Abstract
AIM: To study the distribution pattern of transcription factors NF-κB and AP-1 and their relations with the expression of apoptosis associated-proteins Fas/FasL and ICH-1L/S in human hepatocellular carcinoma (HCC).
METHODS: We performed in situ hybridization and immunohistochemical techniques for NF-κB, AP-1, Fas/FasL and ICH-1 in 40 cases of human HCC along with corresponding nontumoral tissues and 7 cases of normal liver tissues.
RESULTS: Twenty-two (55%) and 25 (62.5%) of 40 cases for NF-κB and AP-1 were presented for nuclear or both nuclear and cytoplastic staining respectively, while less cases were presented for only cytoplastic staining for NF-κB (18%) and AP-1 (10%) in adjacent nontumoral tissues and negative staining in normal liver tissues. There was no statistically significant difference of NF-κB or AP-1 activation between well differentiated tumors and poorly differentiated tumors (P > 0.05). NF-κB activity is positively corresponded to AP-1 activation. The expression of ICH-1L/S was associated with the activation of NF-κB and AP-1 (P < 0.05), but no significant relationship was found between Fas/FasL and NF-κB or AP-1(P > 0.05).
CONCLUSION: Activation of both NF-κB and AP-1 may be required for ICH-1L/S-induced apoptosis in HCC, but not for Fas/FasL-mediated apoptosis. NF-κB and AP-1 may play important roles in the pathogenesis of human HCC.
Keywords: Hepatocellular carcinoma (HCC), Transcription factors, Apoptosis, Protein
INTRODUCTION
Hepatocellular carcinoma (HCC) is one of the most common cancers and cause of mortality in China. Much advanced progresses in the mechanism of hepatocarcinogenesis have been achieved for these years. Many genes such as proto-oncogenes, tumor suppressor genes, apoptosis genes and growth factors genes have been implicated and apoptosis genes may play an important effect in the process of hepatocarcinogenesis[1-6]. Apoptosis-related genes such as bcl-2/bax, Fas/FasL and caspase are involved in the pathogenesis of HCC[3-6]. However, more steps of apoptosis genes in this process remain unknown yet.
Activated protein-1 (AP-1) and nuclear factor κB (NF-κB), two of important transcription factors, play important roles in signal transduction pathways of cell differentiation, proliferation and apoptosis in response to a variety of physiological and pathological stimuli[7-13]. AP-1 consists of homodimers and heterodimers of the Jun family (c-Jun, JunB, JunD), and the Fos family (c-Fos, FosB, Fra1, Fra2). NF-κB is a heterodimeric complex composed of two subunits of the Rel/NF-κB family, factors including NF-κB1 (p50), NF-κB2 (p52), c-Rel, RelA/p65, and RelB[14]. Some investigators have presented aberrant DNA binding activity of AP-1 and NF-κB in various types of human tumor such as HCC, gastric carcinoma, breast carcinoma and so on[15-22]. These findings suggested that AP-1 and NF-κB may be important in the control of cell proliferation and oncogenesis of these tumors.
The molecular mechanisms of NF-κB and AP-1 in the regulation of Fas/FasL mediated apoptosis may be different in T cells, Jurkat cells, hepatocyte-derived cell lines and colon carcinoma cell[23-27]. However, whether NF-κB and AP-1 play important roles in regulation of Fas/FasL and ICH-1L/S expression in human HCC is still not known. In the present study, we undertook to investigate whether AP-1 and NF-κB are constitutively activated in human HCC tissues and to evaluate the relationship between AP-1 or NF-κB activity and the expression of apoptosis associated proteins (Fas/FasL and ICH-1L/S) by in situ hybridization and immunohistochemical techniques.
MATERIALS AND METHODS
Tissue preparation
Forty samples were obtained by surgical resection in our department. All samples were independently reviewed by two pathologists. The cases of HCC were classified according to the criteria described by Edmondson-Steiner and grouped as well differentiated (grade I-II; n = 25) or poorly differentiated (grade III-IV; n = 15). Seven undamaged liver tissues from surgical resection specimens of young adults with minor liver injury who underwent partial hepatectomy were used as normal control. All tissues were fixed in 40 g/L formaldehyde (pH 7.0) for 12-24 h and embedded in paraffin wax and then 4 µm serial sections were cut and mounted on poly-l-lysine coated slides.
Immunohistochemistry staining
Sections were deparaffinized and rehydrated routinely. Antigen was retrieved by heating sections in a microwave oven at 700 W in 10 mmol/L citrate buffer (pH 6.0) for 10 min. After blocking with 0.3% H2O2 and swine serum, specimens were then incubated with the primary antibodies, directed against Fas, FasL, and ICH-1L/S (Santa Cruz product, dilution 1:100) at 4°C overnight. Secondary antibodies were applied according to the manufacturer’s recommendations (Amersham). The staining was performed by streptavidin-peroxidase enzyme conjugate method using a S-P kit (Zymed product). Reaction products were visualized by DAB (diaminobenzidine). The slides were counterstained with hematoxylin before mounting in paramount. Brown-yellow granules in cytoplasm were recognized as positive staining.
In situ hybridization
Sections were deparaffinized and rehydrated routinely. Oligonucleotides containing the consensus sequence of AP-1 (5’-CGCTTGATGAGTCAGCCGGAA-3’) and NF-κB (5’-AGTTGAGGGGACTTTCCCAGGC-3’) were respectively used as probes and 3’-labeled with biotin. Preparations were incubated with the labeled probes (37°C, overnight). Non-specific antigen was blocked with 2% bovine serum and 0.3% Triton X-100, followed by incubation with anti-biotin antibody alkaline phosphates mixture for 1 h. Slides were then visualized with BCIP/ NBT. Purple-blue granules were regarded as positive staining. In general, inactivated NF-κB and AP-1 were located in cytoplasm and nuclei staining scored as activated NF-κB and AP-1.
Negative controls
We used the following negative controls: (a) absence of probes, (b) mutant AP-1 or NF-κB probes 5’-CGCTTGAT-AAATCAGCCGGAA-3’, and 5’-AGTTGAGGCTC-CTTTCCCAGGC-3’, respectively, labeled with biotin, (c) competition assays with a 100-fold excess of unlabeled AP-1 or NF-κB probes, followed by incubation with its respective labeled probe.
Statistical analysis
Statistical significance was calculated by χ2 test. The χ2 test was used to analyze the association between NF-κB/AP-1 and histopathological grades and Fas/FasL and ICH-1L/S. P < 0.05 was regarded as significant difference.
RESULTS
Detection of NF-κB and AP-1 in HCC
In situ hybridization was performed to detect the activity status of NF-κB and AP-1 in all 40 HCCs. NF-κB and AP-1 distribution in nuclear or both nuclear and cytoplasm of cancer cells are illustrated in Figures 1 and 2. Of 40 HCCs, 22 (55%) presented nuclear or both nuclear and cytoplasmic staining of NF-κB by in situ hybridization in which 13/25 (52%) were well differentiated tumors and 9/15 (60%) were poorly differentiated tumors. While 25 (62.5%) of 40 cases for AP-1 positive staining were shown in which 14/25 (56%) were well differentiated tumors and 10/15 (66.7%) were poorly differentiated tumors. There was no statistically significant difference of NF-κB or AP-1 activation between well differentiated tumors and poorly differentiated tumors (P > 0.05). In adjacent non-tumor tissues, cytoplasmic staining of NF-κB or AP-1 was noted in 27(68%) and 23(58%) respectively. But lower nuclear staining for NF-κB (17.5%) and AP-1 (10%) was found in non-tumor tissues than those in HCC with a statistical significance (P < 0.05). Only few hepatocytes showed cytoplasmic staining for NF-κB (1/7) and AP-1 (2/7) in normal liver tissues, but no staining in the nucleus. NF-κB activity positively corresponded to AP-1 activation (Table 1).
Figure 1.
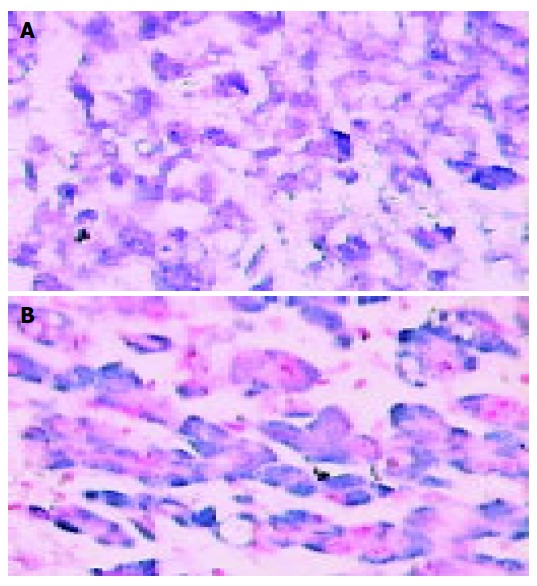
Hepatocellular carcinoma showing nuclear and cytoplasmic positivity positivity for NF-κB (A) and AP-1(B). In situ hybridization (magnification ×200).
Figure 2.
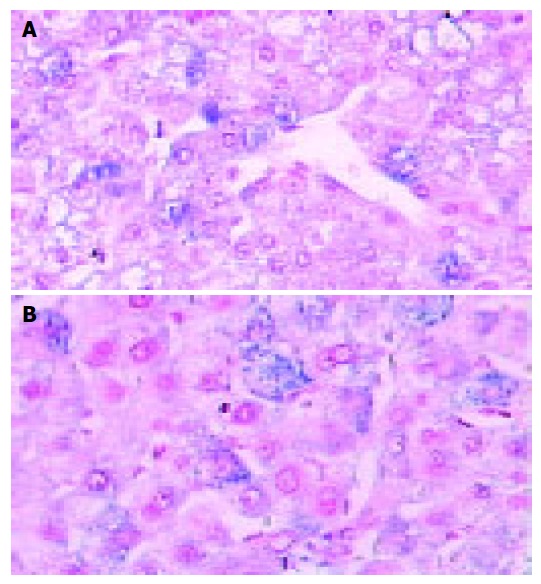
Nontumor liver tissue showing focal and weak cytoplasmic for NF-κB (A) and AP-1 (B). In situ hybridization (magnification ×200).
Table 1.
Detection of NF-κB and AP-1 in HCC
| Group | Cases | NF-κB | AP-1 |
| nucleus/cytoplasm | nucleus/cytoplasm | ||
| + nucleus (+) (%) | + nucleus (+) (%) | ||
| Tumor tissue | 40 | 22 (55)a | 24 (60) |
| Well differentiated | 25 | 13 (52) | 14 (56) |
| Poorly differentiated | 15 | 9 (60) | 10 (66.7) |
| Nontumor tissue | 40 | 7 (17.5) | 4 (10) |
| Normal tissue | 7 | 0 | 0 |
P < 0.05 vs tumor tissue.
Expression of apoptosis proteins in HCC
Table 2 summarizes the results of immunohistochemical studies on apoptosis proteins including Fas, FasL, ICH-1L and ICH-1S. Fas, FasL, ICH-1L, and ICH-1S presented cytoplasmic reactivity in 7 (17.5%), 6 (15%), 15 (37.5%) and 21 (52.5%) of tumors, while in 21 (52.5%), 18 (45%), 29 (72.5%) and 31 (77.5%) of nontumor tissues, in 0%, 0%, 2/7 and 1/7 of normal liver tissues, respectively. There were statistically significant differences of the expression of Fas, FasL, ICH-1L, and ICH-1S between non-tumor tissues and tumor tissues (P < 0.05). The differences of Fas and ICH-1S expression except FasL and ICH-1L were also statistically significant between nontumor tissues and normal liver tissues (P < 0.05, Figure 3).
Table 2.
Expression of Fas, FasL, ICH-1L, and ICH-1S in HCC
| Group | Cases | Fas (%) | FasL (%) | ICH-1L (%) ICH-1S (%) | |
| Tumor tissue | 40 | 7 (17.5)a | 6 (15) | 15 (37.5) | 21 (52.5) |
| Nontumor tissue | 40 | 21 (52.5)1 | 18 (45)2 | 29 (72.5)3 | 31 (77.5)4 |
| Normal tissue | 7 | 05 | 0b | 2 | 16 |
P < 0.05 vs tumor tissue.
χ2 = 10.2692,
χ2 = 8.5714,
χ2 = 9.8990,
χ2 = 5.4945,
χ2 = 4.6890,
χ2 = 8.2398.
Figure 3.
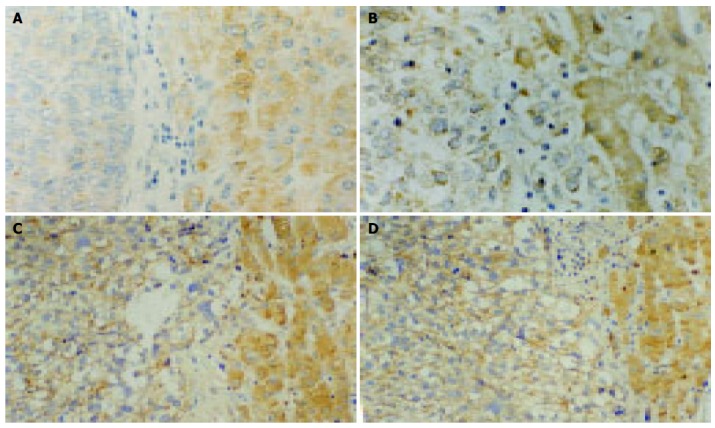
Immunohistochemistry staining showing strong cytoplasmic positivity in adjacent hepatocytes and weak positivity in HCC for Fas (A), FasL (B), ICH- lL (C) and ICH-lS (D) (magnification ×200).
Relationship between activated NF-κB and apoptosis proteins in HCC
The expression of Fas and FasL was more frequent in 22 cases of HCC with activated NF-κB compared with 18 cases of HCC with inactivated NF-κB, but the differences were not significant statistically between cases with activated NF-κB group and with inactivated NF-κB group (P > 0.05). However, there was positive relationship between the expression of ICH-1L and ICH-1S in the cases with activated NF-κB and those with inactivated NF-κB (P < 0.05, Table 3).
Table 3.
Relationship between activated NF-κB and apoptosis proteins in HCC
| NF-κB | Cases |
Apoptosis protein |
|||||||
|
Fas |
FasL |
ICH-1L |
ICH-1S |
||||||
| + | – | + | – | + | – | + | – | ||
| Activated | 22 | 6 | 16 | 5 | 17 | 13 | 12 | 17 | 8 |
| Inactivated | 18 | 1 | 17 | 1 | 17 | 2 | 13 | 4 | 11 |
| χ2 | 1.9048 | 1.4495 | 4.4444 | 4.8722 | |||||
| P | > 0.05 | > 0.05 | < 0.05 | < 0.05 | |||||
Relationship between activated AP-1 and apoptosis proteins in HCC
The expression of ICH-1L and ICH-1S in HCC with activated AP-1 was also more common with a statistical significance as compared with those with inactivated AP-1 respectively (P < 0.05). But there was no significant difference between the expression of Fas and FasL in the cases with activated AP-1 and those with inactivated AP-1 (P > 0.05, Table 4).
Table 4.
Relationship between activated AP-1 and apoptosis proteins in HCC
| AP-1 | Cases |
Apoptosis protein |
|||||||
|
Fas |
Fas L |
ICH-1L |
ICH-1S |
||||||
| + | – | + | – | + | – | + | – | ||
| Activated | 25 | 7 | 18 | 6 | 19 | 13 | 12 | 16 | 9 |
| Inactivated | 15 | 0 | 15 | 0 | 15 | 2 | 13 | 3 | 12 |
| χ2 | 3.3362 | 2.5621 | 4.4444 | 5.6207 | |||||
| P | > 0.05 | > 0.05 | < 0.05 | < 0.025 | |||||
DISCUSSION
Electrophoretic mobility shift assays is a common and useful technique for studying transcription factors. However, the distribution of transcription factors either in nucleus or cytoplasm cannot be shown using this technique. In situ detection using nonradioactive oligonucleotides in paraffin wax-embedded tissues was successfully used for studying NF-κB and AP-1 activation in kidney and injured vessels[28]. In this study, we performed this technique to detect NF-κB and AP-1 in HCC and obtained ideal results. The distribution of NF-κB and AP-1 was presented in nuclear and cytoplasmic types. Positive signals in HCC were mainly located both in nuclei and cytoplasm while cytoplasmic staining mainly in nontumor. We concluded that in situ hybridization is a convenient and efficient tool for studying transcription factors (Table 5).
Table 5.
Relationship between activated NF-κB and apoptosis proteins in nontumor tissues
| NF-κB | Cases |
Adjacent nontumor tissues |
ICH-1S |
Cases |
Normal liver tissues |
|||||||||||||
|
Fas |
FasL |
ICH-1L |
Fas |
FasL |
ICH-1L |
ICH-1S |
||||||||||||
| + | – | + | – | + | – | + | – | + | – | + | – | + | – | + | – | |||
| Activated | 7 | 15 | 7 | 13 | 9 | 19 | 3 | 21 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Inactivated | 33 | 6 | 12 | 5 | 13 | 10 | 8 | 10 | 8 | 7 | 0 | 7 | 0 | 7 | 2 | 5 | 1 | 6 |
| χ2 | 4.8211 | 3.9221 | 4.7130 | 6.8946 | 0 | 0 | 0 | 0 | ||||||||||
| P | < 0.05 | < 0.05 | < 0.05 | < 0.05 | > 0.05 | > 0.05 | > 0.05 | > 0.05 | ||||||||||
Some studies have shown that DNA binding activity of NF-κB and AP-1 was aberrant in HCC[15,16]. Liu et al[15] measured the DNA binding activity of AP-1 and NF-κB in the peritumoral and the tumoral parts of 15 primary liver cancers. AP-1 and NF-κB binding activities were in 73% and 87% of the cases in the peritumoral tissue respectively. A further activation of AP-1 and NF-κB binding in the tumoral parts was detected in 40% and 80% of the cases respectively. Early activation of AP-1 and NF-κB contributes probably to the acquisition of a transformed phenotype during hepatocarcinogenesis, whatever the etiology. Tai et al[16] studied the NF-κB-DNA binding activity and its dimmer, active nuclear RelA and nuclear IkappaB-alpha proteins expression in HCC using electrophoretic mobility shift assay and Western blot analysis. The results showed that nuclear NF-κB DNA binding activity and nuclear RelA protein expression were greater in tumor tissue compared with nontumor tissue. Constitutive activation of NF-κB was found more frequently in tumor tissue compared with nontumor tissue. In our study, the results are similar to that by electrophoretic mobility shift assay. Furthermore, our findings presented positive staining for NF-κB and AP-1 distribution in cytoplasm (inactive from) at 68% and 58% in adjacent liver tissues, 1/7 and 2/7 in normal liver, and in nuclei (active form) at 55% and 62.5% in tumor tissues. The possible activation of NF-κB has been shown in some carcinoma tissues such as human pancreatic carcinoma, gastric carcinoma and breast carcinoma. NF-κB activation was evaluated on the basis of nuclear translocation from inactive form with NF-κB-IκBs complex in cytoplasm[18,20-22]. However, the mechanism concerning AP-1 activation in carcinoma is not available. We further found that NF-κB activity positively corresponded to AP-1 activation. Taken together, these results demonstrated that NF-κB and AP-1 translocated from cytoplasm (inactive form) to nucleus (active form) during hepatocarcinogenesis and suggested that activation of both NF-κB and AP-1 may be required to produce potential biological function and play important roles in the oncogenesis of human HCC.
Fas/FasL system is involved in apoptosis in human HCC[5,6]. In this study, we further investigated whether there is a relationship between NF-κB or AP-1 activity and Fas/ FasL expression in HCC. The results showed that more expression of Fas and FasL was found in cases with activated NF-κB or AP-1 than those with inactivated NF-κB or AP-1 in HCC but the differences were not significant statistically (P > 0.05). These data indicated that NF-κB and AP-1 activation may not be required for Fas/FasL-induced apoptosis in HCC. Similar results have been reported in other different cells. Nagaki et al[23] found NF-κB blocks hepatocyte apoptosis mediated by the TNF receptor, but not by Fas. In T cells, NF-κB signaling pathway is not required for activation-induced FasL expression[24]. Lack of a requirement for AP-1 induction in Fas-mediated death was substantiated with Jurkat cell[25]. However, the results contrary to those stated above have been implicated in some studies. Marusawae et al[26] demonstrated that NF-κB activity is related to Fas signaling in hepatocyte-derived cell lines, HepG2 and Huh-7 cells. Overexpression of kinase-inactive NF-κB-inducing kinase (NIK) and IkappaB kinase (IKK) inhibited the activation of NF-κB introduced by anti-Fas treatment in these cells. Inactivation of NF-κB by the production of IkappaB-alpha protein made these cells more susceptible to apoptosis induced by Fas stimulation. NF-κB and AP-1 are also found involving in the transcriptional regulation of FasL in Fas-mediated thymineless death of colon carcinoma cell[27]. These results suggested NF-κB and AP-1 may play different roles in the regulation of Fas/ FasL-mediated apoptosis in different cells.
ICH-1, a gene related to the C. elegans cell death gene ced-3 and the mammalian homolog of ced-3, interleukin-1 beta-converting enzyme (ICE). Alternative splicing results in two distinct ICH-1 mRNA species. One mRNA species encodes a protein product of 435 amino acids (ICH-1L) that is homologous to both the P20 and P10 subunits of ICE (27% identity) and the entire CED-3 protein (28% identity). The other mRNA encodes a 312 amino acid truncated version of ICH-1L protein (ICH-1S). Overexpression of ICH-1L induces programmed cell death, while ICH-1S suppresses Rat-1 cell death induced by serum deprivation. ICH-1 plays an important role in both positive and negative regulation of programmed cell death in vertebrate animals[29]. In this study, we found that aberrant expression of ICH-1 in HCC and adjacent tissues compared to normal liver. The results also showed that there are statistical differences between expression of ICH-1L or ICH-1S and NF-κB or AP-1 activation. These findings suggested that NF-κB and AP-1 may play a role in mediating ICH-1L/S expression during pathogenesis of HCC.
Footnotes
Science Editor Li WZ Language Editor Elsevier HK
References
- 1.Shao J, Li Y, Li H, Wu Q, Hou J, Liew C. Deletion of chromosomes 9p and 17 associated with abnormal expression of p53, p16/MTS1 and p15/MTS2 gene protein in hepatocellular carcinomas. Chin Med J (Engl) 2000;113:817–822. [PubMed] [Google Scholar]
- 2.Staib F, Hussain SP, Hofseth LJ, Wang XW, Harris CC. TP53 and liver carcinogenesis. Hum Mutat. 2003;21:201–216. doi: 10.1002/humu.10176. [DOI] [PubMed] [Google Scholar]
- 3.Yuen MF, Wu PC, Lai VC, Lau JY, Lai CL. Expression of c-Myc, c-Fos, and c-jun in hepatocellular carcinoma. Cancer. 2001;91:106–112. doi: 10.1002/1097-0142(20010101)91:1<106::aid-cncr14>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 4.Ikeguchi M, Hirooka Y, Kaibara N. Quantitative analysis of apoptosis-related gene expression in hepatocellular carcinoma. Cancer. 2002;95:1938–1945. doi: 10.1002/cncr.10898. [DOI] [PubMed] [Google Scholar]
- 5.Lee SH, Shin MS, Lee HS, Bae JH, Lee HK, Kim HS, Kim SY, Jang JJ, Joo M, Kang YK, et al. Expression of Fas and Fas-related molecules in human hepatocellular carcinoma. Hum Pathol. 2001;32:250–256. doi: 10.1053/hupa.2001.22769. [DOI] [PubMed] [Google Scholar]
- 6.Roskams T, Libbrecht L, Van Damme B, Desmet V. Fas and Fas ligand: strong co-expression in human hepatocytes surrounding hepatocellular carcinoma; can cancer induce suicide in peritumoural cells? J Pathol. 2000;191:150–153. doi: 10.1002/(SICI)1096-9896(200006)191:2<150::AID-PATH612>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 7.Angel P, Karin M. The role of Jun, Fos and the AP-1 complex in cell-proliferation and transformation. Biochim Biophys Acta. 1991;1072:129–157. doi: 10.1016/0304-419x(91)90011-9. [DOI] [PubMed] [Google Scholar]
- 8.Karin M, Liu Zg, Zandi E. AP-1 function and regulation. Curr Opin Cell Biol. 1997;9:240–246. doi: 10.1016/s0955-0674(97)80068-3. [DOI] [PubMed] [Google Scholar]
- 9.Shaulian E, Karin M. AP-1 in cell proliferation and survival. Oncogene. 2001;20:2390–2400. doi: 10.1038/sj.onc.1204383. [DOI] [PubMed] [Google Scholar]
- 10.Beg AA, Sha WC, Bronson RT, Ghosh S, Baltimore D. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-kappa B. Nature. 1995;376:167–170. doi: 10.1038/376167a0. [DOI] [PubMed] [Google Scholar]
- 11.Van Antwerp DJ, Martin SJ, Kafri T, Green DR, Verma IM. Suppression of TNF-alpha-induced apoptosis by NF-kappaB. Science. 1996;274:787–789. doi: 10.1126/science.274.5288.787. [DOI] [PubMed] [Google Scholar]
- 12.Bellas RE, FitzGerald MJ, Fausto N, Sonenshein GE. Inhibition of NF-kappa B activity induces apoptosis in murine hepatocytes. Am J Pathol. 1997;151:891–896. [PMC free article] [PubMed] [Google Scholar]
- 13.Beg AA, Baltimore D. An essential role for NF-kappaB in preventing TNF-alpha-induced cell death. Science. 1996;274:782–784. doi: 10.1126/science.274.5288.782. [DOI] [PubMed] [Google Scholar]
- 14.Siebenlist U, Franzoso G, Brown K. Structure, regulation and function of NF-kappa B. Annu Rev Cell Biol. 1994;10:405–455. doi: 10.1146/annurev.cb.10.110194.002201. [DOI] [PubMed] [Google Scholar]
- 15.Liu P, Kimmoun E, Legrand A, Sauvanet A, Degott C, Lardeux B, Bernuau D. Activation of NF-kappa B, AP-1 and STAT transcription factors is a frequent and early event in human hepatocellular carcinomas. J Hepatol. 2002;37:63–71. doi: 10.1016/s0168-8278(02)00064-8. [DOI] [PubMed] [Google Scholar]
- 16.Tai DI, Tsai SL, Chang YH, Huang SN, Chen TC, Chang KS, Liaw YF. Constitutive activation of nuclear factor kappaB in hepatocellular carcinoma. Cancer. 2000;89:2274–2281. [PubMed] [Google Scholar]
- 17.Fujikawa K, Shiraki K, Sugimoto K, Ito T, Yamanaka T, Takase K, Nakano T. Reduced expression of ICE/caspase1 and CPP32/caspase3 in human hepatocellular carcinoma. Anticancer Res. 2000;20:1927–1932. [PubMed] [Google Scholar]
- 18.Sasaki N, Morisaki T, Hashizume K, Yao T, Tsuneyoshi M, Noshiro H, Nakamura K, Yamanaka T, Uchiyama A, Tanaka M, et al. Nuclear factor-kappaB p65 (RelA) transcription factor is constitutively activated in human gastric carcinoma tissue. Clin Cancer Res. 2001;7:4136–4142. [PubMed] [Google Scholar]
- 19.Luque I, Gélinas C. Rel/NF-kappa B and I kappa B factors in oncogenesis. Semin Cancer Biol. 1997;8:103–111. doi: 10.1006/scbi.1997.0061. [DOI] [PubMed] [Google Scholar]
- 20.Wang W, Abbruzzese JL, Evans DB, Larry L, Cleary KR, Chiao PJ. The nuclear factor-kappa B RelA transcription factor is constitutively activated in human pancreatic adenocarcinoma cells. Clin Cancer Res. 1999;5:119–127. [PubMed] [Google Scholar]
- 21.Sovak MA, Bellas RE, Kim DW, Zanieski GJ, Rogers AE, Traish AM, Sonenshein GE. Aberrant nuclear factor-kappaB/Rel expression and the pathogenesis of breast cancer. J Clin Invest. 1997;100:2952–2960. doi: 10.1172/JCI119848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim DW, Sovak MA, Zanieski G, Nonet G, Romieu-Mourez R, Lau AW, Hafer LJ, Yaswen P, Stampfer M, Rogers AE, et al. Activation of NF-kappaB/Rel occurs early during neoplastic transformation of mammary cells. Carcinogenesis. 2000;21:871–879. doi: 10.1093/carcin/21.5.871. [DOI] [PubMed] [Google Scholar]
- 23.Nagaki M, Naiki T, Brenner DA, Osawa Y, Imose M, Hayashi H, Banno Y, Nakashima S, Moriwaki H. Tumor necrosis factor alpha prevents tumor necrosis factor receptor-mediated mouse hepatocyte apoptosis, but not fas-mediated apoptosis: role of nuclear factor-kappaB. Hepatology. 2000;32:1272–1279. doi: 10.1053/jhep.2000.20239. [DOI] [PubMed] [Google Scholar]
- 24.Rivera-Walsh I, Cvijic ME, Xiao G, Sun SC. The NF-kappa B signaling pathway is not required for Fas ligand gene induction but mediates protection from activation-induced cell death. J Biol Chem. 2000;275:25222–25230. doi: 10.1074/jbc.M000444200. [DOI] [PubMed] [Google Scholar]
- 25.Lenczowski JM, Dominguez L, Eder AM, King LB, Zacharchuk CM, Ashwell JD. Lack of a role for Jun kinase and AP-1 in Fas-induced apoptosis. Mol Cell Biol. 1997;17:170–181. doi: 10.1128/mcb.17.1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marusawa H, Hijikata M, Watashi K, Chiba T, Shimotohno K. Regulation of Fas-mediated apoptosis by NF-kappaB activity in human hepatocyte derived cell lines. Microbiol Immunol. 2001;45:483–489. doi: 10.1111/j.1348-0421.2001.tb02648.x. [DOI] [PubMed] [Google Scholar]
- 27.Harwood FG, Kasibhatla S, Petak I, Vernes R, Green DR, Houghton JA. Regulation of FasL by NF-kappaB and AP-1 in Fas-dependent thymineless death of human colon carcinoma cells. J Biol Chem. 2000;275:10023–10029. doi: 10.1074/jbc.275.14.10023. [DOI] [PubMed] [Google Scholar]
- 28.Hernández-Presa MA, Gómez-Guerrero C, Egido J. In situ non-radioactive detection of nuclear factors in paraffin sections by Southwestern histochemistry. Kidney Int. 1999;55:209–214. doi: 10.1046/j.1523-1755.1999.00226.x. [DOI] [PubMed] [Google Scholar]
- 29.Wang L, Miura M, Bergeron L, Zhu H, Yuan J. Ich-1, an Ice/ced-3-related gene, encodes both positive and negative regulators of programmed cell death. Cell. 1994;78:739–750. doi: 10.1016/s0092-8674(94)90422-7. [DOI] [PubMed] [Google Scholar]


