Abstract
Objective
Preliminary evidence suggests that chronic pain patients complete pain intensity measures using idiosyncratic methods. Our objective was to understand these methods and how they might impact the psychometric properties of the instruments.
Design
A qualitative focus-group based study.
Setting
An academic center in New York City
Subjects
Outpatients (n=36) with chronic low back pain, or neuropathic pain due to diabetes or HIV.
Methods
Participants were divided into three focus groups based on their pain condition, and asked to discuss pain intensity measures (visual analog and numeric rating scales for average pain over 24 hours; Brief Pain Inventory; and McGill Pain Questionnaire). Audio-recordings were transcribed and analyzed using an inductive thematic method.
Results
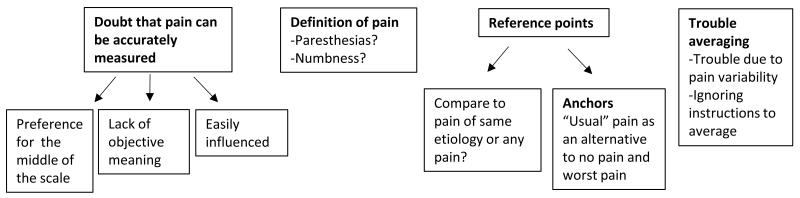
We discovered four main themes, and five sub-themes: 1) doubt that pain can be accurately measured (sub-themes: pain measurement is influenced by things other than pain, the numbers used to rate pain do not have an absolute meaning, and preference for pain intensity ratings “in the middle” of the scale); 2) confusion regarding the definition of pain; 3) what experiences to use as referents (sub-themes: appropriate comparator experiences and the interpretation of the anchors of the scale); and 4) difficulty averaging pain.
Conclusions
The themes discovered suggest that patients include sensations and experiences other than pain intensity in their ratings, experience the rating of pain as a comparative task, and do not use the scale in a linear manner. These themes are relevant to understanding the validity and scale properties of commonly used pain intensity measures.
Keywords: measurement, chronic pain, diabetic neuropathy, HIV, neuropathic pain, low back pain
Introduction
Pain is a subjective experience, which is difficult to accurately measure. It is widely accepted that self-report, as opposed to observation of behavior or diagnostic testing, is currently the best way to measure pain in adults, and that measurement tools should at a minimum measure pain intensity, and ideally other dimensions, for example pain affect, interference, location and pattern.(1)
Many instruments are used to measure pain intensity, however most include some type of Visual Analog Scale (VAS) and/or a Numeric Pain Rating Scale (NPRS), both of which are also used as stand-alone measures. The VAS and NPRS have been the subject of significant quantitative research to determine their psychometric properties, such as validity and whether they possess ratio scale properties. The concept of validity, the extent to which a test measures what it purports to measure, is more nuanced in the case of a subjective experiences such as pain, for which there is no external referent. Investigators have attempted to demonstrate the validity of the VAS and the NPRS quantitatively based on their correlation with other self-report measures of pain intensity,(2,3) and their sensitivity to treatment effects.(4)
The issue of whether the VAS and NPRS have ratio scale properties has been controversial. A ratio scale is one in which the intervals all have equal meaning, (e.g. a change from “one” to “two” is the same as a change from “two” to “three”), and zero has an absolute meaning, which allows for the expression of meaningful ratios, (e.g. the assertion that a “six” on the scale is twice as much as a “three”). Investigators have attempted to demonstrate ratio scale properties of pain intensity measures in two main ways, using experimental painful stimuli, or response to pain medication. In the former approach chronic pain patients and/or healthy controls are exposed to a quantifiable painful stimulus (such as a thermal stimulus) and are asked to rate the resulting pain.(5,6) The pain rating scale is assumed to have ratio scale properties if the derived stimulus-response function can be fitted by a power function. In the latter approach, a patient (usually with acute pain) is asked rate their pain.(7,8) Pain medication is then administered and the patient is then asked to rate their pain again when it is half as strong as baseline. If the second rating is half the first rating, then this is considered supportive of ratio scale properties. Both of these approaches are problematic. In the first approach, it could be argued that the experimental pain being rated is not clearly relevant to the experience of chronic pain. In the latter approach, it is necessary to introduce the idea of “half as much pain” to the patient, which may suggest to them the desired response, i.e. their second pain rating should be half their first.
Recent quantitative studies have explored alternative methods of pain intensity measurement, for example the use of the slope produced by multiple pain intensity measurements over time.(9) However there has been surprisingly little research qualitatively investigating the perception of pain measurement instruments and the process of completing them from the patient’s perspective. This is an unfortunate omission because much could be learned about the validity and scale properties of pain measurement instruments by asking patients how they go about filling them out. A rigorous qualitative methodology, characterized by a conscientious and open approach to gaining a deep understanding of individual participants’ experiences,(10) is ideally suited to this type of research question. For example, if patients understand the VAS and NPRS and are comfortable using them to rate their pain intensity then this would support at least face-validity. On the other hand, if patients are routinely incorporating constructs other than pain intensity into their rating, that would compromise the usefulness of the instrument. Similarly, if patients understand the anchors of the VAS and/or NPRS to have absolute meaning, and can spontaneously articulate that a “five” (or the middle of the line) is a pain that is half way between no pain and worst pain, then that would support ratio scale properties. To our knowledge there has been only one study that has taken this qualitative approach.(11) de C. Williams and colleagues interviewed 78 chronic pain patients in an inpatient pain management program in London. Qualitative analysis of these data found that patients typically had more than one type of pain, and differed as to whether they chose to rate one particular pain or if they attempted to rate them in aggregate. The investigators also found multiple factors patients either consciously included in their pain intensity rating, or felt influenced their rating, including mood, distress, tiredness, and what other people might think. There was also variability in how the patients perceived the anchors of the scale. Some patients treated zero as their usual pain, whereas others just ignored the bottom half of the scale. Conversely some patients did not feel comfortable using the highest part of the scale. This study suggests problems with validity due to inclusion of constructs other that pain intensity, and a lack of ratio scale properties due to idiosyncratic interpretation of the anchors.
We undertook the present study to further explore chronic pain patients’ perception of pain intensity measures and the process of completing them, using qualitative methods in a U.S. outpatient chronic pain population. Our goal was to gather qualitative evidence either for or against their validity and ratio scale properties, and if necessary to generate ideas to improve these properties. Our study design included three focus groups, each consisting of participants with a particular type of chronic pain: chronic low back pain, neuropathic pain due to diabetes, and neuropathic pain due to HIV. We chose to use focus group methodology because it is considered particularly useful for exploring participants’ knowledge and experiences and for allowing participants to pursue their own lines of thought rather than those dictated by investigators.(12) Focus groups have also been shown to facilitate the expression of criticism which might be suppressed in a one-on-one interview, a quality well suited to our aim of generating ideas for improvement of the scales.(12) We chose to focus on three particular types of chronic pain in order to create cohesion in the groups and facilitate the group dynamic. Also we were interested in whether differences between the groups would emerge. We chose these particular pain conditions because they are among the most common seen in our neurology practices.
Methods
Participants
Potential participants learned of the study in one of three ways: 1) from their healthcare provider; 2) newsletter announcements and posters displayed at the Mount Sinai Medical Center; 3) a posting on ResearchMatch.org, an NIH sponsored website linking interested volunteers with research studies. Inclusion criteria for all subjects were: age greater than 18 years; able and willing to provide written informed consent; stable pain treatment regimens during the past 30 days and willingness to continue the same pain treatment regimen during the study period; physically able to attend all study visits. Additional inclusion criteria for the neuropathic pain groups were: the presence of pain, burning, or dysesthesias in both feet for at least two months prior to the screening visit; either absent/diminished ankle reflexes or distal diminution of at least one sensory modality (vibration, pinprick, or temperature) as determined by a study neurologist; documentation of a diagnosis of either HIV-infection or diabetes as the primary cause of peripheral neuropathy, but not both. Additional inclusion criteria for the back pain group were at least two months of pain restricted to the lower back, or associated with radiation to the proximal portion of the lower limb only, as documented by a clinical diagnosis from a treating physician and confirmed at screening by a study neurologist. Exclusion criteria were: history of schizophrenia or bipolar disorder; serious illness requiring hospitalization within the past 90 days or that was expected to preclude successful participation in the study; treatment with any experimental agent within the past 90 days or planning participation in an experimental treatment during the study period; major surgery related to their chronic pain condition within the past 12 months; any neuropathy that was not related to either HIV or diabetes.
Procedures
Study procedures were performed in accordance with a protocol approved by the Institutional Review Board of the Icahn School of Medicine at Mount Sinai. All participants gave written informed consent. There were a total of four study visits. At visits 1, 2 and 4 participants were seen individually, and visit 3 was the focus group. Visit 1 included the informed consent process and screening for eligibility, including examination by a study neurologist. The purpose of the examination was to document the presence of clinical signs of neuropathy in the neuropathic pain groups, and the absence of such signs in the back pain group. At visit 2 participants were asked to complete the following pain questionnaires in order to familiarize them with the scales prior to the focus group: the VAS and NPRS for average pain over the past 24 hours, the Brief Pain Inventory,(13) and the short-form McGill Pain Questionnaire.(14) The instruments were all administered in the standard manner. Visit 3 was the focus group. In order to foster a unifying group dynamic and encourage participants to speak freely, three separate focus groups were conducted, one for each pain condition. Each focus group was led by two trained facilitators (DD and MCG) using a script with pre-defined questions and probes designed to guide the conversation. In addition, the measures being discussed were projected on a screen during the conversation to provide a mutual point of reference. Visit 4 consisted of an audiotaped exit interview designed to record additional impressions of the questionnaires on an individual basis.
Analyses
The audio-recordings were transcribed by a professional transcription service. An inductive thematic analysis was used to identify themes and sub-themes within the data.(15) The data analysis began with detailed reading of the transcripts multiple times by two of the co-investigators independently (JRP and MCG) in order to become immersed in the data. During these readings the investigators initially attempted to identify interesting features of the data that might ultimately form patterns and the basis for themes. With subsequent readings, codes were identified, recorded and organized in an iterative process of moving repeatedly through the data. Commonality in codes was then sought and codes were grouped or combined into themes as appropriate. Themes were then reviewed and refined. The coding and identification of themes and subthemes were discussed and developed collaboratively between JRP and MCG following the initial readings. Quotes supporting the themes and sub-themes were identified by JRP and agreed upon by all co-authors. Qualitative methodology has been described in greater detail by other authors.(16-19)
Results
Participants
There were a total of 36 participants in the study, 12 in the HIV group, 11 in the diabetes group, and 13 in the back pain group. The demographic characteristics of the groups are described in table 1.
Table 1.
Participant Demographics
| HIV group (n=12) | Diabetes group (n=11) | Low Back Pain Group (n=13) | |
|---|---|---|---|
| Gender | |||
| Male, n | 10 | 6 | 6 |
| Female, n | 2 | 5 | 7 |
| Race/ethnicity | |||
| African-American, n | 6 | 2 | 2 |
| Hispanic, n | 3 | 2 | 5 |
| White, n | 3 | 7 | 6 |
| Age, mean (range) | 55, (44-63) | 49, (18-65) | 45, (25-74) |
| Years of education, mean (range) |
14, (8-16) | 17, (13-20) | 15, (12-19) |
Themes
Many themes were common to all three groups. When this was the case we have included relevant quotes from each of the groups. When this was not the case we have indicated so, including the nature of the differences. Each scale was initially presented to the participants separately and the conversation generated by each was initially analyzed separately. However it became apparent that the participants viewed the pain intensity sections of the BPI, the VAS within the McGill, and the stand-alone VAS and NPRS similarly. Many participants (four in the HIV group, three in the diabetes group, and two in the back pain group) spoke of the VAS in terms of 0-10, even though there were no numbers printed on the instrument. These were also the items that generated the bulk of the discussion, with participants tending to spontaneously return to them. Thus in the final analysis these items were considered together. The final analysis also considers data from the focus groups (visit 3) and from the individual interviews (visit 4) together because there were no significant or interesting differences between the views expressed in the groups and those expressed in the one-on-one visits.
Four major themes were identified from the participants’ discussions of the pain intensity instruments (figure 1 and table 2): 1) doubt that pain can be accurately measured; 2) confusion regarding the definition of pain; 3) what experiences to use as referents; and 4) difficulty averaging pain.
Figure 1.
Pain intensity measurement themes
Table 2. Representative quotes grouped by theme/sub-theme and pain condition.
| Doubt that pain can be accurately measured |
| HIV group: “I think it’s confusing, even if I give you an average. It’s very misleading.” |
| Diabetes group: “Trying to quantify something that you…can’t.” |
| Back pain group: “A single line is really not going to tell him what I’m actually feeling.” |
|
|
| Preference for the middle of the scale |
| HIV group: “I know I’m always gonna stay pretty much at the middle pain” |
| Diabetes group: “It’s somewhere in the middle somewhere.” |
| Back pain group: “I kind of go towards the middle.” |
|
|
|
Pain intensity measurement is influenced by other factors (e.g. depression, anticipated response of
the examiner, disability level) |
| HIV group: “Depression affects all of those things.” |
| Diabetes group: “I wouldn’t… tell the doctor that my pain is just mild because it’s like…you don’t really have that much of an issue.” |
| Back pain group: “With no pain (on the VAS), I wouldn’t be so restricted… but it can possibly get worse. I can still walk.” |
|
|
| Lack of objective/absolute meaning |
| HIV group: “I think that it’s very deceptive, because everyone has their own… pain.” |
| Diabetes group: “Everybody’s different… they’re saying eight and seven and he’s saying four.” |
| Back pain group: “How does my… one to ten mean the same thing as his… one to ten?” |
|
|
| Definition of pain (e.g. including paresthesias, numbness, and functional limitations) |
| HIV group: “Pins and needles and numbness and all that.” |
| Diabetes group: “Is numbness a pain?” |
| Back pain group: “The feeling (of pain) I feel during the day, and how much it restricts me.” |
|
|
| Appropriate reference pain (i.e. whether to compare to pain of the same etiology or any pain) |
| HIV group: “I tend to compare to pain in general.” |
| Diabetes group: “Worst possible pain… a herniated disc.” |
| Back pain group: “That peak period… that’s what I’m referencing…” |
|
|
| “Usual,” “normal,” or “average” pain as an alternative anchor to no pain and worst pain |
| HIV group: “I think we all would consider five average.” |
| Diabetes group: “What’s the purpose of these numbers… to make comparison easier to our own pain?” |
| Back pain group: “My average day might not be anything compared to her average day.” |
|
|
| Difficulty averaging related to pain variability |
| HIV group: “Some is severe. Some of it is not.” |
| Diabetes group: “You have an episode (of)… worst possible pain and… at other times… no pain how do you really reflect that?” |
| Back pain group: “Two days… bad and 12 days… weren’t so bad… average it out in the low end. But that’s not going to explain… the high end.” |
Theme 1
The first and most general theme was the perception that it may not even be possible to measure pain in a meaningful way, for example from the diabetes group: “You run into the same dilemma of trying to quantify something that you possibly can’t,” and the back pain group: “At the end of the day a single line is really not going to tell him what I’m actually feeling.” Some participants expressed this sentiment more forcibly and were even angered by the perceived futility of pain measurement, for example from the HIV group: “I’ve had my pain since 1999. And, now when I see this chart it pisses me off… because the person who’s asking me, 99% of the time, they can’t help me. So, why should I put a mark down here? I mean, it don’t mean nothing.”
In addition to these general concerns, three more specific sub-themes were identified: 1a) pain measurement is influenced by things other than pain, 1b) the numbers used to rate pain do not have an absolute meaning, and 1c) preference for pain intensity ratings “in the middle” of the scale, i.e. in the middle of the line for the VAS or the middle numbers for the NPRS.
Sub-theme 1a
Participants offered a variety of factors they thought would influence the report of pain intensity. Examples from the HIV group were depression and events that had occurred immediately before the completion of the instrument: “Depression affects all of those things. So, yeah, I think many times it’s very difficult to understand whether it’s just the pain you’re feeling or whether it’s the combination of the two,” and “So if I did something before, like exercise… Yes, that would affect my answer.” Participants in the diabetes and back pain groups thought that the anticipated response of the examiner was an important influence: “My tendency is to kinda lowball it… I just feel like, oh, they’re busy and I can deal with it,” and “I wouldn’t necessarily want to tell the doctor that my pain is just mild because it’s like okay well, if it’s mild you don’t really have that much of an issue.” Another example from the back pain group of a factor that influenced the pain intensity rating was degree of disability: “I personally would put mine (pain rating) smack dab in the middle… because with no pain, I wouldn’t be so restricted to do certain things, but with worst possible pain, I also wouldn’t be able to do certain things that I can still do.”
Sub-theme 1b
Participants were troubled by the idea that the pain intensity rating did not have an absolute meaning and so the same score could mean different things to different people. For example, from the diabetes group: “It’s also a question of what those numbers mean. They mean different things to different people;” and from the HIV group: “I think that it’s very deceptive, because everyone has their own individual tones and pain;” and from the back pain group: “I have a high tolerance for pain. Not everyone does. So how does my determination on a scale from one to ten mean the same thing as his determination on a scale from one to ten?”
Sub-theme 1c
The preference for the middle of the line for the VAS or the middle numbers for the NPRS was an important sub-theme in the HIV and back pain groups, but was less clearly articulated in the diabetes group. Examples from the HIV group were: “I know I’m always gonna stay pretty much at the middle pain;” and “I always go towards that middle of the line.” Examples from the back pain group were: “I kind of go towards the middle because there is no such thing as non-existent, no pain. But worst possible pain, it can always get worse;” “Anyone who had to complain about their back would always go toward the middle;” and “Do you feel like you default always towards the center?” In the diabetes group one participant said: “But they ask me what is your pain from one to ten… It’s somewhere in the middle somewhere,” but there was no consensus about tending toward the middle.
Theme 2
The second main theme was the definition of pain. This was mostly an issue among the groups with neuropathic pain in whom paresthesias and numbness were bothersome, uncomfortable symptoms, but not necessarily considered pain, for example from the HIV group: “I ain’t got no pain, but I can get the pins and the needles… We talking about pain. For me, pain is hurt,” and “I was always thinking about this chart as pins and needles and numbness and all that.” A participant in the diabetes group said: “I just want to ask people who has said that they have numbness, is numbness a pain?” They also named many different sensations that they attributed to their neuropathy, and may or may not include under the definition of pain, such as: “like hundreds of bees or needles,” “tingling feeling which keeps me awake,” “severe and burning… knifing,” “cramping,” and “just very uncomfortable and it’s numb but it’s – you’re aware of it.” In the back pain group, the definition of pain was not an explicit subject of conversation. However as mentioned above, some participants combined degree of disability and pain in their response: “The feeling (of pain) I feel during the day, and how much it restricts me.”
Theme 3
The third main theme was the points of reference to be used when rating pain. This theme included the sub-themes of: 3a) appropriate comparator experiences, and 3b) the interpretation of the anchors of the scale.
Sub-theme 3a
In terms of the appropriate comparator experiences, participants differed as to whether they would include all types of pain or whether they would just use their experiences with the type of pain that defined their particular focus group. Participants in the neuropathic pain groups tended to use broad comparators, for example from the HIV group: “I tend to compare to pain in general, not just neuropathy,” and “I’ve experienced pain in other parts of my body, in other ways, real serious pain,” and from the diabetes group: “…worst possible pain… a herniated disc and you’ve had nerve death or something like that.” Participants from the back pain group tended to use prior episodes of back pain as comparators, for example: “Mine was caused by accident to me and I had a lot of recovery, so I know what it was like during that peak period. That’s what I’m referencing because it’s the worst I’ve ever had.” Participants in the back pain group also sometimes did not use a comparator experience at all, but rather thought of pain severity in terms of how much medication they took on a particular day: “So if I have to take more than one of those a day… to me that’s a bad day;” or how limited by pain they were: “If I had to think about what’s the worst pain, I would probably be thinking that it had to be at some level where I couldn’t even walk.”
Sub-theme 3b
The appropriate anchors for the pain scales were discussed at length. Many participants thought that anchoring the low end of the scale at “no pain” was inappropriate for their condition, because they always experienced some pain, for example from the back pain group: “No pain to me, that’s non-existent,” and from the HIV group: “Pain… I’m always at this. I’ll always be there.” This was less problematic in the diabetes group where some participants experienced pain free times: “For me last 24 hours I would say I did not have pain all the time continuously. It comes and goes.” Participants who expressed that “no pain” was inappropriate for their condition had different strategies for reconciling this with the pain intensity measures. Some reset the zero value, for example from the HIV group: “The numbing and the tingling part… we all gonna have that for the rest of our lives… so that’s considered no pain anymore.” Others said that they would simply ignore the lower parts of the scale, for example from the back pain group: “We never have no pain. So I would disregard the first 25 percent of the line.” At the high end of the scale, discussion focused on whether actual experience or a theoretical “worst pain” should be used as the reference. This was articulated best by a participant in the back pain group: “He says the worst possible pain he’s ever experienced, I would never go to ten because I would always look at it as, whatever pain I’m in can possibly get worse. Not something I’ve already experienced, but can be worse. So worst possible pain, to me, is non-existent.” Among those who favored a concrete “worst pain” various examples were offered, for example from the HIV group: “child birth pain or biopsy while you’re awake,” and from the diabetes group: “when I fell in the snow and I broke my tibia and fibula.” Others expressed the idea that they would not use the highest numbers on the scale because doing so would indicate a lack of ability to cope with the pain, for example from the HIV group: “I would never go to an eight or nine or a 10. Because, I’m gonna always be, ‘I’m gonna handle this.’”
The idea of “average,” “normal,” or “usual” pain as an alternative anchor and/or comparator emerged strongly in the back pain group. Many participants expressed the idea that they had a normal amount of pain that was very familiar and specific to them, and that they tended to judge pain intensity in reference to this normal pain, leading to the concept of “good days” that are better than normal versus “bad days” that are worse than normal. Examples included: “Today might have been a little bit worse than yesterday – average, maybe a little bit more than average;” “So I look at it as a good day versus a really bad day;” “My average day might not be anything compared to her average day;” “no more (pain) than usual;” “normal wear and tear.” The diabetes and HIV groups expressed similar ideas, albeit with lower frequency. An example from the diabetes group was: “So a number… it can be comparative… It may make it easier for me to say yeah, it was worse today than yesterday so maybe six rather than five.” Some participants in the HIV group thought that “average” as an anchor was a concept already contained within the NPRS: “I think we all would consider five average. 10 worse and a zero, one very low.”
Theme 4
The fourth main theme was difficulty generating an “average” pain score. In contrast to the concept of “average pain” as their normal or usual pain, which participants understood well, the concept of generating a number to represent “average pain” over a given time period was not intuitive. The longer the time period over which they were asked to average, the more difficulty participants had, for example from the back pain group: “I think a month is too large a scale. Especially when you live with pain every single day you tend to just move forward and just keep going. You don’t really think back so much,” and from the HIV group: “I could go back a week. I mean, and I’m really thinking hard. But two weeks?” Few, if any, participants could articulate a clear method for generating an average over any time period. A participant in the diabetes group attempted to explain as follows: “I got stabbed at 11:00 p.m. at night, I got up. At 2:00 a.m. the stabbing came back, I couldn’t go to sleep. I can average it out.” Other participants could articulate a method for averaging to some extent but simultaneously expressed discomfort with the process, for example from the HIV group: “I may have had an extremely sharp pain that lasted three minutes. But, the rest of the day, it was at a three, you know? So, if I tell you it’s a five, it is misleading,” and from the back pain group talking about averaging over two weeks: “Maybe I only had two days that were really bad and 12 days that weren’t so bad so I’m just going to average it out in the low end. But that’s not going to explain anything about the high end.” Thus this difficulty averaging appeared to arise, at least in part, from pain variability.
The idea of variability was expressed primarily by the neuropathic pain groups and included variability in anatomic distribution, type of pain, intensity, and duration. Examples from the HIV group were: “But sometimes with neuropathy… the toes are hurting a lot more than the rest of the foot. Some is severe. Some of it is not,” and “Well, pins and needles is a three, four and five. Pain is seven, eight, nine and 10.” Examples from the diabetes group were: “If during those 24 hours you have an episode in which you have the worst possible pain and if at other times you have no pain how do you really reflect that,” and “We’re talking about the intensity of pain… the length of time the pain persists and… the quality of the pain… so that’s a problem I have with all these scales… they all ask you to say one thing.”
Interestingly, some participants seemed to ignore the instruction to average altogether, and employed other techniques in generating a score, for example from the HIV group: “I don’t even try to go back 24 hours. I just go with immediate,” and “If I felt a bad pain that day, that’s what I’ll put down. I won’t put down a little bit of pain, I’ll put down the bad pain.”
The BPI and McGIll
Discussion of the pain interference section of the BPI was relatively brief, although some participants, particularly from the diabetes and back pain groups, indicated that the construct of interference was relevant to them and easier to conceptualize and rate than intensity. An example from the diabetes group was: “Someone can have a seven (intensity) and it may not interfere with their day but someone else who has seven may… These sort of scales… give you a much more detailed explanation as to how it affects you as opposed to just saying yeah, I have a seven or eight today.” Examples from the back pain group were: “That’s pretty straight forward… So zero does mean something to me… That means doesn’t affect my walking ability,” and “Zero here means something to us because… the things we have to do are the things we have to do. So we will have zero interference.”
Discussion of the verbal descriptors in the McGill, was not very fruitful and mainly consisted of participants commenting on whether they thought the words were applicable to their condition, without arriving at any consensus.
Discussion
In this study we conducted focus groups and individual interviews with participants with chronic low back pain, neuropathic pain related to diabetes, or neuropathic pain related to HIV. Our goal was to qualitatively investigate from the patient perspective, factors that might influence the validity and ratio scale properties of pain intensity measurement tools, and to generate ideas to improve these properties if necessary. We discovered four main themes in the participants’ discussion of the measures, and a total of five sub-themes: 1) doubt that pain can be accurately measured (three sub-themes: 1a) pain measurement is influenced by things other than pain, 1b) the numbers used to rate pain do not have an absolute meaning, 1c) preference for pain intensity ratings “in the middle” of the scale); 2) confusion regarding the definition of pain; 3) what experiences to use as referents (two sub-themes: 3a) appropriate comparator experiences, 3b) the interpretation of the anchors of the scale); and 4) difficulty averaging pain. Themes 1, 2 and 4 are relevant to validity and reliability and are discussed first. Theme three, and two of the sub-themes of theme one are relevant to whether or not the instruments have ratio scale properties and are discussed subsequently.
Theme one (doubt that pain can be accurately measured) suggests a lack of face-validity insofar as participants did not feel that their pain intensity could be adequately captured by the instruments. However this did not appear to be specific to the instruments at hand, but rather a broader objection to the whole notion of quantifying pain. While interesting, this finding does not suggest a clear remedy, since in the absence of a reliable biomarker for pain, we must continue to rely on self-report. Perhaps acknowledging to patients that doing so is difficult and imperfect might be one simple way to address this concern. The sub-theme of “pain measurement is influenced by things other than pain” illustrates a more specific potential threat to validity and reliability, namely that participants were incorporating other dimensions of the pain experience (e.g. affect, interference) into their pain intensity rating, as well as constructs outside of the pain experience altogether (e.g. what the interviewer might think). Theme two (confusion about the definition of pain) was important especially among participants with neuropathic pain, who differed as to whether they considered paresthesias and uncomfortable numbness to be part of their pain syndrome or not. Theme four (difficulty averaging pain) illustrates that patients do not have a clear strategy for reporting an average pain over a given period of time, and that this was mostly due to variability in pain and difficulty with recall. This could have implications for validity because the patients’ report is unlikely to accurately reflect their experience if they don’t understand how to generate an average. Also if whatever strategy they choose to use varies over time, then this could adversely impact reliability, although we did not specifically address this issue. Taken together these results suggest the need for very specific instruction as to what sensations and experiences patients are to include in their pain intensity rating. Furthermore if patients are required to average pain over a period of time, they likely need additional instruction in strategies for doing so.
Theme three (what experiences to use as referents) and its two sub-themes (appropriate comparator experiences and the interpretation of the anchors of the scale) have important implications regarding whether the instruments have ratio scale properties. In the broadest sense, theme three demonstrates that rating pain intensity was an inherently comparative experience for our participants. This has long been recognized, for example by Huskisson who wrote that it is more usual for a patient to say “my pain is a little better” as opposed to “my pain is now moderate.”(20) The subtheme of “interpretation of the anchors of the scale,” included the concept that “no pain” or a “zero” pain intensity was not meaningful due to the constant presence of pain. This is directly relevant to one of the requirements of the ratio scale, which is that zero has absolute meaning. Conversely many participants did not feel comfortable using the highest parts of the VAS/NPRS for various reasons, for example the impression that a pain score of “ten” would indicate that they were overwhelmed or had given up, or the idea that things could always be worse. Both these perceptions are problematic with regard to ratio-scale properties. The former suggests that pain affect may become increasingly relevant at the higher portions of the scale, and therefore have a non-uniform influence on the report of pain intensity. The latter suggests that some participants may effectively view the higher portions of the scale as an infinite amount of pain that can never truly be achieved. In contrast to the anchors of “no pain” and “worst possible pain”, the concepts of “average” or “usual” pain and “good” days and “bad” days were well understood by participants. One participant even assumed that the midpoint or “five” on the scale was meant to indicate average pain. Taken together, these data suggest that our participants did not use the VAS/NPRS as a linear, ratio scale.
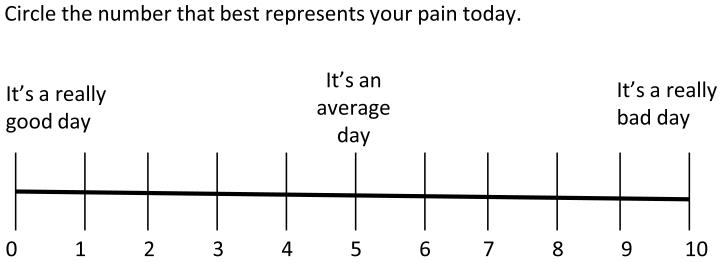
In response to the discussion of anchors (theme four), we conceived a modification (figure 2) of the VAS/NPRS that might approximate our participants’ pain intensity conceptualization more closely. This modification explicitly acknowledges the comparative process of generating a pain score already employed by our participants. The modification also defines the comparator experience to be used as average or usual pain, which was a comparator that was well understood by our participants. The potential benefits of such a scale include: 1) broadening the range of scores used by participants thus enhancing the ability to detect change, and 2) increasing validity by more closely approximating the patients’ conceptualization of their pain. The major drawback would be the lack of ratio scale properties since there is no absolute zero. However it could be argued that patients are already conceptualizing the VAS/NPRS this way anyway and that changing the labels merely makes explicit what is already done.
Figure 2.
Comparative Numeric Pain Rating Scale (CNPRS)
Issues of ratio scale properties are most important in the research setting where pain intensity for different patients at different time points must be analyzed in aggregate. However our findings also have applicability to the one-on-one interactions between patient and provider that are typical of the clinical setting. The provider seeking to improve communication and to better understand a patient’s pain intensity might do well to acknowledge the difficulty of quantifying pain, seek to understand what sensations the patient includes in his/her conceptualization of pain, and discuss intensity as a comparative rather than absolute experience.
There are commonalities between our findings and those of de C. Williams and colleagues who similarly studied chronic pain patients’ perception of the VAS and NPRS using qualitative methods.(11) Our study reproduced several of their themes including the blending of pain intensity and pain distress, the influence of multiple factors on the pain intensity rating, and discomfort with the maximum and minimum values on the scale. These commonalities in two very different populations (inpatient, U.K., mixed pain disorders vs. outpatient, U.S., specific pain disorders), using two different techniques (individual interview vs. focus group), suggest that the findings of this study may be applicable to other settings. In addition, a recent study examining Japanese chronic pain patients found that “worst pain imaginable” is considered difficult to understand by most chronic pain patients, similar to our findings (theme 3b).(21)
In summary our study identified four themes in the participants’ discussion of pain intensity measures: 1) doubt that pain can be accurately measured; 2) confusion regarding the definition of pain; 3) what experiences to use as referents; and 4) difficulty averaging pain. These themes represent barriers patients may encounter in trying to quantify their pain accurately, and are thus relevant to the psychometric properties of pain intensity measurement instruments. Understanding these perceptions could lead to educational approaches to improve pain intensity reporting, and greater insight into the instruments themselves.
Summary.
This article describes a qualitative, focus-group based study of patients’ perceptions of instruments commonly used for measuring chronic pain intensity. We found that patients doubt that pain can be accurately measured; are confused regarding the definition of pain; differ as to what experiences to use as referents; and have trouble arriving at an average pain score. We discuss how these barriers may affect the psychometric properties of the instruments.
Acknowledgments
This research was supported in part by an unrestricted grant from Eli Lilly and Company to the Icahn School of Medicine at Mount Sinai (Project F1J-US-X055; PI: David M. Simpson, MD). Assistance with recruitment of study participants was provided by grant UL1TR000067 from the National Center for Advancing Translational Sciences, National Institutes of Health. Dr. Robinson-Papp receives funding for research from the following sources: National Institutes of Health (K23 NS066789 and UL1 TR000067), The Foundation for Peripheral Neuropathy, Astellas Pharma, and Isis Pharmaceuticals. Dr. Robinson-Papp is a consultant and lecturer for the AIDS Education and Training Centers. Dr. Simpson receives funding for research from the following sources: Allergan, Merz, Ipsen, US Worldmeds, Astellas, Viromed, CSL Behring, and The Foundation for Peripheral Neuropathy. Dr. Simpson is a consultant and/or lecturer for the following entities: Astellas, Merz, Acorda, Depomed, Allergan.
Footnotes
Disclosure:
The authors report no conflicts of interest with regard to the information presented in this manuscript, however the following sources of funding are included for the sake of completeness.
References
- (1).Dworkin RH, Turk DC, Farrar JT, Haythornthwaite JA, Jensen MP, Katz NP, et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005 Jan;113(1-2):9–19. doi: 10.1016/j.pain.2004.09.012. [DOI] [PubMed] [Google Scholar]
- (2).Paice JA, Cohen FL. Validity of a verbally administered numeric rating scale to measure cancer pain intensity. Cancer Nurs. 1997 Apr;20(2):88–93. doi: 10.1097/00002820-199704000-00002. [DOI] [PubMed] [Google Scholar]
- (3).Jensen MP, Karoly P, Braver S. The measurement of clinical pain intensity: a comparison of six methods. Pain. 1986 Oct;27(1):117–126. doi: 10.1016/0304-3959(86)90228-9. [DOI] [PubMed] [Google Scholar]
- (4).Ohnhaus EE, Adler R. Methodological problems in the measurement of pain: a comparison between the verbal rating scale and the visual analogue scale. Pain. 1975 Dec;1(4):379–384. doi: 10.1016/0304-3959(75)90075-5. [DOI] [PubMed] [Google Scholar]
- (5).Price DD, Bush FM, Long S, Harkins SW. A comparison of pain measurement characteristics of mechanical visual analogue and simple numerical rating scales. Pain. 1994 Feb;56(2):217–226. doi: 10.1016/0304-3959(94)90097-3. [DOI] [PubMed] [Google Scholar]
- (6).Price DD, McGrath PA, Rafii A, Buckingham B. The validation of visual analogue scales as ratio scale measures for chronic and experimental pain. Pain. 1983 Sep;17(1):45–56. doi: 10.1016/0304-3959(83)90126-4. [DOI] [PubMed] [Google Scholar]
- (7).Myles PS, Urquhart N. The linearity of the visual analogue scale in patients with severe acute pain. Anaesth Intensive Care. 2005 Feb;33(1):54–58. doi: 10.1177/0310057X0503300108. [DOI] [PubMed] [Google Scholar]
- (8).Myles PS, Troedel S, Boquest M, Reeves M. The pain visual analog scale: is it linear or nonlinear? Anesth Analg. 1999 Dec;89(6):1517–1520. doi: 10.1097/00000539-199912000-00038. [DOI] [PubMed] [Google Scholar]
- (9).Lang EV, Tan G, Amihai I, Jensen MP. Analyzing acute procedural pain in clinical trials. Pain. 2014 Jul;155(7):1365–1373. doi: 10.1016/j.pain.2014.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Davies D, Dodd J. Qualitative research and the question of rigor. Qual Health Res. 2002 Feb;12(2):279–289. doi: 10.1177/104973230201200211. [DOI] [PubMed] [Google Scholar]
- (11).de C Williams AC, Davies HT, Chadury Y. Simple pain rating scales hide complex idiosyncratic meanings. Pain. 2000 Apr;85(3):457–463. doi: 10.1016/S0304-3959(99)00299-7. [DOI] [PubMed] [Google Scholar]
- (12).Kitzinger J. Qualitative research. Introducing focus groups. BMJ. 1995 Jul 29;311(7000):299–302. doi: 10.1136/bmj.311.7000.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Tan G, Jensen MP, Thornby JI, Shanti BF. Validation of the Brief Pain Inventory for chronic nonmalignant pain. J Pain. 2004 Mar;5(2):133–137. doi: 10.1016/j.jpain.2003.12.005. [DOI] [PubMed] [Google Scholar]
- (14).Melzack R. The short-form McGill Pain Questionnaire. Pain. 1987 Aug;30(2):191–197. doi: 10.1016/0304-3959(87)91074-8. [DOI] [PubMed] [Google Scholar]
- (15).Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3:77-77–101. [Google Scholar]
- (16).Corbin J, Strauss A. Basics of Qualitative Research: Techniques and Procedures for Developing Grounded Theory. 4th ed. Sage Publications; Los Angeles: 2014. [Google Scholar]
- (17).Glaser B, Strauss A. The Discovery of Grounded Theory: Strategies for Qualitative Research. Aldine Transaction; New Brunswick: 1999. [Google Scholar]
- (18).Charmaz K. ‘Discovering’ chronic illness: using grounded theory. Soc Sci Med. 1990;30(11):1161–1172. doi: 10.1016/0277-9536(90)90256-r. [DOI] [PubMed] [Google Scholar]
- (19).Denzin N, Lincoln Y, editors. The SAGE Handbook of Qualitative Research. 3rd ed. Sage Publications; Thousand Oaks, CA: 2005. [Google Scholar]
- (20).Huskisson EC. Measurement of pain. Lancet. 1974 Nov 9;2(7889):1127–1131. doi: 10.1016/s0140-6736(74)90884-8. [DOI] [PubMed] [Google Scholar]
- (21).Yokobe J, Kitahara M, Matsushima M, Uezono S. Preference for different anchor descriptors on visual analogue scales among Japanese patients with chronic pain. PLoS One. 2014 Jun 13;9(6):e99891. doi: 10.1371/journal.pone.0099891. [DOI] [PMC free article] [PubMed] [Google Scholar]