Abstract
Kidney transplantation is the treatment of choice in end-stage renal disease, given the better quality of life of transplanted patients when compared with patients on maintenance dialysis. In spite of surgical improvements and new immunosuppressive regimens, parts of transplanted grafts still develop chronic dysfunction. Ultrasonography, both in B-mode and with Doppler ultrasound, is an important diagnostic tool in case of clinical conditions which might impair kidney function. Even though ultrasonography is considered fundamental in the diagnosis of vascular and surgical complications of the transplanted kidney, its role is not fully understood in case of parenchymal complications of the graft. The specificity of Doppler is low both in case of acute complications, such as acute tubular necrosis, drugs toxicity and acute rejection, and in case of chronic conditions, such as chronic allograft nephropathy. Single determinations of resistance indices present low diagnostic accuracy, which is higher in case of successive measurements performed during the follow-up of the graft. Modern techniques such as tissue pulsatility index, maximal fractional area and contrast-enhanced ultrasound increase ultrasonography diagnostic power in case of parenchymal complications of the transplanted kidney.
Electronic supplementary material
The online version of this article (doi:10.1007/s40477-014-0118-1) contains supplementary material, which is available to authorized users.
Keywords: Ultrasonography, Doppler ultrasound, Renal transplantation, Parenchymal complications
Sommario
Il trapianto di rene è il trattamento di scelta nella malattia renale allo stadio terminale, data la migliore qualità di vita dei pazienti trapiantati rispetto a quelli che continuano il trattamento dialitico. Nonostante i miglioramenti chirurgici e nuovi regimi immunosoppressivi, parte dei pazienti trapiantati sviluppano ancora disfunzioni croniche. L’ecografia, sia in B-mode che Doppler, è un importante strumento diagnostico in caso di condizioni cliniche che possono alterare la funzione renale. Anche se l’ecografia è considerata fondamentale nella diagnosi di complicanze chirurgiche e vascolari del rene trapiantato, il suo ruolo non è pienamente conosciuto in caso di complicanze parenchimali. La specificità del Doppler è bassa, sia in caso complicanze acute, quali la necrosi tubulare acuta, la tossicità acuta da farmaci, il rigetto, sia in caso di complicanze croniche, come la nefropatia cronica del trapianto. Singole determinazioni di indici di resistenza presentano bassa accuratezza diagnostica, che è più elevata in caso di misure effettuate successivamente durante il follow-up del trapianto. Tecniche moderne, come l’indice di pulsatilità tissutale, massima frazione di zona ed ecografia con mdc aumentano le potenzialità diagnostiche dell’ecografia in caso di complicanze parenchimali del rene trapiantato.
Electronic supplementary material
The online version of this article (doi:10.1007/s40477-014-0118-1) contains supplementary material, which is available to authorized users.
Introduction
Renal transplantation is believed to be the ideal treatment in end-stage renal disease, both in terms of survival and quality of life. In the past few years, surgical improvements and new immunosuppressive drugs have increased short- and long-term graft survival. However, some patients still develop several complications, which might cause the loss of the graft or its dysfunction, as reported in Table 1.
Table 1.
Most common parenchymal complications in renal transplantation
| Immediate—within the first week |
|---|
| Acute tubular necrosis |
| Hyperacute rejection—accelerated rejection |
| Calcineurin inhibitors’ toxicity |
| Infectious complications (acute graft pyelonephritis) |
| Early—between the first and the twelfth week |
|---|
| Acute rejection |
| Calcineurin inhibitors’ toxicity |
| Infectious complications (acute graft pyelonephritis) |
| Late—after the twelfth week |
|---|
| Chronic rejection |
| Calcineurin inhibitors toxicity |
| Infectious complications (acute graft pyelonephritis) |
| Nephropathy relapse |
Ultrasonography is considered to be the first-level imaging method in the diagnosis of vascular, surgical and urological complications of the transplanted kidney, while the use of Doppler ultrasound (DUS) is not well codified in case of parenchymal complications of the graft [1].
We will discuss the role of DUS in case of parenchymal complications of the graft, referring the reader to a separate analysis of vascular complications. Parenchymal complications of the transplanted kidney can range from disease relapse, drug toxicity, acute and chronic rejection and acute tubular necrosis to infections. Unfortunately, all these conditions present similar characteristics at DUS, thereby accounting for the low specificity of this technique in the differential diagnosis of graft dysfunction.
Method of test performance
The study of the transplanted kidney is easy to perform given the superficial position of the graft. Convex probes with frequencies ranging from 3.5 to 5.0 MHz allow obtaining longitudinal, transversal and oblique scans.
To minimise technical failures, it is worthwhile standardising the working methodology, starting the test in B-mode and identifying the site, the form and the position of the graft.
The morphological and echo-structural parameters observed at the scale of greys test, such as a rapid volumetric increase over 20 %, parenchymal hypoechogenicity, the presence of a hyperchogenous shade between the echoes of the renal sinus and the walls of the renal pelvis, the reduction in volume and the thinning of the parenchyma, might provide a large amount of information concerning a possible damage of the organ [2].
Once the B-mode test is completed, colour Doppler imaging is fundamental for a rapid and clear assessment of arterial and venous perfusion of the organ. The extension of the box colour must be limited, to improve the Doppler analysis capacity and the frame rate (FR). Pulse repetition frequency (PRF) should be set at values of 1.0–1.5 kHz, wall filter at values of 100 Hz and the colour gain should be regulated as well, to optimise the image without aliasing and to avoid colour diffusion to the perivascular tissues (colour bleeding) [3].
Finally, the spectral analysis module should be activated with the positioning of the “sample volume” in the lumen of the interlobular artery and the recording of the velocity/time curve (V/t), which will allow obtaining the resistance index (RI) and the pulsatility index (PI).
Study of diastolic flow
In the healthy kidney, as well as in the well-functioning transplanted graft, the vascular resistances are so low that the blood flow remains constantly anterograde, throughout the duration of the cardiac cycle. The gradual increase in the vascular resistances is reflected in the reduction of the diastolic flow velocity and the consequent increase in the ratio between systolic and diastolic speed in the velocitogram.
The difference between systolic peak velocity (S) and telediastolic velocity (D) can provide the measurement of two important Doppler parameters. The first one derives from the relationship between this difference and the systolic peak velocity. Named resistance index (RI) is the parameter classically adopted in the Doppler analysis performed at the interlobular level (RI = S−D/S). While an RI < 0.70 at interlobar level is certainly within normal range, an RI > 0.90 is pathological, as it is the trend of an initially normal RI which increases progressively.
All these values should be always considered in the clinical context of the patient. The RI values reflect, indeed, the microcirculation conditions in the explored area without any information regarding glomerular function or ongoing pathology. Both in healthy subjects and in transplanted patients, fluximetric indices are extremely sensitive to haemodynamic conditions, such as arterial hypertension, systemic atherosclerosis, tachycardia, dehydration and shock. For this reason, it is essential to standardise the haemodynamic conditions at the moment of the measurement, for a correct and reliable interpretation of the parameters [4].
It is also necessary to remember that RI increases during forced inspiration (Valsalva manoeuvre) or in case of a heart rate <50 bpm. To evaluate the integrity of the graft, in common clinical setting it is useful to constantly monitor renal parenchymal RI during the follow-up and taking into account any changes in their values rather than considering their absolute value. [5].
In case of high vascular resistance, as it happens during acute rejection or acute tubular necrosis, the diastolic flow can stop or even invert, as described by Lockhart et al. [6]. Renal vein thrombosis as well may be associated with increased vascular resistance.
If diastolic flow is absent or inverted, it is not possible to calculate RI. Pulsatility index (PI) may represent a valid alternative. It derives from the relationship between the difference between systolic peak velocity and telediastolic velocity and the mean velocity M (PI = S−D/M).
The detection of reversed diastolic flow has been demonstrated to have a negative prognostic value both for the short-term and long-term functionality of the graft and three different patterns have been so far described [6] (Fig. 1). They appear as three stages of a progressive phenomenon that initially affects only the proto-diastolic phase, then progresses and becomes biphasic. The frequent monitoring of the diastolic flow at the level of interlobar arteries of the transplanted kidney is therefore of particular interest and usefulness, since it allows an extremely early diagnosis of the intrarenal haemodynamic changes, which are expressions of organ damage [7].
Fig. 1.
Patterns of reversed diastolic flow in renal transplantation
In conclusion, the low specificity of Doppler parameters is compensated by considering such values in relation to the onset of any abnormal changes. For instance, at less than 24 h after the transplantation, the sudden onset of an inversion in the diastolic flow must impose the rapid exclusion of a vascular kinking, a renal vein thrombosis or the presence of wide peritransplant haematomas. In the absence of these signs, it is necessary to perform a kidney biopsy to exclude acute tubular necrosis or acute rejection.
Doppler ultrasound and the timing of the complications
The timing of the onset of clinical manifestations related to graft parenchymal (nonobstructive) complications is obviously important for the interpretation of DUS (Table 1).
Parenchymal complications are classified into:
Immediate, if they occur within the first week since the surgical intervention.
Early, if they occur between the first and the twelfth week since the surgical intervention.
Late, if they occur after the twelfth week.
The parenchymal (nonobstructive) complications can be roughly divided into immunological, infective and related to calcineurin inhibitors toxicity. Immediate parenchymal complications are often responsible for a delay in the recovery of the graft function and are related to a higher rate of hospitalisation and a worse graft survival both in the short and in the long period [8]. These complications are essentially represented by hyperacute and accelerated rejection, acute tubular necrosis (ATN), calcineurin inhibitors’ toxicity and pyelonephritis. Besides calcineurin inhibitors’ toxicity, early parenchymal complications include the acute rejection, while the late parenchymal complications include chronic rejection, calcineurin inhibitors’ toxicity, infections and nephropathy relapse. Ultrasonography, both in B-mode and with colour Doppler, plays a fundamental role in the differential diagnosis of these complications. While differential diagnosis is easy between atypical urethral obstruction and vascular thrombosis, cyclosporine nephrotoxicity is difficult to be distinguished from acute tubular necrosis.
Hyperacute and accelerated rejection
Even though new immunosuppressive drugs have dramatically reduced the incidence of hyperacute and accelerated rejection, some patients still develop these complications, which are responsible for the early loss of the graft [9]. Hyperacute rejection is usually diagnosed during surgical intervention, as soon as the kidney suddenly becomes cyanotic and flaccid. The patient develops oliguria and intrarenal blood flow is not detectable with DUS. The role of this technique is limited by the rapid organ destruction related to the thrombotic and inflammatory processes which involve the graft. The prognosis is scarce with a graft loss rate over 60 % [8, 9]. The echographic characteristics are extremely nonspecific and similar to those observed in case of acute rejection and acute tubular necrosis. This latter one is the most frequent cause of delayed graft function and is detected in 20–60 % of deceased donor renal transplantation [10]. It is usually observed during the first 48 h post-transplantion and is related to the reversible ischaemic injury involving tubular renal cells in the pre-transplant period. The main risk factors for acute tubular necrosis include deceased donor renal transplantation, donor hypotension and prolonged warm and cold ischaemia period, especially if >30′ in the first case and >24 h in the second one. In B-mode, the US pattern of acute tubular necrosis is extremely variable. The kidney can often present normal features, while an increase in the size and a reduction in the echogenicity with a loss of the corticomedullary differentiation may be signs of an oedematous graft [11]. Cortical thickness and echogenicity with prominent pyramids have been also described, as in the case of ATN involving the native kidneys [12]. Renal sinus may be compressed or obliterated by the oedema. All these patterns appear to be indistinguishable from those reported in the case of acute rejection.
Acute rejection
Acute rejection is common, affecting 20–30 % of deceased donor renal grafts [13]. In case of acute rejection, the ultrasonographic pattern is similar to that observed in the setting of acute conditions involving the native kidney:
Increased volume of the graft due to oedema (very frequent but non-specific).
Enlarged and hypoechogenous pyramids, due to medullar oedema (early but non-specific).
Thickened and hyperechogenous cortex (rarely hypoechogenous cortex and increased medullar thickness, with consequent loss of the corticomedullary differentiation).
Oedema of the collector system and focal parenchymal hypoechogenicity, expression of infarction.
Perigraft anechogenous collections due to necrosis and/or haemorrhages.
Increased parenchymal thickness with obliteration of the hyperechogenous pyelic sinus.
In conclusion, there are no specific ultrasonographic signs of acute rejection of the graft, even though the presence of these patterns associated with a rapid worsening of renal function may be signs of acute rejection. Moreover, the gradual reduction of the diastolic flow at DUS will represent a further indication for a graft biopsy, which is fundamental to distinguish acute rejection from calcineurin inhibitors’ toxicity and acute tubular necrosis.
Calcineurin inhibitors nephrotoxicity
Calcineurin inhibitors nephrotoxicity is characterised by acute interstitial tubular nephropathy, with consequent delayed functional recovery of the graft. Calcineurin inhibitors may induce an intense and generalised vasoconstriction of the afferent arterioles responsible for a drastic reduction in the filtration fraction [14]. This is reflected in the absence of significant alterations in the diastolic flow and resistance index [14].
Acute graft pyelonephritis
Even though acute graft pyelonephritis (AGP) can complicate any phase of post-transplantation period, it will be discussed in this section. According to the recent literature, the incidence of AGP has been reported to be around 19–23 % [11]. Important risk factors for this complication include immunosuppressive therapy, invasive urological procedures such as bladder catheterism or urethral stenting and post-transplantion urethral bladder reflux (Fig. 2) [15]. AGP should always be suspected if the patient suddenly develops fever of unknown aetiology, leucocytosis and leucocyturia as well as increased levels of C-reactive protein, even though all of them are also present in case of cystopyelitis. Differential diagnosis is fundamental, because only AGP is responsible for parenchymal damage, increasing the risk of scarring, acute rejection and worsening of renal function [15]. The use of B-mode has low sensitivity (11–40 %) and low specificity (50 %) in detecting renal parenchymal lesions in case of AGP [15, 16]. Diagnostic accuracy increases if renal abscess or pyelonephritis with dilation of the excretory path is present [15, 16]. Ultrasounds show an increased volume of the organ, induced by the inflammatory process which involves the graft, while renal sinus thickness appears to be reduced as well as corticomedullary differentiation. Sometimes, transplanted patients may develop focal acute pyelonephritis, which is characterised by hypo or anechogenous and poorly defined areas with faded borders at ultrasonography (Fig. 3). Posterior parietal reinforcement might be present as an expression of non-suppurative parcellar inflammation related to interstitial oedema and/or haemorrhage. DUS has a higher sensitivity (38–63 %) in detecting parenchymal abnormalities in case of AGP. Power Doppler has been shown to have a higher sensitivity (41 %) and specificity (89 %), when compared with colour Doppler [17]. Moreover, we have demonstrated the important role that echocontrastography plays in defining perfusion reduction of the areas affected by acute pyelonephritis (Fig. 4) [16].
Fig. 2.
Transplanted patient with urethral stent
Fig. 3.
Transplanted patient with focal acute pyelonephritis
Fig. 4.

Clear, wedge-shaped area of hypoperfusion due to acute pyelonephritis. a Longitudinal scan. b Axial scan
Chronic rejection
Chronic rejection, or chronic allograft nephropathy (CAN), is characterised by a progressive decline of the renal function, often accompanied by arterial hypertension and proteinuria with no signs of acute rejection and/or nephrotoxicity. The diagnosis of CAN is not easy to perform, since the only alteration is represented by the progressive and slow increase of serum creatinine, which is also present in case of underlying disease recurrence, transplanted renal artery stenosis and calcineurin inhibitor nephrotoxicity [18]. Renal biopsy is often necessary to exclude potentially treatable causes of chronic rejection. In chronic rejection glomerulosclerosis, tubular atrophy, interstitial fibrosis and severe vascular involvement may be observed [19]. These patterns are also found in case of chronic diseases of the native kidney. Ultrasounds show a hyperechogenous cortex and a reduced volume of the graft. In case of long-lasting CAN, intrarenal lymphatic collectors can develop scars and become occluded, thus determining the formation of lymphatic accumulation under renal capsular surface (subcapsular lymphocele). Ultrasonography will show a thin sub-capsular hypoechogenous band/strip [18, 19].
The resistance index is frequently increased, even though in certain patients it might be within the normal range. Its accuracy in the diagnosis of CAN is low, compared with clinical and laboratory data [20, 21]. Rademarcher et al. have demonstrated that a high RI after 3–4 months since the transplantation is a negative predictive factor of long-term graft survival, more than classical risk factors such as donor and recipient age, proteinuria, hypertension and delayed graft function [22].
Some authors have suggested a way to quantify the pixels of colour of the power Doppler in the region of interest (ROI) [23]. According to this technique, parenchymal vascularisation can be quantified in relation to the maximum fractional area (MFA) at the colour Doppler and the distance between the most peripheral colour pixels and the renal capsule (Fig. 5). This method has been demonstrated to have an excellent reproducibility and an elevated diagnostic accuracy. The distance between the peripheral colour pixels and the renal capsule showed a 91 % sensitivity in the diagnosis of CAN and reached 94 % when combined with MFA. Scholbach et al. have suggested the use of tissue Doppler for the diagnosis of CAN to detect the tissue pulsatility index (PI: calculated as the ratio between the differences between systolic mean and diastolic mean in the ROI and the mean velocity), to quantify graft perfusion [24].
Fig. 5.
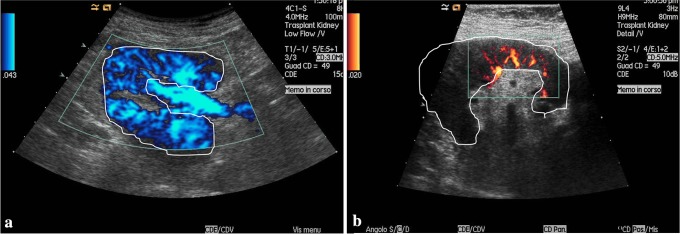
a Normal kidney. b Reduced renal perfusion in a transplanted patient
Conclusions
The use of US allows a continuous, effective, low-cost and risk-free monitoring of the graft status. The use of B-mode, pulsed wave Doppler, colour and power Doppler increases the diagnostic accuracy of this technique and the information they provide should always be considered in the clinical setting of the patient.
Electronic supplementary material
Conflict of interest
Antonio Granata, Pierpaolo Di Nicolò, Viviana R. Scarfia, Monica Insalaco, Paolo Lentini, Massimiliano Veroux, Pasquale Fatuzzo, Fulvio Fiorini declare that they have no conflict of interest.
Informed consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 (5). All patients provided written informed consent for enrolment in the study and to the inclusion in this article of information that could potentially lead to their identification.
Human and animal studies
The study was conducted in accordance with all institutional and national guidelines for the care and use of laboratory animals.
References
- 1.O’Neill WC, Baumgarten DA. Ultrasonography in renal transplantation. Am J Kidney Dis. 2002;39:663–678. doi: 10.1053/ajkd.2002.31978. [DOI] [PubMed] [Google Scholar]
- 2.Granata A, Clementi S, Clementi A, Di Pietro F, Scarfia VR, Insalaco M, Aucella F, Prencipe M, Figuera M, Fiorini F, Sicurezza E. Parenchymal complications of the transplanted kidney: the role of color-Doppler imaging. G Ital Nefrol. 2012;29(S57):S90–S98. [PubMed] [Google Scholar]
- 3.Chaeles V, Zwirewich MD (2007) Renal transplant imaging and intervention: practical aspects. Vancouver Hospital and Health Sciences Center, 1–27
- 4.Drudi FM, Cascone F, Pretagostini R, Ricci P, Trippa F, Righi A, Iannicelli E, Passariello R. Ruolo dell’eco color-Doppler nella diagnostica del rene trapiantato. Radiol Med. 2001;101:243–250. [PubMed] [Google Scholar]
- 5.Schwenger V, Keller T, Hofmann N, Hoffmann O, Sommerer C, Nahm AM, Morath C, Zeier M, Krumme B. Color Doppler indices of renal allografts depend on vascular stiffness of the transplant recipients. Am J Transplant. 2006;6(11):2721–2724. doi: 10.1111/j.1600-6143.2006.01524.x. [DOI] [PubMed] [Google Scholar]
- 6.Lockhart ME, Wells CG, Morgan DE, Fineberg NS, Robbin ML. Reversed diastolic flow in the renal transplant: perioperative implications versus transplants older than 1 month. AJR. 2008;190:650–655. doi: 10.2214/AJR.07.2666. [DOI] [PubMed] [Google Scholar]
- 7.Thalhammer C, Aschwanden M, Mayr M, Koller M, Steiger J, Jaeger KA. Duplex sonography after living donor kidney transplantation: new insights in the early postoperative phase. Ultraschall Med. 2006;27:141–145. doi: 10.1055/s-2006-926560. [DOI] [PubMed] [Google Scholar]
- 8.Akbar SA, Jafri SH, Amendola MA, Madrazzo BL, Salem R, Bis KG. Complications of renal transplantation. Radiographic. 2005;25:1335–1356. doi: 10.1148/rg.255045133. [DOI] [PubMed] [Google Scholar]
- 9.Vella J, Koch MJ, Brennan DC. Acute renal allograft rejection: Diagnosis. UpToDate 1/7/2013
- 10.Irshad A, Ackerman S, Sosnouski D, Anis M, Chavin K, Baliga P. A review of sonographic evaluation of renal transplant complications. Curr Probl Diagn Radiol. 2008;37:67–79. doi: 10.1067/j.cpradiol.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 11.Brown ED, Chen MYM, Wolfman NT, et al. Complications of renal transplantation: evaluation with US and radionuclide imaging. Radiographics. 2000;20:607–622. doi: 10.1148/radiographics.20.3.g00ma14607. [DOI] [PubMed] [Google Scholar]
- 12.Fischer T, Mehrsai A, Salem S, Ahmadi H, Baradaran N, Taherimahmoudi M, Nikoobakht MR, Rezaeidanesh M, Mansoori D, Pourmand G. Role of resistive index measurement in diagnosis of acute rejection episodes following successful kidney transplantation. Transplant Proc. 2009;41(7):2805–2807. doi: 10.1016/j.transproceed.2009.07.050. [DOI] [PubMed] [Google Scholar]
- 13.Krejčí K, Zadražil J, Tichý T, Al-Jabry S, Horčička V, Štrebl P, Bachleda P. Sonographic findings in borderline changes and subclinical acute renal allograft rejection. Eur J Radiol. 2009;71:288–295. doi: 10.1016/j.ejrad.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 14.Dupont PJ, Dooldeniya M, Cook T, Warrens AN. Role of duplex Doppler sonography in diagnosis of acute allograft dysfunction-time to stop measuring the resistive index? Transpl Int. 2003;16:648–652. doi: 10.1007/s00147-003-0601-7. [DOI] [PubMed] [Google Scholar]
- 15.Pellé G, Vimont S, Levy PP, et al. Acute pyelonephritis represents a risk factor impairing long-term kidney graft function. Am J Transplant. 2007;7(4):899–907. doi: 10.1111/j.1600-6143.2006.01700.x. [DOI] [PubMed] [Google Scholar]
- 16.Granata A, Andrulli S, Fiorini F, Basile A, Logias F, Figuera M, Sicurezza E, Gallieni M, Fiore CE. Diagnosis of acute pyelonephritis by contrast-enhanced ultrasonography in kidney transplant patients. Nephrol Dial Transplant. 2011;26(2):715–720. doi: 10.1093/ndt/gfq417. [DOI] [PubMed] [Google Scholar]
- 17.Dell’Atti L, Borea PA, Ughi G, Russo GR. Clinical use of ultrasonography associated with color Doppler in the diagnosis and follow-up of acute pyelonephritis. Arch Ital Urol Androl. 2010;82(4):217–220. [PubMed] [Google Scholar]
- 18.Saracini A, Santarsia G, Latorraca A, Gaudiano V. Early assessment of renal resistance index after kidney transplant can help predict long-term renal function. Nephrol Dial Transplant. 2006;21:2916–2920. doi: 10.1093/ndt/gfl203. [DOI] [PubMed] [Google Scholar]
- 19.Krumme B. Renal Doppler sonography—update in clinical nephrology. Nephron Clin Pract. 2006;103:c24–c28. doi: 10.1159/000090605. [DOI] [PubMed] [Google Scholar]
- 20.Vallejos A, Alperovich G, Moreso F, Cañas C, de Lama ME, Gomà M, Fulladosa X, Carrera M, Hueso M, Hueso M, Grinyó, Serón D. Resistive index and chronic allograft nephropathy evacuate in protocol biopsies as predictors of graft outcome. Nephrol Dial Transpl. 2005;20:2511–2516. doi: 10.1093/ndt/gfi041. [DOI] [PubMed] [Google Scholar]
- 21.Scolari MP, Cappuccilli ML, Lanci N, La Manna G, Comai G, Persici E, Todeschini P, Faenza A, Stefoni S. Predictive factors in chronic allograft nephropathy. Transpl Proc. 2005;37:2482–2484. doi: 10.1016/j.transproceed.2005.06.092. [DOI] [PubMed] [Google Scholar]
- 22.Radermarcher J, Mengel M, Ellis S, Stuht S, Hiss M, et al. The renal arterial resistance index and renal allograft survival. N Engl J Med. 2003;349:115–124. doi: 10.1056/NEJMoa022602. [DOI] [PubMed] [Google Scholar]
- 23.Nankivell BJ, Chapman JR, Gruenewald SM. Detection of chronic allograft nephropathy by quantitative Doppler imaging. Transplantation. 2002;74(1):90–96. doi: 10.1097/00007890-200207150-00016. [DOI] [PubMed] [Google Scholar]
- 24.Scholbach T, Girelli E, Scholbach J. Tissue pulsatility index: a new parameter to evaluate renal transplant perfusion. Transplantation. 2006;81:751–755. doi: 10.1097/01.tp.0000201928.04266.d2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.





