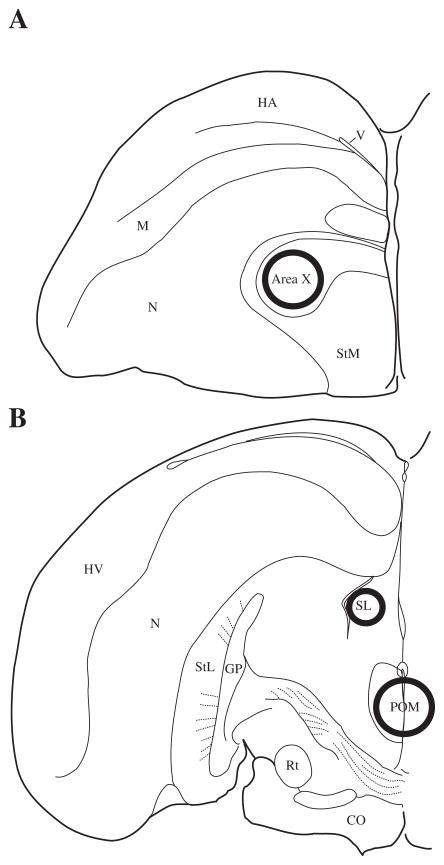
Figure 1.
Location of tissue punches illustrated in one, coronal hemisphere of the starling brain. Approximate punch sizes and locations are represented by circles centered in Area X (1.25mm diameter), POM (1.25mm diameter), and LS (0.75mm diameter). Abbreviations: HA = hyperpallium apicale; M = mesopallium; N = nidopallium; StM = striatum mediale; StL = striatum laterale; GP = globus pallidus; Rt = nucleus rotundus; OC = optic chiasm.
Primers for D1 and D2 receptors (Table 1) were designed using NCBI Gene Database Primer-Blast referencing the zebra finch (Taeniopygia guttata) genome. Two reference genes, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and hypoxanthine-guanine phosophoribosyltransferase (HPRT) were also analyzed with primers used previously in (Bentley et al., 2013) (Table 1). Non template controls were run with each primer pair to check for formation of nonspecific amplification products. All primer runs yielded single peak melt curves indicating amplification of single genes. The qPCR reaction product for each gene (target and reference) was sequenced using Sanger sequencing with both forward and reverse primers at the University of Wisconsin Biotechnology Center and sequences are provided in Table 2. Using NCBI BLAST all sequences match the intended targets.
Table 1.
Primer information for each gene
| Gene | Accession Number | Direction | Sequences | Ta |
|---|---|---|---|---|
| HPRT | NM204848.1 | Forward | GACCTGGACTTGTTCTGCAT | 57 |
| Reverse | ATTTCACGTGCCAGTCTCTC | |||
| GAPDH | NM204305.1 | Forward | AGCAATGCTTCCTGCACTAC | 57 |
| Reverse | CTGTCTTCTGTGTGGCTGTG | |||
| Dopamine receptor 1 | NM_001243833.1 | Forward | ACGAGAGGAAAATGACCCCC | 58 |
| Reverse | GTTGTAGCCTTGTGCCAGTT | |||
| Dopamine receptor 2 | XM_002191611.2 | Forward | TACCAGTCCCCCTGAGAAAG | 58 |
| Reverse | GTAGAGTTGTTGCCCCGATT |
Table 2.
Results of Sanger sequencing of qPCR reaction product for each gene
| Gene | Sequence |
|---|---|
| GAPDH | AGGGCTGGCAGGTATCCATGACACTTTGGCATCGTGGAGGGTCTCATGACCACTGGTCCATGCCATCACAGCCACACAGAAGACAGATA |
| HPRT1 | CCCNGNCGANATTTGGAAAGTCTATATTCCTCATGGGCTCATCATGGGACAGGCACGGNAGAGACTGGCACGNTGAAATTAA |
| DAR1 | GAGGAANACACGGGACAAAGGTCCACGGCCACGCCTGGATCATGGATGAAGGGCTGCCTTGGGGGGTCATTTTCCCTCTT |
| DAR2 | AGNCNANNNNGGGGNTNNTTGGTGCTGTGGGNTTTGAAGGGCCGCTTTCTCAGGGGGACCTGGATAA |
Samples were prepared for analysis in qPCR reaction tubes containing sample cDNA, nuclease free water, forward and reverse primers (5μM; University of Wisconsin), and SsoFast EvaGreen Supermix (Catalog No. 172-5201). Five standards were run with each plate of samples (1:4 serial dilution, starting concentration at 500ng/μl), along with a negative control (nuclease free water substituted for cDNA). Standards and samples were run in triplicate on each plate and all plates were read with the BioRad CFX96 Touch Real-Time PCR Detection System (Catalog No. 185-5195; Bio-Rad, Hercules, CA). Each qPCR run consisted of: an initiation step at 95°C for 30s, followed by 40 cycles of 95°C for 5s, followed by a 30s annealing phase, a 20s elongation phase at 72°C, and a melt curve from 60°C to 88°C, 0.5 degrees for each 5s step. Plates were read following each elongation and melt curve step. Criteria for inclusion in the dataset were: run efficiencies between 90–110%, an R2 of at least 0.990, and a melt curve displaying a single peak indicative of primer specificity.
2.4 Statistical Analyses
Before statistical analyses, mean cycle threshold (Ct; amplification threshold = 200 RFU) values of samples were transformed using the Pfaffl Method (Pfaffl, 2001) to determine relative levels of gene expression (detailed in (Cordes et al., 2014a)). Behavioral data were not normally distributed (Lilliefors test, p < 0.01) and were square root transformed for analysis. Non-specific behavioral measures (feeding, drinking, and preening) were summed for analyses. All data were analyzed with Statistica software (StatSoft 2001, Tulsa, OK).
As part of initial analyses we used one-way ANOVAs to determine if there were any differences in D1 or D2 expression or singing behaviors across aviaries. No significant differences were observed. Similarly when aviary was included as a variable in multiple regression analyses to examine relationships between D1 and D2 receptor expression and song (detailed below) it did not contribute to any model. Thus aviary is not considered further in this paper.
3.0 Results
3.1 Confirmation of reproductive state
Half of the males acquired and defended nesting sites, which is typical in aviaries containing multiple males and only observed in starlings during the breeding season (Riters et al., 2000). T concentrations were also typical of male starlings in the breeding season (mean (pg/ml) = 1191.17; sd 1041.45; range 206.9 – 4060.40). For reference, T values in past studies in male starlings living in stable flocks in captivity range from a mean of 750 – 1600 pg/ml (Gwinner et al., 2002, Stevenson et al., 2009).
3.2 Levels of D1 and D2 receptor expression and singing behavior
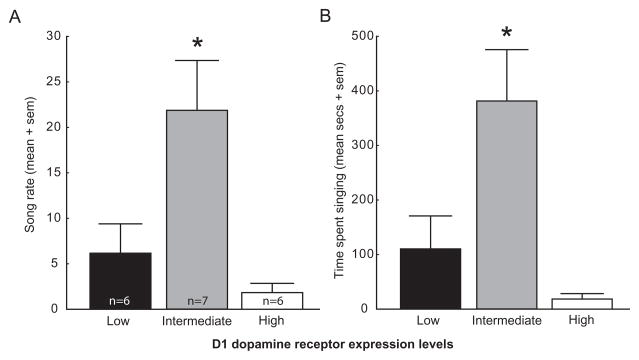
Based on prior data showing that an intermediate level of D1 receptor stimulation in POM is necessary to stimulate song production (reviewed in the introduction), we first examined the possibility that this may be reflected in an inverted-U shaped relationship between song production and D1 receptor expression in POM. To do this, we rank-ordered and divided males into 3 groups based on levels of D1 receptor expression; low (n=6; mean expression = 0.67, median = 0.67, range = 0.49–0.83), intermediate (n=7; mean expression = 0.94, median = 0.90, range = 0.85–1.06), and high (n=6; mean expression = 1.56, median = 1.30, range = 1.22–2.51) and performed one-way ANOVAs with measures of song entered as dependent variables. For these and subsequent analyses of D1 as well as D2 receptors in POM, one bird was removed from analysis because expression levels were well over 2 standard deviations above the mean (D1 expression in POM = 11.82; D2 = 18.92), resulting in n = 19. Both measures of song rate (F2,16 = 7.21, p = 0.006, Figure 2) and time spent singing (F2,16 = 8.84, p = 0.003, Figure 2) significantly differed across D1 categories. Individuals with intermediate D1 expression sang more songs (Fisher’s post-hoc: all p’s < 0.02) and spent more time singing (all p’s < 0.02) than individuals with low or high D1 mRNA values. Song rate (p = 0.40) and time spent singing (p = 0.29) did not significantly differ between low or high D1 expression categories.
Figure 2.
D1 dopamine receptor expression in POM is highest in males singing at intermediate levels. Bar graphs showing mean (+ sem) song rate (A) and time spent singing (B) for males categorized as possessing low, intermediate or high levels of D1 receptor expression (see text for details). Sample sizes for both measures are shown in bars in figure A. * = significantly different from males in the low or high category.
Results were not significant when the same analyses were run for low, intermediate, and high categories of D1 expression in either LS (song rate: p = 0.969; time singing: p = 0.998) or Area X (song rate: p = 0.393; time singing: p = 0.330). Furthermore, no significant results were found when birds were categorized based on levels of D2 expression in POM, LS, or Area X for either song rate or time spent singing (POM D2: song rate, p = 0.123; time singing, p = 0.109; LS: song rate, p = 0.162; time singing, p = 0.186; Area X D2: song rate, p = 0. 345; time singing, p = 0.543). Finally, T concentrations did not differ significantly across birds categorized based on D1 or D2 expression levels in any brain region.
3.3 Statistical contributions of D1 and D2 mRNA expression to singing behavior
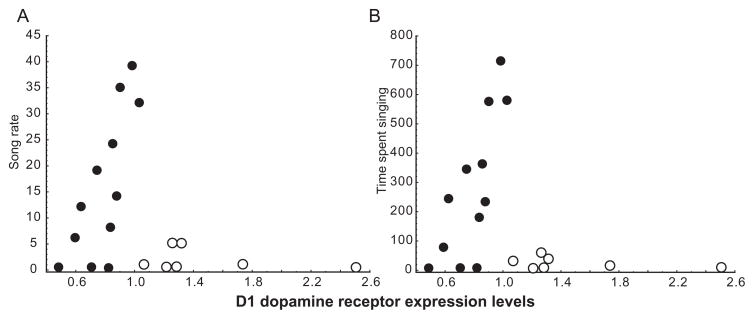
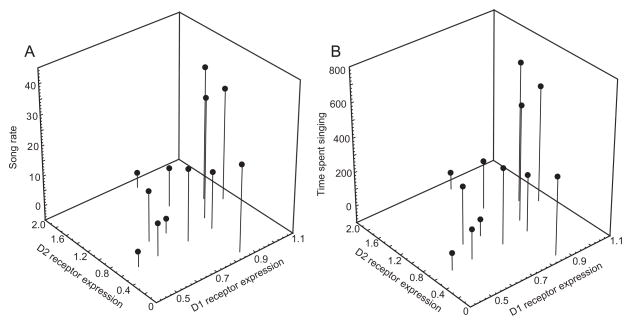
Based on past research showing that both D1 and D2 receptors in the medial preoptic area influence sexually-motivated behavior, in some cases in opposing ways (reviewed in the introduction), we next used forward stepwise multiple regression analyses to explore statistical contributions of both D1 and D2 mRNA expression in POM to measures of singing behavior. Results of a first set of analyses in which D1 and D2 expression measures in POM as well as T concentrations were entered as predictor variables and song rate and time spent singing were entered as dependent variables (in separate analyses) revealed no significant contributions of D1 or D2 expression or T concentrations to either measure of singing behavior (song rate: adj R2 = 0.06, p = 0.169; song duration: adj R2 = 0.05, p = 0.174). Consistent with the categorical analysis, a closer examination of the data revealed that in POM measures of song increased linearly in association with D1 receptor expression to a point (which corresponded to the statistical mean) after which singing behavior dropped abruptly (Figure 3; a similar pattern was not observed for D2 receptors). This finding suggests a threshold may exist after which D1 expression is associated with a lack of singing behavior but that before this point D1 expression relates linearly to singing behavior. To examine the latter possibility we removed from analysis the 7 birds with the highest levels of D1 expression in POM and associated low rates of singing behavior (Figure 3) and ran a second set of multiple regression analyses as described above. Results of a stepwise forward multiple regression analysis on song rate were significant (adj R2 = 0.61, p = 0.006). D1 expression in POM positively contributed to variation in song rate (beta = 0.82, se of beta = 0.20, t(9) = 4.18, p = 0.002); whereas, D2 expression contributed negatively (beta = −0.47, se of beta = 0.20, t(9) = 2.42, p = 0.039; Figure 4). An additional model suggested similar findings for time spent singing (adj R2 = 0.56, p = 0.011), with variation positively explained by D1 expression (beta = 0.80, se of beta = 0.21, t(9) = 3.83, p = 0.004, and a trend for a negative contribution by D2 expression (beta = −0.42, se of beta = 0.21, t(9) = 2.02, p = 0.074; Figure 4). T concentrations did not contribute significantly to any model.
Figure 3.
Singing behavior correlates positively with D1 expression in POM to a point after which high expression is associated low levels of singing behavior. Scatterplots illustrating relationships between song rate (A) and time spent singing (B) and D1 dopamine receptor expression in POM. Each point represents data from a single individual. Filled points highlight individuals for which singing behavior and D1 receptors in POM correlated positively in multiple linear regression analyses. Open points indicate individuals not included in linear analyses. Analyses were run on square root transformed singing data, but raw data are shown here.
Figure 4.
Singing behavior relates positively to D1 expression and negatively to D2 expression in POM. 3-D Scatterplots illustrating relationships between song rate (A) and time spent singing (B) and D1 and D2 dopamine receptor expression in POM. Each point represents data from a single individual (the same individuals highlighted by filled points in Figure 3). Analyses were run on square root transformed singing data, but raw data are shown here.
The same analyses were run to examine relationships between D1 and D2 expression in LS and Area X. Datasets for these regions did not violate the assumption of linearity; therefore, all individuals were included in multiple regression analyses. No models were returned examining relationships between song production and D1 and D2 expression in LS or Area X.
3.4 Non-song behaviors
To determine whether significant relationships identified above between D1 and D2 expression in POM and behavior were specific to singing behavior, we ran the same ANOVAs and multiple regression analyses described above but entered the sum of eating, drinking, and preening in place of measures of song. Results of ANOVAs examining birds with low, intermediate, and high measures of D1 receptors were not significant (F2,16 = 0.57, p = 0.578). Similarly, multiple regression analysis revealed no significant statistical contributions of D1 or D2 expression to non-song behavior (adj R2 = 0.15, p = 0.11).
4.0 Discussion
Data from this study support the hypothesis that an optimal level of D1 receptor activity in the POM facilitates sexually-motivated song in male starlings. Results additionally suggest a potentially inhibitory role for D2 receptors in the POM in this sexually-motivated behavioral context. No significant relationships were identified between non-song behaviors (preening plus feeding and drinking) and measures of D1 or D2 expression in POM, indicating that differences in D1 and D2 expression were tightly coupled to singing behavior, rather than general motor behaviors. The results suggest that individual differences in the production of sexually-motivated song may in part be explained by individual differences in the ratios of D1 and D2 receptors in the POM Given that pharmacological manipulations of D1 and D2 receptors dramatically alter male sexual behaviors, we interpret our data as consistent with alterations in D1 and D2 receptors causing individual differences in singing behavior. However another possible and non-mutually exclusive interpretation of our results is that singing induces differences in D1 and D2 receptor expression or possibly another variable (such as testosterone concentrations or dominance status) influences all three. In the present study, neither testosterone concentrations, nor social status (i.e., whether or not a male possessed a nesting site) related statistically to singing behavior or dopamine receptor expression, suggesting that these variables were not influential in the present study. However, the directionality of the relationships reported here and additional variables must be examined in future work.
4.1 Optimal POM D1 receptor expression and sexually-motivated song
Past studies indicate that D1 receptor stimulation can have excitatory or inhibitory effects depending on current receptor occupancy, resulting in inverted U-shaped effects on behavioral performance (reviewed in (Williams and Castner, 2006)). In the present study, singing behavior rose linearly in association with increasing levels of D1 expression but then dropped abruptly, such that individuals with D1 expression values higher than the mean sang much less. This suggests that there may be a D1 receptor threshold after which singing drops. The finding that male starlings with the highest and lowest D1 expression in the POM sang significantly less than males with intermediate levels indicates that dopamine’s impact on sexually-motivated behavior may be regulated at the level of POM D1 receptor gene expression. This nicely corroborates recent findings that show that an intermediate level of D1 receptor occupancy in the POM most efficiently facilitated sexually-motivated song in male starlings (Riters et al., 2014). Similar results were also reported for male Japanese quail, in which intracerebroventricular injections of D1 receptor agonist near the POM resulted in an inverted-U shaped effect for male sexually motivated behavior (Kleitz-Nelson et al., 2010a). Receptor occupancy is limited by receptor availability, which suggests that receptor gene expression might provide a mechanism for modifying sexually-motivated behavior. Finally, as discussed above, it is also possible that D1 expression was altered as a consequence of singing behavior or an unidentified third variable.
4.2 A potential inhibitory role for D2 receptor expression in the POM in sexually-motivated song
The role of D2 receptors in the performance of sexually-motivated behavior has not been as well studied as the role of D1 receptors, but should be considered since D2 and D1 receptors can have similar or opposing effects on behavior (reviewed in the introduction). The results of our multiple regression analysis demonstrated that whereas D1 receptor expression in POM was positively related to song rate and time spent singing, D2 receptor expression related negatively to song rate with a similar trend observed for time spent singing. Such results suggest that the level of D2 receptors expressed in the POM could have an inhibitory effect on sexually-motivated singing behavior or alternatively that singing behavior inhibits D2 receptor expression in POM. Although no relationships between song production and D2-like receptors were found in a prior study with male starlings (Heimovics et al., 2009), studies in rats suggest that increasing the ratio of D2/D1 receptors in the medial preoptic nucleus can inhibit the performance of some sexual behaviors (Hull et al., 1989). Receptors in the D1 and D2 families (i.e., D1- and D2-like receptors) are G protein -coupled receptors that differ in the second messenger pathways each type initiates. Generally, D1-like receptors activate adenylate cyclase (AC) and stimulate downstream neuronal responses; whereas, D2 receptors inhibit AC and neuronal responses in mammals (reviewed in (Stoof and Kebabian, 1984, Jaber et al., 1996, Vallone et al., 2000) as well as songbirds (Ding and Perkel, 2002). Dopamine receptor subtypes also have different binding affinities for dopamine with D2-like receptors having the highest binding affinity followed by the D1-like receptors (Richfield et al., 1989, Callier et al., 2003). Differential effects may also depend on whether D1 and D2 receptors are located in the same or distinct neurons, which we did not investigate in the present study. Thus the proportion of D1 and D2 receptors, the amount of dopamine released into the synapse, and where receptors are located can all contribute to whether dopamine facilitates or inhibits neuronal activity and behavior. The specific mechanisms by which D1 and D2 receptors in POM modify (or are modified by) singing behavior warrants further investigation.
4.3 D1 and D2 expression in LS and Area X did not relate to sexually-motivated song
No significant relationships were identified between D1 or D2 expression in either LS or Area X. We assume in the present study that songs were predominately sexually-motivated because we recorded song after releasing a female into the aviary, and past studies show mate attraction to be the primary function of male starling song (Eens et al., 1990, 1993). Thus we interpret findings for LS as consistent with past data that also showed no relationships between D1 receptors in LS and sexually-motivated song (Heimovics et al., 2009). In contrast, past data show negative correlations between D1 (but not D2) receptors in LS and presumed agonistically-motivated song produced in the presence of other males (Heimovics et al., 2009). It is possible that had we examined agonistically-motivated song produced in response to introducing a male into the aviaries, a relationship between D1 receptors in LS and song may have been identified. Further work is needed to examine the type of song modulated by dopamine receptor stimulation in the POM versus the LS.
The lack of significant relationships between D1 and D2 receptors in Area X was somewhat unexpected. Area X is rich in dopamine and contains both D1 and D2 receptors and TH (Casto and Ball, 1994, Heimovics and Riters, 2008, Kubikova et al., 2010, Heimovics et al., 2011). In male starlings, dopamine concentrations in Area X correlated positively with the production of sexually-motivated song (Heimovics et al., 2011). In male zebra finches D1 receptor stimulation increases whereas D2 stimulation inhibits neuronal activity in Area X (Ding and Perkel, 2002); and dopamine levels in Area X measured using microdialysis are higher during sexually-motivated female-directed than undirected song (Sasaki et al., 2006). In Area X a higher proportion of neurons that expressed D1 receptors were active (as measured using immediate early gene egr1 expression) during sexually-motivated female-directed song compared to undirected song (Kubikova et al., 2010). Furthermore, pharmacological blockade of dopamine D1 receptors in Area X disrupts context-dependent changes in song variability in male zebra finches singing female-directed compared to undirected song (Leblois and Perkel, 2012). The present lack of relationship between D1 and D2 expression and sexually-motivated song in starlings suggests that expression of these receptors in Area X is not linked linearly to sexually-motivated song in male starlings, but certainly does not preclude a role for these receptors in modifying sensory motor aspects of song production.
4.4 Limitations
qPCR stands out for providing highly quantifiable information, and the present study yields promising implications for the role of dopamine receptor gene expression in the production of sexually-motivated song. As with any technique there are technical limitations to qPCR. First, it is possible that the neural locations of D1/D2 receptor transcription are not the same as the final location of the translated protein. This may be one reason that D1 and D2 receptors in Area X did not relate to singing behavior (i.e., transcription may have occurred outside Area X but the resulting receptor proteins may then be transported within neurons to Area X). It is also possible that the protein resulting from receptors transcribed in POM may be transported to other target regions. Protein measures and site-directed pharmacological manipulations are now needed to identify the sites in which these proteins are expressed and active.
4.5 Future Directions and Conclusions
This study supports recent work that indicates that an optimal level of D1 receptor stimulation in the POM best facilitates sexually-motivated singing behavior. Here we show that D1 and D2 receptor transcription (and presumed translation into available receptors) in the POM, in addition to D1 receptor occupancy, may modulate such behavior. Furthermore, this study indicates that D2 receptors might have an inhibitory effect on sexually-motivated communication, as has been suggested for other sexually-motivated male behavior (Hull et al., 1989, Kleitz-Nelson et al., 2010a). Our data also support the non-mutually exclusive possibility that singing behavior leads to changes in D1 and D2 receptors in the POM. Site-directed pharmacological manipulations of D1 and D2 receptors or gene silencing techniques are now needed to directly test these predictions.
Highlights.
Past data show that dopamine in the medial preoptic nucleus (POM) modulates vocal production in songbirds
Male starlings with intermediate levels of D1 expression in POM sang more than males with low or high levels of expression
Singing correlated positively with POM D1 expression in males with low and intermediate levels of D1 expression
Singing correlated negatively with POM D2 expression in males with low and intermediate levels of D1 expression
An optimal level of POM D1 expression may facilitate sexually-motivated song, and D2 receptors may have inhibitory effects
Acknowledgments
This work was supported by NIMH grant R01 MH080225 to L. V. Riters. The authors gratefully acknowledge Devin Merullo and Jeremy Spool for helpful discussion of manuscript content, Chris Elliott for animal care, and two anonymous reviewers for useful suggestions to improve the quality of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
M. S. DeVries, Email: msdevries@wisc.edu.
M.A. Cordes, Email: macordes@wisc.edu.
S.A. Stevenson, Email: sstevenson@wisc.edu.
L.V. Riters, Email: LVRiters@wisc.edu.
References
- Alger SJ, Maasch SN, Riters LV. Lesions to the medial preoptic nucleus affect immediate early gene immunolabeling in brain regions involved in song control and social behavior in male European starlings. The European journal of neuroscience. 2009;29:970–982. doi: 10.1111/j.1460-9568.2009.06637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alger SJ, Riters LV. Lesions to the medial preoptic nucleus differentially affect singing and nest box-directed behaviors within and outside of the breeding season in European starlings (Sturnus vulgaris) Behav Neurosci. 2006;120:1326–1336. doi: 10.1037/0735-7044.120.6.1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balthazart J, Ball GF. Topography in the preoptic region: differential regulation of appetitive and consummatory male sexual behaviors. Frontiers in neuroendocrinology. 2007;28:161–178. doi: 10.1016/j.yfrne.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balthazart J, Castagna C, Ball GF. Differential effects of D1 and D2 dopamine-receptor agonists and antagonists on appetitive and consummatory aspects of male sexual behavior in Japanese quail. Physiology & behavior. 1997;62:571–580. doi: 10.1016/s0031-9384(97)00163-7. [DOI] [PubMed] [Google Scholar]
- Bentley GE, Perfito N, Calisi RM. Season- and context-dependent sex differences in melatonin receptor activity in a forebrain song control nucleus. Horm Behav. 2013;63:829–835. doi: 10.1016/j.yhbeh.2012.11.015. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Arnsten AF. Psychostimulants and motivated behavior: arousal and cognition. Neuroscience and biobehavioral reviews. 2013;37:1976–1984. doi: 10.1016/j.neubiorev.2012.11.005. [DOI] [PubMed] [Google Scholar]
- Bradbury JW, Vehrencamp SL. Principles of animal communication. Sunderland, Mass: Sinauer Associates; 2011. [Google Scholar]
- Callier S, Snapyan M, Le Crom S, Prou D, Vincent JD, Vernier P. Evolution and cell biology of dopamine receptors in vertebrates. Biol Cell. 2003;95:489–502. doi: 10.1016/s0248-4900(03)00089-3. [DOI] [PubMed] [Google Scholar]
- Casto JM, Ball GF. Characterization and localization of D1 dopamine receptors in the sexually dimorphic vocal control nucleus, area X, and the basal ganglia of European starlings. J Neurobiol. 1994;25:767–780. doi: 10.1002/neu.480250703. [DOI] [PubMed] [Google Scholar]
- Cordes MA, Stevenson SA, Driessen TM, Eisinger BE, Riters LV. Sexually-motivated song is predicted by androgen-and opioid-related gene expression in the medial preoptic nucleus of male European starlings (Sturnus vulgaris) Behav Brain Res. 2014a;278C:12–20. doi: 10.1016/j.bbr.2014.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordes MA, Stevenson SA, Riters LV. Status-appropriate singing behavior, testosterone and androgen receptor immunolabeling in male European starlings (Sturnus vulgaris) Horm Behav. 2014b;65:329–339. doi: 10.1016/j.yhbeh.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews D. Evolution of neuroendocrine mechanisms that regulate sexual behavior. Trends in endocrinology and metabolism: TEM. 2005;16:354–361. doi: 10.1016/j.tem.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Dawson A, King VM, Bentley GE, Ball GF. Photoperiodic control of seasonality in birds. Journal of Biological Rhythms. 2001;16:365–380. doi: 10.1177/074873001129002079. [DOI] [PubMed] [Google Scholar]
- Ding L, Perkel DJ. Dopamine modulates excitability of spiny neurons in the avian basal ganglia. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:5210–5218. doi: 10.1523/JNEUROSCI.22-12-05210.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eens M, Pinxten R, Verheyen RF. On the function of singing and wing-waving in the European Starling Sturnus vulgaris. Bird Study. 1990;37:48–52. [Google Scholar]
- Eens M, Pinxten R, Verheyen RF. Function of the Song and Song Repertoire in the European Starling (Sturnus Vulgaris): an Aviary Experiment. Behaviour. 1993;125:51–66. [Google Scholar]
- Gwinner H, Van’t Hof T, Zeman M. Hormonal and behavioral responses of starlings during a confrontation with males or females at nest boxes during the reproductive season. Horm Behav. 2002;42:21–31. doi: 10.1006/hbeh.2002.1795. [DOI] [PubMed] [Google Scholar]
- Heimovics SA, Cornil CA, Ball GF, Riters LV. D1-like dopamine receptor density in nuclei involved in social behavior correlates with song in a context-dependent fashion in male European starlings. Neuroscience. 2009;159:962–973. doi: 10.1016/j.neuroscience.2009.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimovics SA, Riters LV. Evidence that dopamine within motivation and song control brain regions regulates birdsong context-dependently. Physiology & behavior. 2008;95:258–266. doi: 10.1016/j.physbeh.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimovics SA, Salvante KG, Sockman KW, Riters LV. Individual differences in the motivation to communicate relate to levels of midbrain and striatal catecholamine markers in male European starlings. Horm Behav. 2011;60:529–539. doi: 10.1016/j.yhbeh.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull EM, Dominguez JM. Getting his act together: roles of glutamate, nitric oxide, and dopamine in the medial preoptic area. Brain Res. 2006;1126:66–75. doi: 10.1016/j.brainres.2006.08.031. [DOI] [PubMed] [Google Scholar]
- Hull EM, Warner RK, Bazzett TJ, Eaton RC, Thompson JT, Scaletta LL. D2/D1 ratio in the medial preoptic area affects copulation of male rats. The Journal of pharmacology and experimental therapeutics. 1989;251:422–427. [PubMed] [Google Scholar]
- Jaber M, Robinson SW, Missale C, Caron MG. Dopamine receptors and brain function. Neuropharmacology. 1996;35:1503–1519. doi: 10.1016/s0028-3908(96)00100-1. [DOI] [PubMed] [Google Scholar]
- Kelm CA, Forbes-Lorman RM, Auger CJ, Riters LV. Mu-opioid receptor densities are depleted in regions implicated in agonistic and sexual behavior in male European starlings (Sturnus vulgaris) defending nest sites and courting females. Behav Brain Res. 2011;219:15–22. doi: 10.1016/j.bbr.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleitz-Nelson HK, Cornil CA, Balthazart J, Ball GF. Differential effects of central injections of D1 and D2 receptor agonists and antagonists on male sexual behavior in Japanese quail. The European journal of neuroscience. 2010a;32:118–129. doi: 10.1111/j.1460-9568.2010.07257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleitz-Nelson HK, Dominguez JM, Ball GF. Dopamine release in the medial preoptic area is related to hormonal action and sexual motivation. Behav Neurosci. 2010b;124:773–779. doi: 10.1037/a0021490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleitz HK, Cornil CA, Balthazart J, Ball GF. Species differences in the relative densities of D1- and D2-like dopamine receptor subtypes in the Japanese quail and rats: an in vitro quantitative receptor autoradiography study. Brain, behavior and evolution. 2009;73:81–90. doi: 10.1159/000209864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubikova L, Wada K, Jarvis ED. Dopamine receptors in a songbird brain. The Journal of comparative neurology. 2010;518:741–769. doi: 10.1002/cne.22255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblois A, Perkel DJ. Striatal dopamine modulates song spectral but not temporal features through D1 receptors. The European journal of neuroscience. 2012;35:1771–1781. doi: 10.1111/j.1460-9568.2012.08095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowski VP, Eaton RC, Lumley LA, Moses J, Hull EM. A D1 agonist in the MPOA facilitates copulation in male rats. Pharmacology, biochemistry, and behavior. 1994;47:483–486. doi: 10.1016/0091-3057(94)90147-3. [DOI] [PubMed] [Google Scholar]
- Paredes RG, Agmo A. Has dopamine a physiological role in the control of sexual behavior? A critical review of the evidence. Progress in neurobiology. 2004;73:179–226. doi: 10.1016/j.pneurobio.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT PCR. Nucleic acids research. 2001;29:e45–e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richfield EK, Penney JB, Young AB. Anatomical and affinity state comparisons between dopamine D1 and D2 receptors in the rat central nervous system. Neuroscience. 1989;30:767–777. doi: 10.1016/0306-4522(89)90168-1. [DOI] [PubMed] [Google Scholar]
- Riters LV. Pleasure seeking and birdsong. Neuroscience and biobehavioral reviews. 2011;35:1837–1845. doi: 10.1016/j.neubiorev.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riters LV. The role of motivation and reward neural systems in vocal communication in songbirds. Frontiers in neuroendocrinology. 2012;33:194–209. doi: 10.1016/j.yfrne.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riters LV, Ball GF. Lesions to the medial preoptic area affect singing in the male European starling (Sturnus vulgaris) Horm Behav. 1999;36:276–286. doi: 10.1006/hbeh.1999.1549. [DOI] [PubMed] [Google Scholar]
- Riters LV, Eens M, Pinxten R, Duffy DL, Balthazart J, Ball GF. Seasonal Changes in Courtship Song and the Medial Preoptic Area in Male European Starlings (Sturnus vulgaris) Hormones and Behavior. 2000;38:250–261. doi: 10.1006/hbeh.2000.1623. [DOI] [PubMed] [Google Scholar]
- Riters LV, Pawlisch BA, Kelm-Nelson CA, Stevenson SA. Inverted-U shaped effects of D1 dopamine receptor stimulation in the medial preoptic nucleus on sexually motivated song in male European starlings. The European journal of neuroscience. 2014;39:650–662. doi: 10.1111/ejn.12429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riters LV, Schroeder MB, Auger CJ, Eens M, Pinxten R, Ball GF. Evidence for Opioid Involvement in the Regulation of Song Production in Male European Starlings (Sturnus vulgaris) Behavioral neuroscience. 2005;119:245. doi: 10.1037/0735-7044.119.1.245. [DOI] [PubMed] [Google Scholar]
- Sasaki A, Sotnikova TD, Gainetdinov RR, Jarvis ED. Social context-dependent singing-regulated dopamine. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:9010–9014. doi: 10.1523/JNEUROSCI.1335-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder MB, Riters LV. Pharmacological manipulations of dopamine and opioids have differential effects on sexually motivated song in male European starlings. Physiology & behavior. 2006;88:575–584. doi: 10.1016/j.physbeh.2006.05.011. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Yang CR. The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Progress in neurobiology. 2004;74:1–58. doi: 10.1016/j.pneurobio.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Stevenson TJ, Bernard DJ, Ball GF. Photoperiodic condition is associated with region-specific expression of GNRH1 mRNA in the preoptic area of the male starling (Sturnus vulgaris) Biol Reprod. 2009;81:674–680. doi: 10.1095/biolreprod.109.076794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolzenberg DS, Numan M. Hypothalamic interaction with the mesolimbic DA system in the control of the maternal and sexual behaviors in rats. Neuroscience and biobehavioral reviews. 2011;35:826–847. doi: 10.1016/j.neubiorev.2010.10.003. [DOI] [PubMed] [Google Scholar]
- Stoof JC, Kebabian JW. Two dopamine receptors: biochemistry, physiology and pharmacology. Life Sci. 1984;35:2281–2296. doi: 10.1016/0024-3205(84)90519-8. [DOI] [PubMed] [Google Scholar]
- Vallone D, Picetti R, Borrelli E. Structure and function of dopamine receptors. Neuroscience and biobehavioral reviews. 2000;24:125–132. doi: 10.1016/s0149-7634(99)00063-9. [DOI] [PubMed] [Google Scholar]
- Williams GV, Castner SA. Under the curve: critical issues for elucidating D1 receptor function in working memory. Neuroscience. 2006;139:263–276. doi: 10.1016/j.neuroscience.2005.09.028. [DOI] [PubMed] [Google Scholar]
- Zeigler HP, Marler P. Neuroscience of Birdsong. Cambridge University Press; 2008. [Google Scholar]