Abstract
Several inflammatory diseases including scleroderma and atopic dermatitis display dermal thickening, epidermal hypertrophy, or excessive accumulation of collagen. Factors that might promote these features are of interest for clinical therapy. We previously reported that LIGHT, a TNF superfamily molecule, mediated collagen deposition in the lungs in response to allergen. We therefore tested whether LIGHT might similarly promote collagen accumulation and features of skin fibrosis. Strikingly, injection of recombinant soluble LIGHT into naïve mice, either subcutaneously or systemically, promoted collagen deposition in the skin, and dermal and epidermal thickening. This replicated the activity of bleomycin, an antibiotic that has been previously used in models of scleroderma in mice. Moreover skin fibrosis induced by bleomycin was dependent on endogenous LIGHT activity. The action of LIGHT in vivo was mediated via both of its receptors, HVEM and LTβR, and was dependent on the innate cytokine TSLP and TGF-β. Furthermore, we found that HVEM and LTβR were expressed on human epidermal keratinocytes, and that LIGHT could directly promote TSLP expression in these cells. We reveal an unappreciated activity of LIGHT on keratinocytes and suggest that LIGHT may be an important mediator of skin inflammation and fibrosis in diseases such as scleroderma or atopic dermatitis.
INTRODUCTION
Fibrosis and thickening of the skin is a characteristic of several inflammatory and autoimmune diseases including scleroderma and atopic dermatitis (Boin and Wigley, 2009; Rosenbloom et al., 2010; Wynn and Ramalingam, 2012; Yamamoto, 2009). Current treatments involve non-selective immunotherapy with corticosteroids, D-penicillamine, methotrexate, or cyclophosphamide, but defining new targets for intervention of fibrosis in the skin is important. Fibrosis is a feature that is shared with other diseases such as severe asthma, and autoimmune diseases like RA, Crohn’s disease, and SLE, but whether there are common molecules that promote clinical symptoms across these syndromes is not clear. Recent studies of thymic stromal lymphopoietin (TSLP) have suggested it may be central to fibrosis in a number of different tissues (Comeau and Ziegler, 2010; Yoo et al., 2005; Ziegler and Artis, 2010). Transgenic expression of TSLP in keratinocytes resulted in spontaneous development of features of atopic dermatitis or scleroderma, and transgenic expression of TSLP in lung epithelial cells promoted lung fibrosis characteristic of severe asthma (Yoo et al., 2005; Zhou et al., 2005). Furthermore, human keratinocytes in the skin of patients with atopic dermatitis or systemic sclerosis/scleroderma express high levels of TSLP, and elevated TSLP is also seen in asthmatics (Alysandratos et al., 2010; Lee et al., 2010; Sano et al., 2013; Shikotra et al., 2012; Yao et al., 2013; Ying et al., 2008). This suggests that there may be shared mechanisms that drive some of the features of these diseases.
In this regard, we showed that TNF superfamily member 14 (TNFSF14), known as LIGHT or CD258 (Ware, 2005, 2009), strongly promoted fibrosis in the lungs of mice chronically challenged with allergen (Doherty et al., 2011). Here, we now show that LIGHT can similarly promote fibrotic features in the skin. Direct injection of recombinant LIGHT into naïve mice promoted dermal and epidermal thickening. Additionally, fibrotic features in the skin were abrogated in LIGHT-deficient mice treated with bleomycin in a mouse model that produces symptoms reminiscent of those exhibited in scleroderma (Yamamoto, 2006; Yamamoto and Nishioka, 2005). Importantly, the action of LIGHT was dependent on activity of TSLP, and also TGF-β, and stimulation of keratinocytes with recombinant LIGHT promoted TSLP expression. These data further the contention that LIGHT may be central to fibrosis in multiple tissues and may be amenable to clinical targeting in diseases of the skin.
Results
Soluble recombinant LIGHT induces features of skin fibrosis
Our prior studies in mice chronically challenged with allergen, in models of asthma, showed that LIGHT was endogenously produced in the lungs and contributed to lung fibrosis and tissue remodeling. Moreover, intratracheal injection of an arbitrary amount of 10 μg of recombinant LIGHT, into acutely allergen-challenged animals, mimicked tissue remodeling in the lungs of chronically allergen-challenged animals (Doherty et al., 2011). We therefore asked whether recombinant LIGHT was able to drive similar fibrotic features in the skin, and whether this could occur in the absence of other inflammatory stimuli. We injected recombinant LIGHT or PBS twice, either subcutaneously (SC) or intratracheally (IT). The latter route was used based on our prior results in the lung studies (Doherty et al., 2011), and with the notion that LIGHT might become systemic and lead to activity in the skin, of possible relevance to the link that has been described in humans between allergic responses in the lungs and skin.
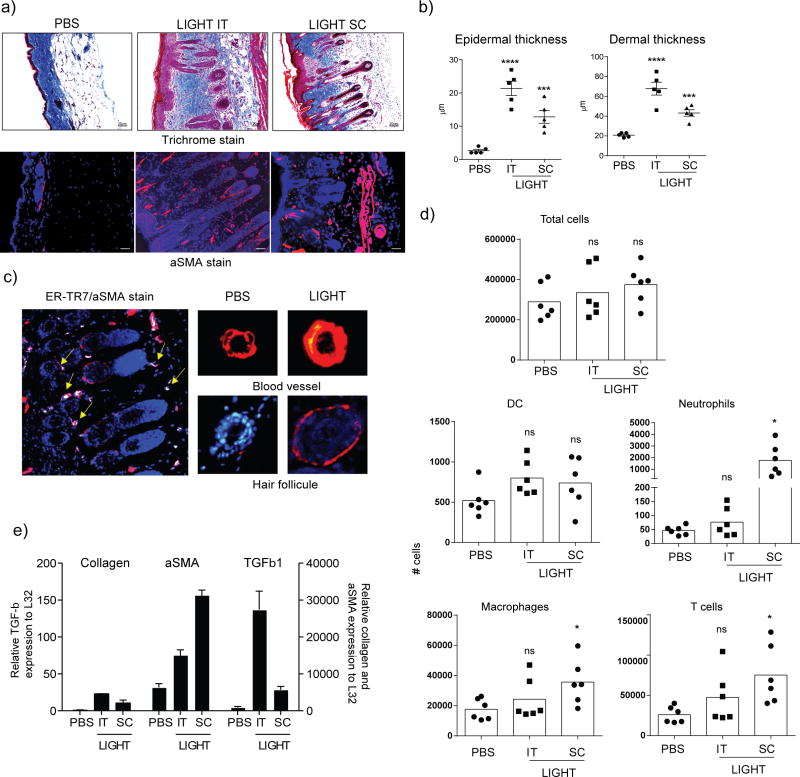
Impressively, LIGHT injection led to collagen deposition in the skin, as assessed with Masson’s trichrome stain, penetrating into the adipose layer, regardless of whether the SC or IT route was used (Fig. 1a). In line with this, we found that total skin collagen, as assessed by sircol assay, was increased by an average of 34% in mice injected with LIGHT when compared to mice injected with PBS (data not shown). Epidermal activity was also obvious with pronounced keratinocyte hypertrophy (Fig. 1a), and quantitation of epidermal and dermal thickening further confirmed a pronounced activity of LIGHT (Fig. 1b). A strong increase in staining of alpha smooth muscle actin (aSMA) was also seen in skin sections, particularly in cells with the morphology of basal keratinocytes in the hair follicles (Fig. 1a and 1c), in line with prior reports that these cells can express aSMA (Jahoda et al., 1991; Latorre et al., 2013; Li et al., 2014). Other cells that were likely myofibroblasts also expressed aSMA, based on staining for the marker ER-TR7 (Fig. 1c). Some vascular changes were additionally observed with the endothelial wall of skin blood vessels also expressing increased aSMA in animals injected with LIGHT (Fig. 1a and 1c). We additionally observed a low level of immune cellular infiltration into the skin in some mice, including macrophages, neutrophils, and T cells, although overall this was only statistically significant when LIGHT was injected subcutaneously (Fig 1d). qPCR analysis confirmed up-regulation of collagen and aSMA transcripts, as well as TGF-β1 that is often associated with fibrotic activity, with both SC and IT injection (Fig. 1e).
Figure 1. Recombinant LIGHT induces skin fibrosis in naïve mice.
Naïve WT mice were injected with 10 μg of soluble rmLIGHT or PBS alone, administered subcutaneously (SC, on the back between the ears) or intratracheally (IT) on days 1 and 2. Skin inflammation and fibrosis were assessed 24hr later on day 3. (a–c) (a) Skin sections (magnification 20x) were stained with Masson’s trichrome (top, blue) or an antibody to aSMA (bottom, red, DAPI blue). Dashed line in immunofluorescent images delineates the basement membrane. (b) Quantification of epidermal and dermal thickening. Values from individual tissues from 5 mice. (c) Higher power images of: Left; LIGHT-injected skin section co-stained with ER-TR7 (green) and aSMA (red). Yellow arrows indicate ER-TR7/aSMA positive cells (white). Right top; Hair follicle in LIGHT-injected skin stained with aSMA (red). Right bottom; blood vessels from PBS or LIGHT-injected skin sections stained for aSMA (red). Scale bar = μm. Data are representative of 6 experiments. (d) Total CD45+ cells, dendritic cells (DC), neutrophils, macrophages, and T cells, were enumerated in skin biopsies that included both dermis and epidermis. Values are from individual tissues from 6 mice. (e) qPCR analysis of skin samples for mRNA of collagen, aSMA, and TGF-β calculated relative to L32. Values are mean ± SEM from 2 to 4 mice per condition. ns, not significant; *p < 0.05; **, p < 0.1; ***, p < 0.01; ****, p < 0.001.
Reduced skin fibrotic activity in LIGHT-deficient mice
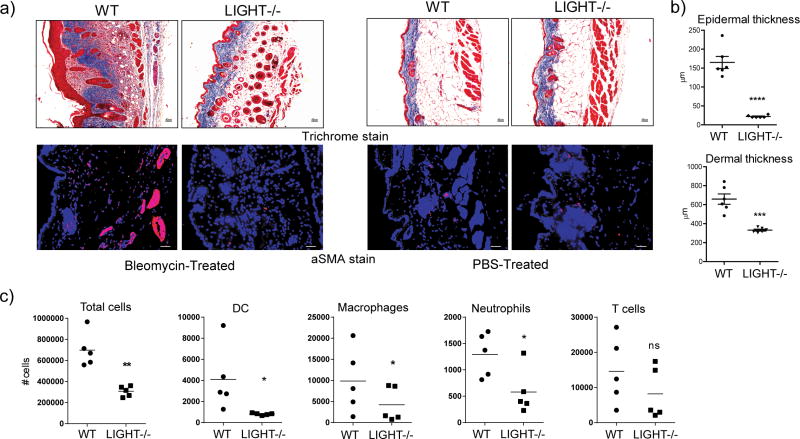
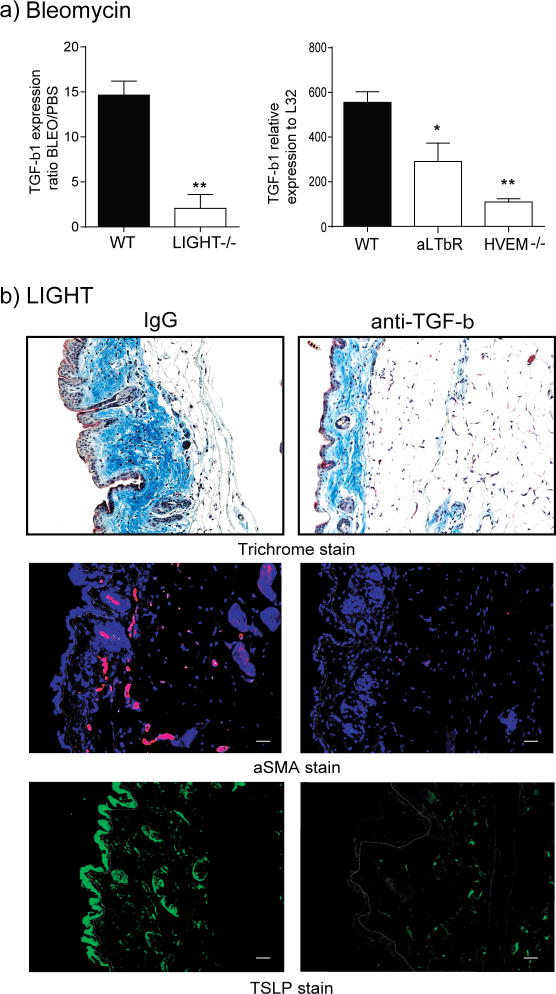
To determine whether endogenous LIGHT activity could contribute to fibrotic features in the skin, we used an established model with the antibiotic bleomycin that produces symptoms in mouse skin that have features in common with skin of patients with scleroderma (Yamamoto, 2006; Yamamoto and Nishioka, 2005). Interestingly, the phenotype in the skin of WT mice promoted by bleomycin was histologically similar to that induced by injection of recombinant LIGHT. Importantly, bleomycin-treated LIGHT−/− mice failed to accumulate significant amounts of collagen with little evidence of trichrome stain in the sub-dermal adipose layer (Fig. 2a). Total skin collagen, based on sircol assay, was increased with a mean of 38% in WT mice given bleomycin over those given PBS, whereas a mean increase of only 13% was seen in LIGHT−/− mice. Alpha smooth muscle actin expression was also not upregulated to any great extent in LIGHT−/− mice (Fig. 2a). Moreover, quantitation of both dermal and epidermal thickening revealed a very pronounced activity of bleomycin in WT mice with markedly less in LIGHT−/− mice (Fig. 2b). Cellular infiltration was moderately reduced in LIGHT-deficient mice compared to WT mice with an overall trend to fewer dendritic cells, macrophages, neutrophils, and T cells, although not statistically significant in all cases (Fig. 2c). This data demonstrates a primary role for endogenously produced LIGHT in driving skin fibrosis, and also substantiates the conclusion that the prior results with injection of recombinant LIGHT into naïve mice (Fig. 1) produced a physiologically relevant effect.
Figure 2. LIGHT-deficient mice exhibit decreased skin fibrosis induced by bleomycin.
WT and LIGHT−/− mice were administered 0.2U bleomycin/mouse. Mice were sacrificed on day 7. (a) Skin fibrosis was assessed by analyzing trichrome (mag. 10x) and aSMA stained sections (mag. 20x). Dashed line delineates the basement membrane. Scale bar = μm. (b) Dermal and epidermal thickness was quantitated. Values from individual tissues from 6 mice. Data are representative of 6 experiments. ***, p < 0.01; ****, p < 0.001. (c) Total CD45+ cells, dendritic cells (DC), neutrophils, macrophages, and T cells, were enumerated in skin samples. Values are from individual tissues from 5 mice.
Both LTβR and HVEM contribute to skin fibrosis and thickening
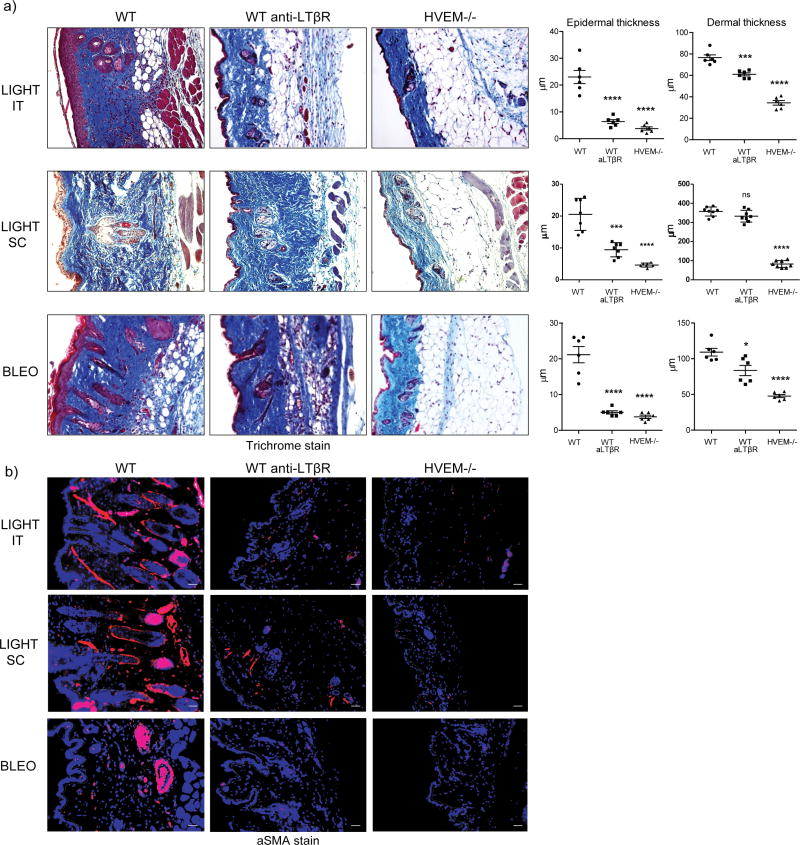
LIGHT binds to two receptors, LTβR/TNFRSF3 and HVEM/TNFRSF14. To understand whether one or both of these molecules were involved, we assessed responses in WT mice treated with an antibody that selectively neutralizes LIGHT-LTβR interactions and also tested responses in HVEM−/− mice. In naïve mice injected with recombinant LIGHT, epidermal thickening and hypertrophy of keratinocytes was almost completely abrogated with deletion of HVEM or with blocking LTβR. In contrast, dermal fibrosis was reduced to a much greater extent in the absence of HVEM interactions. Blocking LIGHT-LTβR still reduced dermal and sub-dermal collagen slightly but to a markedly lesser degree (Fig. 3a). aSMA expression was also reduced when either LIGHT-HVEM or LIGHT-LTβR interaction was neutralized (Fig. 3b). Importantly, almost identical results were obtained in mice treated with bleomycin with epidermal thickening and aSMA accumulation being regulated by both LTβR and HVEM, and dermal thickening more dependent on HVEM (Fig. 3a and 3b).
Figure 3. HVEM and LTβR differentially contribute to skin fibrosis.
WT and HVEM−/− mice were compared to WT mice treated with 200 μg neutralizing anti-LTβR. Mice were either administered 10 μg rmLIGHT SC or IT on days 1 and 2 before sacrificing the mice at day 3, or were given 0.2U bleomycin/mouse and sacrificed on day 7. Fibrotic activity in the skin was assessed as before. (a) Trichrome staining. (b) aSMA staining (mag. 20x). Dashed line delineates the basement membrane. Scale bar = μm. Quantification from individual tissues from 6 mice. Data representative of 2 experiments. ***, p < 0.01; ****, p < 0.001.
LIGHT upregulates TSLP in vivo to promote skin fibrosis
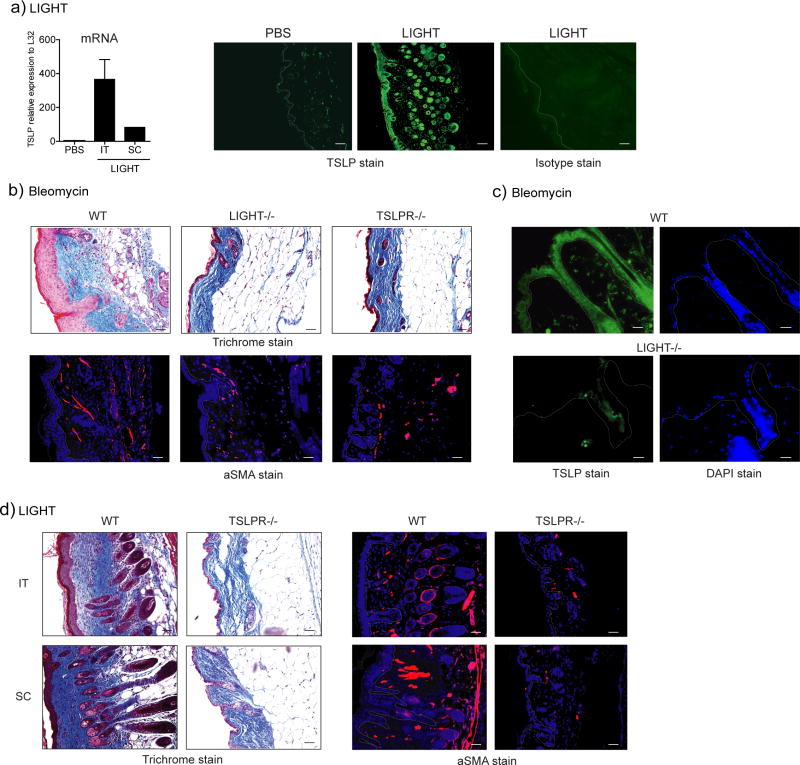
Given the prominent role suggested for TSLP in promoting skin fibrosis when over-expressed in transgenic mice (Yoo et al., 2005) and data that found TSLPR−/− mice did not develop pronounced skin fibrosis induced by subcutaneous injection of bleomycin (Usategui et al., 2013), we determined whether LIGHT might control TSLP expression. Significantly, in naïve mice injected with rLIGHT we found upregulated expression of TSLP mRNA within 2 days in the skin (Fig. 4a). Again, this was regardless of administration SC or IT. Importantly, induction of TSLP protein was easily visualized by immunohistochemistry in the skin, largely expressed in keratinocytes in both the epidermis and hair follicles (Fig. 4a). To extend this, we found a similar lack of the skin fibrotic phenotype in TSLPR−/− mice as observed in LIGHT−/− mice (Fig. 4b), and found a dramatic reduction in TSLP protein in the skin of bleomycin-treated LIGHT−/− mice (Fig. 4c). We further showed that essentially all of the hallmarks of disease, including collagen deposition and dermal and epidermal thickening, as well as aSMA accumulation, were abrogated when LIGHT was injected into TSLPR−/− mice (Fig. 4d).
Figure 4. LIGHT promotes skin fibrosis dependent on TSLP.
(a) WT mice were treated SC or IT with 10 μg of rmLIGHT or PBS on days 1 and 2. TSLP expression was measured in the skin by immunofluorescence (green, mag. 20x, versus Isotype control stain) or PCR on day 3. Dashed line delineates the basement membrane. Scale bar = μm. (b) WT, LIGHT−/−, and TSLPR−/− mice were compared for skin fibrosis after treatment with bleomycin. WT mice were assessed at day 7, whereas gene-deficient mice were analyzed on day 14. WT mice had to be sacrificed before day 14 due to excessive weight loss. (c) WT and LIGHT−/− mice were treated with bleomycin and skin was assessed for TSLP expression on day 7 (green; DAPI blue) (mag. 20x). (d) WT and TSLPR−/− mice were administered 10 μg of rmLIGHT on days 1 and 2 and skin fibrosis assessed on day 3. All data are representative of 4 experiments.
LIGHT synergizes with TGF-β in vivo to promote TSLP expression and skin fibrosis
Recombinant LIGHT promoted TGF-β expression in the skin (Fig. 1). In accordance, we also found reduced TGF-β mRNA levels in the skin of LIGHT−/− mice, WT mice treated with anti-LTβR, and HVEM−/− mice, injected with bleomycin (Fig. 5a). As this cytokine has been implicated in fibrotic activity in many situations, we then neutralized TGF-β signaling in vivo. We observed a significant reduction in collagen deposition and aSMA accumulation, as well as interestingly a lower level of TSLP expression in the skin (Fig. 5b). This suggests that LIGHT and TGF-β synergized to promote TSLP, or LIGHT indirectly promoted TSLP through TGF-β.
Figure 5. TGF-β is required for LIGHT driven skin fibrosis.
(a) WT, LIGHT−/−, HVEM−/−, and WT mice treated with anti-LTβR, were challenged with intratracheal bleomycin, and after 7 days skin expression of TGF-β1 mRNA was assessed. Values are mean ± SEM of 3 to 4 mice per group. (b) WT mice, treated with control IgG or anti-TGF-β, were administered 10 μg of rmLIGHT on days 1 and 2. Skin fibrosis and TSLP expression was assessed as before on day 3. Dashed line delineates the basement membrane. Scale bar = μm. Data are representative of 3 individual mice analyzed per group.
LIGHT directly induces TSLP in keratinocytes independently of TGF-β
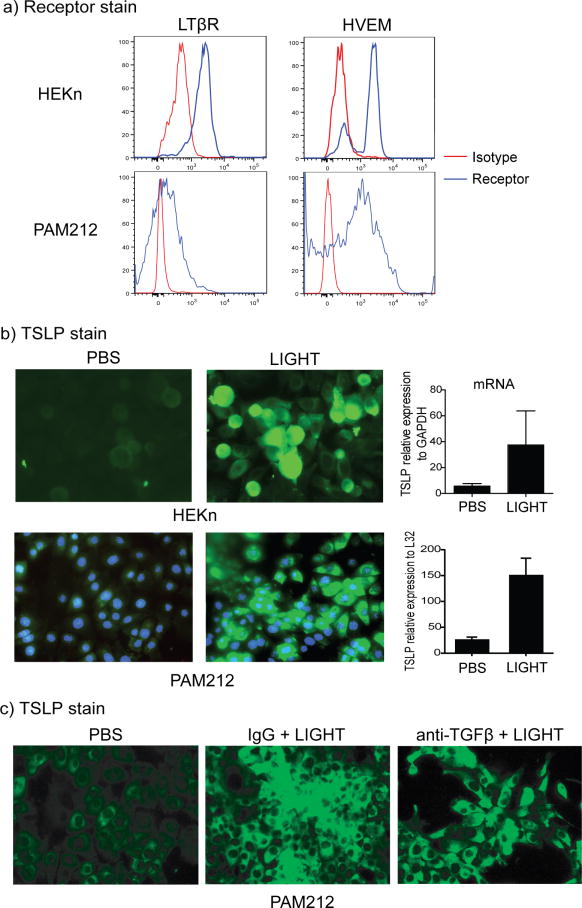
Because the primary source of TSLP in the skin appeared to be keratinocytes, we then asked whether human and mouse keratinocytes expressed the receptors for LIGHT and might directly respond by producing TSLP. Significantly, LTβR and HVEM were constitutively expressed on normal human epidermal keratinocytes and a mouse keratinocyte cell line (Fig. 6a). Furthermore, recombinant human LIGHT or mouse LIGHT strongly induced the expression of TSLP mRNA in the human and mouse keratinocytes, and TSLP protein was also detected by immunofluorescence (Fig. 6b). Moreover, blocking TGF-β in these cultures did not ablate TSLP expression by keratinocytes (Fig. 6c). Together with the in vivo data in Fig. 5, this suggests that LIGHT can both directly induce TSLP from keratinocytes and can also co-operate with TGF-β to further control TSLP production.
Figure 6. LIGHT induces TSLP in keratinocytes.
(a) Human epidermal keratinocytes (HEKn) and mouse PAM212 keratinocytes were stained for LTβR and HVEM expression by flow cytometry. Isotype control in red. (b) HEKn and PAM212 cells were stimulated in vitro with 20 ng/ml of soluble rhLIGHT or rmLIGHT for 72h. TSLP expression was evaluated by immunofluorescence (TSLP, green; DAPI, blue), and by qPCR. Values are mean ± SEM of triplicate samples per condition. (c) PAM212 cells were stimulated as in (b) in the presence of control IgG or anti-TGF-β, and TSLP expression analyzed by IHC. Scale bar = μm. All data are representative of 4 experiments.
Lastly, as LIGHT promoted epidermal thickening in vivo, we asked if it could directly induce keratinocyte proliferation/hyperplasia or hypertrophy. Staining of skin tissue sections for Ki67, a protein associated with cell division, did reveal that some, although not all, epidermal keratinocytes, expressed this molecule in mice injected with rLIGHT (not shown), implying epidermal thickening was likely the result of both hypertrophy and hyperplasia. However, BrdU and 7AAD analysis of keratinocytes did not show any evidence of enhanced proliferation when stimulated with LIGHT in vitro, and no obvious increase in cell size (not shown). As data in TSLPR−/− mice, and with blocking TGF-β showed a lack of epidermal thickening (Figs. 4 and 5), this suggests that LIGHT primarily acted indirectly through these or other cytokines to drive hypertrophy and hyperplasia of keratinocytes in vivo. Nevertheless, we cannot exclude the possibility that direct LIGHT signaling in keratinocytes might promote these activities in synergy with another molecule.
Discussion
Understanding the molecules that directly promote fibrosis or might drive activity of other fibrotic factors may prove useful for future therapeutic approaches. We now show that LIGHT as a soluble molecule can very rapidly induced a fibrotic phenotype in the skin even in the absence of any other stimulus, and can promote the scleroderma- or atopic dermatitis-like phenotype that is characteristic of skin disease driven by bleomycin. Moreover, LIGHT controlled the production of TSLP and TGF-β in the skin and directly induced TSLP expression by keratinocytes.
Our finding that recombinant LIGHT alone could induce fibrosis in the skin, and LIGHT-deficient mice displayed defective skin fibrotic activity, suggest it is an important mediator of the complex inflammatory response that can result in this organ. Moreover, the fact that the activity of LIGHT was in part mediated by controlling TSLP expression provides more evidence of the potential importance of LIGHT in clinical skin disease. TSLP was shown to be a central regulator or inducer of allergic inflammation some time ago, and a strong role of TSLP in the generation of Th2-type responses has been documented in many models (Comeau and Ziegler, 2010; Ziegler, 2010; Ziegler and Artis, 2010). Blocking TSLP, or studies of mice deficient in TSLPR, have shown roles in the development of inflammatory responses in the lung and skin implying commonalities in immune regulation between these tissues, with the primary sources of TSLP being thought to be epithelial cells. In line with this, transgenic mice where TSLP was over-expressed in bronchial epithelium or in keratinocytes exhibited tissue remodeling in those organs (Yoo et al., 2005; Zhou et al., 2005). Furthermore, neutralization of TSLP signaling in a model of atopic dermatitis also resulted in a strong reduction in skin fibrosis (Zhu et al., 2011) and the data here and elsewhere (Usategui et al., 2013) show its important role in promoting skin fibrosis triggered by bleomycin. Given the connection we now show between LIGHT and TSLP, it will then be important in the future to determine if LIGHT is elevated together with TSLP in samples from patients with skin or lung fibrotic disorders. Recently, it was found that TSLP was highly expressed in skin lesions of patients with systemic sclerosis (Usategui et al., 2013), and a GWAS found an association between genetic variants in TSLP and atopic dermatitis (Gao et al., 2010). LIGHT was shown to be upregulated in the plasma of atopic dermatitis patients, and levels were reduced coincident with improvement in clinical dermatitis symptoms in patients treated with topical steroids (Kotani et al., 2012). Additionally, microarray analysis found that LIGHT was upregulated in patients with Alopecia areata, a syndrome to which atopic dermatitis patients have an increased risk (Coda and Sinha, 2011). However, more expression and association data will be essential for substantiating the notion that production of LIGHT is characteristic of human scleroderma or atopic dermatitis.
The sources of LIGHT that drive fibrotic activity are not known at present. Similar to many molecules in the TNF superfamily, LIGHT is not constitutively expressed. It was first described as a product of activated T cells (Mauri et al., 1998), but has also been found to be made by NK cells, dendritic cells, and macrophages in some conditions (Ware, 2005, 2009). LIGHT might also be produced by other, as yet unidentified, cells. Bleomycin induces tissue injury and has been shown to induce modification of self-antigens, such as cleavage of topoisomerase I (Yamamoto, 2006, 2009; Yamamoto and Nishioka, 2005). Moreover, apoptosis is detected in the skin of human systemic sclerosis patients associated with the severity of tissue damage (Yamamoto, 2009; Yamamoto and Nishioka, 2005; Yoshizaki et al., 2010). Therefore, many of the above immune cell types could be activated to produce LIGHT during the development of a skin inflammatory response.
Our results suggest that the action of LIGHT in promoting collagen and alpha smooth muscle actin accumulation in the skin may be largely indirect through enhancing TSLP and TGF-β expression. For LIGHT to be involved in a fibrotic response in the skin, its receptors obviously need to be expressed. T cells, dendritic cells, and stromal cells are well known to express either HVEM and/or LTβR. However, most relevant to fibrosis in the skin may be either resident immune cells, such as dermal dendritic cells, macrophages, and fibroblasts, or as we focus on in this study keratinocytes. By identifying TSLP as a downstream product of LIGHT activity in keratinocytes, and TSLP as being a major mediator of the pro-fibrotic effects of LIGHT in vivo, this then strongly suggests there will be a central role of LTβR or HVEM expressed in the epidermis in contributing to the clinical manifestations of skin fibrosis. Future studies of mice where LTβR or HVEM are conditionally deleted in keratinocytes will however be required to directly show the importance of these molecules on this cell type in vivo.
Our current results also reveal that LIGHT additionally promoted TGF-β expression in the skin, and that TGF-β was essential for the fibrotic activity of LIGHT as well as contributing to the expression of TSLP in vivo. However, LIGHT induction of TSLP from keratinocytes was TGF-β independent, suggesting another source of TGF-β is likely to synergize with LIGHT in vivo. Previous work from our laboratory found that LIGHT could promote TGF-β expression in lung macrophages that was associated with tissue remodeling in the lungs (Doherty et al., 2011). Thus, macrophages in the skin may also be a primary source of TGF-β although other cell types could equally be receptive to LIGHT signals. Fibroblasts can additionally produce TGF-β, and dermal fibroblasts were shown to express HVEM in cutaneous T cell lymphoma patients (Miyagaki et al., 2012), and LTβR expression has been seen on lung fibroblasts or synovial fibroblasts (Ishida et al., 2008; Mackay et al., 1996; Pierer et al., 2007). Therefore, dermal fibroblasts may also be a source of TGF-β directly induced by LIGHT.
Likewise, TSLP may have multiple targets in contributing to skin fibrosis and its action could be both direct and indirect. TSLPR can be expressed on dendritic cells, T cells, natural killer T cells, eosinophils, mast cells, and innate lymphoid cells (Ziegler and Artis, 2010). TSLPR was also found on fibrocytes/fibroblasts, and when stimulated with TSLP they produced collagen. Neutralization of TSLP or genetic deletion of TSLPR in IL-13 transgenic mice also resulted in a significant reduction in the number of fibrocytes and in skin fibrosis (Oh et al., 2011; Zhu et al., 2011). Furthermore, in a model with transgenic expression of TSLP in keratinocytes (Yoo et al., 2005a), upregulation of other molecules that have been found to contribute to fibrotic activity, such as IL-4, IL-13, and eotaxin-2, was TSLP-dependent. Collectively, this suggests that there is likely to be a very complex interplay between many cytokines that ultimately contribute to symptoms associated with disease of the skin, including that between LIGHT, TSLP, and TGF-β.
LIGHT is currently being targeted with a human antibody (SAR252067) in phase II clinical trials for inflammatory bowel disease (Croft et al., 2013). The data here complement our previous studies which suggested that LIGHT might be an attractive target for the therapy of lung fibrosis associated with asthma, and they expand the potential indications for LIGHT neutralization to diseases of the skin that are characterized by fibrosis such as scleroderma and atopic dermatitis.
Material and Methods
Mice
Six- to 8-week-old female WT, LIGHT−/− (from Dr. K. Pfeffer (Scheu et al., 2002)), HVEM−/− (from Dr. K. Pfeffer (Wang et al., 2005)) or TSLPR−/− mice (from Dr. S. Ziegler (Carpino et al., 2004)) were bred in house on the C57BL/6 background. All experiments were in compliance with the regulations of the La Jolla Institute for Allergy and Immunology Animal Care Committee.
Experimental protocols
Mice were given 10 μg of recombinant mouse LIGHT (1794-LT/CF, R&D Systems) or PBS subcutaneously or intratracheally on days 1 and 2 and sacrificed one day later on day 3. Alternatively, mice were challenged with bleomycin (Sigma), 0.2U/mouse, given intratracheally once, and monitored for skin inflammation and fibrosis after 7 or 14 days. For neutralization of LIGHT-LTβR interactions, mice were administered 200 μg of anti-LTβR mAb (LLTB2) (Anand et al., 2006) given i.v. one day prior to injection of bleomycin or rLIGHT and every other day until the end of the experiment. The anti-LTβR mAb (LLTB2) specifically blocks binding of LIGHT to LTβR (Anand et al., 2006). For neutralization of TGF-β, mice were administered 300 μg of anti-TGF-β mAb (1D11, BioXCell) given i.p. one day prior to injection of rLIGHT and every other day thereafter.
Quantitative real-time PCR
Total RNA was isolated using TRIzol (Invitrogen). To further purify RNA from skin samples, we used RNeasy Fibrous Tissue mini kit (Qiagen, 74704). Single-strand cDNA was prepared by reverse transcribing 5 μg of total RNA using Transcriptor First Strand cDNA kit (Roche). Samples were amplified in IQ SYRB Green Supermix (Bio-Rad Laboratories) using the primer pairs: LIGHT: forward, 5′-ACA GCC TTC AGT GTT TGT GGT G-3′; reverse, 5′-TCC GGT GGT TCT GTT CCA G-, Collagen: forward, 5′-GAG CCC TCG CTT CCG TAC TC-3′; reverse, 5′-TGT TCC CTA CTC AGC CGT CTG T-3′, aSMA: forward, 5′-TCT CTA TGC TAA CAA CGT CCT GTCA-3′; reverse, 5′-CCA CCG ATC CAG ACA GAG TAC TT-3′, TGF-β1: forward, 5′-CCC TAT ATT TGG AGC CTG GA-3′; reverse, 5′-GGA AGC TTC GGG ATT TAT GG-3′, muTSLP: forward, 5′-TCG AGG ACT GTG AGA GCA AGC CAG-3′; reverse, 5′-CTG GAG ATT GCA TGA AGG AAT ACC-3′, huTSLP: forward, 5′-TAT GAG TGG GAC CAA AAG TAC CG-3′; reverse, 5′-GGG ATT GAA GGT TAG GCT CTG G-3′. All samples were run in triplicate, and the mean values were used for quantification.
Collagen and skin thickness measurements
Formalin (4% in PBS) fixed skin sections were stained with Masson’s Trichrome to evaluate collagen using an image analysis system (Image-Pro Plus; Media Cybernetics) (Cho et al., 2004). Epidermal thickness as well as dermal thickening was quantitated using Image Pro Analyser 7 software.
Immunohistochemical staining
Skin samples were de-paraffinized by sequential placement in xylene and ethanol. Sections were treated with Fc block in 10% Donkey serum (in PBS) and stained with (i) rabbit polyclonal antibody to alpha smooth muscle actin (clone 1A4, ab7817-abcam) at 1:200 concentration followed by Rhodamine Red-X AffiniPure Donkey anti-rabbit (Jackson Immunoresearch, 711295152) at 1:500; (ii) Goat polyclonal antibody to TSLP (clone L18, Santa Cruz Biotechnology) at 1:200 followed by Donkey anti-goat IgG-FITC (sc-2024, Santa Cruz Biotechnology) at 1:500. Slides were read on a Nikon 80i microscope and analyzed by LSM-Image software.
Analysis of skin immune cell infiltrates
Skin biopsy, including both dermis and epidermis, was minced in the presence of trypsin/dispase, and cellular infiltration was assessed by flow on isolated skin cells using: CD45.2-BV711 (Clone 104; BD), CD3e-BV650 (Clone 145-2C11; BD), Mac3-Alexa Fluor 647 (Clone M4/84; Biolegend), Ly6-G/Ly6-G-Alexa Fluor 700 (Gr-1, clone RB6-8C5; Biolegend), SiglecF-PE-CF594 (Clone E50-2440; BD), CD11b-BB515 (Clone M1/70; BD), and CD11c-Brilliant Violet 785 (Clone N418; Biolegend). Macrophages were gated as CD45+, autofluorescent high, Mac3+, CD11c+ and SiglecF+; Dendritic cells as CD45+, CD11c+, CD11b+; Neutrophils as CD45+, GR1+, CD11b+ and Siglec F−; T cells as CD45+, CD3+.
Stimulation of keratinocytes
Human Epidermal Keratinocytes from neonates (HEKn) (from Dr. Wendy Havran) or a mouse keratinocyte cell line, PAM212, were stimulated with 20 ng/ml of recombinant human LIGHT (R&D, 664-LI/CF) or mouse LIGHT for 72h in Epilife media (Life technologies). TSLP was measured by immunostaining, using anti-hTSLP mAb (clone AF1398, from R&D) or anti-mTSLP (clone 18, Santa Cruz Biotechnology), and qPCR analyses. For TGF-β neutralization, 30 μg/ml of anti-TGF-β mAb (1D11) or its Isotype control were added into culture. LIGHT receptors were visualized using anti-LTβR and anti-HVEM (Biolegend).
Statistical analyses
Statistical analysis was performed using GraphPad Prism software. A nonparametric t test or Mann-Whitney test was used where indicated. A P value < 0.05 was considered statistically significant.
Acknowledgments
We thank Klaus Pfeffer and Steve Ziegler for originally making available mice deficient in LIGHT, HVEM, and TSLPR. This work was supported by NIH grants AI070535 and AI100905 to M.C. This is manuscript number # 1735 from the La Jolla Institute for Allergy and Immunology.
Footnotes
Conflict of interest
All authors concur with submission of the manuscript. The subject material is original research, has not been previously published, and will not be submitted for publication elsewhere while under consideration by the Journal of Investigative Dermatology. There is no conflicting financial interest associated with this manuscript.
References
- Alysandratos KD, Angelidou A, Vasiadi M, et al. Increased affected skin gene expression and serum levels of thymic stromal lymphopoietin in atopic dermatitis. Ann Allergy Asthma Immunol. 2010;105:403–4. doi: 10.1016/j.anai.2010.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand S, Wang P, Yoshimura K, et al. Essential role of TNF family molecule LIGHT as a cytokine in the pathogenesis of hepatitis. Journal of Clinical Investigation. 2006;116:1045–51. doi: 10.1172/JCI27083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boin F, Wigley F. Connective tissue diseases: Immunosuppressive therapy in SSc: what is the target? Nat Rev Rheumatol. 2009;5:357–8. doi: 10.1038/nrrheum.2009.108. [DOI] [PubMed] [Google Scholar]
- Carpino N, Thierfelder WE, Chang MS, et al. Absence of an essential role for thymic stromal lymphopoietin receptor in murine B-cell development. Mol Cell Biol. 2004;24:2584–92. doi: 10.1128/MCB.24.6.2584-2592.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho JY, Miller M, Baek KJ, et al. Inhibition of airway remodeling in IL-5-deficient mice. Journal of Clinical Investigation. 2004;113:551–60. doi: 10.1172/JCI19133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coda AB, Sinha AA. Integration of genome-wide transcriptional and genetic profiles provides insights into disease development and clinical heterogeneity in alopecia areata. Genomics. 2011;98:431–9. doi: 10.1016/j.ygeno.2011.08.009. [DOI] [PubMed] [Google Scholar]
- Comeau MR, Ziegler SF. The influence of TSLP on the allergic response. Mucosal Immunol. 2010;3:138–47. doi: 10.1038/mi.2009.134. [DOI] [PubMed] [Google Scholar]
- Croft M, Benedict CA, Ware CF. Clinical targeting of the TNF and TNFR superfamilies. Nat Rev Drug Discov. 2013;12:147–68. doi: 10.1038/nrd3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty TA, Soroosh P, Khorram N, et al. The tumor necrosis factor family member LIGHT is a target for asthmatic airway remodeling. Nat Med. 2011;17:596–603. doi: 10.1038/nm.2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao PS, Rafaels NM, Mu D, et al. Genetic variants in thymic stromal lymphopoietin are associated with atopic dermatitis and eczema herpeticum. J Allergy Clin Immunol. 2010;125:1403–7 e4. doi: 10.1016/j.jaci.2010.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida S, Yamane S, Ochi T, et al. LIGHT induces cell proliferation and inflammatory responses of rheumatoid arthritis synovial fibroblasts via lymphotoxin beta receptor. J Rheumatol. 2008;35:960–8. [PubMed] [Google Scholar]
- Jahoda CA, Reynolds AJ, Chaponnier C, et al. Smooth muscle alpha-actin is a marker for hair follicle dermis in vivo and in vitro. J Cell Sci. 1991;99 (Pt 3):627–36. doi: 10.1242/jcs.99.3.627. [DOI] [PubMed] [Google Scholar]
- Kotani H, Masuda K, Tamagawa-Mineoka R, et al. Increased plasma LIGHT levels in patients with atopic dermatitis. Clinical and experimental immunology. 2012;168:318–24. doi: 10.1111/j.1365-2249.2012.04576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latorre V, Sevilla LM, Sanchis A, et al. Selective ablation of glucocorticoid receptor in mouse keratinocytes increases susceptibility to skin tumorigenesis. J Invest Dermatol. 2013;133:2771–9. doi: 10.1038/jid.2013.255. [DOI] [PubMed] [Google Scholar]
- Lee EB, Kim KW, Hong JY, et al. Increased serum thymic stromal lymphopoietin in children with atopic dermatitis. Pediatr Allergy Immunol. 2010;21:e457–60. doi: 10.1111/j.1399-3038.2009.00919.x. [DOI] [PubMed] [Google Scholar]
- Li M, Ti D, Han W, et al. Microenvironment-induced myofibroblast-like conversion of engrafted keratinocytes. Sci China Life Sci. 2014;57:209–20. doi: 10.1007/s11427-014-4613-6. [DOI] [PubMed] [Google Scholar]
- Mackay F, Majeau GR, Hochman PS, et al. Lymphotoxin beta receptor triggering induces activation of the nuclear factor kappaB transcription factor in some cell types. J Biol Chem. 1996;271:24934–8. doi: 10.1074/jbc.271.40.24934. [DOI] [PubMed] [Google Scholar]
- Mauri DN, Ebner R, Montgomery RI, et al. LIGHT, a new member of the TNF superfamily, and lymphotoxin alpha are ligands for herpesvirus entry mediator. Immunity. 1998;8:21–30. doi: 10.1016/s1074-7613(00)80455-0. [DOI] [PubMed] [Google Scholar]
- Miyagaki T, Sugaya M, Suga H, et al. Low herpesvirus entry mediator (HVEM) expression on dermal fibroblasts contributes to a Th2-dominant microenvironment in advanced cutaneous T-cell lymphoma. J Invest Dermatol. 2012;132:1280–9. doi: 10.1038/jid.2011.470. [DOI] [PubMed] [Google Scholar]
- Oh MH, Oh SY, Yu J, et al. IL-13 induces skin fibrosis in atopic dermatitis by thymic stromal lymphopoietin. J Immunol. 2011;186:7232–42. doi: 10.4049/jimmunol.1100504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierer M, Brentano F, Rethage J, et al. The TNF superfamily member LIGHT contributes to survival and activation of synovial fibroblasts in rheumatoid arthritis. Rheumatology (Oxford) 2007;46:1063–70. doi: 10.1093/rheumatology/kem063. [DOI] [PubMed] [Google Scholar]
- Rosenbloom J, Castro SV, Jimenez SA. Narrative review: fibrotic diseases: cellular and molecular mechanisms and novel therapies. Ann Intern Med. 2010;152:159–66. doi: 10.7326/0003-4819-152-3-201002020-00007. [DOI] [PubMed] [Google Scholar]
- Sano Y, Masuda K, Tamagawa-Mineoka R, et al. Thymic stromal lymphopoietin expression is increased in the horny layer of patients with atopic dermatitis. Clinical and experimental immunology. 2013;171:330–7. doi: 10.1111/cei.12021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheu S, Alferink J, Potzel T, et al. Targeted disruption of LIGHT causes defects in costimulatory T cell activation and reveals cooperation with lymphotoxin beta in mesenteric lymph node genesis. Journal of Experimental Medicine. 2002;195:1613–24. doi: 10.1084/jem.20020215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shikotra A, Choy DF, Ohri CM, et al. Increased expression of immunoreactive thymic stromal lymphopoietin in patients with severe asthma. J Allergy Clin Immunol. 2012;129:104–11. e1–9. doi: 10.1016/j.jaci.2011.08.031. [DOI] [PubMed] [Google Scholar]
- Usategui A, Criado G, Izquierdo E, et al. A profibrotic role for thymic stromal lymphopoietin in systemic sclerosis. Ann Rheum Dis. 2013;72:2018–23. doi: 10.1136/annrheumdis-2012-202279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Subudhi SK, Anders RA, et al. The role of herpesvirus entry mediator as a negative regulator of T cell-mediated responses. Journal of Clinical Investigation. 2005;115:711–17. doi: 10.1172/JCI200522982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware CF. Network communications: lymphotoxins, LIGHT, and TNF. Annual Review of Immunology. 2005;23:787–819. doi: 10.1146/annurev.immunol.23.021704.115719. [DOI] [PubMed] [Google Scholar]
- Ware CF. Targeting the LIGHT-HVEM pathway. Adv Exp Med Biol. 2009;647:146–55. doi: 10.1007/978-0-387-89520-8_10. [DOI] [PubMed] [Google Scholar]
- Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med. 2012;18:1028–40. doi: 10.1038/nm.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T. The bleomycin-induced scleroderma model: what have we learned for scleroderma pathogenesis? Arch Dermatol Res. 2006;297:333–44. doi: 10.1007/s00403-005-0635-z. [DOI] [PubMed] [Google Scholar]
- Yamamoto T. Scleroderma--pathophysiology. Eur J Dermatol. 2009;19:14–24. doi: 10.1684/ejd.2008.0570. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Nishioka K. Cellular and molecular mechanisms of bleomycin-induced murine scleroderma: current update and future perspective. Exp Dermatol. 2005;14:81–95. doi: 10.1111/j.0906-6705.2005.00280.x. [DOI] [PubMed] [Google Scholar]
- Yao W, Zhang Y, Jabeen R, et al. Interleukin-9 is required for allergic airway inflammation mediated by the cytokine TSLP. Immunity. 2013;38:360–72. doi: 10.1016/j.immuni.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying S, O’Connor B, Ratoff J, et al. Expression and cellular provenance of thymic stromal lymphopoietin and chemokines in patients with severe asthma and chronic obstructive pulmonary disease. J Immunol. 2008;181:2790–8. doi: 10.4049/jimmunol.181.4.2790. [DOI] [PubMed] [Google Scholar]
- Yoo J, Omori M, Gyarmati D, et al. Spontaneous atopic dermatitis in mice expressing an inducible thymic stromal lymphopoietin transgene specifically in the skin. J Exp Med. 2005;202:541–9. doi: 10.1084/jem.20041503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizaki A, Yanaba K, Iwata Y, et al. Treatment with rapamycin prevents fibrosis in tight-skin and bleomycin-induced mouse models of systemic sclerosis. Arthritis Rheum. 2010;62:2476–87. doi: 10.1002/art.27498. [DOI] [PubMed] [Google Scholar]
- Zhou B, Comeau MR, De Smedt T, et al. Thymic stromal lymphopoietin as a key initiator of allergic airway inflammation in mice. Nature immunology. 2005;6:1047–53. doi: 10.1038/ni1247. [DOI] [PubMed] [Google Scholar]
- Zhu Z, Oh MH, Yu J, et al. The Role of TSLP in IL-13-induced atopic march. Sci Rep. 2011;1:23. doi: 10.1038/srep00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler SF. The role of thymic stromal lymphopoietin (TSLP) in allergic disorders. Curr Opin Immunol. 2010;22:795–9. doi: 10.1016/j.coi.2010.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler SF, Artis D. Sensing the outside world: TSLP regulates barrier immunity. Nature immunology. 2010;11:289–93. doi: 10.1038/ni.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]