Abstract
Cancer stem cells are thought to be responsible for rapid tumor growth, metastasis and enhanced tumor survival following drug treatment. For this reason, there is a major emphasis on identifying proteins that can be targeted to kill cancer stem cells or control their growth and transglutaminase type II (TGM2/TG2) is such a target in epidermal squamous cell carcinoma. TG2 was originally described as a transamidase in the extracellular matrix (ECM) that crosslinks proteins by catalyzing ε-(γ-glutamyl)lysine bonds. However, subsequent studies have shown that TG2 is a GTP binding protein that plays an important role in cell signaling and survival. In the present study, TG2 shows promise as a target for anti-cancer stem cell therapy in human squamous cell carcinoma. TG2 was determined to be highly elevated in epidermal cancer stem cells (ECS cells) and TG2 knockdown or suppression of TG2 function with inhibitors reduced ECS cell survival, spheroid formation, matrigel invasion and migration. The reduction in survival is associated with activation of apoptosis. Mechanistic studies, using TG2 mutants revealed that the GTP-binding activity is required for maintenance of ECS cell growth and survival, and that the action of TG2 in ECS cells is not mediated by NFκB signaling.
Implications
This study suggests that TG2 has an important role in maintaining cancer stem cell survival, invasive and metastatic behavior, and is an important therapeutic target to reduce survival of cancer stem cells in epidermal squamous cell carcinoma.
Keywords: squamous cell carcinoma, type II transglutaminase, GTP-binding, G-protein, stem cell, cancer, TG2
Introduction
Epidermal squamous cell carcinoma (SCC) is a common form of skin cancer that develops in response to UV light exposure (1). SCC can often be treated by surgical excision, but the recurrence rate ranges to thirty percent (1). The worldwide incidence of SCC is increasing because of population aging and because of increased exposure to ultraviolet light (2).
Increasing evidence suggests the existence of cancer stem cells that have a role in tumor formation, and facilitate cancer recurrence and metastasis in epithelial-derived cancers (3–8). We recently characterized epidermal cancer stem cells (ECS cells) and showed that ECS cells express stem cell markers characteristic of normal epidermal stem cells and embryonic stem cells (9). These ECS cells are able to generate tumors in immune-compromised mice following subcutaneous injection of as few as 100 cells (9). ECS cells express pluripotent markers, which are also expressed in esophageal and head/neck cancer stem cells (6, 10, 11).
As part of a search for stem cell survival proteins, we identified transglutaminase type 2 (TG2) as highly elevated in ECS cells as compared to non-stem cancer cells. TG2 is a multifunctional protein, with both enzymatic and scaffold functions, that is involved in inflammation, tissue repair and cancer (12, 13). TG2 catalyzes a number of reactions including calcium dependent protein crosslinking (TGase activity), GTP binding activity, protein disulfide isomerase activity, serine/threonine kinase activity (14) and also serves as a scaffold protein (15). Among these activities, the best characterized and most important are the transamidase (TGase) and the GTP binding activities.
TG2 is expressed in the basal epidermal layers where, we propose, it has a survival role. We now show that TG2 is markedly elevated in epidermal squamous cell carcinoma and is selectively and highly enriched in ECS cells, suggesting it may have a role in ECS cell survival. Indeed, knockdown and inhibitor studies show that TG2 is required for ECS cell survival, spheroid formation, migration and invasion. Moreover, inhibition of TG2 activates ECS cell apoptosis. Studies with TG2 mutants indicate that GTP binding/G-protein related activity is required for ECS cell survival, but that TG2 transamidase activity is not. Thus, our results suggest that agents that modulate TG2 signalling may be useful cancer prevention and treatment agents.
Materials and Methods
Antibodies and reagents
Dulbecco’s modified Eagle’s medium (11960-077), sodium pyruvate (11360-070), L-Glutamine (25030-164) and 0.25% trypsin-EDTA (25200-056) were purchased from Gibco (Grand Island, NY). Heat-inactivated fetal calf serum (FCS, F4135), anti-β-actin (A5441) and A23187 ionophore (C7522), trypan blue (T8154) were purchased from Sigma (St. Louis, MO). Cell lysis Buffer (9803) was purchased from Cell Signaling Technology (Danvers, MA). Anti-TG2 (MAB3839) was purchased from EMD Milipore (Bedford, MA). Antibody for Sox2 (ab15830-100) was purchased from Abcam. Antibodies for caspase-3 (9665) and Nanog (4839), and NFκB-p65 siRNA (6261) were purchased from Cell Signaling Technologies. Anti-Oct4 (611203) was purchased from BD Transduction Laboratories (San Jose, CA). Peroxidase-conjugated anti-mouse IgG (NXA931) and anti-rabbit IgG (NA934V) were obtained from GE Healthcare (Buckinghamshire, UK). Production of NC9 was described previously (16). TG2- (sc-37514) and control-siRNA (sc-37007) were purchased from Santa Cruz (Dallas, TX). Anti-TG1 (SC-166467) and anti-NFκB-p65 (sc-109) were purchased from Santa Cruz (Dallas, TX). Anti-FXIIIa (ab79759) was purchased from Abcam. Fluorescein cadaverine (FC) was purchased from Life Technologies. BD Biocoat cell inserts (353097) and Matrigel (354234) were purchased from BD Biosciences. Proteins were detected by immunoblot (17, 18).
Plasmids
Plasmids encoding wild-type TG2 and TG2(C277S) cloned in EC1214 vector were provided by Dr. Kapil Mehta. Plasmids encoding TG2(R580A), TG2(Y526F0, TG2(W241A), cloned in pcDNA3.1 were provided by Dr. Gail Johnson (14, 19, 20).
Lentivirus production
Lentiviruses were packaged using 293T cells which were maintained in DMEM containing 1 mM sodium pyruvate, 1 mM L-glutamine and 10% fetal calf serum (FCS). The cells were harvested and plated in 100 mm dishes at 60% confluence 24 h prior to transfection. The serum-containing medium was removed and the cultures washed with Hank’s Balanced Salt Solution prior to co-transfection with 1 μg pCMV-VSVG, 0.5 μg pCMV-dr8.91 and 0.5 μg shRNA encoding plasmid in serum-free medium. pCMV-VSVG (8454) and pCMV-dr8.91 were purchased from Addgene and kindly provided by Dr. CY Lin. After 3 h the medium was supplemented with 10% FCS and after an additional 72 h the medium was collected, centrifuged at 1500 rpm for 15 min, forced through a 22 μm filter, aliquoted at 1 ml/tube and stored frozen at −80 C. The lentivirus plasmids, pLKO.1-Puro-NT-shRNA (Control) and pLKO.1-Puro-hTGM2-shRNA (TRCN-0000272760), were purchased from Sigma-Aldrich (St. Louis, MO).
Production of TG2 knockdown stable cell lines
SCC-13 cells (1 × 105) were allowed to attach overnight in 24 well cluster plates and then infected with TG2-shRNA encoding lentivirus in serum-free growth media for 5 h at 37 C. The serum-free growth media contained 8 μg/ml polybrene. The medium was then replaced with 5% fetal calf serum supplemented growth media and near-confluent cells were harvested, plated at low density in 100 mm dishes and selected for two weeks in the presence of 0.25 μg/ml puromycin. These cells were then infected a second time with the same virus and reselected. The resulting cells are a non-clonal population of cells we call SCC13-TG2-shRNA2. A control population of cells (SCC13-Control-shRNA) was derived by double infection with control-shRNA (scrambled) encoding lentivirus using an identical protocol.
Spheroid formation assay
Spheroid formation assays were exactly as outlined in our previous report (9), except that the spheroids were grown in six well ultra-low attachment Costar cluster dishes (4371, Corning, Tewksbury, MA).
Electroporation of nucleic acids
Cells were electroporated exactly as outlined (21). In applications using siRNA, the cells were harvested 72 h post-electroporation and electroporated a second time following the same protocol (double-electroporation). This resulted in sustained knockdown of the target transcript.
In situ TG2 activity assay
Cells (40,000) were plated in 24 well attachment dishes in spheroid media and grown until 50% confluent. Fluorescein cadaverine (FC) was added in 2 ml of serum-free medium at a final concentration of 20 μM and incubated for 4 h. The wells were washed twice with serum-free spheroid medium, and then 2 ml of fresh serum-free medium was added containing 0 – 20 μM NC9. After 30 min, the wells were supplemented with 10 μM A23187. Cells were incubated for an additional 90 min and then washed three times with Ca++/Mg++-free HBSS, fixed with formalin, washed with phosphate-buffered saline and imaged to detect fluorescein.
Trypan Blue viability assay
SCC-13 cells were grown as spheroids in six well cluster dishes in spheroid medium for 8 d and then treated with 0 – 20 μM NC9. At 0, 24, 48, and 72 h post-NC9 treatment, spheroids were counted, and all cells in the well were collected to prepare a single cell suspension in Hank’s Balanced Salt Solution. Trypan Blue solution (0.5 ml, 0.4%) was added to a 15 ml conical tube with 0.3 ml of Hank’s Balanced Salt Solution and 0.2 ml of cell suspension. After 10 min, 8 μl of the mixture was transferred to a hemocytometer to count viable and total cell number.
Invasion assay
Matrigel (BD Biolabs) was diluted into 2 ml of 0.01 M Tris-HCl/0.7% NaCl to a final concentration of 300 μg/ml, filter sterilized and 0.1 ml was added per BD BioCoat cell insert. After 2 h, near-confluent SCC13-TG2-shRNA2 and SCC13-Control-shRNA cells were harvested and 25,000 cells were plated in 100 μl of growth media containing 1% FCS atop the Matrigel layer. Growth medium containing 10% FCS was added to the bottom chamber followed by an overnight incubation at 37 C. The following day a cotton swab was used to remove cells from the upper side of the membrane, the membrane was rinsed with phosphate-buffered saline, fixed with 4% paraformaldehyde for 10 min, washed again, and stained with 1 μg/ml DAPI for 10 min. The underside of the membrane was viewed with an inverted fluorescent microscope and nuclei were counted.
Results
TG2 is required for ECS cell survival and spheroid formation
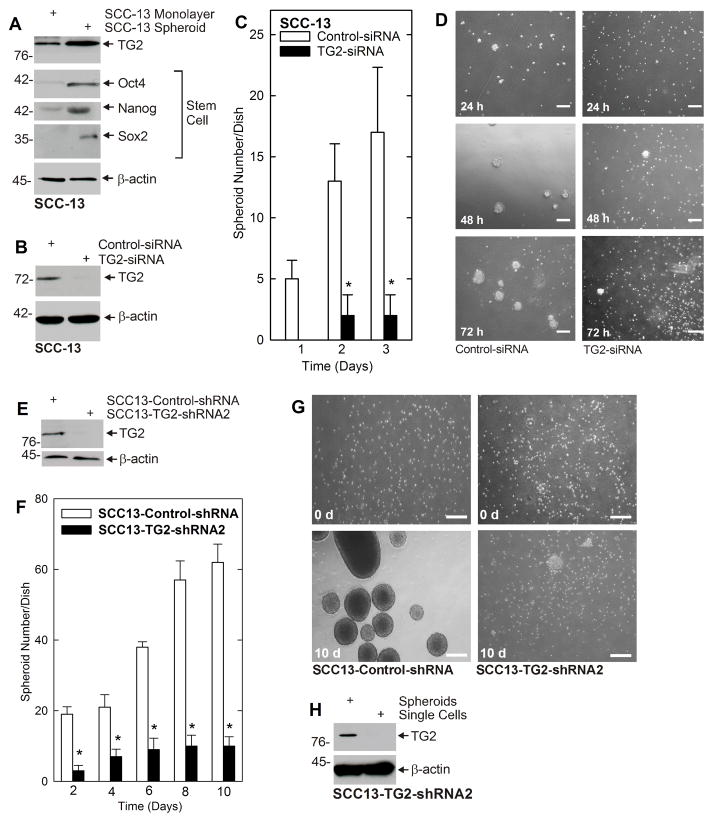
Our previous studies show that ECS cells, which comprise less than 0.2% of the total cancer cell population, can be isolated from bulk SCC-13 cancer cells by growth as spheroids in non-attached conditions (9). An important finding is that ECS cells (spheroids) are highly enriched for expression of TG2 as compared to non-stem cell (monolayer) cultures (Fig. 1A) and that this expression is associated with expression of stem cell markers, including Oct4, Nanog and Sox2 (Fig. 1A). To determine whether TG2 has a role in ECS cell maintenance and spheroid formation, we treated SCC-13 cells with control- or TG2-siRNA to knockdown TG2 (Fig. 1B) and monitored ability to form spheroids. Markedly fewer spheroids are formed by TG2 knockdown cells (Fig. 1C). Moreover, loss of spheroid formation, following TG2 knockdown, is associated with accumulation of single cells (Fig. 1D). To confirm this finding, we prepared TG2-negative cell lines by infection of SCC-13 cells with TG2-shRNA encoding lentivirus. Fig. 1E confirms the SCC13-TG2-shRNA2 cells, which have reduced TG2 expression (Fig. 1F), form spheroids less efficiently than SCC13-control-shRNA cells. Most SCC13-TG2-shRNA2 cells remain as single cells (Fig. 1G). However, some spheroid formation is observed in SCC13-TG2-shRNA2 cultures and we wondered whether this is due to TG2 re-expression. To test this, we grew SCC13-TG2-shRNA2 cells as non-attached spheroids for 10 d and then isolated spheroids and single cells for preparation of extracts. Fig. 1H reveals that the few spheroids that do form express TG2, further suggesting that TG2 is required for spheroid formation.
Fig. 1. TG2 is enriched in ECS cells and is required for spheroid formation.
A ECS cells are enriched for expression of TG2. SCC-13 cells (40,000 per well) were grown as monolayers (non-stem cells) or as unattached spheroids (ECS cells) in spheroid medium for 10 d before extracts were prepared. B ECS cells were electroporated with control- or TG2-shRNA and after 72 h extracts were prepared to assay TG2 level. C TG2 is required for spheroid formation. SCC-13 cells were electroporated with 3 μg of control- or TG2-siRNA and then plated at 40,000 cells per well in 35 mm dishes and grown as spheroids and then counted. D Cells were photographed at 24, 48 and 72 h after plating. E SCC13-Control-shRNA and SCC13-TG2-shRNA2 cells were harvested and extracts were prepared for immunoblot detection of TG2 and β-actin. F TG2 is required for spheroid growth. Cells were plated at 40,000 per 35 mm dish in spheroid medium and spheroid formation was monitored. G Cells from panel B were photographed at 0 and 10 d after plating. H SCC13-TG2-shRNA2 cell spheroids and non-spheroids (single cells) were collected at 10 d and assayed for TG2 level. Spheroid formation is associated with restoration of TG2 expression. In all panels, the values are mean ± SEM, n = 3, p < 0.05 and the bars = 125 μm.
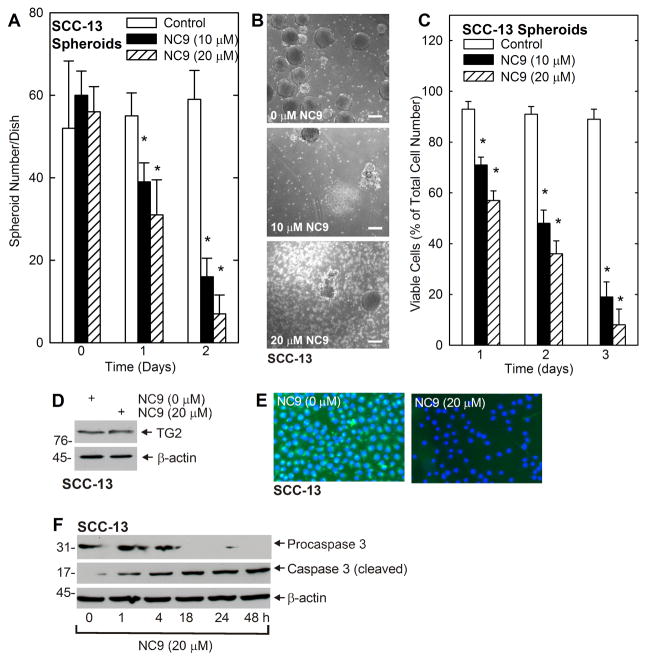
We next studied the impact of TG2 inhibitor on spheroid formation. Spheroids were grown for 8 d and then treated with NC9, an irreversible inhibitor of TG2 transamidase (TGase, crosslinking) activity (16). NC9 treatment reduced spheroid number (Fig. 2A) which is associated with accumulation of spheroid fragments and single cells (Fig. 2B). A key issue is whether the NC9 treatment reduces cell viability. As shown in Fig. 2C, as assessed by ability to exclude trypan blue, NC9 causes a concentration-dependent reduction in viable cell number. Fig 2D shows that the reduction in TG2 activity is not associated with reduced TG2 level. This indicates that TG2 knockdown (Fig. 1) or inhibition of activity (Fig. 2) reduces ECS cell survival. To confirm that NC9 inhibits TG2 transamidase (TGase) activity, we loaded cells with fluorescein cadaverine (FC), a known transglutaminase substrate, added 0 or 20 μM NC9, activated TG2 TGase activity by treatment with calcium ionophore and monitored for intracellular incorporation of the fluorescent label. This experiment shows that TG2 activity is reduced by NC9 treatment (Fig. 2E). To gain some insight regarding the mechanism, we monitored for cleavage of procaspase 3. Fig. 2F shows that the response to NC9 includes cleavage of procaspase 3 and that this cleavage is initiated within 1 h after NC9 addition.
Fig. 2. NC9 causes fragmentation of pre-formed spheroids.
A SCC-13 cells (40,000) were plated in non-adherent six well dished, grown for 8 d in spheroid medium, and then NC9 was added and spheroid number was monitored at 0 – 48 h. B Spheroids were grown for 10 d, treated for 48 h with indicated level of NC9 and photographed. C SCC-13 cells were plated at 40,000 cells per well in non-adherent six well dishes and after 10 d the spheroids were treated with 0 – 20 μM NC9 for 0 – 72 h before trypan blue exclusion viability assay. D SCC-13 spheroids were grown for 10 d, treated with 0 or 20 μM NC9 for 48 h, and TG2 level was assayed by immunoblot. E SCC-13 cells were grown as monolayers in spheroid medium and TG2 activity was monitored by FC incorporation assay as outlined in Materials and Methods. F SCC-13 cell 8 d spheroids were treated with 20 μM NC9 and cells were harvested at the indicated times for immunoblot detection of procaspase 3 and cleaved caspase 3. In all panels, the values are mean ± SEM, n = 3, p < 0.05.
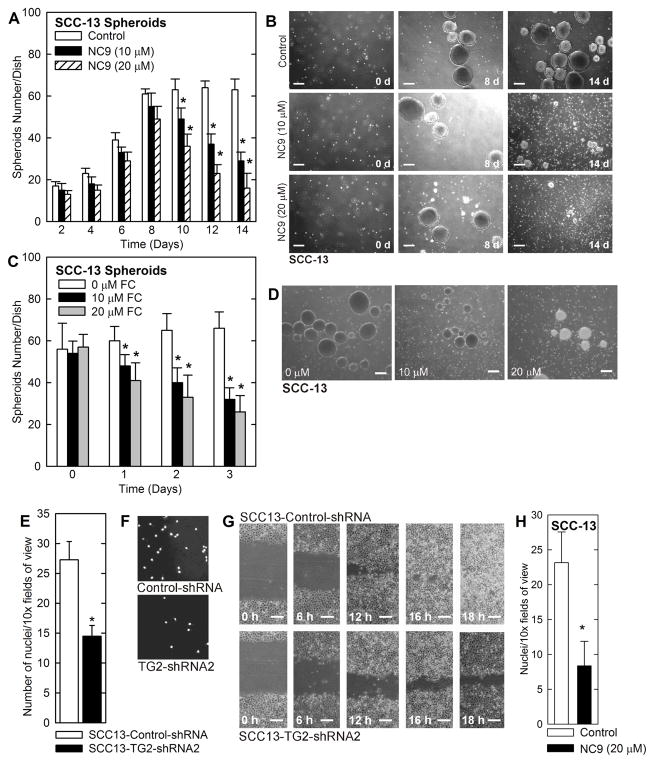
We next assessed whether NC9 can prevent spheroid formation initiated from single cells. We seeded SCC-13 single cells in spheroid growth conditions and after 12 h added NC9, and monitored spheroid number over fourteen days. We did not replenish the medium or add fresh NC9 during this time course. Spheroid number increases in all groups until day eight, but then selectively declines in the NC9 treated groups (Fig. 3A). This is associated with morphological survival of the spheroids until 8 d and progressive destruction from 8 to 14 d (Fig. 3B) indicating that spheroids can form in the presence of NC9, but that fully formed spheroids undergo NC9-associated destruction. This is consistent with NC9-related destruction of mature spheroids shown in Fig. 2. In addition, the findings shown in Fig. 3 suggest that a single treatment with NC9 produces long-lasting effects on ECS cell survival and spheroid formation. We next monitored the impact of another transglutaminase inhibitor, fluorescein cadaverine (FC), on spheroid formation. FC acts as a competitive substrate inhibitor of transglutaminase that prevents TG2 interaction with intracellular targets (22, 23). Spheroids were grown for 8 d and then treated with FC. FC treatment reduces spheroid number and promotes accumulation of spheroid fragments and dissociated single cells (Fig. 3C and D).
Fig. 3. Impact of NC9 and TG2 knockdown on spheroid formation, invasion and wound closure.
A SCC-13 cells (40,000) were plated in spheroid growth conditions and after 18 h, 0, 10 or 20 μM NC9 was added. Incubation was continued for 0 – 14 d without addition of fresh NC9 or medium, and spheroid number was counted at each time point. B Cells were treated as in panel A and images were captured at 0, 8 and 14 d. Similar results were observed in each of three experiments. C SCC-13 cells were seeded in six well non-attachment plates and after 8 d spheroids were treated with 0 - 20 μM FC for 72 h. D Images of spheroids following 2 d treatment with various concentrations of FC. In all panels, the values are mean ± SEM, n = 3, p < 0.05. E SCC13-Control-shRNA and SCC13-TG2-shRNA2 cells (25,000) were seeded on top of matrigel in 1 ml of growth medium in a Millicell chamber. After 24 h, the membrane was rinsed with phosphate buffered saline and fixed in 4% paraformaldehyde and then stained with DAPI. The underside of chambers were viewed with an inverted fluorescent microscope and nuclei counted. F Images of the DAPI-stained membrane. G SCC13-Control-shRNA and SCC13-TG2-shRNA2 cells (2 million) were plated on 100 mm dishes in growth medium in monolayer conditions and confluent monolayers were “wounded” with a 10 μl pipette. Images were collected at 0 – 18 h after wounding to assess closure. Cell proliferation does not account for the difference in wound closure rate (not shown). H SCC-13 cells were pretreated for 1 h with 0 or 20 μM NC9 and then 25,000 cells were seeded on Matrigel in six Millicell chambers per treatment. After 24 h, the chambers were harvested, cleaned and cells that had migrated through to the membrane inner surface were visualized using DAPI. The values are mean ± SEM, n = 6, p < 0.05.
TG2 is required for ECS cell migration
We recently demonstrated that epidermal cancer-derived ECS cells migrate more efficiently than non-stem cancer cells (9). To determine whether this requires TG2, we monitored the ability of TG2 knockdown cells to invade Matrigel and close a scratch wound. Fig. 3E/F shows that TG2 loss reduces cell migration through Matrigel. Fig. 3G shows that ability of cells to close a scratch wound is also reduced in the absence of TG2 (Fig. 3G). Moreover, Fig. 3H shows that pretreating the cells with NC9 reduces matrigel invasion.
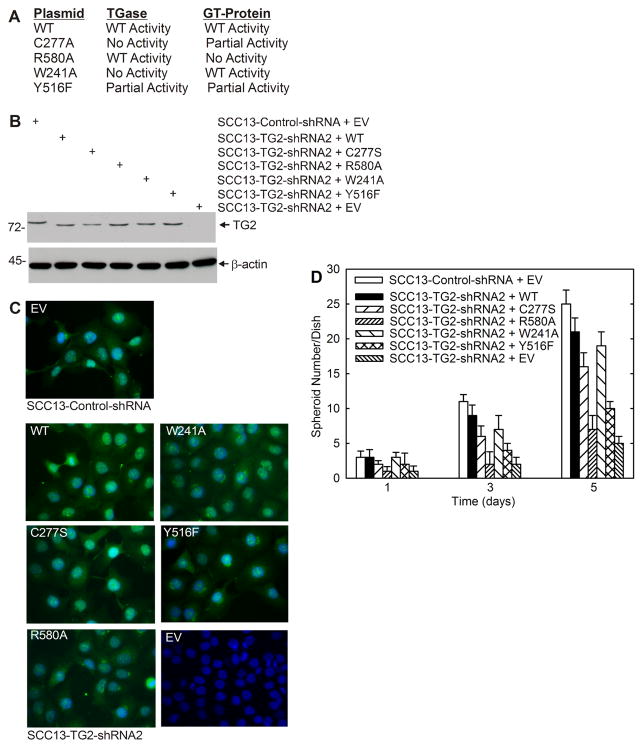
TG2 GTP binding function is required for activity
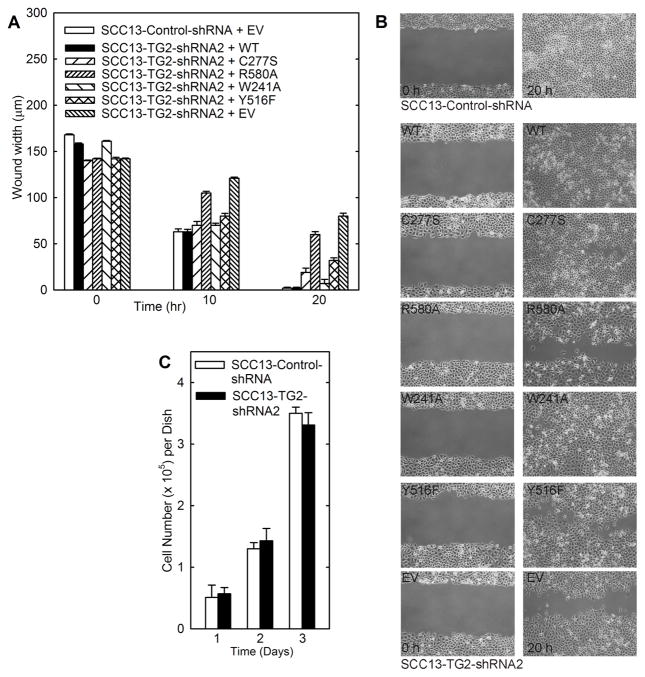
The two most important enzymatic functions of TG2 are the TGase and GTP binding activities (14). We therefore examined which activity is required for ECS cell spheroid formation. To accomplish this, we measured the ability of wild-type and mutant TG2 to restore SCC13-TG2-shRNA2 cell spheroid formation. Plasmids encoding wild-type and mutant TG2 forms were delivered by electroporation. The mutants include C277S (no TGase activity, partial GTP binding activity), R580A (wild-type TGase activity, no GTP binding activity), W241A (no TGase activity, wild-type GTP binding activity) and Y516F (partial TGase activity, partial GTP binding activity) (Fig. 4A) (19). Fig. 4B shows that each electroporated mutant is expressed at a comparable level. We also show (Fig. 4C) that each mutant localizes in a pattern which resembles that of wild-type endogenous TG2 (SCC13-Control-shRNA, EV) and that SCC13-TG2-shRNA2 cells express reduced levels of TG2.
Fig. 4. Expression of TG2 mutants in SCC13-TG2-shRNA2 cells.
A TG2 mutants and impact on TGase and GTP-binding/G-protein-related activity. B SCC13-TG2-shRNA2 cells (TG2 knockdown) were electroporated with plasmids encoding wild-type or mutant TG2, or empty vector (EV) and grown as monolayers in spheroid growth medium. After three days, the cells were harvested for immunoblot with anti-TG2. C SCC13-TG2-shRNA2 cells were electroporated with plasmids encoding the indicated plasmids, plated on attachment plates in spheroid medium and after 3 d the cells were fixed, permeabilized and stained with anti-TG2. As a control, SCC13-Control-shRNA cells were electroporated with empty vector and stained in parallel. TG2 detected in these cells is endogenous. Similar findings were observed in each of three repeated experiments. D SCC13-Control-shRNA cells were electroporated with 3 μg of empty vector (EV) and SCC13-TG2-shRNA2 cells were electroporated with plasmid encoding TG2-wt, TG2 mutants (C277S, R580A, Y526F or W241A) or empty vector. After electroporation, the cells were seeded at 40,000 cells in each of six low-attachment wells in 2.5 ml of spheroid media. Spheroids were counted on days 1, 3 and 5.
We next assessed the ability of wild-type and mutant TG2 to drive spheroid formation. The time course in Fig. 4D shows that SCC13-TG2-shRNA2 cells (TG2 knockdown) form 80% fewer spheroids than empty vector (EV)-electroporated SCC13-Control-shRNA cells when measured at 5 day. We next examined the ability of wild-type TG2 and TG2 mutants to restore SCC13-TG2-shRNA2 cell spheroid formation (Fig. 4D). The general trend is the same at all times. Considering the 5 d time point, expression of wild-type TG2 restores spheroid number to approximately 80% of control. Mutants C277S and W241A, which retain partial and full GTP binding function, respectively, also largely restore spheroid formation. In contrast, Y516F, which retains partial GTP binding activity, slightly restores spheroid formation, and R580A, which lacks GTP binding function, does not restore spheroid formation. The fact that C277S and W241A, which lack TGase activity, restore spheroid formation, suggests that TGase activity is not required.
We also examined the impact of wild-type TG2 and mutants on cell migration and wound closure. SCC13-TG2-shRNA2 cells (TG2 knockdown) have reduced efficiency of wound closure compared to SCC13-Control-shRNA cells which express normal endogenous levels of TG2 (Fig. 5A). Mutants C277S and W241A, which retain partial and full GTP binding function, respectively, largely restore wound closure. Y516F, which retains partial GTP binding activity, is also effective, but R580A, which lacks GTP binding function, is largely ineffective (Fig. 5A). The fact that C277S and W241A, which lack TGase activity, enhance closure, suggests that TGase activity is not required. Fig. 5B shows the wound images at the 20 h time point for a representative experiment. Fig. 5C shows that these cell lines show no difference in cell number over three days of growth, suggesting that differences in cell proliferation rate cannot explain the marked difference in wound closure and invasions rates.
Fig. 5. TG2 mutant impact on migration and proliferation in SCC13-TG2-shRNA2 cells.
A SCC13-Control-shRNA cells were electroporated with 3 μg of empty vector (EV) and SCC13-TG2-shRNA2 cells were electroporated with plasmid encoding TG2-wt, TG2 mutants (C277S, R580A, Y526F or W241A) or empty vector. After electroporation, the cells were seeded at high density in 6-well cluster conventional attachment plates in 2.5 ml of spheroid media. Uniform wounds were created using a pipette and wound width was monitored at 0, 10 and 20 h. An immunoblot confirming mutant expression is shown in Fig. 5B. B Wound images at 20 h. C TG2 knockdown does not alter SCC-13 cell proliferation. SCC13-Control-shRNA and SCC13-TG2-shRNA cells were plated at equal density in attachment plates in spheroid medium and growth was monitored over a period of three days. Values are mean ± SEM, n = 3, p < 0.05.
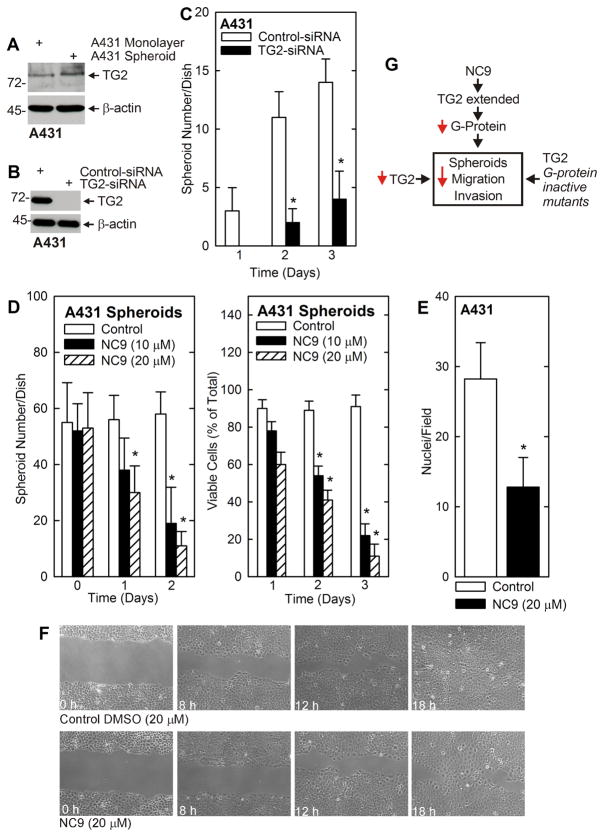
TG2 is also required for survival and migration of A431 ECS cells
We wanted to compare these findings in another squamous cell carcinoma cell type. We chose A431 cells, as we have previously shown that spheroid-forming ECS cells comprise 0.03% of this cell population (9). These cells are derived from human vulvar skin. Fig. 6A reveals that TG2 level is substantially elevated in A431-derived ECS cells. We next examined the impact of TG2 knockdown or treatment with NC9 on spheroid formation. Electroporation of A431 ECS cells with TG2-siRNA reduced TG2 level (Fig. 6B). As shown in Fig. 6C, TG2 loss reduces A431 cell spheroid formation by seventy percent. Moreover, the ability of A431 ECS cells to invade Matrigel is reduced by 50% in the presence of NC9, as is ability to close a scratch wound (Fig. 6E and F). Fig. 6G will be explained in the discussion section.
Fig. 6. Role of TG2 in A431 cells.
A A431 cells were grown as monolayers or in ultralow attachment plates (spheroids) in spheroid medium. After 8 d, extracts were prepared and assayed for expression of TG2 by immunoblot. Similar results were observed in four separate experiments. B A431 cells were electroporated with control- or TG2-siRNA and after 72 h cell extracts were prepared to detect TG2. C A431 cells were electroporated with the indicated siRNA and 40,000 cells were seeded into low-attachment six well dishes at time zero. Spheroid number was counted on days 1, 2 and 3. D A431 cells were seeded at 40,000 cells per six well cluster dish and after 12 h NC9 was added at time zero. Spheroid number and trypan-blue viable cell number were determined at the indicated times following NC9 addition. The values are mean ± SEM, n = 3, p < 0.05. E A431 cells, maintained as spheroids, were treated with 0 (Control) or 20 μM NC9 for 1 h and then plated atop Matrigel in 1 ml of spheroid medium in a Millicell chamber. After 24 h, the chambers were collected and stained with DAPI to detect cells that had migrated through the Matrigel to the inner surface of the membrane. The values are mean ± SEM, n = 6, p < 0.05. F A431-derived ECS cells were seeded at confluence as monolayer cultures. A wound was created and ability of the cells to close the wound was monitored with time. G Model describing regulation of spheroid formation, migration and invasion by TG2. Reduction in TG2 level, or loss of TG2 GTP binding/G-protein function, reduces ECS cell function (survival, spheroid formation, invasion and migration). NC9 inactivates TG2 TGase activity forcing it into an extended conformation to indirectly reduce GTP binding activity.
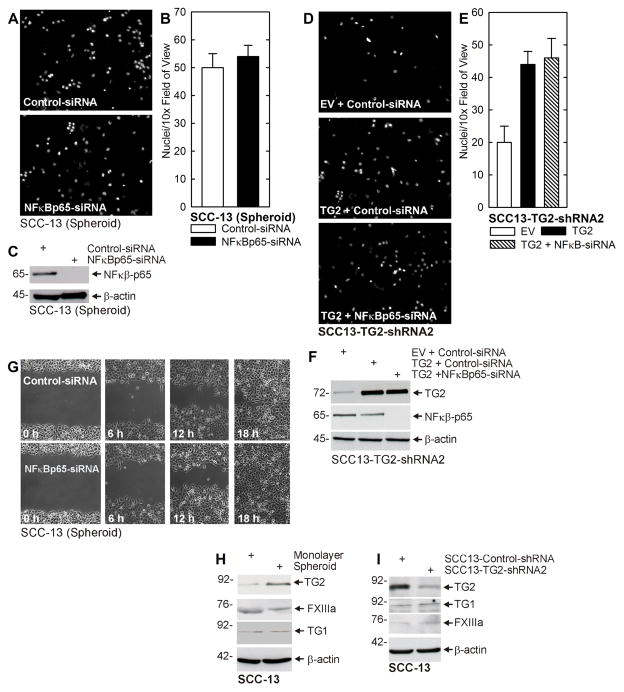
Role of NFκB
Experiments in Fig. 3E/F shows that TG2 is required for ECS cell invasion through matrigel. Previous studies have implicated NFκB as a mediator of TG2-dependent EMT-related processes in several cancer cell types (24–29). We therefore examined whether NFκB plays a role in mediating these processes in SCC-13 cells. ECS cells were electroporated with control- or NFκB-siRNA before plating on matrigel. Fig. 7A/B shows that NFκB knockdown does not reduce ECS cell invasion, and Fig. 7C confirms the knockdown. We next determined whether NFκB knockdown attenuates TG2 stimulation of invasion. SCC13-TG2-shRNA2 cells were electroporated with plasmid encoding wild-type TG2 in the presence or absence of NFκB-siRNA. Fig. 7D/E shows that expression of TG2 enhances invasion, and that TG2-simulated invasion is not reduced by NFκB knockdown. Fig. 7F confirms the elevation of TG2 and the knockdown of NFκB. We also examined the impact of NFκB knockdown on ECS cell migration. Fig. 7G shows that NFκB knockdown does not alter the rate of ECS cell wound repair (migration).
Fig. 7.
NFκB does not mediate TG2-dependent invasion or migration. A/B ECS cells were electroporated with the indicated siRNA and permitted to recover for 24 h. The cells (25,000 per well) were seeded on top a matrigel-coated membrane in 1 ml of spheroid growth medium in a Millicell chamber. After 24 h, the membrane was rinsed with phosphate buffered saline, fixed in 4% paraformaldehyde and stained with DAPI. The underside of the membranes were viewed with an inverted fluorescent microscope and nuclei counted. C ECS cells were harvested at the end of migration to assay NFκB level. D/E ECS cells were electroporated with empty vector (EV) or TG2-encoding (TG2) expression plasmid in the presence of control- or NFκB-siRNA. After 24 h, the membranes were process to visualize the nuclei of migrated cells. F ECS cells were harvested at the end of migration to assay to monitor TG2 and NFκB level. G ECS cells were electroporated with control- or NFκB-siRNA, seeded at confluent density. After attachment, uniform wounds were prepared, and cell migration to fill the wound was monitored from 0 – 18 h. Cell division does not significantly contribute to wound closure under these conditions (not shown). H Extracts were prepared from ECS cells (8 d spheroids) and non-stem cancer cells derived from monolayer cultures. Extracts were electrophoresed for immunoblot detection of the indicated epitopes. I ECS cells were treated with control- or TG2-siRNA and maintained in spheroid medium in non-attachment plates. After 48 h extracts were prepared for assay of the indicated epitopes. Similar results were observed in each of three experiments.
TG1 and FXIIIa
We also assessed the likelihood that other transglutaminase forms may be involved in the regulation. Transformed keratinocytes express relatively low levels of TG1 and FXIIIa. Fig. 7H shows that ECS cells (spheroids) express elevated levels of TG; however, there is not change in FXIIIa or TG1 levels. Fig. 7I shows that knockdown of TG2 in ECS cells is associated with a slight increase in TG1 and FXIIIa level. The fact that these enzymes change minimally in level in ECS cells as compared to non-stem cancer cells, and are minimally impacted by TG2 knockdown, suggest they do not play a role in the observed loss of ECS cell properties observed following TG2 loss.
Discussion
The cancer stem cell model was predicted over 150 years ago, but only recently have technologies evolved to test this model. This concept predicts that tumors originate from tissue stem cells, and that tumors retain a subpopulation of cancer stem cells that undergo aberrant differentiation that generate cellular heterogeneity in the tumor (30). These tumor stem cells display specific properties including the capacity for self-renewal, ability to differentiate, active telomerase expression, activation of anti-apoptotic pathways, increased membrane transporter activity, ability to migrate and metastasize and expression of stem cell markers (30). This hypothesis has important implications for cancer risk assessment, detection, prevention and treatment. It is also important to consider that the present cancer therapies appear to kill differentiated cancer cells but spare the cancer stem cell population (31, 32). This suggests that developing cancer treatment strategies that target the cancer stem cell population will be beneficial.
We have characterized putative epidermal cancer stem cells established from epidermal squamous cell carcinoma cells. These ECS cells can be selected by growth in non-attached conditions (9). Under these conditions, < 0.2% of the cells form spheroids and express a host of epidermal and embryonic stem cell markers (9). When injected into immune compromised mice, these cells form large, rapidly-growing, aggressive, invasive and highly-vascularized tumors (9). This is in contrast to the small and non-vascularized tumors that form upon injection of cell population comprised largely of non-stem cells. Moreover, injection of as few as one-hundred ECS cells can drive formation of large vascularized tumors. These cells also display enhanced mobility and invasion of Matrigel (9). The fact that these cells are readily able to form aggressive tumors, as compared to non-stem cells, confirms that they are important targets for cancer prevention and therapy.
An important strategy for understanding ECS cell properties, and designing anti-ECS cell therapy, is identifying survival proteins that are highly enriched in ECS cells as compared to non-stem cells. Based on the demonstrated importance of TG2 in several epithelial cancers (33–37), we examined expression of TG2 in skin cancer. Our studies show that TG2 levels are markedly elevated in ECS cells as compared to bulk cultures of cancer cells. Moreover, knockdown of TG2 using siRNA or by creating stable TG2 knockdown cell lines results in a marked reduction in ECS cell survival and spheroid formation. Inhibition of TG2 results in destruction of pre-established ECS cell spheroids and suppression of spheroid growth. Moreover, TG2 knockdown cells display a reduced ability to form spheroids.
We also examined the impact of TG2 inhibitors on ECS cell survival and spheroid formation. NC9 is an active site-directed irreversible inhibitor that inactivates TG2 TGase activity and in the process locks TG2 in an extended conformation (38–40). Treating ECS cells with NC9 inhibits spheroid formation and also promotes destruction of pre-existing spheroids. This is associated with inhibition of TGase activity. We also performed structural studies which suggest that NC9 produces an open TG2 structure in which the GTP binding site is moved to an inactive conformation (not shown). Thus, we propose that NC9 inhibits both GTP binding and TGase activity. Ultimately, NC9 causes ECS cell apoptosis and reduced viability. This is particularly interesting, as stem cells are known to suppress apoptotic signaling pathways to enhance survival, and it is possible that TG2 has an important role in mediating this activity (26, 27, 41). Fluorescein cadaverine, a competitive substrate inhibitor of TG2 (42), also suppresses spheroid growth. This agent also inhibits migration through Matrigel, but has a minimal effect on the rate of scratch wound closure (not shown). Overall, these observations suggest that inhibiting TG2 disrupts multiple ECS cell processes. In particular, TG2 appears to be required for ECS cell spheroid formation, survival, migration and invasion. Survival, migration and invasion are properties known to be required for tumor cell extravasation during metastasis, suggesting that TG2 may be required for in vivo metastasis (43–47). Indeed, such a role has been documented in other cancer types (48–50).
Recent studies suggest that in some cancer cell types TG2 activates NFκB to promote cancer cell survival (24–29). We therefore tested whether NFκB mediates TG2 action in ECS cells. It is interesting that knockdown of TG2 does not impair TG2 regulation of invasion or migration (Fig. 7) or spheroid formation or EMT (not shown). NFκB has been described as having a unique role in epidermal cells where it actually inhibits cell proliferation (51). This difference in properties may explain the lack of a role for NFκB as a TG2 mediator in ECS cells.
TG2 is a multifunctional enzyme expressed in many tissues (52). In addition to transamidase (TGase) activity, which is activated by calcium (14), TG2 binds and hydrolyzes GTP (53). GTP bound TG2 functions in G-protein signaling (54, 55). TG2 also functions as a protein disulfide isomerase (56, 57), protein kinase (58, 59), protein scaffold (60, 61) and as a DNA hydrolase (62). The TG2 TGase and GTP binding activities are the best studied and appear to be the most important (14). To understand the role of these activities in maintaining ECS cell function, we studied the ability of TG2 mutants to restore spheroid formation, invasion, and migration, in TG2 knockdown cells. These studies show that wild-type TG2, and mutants (Fig. 4A) that retain partial (C277S, Y526F) or full (W241A) GTP binding function, can partially or near-fully restore spheroid formation. In contrast, R580A, which lacks GTP binding, does not restore activity. Conversely, these same studies show that mutants (C277A, W241A), which lack TGase activity, are able to form spheroids. This genetic evidence confirms a role for the TG2 GTP binding activity in driving ECS cell spheroid formation, invasion and migration.
We propose that the TG2 mutant data unequivocally demonstrates that GTP binding is required for ECS cell function and that the inhibitor data also supports this hypothesis (Fig. 6G). NC9 is an irreversible inhibitor that covalently binds to TG2 to inactivate TGase activity (16). However, NC9 also locks TG2 into an extended conformation (38) which is associated with inactivation of GTP binding (63), as TG2 GTP binding requires a closed configuration (63). In silico structural modeling studies indicate that TG2 GTP activity is inactive when bound to NC9 (not shown). Thus, we propose that NC9 treatment inhibits both TG2 TGase and TG2 GTP binding/G-protein function in ECS cells. Based on these findings we conclude that TG2 is essential for cancer stem cell survival in epidermal squamous cell carcinoma and is likely to contribute to tumor and metastasis formation in squamous cell carcinoma.
Acknowledgments
This work was supported by National Institutes of Health R01-CA131064 (RLE) and an American Cancer Society investigator award from the University of Maryland Greenebaum Cancer Center (CK). We thank Drs. Kapil Mehta and Gail Johnson for graciously providing the TG2 mutant constructs.
Footnotes
Conflict of Interest: The authors indicate no conflict of interest.
References
- 1.Lansbury L, Bath-Hextall F, Perkins W, Stanton W, Leonardi-Bee J. Interventions for non-metastatic squamous cell carcinoma of the skin: systematic review and pooled analysis of observational studies. BMJ. 2013;347:f6153. doi: 10.1136/bmj.f6153.:f6153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lomas A, Leonardi-Bee J, Bath-Hextall F. A systematic review of worldwide incidence of nonmelanoma skin cancer. Br J Dermatol. 2012;166:1069–80. doi: 10.1111/j.1365-2133.2012.10830.x. [DOI] [PubMed] [Google Scholar]
- 3.Thenappan A, Li Y, Shetty K, Johnson L, Reddy EP, Mishra L. New Therapeutics Targeting Colon Cancer Stem Cells. Curr Colorectal Cancer Rep. 2009;5:209. doi: 10.1007/s11888-009-0029-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carpentino JE, Hynes MJ, Appelman HD, Zheng T, Steindler DA, Scott EW, et al. Aldehyde dehydrogenase-expressing colon stem cells contribute to tumorigenesis in the transition from colitis to cancer. Cancer Res. 2009;69:8208–15. doi: 10.1158/0008-5472.CAN-09-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–8. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li C, Yan Y, Ji W, Bao L, Qian H, Chen L, et al. OCT4 positively regulates Survivin expression to promote cancer cell proliferation and leads to poor prognosis in esophageal squamous cell carcinoma. PLoS ONE. 2012;7:e49693. doi: 10.1371/journal.pone.0049693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee CJ, Dosch J, Simeone DM. Pancreatic cancer stem cells. J Clin Oncol. 2008;26:2806–12. doi: 10.1200/JCO.2008.16.6702. [DOI] [PubMed] [Google Scholar]
- 8.Saini V, Shoemaker RH. Potential for therapeutic targeting of tumor stem cells. Cancer Sci. 2010;101:16–21. doi: 10.1111/j.1349-7006.2009.01371.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adhikary G, Grun D, Kerr C, Balasubramanian S, Rorke EA, Vemuri M, et al. Identification of a population of epidermal squamous cell carcinoma cells with enhanced potential for tumor formation. PLoS One. 2013;8:e84324. doi: 10.1371/journal.pone.0084324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsai LL, Hu FW, Lee SS, Yu CH, Yu CC, Chang YC. Oct4 Mediates Tumor Initiating Properties in Oral Squamous Cell Carcinomas through the Regulation of Epithelial-Mesenchymal Transition. PLoS ONE. 2014;9:e87207. doi: 10.1371/journal.pone.0087207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang CF, Xu XR, Wu TF, Sun ZJ, Zhang WF. Correlation of ALDH1, CD44, OCT4 and SOX2 in tongue squamous cell carcinoma and their association with disease progression and prognosis. J Oral Pathol Med. 2014;10 doi: 10.1111/jop.12159. [DOI] [PubMed] [Google Scholar]
- 12.Kim SY. Transglutaminase 2 in inflammation. Front Biosci. 2006;11:3026–35. doi: 10.2741/2030. [DOI] [PubMed] [Google Scholar]
- 13.Siegel M, Khosla C. Transglutaminase 2 inhibitors and their therapeutic role in disease states. Pharmacol Ther. 2007;115:232–45. doi: 10.1016/j.pharmthera.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gundemir S, Colak G, Tucholski J, Johnson GV. Transglutaminase 2: a molecular Swiss army knife. Biochim Biophys Acta. 2012;1823:406–19. doi: 10.1016/j.bbamcr.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park D, Choi SS, Ha KS. Transglutaminase 2: a multi-functional protein in multiple subcellular compartments. Amino Acids. 2010;39:619–31. doi: 10.1007/s00726-010-0500-z. [DOI] [PubMed] [Google Scholar]
- 16.Keillor J, chica R, Chabot N, Vinci V, Pardin C, Fortin E, et al. The bioorganic chemistry of transglutaminase - from mechanism to inhibition and engineering. Can J Chem. 2008;86:271–6. [Google Scholar]
- 17.Efimova T, LaCelle P, Welter JF, Eckert RL. Regulation of human involucrin promoter activity by a protein kinase C, Ras, MEKK1, MEK3, p38/RK, AP1 signal transduction pathway. J Biol Chem. 1998;273:24387–95. doi: 10.1074/jbc.273.38.24387. [DOI] [PubMed] [Google Scholar]
- 18.Efimova T, Deucher A, Kuroki T, Ohba M, Eckert RL. Novel protein kinase C isoforms regulate human keratinocyte differentiation by activating a p38 delta mitogen-activated protein kinase cascade that targets CCAAT/enhancer-binding protein alpha. J Biol Chem. 2002;277:31753–60. doi: 10.1074/jbc.M205098200. [DOI] [PubMed] [Google Scholar]
- 19.Gundemir S, Johnson GV. Intracellular localization and conformational state of transglutaminase 2: implications for cell death. PLoS One. 2009;4:e6123. doi: 10.1371/journal.pone.0006123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruan Q, Tucholski J, Gundemir S, Johnson Voll GV. The Differential Effects of R580A Mutation on Transamidation and GTP Binding Activity of Rat and Human Type 2 Transglutaminase. Int J Clin Exp Med. 2008;1:248–59. [PMC free article] [PubMed] [Google Scholar]
- 21.Adhikary G, Chew YC, Reece EA, Eckert RL. PKC-delta and -eta, MEKK-1, MEK-6, MEK-3, and p38-delta Are Essential Mediators of the Response of Normal Human Epidermal Keratinocytes to Differentiating Agents. J Invest Dermatol. 2010 doi: 10.1038/jid.2010.108. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 22.Sturniolo MT, Chandraratna RA, Eckert RL. A novel transglutaminase activator forms a complex with type 1 transglutaminase. Oncogene. 2005;24:2963–72. doi: 10.1038/sj.onc.1208392. [DOI] [PubMed] [Google Scholar]
- 23.Sturniolo MT, Dashti SR, Deucher A, Rorke EA, Broome AM, Chandraratna RA, et al. A novel tumor suppressor protein promotes keratinocyte terminal differentiation via activation of type I transglutaminase. J Biol Chem. 2003;278:48066–73. doi: 10.1074/jbc.M307215200. [DOI] [PubMed] [Google Scholar]
- 24.Kim JM, Voll RE, Ko C, Kim DS, Park KS, Kim SY. A new regulatory mechanism of NF-kappaB activation by I-kappaBbeta in cancer cells. J Mol Biol. 2008;384:756–65. doi: 10.1016/j.jmb.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 25.Kim DS, Han BG, Park KS, Lee BI, Kim SY, Bae CD. I-kappaBalpha depletion by transglutaminase 2 and mu-calpain occurs in parallel with the ubiquitin-proteasome pathway. Biochem Biophys Res Commun. 2010;399:300–6. doi: 10.1016/j.bbrc.2010.07.078. [DOI] [PubMed] [Google Scholar]
- 26.Yakubov B, Chelladurai B, Schmitt J, Emerson R, Turchi JJ, Matei D. Extracellular tissue transglutaminase activates noncanonical NF-kappaB signaling and promotes metastasis in ovarian cancer. Neoplasia. 2013;15:609–19. doi: 10.1593/neo.121878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kumar S, Mehta K. Tissue Transglutaminase Constitutively Activates HIF-1alpha Promoter and Nuclear Factor-kappaB via a Non-Canonical Pathway. PLoS One. 2012;7:e49321. doi: 10.1371/journal.pone.0049321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mann AP, Verma A, Sethi G, Manavathi B, Wang H, Fok JY, et al. Overexpression of tissue transglutaminase leads to constitutive activation of nuclear factor-kappaB in cancer cells: delineation of a novel pathway. Cancer Res. 2006;66:8788–95. doi: 10.1158/0008-5472.CAN-06-1457. [DOI] [PubMed] [Google Scholar]
- 29.Cao L, Petrusca DN, Satpathy M, Nakshatri H, Petrache I, Matei D. Tissue transglutaminase protects epithelial ovarian cancer cells from cisplatin-induced apoptosis by promoting cell survival signaling. Carcinogenesis. 2008;29:1893–900. doi: 10.1093/carcin/bgn158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Strieth S, Hartschuh W, Pilz L, Fusenig NE. Angiogenic switch occurs late in squamous cell carcinomas of human skin. Br J Cancer. 2000;82:591–600. doi: 10.1054/bjoc.1999.0969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Al-Hajj M, Becker MW, Wicha M, Weissman I, Clarke MF. Therapeutic implications of cancer stem cells. Curr Opin Genet Dev. 2004;14:43–7. doi: 10.1016/j.gde.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 32.Dontu G, Al-Hajj M, Abdallah WM, Clarke MF, Wicha MS. Stem cells in normal breast development and breast cancer. Cell Prolif. 2003;36 (Suppl 1):59–72. doi: 10.1046/j.1365-2184.36.s.1.6.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Agnihotri N, Kumar S, Mehta K. Tissue transglutaminase as a central mediator in inflammation-induced progression of breast cancer. Breast Cancer Res. 2013;15:202. doi: 10.1186/bcr3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Akar U, Ozpolat B, Mehta K, Fok J, Kondo Y, Lopez-Berestein G. Tissue transglutaminase inhibits autophagy in pancreatic cancer cells. Mol Cancer Res. 2007;5:241–9. doi: 10.1158/1541-7786.MCR-06-0229. [DOI] [PubMed] [Google Scholar]
- 35.Chen JS, Agarwal N, Mehta K. Multidrug-resistant MCF-7 breast cancer cells contain deficient intracellular calcium pools. Breast Cancer Res Treat. 2002;71:237–47. doi: 10.1023/a:1014461832403. [DOI] [PubMed] [Google Scholar]
- 36.Mehta K, Kumar A, Kim HI. Transglutaminase 2: a multi-tasking protein in the complex circuitry of inflammation and cancer. Biochem Pharmacol. 2010;80:1921–9. doi: 10.1016/j.bcp.2010.06.029. [DOI] [PubMed] [Google Scholar]
- 37.Verma A, Guha S, Wang H, Fok JY, Koul D, Abbruzzese J, et al. Tissue transglutaminase regulates focal adhesion kinase/AKT activation by modulating PTEN expression in pancreatic cancer cells. Clin Cancer Res. 2008;14:1997–2005. doi: 10.1158/1078-0432.CCR-07-1533. [DOI] [PubMed] [Google Scholar]
- 38.Caron NS, Munsie LN, Keillor JW, Truant R. Using FLIM-FRET to measure conformational changes of transglutaminase type 2 in live cells. PLoS One. 2012;7:e44159. doi: 10.1371/journal.pone.0044159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Al-Jallad HF, Myneni VD, Piercy-Kotb SA, Chabot N, Mulani A, Keillor JW, et al. Plasma membrane factor XIIIA transglutaminase activity regulates osteoblast matrix secretion and deposition by affecting microtubule dynamics. PLoS One. 2011;6:e15893. doi: 10.1371/journal.pone.0015893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Colak G, Keillor JW, Johnson GV. Cytosolic guanine nucledotide binding deficient form of transglutaminase 2 (R580a) potentiates cell death in oxygen glucose deprivation. PLoS One. 2011;6:e16665. doi: 10.1371/journal.pone.0016665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Verma A, Guha S, Wang H, Fok JY, Koul D, Abbruzzese J, et al. Tissue transglutaminase regulates focal adhesion kinase/AKT activation by modulating PTEN expression in pancreatic cancer cells. Clin Cancer Res. 2008;14:1997–2005. doi: 10.1158/1078-0432.CCR-07-1533. [DOI] [PubMed] [Google Scholar]
- 42.Gray AC, Garle MJ, Clothier RH. Fluorescein cadaverine incorporation as a novel technique for the characterization of terminal differentiation in keratinocytes. Toxicol In Vitro. 1999;13:773–8. doi: 10.1016/s0887-2333(99)00023-5. [DOI] [PubMed] [Google Scholar]
- 43.Budillon A, Carbone C, Di GE. Tissue transglutaminase: a new target to reverse cancer drug resistance. Amino Acids. 2011 doi: 10.1007/s00726-011-1167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kumar A, Xu J, Brady S, Gao H, Yu D, Reuben J, et al. Tissue transglutaminase promotes drug resistance and invasion by inducing mesenchymal transition in mammary epithelial cells. PLoS ONE. 2010;5:e13390. doi: 10.1371/journal.pone.0013390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shao M, Cao L, Shen C, Satpathy M, Chelladurai B, Bigsby RM, et al. Epithelial-to-mesenchymal transition and ovarian tumor progression induced by tissue transglutaminase. Cancer Res. 2009;69:9192–201. doi: 10.1158/0008-5472.CAN-09-1257. [DOI] [PubMed] [Google Scholar]
- 46.Park MK, You HJ, Lee HJ, Kang JH, Oh SH, Kim SY, et al. Transglutaminase-2 induces N-cadherin expression in TGF-beta1-induced epithelial mesenchymal transition via c-Jun-N-terminal kinase activation by protein phosphatase 2A down-regulation. Eur J Cancer. 2013;49:1692–705. doi: 10.1016/j.ejca.2012.11.036. [DOI] [PubMed] [Google Scholar]
- 47.Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011;147:275–92. doi: 10.1016/j.cell.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mehta K, Han A. Tissue Transglutaminase (TG2)-Induced Inflammation in Initiation, Progression, and Pathogenesis of Pancreatic Cancer. Cancers (Basel) 2011;3:897–912. doi: 10.3390/cancers3010897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Satpathy M, Shao M, Emerson R, Donner DB, Matei D. Tissue transglutaminase regulates matrix metalloproteinase-2 in ovarian cancer by modulating cAMP-response element-binding protein activity. J Biol Chem. 2009;284:15390–9. doi: 10.1074/jbc.M808331200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tovar-Vidales T, Fitzgerald AM, Clark AF, Wordinger RJ. Transforming growth factor-beta2 induces expression of biologically active bone morphogenetic protein-1 in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2013;54:4741–8. doi: 10.1167/iovs.13-12203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang JY, Green CL, Tao S, Khavari PA. NF-kappaB RelA opposes epidermal proliferation driven by TNFR1 and JNK. Genes Dev. 2004;18:17–22. doi: 10.1101/gad.1160904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fesus L, Piacentini M. Transglutaminase 2: an enigmatic enzyme with diverse functions. Trends Biochem Sci. 2002;27:534–9. doi: 10.1016/s0968-0004(02)02182-5. [DOI] [PubMed] [Google Scholar]
- 53.Begg GE, Carrington L, Stokes PH, Matthews JM, Wouters MA, Husain A, et al. Mechanism of allosteric regulation of transglutaminase 2 by GTP. Proc Natl Acad Sci U S A. 2006;103:19683–8. doi: 10.1073/pnas.0609283103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nakaoka H, Perez DM, Baek KJ, Das T, Husain A, Misono K, et al. Gh: a GTP-binding protein with transglutaminase activity and receptor signaling function. Science. 1994;264:1593–6. doi: 10.1126/science.7911253. [DOI] [PubMed] [Google Scholar]
- 55.Vezza R, Habib A, FitzGerald GA. Differential signaling by the thromboxane receptor isoforms via the novel GTP-binding protein, Gh. J Biol Chem. 1999;274:12774–9. doi: 10.1074/jbc.274.18.12774. [DOI] [PubMed] [Google Scholar]
- 56.Hasegawa G, Suwa M, Ichikawa Y, Ohtsuka T, Kumagai S, Kikuchi M, et al. A novel function of tissue-type transglutaminase: protein disulphide isomerase. Biochem J. 2003;373:793–803. doi: 10.1042/BJ20021084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mastroberardino PG, Farrace MG, Viti I, Pavone F, Fimia GM, Melino G, et al. “Tissue” transglutaminase contributes to the formation of disulphide bridges in proteins of mitochondrial respiratory complexes. Biochim Biophys Acta. 2006;1757:1357–65. doi: 10.1016/j.bbabio.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 58.Mishra S, Murphy LJ. Tissue transglutaminase has intrinsic kinase activity: identification of transglutaminase 2 as an insulin-like growth factor-binding protein-3 kinase. J Biol Chem. 2004;279:23863–8. doi: 10.1074/jbc.M311919200. [DOI] [PubMed] [Google Scholar]
- 59.Mishra S, Saleh A, Espino PS, Davie JR, Murphy LJ. Phosphorylation of histones by tissue transglutaminase. J Biol Chem. 2006;281:5532–8. doi: 10.1074/jbc.M506864200. [DOI] [PubMed] [Google Scholar]
- 60.Akimov SS, Belkin AM. Cell-surface transglutaminase promotes fibronectin assembly via interaction with the gelatin-binding domain of fibronectin: a role in TGFbeta-dependent matrix deposition. J Cell Sci. 2001;114:2989–3000. doi: 10.1242/jcs.114.16.2989. [DOI] [PubMed] [Google Scholar]
- 61.Akimov SS, Belkin AM. Cell surface tissue transglutaminase is involved in adhesion and migration of monocytic cells on fibronectin. Blood. 2001;98:1567–76. doi: 10.1182/blood.v98.5.1567. [DOI] [PubMed] [Google Scholar]
- 62.Takeuchi Y, Ohashi H, Birckbichler PJ, Ikejima T. Nuclear translocation of tissue type transglutaminase during sphingosine-induced cell death: a novel aspect of the enzyme with DNA hydrolytic activity. Z Naturforsch C. 1998;53:352–8. doi: 10.1515/znc-1998-5-609. [DOI] [PubMed] [Google Scholar]
- 63.Pinkas DM, Strop P, Brunger AT, Khosla C. Transglutaminase 2 undergoes a large conformational change upon activation. PLoS Biol. 2007;5:e327. doi: 10.1371/journal.pbio.0050327. [DOI] [PMC free article] [PubMed] [Google Scholar]