Abstract
Improvements in the care of kidney transplant recipients and advances in immunosuppressive therapy have reduced the incidence of graft rejection. As a result, other types of kidney transplant complications, such as surgical, urologic, parenchymal, and vascular complications, have become more common. Although vascular complications account for only 5–10 % of all post-transplant complications, they are a frequent cause of graft loss. Ultrasonography, both in B-mode and with Doppler ultrasound, is a fundamental tool in the differential diagnosis of renal allograft dysfunction. Doppler ultrasound is highly specific in cases of transplanted renal artery stenosis, pseudoaneurysms, arteriovenous fistulas, and thrombosis with complete or partial artery or vein occlusion. A single measurements of color Doppler indexes display high diagnostic accuracy and in particular cases are more useful during the post-transplantation follow-up period. More recent techniques, such as contrast-enhanced ultrasound, undoubtedly increase the accuracy of ultrasonography in the diagnosis of vascular complications involving the transplanted kidney.
Keywords: Ultrasonography, Doppler ultrasound, Renal transplant, Vascular complications
Riassunto
La progressiva riduzione dell’incidenza del rigetto ha reso più frequenti le complicanze urologiche, chirurgiche, parenchimali e vascolari. Queste ultime, pur rappresentando soltanto il 5–10 % di tutte le complicanze post-trapianto, sono frequente causa di perdita del graft. L’esame ultrasonografico, sia in B-mode che con l’ausilio del color Doppler, è fondamentale nella diagnosi differenziale delle cause che possono innescare una disfunzione del graft. Sebbene sia ormai indiscussa la sua utilità nella diagnosi di complicanze parenchimali, chirurgiche e urologiche, non è ancora consolidato il suo ruolo in caso di complicanze a carico dell’asse vascolare renale. L’ecocolor-Doppler, in particolare, possiede una specificità tale da poter essere considerato uno strumento diagnostico nella maggior parte delle complicanze vascolari del rene trapiantato, sia acute (occlusione parziale o totale dei vasi renali) che croniche (stenosi dell’arteria renale, pseudo aneurisma e fistola artero-venosa) Gli indici color-Doppler possiedono, infatti, una alta accuratezza diagnostica nella loro singola determinazione, risultando in casi particolari più utili nel follow-up. L’utilizzo di tecniche più moderne, come il mezzo di contrasto ecografico, consente indubbiamente di aumentare l’accuratezza diagnostica dell’esame ultrasonografico nel caso delle complicanze vascolari del rene trapiantato.
Introduction
Over the past 20 years, the availability of calcineurin inhibitors and other new classes of immunosuppressive drugs has significantly reduced the incidence of rejection-related loss of renal transplants and pushed other types of complications to the forefront [1]. The latter consist mainly of surgical/urological events and parenchymal complications, which occur in 45–60 and 25–30 % of all cases, respectively [2]. Although vascular problems account for only 5–10 % of all post-transplant complications, they can be a frequent cause of graft loss [1]. The principal vascular complications are stenosis and/or thrombosis of the renal artery and/or vein, segmental renal infarction, dissection of the iliac and/or renal artery, and the development of arteriovenous fistulas (mainly after biopsy) and pseudoaneurysms (Table 1).
Table 1.
Complications of the transplanted kidney
| Early complications | 50–60 % |
| • Acute tubular necrosis (10–30 %) | |
| • Acute rejection (20–40 %) | |
| • Renal artery thrombosis (rare) | |
| • Renal vein thrombosis (rare) | |
| • Urinary obstruction | |
| • Urinary hemorrhage and/or leak (6 %) | |
| • Fluid collections (abscess, hematoma, lymphocele, urinoma) | |
| • Cyclosporine and tacrolimus toxicity | |
| • Infections | |
| • Disease relapse | |
| Late complications | 10–20 % |
| • Transplanted renal artery stenosis (10–12 %) | |
| • Renal infarction | |
| • Cyclosporine and tacrolimus toxicity | |
| • Infections | |
| • Arteriovenous fistulas (10 %) | |
| • Obstruction | |
| • Transplanted renal vein stenosis | |
| • Chronic allograft nephropathy | |
| Long-term complications | 1–2 % |
| • De novo glomerulonephritis | |
| • Recurrent disease | |
| • Other complications (1 %) | |
Evaluation of the vascular axis of the transplanted kidney: examination technique
Color Doppler ultrasonography (CDUS) of the transplanted renal artery and the rest of the graft’s vascular axis should include full length of the vessels. To minimize the risk of technical failures, the examination protocol should be standardized. The examination should begin with a B-mode assessment of the aorta, the aortoiliac axis, the external iliac artery, and the origins of the transplanted vessels. Before starting, it is useful to read the operative report to get a clear idea of the procedure used and the number of arteries transplanted [3]. For the color Doppler analysis, the color box area should be restricted to the region to be examined to improve the frame rate (FR). The clinician should adjust the pulse repetition frequency (PRF) (approximate range 1.5–3 kHz), the wall filter (100 Hz), and the color gain to obtain an optimal image, i.e., a uniform red or blue map with no aliasing or color diffusion effects into the perivascular tissues (color bleed) [4]. Finally, the spectral analysis module is activated: the sample volume is positioned in the lumen of the renal artery and the velocity/time (V/t) curve recorded. The size of the sample volume (2–4 mm) should be adjusted to allow homogeneous insonation of the vessel without producing oversampling/undersampling artifacts [4]. The V/t curve should be recorded several times with optimal insonation angles (<60°) because the diagnosis of renal artery stenosis in the graft is based exclusively on absolute velocity values [measurement of the peak systolic velocity (PSV)] (Fig. 1).
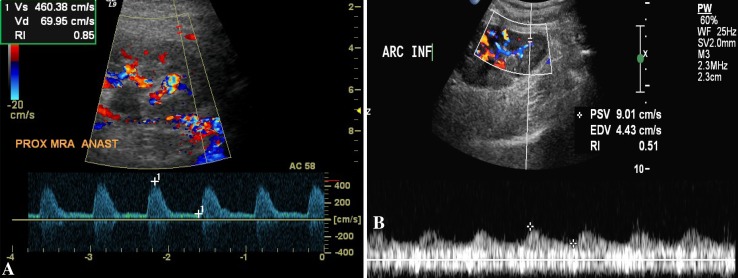
Fig. 1.
Renal artery stenosis. a Color Doppler study shows mosaic pattern at the site of the anastomosis and a significant increase in the peak systolic velocity (460 cm/s). b Spectral analysis shows a “tardus-parvus” waveform at the level of the arcuate artery
Transplanted renal artery stenosis (TRAS)
Transplanted renal artery stenosis accounts for 75 % of all vascular complications. It is generally observed 3–24 months after the surgical procedure although it can really occur at any time [3].
Transplanted renal artery stenosis is a relatively frequent complication, with a prevalence that ranges from 1.5 to 4 % [5] and an incidence between 1 and 23 % [6]. The wide variability of reported incidence rates reflects a lack of consensus on the definition of hemodynamically significant TRAS. The definitions reported in the literature vary from 50 to 80 %, and there are also differences in the characteristics of the study population (presence/absence of chronic renal failure, arterial hypertension, etc.). Moreover, in several cases angiographic documentation of the stenosis is also lacking. One of the best-designed studies in terms of the methods used revealed an incidence of 12.4 % [7].
Risk factors for TRAS include atherosclerotic disease in a donor vessel, cytomegalovirus infection, delayed restoration of renal function, and transplantation of a pediatric kidney in an adult recipient [7]. TRAS should be suspected when the patient has poorly controlled blood pressure and/or a progressive decline in renal function that cannot be attributed to other obvious causes (rejection, obstruction, infection) or that follows the administration of ACE inhibitors or angiotensin receptor blockers [3].
In about half of all TRAS cases, the stenosis involves the anastomosis site, and it is iatrogenic, i.e., caused by scarring related to the explanation, clamping, and/or anastomosis of the vessel with the iliac artery axis [8]. Less commonly multiple segments of the artery (or even the entire vessel) are stenotic. In these cases, the stenosis is generally the result of catheter-related trauma to the intima during the phase of cold ischemia, but it may also be caused by torsion and/or kinking after surgical implantation [8].
Since TRAS is a major cause of graft dysfunction and/or loss, prompt diagnosis and treatment can significantly improve graft survival. Ultrasound studies, particularly color Doppler imaging, play important roles in the screening, diagnosis, and follow-up stages although angiography is still the gold-standard in this setting [3, 9]. The increased use of CDUS and/or other, more complex imaging studies, such as computed tomography (CT) or magnetic resonance imaging (MRI) has led to an increase in the incidence of asymptomatic cases of TRAS [10].
Color Doppler ultrasound criteria for the diagnosis of TRAS
Difficulties can arise during CDUS examination of the transplanted renal artery. Marked tortuosity of the vessels can lead to erroneous insonation angles, reducing the accuracy of PSV measurements; other sources of error are renal artery stretching and/or kinking, which can cause false acceleration [11]. Renal blood flow is strongly dependent on renal function, so defining a precise PSV threshold for the diagnosis of TRAS (unlike stenosis of the native renal artery) is not possible. In the absence of hemodynamically significant stenosis, the PSV of the RA in a hypertrophic, well-functioning transplanted kidney may exceed 250–300 cm/s along the full length of the artery. In contrast, in the presence of chronic graft dysfunction with reduced organ volume, a focal PSV of 180–200 cm/s may be suggestive of significant TRAS, particularly when the other segments of the artery exhibit markedly lower PSVs (40–50 cm/s). Therefore, focal acceleration of flow that is 2.5 times higher than the pre- or post-stenotic velocity (e.g., PSV 250–270 vs. 80–120 cm/s) represents a direct criterion for the diagnosis of TRAS. The PSV threshold for defining hemodynamically significant stenosis varies somewhat from author to author [12, 13], but there is a reasonable level of consensus that a value of 250 cm/s is still within the normal range, whereas higher velocities predict significant stenosis with high sensitivity and specificity (Table 2) [14, 15]. Some authors have pointed out that the PSV threshold for stenosis may vary depending on the type of anastomosis (end-to-side vs. end-to-end) created between the renal artery and iliac vessels. They also maintain that the best Doppler criterion for diagnosis of TRAS, regardless of the type of anastomosis, is a stenotic renal artery PSV that is >13 times higher than that of an interlobar artery [16, 17] (Table 3). A limited number of studies have attempted to identify the presence of TRAS using contrast-enhanced ultrasound, but the role of this technique is still unclear [18].
Table 2.
Diagnostic accuracy of CDUS in the diagnosis of TRAS
| PSV | ≥2.0 m/s | ≥2.5 m/s | ≥3.0 m/s |
|---|---|---|---|
| Specificity | 67 % (55–77 %) | 79 % (65–82 %) | 93 % (77–96 %) |
| Sensitivity | 100 % (46–100 %) | 100 % (46–100 %) | 80 %(29–98 %) |
| Accuracy | 68 % | 81 % | 92 % |
Table 3.
Sensitivity of Doppler indexes in the diagnosis of severe TRAS
| TRAS > 80 % | |
|---|---|
| PSV ra/PSV ia > 13 | 100 % |
| PSV ra > 300 cm/s | 80 % |
| AT > 0.06 s | 93 % |
| RI < 0.5 | 50 % |
ra renal artery, ia interlobar artery, at acceleration time, ri resistance index
In contrast to the diagnosis of native renal artery stenosis, indirect signs are of little use in the diagnosis of TRAS because the transplanted artery can be directly visualized in most cases, its tardus-parvus waveform cannot be compared with that of the contralateral kidney, and because the resistance to flow is influenced by numerous variables [19] except in particular cases (Fig. 1). Acceleration time is the only indirect index that displays high sensitivity in the diagnosis of TRAS, but only when the degree of stenosis exceeds 80 % [19] (Table 3). Secondary effects, such as turbulence, reverse flow, and spectral dispersion, can be evaluated in the segment immediately downstream from the stenosis.
In clinical practice, it can be very difficult to distinguish between a true stenotic lesion and torsion/curving of the renal artery, which also exerts hemodynamic effects on blood flow and the maximum PSV (Peak Systolic Velocity max); Power Doppler imaging can be useful in these cases. The iliac arteries should also be examined to evaluate the preanastomotic PVSmax because iliac stenosis can lead to reduced renal function similar to that observed in the presence of TRAS. For this reason, the recipient’s iliac vessels must also be subjected thorough pretransplant color Doppler exploration. Narrowing of arterial vessels within the graft has been reported, but it is difficult to visualize with CDUS or with angiography. CDUS is also particularly useful during follow-up and in the diagnosis of recurrence of TRAS after treatment [19].
Thrombosis of the transplanted renal artery
Frank occlusion of the renal artery is a very rare occurrence with negative prognostic implications: it occurs very early during the postoperative period and leads inexorably to graft loss. The sensitivity and specificity of CDUS in the diagnosis of renal artery thrombosis are close to 100 %. Occlusion of the main renal artery is reflected on CDUS by the absence of arterial flow within the kidney (distal to the site of occlusion) along with the complete absence of venous flow [19]. Occlusion involving a segmental artery leads to segmental infarction, which is reflected by the absence of arteriovenous flow only in the affected segment. Power Doppler imaging can be helpful in cases of this type because of its excellent capacity for identifying low-flow vessels, but in certain cases, angiographic confirmation of the diagnosis is still necessary [20].
Thrombosis and stenosis of the transplanted renal vein
Thrombosis of the transplanted renal vein (TTRV) is also a rare event: it occurs in approximately 4 % of cases, generally during the immediate postoperative period. It can be complete, in which case it leads to loss of the graft, or partial [21, 22]. Surgical problems (complications, technical errors), hypovolemia, thrombosis of the iliac axis, and compression caused by perinephric fluid collections (lymphocele, urinoma, etc.) are among the most common causes (Fig. 3). TTRV should be suspected when there is a sudden drop in urine output and enlargement of the graft with tenderness, swelling, proteinuria, and deteriorating renal function [19].
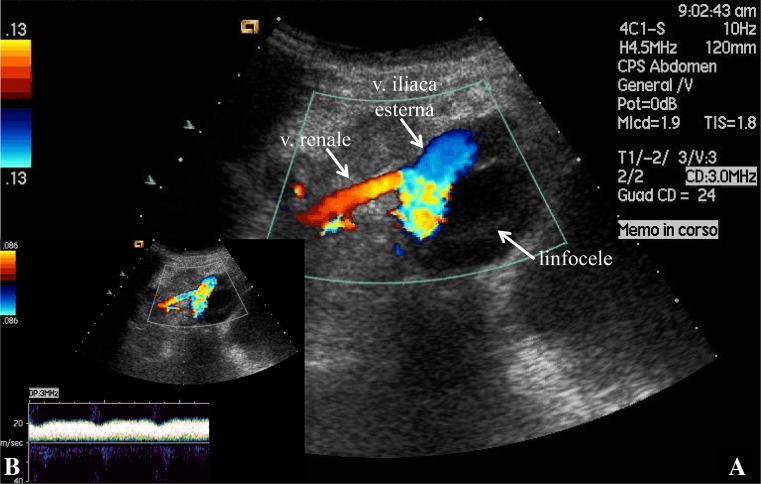
Fig. 3.
Stenosis of the transplanted renal vein. a Color Doppler ultrasound shows external compression of the homolateral external iliac vein and b a significantly increased PSV (200 cm/s)
Color Doppler ultrasonography plays an important role in the diagnosis and follow-up of this complication. In the presence of complete TTRV, the vessel is characterized by scarce or absent compressibility, and the B-mode examination reveals renal enlargement, reduced parenchymal echogenicity, diminished/absent corticomedullary differentiation, and disappearance of the renal sinus and collecting system (all of which are nonspecific). The two most important CDUS findings are the absence of the venous color signal (reflecting absence of vascularization) and reverse diastolic flow within the renal artery [22] (Fig. 2). In the presence of complete TTRV, it is also impossible to demonstrate the intrarenal veins at the level of the hilum, and the high resistance caused by the renal vein thrombosis results in pathognomonic bidirectional flow in the intrarenal arteries (the arterial waveform will be positive during systole and negative during diastole [23]. The net flow across the kidney drops to zero, as does the mean flow velocity for each cardiac cycle. This bidirectional flow is so specific for TTRV that it is regarded as an indication for immediate surgical revision of the graft without further diagnostic confirmation.
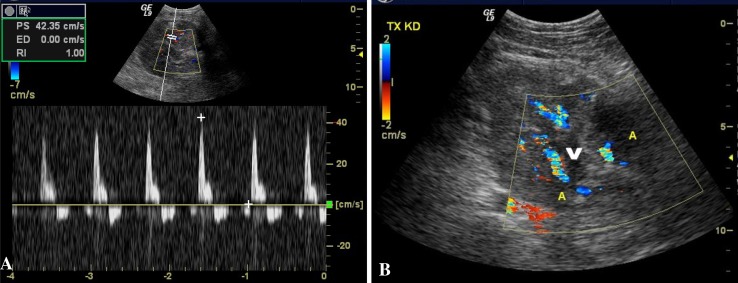
Fig. 2.
Thrombosis of the transplanted renal vein. a Color Doppler ultrasound shows “reverse” diastolic flow (below the baseline); b color imaging of the hilar region shows two segmental arteries (A) and no flow in the renal vein (V)
Stenosis of the transplanted renal vein is rare. It may be the result of compression of the vein by a perinephric fluid collection (Fig. 3) or perivascular fibrosis. The CDUS findings are not as conclusive as they are in cases of TTRV. Indeed, the parenchyma may appear normal or mildly hypoechoic. A 3–4-fold increase in the PSV between the stenotic and prestenotic segments is regarded as highly suggestive of focal stenosis [19].
Angiography should be used only to confirm or treat stenosis in patients with positive CDUS findings or in cases in which there is a strong clinical suspicion of stenosis despite the indeterminate or equivocal imaging findings.
Arteriovenous fistulas and pseudoaneurysms
These two lesions are reported mainly as biopsy complications. Their incidence ranges from 1 to 2 %. Arteriovenous fistulas (AVFs) develop as a result of damage to the walls of an artery and a vein during needle biopsy of the kidney. In contrast, pseudoaneurysms can develop when the damage is limited to the arterial wall [19]. Both lesions are generally small, clinically silent, and likely to resolve spontaneously [19, 24].
On ultrasound, pseudoaneurysms appear as small cyst-like anechoic areas containing finely hyperechoic material representing internal thrombi. CDUS reveals turbulence and “to-and-fro” flow resembling to that seen in pseudoaneurysms involving the arterial extremities [24].
Arteriovenous fistulas can be visualized in B-mode only if they are large. On CDUS, they appear as focal areas with both arterial and venous flow (color mosaic pattern) (Fig. 4): this pattern can be differentiated from high flow patterns by increasing the PRF until only the area with anomalous, accelerated flow inside the AVF is visualized. This maneuver almost always produces diagnostic results. Spectral analysis may show increased systodiastolic flow in the area of interest with resistive and pulsatility indexes that are often normal or lower than those of nearby vessels, whereas venous flow may be normal or turbulent, and pulsatile acceleration is present in around 33 % of all cases [25]. In rare cases, AVFs are large enough to reduce renal perfusion and cause graft ischemia; pseudoaneurysms can cause complications if they rupture. Patients with large AVFs, who undergo repeated renal biopsies are at increased risk for hemorrhagic complications. For AVFs, the need for treatment is related to the size of the lesion. With pseudoaneurysms, there is always the risk of rupture, and for this reason, they should always be treated.
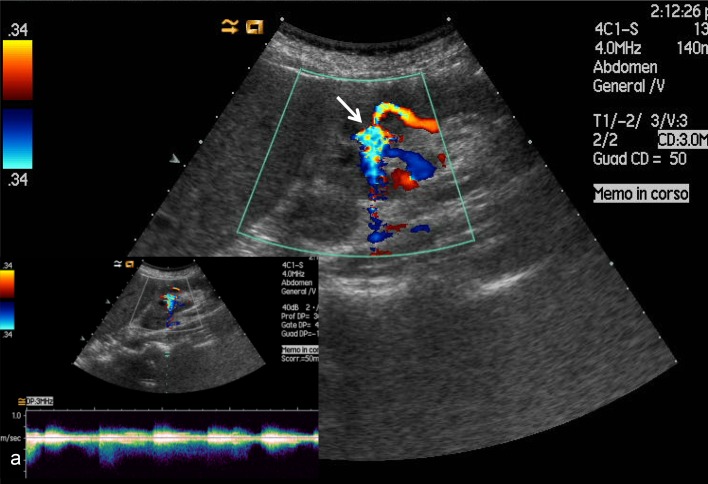
Fig. 4.
Color Doppler ultrasound shows large-caliber interlobar artery and vein. The resistance index of the nutrient artery is 0.50 (vs. approximately 0.80 in other regions of the graft). The venous spectrum reflects markedly pulsatile flow that cannot be found in other interlobar veins. Post-biopsy arteriovenous fistula (arrow); a Doppler shows alternating flow
Rare vascular complications
Torsion of the vascular pedicle of the transplanted kidney is extremely rare. It is caused by intraperitoneal placement of the graft (which occurs during combined renal–pancreatic transplants) [19]. Because of its increased mobility, the kidney may rotate around the vascular pedicle, causing vascular occlusion that will lead to parenchymal necrosis and graft loss if not identified promptly. The clinical presentation varies: it may resemble acute rejection or renal vein thrombosis. Ultrasonography can facilitate the diagnosis by documenting a change in the orientation of the kidney, so that the hilum is anterior rather than posterior [26]. Color Doppler ultrasound findings are variable with low diagnostic accuracy. Dissection of the iliac artery and renal artery are extremely rare events, which are caused by dissection of the aorta and present the same features on CDUS.
Conclusions
In expert hands, color Doppler ultrasound can be a valid tool for the diagnosis and follow-up assessment of the vascular complications of renal transplantation. However, additional, well-designed studies are needed to further validate its role in these settings, and the nephrologist responsible for the transplant recipient must be well versed in both the theory and practice of ultrasonography.
Conflict of interest
Antonio Granata, Silvia Clementi, Francesco Londrino, Giulia Romano, Massimiliano Veroux, Fulvio Fiorini, Pasquale Fatuzzo declare that they have no conflict of interest.
Human and animal studies
The study described in this article did not include any procedures involving humans or animals.
References
- 1.Ghazanfar A, Tavakoli A, Augustine T, Pararajasingam R, Riad H, Chalmers N. Management of transplant artery stenosis and its impact on long-term allograft survival: a single-centre experience. Nephrol Dial Transpl. 2011;26:336–343. doi: 10.1093/ndt/gfq393. [DOI] [PubMed] [Google Scholar]
- 2.O’Neill WC, Baumgarten DA. Ultrasonography in renal transplantation. Am J Kidney Dis. 2002;39:663–678. doi: 10.1053/ajkd.2002.31978. [DOI] [PubMed] [Google Scholar]
- 3.Mangray M, Vella JP. Hypertension after kidney transplant. Am J Kidney Dis. 2011;57:331–341. doi: 10.1053/j.ajkd.2010.10.048. [DOI] [PubMed] [Google Scholar]
- 4.Granata A, Floccari F, Lentini P, Vittoria S, Di Pietro F, Zamboli P, et al. Vascular complications following kidney transplant: the role of color-Doppler imaging. G Ital Nefrol. 2012;29(S57):S99–S105. [PubMed] [Google Scholar]
- 5.Patel U, Khaw KK, Hughes NC. Doppler ultrasound for detection of renal transplant artery stenosis-threshold peak systolic velocity needs to be higher in a low-risk or surveillance population. Clin Radiol. 2003;58:772–777. doi: 10.1016/S0009-9260(03)00211-3. [DOI] [PubMed] [Google Scholar]
- 6.Ferveza FC, Lafayette RA, Alfrey EJ, Petersen J. Renal artery stenosis in kidney transplant. Am J Kidney Dis. 1998;31:142–148. doi: 10.1053/ajkd.1998.v31.pm9428466. [DOI] [PubMed] [Google Scholar]
- 7.Weir MR, Salzberg DJ. Management of hypertension in the transplant patient. J Am Soc Hypertens. 2011;5:425–432. doi: 10.1016/j.jash.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 8.Irshad A, Ackerman SJ, Campbell AS, Anis M. An overview of renal transplantation: current practice and use of ultrasound. Semin Ultrasound CT MR. 2009;30(4):298–314. doi: 10.1053/j.sult.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 9.Loubeyre P, Abidi H, Cahen R, Tran Minh VA. Transplanted renal artery: detection of stenosis with color Doppler US. Radiology. 1997;203:661–665. doi: 10.1148/radiology.203.3.9169685. [DOI] [PubMed] [Google Scholar]
- 10.Spinosa DJ, Isaacs RB, Matsumoto AH, Angle JF, Hagspiel KD, Leung DA. Angiographic evaluation and treatment of transplant renal artery stenosis. Curr Opin Urol. 2001;11:197–205. doi: 10.1097/00042307-200103000-00012. [DOI] [PubMed] [Google Scholar]
- 11.Sharfuddin A. Imaging evaluation of kidney transplant recipients. Semin Nephrol. 2011;31(3):259–271. doi: 10.1016/j.semnephrol.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 12.Helenon O, el Rody F, Correas JM, Melki P, Chaveau D, Chrétien Y, et al. Color Doppler US of renovascular disease in native kidneys. Radiographics. 1995;15:833–854. doi: 10.1148/radiographics.15.4.7569132. [DOI] [PubMed] [Google Scholar]
- 13.Granata A, Fiorini F, Andrulli S, Logias F, Lo Piccolo, Sicurezza E (2009) L’ecocolorDoppler nella patologia vascolare del rene trapiantato. Cap. 15. In: Accademia Nazionale di Medicina Granata A, Fiorini F, D’Amelio A, Logias F, Andrulli S (ed) L’ecocolorDoppler nella pratica clinica nefrologica, vol 2, pp 187–196
- 14.Akbar SA, Jafri SZ, Amendola MA, Madrazo BL, Salem R, Bis KG. Complications of renal transplantation. Radiographics. 2005;25(5):1335–1356. doi: 10.1148/rg.255045133. [DOI] [PubMed] [Google Scholar]
- 15.Baxter GM, Ireland H, Moss JG, Harden PN, Junor BJ, Rodger RS, et al. Colour Doppler ultrasound in renal transplant artery stenosis: which Doppler index? Clin Radiol. 1995;50:618–622. doi: 10.1016/S0009-9260(05)83291-X. [DOI] [PubMed] [Google Scholar]
- 16.Gao J, Li JC, Xiao MS, Ng A, Trost D, Goldstein M, et al. Color duplex sonography in severe transplant renal artery stenosis: a comparison of end-to-end and end-to-side arterial anastomoses. Clin Imaging. 2009;33:116–122. doi: 10.1016/j.clinimag.2008.08.020. [DOI] [PubMed] [Google Scholar]
- 17.Li JC, Ji ZG, Cai S, Jiang YX, Dai Q, Zhang JX. Evaluation of severe transplant renal artery stenosis with Doppler sonography. J Clin Ultrasound. 2005;33:261–269. doi: 10.1002/jcu.20129. [DOI] [PubMed] [Google Scholar]
- 18.Gao J, Ng A, Shih G, Goldstein M, Kapur S, Wang J, et al. Intrarenal color duplex ultrasonography: a window to vascular complications of renal transplants. J Ultrasound Med. 2007;26(10):1403–1418. doi: 10.7863/jum.2007.26.10.1403. [DOI] [PubMed] [Google Scholar]
- 19.Brown ED, Chen MY, Wolfman NT, Ott DJ, Watson NE., Jr Complications of renal transplantation: evaluation with US and radionuclide imaging. Radiographics. 2000;20:607–622. doi: 10.1148/radiographics.20.3.g00ma14607. [DOI] [PubMed] [Google Scholar]
- 20.Cosgrove DO, Chan KE. Renal transplants: what ultrasound can and cannot do. Ultrasound Q. 2008;24(2):77–87. doi: 10.1097/RUQ.0b013e31817c5e46. [DOI] [PubMed] [Google Scholar]
- 21.Lockhart ME, Robbin ML. Renal vascular imaging: ultrasound and other modalities. Ultrasound Q. 2007;23:279–292. doi: 10.1097/ruq.0b013e31815adf4c. [DOI] [PubMed] [Google Scholar]
- 22.Drudi FM, Cascone F, Pretagostini R, Ricci P, Trippa F, Righi A, et al. Role of Color Doppler US in the evaluation of renal transplant. Radiol Med. 2001;101:243–250. [PubMed] [Google Scholar]
- 23.Elsayes KM, Menias CO, Willatt J, Azar S, Harvin HJ, Platt JF. Imaging of renal transplant: utility and spectrum of diagnostic findings. Curr Probl Diagn Radiol. 2011;40(3):127–139. doi: 10.1067/j.cpradiol.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 24.Martinoli C, Bertolotto M, Crespi G, Pretolesi F, Valle M, Derchi LE. Duplex Doppler analysis of interlobular arteries in transplanted kidneys. Eur Radiol. 1998;8:765–769. doi: 10.1007/s003300050469. [DOI] [PubMed] [Google Scholar]
- 25.Krumme B. Renal Doppler sonography-update in clinical nephrology. Nephron Clin Pract. 2006;103:c24–c28. doi: 10.1159/000090605. [DOI] [PubMed] [Google Scholar]
- 26.Dupont PJ, Dooldeniya M, Cook T, Warrens AN. Role of duplex Doppler sonography in diagnosis of acute allograft dysfunction-time to stop measuring the resistive index? Transpl Int. 2003;16:648–652. doi: 10.1007/s00147-003-0601-7. [DOI] [PubMed] [Google Scholar]