Abstract
The precise molecular mechanisms underlying priapism associated with sickle cell disease remain to be defined. However, there is increasing evidence that up-regulated activity of the opiorphin and adenosine pathways in corporal tissue, resulting in heighted relaxation of smooth muscle, play an important role in development of priapism. A key enzyme in the adenosine pathway is CD73, an ecto-5-prime-nucleotidase (5-prime-ribonucleotide phosphohydrolase; EC 3.1.3.5) which catalyzes the conversion of adenosine mononucleotides to adenosine. In the present study we investigated how sickle cell disease and hypoxia regulate the interplay between opiorphin and CD73. In the corpora of sickle cell mice we observed significantly elevated expression of both the mouse opiorphin homologue mSmr3a (14-fold) and CD73 (2.2-fold) relative to non-sickle cell controls at a life-stage prior to the exhibition of priapism. Sickle cell disease has a pronounced hypoxic component, therefore we determined if CD73 was also modulated in in vitro corporal smooth muscle (CSM) models of hypoxia. Hypoxia significantly increased CD73 protein and mRNA expression by 1.5-fold and 2-fold, respectively. We previously demonstrated that expression of another component of the adenosine signaling pathway, the adensosine 2B receptor, can be regulated by sialorphin (the rat opiorphin homolologue), and we demonstrate that sialorphin also regulates CD73 expression in a dose and time dependent fashion. Using siRNA to knock-down sialorphin mRNA expression in CSM cells in vitro, we demonstrate that the hypoxic up-regulation of CD73 is dependent on the up-regulation of sialorphin. Overall our data provides further evidence to support a role for opiorphin in CSM in regulating the cellular response regulating response to hypoxia or sickle cell disease by activating smooth muscle relaxant pathways.
Introduction
Priapism is a prolonged penile erection which can result in tissue damage and erectile dysfunction. The condition is often associated with patients with sickle cell disease, where 29-42% of patients report a priapic episode during their life time (1). The molecular mechanisms involved in the development of priapism are complex and poorly understood which to date has prevented the development of any effective pharmacological intervention for its prevention or treatment. Understanding the association between different pathways resulting in priapism may lead to novel pharmacological strategies for its treatment.
Penile erection is a neurovascular process in which relaxation of the corporal smooth muscle (CSM) tissue allows increased blood flow into the penis (2). Erectile dysfunction (ED) results when the biochemical pathways mediating CSM relaxation are perturbed, and priapism results when relaxant pathways are excessively activated. Pioneering work conducted in 1992 established a central role for nitric oxide (NO) as a physiologic mediator of penile erection (3). Although NO remains recognized as the principal mediator of CSM relaxation (3, 4), several other signaling pathways are known to play a role in relaxing of CSM and thereby are potentially involved in erectile function and priapism. Recently, adenosine (5) and opiorphin (6, 7) signaling pathways have received attention as possible mediators of excessive CSM relaxation that result in priapism.
Adenosine has been implicated in the regulation of penile erection almost as long as NO (8). The adenosine signaling pathways were shown to potentially play a role in priapism when mice lacking adenosine deaminase (ADA), an enzyme necessary for the breakdown of adenosine, displayed unexpected priapic activity (9). In mouse models which mimic the priapic condition associated with human sickle cell disease, mouse corporal tissue exhibited elevated adenosine levels and adenosine 2B receptor (A2B) expression(9). It has recently been suggested that excess adenosine A2B receptor signaling both in sickle cell and ada–/– mice models contributes to priapism through a mechanism in which there is Hif-1a mediated reduction of PDE5 gene expression (10).
Adenosine is derived from extracellular adenine nucleotides which are dephosphorylated by an ectonucleotidase (CD39), which hydrolyzes ATP to ADP and ADP to AMP. CD73 an ecto-5′-nucleotidase (also known as 5-prime-ribonucleotide phosphohydrolase; EC 3.1.3.5) then catalyzes the dephosphorylation of AMP to adenosine and is generally the rate-limiting step in the formation of adenosine from extracellular adenine nucleotides. Histological analysis has demonstrated that CD73 is widely expressed in penile tissue, with remarkably high levels in the nerve bundles, smooth muscle, and endothelium (11). Additional genetic and pharmacological studies demonstrate that CD73-mediated adenosine production contributes to the initiation and maintenance of penile erection following cavernous nerve stimulation(11).
In contrast to adenosine and NO, evidence for the involvement of opiorphins in penile erection is relatively recent. The opiorphins were shown to be amongst the most down-regulated genes in corporal tissue from animal models of ED (12, 13) and are also down regulated in the corpora of patients with ED (14). Overexpression of opiorphins by gene transfer has been shown to cause a priapic-like condition in rat models and the opiorphin genes of mice are significantly up-regulated in the corporal tissue of sickle cell mice compared to controls (6). It was recently proposed that up-regulation of opiorphins in corporal tissue might occur as a response to low levels of oxygen resulting from sickle cell disease (7). Although the opiorphins can directly mediate CSM relaxation (15) they also appear to be involved in regulation of gene expression that may be involved in smooth muscle relaxation pathways, such as HIF-1a, A2BR(7), G-protein coupled receptors and members of the ornithine decarboxylase pathway (6, 16).
Given that both opiorphin and CD73 appear to play an important role in development of priapism in the present study, we studied their potential inter-relationship and factors that may regulate their expression in sickle cell disease.
Materials and Methods
Animals, cell isolation and culture
Five-week old Berk transgenic sickle cell mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). They were maintained in the Albert Einstein Animal Facility till the life stage to be investigated (5 and 12 weeks old). Age-matched C57BL/6 animals were used as controls. Numbers of animals for each experiment are described in figure legends.
For preparation of CSM cells, corpus cavernosum tissue was harvested from 8-week-old male Sprague-Dawley rats (Charles River, Wilmington, MA, USA) under euthanasia. After dissection and removal of the urethra, the corpus cavernosum tissues were obtained excluding the glans, cartilaginous portion of the penis, and the part of overlying ischiocavernous muscle. The corpus cavernosum tissue was washed in phosphate-buffered saline (PBS) several times. The sample was cut into fragments. The tissue fragments were then placed in culture dish and incubated for 10 days. The sprouting cells were subcultured in Dulbecco's modified eagle medium (DMEM) containing 10% fetal bovine serum (FBS), 100 U/ml penicillin, 100 g/ml streptomycin. All animal studies were approved by the Institute of Animal Studies at Albert Einstein College of Medicine.
Chemicals
Sialorphin (Sigma, St. Louis, USA) was dissolved in H2O at concentration of 10 mM and stored at −20°C until use.
Establishment of in vitro hypoxic conditions
In order to cause in vitro chemical hypoxia CoCl2 was added to DMEM to a final concentration of 100 μMol as previously described (17, 18). Hypoxic conditions due to low oxygen levels was induced by culture of CSM cells in 1% O2, 5% CO2, and 94% N2 in a 95% humidified atmosphere as previously described (19). Control cells were kept under normoxic conditions (20% O2). In other experiments sialorphin inhibitor was added to the media at the concentrations and for the time indicated in the figure legends.
Immunoblotting analysis
Cells were washed with cold PBS and lysed in lysis buffer (Cell Signaling) using a standard protocol. The protein concentration was analyzed by a BCA protein assay kit with bovine serum albumin standards according to the manufacturer's instructions (Thermo,MA,U.S.A). Cell lysate was separated by SDS-PAGE and transferred onto a nitrocellulose membrane (Hybond-C, Amersham Pharmacia Biotech, Inc., Piscataway, NJ). Following blocking with PBS-Tween-20 containing 5% nonfat dry milk for 1 h, membranes were incubated overnight at 4°C with anti–CD73 antibody(Cell Signaling Technology, Denvers, MA, USA) followed by incubation with horseradish peroxidase– conjugated secondary antibody. GAPDH (Fitzgerald, Acton, MA, USA) was used as loading control. Immunoreactive bands were detected by an enhanced chemiluminescence kit. (PerkinElmer, Waltham, MA, USA). The densitometry results were normalized by GAPDH expression and finally normalized by control. The intensities of the resulting bands were quantified using the software ImageJ, version 1.47q (National Institutes of Health, Bethesda, MD).
Quantitative RT-PCR
The total RNA was extracted from frozen tissue with TRIzol according to the manufacturer's instruction. Briefly, ≈ 50 mg tissue was added to 1 mL of TRIzol reagent and homogenized using a polytron homogenizer (Brinkman, Westbury, NY, USA) for 30 s. The homogenized tissues were incubated for 5 min at room temperature followed by adding 200 μL of chloroform. After mixing, the aqueous phases were separated by centrifugation (12,000 g for 15 min) at 4°C and then were transferred to a clean tube. The RNA was precipitated from the aqueous phase by addition of isopropyl alcohol and pelleted by centrifugation at 12,000g for 10 min at 4°C, washed once with 75% ethanol, and again pelleted at 7,500 g for 5 min. Then ethanol was aspirated and the RNA pellet was dissolved in sterile water; 4 μg of total RNA was reverse-transcribed to first-strand cDNA primed with Oligo(dT) using the Superscript (Invitrogen) First-Strand Synthesis System for real-time PCR. RNA was denatured for 5 min at 65°C and immediately cooled on ice. Then RNA was combined with reaction buffer and DTT for min at 42°C, then RNA was combined with Superscript II RT. cDNA was synthesized for 50 min at 42°C, then terminate the reaction at 70 for 15min and chill the samples on ice. RT products then were amplified using Sybr Green 2 ×PCR Master Mix (PE Applied Biosystems, Warrington, UK). Real-time quantitative PCR analysis was performed using the 7300 real-time PCR system (Applied Biosystems, Foster City, CA, USA). The primers used are described in Table 1. The PCR reactions for all samples were performed in 96-well plates, with 2 μ L cDNA, 100 n M of each primer, and 12.5 μ L of Sybr Green in a 25- μ L reaction volume. The cycling conditions were: activation of Sybr Green DNA polymerase at 95°C for 10 min, 40 cycles of denaturation at 95°C for 15 s, annealing/ extension at 60°C for 1 min. Results from real-time PCR were presented as threshold cycles normalized to that of the housekeeping gene RPL24. The expression of transcripts was analyzed by the comparative crossing threshold (C t) method (also known as the 2 −(ΔΔ)Ct method).
Table 1.
Primers used in studies.
| Rat Gene | Forward primer | Reverse Primer |
|---|---|---|
| vcsa1 | GGGCTACCAAAGATGAAGTC | TGCCACCACCTTCAAAAATA |
| Cd73 | AACGGTGTGGAAGGACTGAT | CACCACCGACAGAGAGAACT |
| rpl24 | TCGAGCTGTGCAGTTTTAGTGG | GCGGACTCACATTTGGCATTA |
| Mouse Genes | ||
| mSmr3a | CTCCTACCGGACCTCCTACCACA | GGCAGCTGGCATTGTAGTTGCTTG |
| mSmr2 | CTC AAC CAG ACA ATA TCC CC | GGC AGC TGG TGT TGC ATT TG |
| CD73 | ACGGTGTGGAAGGACTGATT | TGTTTGCGCTCAGAATTGGA |
| rpl19 | TCGCCAATGCCAACTCCCGT | GGCCAGGGTGTTTTTCCGGC |
Results
In corporal tissue of sickle cell mice there is up-regulation of genes encoding the mouse opiorphin homologues and CD73
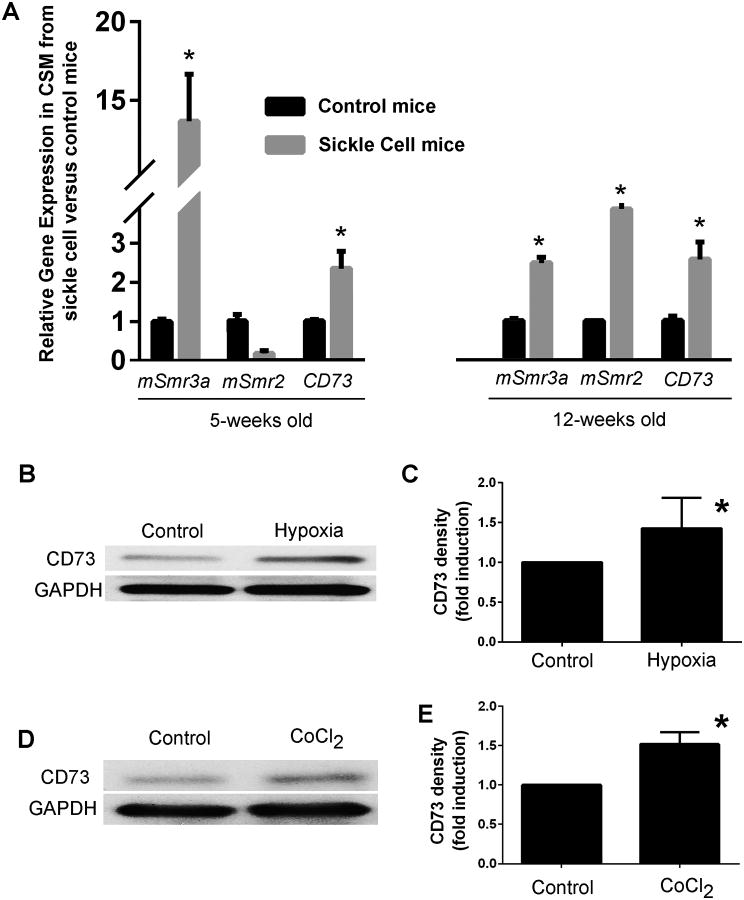
In order to understand the co-regulation of opiorphins and adenosine pathways we compared gene expression of the mouse opiorphin homologues (mSmr3a and mSmr2) with CD73 in the corpora of sickle cell mice to controls at a life stage which does not exhibit any physiological evidence of priapism (5-weeks old), and at a later life stage (12-weeks) when mice have been shown to present physiological evidence of priapism (6) (Figure 1A). There was up-regulation of the opiorphin homologue mSmr3a and CD73 (14-fold and 2.2-fold, respectively) in the corporal tissue of a 5-week old sickle cells animal compared to a control, and in a 12 week old animal there is up-regulation of both mouse opiorphins homologues (mSmr3a and mSmr2) and CD73 (2.5-fold, 4.1-fold and 2.6-fold, respectively).
Figure 1. (A) Upregulation of genes encoding the mouse opiorphin homologues and CD73 in corporal tissue of sickle cell mice.
Expression profiles of mSmr3a, mSmr2, and CD73 in corporal tissue in control and sickle mice at the age of 5 weeks and 12 weeks, normalized to the house-keeping gene rpl19. Bars represent the mean change in gene expression in sickle cell mice compared to controls. 5 animals were used in each age group. *=P<0.05 significantly increased fold-change in gene expression compared to non-sickle cell control. (B) A representative immunoblot to analyze protein expression of CD73 in CSM cells incubated for 24 hours in low oxygen tension (1% O2, 5% CO2, and 94% N2, hypoxia) or normoxic conditions (20% O2, control). (C) Densitometric analysis was performed from three separate experiments to determine expression of CD73 and GAPDH. Expression of CD73 was normalized to the housekeeping gene GAPDH. The mean fold-change (± Std. Dev.) in expression of CD73 caused by hypoxia relative to the non-hypoxic controls are shown. *=P<0.05 significantly increased fold-change in protein expression compared to control. (D) A representative immunoblot is shown for analysisof CD73 protein expression in rat CSM cells with or without 100 μM CoCl2 incubation for 24 hours. (E) Densitometric analysis was performed from three separate experiments to determine expression of CD73 and GAPDH. Expression of CD73 was normalized to the housekeeping gene GAPDH. The mean fold-change (± Std. Dev.) in expression of CD73 caused by CoCl2-induced hypoxia relative to the non-hypoxic controls are shown. *=P<0.05 significantly increased fold-change in protein expression compared to control.
Sickle cell disease can be viewed as a disease of hypoxia (20). We have previously shown that in two in vitro models of hypoxia (low oxygen tension and chemical treatment with CoCl2) the rat opiorphin homologue (sialorphin) is up-regulated in rat CSM cells. We extended these studies to demonstrate that hypoxia also regulates CD73 expression in rat CSM cells in vitro. As shown in Figure 1B and 1C, both types of hypoxia caused a significant increase in the level of CD73 protein expressed, and as shown in Figure 3B there was a significant increase in CD73 mRNA when cells were incubated with CoCl2 to mimic hypoxia.
Figure 3. Up regulation of CD73 expression by hypoxia is dependent on sialorphin.
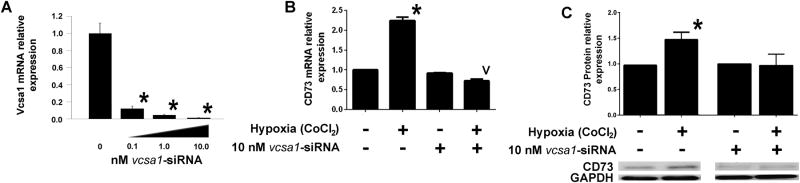
(A) In preliminary experiments CSM cells were treated for 48 hours with different concentrations of vcsa1-siRNA for 48 hours and expression of vcsa1 determined by quantitative-RT-PCR and expressed as fold change relative to the untreated cells. All concentrations gave a significant decrease in vcsa1 expression (*= P<0.05). (B) CSM cells were pretreated for 48 hours with 10 nM control-siRNA or 10 nM vcsa1-siRNA, and cells were then exposed for 8 hours to CoCl2 to mimic hypoxia. Gene expression of CD73 and rpl24 was determined in the presence or absence of CoCl2, in CSM cells pre-treated with control-siRNA or vcsa1-siRNA. Three separate experiments were performed with each gene being measured in triplicate. Data were normalized to the house keeping gene, rpl24, and expressed relative to the non-hypoxic CSM cells treated with control-siRNA. Data is expressed as mean ± Std. Dev. *=P<0.05 significantly increased, v= P<0.05 significantly decreased fold-change in gene expression compared to control (non-hypoxic CSM cells treated with control-siRNA). (C, lower panel) A representative immunoblot for the expression of CD73 and GAPDH. (C, upper panel) Densitometric analysis was performed from three separate experiments to determine expression of CD73 and GAPDH. Expression of CD73 was normalized to the housekeeping gene, GAPDH. The mean fold-change (± Std. Dev.) in expression of CD73 was compared between cells in hypoxic conditions with non-hypoxic cells for each of the treatment conditions *=P<0.05 significantly increased fold-change in protein expression compared to non-hypoxic controls.
Sialorphin regulates CD73 expression in a dose and time dependent manner
We have previously shown that sialorphin can modulate expression of several genes involved in regulation of smooth muscle tone (6, 7). Therefore we investigated if sialorphin might also regulate CD73 expression. We analyzed both the temporal and dose dependence effect of sialorphin on CD73 expression in rat CSM. As shown in Figure 2A, doses of sialorphin from 100-800 nM significantly increased CD73 expression in rat CSM cells. In Figure 2B, 400 nM sialorphin significantly increased CD73 expression in a time-dependent manner (determined at 2,4 and 8 hours) and reached a maximum at 8 hours.
Figure 2. Sialorphin regulates expression of CD73 in a dose and time-dependent manner.
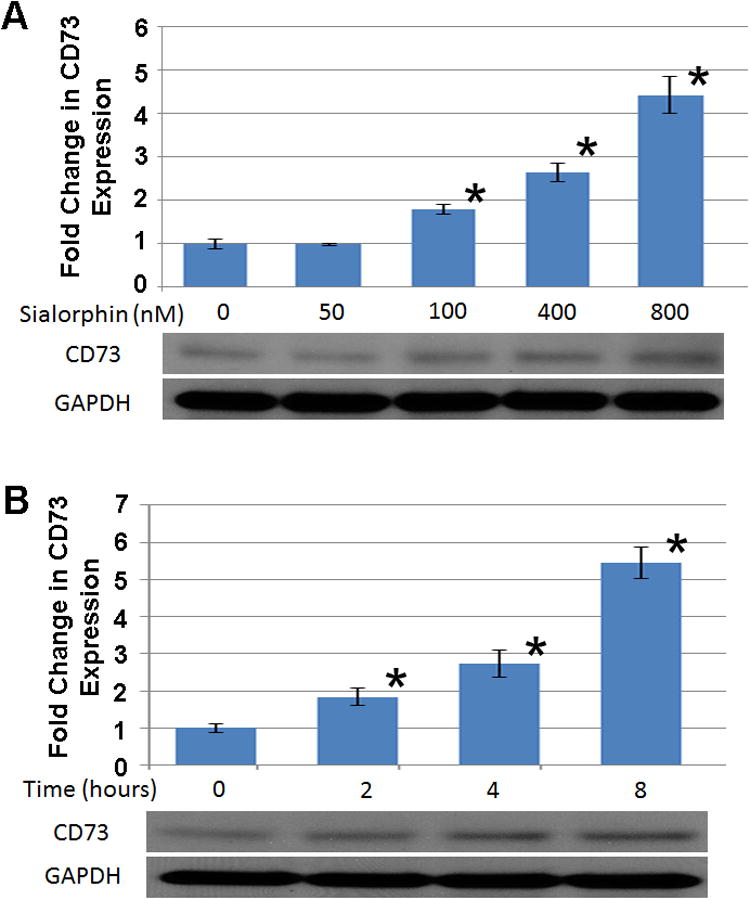
(A, lower panel) A representative immunoblot to analyze CD73 protein expression in rat CSM after 8 hours incubation with increasing concentrations of sialorphin. (A, upper panel) Densitometry analysis of ratio of CD73 to GAPDH protein expression with increasing sialorphin concentrations. Densitometric analysis was performed from three separate experiments to determine expression of CD73 and GAPDH. Expression of CD73 was normalized to the housekeeping gene GAPDH. The mean fold-change (± Std. Dev.) in expression of CD73 caused by increasing sialorphin concentration relative to untreated controls are shown. *=P<0.05 significantly increased fold-change in protein expression compared to untreated control. (B, lower panel) A representative immunoblot to analyze time dependent protein expression ofCd73 in rat corporal cells with 400 nM sialorphin. (B, upper panel) Densitometric analysis was performed from three separate experiments to determine expression of CD73 and GAPDH. Expression of CD73 was normalized to the housekeeping gene GAPDH. The mean fold-change (± Std. Dev.) in expression of CD73 at different time points, compared to protein expression at baseline (T=0), are shown. *=P<0.05 significantly increased fold-change in protein expression compared to baseline.
Hypoxic upregulation of CD73 expression is dependent on sialorphin expression
We have previously used siRNA to knock-down the gene encoding sialorphin (vcsa1) expression in CSM cells (7, 16). We demonstrate in Figure 3A incubation of CSM with increasing amounts of vcsa1-siRNA causes significant down-regulation of vcsa1, such that 10 nM vcsa1-siRNA reduced expression by >97% of normal levels. In order to determine that the hypoxic up-regulation of CD73 is dependent on sialorphin, we first knocked-down vcsa1 expression and then incubated cells in hypoxic conditions simulated by CoCl2. As shown in Figure 3B and 3C pre-incubation of CSM cells with 10 nM vcsa1-siRNA to knock-down vcsa1 expression for 48 hours prior to exposure to hypoxic conditions for 8 hours prevents the up-regulation of both CD73 mRNA (Figure 3B) and protein (Figure 3C).
Discussion
For the first time we show that both mouse opiorphin homologues and CD73 expression are up-regulated in the CSM of sickle cell mice at life-stages both prior to and during the exhibition of priapism. Sickle cell disease is a disease of hypoxia; because of insufficient numbers of erythrocytes for oxygen delivery, sickle cell patients constantly face hypoxic conditions (20). Therefore we investigated if similar molecular changes would occur in CSM cells in in vitro models of hypoxia. We demonstrated that hypoxic conditions up-regulate CD73 expression in rat CSM cells, conditions that we have also documented as causing an increase in the expression of the rat opiorphin homologue, sialorphin (7). We demonstrated that the hypoxic up-regulation of CD73 is dependent on the up-regulation of vcsa1, by using vcsa1-siRNA knock-down expression of vcsa1 expression prior to the exposure of CSM cells to hypoxic conditions.
There is increasing evidence that at least in CSM tissue opiorphin is one of the prime regulators of the hypoxic response. Several of the pathways/genes that have previously been shown to be regulated by opiorphin (such as the polyamine pathway(6), A2B and Hif-1a(7)) are related to control of smooth muscle tone. We have proposed that the up-regulation of these genes in response to hypoxia has as the physiologic goal increasing the blood flow into the penis, and thereby reducing hypoxia(7). We propose that it is the excessive activation of these relaxant pathways which leads to priapism. CD73 has previously been shown to be up-regulated by hypoxia in intestinal epithelial cells in a Hif-1a dependent manner (21). As described above one of the downstream targets of opiorphin in CSM cells is Hif-1a (7), suggesting that opiorphin activation of CD73 may at least partially be mediated through Hif-1a.
Adenosine accumulates during ischemia and inflammation, a process which is believed to protect tissues from injury (22). Although several mechanisms might contribute to the protective role of adenosine in hypoxia, adenosine is known to relax vascular smooth muscle tissue causing increased microcirculatory blood flow and thereby oxygenation (23, 24). Increased adenosine levels are primarily thought to occur through two mechanisms; inhibition of adenosine kinase and rapid conversion of adenine nucleotides released from cells by ecto-nucleotidases including CD73. The up-regulation of CD73 we observe both in corpora of animal models of sickle cell disease, and in CSM cells in response to hypoxia, would result in increased local levels of adenosine, which in turn would activate smooth muscle relaxant pathways.
There are 4 adenosine G-protein coupled receptors which bind adenosine, designated A1, A2A, A2B, and A3. While A1 and A3 are associated with Gi and inhibit adenylate cyclase activity (25), A2A and A2B are coupled to Gs and activate adenylate cyclase (26). Several studies have established that A2A and A2B play an important role in erectile function in both patients and animal models (27) and that activation A2B expression is associated with priapism (9, 10).
In conclusion, the up-regulation of CD73 in the corpora of sickle cell mice, which we report here, would result in higher local levels of adenosine, and when factored with the previously documented activation of A2B (7) (which would lead to greater sensitivity to adenosine levels) would lead to heighted relaxation of CSM tissue. The increased relaxation of the CSM caused by the activation of these pathways would result in increased intracorporal blood-flow and thereby the excessive erectile tendencies associated with priapism.
Supplementary Material
Acknowledgments
This work was supported by grants R01DK087872 from the NIH/NIDDK to Kelvin P. Davies and Sexual Medicine Society of North America to Shibo Fu.
References
- 1.Emond AM, Holman R, Hayes RJ, Serjeant GR. Priapism and impotence in homozygous sickle cell disease. Arch Intern Med. 1980;140(11):1434–7. [PubMed] [Google Scholar]
- 2.Andersson KE. Erectile physiological and pathophysiological pathways involved in erectile dysfunction. J Urol. 2003;170(2 Pt 2):S6–13. doi: 10.1097/01.ju.0000075362.08363.a4. discussion S-4. [DOI] [PubMed] [Google Scholar]
- 3.Burnett AL, Lowenstein CJ, Bredt DS, Chang TS, Snyder SH. Nitric oxide: a physiologic mediator of penile erection. Science. 1992;257(5068):401–3. doi: 10.1126/science.1378650. [DOI] [PubMed] [Google Scholar]
- 4.Lagoda G, Sezen SF, Hurt KJ, Cabrini MR, Mohanty DK, Burnett AL. Sustained nitric oxide (NO)-releasing compound reverses dysregulated NO signal transduction in priapism. Faseb J. 2013 doi: 10.1096/fj.13-228817. Epub 2013/10/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dai Y, Zhang Y, Phatarpekar P, Mi T, Zhang H, Blackburn MR, et al. Adenosine signaling, priapism and novel therapies. J Sex Med. 2009;6(Suppl 3):292–301. doi: 10.1111/j.1743-6109.2008.01187.x. [DOI] [PubMed] [Google Scholar]
- 6.Kanika ND, Tar M, Tong Y, Kuppam DS, Melman A, Davies KP. The mechanism of opiorphin-induced experimental priapism in rats involves activation of the polyamine synthetic pathway. Am J Physiol Cell Physiol. 2009;297(4):C916–27. doi: 10.1152/ajpcell.00656.2008. Epub 2009/08/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fu S, Tar MT, Melman A, Davies KP. Opiorphin is a master regulator of the hypoxic response in corporal smooth muscle cells. Faseb J. 2014 doi: 10.1096/fj.13-248708. Available On-line ahead of print. Epub 2014/05/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takahashi Y, Ishii N, Lue TF, Tanagho EA. Effects of adenosine on canine penile erection. J Urol. 1992;148(4):1323–5. doi: 10.1016/s0022-5347(17)36901-x. Epub 1992/10/01. [DOI] [PubMed] [Google Scholar]
- 9.Mi T, Abbasi S, Zhang H, Uray K, Chunn JL, Xia LW, et al. Excess adenosine in murine penile erectile tissues contributes to priapism via A2B adenosine receptor signaling. J Clin Invest. 2008;118(4):1491–501. doi: 10.1172/JCI33467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ning C, Wen J, Zhang Y, Dai Y, Wang W, Zhang W, et al. Excess adenosine A2B receptor signaling contributes to priapism through HIF-1alpha mediated reduction of PDE5 gene expression. Faseb J. 2014 doi: 10.1096/fj.13-247833. Available On-line ahead of print. Epub 2014/03/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wen J, Dai Y, Zhang Y, Zhang W, Kellems RE, Xia Y. Impaired erectile function in CD73-deficient mice with reduced endogenous penile adenosine production. J Sex Med. 2011;8(8):2172–80. doi: 10.1111/j.1743-6109.2011.02316.x. Epub 2011/05/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.User HM, Zelner DJ, McKenna KE, McVary KT. Microarray analysis and description of SMR1 gene in rat penis in a post-radical prostatectomy model of erectile dysfunction. J Urol. 2003;170(1):298–301. doi: 10.1097/01.ju.0000060882.75475.5a. [DOI] [PubMed] [Google Scholar]
- 13.Tong Y, Tar M, Davelman F, Christ G, Melman A, Davies KP. Variable coding sequence protein A1 as a marker for erectile dysfunction. BJU Int. 2006;98(2):396–401. doi: 10.1111/j.1464-410X.2006.06247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tong Y, Tar M, Monrose V, DiSanto M, Melman A, Davies KP. hSMR3A as a marker for patients with erectile dysfunction. J Urol. 2007;178(1):338–43. doi: 10.1016/j.juro.2007.03.004. Epub 2007/05/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davies KP, Tar M, Rougeot C, Melman A. Sialorphin (the mature peptide product of Vcsa1) relaxes corporal smooth muscle tissue and increases erectile function in the ageing rat. BJU Int. 2007;99(2):431–5. doi: 10.1111/j.1464-410X.2006.06577.x. Epub 2006/10/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tong Y, Tiplitsky SI, Tar M, Melman A, Davies KP. Transcription of G-protein coupled receptors in corporeal smooth muscle is regulated by the endogenous neutral endopeptidase inhibitor sialorphin. J Urol. 2008;180(2):760–6. doi: 10.1016/j.juro.2008.03.187. Epub 2008/06/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang YF, Chen Z, Hu SL, Hu J, Li B, Li JT, et al. Interleukin-1 receptor associated kinases-1/4 inhibition protects against acute hypoxia/ischemia-induced neuronal injury in vivo and in vitro. Neuroscience. 2011;196:25–34. doi: 10.1016/j.neuroscience.2011.08.059. Epub 2011/09/20. [DOI] [PubMed] [Google Scholar]
- 18.Goldberg MA, Schneider TJ. Similarities between the oxygen-sensing mechanisms regulating the expression of vascular endothelial growth factor and erythropoietin. J Biol Chem. 1994;269(6):4355–9. Epub 1994/02/11. [PubMed] [Google Scholar]
- 19.Roemeling-van Rhijn M, Mensah FK, Korevaar SS, Leijs MJ, van Osch GJ, Ijzermans JN, et al. Effects of Hypoxia on the Immunomodulatory Properties of Adipose Tissue-Derived Mesenchymal Stem cells. Frontiers in immunology. 2013;4:203. doi: 10.3389/fimmu.2013.00203. Epub 2013/07/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun K, Xia Y. New insights into sickle cell disease: a disease of hypoxia. Current opinion in hematology. 2013;20(3):215–21. doi: 10.1097/MOH.0b013e32835f55f9. Epub 2013/04/04. [DOI] [PubMed] [Google Scholar]
- 21.Synnestvedt K, Furuta GT, Comerford KM, Louis N, Karhausen J, Eltzschig HK, et al. Ecto-5′-nucleotidase (CD73) regulation by hypoxia-inducible factor-1 mediates permeability changes in intestinal epithelia. J Clin Invest. 2002;110(7):993–1002. doi: 10.1172/JCI15337. Epub 2002/10/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Linden J. Molecular approach to adenosine receptors: receptor-mediated mechanisms of tissue protection. Annu Rev Pharmacol Toxicol. 2001;41:775–87. doi: 10.1146/annurev.pharmtox.41.1.775. Epub 2001/03/27. [DOI] [PubMed] [Google Scholar]
- 23.Nagaoka T, Sakamoto T, Mori F, Sato E, Yoshida A. The effect of nitric oxide on retinal blood flow during hypoxia in cats. Investigative ophthalmology & visual science. 2002;43(9):3037–44. Epub 2002/08/31. [PubMed] [Google Scholar]
- 24.Segal S. Communication among endothelial and smooth muscle cells coordinates blood flow control during exercise. News Physiological Science. 1992;7:152–6. [Google Scholar]
- 25.Tager AM, LaCamera P, Shea BS, Campanella GS, Selman M, Zhao Z, et al. The lysophosphatidic acid receptor LPA1 links pulmonary fibrosis to lung injury by mediating fibroblast recruitment and vascular leak. Nat Med. 2008;14(1):45–54. doi: 10.1038/nm1685. Epub 2007/12/11. [DOI] [PubMed] [Google Scholar]
- 26.Fredholm BB, AP IJ, Jacobson KA, Klotz KN, Linden J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev. 2001;53(4):527–52. Epub 2001/12/06. [PMC free article] [PubMed] [Google Scholar]
- 27.Phatarpekar PV, Wen J, Xia Y. Role of adenosine signaling in penile erection and erectile disorders. J Sex Med. 2010;7(11):3553–64. doi: 10.1111/j.1743-6109.2009.01555.x. Epub 2009/11/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.