Abstract
Background
Given the increasing evidence of safe application of elevated temperature in other clinical contexts, we consider the potential for supplemental hyperthermia to augment the effects of vancomycin against staphylococci, a major source of post-operative and post-traumatic sepsis.
Methods
Laboratory reference strains and libraries of clinical blood isolates of S. epidermidis and methicillin resistant S. aureus, both as planktonic cells and as established biofilms, were assessed for thermosensitivity and increased susceptibility to vancomycin in the setting of thermal treatment. In addition to viability measures, patterns of stress gene expression were assessed with qPCR and structural changes were measured using quantitative transmission electron microscopy.
Results
Laboratory strains of both species had reduced growth and biofilm viability at 45°C, a temperature commonly employed in other domains such as adjuvant treatments of malignancy. Blood isolates of S. epidermidis were consistent in this regard as well, but significant between-isolate variability in thermosensitivity was seen in blood isolates of S. aureus. Expression profiling and ultrastructural measurements confirmed that elevated temperature was a substantial stressor with or without vancomycin treatment.
Conclusions
Our findings suggest that temperature elevations shown to be tolerated in humans in other settings hold the potential to be used as an adjuvant to antibiotic therapy against staphylococcal biofilms.
Keywords: Central line associated bloodstream infections, postoperative wound infection, medical device infection
INTRODUCTION
Implanted materials and devices are an essential element of the surgical treatment of trauma, shock, and sepsis. While the composition and location of these tools is highly diverse, contamination and infection is a common and sustained clinical problem. It is believed that each year in the United States, 2 million instances of nosocomial infection related to devices and materials occur, with an estimated economic impact of more than 8 billion dollars annually (1–3). Beyond the financial implications, the resilience of device-associated bacterial biofilms to conventional antibiotic treatment very often results in the need to remove and replace the degraded device, a procedure which may vary from inconvenient to life threatening (1, 3). Over the past two decades, tremendous effort has been exerted on reducing the incidence of device infections through a combination of improved device surface characteristics and changes in clinical practice (4–6), and it is clear that strides have been made. For example, central line associated bloodstream infections (CLABSI) declined by 14.1% between 2008 and 2012, while the incidence rate of methicillin resistant Staphylococcus aureus (MRSA) infection fell by an average of 8.7% per year (7). However, despite these advances, device-related infections continue to be a part of daily medical practice for many care providers (8). What the next significant advance in reducing device infections will be is not clear, which has prompted some investigators to consider better strategies for treating the seemingly inevitable instances of infection that arise in contemporary care (9).
Heat is an important tool in managing microbial contamination in medical and nonmedical contexts. Autoclaving and pasteurization are ubiquitous examples of this strategy (10). The use of supplemental heat for infection is reported but not common – treatment of infected cutaneous lesions is a recent promising development (11, 12). A mouse model of heat as an adjunctive therapy has also been described (13).
Recently, applied heat (extrinsically by way of microwave irradiation, internally by way of modified cardiopulmonary bypass) has found some utility in the treatment of advanced metastatic malignancy, where evidence suggests it may have a role as a ‘second hit’ sensitizing cancer cells to chemotherapy. Remarkably, the warming of tissue by either strategy appears well-tolerated (14). The appearance of new applications and technologies for thermal therapy prompted us to consider a possible role for this technique in the treatment of medical device infection.
In the current report, we consider the use of supplemental thermal treatment as a means of augmenting the killing of Staphylococcus aureus and Staphylococcus epidermidis by vancomycin. We specifically evaluated the temperature sensitivity of laboratory and clinical isolates in terms of growth and antibiotic susceptibility, stress response as measured by heat shock and cell wall reparative gene expression, cell morphology, and biofilm persistence. Our results support further exploration of therapeutic hyperthermia as an adjunctive means to manage infected medical devices.
MATERIALS AND METHODS
Strains, media, and antibiotics
Strains used in this study are summarized in Table I. Staphylococcus epidermidis RP62A and Staphylococcus aureus 27660 were obtained from American Type Culture Collection (Manassas, VA). Clinical blood isolates of both species were obtained from the University of Michigan Hospital Clinical Microbiology Laboratory. For S. epidermidis isolates, we used only MLST-2 and -5, the types most strongly associated with clinical infection (15). All S. aureus strains were methicillin resistant by standard clinical methods. On the day prior to use, cultures were grown in tryptic soy broth with added 1% glucose (TSB-G). Media were supplemented with vancomycin where indicated.
Table I.
Strains and primers used in this report.
| Laboratory Strains | Characteristics/Comments | References | ||
|---|---|---|---|---|
| S. epidermidis RP62A | (26) | |||
| S. aureus ATCC 27660 | Methicillin Sensitive | This report | ||
| Clinical strains | ||||
| S. epidermidis | Five each of MLST-5 and MLST-2 | (15) | ||
| S. aureus | mecA+ | This report | ||
| Primers | Forward (5’ to 3’) | Reverse (5’ to 3’) | ||
| S. epidermidis | ||||
| hsp60 | TCTTAAGAATGTTACAAGTGGTGCAA (Accession # AF029245) |
AATCTCATGGAGCGCTTCTATAGC (Accession # AF029245) |
(27) | |
| murAB | TAAGTGTTGGAGCGACGATT (Accession # NC_002976) |
TCCTCTAATATCAGCACCCA (Accession # NC_002976) |
This report | |
| dhfr | GGGAAACCATTGCCAAATAGAC (Accession # Z48233) |
CGAATAACGTTTGTCCTCCAAATA (Accession # Z48233) |
(27, 28) | |
| S. aureus | ||||
| hsp60 | TTGCTGGTGACGGTACGACAA (Accession # CP002388) |
TAACGCTTCAACAGCAACTT (Accession # CP002388) |
This report | |
| murZ | AAGAGGTGGACGCACACTAA (Accession # CP007454) |
ATCAGAGATTTGCGGTAACCCTT (Accession # CP007454) |
This report | |
| dhfr | ACCGAATCGTCGAAATGTTGT (Accession # NC_002758) |
GAAAACATGGCCCGGTAGTTG (Accession # NC_002758) |
This report | |
Thermal sensitivity of planktonic cells and effect of vancomycin and serum
Organisms were grown on an orbital shaker at 200 rpm at temperatures from 27°C to 50°C. Turbidity was measured at 600 nm every 30 minutes and a first order exponential growth rate k (i.e., Bt = B0ekt) was determined by least squares. Clinical isolates were tested only at 37°C and 45°C. In some experiments, vancomycin was added at various concentrations spanning at least 10-fold above and below the minimal inhibitory concentration (MIC). In additional experiments, cells were treated with heat for 2 hours, then exposed to normal human serum for 30 minutes. In vancomycin and serum exposure experiments, CFU before and after treatment, rather than growth kinetics, were the measured endpoints.
Heat shock and cell wall repair gene expression
Following lysostaphin lysis, proteinase K digestion, and mechanical disruption (micro-MiniBeadbeater, Biospec, Bartlesville, OK), total RNA was isolated (RNeasy Mini Kit, Qiagen, Valencia, CA). Quantitative PCR (qPCR) was carried out for hsp60, murAB (in S. epidermidis) and murZ (in S. aureus), using dihydrofolate reductase (dhfr) as the endogenous control. Primers are shown in Table 1. cDNA was synthesized using the QuantiTect Reverse Transcription Kit (Qiagen). qPCR was performed using SYBR Green reagents (QuantiTect SYBR Green PCR Kit, Qiagen) and the Applied Biosystems StepOne Plus (Life Technologies, Carlsbad, CA).
Thermal and vancomycin sensitivity of established biofilms
Exponential phase organisms were seeded into 96-well cell culture-treated plates. The plates were incubated overnight (18 hours) at 37°C without shaking. Thusly formed biofilms were then subjected to 2 additional hours of 37°C or 45°C treatment with or without vancomycin. Post-treatment biofilm viability was assessed using the FilmTracer LIVE/DEAD Biofilm Viability Kit (Life Technologies) according to manufacturer instructions. Fluorescence intensity was measured with the Enspire 2300 Multimode Plate Reader (Perkin Elmer, Waltham, Massachusetts). For some biofilms, biofilm extent and cellular viability were confirmed with confocal microscopy as we have previously described (16, 17).
Cell wall integrity
Cells were incubated at 37°C or 45°C for 2 hours with or without vancomycin at MIC level, then washed once with 0.1 M Sorensen’s Buffer and resuspended in 2.5% glutaraldehyde. These were embedded in EMbed-812 resin (Electron Microscopy Sciences, Hatfield PA). Ultra-thin sections, 70 nm in thickness, were mounted on copper grids for transmission electron microscopy (TEM). Grids were post-stained with uranyl acetate and Reynolds lead citrate. Sections were examined using a Philips CM100 Transmission Electron Microscope at 60 kV. Images were recorded digitally using a Hamamatsu ORCA-HR digital camera system, operated using AMT software (Advanced Microscopy Techniques Corp., Danvers, MA). The cell wall thickness was determined in ImageJ by identifying the geometric center of each cell and constructing a radius through a representative region of cell wall. The length of radius passing from the cell membrane to the outer edge of the cell wall was defined as the cell wall thickness.
Statistical methods
Standard parametric methods were used throughout. All analyses were performed in R 3.02.
RESULTS
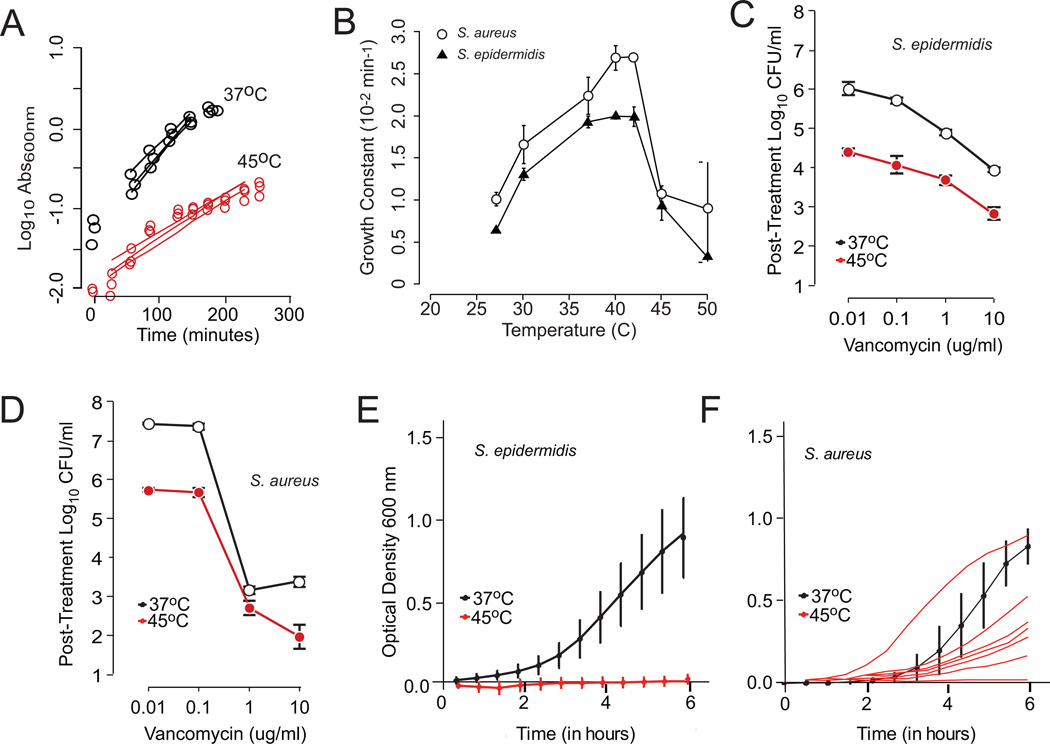
Both S. aureus and S. epidermidis reference strains demonstrated a sharp reduction in growth at temperatures above 40°C. (Figure 1, panels A and B). As 45°C is the highest previously reported temperature for use in vivo (18), this temperature was chosen for all other experiments. In planktonic cultures of both species, incubation at 45°C synergized with vancomycin and imparted an additional 1.5 log killing (Figure 1, panels C and D). The S. epidermidis blood isolates all showed remarkable temperature sensitivity. However, the S. aureus isolates were less sensitive overall, with 7 out of 10 isolates showing some degree of thermotolerance at 45°C (Figure 1, Panels E and F). When examined at 37°C, all strains were resistant to human serum-mediated cell lysis, and this did not change after growth at 45°C (data not shown).
Figure 1. Thermal sensitivity of planktonic S. epidermidis and S. aureus.
Panel A. Representative turbidometric growth curves obtained at temperatures ranging from 27°C to 50°C (S. epidermidis shown in this case). These were converted into first order growth constants (slope of fitted line). Panel B. Growth constants as a function temperature. Shown are the detailed temperature sensitivities of the reference strains S. epidermidis RP62a and S. aureus 27660. Measurements at each temperature were repeated at least 5 times and are shown as mean and standard deviation. From these data, the 45°C temperature point was selected as a compromise between bacterial inhibition and potential host tissue injury. Panels C and D. Synergism between vancomycin and increased temperature for S. epidermidis and S. aureus. For both species, treatment at 45°C significantly reduced post-treatment cell viability at all concentrations of vancomycin studied (p < 0.05 by two-way ANOVA). Each plot represents the mean of at least 5 replicates with associated standard deviation bars. Panels E and F. Thermal susceptibility to clinical blood isolates of S. epidermidis and S. aureus. As with the reference strain, all S. epidermidis isolates were exquisitely sensitive to elevated temperature, with none showing optically detectable growth at 45°C. This was not the case with S. aureus clinical isolates, where a majority demonstrated some capacity to grow at elevate temperature. The individual growth curves for S. aureus isolates are shown to clarify the variability in behavior.
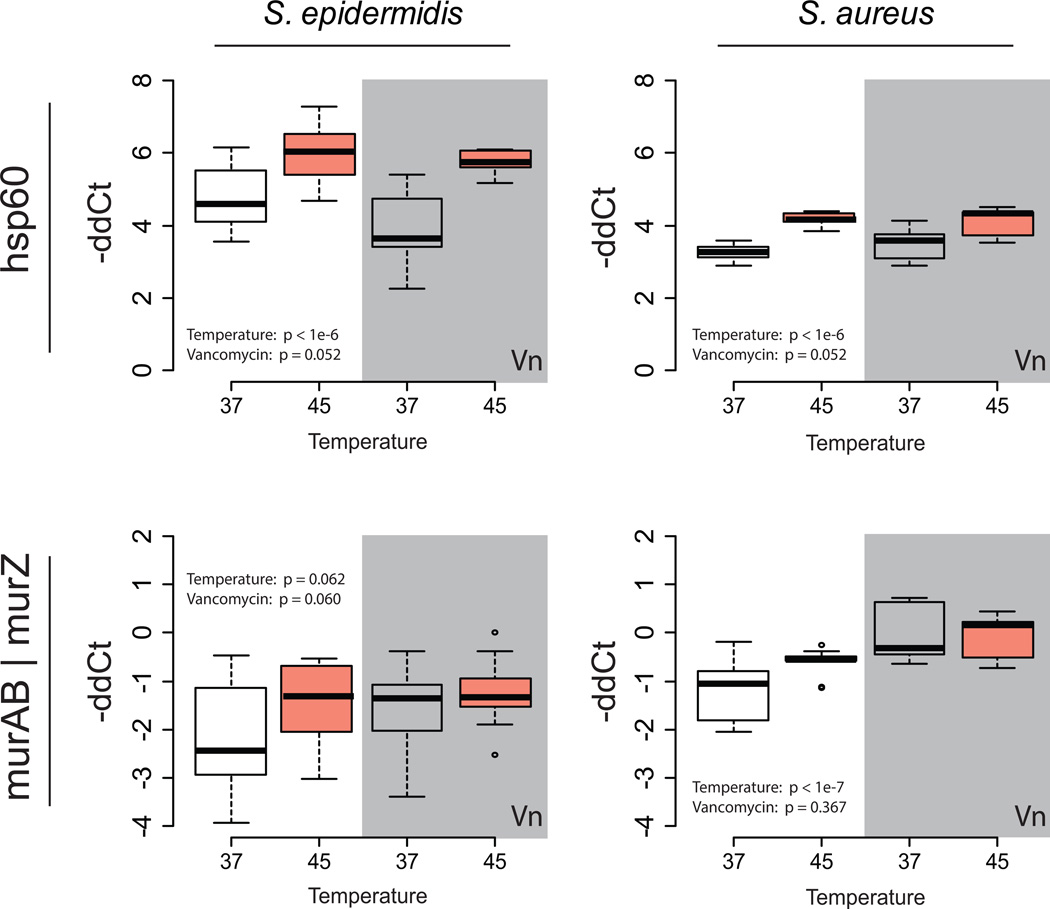
Two stress response genes (hsp60, which contributes to the misfolded protein response, and murAB/Z, which is the first committed step in staphylococcal cell wall synthesis and is known to participate in cell wall repair), were upregulated at elevated temperature (Figure 2). Expression across both temperatures and the presence or absence of vancomycin was compared with two-way ANOVA. Of the two interventions, temperature had the strongest statistical effect on gene expression. These results were interpreted (especially in light of the transmission electron micrographic results) as reflecting significant activation of heat-defensive mechanisms via hsp60. These stress responses were not seen as strongly with vancomycin therapy alone, and based on the several log-fold reduction in cell viability, were not sufficient to protect staphylococci from the combined antibiotic and thermal attack.
Figure 2. Effect of heat on stress gene expression.
qPCR was performed on planktonic cells after two hours of heat treatment, assessing heat shock protein 60 (hsp60, upper panels) and a cell wall synthetic enzyme (murAB/murZ, lower panels), and expressed as −ddCT normalized to dihydrofolate reductase (dhfr) expression as the housekeeping gene. White and gray backgrounds represent treatment in the absence or presence, respectively, of MIC concentrations of vancomycin for each strain. Conditions were compared using two-factor analysis of variance, with the p value for each term shown in each plot. Data plotted as 5th, 25th, median, 75th, and 95th percentiles of 5 more or measurements in each case. Because of differences in PCR efficiency between the primer sets used for S. epidermidis and S. aureus, comparisons between conditions should only be made within species, not between.
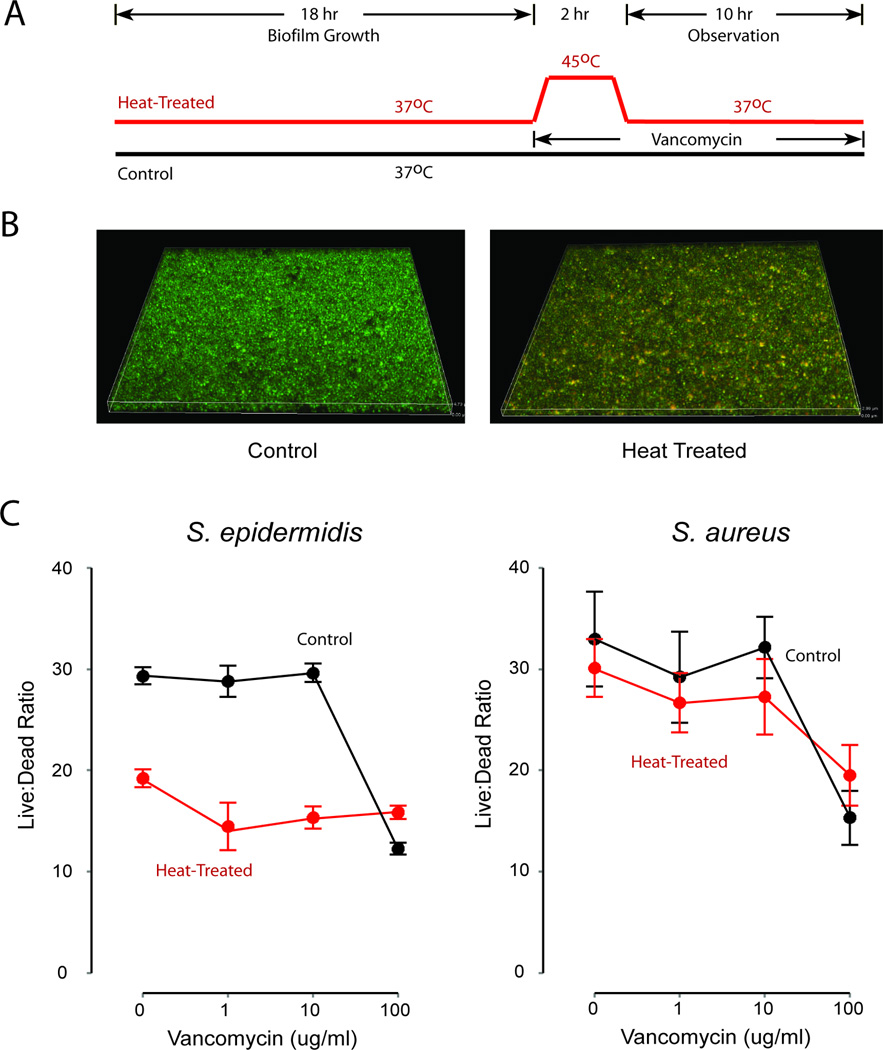
Viability of staphylococcal biofilms was assessed quantitatively using LIVE/DEAD intravital staining to evaluate one round of an imagined clinical treatment regimen (Figure 3a). Following 18 hours of development, biofilms were re-fed with fresh media and treated with vancomycin at concentrations ranging from 0 to 100 ug/ml and temperatures of 37°C or 45°C for 2 hours. All biofilms were then returned to 37°C and observed for an additional 10 hours (for a total of 12 hours of antibiotic treatment). Representative confocal microscopic images of biofilms fluorescently stained for cell viability are shown in Figure 3b, and qualitatively indicate diffuse disruption of cell membranes in heat-treated biofilms. Quantitatively, as in planktonic culture, S. epidermidis biofilms grown in this system were very sensitive to elevated temperature, with the post-treatment viability of cultures treated with 1 ug/ml vancomycin and 45°C showing killing comparable to that achieved with 100 ug/ml vancomycin in the absence of increased temperature. Also as in planktonic measurements, the response of S. aureus was not as pronounced, although statistically significant (p < 0.05).
Figure 3. Thermal sensitivity of S. epidermidis and S. aureus biofilms.
Panel A. Design of experiment. Heat-treated and control biofilms were established over 18 hours, then treated with vancomycin. Heat-treated biofilms received two hours of 45°C heating and then returned to 37°C for the duration of the experiment.
Panel B. Representative examples of treated and untreated biofilms as assessed by confocal microscopy. Biofilms were treated using the protocol in Figure 3 Panel A. In these images, cells staining green represent viable staphylococci, while those staining yellow have disrupted cell membrane integrity, indicating compromised viability. Panel C. Biofilm viability following treatment. Treated biofilms were assessed at 30 hours (10 hours after the conclusion of heat treatment) for viability as measured by live:dead (SYTO9:propidium iodide) staining under fluorescence microscopy. Both species showed increased vancomycin sensitivity in the presence of heat treatment (p < 0.05 by two-way ANOVA), but the effect was only modest in S. aureus. Among S. epidermidis biofilms treated, the impact of heat on cell viability was comparable to vancomycin treatment at 100x MIC. Data plotted as mean and standard deviation of at least 5 replicates.
TEM images revealed easily detectable morphological changes in the cell walls of cells exposed to two hours of elevated temperature (Figure 4). In both species, cell walls became significantly thicker and irregular, and overall cell shape also showed greater irregularities. Using formal image processing, cell wall thickness was found to be noticeably increased at 45°C in both species. The magnitude of this effect was greatest with S. epidermidis. Corroborating the above described gene expression measurements, increases in cell wall thickness were mostly driven by differences in temperature, not vancomycin treatment.
Figure 4. Effect of heat on cell wall morphology.
Planktonic cultures of S. epidermidis and S. aureus were grown at 37°C and 45°C for two hours then preserved and visualized with transmission electron microscopy. Panel A. Representative sections from both species. At 37°C, S. epidermidis had a thicker cell wall and its response to heat was more pronounced. Panel B. Formal cell wall thickness measurements for both experimental conditions. Data are shown as the 5th, 25th, 50th, 75th, and 95th percentile of more than 100 cell wall measurements per condition. P values are reported from two factor analysis of variance.
DISCUSSION
With one- to two-percent of all implanted medical devices ultimately becoming infected, managing contaminated devices is a prominent clinical problem with no obvious end in sight (19). Advances in clinical practice and antimicrobial surface coatings have produced improvements, but the problem remains ubiquitous in clinical practice. In most instances, infection cannot be managed successfully without explantation of the compromised device. Microorganisms sequestered on an artificial surface form structures known as bacterial biofilms which provide a physical and metabolic niche from which antibiotics are notoriously excluded (20, 21). Removal may be technically trivial (e.g., most peripherally inserted central catheters) or life threatening (e.g., prosthetic heart valves). As the consequences and costs of removal and replacement increase, questions may arise about the possibility of leaving the device in place and ‘treating through’ the acute infection. This plan is successful in a minority of cases, presumably because of the array of persistence mechanisms brought to bear by the inciting bacterial biofilm (22).
In the current work, we began to examine a potential adjuvant therapy for infected devices, namely the application of low level heating to augment the susceptibility of staphylococcal biofilms to vancomycin. The evidence for efficient killing of microbial organisms by means of thermal treatment dates to the nineteenth century (23). Industrial interest in both the use of heat to protect the food supply and in understanding heat’s effects on bacterial viability and detectability via production surveillance remains as high today as ever. However, the possible utility of thermal augmentation of antibiotics against biofilms, particularly in the context of in situ treatment of infected medical devices, remains largely unexplored. The human in vivo application of heat is a more recent phenomenon, and may seek tissue destruction – such as in radio frequency induced coagulation of abnormal conductive tissue in the heart – or simply sensitization of abnormal tissue to other therapies – such as in treatment of hepatocellular carcinoma (24). In the case of tissue ablation, tissue destruction is achieved at temperatures in excess of 57°C. For adjuvant therapy in cancer, temperature targets are more modest, frequently in the range of 41°C to 45°C (18).
We found that 45°C treatment, alone and especially in conjunction with vancomycin, was strongly inhibitory of planktonic growth and of biofilm viability. This level of temperature elevation provided at least 10-fold greater bacterial killing at vancomycin doses from 0.1 to 10 ug/ml in planktonic cells. Comparable effects were seen in established biofilms pulsed with heat for 2 hours and returned to physiologic temperature. The effect was most marked with S. epidermidis and was found consistently across ten blood isolates from patients with confirmed coagulase-negative staphylococcal bacteremia (15). In the laboratory reference strain of S. aureus that we examined, neither the impact of elevated temperature nor the combination of heat and antibiotics was as pronounced, and there was variability among clinical isolates as to the effectiveness of heat on either growth or biofilm viability.
Exposure to elevated temperature is known to elicit a number of responses in staphylococci and this was observed here. Quantitative assessment of gene expression showed increased presence of cell wall synthetic enzyme transcripts, corresponding to ultrastructural evidence of increased cell wall thickness and corroborating previous reports of increased muropeptide production during heat stress by S. aureus (25). These responses are recognized to have complex origins (13, 26, 27), to be tied to temperature-variant susceptibility to antibiotics (28), and, at least among isolates of S. aureus, to vary between isolates. From previous reports, as here, it is not clear whether these represent an adaptive or maladaptive response. In a recent report, we have noted that elevated temperature may have other beneficial effects against staphylococcal biofilms. Temperature increases similar to those studied in this report appear to mechanically destabilize intact biofilms (17, 29). Thus, it is possible that elevated temperature, through a combination of decreased cellular viability and degraded mechanical durability, could represent a significant adjuvant role in the in situ treatment of infected devices. Beyond its application in infected implanted material, we note that approximately 95% of all staphylococcal infections are focal (skin and soft tissue, respiratory, urinary) and that these sites are all biofilm-associated (30). The use of focal hyperthermia more broadly against intransigent staphylococcal infections warrants consideration.
As technologies to achieve focal internal heating are in clinical use currently, our results hold some promise for an accelerated path to clinical application. However, they also prompt several important questions that must be addressed. The first is tissue tolerability. Staphylococcal infections are well recognized to promote apoptotic changes similar to those seen during hyperthermic cancer therapy (31). In some tissues, sustained temperatures comparable to those studied here are sufficient to induce apoptosis and, presumably, injury in the vicinity of the device. We do note that the important clinical question is not, ‘Will elevated temperature damage tissue?’ but rather, ‘Will elevated temperature injure tissue more than what is being experienced as a result of the infected device?’ The answer to this question will certainly vary from site to site not just because of differences in tissue composition, but also in the local heat transport characteristics. For example, the rate at which excess heat is carried away from a dialysis catheter located in the superior vena cava would be expected to be much greater than the rate of thermal dissipation in proximity to a sheet of infected mesh in a previously repaired ventral hernia. Thus host thermotolerance will likely vary from one clinical application to the next.
A related question is precisely how intensely and for how long does temperature need to be applied to achieve therapeutic goals. In the current report we used two hours of sustained hyperthermia. Our data in Figure 1 suggest that this temperature may be necessary to achieve bacterial killing and biofilm devitalization. We are curious if the use of intermittent pulses of heat to 45°C or greater might have similar effects while sparing surrounding tissue. Complex temperature profiles might provide a broader therapeutic window and would likely drive the particulars of heaters to be used. Before implementation of thermal augmentation treatments, highly optimized therapies for in vitro clearance of experimental biofilms will also need to be developed. However, the next priority for developing clinical treatments is to understand the trade-offs in bacterial clearance and tissue insult at elevated temperature, a process that will necessarily be iterative and in vivo.
Lastly and importantly, our observations suggest that S. epidermidis isolates were much more consistently affected by temperature than isolates of S. aureus. The latter organism is typically more virulent, but from the standpoint of device infection, both organisms are very common; even if therapy was only effective against coagulase-negative staphylococci, heat therapy could still have an importantly clinical impact. Additional understanding of between-species differences is needed, as well as experience with the other typical Gram-positive and Gram-negative organisms encountered in this clinical setting.
CONCLUSIONS
In the current work, we demonstrated a significant susceptibility of staphylococci to elevated temperatures that have been safely achieved in human experience. This level of hyperthermia significantly augmented the effectiveness of vancomycin against both planktonic and biofilm-embedded organisms. A role for hyperthermia may exist in the in situ treatment of focal staphylococcal infections.
ACKNOWLEDGMENTS
The authors would like to thank Jeff Harrison, Dotty Sorensen, and Sasha Meshinchi of the University of Michigan Microscopy and Imaging Laboratory for their expertise and manual labor in the processing and imaging of bacterial samples for electron microscopy.
Funding: This work was supported by the National Institute of General Medical Sciences grant R01GM081702 (JGY, MJS) and an MCubed innovation award from the University of Michigan.
Footnotes
Conflicts of interest: The authors of this manuscript certify no affiliations, financial or otherwise, posing a conflict of interest.
REFERENCES
- 1.Bustos C, Aguinaga A, Carmona-Torre F, Del Pozo JL. Long-term catheterization: current approaches in the diagnosis and treatment of port-related infections. Infect Drug Resist. 2014;7:25–35. doi: 10.2147/IDR.S37773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zimlichman E, Henderson D, Tamir O, Franz C, Song P, Yamin CK, Keohane C, Denham CR, Bates DW. Health care-associated infections: a meta-analysis of costs and financial impact on the US health care system. JAMA Intern Med. 2013;173(22):2039–2046. doi: 10.1001/jamainternmed.2013.9763. [DOI] [PubMed] [Google Scholar]
- 3.Darouiche RO. Treatment of infections associated with surgical implants. N Engl J Med. 2004;350(14):1422–1429. doi: 10.1056/NEJMra035415. [DOI] [PubMed] [Google Scholar]
- 4.Yokoe DS, Mermel LA, Anderson DJ, Arias KM, Burstin H, Calfee DP, Coffin SE, Dubberke ER, Fraser V, Gerding DN, Griffin FA, Gross P, Kaye KS, Klompas M, Lo E, Marschall J, Nicolle L, Pegues DA, Perl TM, Podgorny K, Saint S, Salgado CD, Weinstein RA, Wise R, Classen D. A Compendium of Strategies to Prevent Healthcare-Associated Infections in Acute Care Hospitals. Infection Control and Hospital Epidemiology. 2008;29:S12–S21. doi: 10.1086/591060. [DOI] [PubMed] [Google Scholar]
- 5.Campoccia D, Montanaro L, Arciola CR. A review of the biomaterials technologies for infection-resistant surfaces. Biomaterials. 2013;34(34):8533–8554. doi: 10.1016/j.biomaterials.2013.07.089. [DOI] [PubMed] [Google Scholar]
- 6.Ratner BD, Bryant SJ. Biomaterials: Where we have been and where we are going. Annual Review of Biomedical Engineering. 2004;6:41–75. doi: 10.1146/annurev.bioeng.6.040803.140027. [DOI] [PubMed] [Google Scholar]
- 7.C. f. D. C. a. P. (CDC) CDC National Health Report: Leading Causes of Morbidity and Mortality and Associated Behavioral Risk and Protective Factors—United States, 2005–2013. 2014 Oct 31; [PubMed] [Google Scholar]
- 8.Yousif A, Jamal MA, Raad I. Biofilm-based central line-associated bloodstream infections. Adv Exp Med Biol. 2015;830:157–179. doi: 10.1007/978-3-319-11038-7_10. [DOI] [PubMed] [Google Scholar]
- 9.Vergidis P, Patel R. In: Novel approaches to the diagnosis, prevention, and treatment of medical device-associated infections. Moellering RC, editor. Vol. 26. Philadelphia, PA: Elsevier Inc.; 2012. pp. 173–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rutala WA, Weber DJ the Healthcare Infection Control Practices Advisory Committe (HICPAC) In: Guideline for Disinfection and Sterilization in Healthcare Facilities. U. S. D. o. H. a. H. Services, editor. 2008. pp. 13–32. 2008. [Google Scholar]
- 11.Aronson NE, Wortmann GW, Byrne WR, Howard RS, Bernstein WB, Marovich MA, Polhemus ME, Yoon IK, Hummer KA, Gasser RA, Oster CN, Benson PM. A Randomized Controlled Trial of Local Heat Therapy Versus Intravenous Sodium Stibogluconate for the Treatment of Cutaneous Leishmania major Infection. Plos Neglected Tropical Diseases. 2010;4(3) doi: 10.1371/journal.pntd.0000628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim MH, Yamayoshi I, Mathew S, Lin H, Nayfach J, Simon SI. Magnetic nanoparticle targeted hyperthermia of cutaneous Staphylococcus aureus infection. Ann Biomed Eng. 2013;41(3):598–609. doi: 10.1007/s10439-012-0698-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krieg RE, Wolcott JH, Meyers WM. Mycobacterium ulcerans infection: treatment with rifampin, hyperbaric oxygenation, and heat. Aviat Space Environ Med. 1979;50(9):888–892. [PubMed] [Google Scholar]
- 14.Bai JF, Liu P, Xu L. Recent Advances in Thermal Treatment Techniques and Thermally Induced Immune Responses Against Cancer. IEEE Trans Biomed Eng. 2014 doi: 10.1109/TBME.2014.2314357. [DOI] [PubMed] [Google Scholar]
- 15.Sharma P, Satorius AE, Raff MR, Rivera A, Newton DW, Younger JG. Multilocus Sequence Typing for Interpreting Blood Isolates of Staphylococcus epidermidis. Interdiscip Perspect Infect Dis. 2014;2014:787458. doi: 10.1155/2014/787458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stewart EJ, Satorius AE, Younger JG, Solomon MJ. Role of environmental and antibiotic stress on Staphylococcus epidermidis biofilm microstructure. Langmuir. 2013;29(23):7017–7024. doi: 10.1021/la401322k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pavlovsky L, Sturtevant RA, Younger JG, Solomon MJ. Effects of Temperature on the Morphological, Polymeric, and Mechanical Properties of Staphylococcus epidermidis Bacterial Biofilms. Langmuir. 2015 doi: 10.1021/la5044156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rao W, Deng ZS, Liu J. A review of hyperthermia combined with radiotherapy/chemotherapy on malignant tumors. Crit Rev Biomed Eng. 2010;38(1):101–116. doi: 10.1615/critrevbiomedeng.v38.i1.80. [DOI] [PubMed] [Google Scholar]
- 19.Zimmerli W, Trampuz A. In: Biomaterial-Associated Infection: A Perspective from the Clinic. Moriarty TF, Zaat SAJ, Busscher HJ, editors. New York: Springer; 2013. pp. 3–24. [Google Scholar]
- 20.Satorius AE, Szafranski J, Pyne D, Ganesan M, Solomon MJ, Newton DW, Bortz DM, Younger JG. Complement c5a generation by staphylococcal biofilms. Shock. 2013;39(4):336–342. doi: 10.1097/SHK.0b013e31828d9324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stewart PS, William Costerton J. Antibiotic resistance of bacteria in biofilms. The Lancet. 2001;358(9276):135–138. doi: 10.1016/s0140-6736(01)05321-1. [DOI] [PubMed] [Google Scholar]
- 22.Weber DJ, Rutala WA. Central line-associated bloodstream infections: prevention and management. Infect Dis Clin North Am. 2011;25(1):77–102. doi: 10.1016/j.idc.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 23.Hotchkiss JH. Lambastiug Louis: Lessons from pasteurization. Genetically Modified Food and the Consumer. 2001;13:51–68. [Google Scholar]
- 24.Milleron RS, Bratton SB. 'Heated' debates in apoptosis. Cell Mol Life Sci. 2007;64(18):2329–2333. doi: 10.1007/s00018-007-7135-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sieradzki K, Chung M, Tomasz A. Role of a sodium-dependent symporter homologue in the thermosensitivity of beta-lactam antibiotic resistance and cell wall composition in Staphylococcus aureus. Antimicrob Agents Chemother. 2008;52(2):505–512. doi: 10.1128/AAC.00504-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shirazi F, Pontikos MA, Walsh TJ, Albert N, Lewis RE, Kontoyiannis DP. Hyperthermia sensitizes Rhizopus oryzae to posaconazole and itraconazole action through apoptosis. Antimicrob Agents Chemother. 2013;57(9):4360–4368. doi: 10.1128/AAC.00571-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Silva Laport M, da Silva MR, Costa Silva C, do Carmo de Freire Bastos M, Giambiagi-deMarval M. Heat-resistance and heat-shock response in the nosocomial pathogen Enterococcus faecium. Curr Microbiol. 2003;46(5):313–317. doi: 10.1007/s00284-002-3828-0. [DOI] [PubMed] [Google Scholar]
- 28.Qoronfleh MW, Streips UN, Wilkinson BJ. Basic Features of the Staphylococcal Heat-Shock Response. Antonie Van Leeuwenhoek International Journal of General and Molecular Microbiology. 1990;58(2):79–86. doi: 10.1007/BF00422721. [DOI] [PubMed] [Google Scholar]
- 29.Pavlovsky L, Younger JG, Solomon MJ. rheology of bacterial biofilms. Soft Matter. 2013;9(1):122–131. doi: 10.1039/C2SM27005F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davis SL, Perri MB, Donabedian SM, Manierski C, Singh A, Vager D, Haque NZ, Speirs K, Muder RR, Robinson-Dunn B, Hayden MK, Zervos MJ. Epidemiology and outcomes of community-associated methicillin-resistant Staphylococcus aureus infection. J Clin Microbiol. 2007;45(6):1705–1711. doi: 10.1128/JCM.02311-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perrone EE, Jung E, Breed E, Dominguez JA, Liang Z, Clark AT, Dunne WM, Burd EM, Coopersmith CM. Mechanisms of methicillin-resistant Staphylococcus aureus pneumonia-induced intestinal epithelial apoptosis. Shock. 2012;38(1):68–75. doi: 10.1097/SHK.0b013e318259abdb. [DOI] [PMC free article] [PubMed] [Google Scholar]