Abstract
Introduction
Parenteral nutrition (PN) in preterm infants leads to PN-associated liver disease (PNALD). PNALD has been linked to serum accumulation of phytosterols that are abundant in plant oil but absent in fish oil emulsions.
Hypothesis
Whether modifying the phytosterol and vitamin E composition of soy and fish oil lipid emulsions affects development of PNALD in preterm pigs.
Methods
We measured markers of PNALD in preterm pigs that received 14 days of PN that included 1 of the following: (1) Intralipid (IL, 100% soybean oil), (2) Intralipid + vitamin E (ILE, d-α-tocopherol), (3) Omegaven (OV, 100% fish oil), or (4) Omegaven + phytosterols (PS, β-sitosterol, campesterol, and stigmasterol).
Results
Serum levels of direct bilirubin, gamma glutamyl transferase, serum triglyceride, low-density lipoprotein, and hepatic triglyceride content were significantly lower (P < .05) in the ILE, OV, and PS compared to IL. Hepatic cholesterol 7-hydroxylase and organic solute transporter–α expression was lower (P < .05) and portal plasma FGF19 higher in the ILE, OV, and PS vs IL. Hepatic expression of mitochondrial carnitine palmitoyltransferase 1A and microsomal cytochrome P450 2E1 fatty acid oxidation genes was higher in ILE, OV, and PS vs IL. In vivo 13C-CDCA clearance and expression of pregnane X receptor target genes, cytochrome P450 3A29 and multidrug resistance-associated protein 2, were higher in ILE, OV, and PS vs IL.
Conclusions
α-tocopherol in Omegaven and added to Intralipid prevented serum and liver increases in biliary and lipidemic markers of PNALD in preterm piglets. The addition of phytosterols to Omegaven did not produce evidence of PNALD.
Keywords: neonates, life cycle, liver disease, research and diseases, phytosterols, nuclear receptors, cholestasis
Introduction
PN allows patients who cannot tolerate enteral feeding to obtain their nutrition requirements intravenously (IV). PN is especially important in neonatal infants with congenital or acquired gastrointestinal disorders and requiring small bowel resection who are exposed to longer periods of PN. Unfortunately, prolonged PN use is associated with PNALD, including conditions of fatty liver, cholestasis, and, in worst cases, fibrosis and liver failure; in short-bowel syndrome conditions, it is referred to as intestinal failure–associated liver disease.1–3 The pathogenesis and mechanism of injury leading to PNALD are unknown, but it is likely multifactorial.
The etiology of PNALD has been especially linked to exposure to parenteral lipid load and differences in PN and lipid composition.4–6 In the past decade, several lipid emulsions and their constituents have been evaluated as a possible causal factor in liver disease. In the United States, the primary emulsion available is the soybean oil–based lipid emulsion (Intralipid, Fresenius Kabi, Bad Homburg, Germany).4 In Europe, there are new-generation lipid emulsions, such as the pure fish oil–containing emulsion Omegaven (Fresenius Kabi); SMOFlipid (Fresenius Kabi), which contains soybean oil, medium-chain triglycerides (MCT) oil, olive oil, and fish oil; and Lipoplus (B. Braun, Bethlehem, PA), which contains soybean oil, MCT oil, and fish oil. In the United States, Omegaven is currently available for use only under an investigational new drug application for compassionate use in the treatment of pediatric cholestasis. Omegaven was designed to be used in combination with other lipid emulsions and not as a monotherapy and is not approved for pediatric use in any country. Omegaven was introduced in the United States originally based on evidence showing that it reversed cholestasis in 2 infants with intestinal failure and subsequent PNALD.7 Subsequent studies with larger cohorts have demonstrated that Omegaven has hepatoprotective effects that reverse PNALD.8,9 Early critics of these trials argued that it was merely the lower lipid load used that reversed cholestasis, but evidence from recent clinical studies to support this idea has been mixed.10–14 These recent clinical reports have focused attention on the lipid constituents as a casual factor in the etiology of PNALD.
Considerable evidence has demonstrated a strong correlation between the accumulation of circulating phytosterols and PNALD in infants.15–20 Phytosterols are plant sterols that are abundant in pure soybean and olive oils (Intralipid and ClinOleic) but also present in lower amounts in some new-generation emulsions containing these oils (SMOFlipid and Lipoplus).21 Phytosterols have been implicated in bile acid dysfunction, given their structural similarity to bile acids and their precursor, cholesterol.15,22 Studies supporting this using cultured hepatocytes showed that phytosterols antagonize the bile acid activation of bile salt export pump (BSEP) expression and farnesoid X receptor (FXR) promoter activity.22,23 Direct experimental evidence that phytosterols promote liver injury via activation of Kupffer cell inflammation and downregulation of hepatocyte sterol transporters was recently demonstrated in a mouse model of PNALD.24 In neonatal piglet models, the data are less consistent; Clayton et al treated piglets with direct injections of a mixed phytosterol solution for 14 days and observed elevated serum phytosterol concentrations and elevated serum bile acids.25 However, in our recent study in premature pigs receiving PN for 14 days, administration of both Omegaven and SMOFlipid protected piglets from PNALD, despite the lower infusion rate and plasma accumulation of phytosterols in pigs given SMOFlipid.23 These results suggest that in a neonatal model, phytosterols supplied in a commercial lipid emulsion, as compared to direct administration, may not be directly involved in the development of PNALD.
The protective effects of Omegaven and other new-generation lipid emulsions against PNALD have also been ascribed to other ingredients, including vitamin E and n-3 long chain polyunsaturated fatty acids (PUFAs), docosahexaenoic acid (DHA), and eicosapentaenoic acids. Vitamin E, especially α-tocopherol, is abundant in pure fish oil and new-generation emulsion blends.26 The soy-based emulsion, Intralipid, contains predominantly the γ-tocopherol form of vitamin E, which has a much lower bioactivity than does α-tocopherol.27 Vitamin E is the major lipid-soluble antioxidant that protects unsaturated fatty acid acyl chains in the cell membranes from oxidative damage and thus is added as α-tocopherol to lipid emulsions with high PUFA content to prevent peroxidation.28 Clinical studies have tested the therapeutic effect of dietary vitamin E to prevent nonalcoholic fatty liver disease with mixed success.29,30 However, the evidence and mechanisms that explain any possible benefits of the vitamin E or n-3 PUFA have not been shown.
The aim of the current study was to test whether modifying the phytosterol and vitamin E composition of soy and fish oil lipid emulsions affects the development of PNALD in preterm pigs. We accomplished this by adding phytosterols to Omegaven and α-tocopherol to Intralipid and comparing these to their parent emulsions in preterm pigs.
Material and Methods
The protocol was approved by the Animal Protocol Review Committee of Baylor College of Medicine and conducted in accordance with the Guide for the Care and Use of Laboratory Animals (Department of Health and Human Services publication no. 85–23, revised 1985, Office of Science and Health Reports, NIH, Bethesda, MD).
Animals and Surgery
Pregnant crossbred sows were obtained from the Texas Department of Criminal Justice (Huntsville, TX) and housed in the Children’s Nutrition Research Center. At gestation day 108, piglets were delivered 7 days preterm by cesarean section and immediately placed in cages at 31–32°C. On the first day of life, each pig underwent surgery for jugular vein catheter placement under general anesthesia with isoflurane as described previously.23 During the first 24 hour of life, each piglet received maternal serum (16 mL/kg IV) for passive immunological protection. The piglets also received antibiotics (enrofloxacin 5 mg/kg IV) on alternating days during the 14-day study.
Nutritional Regimen and Study Design
After birth and implantation of catheters, piglets delivered from each of 4 sows were assigned to 1 of the 4 treatment groups based on body weight: (1) PN + Intralipid (IL, 100% soybean oil), (2) PN + Intralipid + vitamin E (ILE), (3) PN + Omegaven (OV, 100% fish oil), or (4) PN + Omegaven + phytosterols (PS). All pigs received via jugular infusion PN that consisted of an elemental nutrition solution, containing a complete nutrient mixture of amino acids, glucose, electrolytes, vitamins, and trace minerals, and a parenteral lipid solution, which was infused separately. The PN intake was increased gradually from 50–100% over 7 days. At day 7, all PN pigs received full amounts of nutrition per kilogram body weight: fluid 240 mL, energy 195 kcal, protein 14 g, and lipid 5 g.
The 3 principal phytosterols found in Intralipid (campesterol, β-sitosterol, stigmasterol) were added to Omegaven in the PS group to match the total phytosterol concentration in Intralipid we had measured previously23 as follows: β-sitosterol (78.6%, containing campesterol) and stigmasterol (96.5%, Sigma-Aldrich, St Louis, MO) were dissolved in ethanol as 200× concentrate at 50°C. Then, under gentle stirring, Omegaven (1000 mL) was heated to 50°C before 5 mL of warm phytosterol concentrate was added. The resulting mixture (milligram per 1,000 mL: 153 β-sitosterol, 29 stigmasterol) was maintained at 50°C for another 15 minutes. All other lipid emulsions (IL, ILE, OV) were treated similarly by adding 5 mL of ethanol per 1,000 mL at 50°C.
Vitamin E was added to Intralipid in the ILE group to match the concentration contained in Omegaven with a similar biological activity of d-α-tocopherol equivalents. Briefly, 1.25 mL of natural d-α-tocopherol (201 mg/mL, Sparhawk Laboratories, Lanexa, KS) was added to 1,000 mL Intralipid at 50°C during the procedure described above, resulting in a final concentration of 251 mg d-α-tocopherol/L emulsion. At the end of the 15-minute heating period, vitamins A, D, and K were added to all lipid emulsions (IL, ILE, OV, PS) according to the nutritional requirements as follows: vitamin A at 0.1 µL/g lipid (500,000 IU/mL), vitamin D3 at 0.1 µL/g lipid (75,000 IU/mL), and vitamin K1 at 2.5 µL/g lipid (10 mg/mL).
Pigs were weighed every other day, and PN infusion rates were adjusted to maintain targeted nutrient delivery rates. Pigs were subjected to an in vivo 13C-CDCA disposal kinetics study on days 12–14. Immediately after the last blood sample was taken at day 14, the animals were euthanized with IV injection of pentobarbital sodium (50 mg/kg) and phenytoin sodium (5 mg/kg, Beuthanasia-D, Schering-Plough Animal Health, Kenilworth, NJ), and portal blood and bile samples were collected rapidly. The liver was removed and weighed, and tissue samples were frozen immediately in liquid nitrogen and stored at −80°C until analysis.
13C-CDCA Disposal Kinetics
To determine how each lipid treatment affects bile acid clearance from blood, we employed a stable isotopic tracer approach. On day 12, each piglet received [24-13C]chenode-oxycholic acid (13C-CDCA, 99%, Cambridge Isotope Laboratories, Inc, Andover, MA) as a bolus injection IV (25 mg/kg body weight at 100 mg/mL ethanol). Blood samples (0.5 mL each) were taken before (time 0 hours) and at 3, 6, 9, 12, 18, 24, 36, and 48 hours after the injection. Plasma samples were obtained as described below and stored at −80°C until analysis.
Sample Preparation and Analysis
Blood samples were collected in sodium-EDTA tubes and centrifuged at 1,500g at 4°C for 10 minutes. The plasma portion was stored at −80°C until analysis. Blood samples for serum biochemistry panel were collected in serum tubes, after 3 hours centrifuged at 1,500g for 10 minutes, and serum was stored at −80°C until analysis.
Serum analysis for aspartate aminotransferase, alanine aminotransferase (ALT), gamma glutamyl transferase (GGT), triglyceride, very low density lipoprotein (VLDL), and bilirubin (total, direct, and indirect) was performed on a Cobas Integra 400 plus analyzer (Roche Diagnostics Ltd, Rotkreuz, Switzerland). Total plasma bile acids were determined using a total bile acid kit (BQ Kits). Plasma bile acid pool was calculated as the product of total plasma bile acid concentration (micromolar) and blood volume assuming 80 mL/kg body weight. Total liver bile acid pool was calculated as the product of liver tissue total bile acid concentration (µmol/g) and liver weight (g liver/kg body weight).
Chenodeoxycholic acid analysis
Isotopic enrichments in plasma were measured by liquid chromatography tandem mass spectrometry (LC-MS/MS) as described previously.31 Sample preparation for bile acids was performed by solid-phase extraction (SPE) with 200 mg Bond Elut cartridges (Agilent Technologies, Santa Clara, CA) that were preconditioned by passing 2 mL of methanol followed by 2 mL LCMS grade water and 2 ml of 100 mmol/L ammonium carbonate buffer at pH 9.3, after which the prepared plasma sample is loaded on to the column. The plasma samples were prepared by mixing with an appropriate amount of internal standard (2H5 -CDCA to determine the concentration of CDCA by isotope dilution), 100 µL of 100 mmol/L ammonium carbonate buffer (pH 9.3), and 500 µL of LCMS-grade water. After sample loading on the SPE column and subsequent elution by gravity, the columns were washed with 2 mL LCMS-grade water followed by drying of the SPE columns under vacuum. The retained compounds were eluted with 3 mL methanol followed by drying and reconstitution with 60 µL of LCMS-grade methanol and 30 µL of ammonium acetate buffer 10 mM (pH 6.5) before being analyzed by LC-MS/MS.
Phytosterol analysis
Quantitation of phytosterols was achieved using LC-MS/MS modified from earlier reported methods.32,33 All analyses were performed using a high mass resolution mass spectrometer (Exactive Orbitrap, Thermo Fisher Scientific, Waltham, MA) equipped with an APCI probe in positive ion mode. The chromatographic separation of phytosterols were achieved using Accela 1200 LC pump and a 100 × 3.0-mm Kinetex, 2.6u XB-C18 100A, analytical column. The mobile phase consisted of acetonitrile:methanol (80:20), at an isocratic flow rate of 0.5 mL/min. The concentrations of free and total phytosterols in plasma/emulsions were determined before and after hydrolysis, respectively, by using the reverse isotope dilution methodology after adding appropriate amount of internal standards of the corresponding phytosterols. Sample preparation prior to LC-MS/MS analysis was performed by SPE with 200 mg Bond Elut cartridges (Agilent Technologies). Briefly, after the preconditioning of the C18 SPE columns with 2 mL of methanol followed by 2 mL of LCMS-grade water, prepared plasma/emulsion samples before or after hydrolysis (for free or total phytosterol concentration) were loaded on to the SPE columns and allowed to elute by gravity. After washing the column with 2 mL LCMS-grade water, SPE columns were dried under vacuum. The retained phytosterols were eluted by 3 mL of methanol and dried under vacuum, and the residues were reconstituted in 100 µL of the mobile phase (80:20 acetonitrile:methanol) before being analyzed by LC-MS/MS.
Vitamin E analysis
Vitamin E (α-tocopherol and γ-tocopherol) in plasma and liver was analyzed by HPLC as described previously.34,35 In brief, liver tissue was homogenized in 2 volumes of ethanol with an Ultra-Turrax homogenizer on ice. Aliquots of the homogenates corresponding to 200 mg liver were saponified in a mixture of ethanol, methanol, ascorbic acid (20% w/v), and KOH-water (1:1 w/v) at 80°C for 30 minutes, subsequently cooled, and extracted twice with 5 mL of heptane. The HPLC column for determination of tocopherol consisted of a 4.0 × 125-mm Perkin-Elmer HS-5-Silica column (D-7770, Perkin-Elmer GmbH, Überlingen, Germany). The mobile phase consisted of heptane containing 2-propanol (3.0 mL/L) and degassed with helium. The flow rate was 3.0 mL/min. A comparison of retention time and peak areas with Merck (D-6100, Damstadt, Germany) external standards were used to obtain the identification and quantification of the tocopherol. Fluorescence detection was performed with an excitation wavelength of 290 nm and an emission wavelength of 327 nm.
Tissue Gene Expression
Quantitative real-time polymerase chain reaction (qPCR) was performed on frozen liver samples. Total RNA was first isolated from approximately 100 mg of liver tissue with Trizol and RNeasy Mini kits (Qiagen, Hilden, Germany). RNA concentration was measured via a Nanodrop spectrophotometer (Thermo Fisher Scientific). Isolated RNA was used for the reverse transcription reactions using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Thermo Fisher Scientific). Reverse transcription was performed in our thermal cycler after the master mix and RNA samples were combined per protocol in 700 µL tubes. cDNA products were subsequently stored in −4°C prior to real-time qPCR. Real-time qPCR was performed in 96-well format using commercially available kits made by Invitrogen (Superscript III Platinum Two-Step qPCR Kit with SYBR Green, Invitrogen, Burlington, ON, Canada) on Bio Rad (CFX96) PCR machine. Primers were designed using the Web-based assay design software from NCBI Primer BLAST (Table 1). Relative quantification of target mRNA expression was calculated and normalized to beta-actin expression. All reactions were performed in triplicate wells for each animal cDNA sample under the following thermal cycling conditions: 10 minutes at 95°, followed by 40 cycles of 95° for 15 seconds and 60° for 60 seconds. The relative quantification of target mRNA expression was calculated and normalized to beta-actin expression using the 2−ΔΔCT-method and expressed as fold change relative to the IL group.
Table 1.
Forward and Reverse Primers for Porcine Gene Quantification by Quantitative Real-Time Polymerase Chain Reaction.
| Gene | Primer | Accession Number |
|---|---|---|
| ACOX | 5’TCCAAACGACTGGAGATCA 5’CACAGTAGGGCTATTGAGAA |
JQ087330.1 |
| BSEP | 5’ TTTCATTCAGCGCCTGACCA 5’ ACTCCAATGAGAGGGCTGAC |
XM_003133457 |
| CPT1A | 5’ GGGACAAAAGGCCCTTGTTT 5’ CCAGCACATCTGCACTCAAA |
NM_001129805 |
| CYP2E1 | 5’ CTCCAACCTGCCACATGAAGC 5’ GGAATTACCACTGTGCCCTTG |
NM_214421.1 |
| CYP3A29 | 5’GTGGAGTGTTACATACGGGC 5’AGGTGATACTAGGTGGGGGT |
NM_214423 |
| CYP7A1 | 5’GAAAGAGAGACCACATCTCGG 5’GAATGGTGTTGGCTTGCGAT |
NM_001005352 |
| FGF19 | 5’AAGATGCAAGGGCAGACTCA 5’AGATGGTGTTTCTTGGACCAGT |
ENSSSCG00000012871 |
| FXR | 5’TTTGTGTCGTTTGCGGAGAG 5’GTTGCCCCCATTTTTACACTTG |
XM_003481738 |
| MRP2 | 5’ GAACAGGTTTGCTGGCGATATT 5’ ACCCTGGATGACACCCTCCCC |
DQ530510 |
| OSTα | 5’ TGTACAAGAACACTCGCTGC 5’ GAACACACACACTATCGTGGG |
NM_001244266XM_003132614 |
| SHP | 5’ GCCTACCTGAAAGGGACCAT 5’ CAACGGGTGTCAAGCCTTTA |
DQ002896 |
ACOX, acyl-CoA oxidase; BSEP, bile salt export pump; CPT1A, carnitine palmitoyltransferase 1A; CYP2E1, cytochrome P450 2E1; CYP3A29, cytochrome P450 3A29; CYP7A1, cholesterol 7-hydroxylase; FXR, farnesoid X receptor; MRP2, multidrug resistance-associated protein 2; OST, organic solute transporter; SHP, small heterodimer partner.
Immunoblot analysis
Western immunoblotting of tissue samples was described previously.21 Briefly, frozen liver was homogenized in buffer containing 50 mM HEPES (pH 7.4), 1 mM EDTA, 1 mM dithiothreitol, 5 mg/L phenylmethylsulfonyl-fluoride, 5 mg/L aprotinin, 5 mg/L chymostatin, and 5 mg/L pepstatin. The homogenate was sonicated and centrifuged at 12,000 g for 15 minutes at 4°C. The extracts (120 µg protein/lane) were separated via 10% SDS-PAGE; transferred to nitrocellulose membranes; and, after blocking with 5% nonfat milk in tris-buffered saline (20 mM Tris, 150 mM NaOH, pH 7.4), incubated with a primary antibody diluted in 5% nonfat milk in tris-buffered saline + 0.1% Tween-20. The membranes were probed with anticholesterol 7-hydroxylase (cholesterol 7-hydroxylase [CYP7A1]) (1:1,000, mouse polyclonal antibody, Cosmo Bio, Carlsbad, CA), which produced a single band at approximately molecular weight 50 kDa. The loading control was anti-alpha-tubulin (1:10,000 dilution, mouse monoclonal antibody, Sigma-Aldrich). Membranes were incubated with a secondary antibody (goat anti-mouse IgG-HRP, 1:5,000, Santa Cruz Biotechnology, Dallas, TX). Signal was developed by ECL-plus (Amersham Biosciences, Piscataway, NJ) and then detected by Bio-Rad Chemi Doc (Bio-Rad Laboratories, Hercules, CA), and the image was quantified by ImageQuant 5.0 software (Molecular Dynamics, Sunnyvale, CA). We expressed the abundances of specific target proteins relative to that of tubulin measured after stripping and reprobing membranes. All Western blots were run with 6 samples from each treatment group and used for statistical analysis. The ratios of each protein relative to tubulin are shown relative to the Intralipid control group.
Porcine pregnane X receptor (PXR) luciferase reporter assay
Porcine PXR was cloned into pcDNA3.1/V5-His TOPO vector (Invitrogen) as previously described.36 HepG2 cells were cultured in Eagle MEM (Earle’s balanced salt solution, nonessential amino acids, 1mM sodium pyruvate, 2mM L-glutamine, 1500 mg sodium bicarbonate/L) (ATCC, Manassas, VA) supplemented with 10% FBS and 1% v/v penicillin/streptomycin (Invitrogen). Cells were plated in 24-well plates at a seeding density of 1.0 × 106 cells per well. Twenty-four hours after plating, cells were transfected with a PXR expression plasmid (250 ng/ well), a pRL-tk control plasmid (5 ng/well), and an XREM-3A4-tk-luciferase reporter plasmid for PXR37 (250 ng/well), using Lipofectamine 2000 (Invitrogen) following the manufacturer’s instructions. Twenty-four hours after transfection, cells were treated with rifampicin (Sigma-Aldrich) or with vitamin E or lipid emulsions (IL or OV). Rifampicin and vitamin E stocks were made in DMSO and then diluted to final concentrations in culture media. A DMSO control treatment was also used, with DMSO at 0.05% (v/v) in culture media. Twenty-four hours after ligand treatment, the media were removed, cells were washed in 1× phosphate-buffered saline, and cells were lysed following the Dual-Luciferase Assay protocol (Promega, Madison, WI). The Dual-Luciferase assay was carried out following manufacturer’s instructions using a Sirius single-tube luminometer with dual injectors (Berthold Detection Systems, Oak Ridge, TN).
Statistics
Statistical analyses were performed using SPSS 17 and SAS software. One-way analysis of variance, Kruskal-Wallis, and Mann-Whitney U tests were used to compare differences between the treatment groups. P values of <.05 were considered significant. The kinetics of 13C-CDCA clearance were estimated using an exponential model, and goodness of fit of the model was determined by graphical residual analysis (PROC NLIN, SAS v. 9.2, SAS Institute Inc, Cary, NC). The terminal half-life after reaching pseudo-equilibrium and mean residence time were calculated as described previously.38 Results are presented as mean ± standard error of the mean (SEM).
Results
Body weight gain from birth to day 14 was not statistically different among the 4 groups (mean ± SEM in g/[kg·d]): IL, 51.8 ± 1.4; ILE, 47.4 ± 1.1; OV, 55.8 ± 1.8; PS, 57.1 ± 4.5). Liver weight was highest in the IL pigs but not statistically different when compared to the other 3 groups (mean liver weight ± SEM in g/kg body weight): IL, 51.1 ± 1.6; ILE, 44.9 ± 2.3; OV, 45.4 ± 1.6; PS, 46.9 ± 1.4).
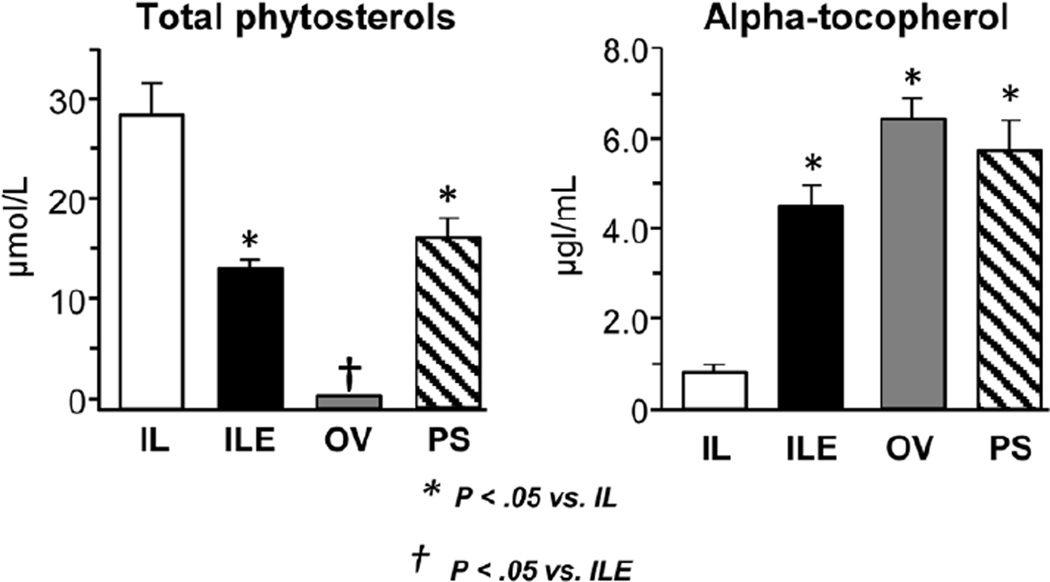
Figure 1 shows the plasma concentrations of total phytosterols and α-tocopherol. As expected, the IL group had the highest total phytosterol concentration, whereas OV pigs had undetectable levels. The plasma concentrations of the 3 principal phytosterols, β-sitosterol, campesterol, and stigmasterol, were in a similar proportion to that found in the 4 lipid emulsion preparations (see Table 2). Interestingly, the plasma phytosterol concentrations in ILE and PS groups were lower (P < .05) than in the IL group despite the fact that the total phytosterol load infused was not significantly different among these 3 groups. The plasma tocopherol infusion loads and the plasma and liver concentrations are shown in Figure 1 and Table 3. The IL and ILE emulsion γ-tocopherol concentrations (not shown) and daily intakes of γ-tocopherol were higher (P < .05) than in OV and PS pigs; γ-tocopherol was not detectable in the OV and PS emulsions. The total α-tocopherol concentration and daily intakes were higher (P < .05) in ILE, OV, and PS groups vs IL group. The higher intake of γ-tocopherol in the IL and ILE groups was reflected in its presence in the liver, but it was not detected in plasma or liver from OV and PS pigs. The plasma (~4–6 fold) and liver tissue (30–48 fold) concentrations of total α-tocopherols were significantly higher (P < .05) in the ILE, OV, and PS groups compared to the IL pigs; the total α-tocopherols in liver were higher in ILE and OV than in PS. Approximately 4,000 µg of γ-tocopherol was infused daily in IL and ILE groups, yet the liver content was 6–9 µg/g tissue, whereas for α-tocopherol the infusion rates ranged from 983 (IL) to as high as 5,900 (ILE), and the liver tissue contents were much higher (20–241 µg/g tissue) on average, suggesting more efficient hepatic uptake of α-tocopherol vs γ-tocopherol. The stereoisomer composition of α-tocopherols in plasma and liver tissue in the ILE vs OV and PS groups reflected the difference in the chemical forms (natural vs synthetic) of α-tocopherol added to these emulsions. The natural d-α-tocopherol we added to the IL emulsion to produce the ILE emulsion was 100% RRR isomer, whereas the synthetic α-tocopherol added to OV by the manufacturer was a mixture of isomers in the form of all-rac-α-tocopherol.
Figure 1.
Plasma concentrations of total phytosterols and total alpha-tocopherol in preterm piglets after 14 days of treatment with PN plus the following: Intralipid (IL), Intralipid plus vitamin E (ILE), Omegaven (OV), or Omegaven plus phytosterols (PS). Values are means ± SEM; n = 8–10 pigs/group. Differences between treatment groups were analyzed using ANOVA, Kruskal-Wallis, and Mann-Whitney U tests. *P < .05 vs IL. ANOVA, analysis of variance; PN, parenteral nutrition; SEM, standard error of the mean.
Table 2.
Daily Intake and Plasma Concentrations for Individual Phytosterols, β-Sitosterol, Campesterol, and Stigmasterol, in Preterm Pigs After 14 Days of Treatment.
| Variable | IL | ILE | OV | PS |
|---|---|---|---|---|
| Lipid intake, g/(kg·d) | 5 | 5 | 5 | 5 |
| Phytosterol intake, µmoL/(kg·d)1 | ||||
| β-sitosterol | 18.96 | 18.96 | nd | 27.96 |
| Campesterol | 3.63 | 3.63 | nd | 1.58 |
| Stigmasterol | 9.36 | 9.36 | nd | 9.30 |
| Total phytosterol | 31.94 | 31.94 | nd | 38.84 |
| Plasma phytosterol, µM | ||||
| β-sitosterol | 16.89 ± 2.09a | 7.83 ± 0.34 | nd | 11.41 ± 1.57 |
| Campesterol | 5.14 ± 0.55a | 2.63 ± 0.12 | nd | 1.46 ± 0.16 |
| Stigmasterol | 6.42 ± 0.84a | 2.25 ± 0.19 | nd | 2.80 ± 0.47 |
| Total phytosterol | 28.45 ± 3.40a | 12.71 ± 0.65 | nd | 15.66 ± 2.19 |
Values are means ± SEM; n = 8–10 pigs/group. Differences between treatment groups in plasma values were analyzed using ANOVA, Kruskal-Wallis, and Mann-Whitney U tests. Values in the same row with different superscripts differ P < .05. Groups are parenteral nutrition plus the following: Intralipid (IL), Intralipid plus vitamin E (ILE), Omegaven (OV), or Omegaven plus phytosterols (PS). ANOVA, analysis of variance; nd, not detected; SEM, standard error of the mean.
Table 3.
Daily Intake and Plasma and Liver Concentrations for Individual Vitamin E Forms in Preterm Pigs After 14 Days of Treatment.1
| Variable | IL | ILE | OV | PS |
|---|---|---|---|---|
| Vitamin E intake, µg/(kg·d) | ||||
| γ-tocopherol | 3,950 | 3,950 | nd | nd |
| α-tocopherol-2S | 0 | 0 | 4,432 | 4,432 |
| α-tocopherol-RSS | 0 | 0 | 1,099 | 1,099 |
| α-tocopherol-RRS | 0 | 0 | 998 | 998 |
| α-tocopherol-RRR | 983 | 5,900 | 1,594 | 1,594 |
| α-tocopherol-RSR | 0 | 0 | 1,594 | 1,594 |
| Total α-tocopherol | 983 | 5,900 | 9,717 | 9,717 |
| Total tocopherol | 4,933 | 9,850 | 9,717 | 9,717 |
| Plasma vitamin E isomers, µg/mL | ||||
| α-tocopherol-2S | nd | nd | 1.781 ± 0.108 | 1.461 ± 0.118 |
| α-tocopherol-RSS | nd | nd | 1.187 ± 0.072 | 0.974 ± 0.078 |
| α-tocopherol-RRS | nd | nd | 1.261 ± 0.077 | 1.034 ± 0.083 |
| α-tocopherol-RRR | 0.999 ± 0.043a | 4.647 ± 0.568b | 0.264 ± 0.016a | 0.216 ± 0.017a |
| α-tocopherol-RSR | nd | nd | 0.960 ± 0.058 | 0.788 ± 0.063 |
| Total α-tocopherol | 0.999 ± 0.043a | 4.647 ± 0.568b | 6.670 ± 0.406b | 5.471 ± 0.440b |
| Liver vitamin E, µg/g tissue | ||||
| γ-tocopherol | 9.57 ± 1.02 | 6.25 ± 0.96 | nd | nd |
| α-tocopherol-2S | nd | nd | 62.0 ± 7.0 | 53.1 ± 3.5 |
| α-tocopherol-RSS | nd | nd | 33.4 ± 3.8 | 22.7 ± 1.5 |
| α-tocopherol-RRS | nd | nd | 29.2 ± 3.3 | 21.0 ± 1.4 |
| α-tocopherol-RRR | 6.12 ± 0.72a | 241.9 ± 12.8b | 48.4 ± 5.5c | 33.9 ± 2.2c |
| α-tocopherol-RSR | nd | nd | 32.3 ± 3.7 | 23.1 ± 1.5 |
| Total α-tocopherol | 6.12 ± 0.72a | 241.9 ± 12.8b | 205.3 ± 23.2b | 153.8 ± 10.1c |
Values are means ± SEM; n = 8–10 pigs/group. Differences between treatment groups in plasma and liver values were analyzed using ANOVA, Kruskal-Wallis, and Mann-Whitney U tests. Values in the same row with different superscript differ P < .05. Groups are parenteral nutrition plus the following: Intralipid (IL), Intralipid plus vitamin E (ILE), Omegaven (OV), or Omegaven plus phytosterols (PS). ANOVA, analysis of variance; nd, not detected; SEM, standard error of the mean.
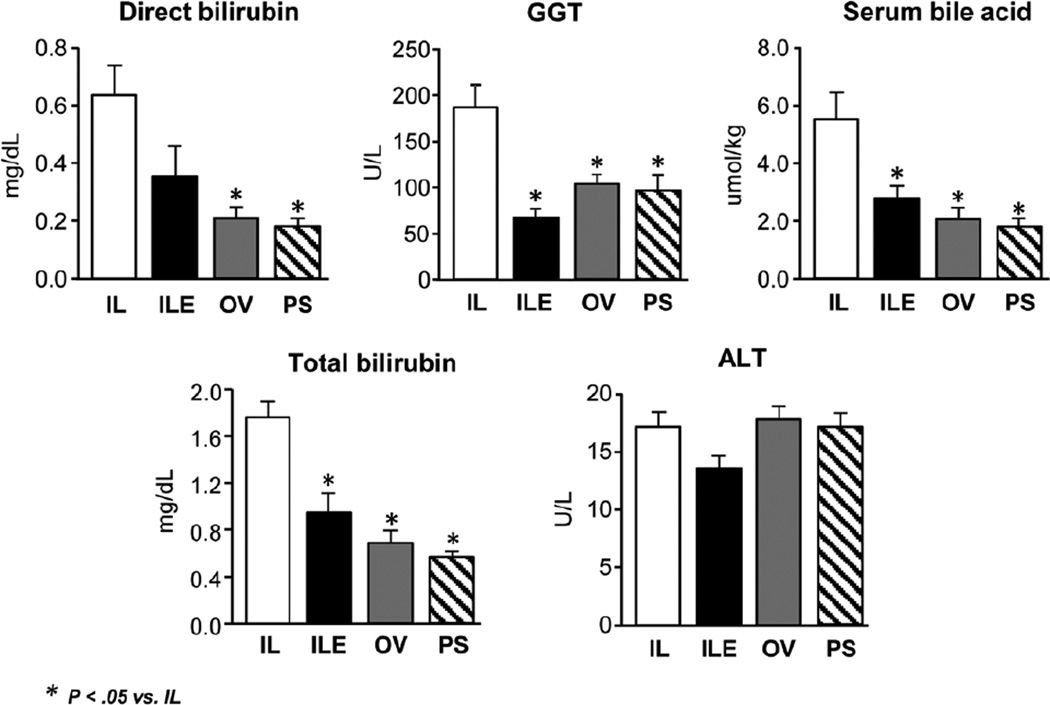
Serum biomarkers measured after 14 days of treatment showed that serum GGT was highest (P < .05) in the IL group vs ILE, OV, and PS (which were similar) (Figure 2). It is important to note that the mean GGT in the IL group was more than twice the mean of the ILE group. Similarly, the IL group had the highest direct and total bilirubin levels among the 4 groups (P < .05). Despite these changes, serum ALT was similar among the 4 treatment groups. These findings mirror the results from our previous work, which again demonstrated biliary injury but not hepatocellular injury in this porcine model.23 We measured the bile acid pools, which indicated that the serum bile acid pool was largest in the IL group vs the ILE, OV, and PS groups (P < .05) (Figure 2). Conversely and to our surprise, the liver bile acid pool was lowest in the IL group as compared to the other 3 groups. This may suggest an adaptive response to redistribute the bile acid pool when exposed to different parenteral diets.
Figure 2.
Serum markers of hepatic injury and cholestasis including direct bilirubin, total bilirubin, bile acids, GGT, and ALT in preterm piglets after 14 days of treatment PN plus the following: Intralipid (IL), Intralipid plus vitamin E (ILE), Omegaven (OV), or Omegaven plus phytosterols (PS). Values are means ± SEM; n = 8–10 pigs/group. Differences between treatment groups were analyzed using ANOVA, Kruskal-Wallis, and Mann-Whitney U tests. *P < .05 vs IL. ALT, alanine aminotransferase; ANOVA, analysis of variance; GGT, gamma glutamyl transferase; PN, parenteral nutrition; SEM, standard error of the mean.
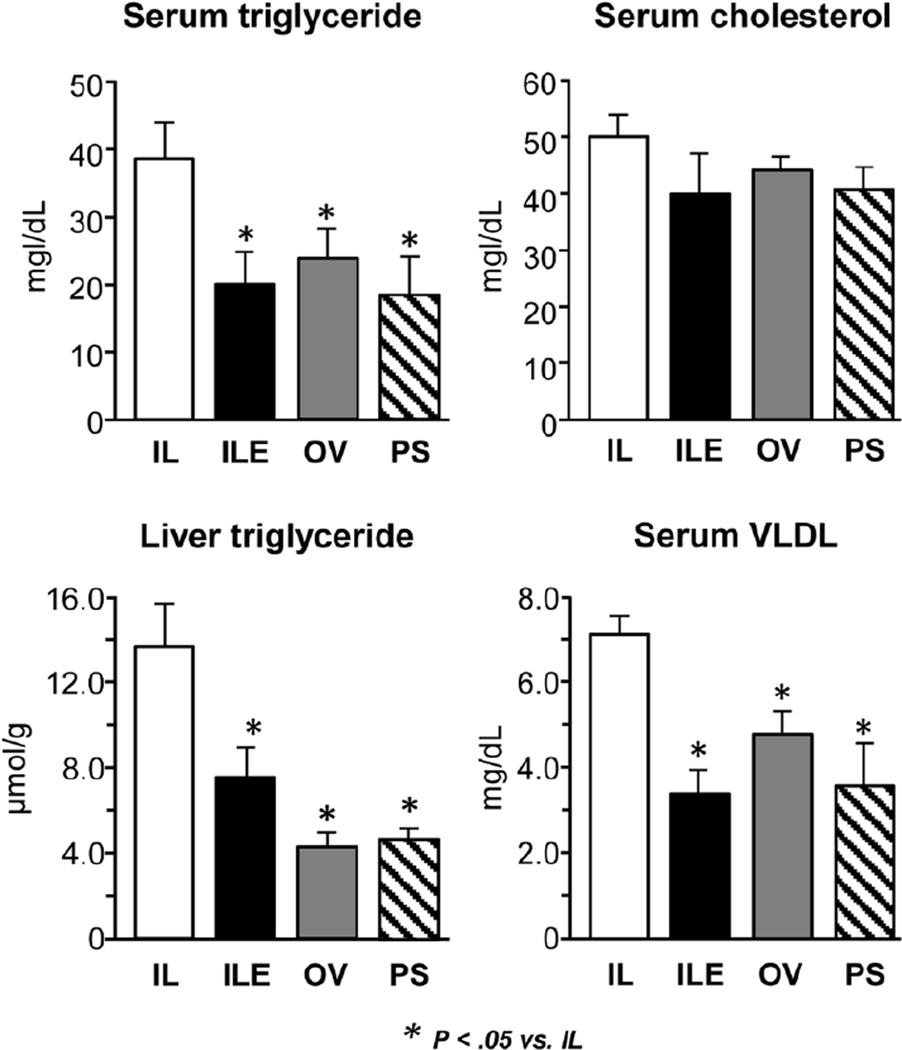
To assess other markers of hepatic dysfunction, we measured liver and serum triglyceride along with VLDL (Figure 3). Both liver and serum triglyceride concentrations were higher in the IL group vs the ILE, OV, and PS groups (P < .05). Similarly, the VLDL level in the IL pigs was higher compared to the other 3 diet groups (P < .05).
Figure 3.
Measurements of liver and serum triglycerides in preterm piglets after 14 days of treatment with PN plus the following: Intralipid (IL), Intralipid plus vitamin E (ILE), Omegaven (OV), or Omegaven plus phytosterols (PS). Values are means ± SEM; n = 8–10 pigs/group. Differences between treatment groups were analyzed using ANOVA, Kruskal-Wallis, and Mann-Whitney U tests. *P < .05 vs IL. ANOVA, analysis of variance; PN, parenteral nutrition; SEM, standard error of the mean.
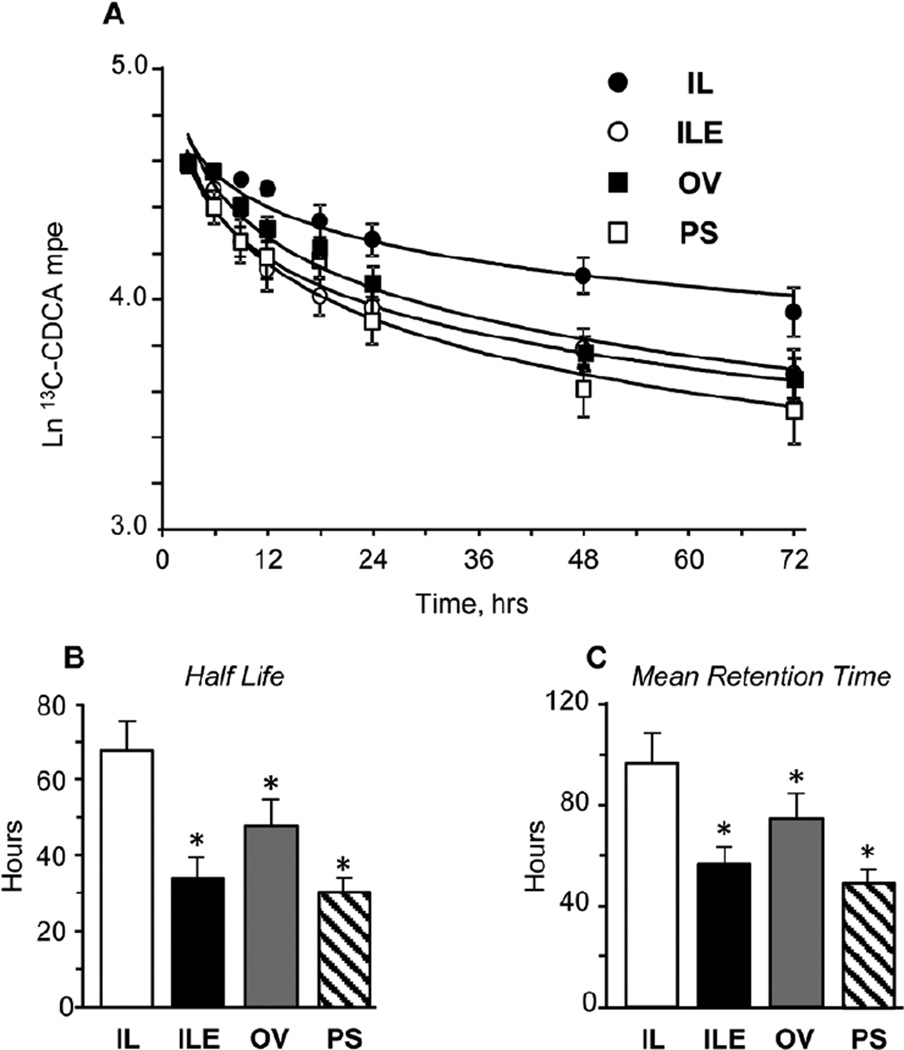
The differences in the serum bile acid concentrations among the 4 groups were further probed by measuring the kinetics of plasma 13C-CDCA clearance as shown in Figure 4. Panel A illustrates the changes in the isotopic enrichments in the 72-hour period during which clearance was measured; the values are expressed as the natural log of the measured enrichment in mole percentage excess. Half-life and mean retention time parameters of plasma 13C-CDCA clearance were estimated and are shown in panels B and C. The results show that in all 3 groups (ILE, OV, and PS) given lipid emulsions enriched with vitamin E, the rates of 13C-CDCA clearance were higher and both the half-life and mean retention times lower than in IL pigs. The half-life and mean retention times ranged from 30–90 hours among the 4 groups.
Figure 4.
Kinetics of plasma 13C-CDCA clearance measured between days 11–14 in preterm piglets given treatment of PN plus the following: Intralipid (IL), Intralipid plus vitamin E (ILE), Omegaven (OV), or Omegaven plus phytosterols (PS). Values are means ± SEM; n = 8–10 pigs/group. *P < .05 vs IL. Panel A shows time-dependent changes in 13C-CDCA enrichment expressed as natural log of mole percentage excess (Ln mpe) during the 72-hour period; panels B and C show the parameters of whole-body half-life and mean retention time of 13C-CDCA calculated as described in the “Statistics” section. PN, parenteral nutrition; SEM, standard error of the mean.
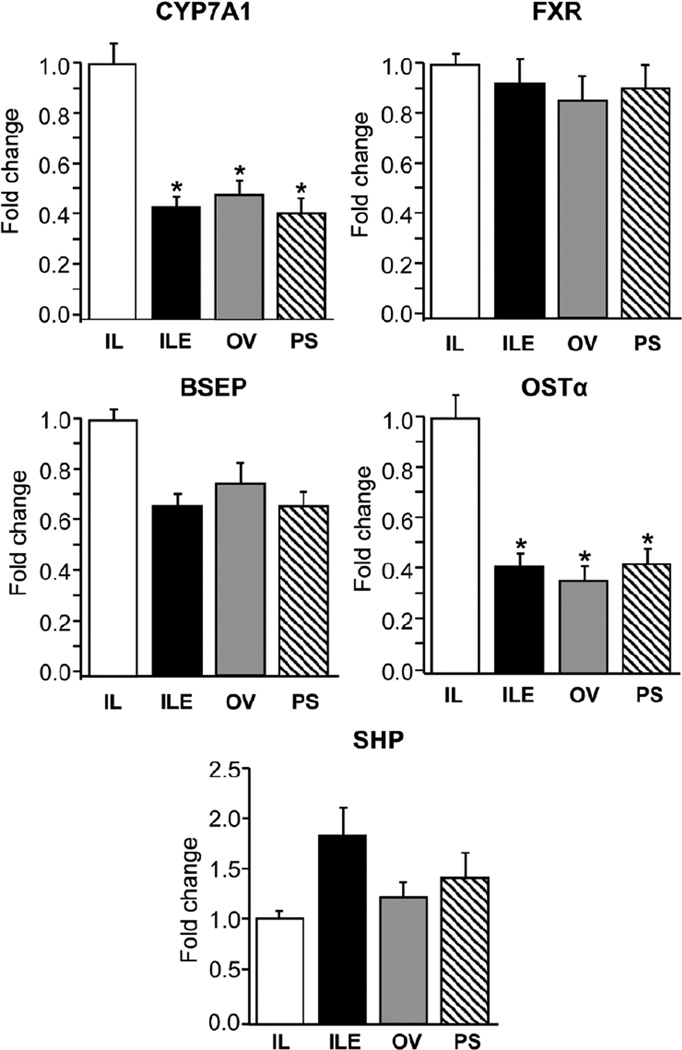
The differences in CDCA clearance prompted us to further explore the hepatic expression of important downstream FXR target genes involved in bile acid homeostasis (Figure 5). Hepatic expression of CYP7A1 mRNA, the rate-limiting enzyme involved in bile acid synthesis, was lower (P < .05) in the ILE, OV, and PS groups vs IL; however, the CYP7A1 protein abundance was similar among all 4 groups (results not shown). The hepatic expression of BSEP, a canalicular bile acid export protein, was slightly lower (P > .05) in the ILE, OV, and PS pigs vs IL. However, expression of organic solute transporter-α (OSTα), the sinusoidal bile acid export protein, was lower (P < .05) in the ILE, OV, and PS groups vs IL. There were no differences in either FXR or small heterodimer partner (SHP) expression among the 4 groups.
Figure 5.
Hepatic expression of genes involved in sensing (FXR, SHP), transport (BSEP, OSTα), and synthesis (CYP7A1) of bile acids in preterm piglets after 14 days of treatment with PN plus the following: Intralipid (IL), Intralipid plus vitamin E (ILE), Omegaven (OV), or Omegaven plus phytosterols (PS). Quantitative RT-PCR analysis showing relative transcript levels of BSEP, OSTα, CYP7A1, FXR, and SHP mRNA. Values are means ± SEM; n = 8–10 pigs/group. Differences between treatment groups were analyzed using ANOVA, Kruskal-Wallis, and Mann-Whitney U tests; *P < .05 vs IL. ANOVA, analysis of variance; BSEP, bile salt export pump; CYP7A1, cholesterol 7-hydroxylase; FXR, farnesoid X receptor; OST, organic solute transporter; PN, parenteral nutrition; RT-PCR, real-time polymerase chain reaction. SEM, standard error of the mean; SHP, small heterodimer partner.
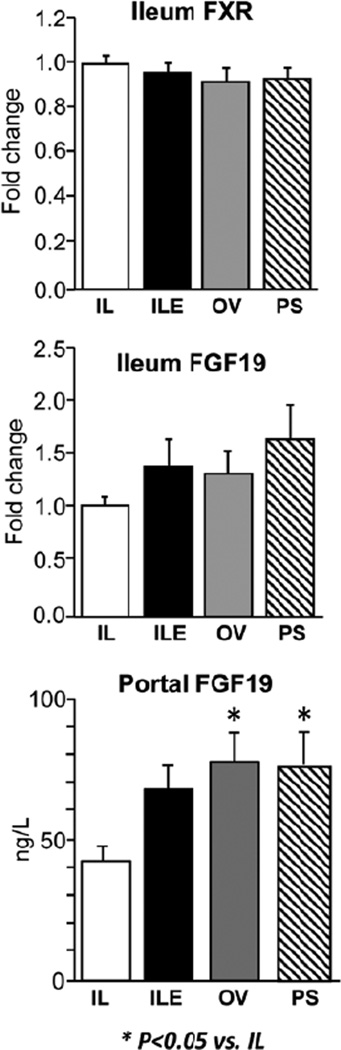
The hepatic expression of CYP7A1 is regulated by FGF19, and so we sought to test whether this was involved in the suppression of CYP7A1 among the various groups. The intestinal expression of FXR and its target, FGF19, was not statistically different among the 4 groups (Figure 6). However, the portal plasma FGF19 concentration was higher (P < .05) in the ILE, OV, and PS groups.
Figure 6.
Ileum expression of bile acid homeostatic genes (FXR, FGF19) and hormones (FGF19) in preterm piglets after 14 days of treatment with PN plus the following: Intralipid (IL), Intralipid plus vitamin E (ILE), Omegaven (OV), or Omegaven plus phytosterols (PS). Quantitative RT-PCR analysis shows relative transcript levels of FXR and FGF19 mRNA and plasma concentrations of FGF19 as described previously.23 Values are means ± SEM; n = 8–10 pigs/group. Differences between treatment groups were analyzed using ANOVA, Kruskal-Wallis, and Mann-Whitney U tests; *P < .05 vs IL. ANOVA, analysis of variance; FXR, farnesoid X receptor; PN, parenteral nutrition; RT-PCR, real-time polymerase chain reaction; SEM, standard error of the mean.
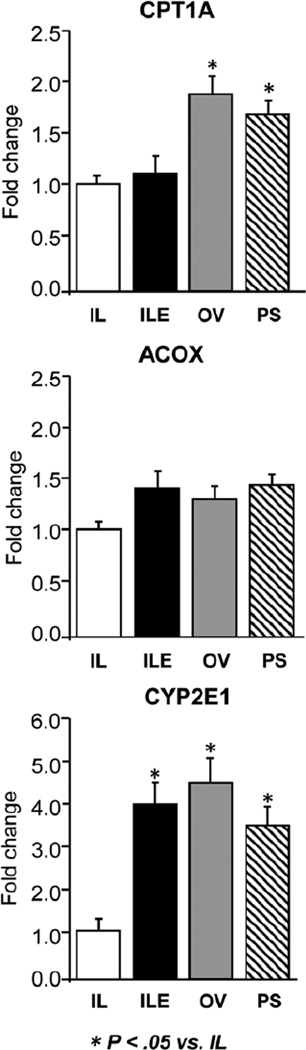
To explore potential underlying mechanisms to explain the changes in serum and hepatic lipidemia in the ILE, OV, and PS groups, we measured the hepatic expression of key genes involved in mitochondrial (carnitine palmitoyltransferase 1A [CPT1A]), peroxisomal (acyl-CoA oxidase [ACOX]), and microsomal (cytochrome P450 2E1 [CYP2E1]) fatty acid oxidation (Figure 7). The expression of all 3 genes tended to be lower in IL vs ILE, OV, and PS groups, and this was significant for CPT1A and CYP2E1.
Figure 7.
Hepatic expression of genes involved in mitochondrial (CPT1A), peroxisomal (ACOX), and microsomal (CYP2E1) fatty acid metabolism in preterm piglets after 14 days of treatment with PN plus the following: Intralipid (IL), Intralipid plus vitamin E (ILE), Omegaven (OV), or Omegaven plus phytosterols (PS). Quantitative RT-PCR analysis shows relative transcript levels of mRNA. Values are means ± SEM; n = 8–10 pigs/group. Differences between treatment groups were analyzed using ANOVA, Kruskal-Wallis, and Mann-Whitney U tests; *P < .05 vs IL. ACOX, acyl-CoA oxidase; ANOVA, analysis of variance; CPT1A, carnitine palmitoyltransferase 1A; CYP2E1, Cytochrome P450 2E1; PN, parenteral nutrition; RT-PCR, real-time polymerase chain reaction; SEM, standard error of the mean.
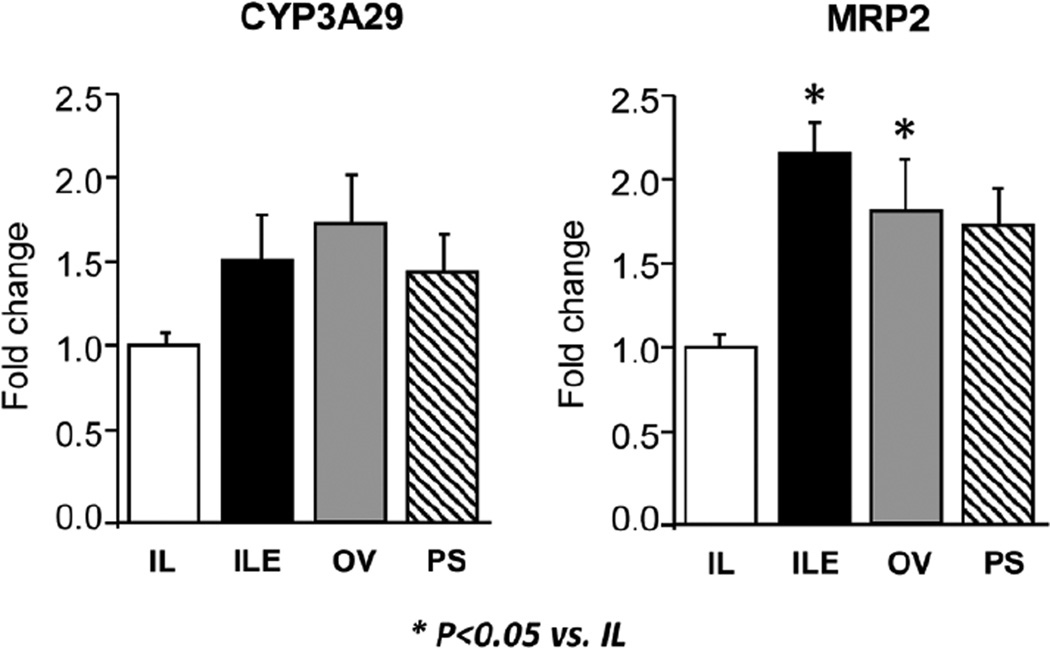
Finally, we measured the hepatic expression of 2 target genes of the xenobiotic sensing, nuclear receptors, PXR (Figure 8). Hepatic cytochrome P450 3A29 (CYP3A29) is the analog of human CYP3A and is involved in hydroxylation as the first step in detoxification of compounds including bile acids. CYP3A29 expression tended (P = .08) to be higher in ILE, OV, and PS groups. However, the expression of multidrug resistance-associated protein 2 (MRP2), involved in bilirubin transport into bile, was higher in ILE and OV pigs vs the IL group.
Figure 8.
Hepatic expression of PXR target genes involved in microsomal bile acid metabolism (CYP3A29) and organic ion efflux (MRP2) in preterm piglets after 14 days of treatment with PN plus the following: Intralipid (IL), Intralipid plus vitamin E (ILE), Omegaven (OV), or Omegaven plus phytosterols (PS). Quantitative RT-PCR analysis shows relative transcript levels of mRNA. Values are means ± SEM; n = 8–10 pigs/group. Differences between treatment groups were analyzed using ANOVA, Kruskal-Wallis, and Mann-Whitney U tests; *P < .05 vs IL. ANOVA, analysis of variance; CYP3A29, cytochrome P450 3A29; MRP2, multidrug resistance-associated protein 2; PN, parenteral nutrition; PXR, pregnane X receptor; RT-PCR, real-time polymerase chain reaction; SEM, standard error of the mean.
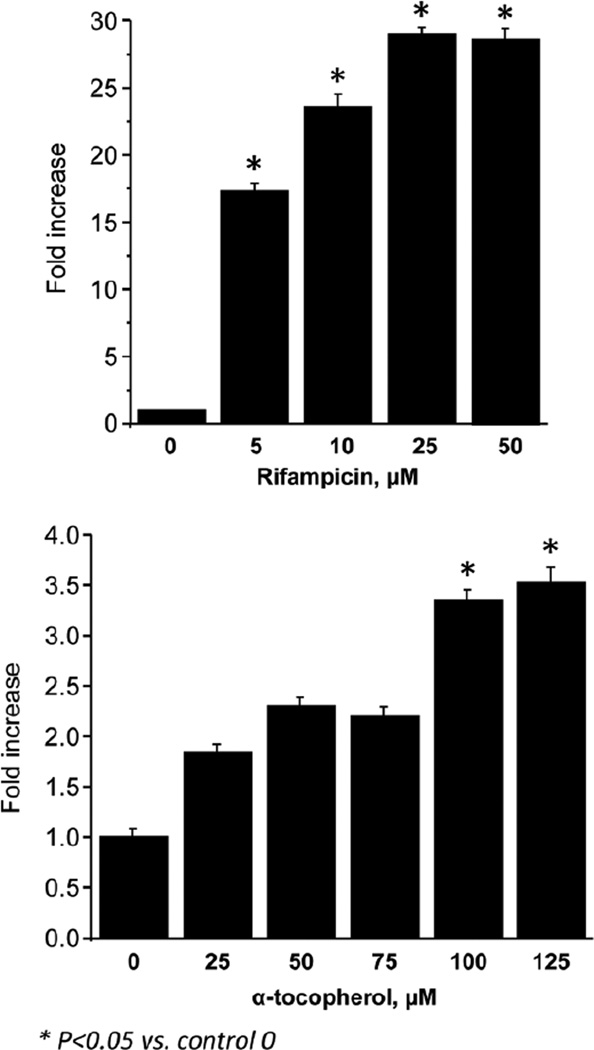
Because these genes are targets of PXR, we examined whether the porcine PXR promoter was sensitive to rifampicin and α-tocopherol. We used HepG2 cells transiently transfected with the porcine PXR promoter and then treated them with increasing concentrations of both rifampicin and α-tocopherol for 24 hours. Results from these luciferase assays with HepG2 cells showed that porcine PXR promoter was markedly induced by rifampicin and to a lesser degree but significantly also by α-tocopherol (Figure 9).
Figure 9.
Fold-change in PXR luciferase assay in which HepG2 cells were transiently transfected with porcine PXR constructs, and luciferase activity was measured after 24 hours of treatment with PXR agonist rifampicin (0–50 µM) or natural d-α-tocopherol (0–125 µM) as described in detail in the “Material and Methods” section. All treated groups were compared statistically to control incubations with no treatment using ANOVA; *P < .05 vs control; regression analysis showed significant (P < .01) linear dose effects for both rifampicin and α-tocopherol. ANOVA, analysis of variance; PXR, pregnane X receptor.
Discussion
PN is a lifesaving nutritional modality, especially for support of preterm infants who are dependent on partial or full PN for an extended period of time. Unfortunately, prolonged PN use can lead to PNALD, and there is increasing evidence that the lipid component of PN is a key contributing cause.6 Recent clinical trials have suggested parenteral lipid load reduction as a therapeutic strategy,10,13 but there is a growing body of work implicating different parenteral lipid emulsion ingredients in the etiology of PNALD, especially phytosterols and vitamin E5. In the current study, we show important new evidence that vitamin E, especially α-tocopherol, present in Omegaven and when added to Intralipid is a key nutrient involved in prevention of PNALD in preterm pigs. Importantly, our results also show that the addition of the 3 major phytosterols (β-sitosterol, campesterol, and stigmasterol) to Omegaven did not increase biomarkers of PNALD.
A major aim of the study was to test the relative effects of both phytosterols and vitamin E present in commercial lipid emulsions on the development of PNALD in our preterm PN-fed piglet model. We were interested in phytosterols given the numerous reports that they accumulate in blood of pediatric liver disease patients given IV PN with soy-based lipid emulsions for a prolong period (several weeks or months).15–20 Soybean oil–based lipid emulsions, such as Intralipid, have an abundance of phytosterols (plant sterols) that are structurally similar to cholesterol and bile acids and, therefore, have been studied as a possible cause of PNALD.21 In contrast, the fish oil lipid emulsion, Omegaven, is devoid of phytosterols. Not only is phytosterolemia associated with elevated liver injury markers, but Carter et al have shown in hepatocytes that stigmasterol can negatively affect bile acid homeostasis via FXR.22 Our previous work using pig hepatocytes also has demonstrated that phytosterols antagonize bile acid–induced FXR signaling and therefore may contribute to the cholestatic phenotype.23 Moreover, the recent work by El Kasmi et al24 shows compelling evidence that stigmasterol when added to Omegaven induces liver injury in PN-fed mice via a cellular mechanism involving Kupffer cell–mediated inflammation.
In the current study, we attempted to reproduce the approach of El Kasmi et al24 in preterm piglets by adding all 3 principal phytosterols to Omegaven to simulate the same total load and profile of phytosterols present in Intralipid. Our analysis of the PS emulsion showed that we generally achieved this goal. Serum biochemical analysis revealed that after 14 days, piglets given PN using lipid emulsions with a higher vitamin E concentration had lower serum bilirubin, GGT, and bile acids when compared to Intralipid. We also observed that ALT levels were comparable among the 4 groups. We conclude that biliary health is maintained when piglets are fed with a high vitamin E load, regardless of whether the emulsion is fish oil or soybean oil derived. These findings mirror our previous work23 and strongly support the idea that biliary injury may precede hepatocellular injury in PNALD.
A major finding from this study is that we did not observe any signs of hepatotoxicity in the pigs given the PS emulsion where phytosterols were added to Omegaven. This is in contrast to the findings in the mouse model of PNALD by El Kasmi et al.24 Another important novel finding from this study is that the circulating concentrations of phytosterols in the ILE and PS groups were significantly lower than in the IL group despite the same daily intake of phytosterols. We speculate that the increased α-tocopherol intake in the ILE and PS vs IL pigs increased the plasma clearance and perhaps metabolism of phytosterols, which may explain why we did not observe signs of hepatotoxicity in these 2 groups. An explanation for the difference in our current study in preterm pigs and that of El Kasmi et al in mice may be due to species and the marked difference in stage of development and/or Kupffer cell function. The definitive approach to test the toxicity of phytosterols in Intralipid or other soy oil emulsions will require their selective removal by extraction and further animal testing in vivo.
Our second major aim of this study was to examine the role of vitamin E since several fish oil–containing emulsions have added synthetic vitamin E to protect against lipid peroxidation due to the high n-3 PUFA content. Molecules having vitamin E antioxidant activity include 4 tocopherols (α-, β-, γ-, δ-) and 4 tocotrienols (α-, β-, γ-, δ-). Alpha-tocopherol is the compound demonstrating the highest vitamin E activity, and the natural form available in plants is always RRR-α-tocopherol, whereas the more commonly used form is synthetic manufactured all-rac-α-tocopheryl acetate. Due to the occurrence of 3 chiral carbons in the phytyl part 23, possible confirmations of α-tocopherol exist. Thus, the commercially available synthetic α-tocopherol preparations consists of a racemic mixture of all 8 possible stereoisomers (RRR, RSR, RRS, SRR, SRR, SRS, SSS) differing in configuration of the side chain. The antioxidant activity of these 8 stereoisomer forms is equal, but those with 2R-configuration have the highest biological activity. In our attempt to match the α-tocopherol equivalents of the ILE preparation with Omegaven, we added a natural d-α-tocopherol form. Our analysis of the total α-tocopherol content of the ILE, OV, and PS emulsions as well as the plasma and liver tissue concentrations showed clear differences in the stereoisomer profiles that reflected the natural vs synthetic vitamin E added to the lipid emulsion. Despite these differences in the stereo-isomer profile, the striking finding from the study was the marked suppression of hepatotoxicity and prevention of PNALD in all 3 groups given increased intakes of α-tocopherol. We find this especially intriguing since vitamin E is known to have diverse biological functions, including suppression of oxidant stress and induction of activation of bile acid and xenobiotic metabolism.28,39
Bile acid homeostasis is governed by multiple nuclear receptors (such as FXR, PXR, and constitutive androstane receptor [CAR]),40–42 which are believed to function in coordination to maintain hepatocyte health. FXR is well described as the main bile acid sensor in hepatocytes, but PXR and CAR also share some common target genes involved in bile acid synthesis, transport, and metabolism. More important, both PXR and CAR are key receptors involved in xenobiotic metabolism.43 Given the striking protective effect of vitamin E, we hypothesized that α-tocopherol leads to activation of PXR and thereby upregulates hepatoprotective pathways involved in bile acid metabolism. Some have suggested that vitamin E may function via PXR, and one report has shown that tocopherols and tocotrienols can activate PXR promoter activity in HepG2 cells.39,44–46
We observed that piglets treated with lipid emulsions fortified with α-tocopherol had decreased expression of CYP7A1, which is the rate-limiting enzyme involved in bile acid synthesis. Furthermore, these piglets had increased expression of MRP2, which are PXR target genes involved in bile acid metabolism via hydroxylation and hepatocyte bilirubin transport into bile, respectively. Although CYP7A1 is well known to be an indirect downstream target of FXR via SHP, CYP7A1 has also been shown to be a downstream target of PXR suppression.47 We speculate that the higher vitamin E status found in the ILE, OV, and PS pigs may have upregulated PXR, resulting in decreased CYP7A1 and increased MRP2 expression, which in combination would explain the lower serum bile acid levels via reduced synthesis and the increased hepatic bile acid efflux into the biliary tract.
We have previously reported reduced plasma FGF19 concentrations associated with PN vs enteral feeding, implicating this enterokine signaling pathway in dysregulation of bile acid homeostasis.48 The enterohepatic FXR-FGF19 signaling pathway has been shown to be a key negative feedback loop to suppress hepatic CYP7A1 expression and limit hepatic bile acid synthesis.49,50 In the present study, we observed a novel finding where portal FGF19 levels were increased in all 3 vitamin E–enriched emulsion groups and thus could be involved in the reduced CYP7A1 expression in these groups. This induction of portal plasma FGF19 occurred despite no change in either intestinal FXR or FGF19 expression, suggesting a possible FXR-independent mechanism, and it warrants further study to test whether vitamin E directly mediated this response.
In addition to the reduced markers of cholestasis, we found that key markers of lipidemia (serum VLDL and TG and hepatic TG) were reduced in all groups receiving enriched vitamin E lipid emulsions (ILE, OV, PS). We measured expression of genes involved in hepatic fatty acid oxidation and found evidence of increased mitochondrial (CPT1A) and microsomal (CYP2E1) oxidation among the vitamin-enriched IL and OV groups, whereas ACOX was similar in all groups. The induction of CPT1A by OV groups but not the ILE group could be mediated specifically by n-3 long-chain PUFA, especially DHA. However, the induction of CYP2E1 was intriguing and may further suggested a potential link between vitamin E because this microsomal enzyme is involved in the metabolism of both vitamin E and n-3 long-chain PUFA. Although intriguing, it is hard to firmly state that PXR is the primary nuclear receptor affected by PN in light of the fact that PXR and CAR have overlapping downstream targets and are viewed as collaborators in xenobiotic metabolism.
Finally, this raises important clinical questions regarding vitamin E nutritional status in PN-fed pediatric patients, especially those who are premature and require extended PN support. The current study in premature piglets suggest that α-tocopherol but not γ-tocopherol provides the protection against PNALD because IL is relative rich in the latter. It is also important to note that based on the daily infusion rate and liver tissue content, the α-tocopherol was incorporated more efficiently than was the γ-tocopherol into the liver tissue in all 3 vitamin E–enriched emulsion groups. This suggests that the more biologically active forms of parenterally administered vitamin E, α-tocopherol vs γ-tocopherol, are selectively enriched in the liver, which is the focal organ in the pathogenesis of PNALD. Recent recommendations for parenteral vitamin E in premature infants suggest a dose that does not increase the plasma concentration higher than 80 µmol/L (35 mg/L).27 The American Academy of Pediatrics Committee on Nutrition has suggested that safe and effective blood levels are between 23–46 µmol/L (10–20 mg/L). Importantly, the changes in total plasma vitamin E we found to prevent PNALD ranged from 4–7 mg/L, which is well within the safe level of vitamin E in human infants. This suggests that the vitamin E loads given in this study either as a supplement to Intralipid or as enriched Omegaven are not pharmacological and well within the normal range of nutritional needs for infants. It is important to highlight that SMOFlipid and Omegaven have similar concentrations of vitamin E, yet SMOFlipid has less than half the amount of phytosterol found in Intralipid.23 The question remains whether infants given Intralipid alone without multivitamin supplementation may develop marginal vitamin E deficiency that contributes to PNALD. Our work warrants further examination of the prevailing vitamin E levels in pediatric liver disease patients, especially those given exclusive soy-based lipid emulsions.
In conclusion, this study shows compelling novel evidence that vitamin E plays an important hepatoprotective role in preventing PNALD. In all premature piglets given lipid emulsions enriched with α-tocopherol, indices of both cholestasis and lipidemia were markedly reduced compared to those given Intralipid alone with no α-tocopherol. We also show evidence that the underlying protective action of vitamin E may involve activation of both microsomal bile acid and fatty acid oxidation pathways controlled by xenobiotic nuclear transcription factors, namely PXR. The other major finding in this study was that addition of a clinically relevant dose of IV phytosterols to Omegaven did not produce evidence of cholestasis or liver injury. This finding contrasts with the current prevailing theory that the accumulation of serum phytosterols is directly linked and an important causative agent contributing to liver injury and cholestasis in PN-fed infants. We think it is possible that phytosterols did not induce PNALD in the ILE and PS groups because they were delivered along with enriched vitamin E. This protective effect of vitamin E in the presence of phytosterols provides an explanation for our recent finding that SMOFlipid protects against PNALD, although the phytosterol content of SMOFlipid is substantial. Thus, we suggest that additional preclinical studies in relevant pediatric models are needed to show conclusive proof that phytosterols induce injury in the absence of the protective ingredients, namely vitamin E, found in new-generation lipid emulsions. This study also further strengthens the idea that lipid composition plays an important role in PNALD and warrants further study into the metabolic effects of new-generation lipid emulsions in the health and prevention of disease in pediatric populations.
Clinical Relevancy Statement.
Parenteral nutrition (PN) is important in neonatal infants yet prolonged use is associated with PN-associated liver disease (PNALD), including conditions of fatty liver and cholestasis. The current study examines the impact of phytosterols and vitamin E, both key ingredients in parenteral lipid emulsions, on the incidence of PNALD in preterm piglets given PN.
Acknowledgments
The authors wish to acknowledge Liwei Cui, Berthe Oosterloo, and the staff of the Texas Medical Center Digestive Disease Center Cellular and Molecular Morphology Core for their technical assistance.
Financial disclosure: This work is a publication of the USDA/ARS Children’s Nutrition Research Center, Department of Pediatrics, Baylor College of Medicine and Texas Children’s Hospital, Houston, Texas. This work was supported in part by federal funds from the U.S. Department of Agriculture, Agricultural Research Service under Cooperative Agreement Number 58-6250-6-001, the American Society for Parenteral and Enteral Nutrition (to K.N.), NIH Grant DK-094616 (D.G.B.), and the Texas Medical Center Digestive Diseases Center (NIH Grant P30 DK-56338). The contents of this publication do not necessarily reflect the views or policies of the U.S. Department of Agriculture, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. government. Kenneth Ng was supported by a research fellowship award from the American Liver Foundation. Miguel Saenz de Pipaon was supported by a grant from the Instituto de Salud Carlos III, BA12/00086.
References
- 1.Carter BA, Karpen SJ. Intestinal failure-associated liver disease: management and treatment strategies past, present, and future. Semin Liver Dis. 2007;27:251–258. doi: 10.1055/s-2007-985070. [DOI] [PubMed] [Google Scholar]
- 2.Christensen RD, Henry E, Wiedmeier SE, Burnett J, Lambert DK. Identifying patients, on the first day of life, at high-risk of developing parenteral nutrition-associated liver disease. J Perinatol. 2007;27:284–290. doi: 10.1038/sj.jp.7211686. [DOI] [PubMed] [Google Scholar]
- 3.Carter BA, Shulman RJ. Mechanisms of disease: update on the molecular etiology and fundamentals of parenteral nutrition associated cholestasis. Nat Clin Pract Gastroenterol Hepatol. 2007;4:277–287. doi: 10.1038/ncpgasthep0796. [DOI] [PubMed] [Google Scholar]
- 4.Waitzberg DL, Torrinhas RS. Fish oil lipid emulsions and immune response: what clinicians need to know. Nutr Clin Pract. 2009;24:487–499. doi: 10.1177/0884533609339071. [DOI] [PubMed] [Google Scholar]
- 5.Burrin DG, Ng K, Stoll B, Sáenz De Pipaón M. Impact of new-generation lipid emulsions on cellular mechanisms of parenteral nutrition-associated liver disease. Adv Nutr. 2014;5:82–91. doi: 10.3945/an.113.004796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nandivada P, Carlson SJ, Chang MI, Cowan E, Gura KM, Puder M. Treatment of parenteral nutrition-associated liver disease: the role of lipid emulsions. Adv Nutr. 2013;4:711–717. doi: 10.3945/an.113.004770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gura KM, Duggan CP, Collier SB, et al. Reversal of parenteral nutrition-associated liver disease in two infants with short bowel syndrome using parenteral fish oil: implications for future management. Pediatrics. 2006;118:e197–e201. doi: 10.1542/peds.2005-2662. [DOI] [PubMed] [Google Scholar]
- 8.Le HD, de Meijer VE, Robinson EM, et al. Parenteral fish-oil-based lipid emulsion improves fatty acid profiles and lipids in parenteral nutrition-dependent children. Am J Clin Nutr. 2011;94:749–758. doi: 10.3945/ajcn.110.008557. [DOI] [PubMed] [Google Scholar]
- 9.Premkumar MH, Carter BA, Hawthorne KM, King K, Abrams SA. Fish oil-based lipid emulsions in the treatment of parenteral nutrition-associated liver disease: an ongoing positive experience. Adv Nutr. 2014;5:65–70. doi: 10.3945/an.113.004671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanchez SE, Braun LP, Mercer LD, Sherrill M, Stevens J, Javid PJ. The effect of lipid restriction on the prevention of parenteral nutrition-associated cholestasis in surgical infants. J Pediatr Surg. 2013;48:573–578. doi: 10.1016/j.jpedsurg.2012.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levit OL, Calkins KL, Gibson LC, et al. Low-dose intravenous soybean oil emulsion for prevention of cholestasis in preterm neonates. JPEN J Parenter Enteral Nutr. In press doi: 10.1177/0148607114540005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nehra D, Fallon EM, Carlson SJ, et al. Provision of a soy-based intravenous lipid emulsion at 1 g/kg/d does not prevent cholestasis in neonates. JPEN J Parenter Enteral Nutr. 2013;37:498–505. doi: 10.1177/0148607112453072. [DOI] [PubMed] [Google Scholar]
- 13.Cober MP, Killu G, Brattain A, Welch KB, Kunisaki SM, Teitelbaum DH. Intravenous fat emulsions reduction for patients with parenteral nutrition-associated liver disease. J Pediatr. 2012;160:421–427. doi: 10.1016/j.jpeds.2011.08.047. [DOI] [PubMed] [Google Scholar]
- 14.Colomb V, Jobert-Giraud A, Lacaille F, Goulet O, Fournet JC, Ricour C. Role of lipid emulsions in cholestasis associated with long-term parenteral nutrition in children. JPEN J Parenter Enteral Nutr. 2000;24:345–350. doi: 10.1177/0148607100024006345. [DOI] [PubMed] [Google Scholar]
- 15.Clayton PT, Bowron A, Mills KA, Massoud A, Casteels M, Milla PJ. Phytosterolemia in children with parenteral nutrition-associated choles-tatic liver disease. Gastroenterology. 1993;105:1806–1813. doi: 10.1016/0016-5085(93)91079-w. [DOI] [PubMed] [Google Scholar]
- 16.Kurvinen A, Nissinen MJ, Andersson S, et al. Parenteral plant sterols and intestinal failure-associated liver disease in neonates. J Pediatr Gastroenterol Nutr. 2012;54:803–811. doi: 10.1097/MPG.0b013e3182474118. [DOI] [PubMed] [Google Scholar]
- 17.Kurvinen A, Nissinen MJ, Gylling H, et al. Effects of long-term paren-teral nutrition on serum lipids, plant sterols, cholesterol metabolism, and liver histology in pediatric intestinal failure. J Pediatr Gastroenterol Nutr. 2011;53:440–446. doi: 10.1097/MPG.0b013e3182212130. [DOI] [PubMed] [Google Scholar]
- 18.Ellegard L, Sunesson A, Bosaeus I. High serum phytosterol levels in short bowel patients on parenteral nutrition support. Clin Nutr. 2005;24:415–420. doi: 10.1016/j.clnu.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 19.Savini S, D’Ascenzo R, Biagetti C, et al. The effect of 5 intravenous lipid emulsions on plasma phytosterols in preterm infants receiving parenteral nutrition: a randomized clinical trial. Am J Clin Nutr. 2013;98:312–318. doi: 10.3945/ajcn.112.056556. [DOI] [PubMed] [Google Scholar]
- 20.Mutanen A, Nissinen MJ, Lohi J, Heikkilä P, Gylling H, Pakarinen MP. Serum plant sterols, cholestanol, and cholesterol precursors associated with histological liver injury in pediatric onset intestinal failure. Am J Clin Nutr. 2014;100:1085–1094. doi: 10.3945/ajcn.114.088781. [DOI] [PubMed] [Google Scholar]
- 21.Xu Z, Harvey KA, Pavlina T, et al. Steroidal compounds in commercial parenteral lipid emulsions. Nutrients. 2012;4:904–921. doi: 10.3390/nu4080904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carter BA, Taylor OA, Prendergast DR, et al. Stigmasterol, a soy lipid-derived phytosterol, is an antagonist of the bile acid nuclear receptor FXR. Pediatr Res. 2007;62:301–306. doi: 10.1203/PDR.0b013e3181256492. [DOI] [PubMed] [Google Scholar]
- 23.Vlaardingerbroek H, Ng K, Stoll B, et al. New generation lipid emulsions prevent PNALD in chronic parenterally fed preterm pigs. J Lipid Res. 2014;55:466–477. doi: 10.1194/jlr.M044545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.El Kasmi KC, Anderson AL, Devereaux MW, et al. Phytosterols promote liver injury and Kupffer cell activation in parenteral nutrition-associated liver disease. Sci Transl Med. 2013;5 doi: 10.1126/scitranslmed.3006898. 206ra137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clayton PT, Whitfield P, Iyer K. The role of phytosterols in the patho-genesis of liver complications of pediatric parenteral nutrition. Nutrition. 1998;14:158–164. doi: 10.1016/s0899-9007(97)00233-5. [DOI] [PubMed] [Google Scholar]
- 26.Wanten GJ, Calder PC. Immune modulation by parenteral lipid emulsions. Am J Clin Nutr. 2007;85:1171–1184. doi: 10.1093/ajcn/85.5.1171. [DOI] [PubMed] [Google Scholar]
- 27.Biesalski HK. Vitamin E requirements in parenteral nutrition. Gastroenterology. 2009;137:S92–S104. doi: 10.1053/j.gastro.2009.07.073. [DOI] [PubMed] [Google Scholar]
- 28.Traber MG. Vitamin E regulatory mechanisms. Annu Rev Nutr. 2007;27:347–362. doi: 10.1146/annurev.nutr.27.061406.093819. [DOI] [PubMed] [Google Scholar]
- 29.Lavine JE, Schwimmer JB, Molleston JP, et al. Treatment of nonalcoholic fatty liver disease in children: TONIC trial design. Contemp Clin Trials. 2010;31:62–70. doi: 10.1016/j.cct.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanyal AJ, Chalasani N, Kowdley KV, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675–1685. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steiner C, von Eckardstein A, Rentsch KM. Quantification of the 15 major human bile acids and their precursor 7alpha-hydroxy-4-cholesten-3-one in serum by liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878:2870–2880. doi: 10.1016/j.jchromb.2010.08.045. [DOI] [PubMed] [Google Scholar]
- 32.Nagy K, Jakab A, Pollreisz F, et al. Analysis of sterols by high-performance liquid chromatography/mass spectrometry combined with chemometrics. Rapid Commun Mass Spectrom. 2006;20:2433–2440. doi: 10.1002/rcm.2606. [DOI] [PubMed] [Google Scholar]
- 33.Bedner M, Schantz MM, Sander LC, Sharpless KE. Development of liquid chromatographic methods for the determination of phytosterols in standard reference materials containing saw palmetto. J Chromatogr A. 2008;1192:74–80. doi: 10.1016/j.chroma.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 34.Jensen SK, Engberg RM, Hedemann MS. All-rac-alpha-tocopherol acetate is a better vitamin E source than all-rac-alpha-tocopherol succinate for broilers. J Nutr. 1999;129:1355–1360. doi: 10.1093/jn/129.7.1355. [DOI] [PubMed] [Google Scholar]
- 35.Jensen SK, Nielsen KN. Tocopherols, retinol, beta-carotene and fatty acids in fat globule membrane and fat globule core in cows’ milk. J Dairy Res. 1996;63:565–574. doi: 10.1017/s0022029900032106. [DOI] [PubMed] [Google Scholar]
- 36.Gray MA, Pollock CB, Schook LB, Squires EJ. Characterization of porcine pregnane X receptor, farnesoid X receptor and their splice variants. Exp Biol Med (Maywood) 2010;235:718–736. doi: 10.1258/ebm.2010.009339. [DOI] [PubMed] [Google Scholar]
- 37.Goodwin B, Hodgson E, Liddle C. The orphan human pregnane X receptor mediates the transcriptional activation of CYP3A4 by rifampicin through a distal enhancer module. Mol Pharmacol. 1999;56:1329–1339. doi: 10.1124/mol.56.6.1329. [DOI] [PubMed] [Google Scholar]
- 38.Marini JC, Didelija IC, Fiorotto ML. Extrarenal citrulline disposal in mice with impaired renal function. Am J Physiol Renal Physiol. 2014;307:F660–F665. doi: 10.1152/ajprenal.00289.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Traber MG. Vitamin E, nuclear receptors and xenobiotic metabolism. Arch Biochem Biophys. 2004;423:6–11. doi: 10.1016/j.abb.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 40.Goodwin B, Kliewer SA. Nuclear receptors, I: nuclear receptors and bile acid homeostasis. Am J Physiol Gastrointest Liver Physiol. 2002;282:G926–G931. doi: 10.1152/ajpgi.00044.2002. [DOI] [PubMed] [Google Scholar]
- 41.Fiorucci S, Zampella A, Distrutti E. Development of FXR, PXR and CAR agonists and antagonists for treatment of liver disorders. Curr Top Med Chem. 2012;12:605–624. doi: 10.2174/156802612799436678. [DOI] [PubMed] [Google Scholar]
- 42.Gonzalez FJ. Nuclear receptor control of enterohepatic circulation. Compr Physiol. 2012;2:2811–2828. doi: 10.1002/cphy.c120007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Willson TM, Kliewer SA. PXR, CAR and drug metabolism. Nat Rev Drug Discov. 2002;1:259–266. doi: 10.1038/nrd753. [DOI] [PubMed] [Google Scholar]
- 44.Mustacich DJ, Gohil K, Bruno RS, et al. Alpha-tocopherol modulates genes involved in hepatic xenobiotic pathways in mice. J Nutr Biochem. 2009;20:469–476. doi: 10.1016/j.jnutbio.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mustacich DJ, Leonard SW, Devereaux MW, Sokol RJ, Traber MG. Alpha-tocopherol regulation of hepatic cytochrome P450s and ABC transporters in rats. Free Radic Biol Med. 2006;41:1069–1078. doi: 10.1016/j.freeradbiomed.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 46.Landes N, Pfluger P, Kluth D, et al. Vitamin E activates gene expression via the pregnane X receptor. Biochem Pharmacol. 2003;65:269–273. doi: 10.1016/s0006-2952(02)01520-4. [DOI] [PubMed] [Google Scholar]
- 47.Li T, Chiang JY. Mechanism of rifampicin and pregnane X receptor inhibition of human cholesterol 7 alpha-hydroxylase gene transcription. Am J Physiol Gastrointest Liver Physiol. 2005;288:G74–G84. doi: 10.1152/ajpgi.00258.2004. [DOI] [PubMed] [Google Scholar]
- 48.Jain AK, Stoll B, Burrin DG, Holst JJ, Moore DD. Enteral bile acid treatment improves parenteral nutrition-related liver disease and intestinal mucosal atrophy in neonatal pigs. Am J Physiol Gastrointest Liver Physiol. 2012;302:G218–G224. doi: 10.1152/ajpgi.00280.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Inagaki T, Choi M, Moschetta A, et al. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2005;2:217–225. doi: 10.1016/j.cmet.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 50.Kir S, Kliewer SA, Mangelsdorf DJ. Roles of FGF19 in liver metabolism. Cold Spring Harb Symp Quant Biol. 2011;76:139–144. doi: 10.1101/sqb.2011.76.010710. [DOI] [PubMed] [Google Scholar]