Abstract
AIM: To investigate the value of spiral CT pneumocolon in preoperative colorectal carcinoma.
METHODS: Spiral CT pneumocolon was performed prior to surgery in 64 patients with colorectal carcinoma. Spiral CT images were compared to specimens from the resected tumor.
RESULTS: Spiral CT depicted the tumor in all patients. Comparison of spiral CT and histologic results showed that the sensitivity and specificity were 95.2%, 40.9% in detection of local invasion, and 75.0%, 90.9% in detection of lymph node metastasis. Compared to the Dukes classification, the disease was correctly staged as A in 6 of 18 patients, as B in 18 of 23, as C in 10 of 15, and as D in 7 of 8. Overall, spiral CT correctly staged 64.1% of patients.
CONCLUSION: Spiral CT pneumocolon may be useful in the preoperative assessment of patients with colorectal carcinoma as a means for assisting surgical planning.
Keywords: Tomography, X-ray compute, Pneumocolon, Preoperative staging, Colorectal cancer
INTRODUCTION
With the development of high-resolution scanners, technical refinements in obtaining better quality studies, and the accumulated clinical experience leading to better interpretation, the role, indications, and accuracy of CT of the colon have dramatically enlarged and improved[1-3]. Reliable preoperative determination of the extent of spread of a colorectal carcinoma not only indicates the expected prognosis but also assists management. For obtaining reliable results from CT scan, preparation of the patients, especially complete distention of the colon using water or air as contrast agent, is the most important precondition. Otherwise, collapse of the colon and feces can easily be misinterpreted as tumor. Many studies have shown that water enema spiral CT is a useful modality for preoperative staging of patients with colorectal carcinoma[4-7]. However, water enema can be difficult and distressing in frail elderly patients and has risk of water incontinence. Air insufflation for the colon can be achieved easily and rapidly and is well tolerated by the patients, and air provides an excellent CT contrast medium[8]. There have been few reports concerning the preoperative staging of colorectal carcinoma with spiral CT pneumocolon. Therefore, this study aimed to assess the value of spiral CT pneumocolon in preoperative colorectal carcinoma.
MATERIALS AND METHODS
Patients
From August 1998 to December 2002, 64 patients with colorectal carcinoma, who were operated on at our institution, underwent spiral CT pneumocolon. There were 40 men and 24 women, ranging in age from 32 to 88 years (mean 59 years). Among the 64 patients who had a prior colonoscopy, 4 of 15 patients had an incomplete barium enema due to barium incontinence, and 19 of 64 patients had incomplete colonoscopy due to inability to cross a distal stricture.
Technique
All patients were fasted for at least 12 h before the study and given an oral colon cleansing preparation the night before CT scan. Nine-hundred milliliters of 3% diluted gastrografin solution was given orally 45 min before, so that small bowel loops were opacified. Anisodamine hydrochloride (10 mg, IM) was used to control peristaltic artifact and relax the colon. The patient was previously instructed not to void. As the study progressed, interpretation was more straightforward when the bladder was full.
The patients were positioned on the CT table in supine position. A Foley catheter was inserted into the rectum and 1 500-2 000 mL room air was administered per rectum to distend the colon. The enema was stopped if the patient experienced abdominal discomfort. Adequate distention of the whole colon was confirmed on the scanogram.
Studies were performed on a Toshiba Xpress/SX spiral CT scanner with a 10-mm collimation and pitch of 1-2, at 120 kV and 200 mA. After plain scanning, 1.5 mL/kg of non-ionic iodinated contrast medium (iopromide, Ultravist 300; Schering, Berlin, Germany) was administered via the antecubital vein at a rate of 3 mL/s using an autoinjector, and scanning commenced 60 s after start of the injection from the dome of the liver to the anal verge. The time between CT scan and surgery ranged 1-8 d (mean 4.7 d).
Evaluating criteria
Based on previous reports[5,7,8] and our own experience, the following three parameters were established and evaluated:
(1) local extramural invasion (irregularly serrated or speculated outer contour, tumor mass or strands of soft tissue extending out, and/or indistinctly increased density of the pericolonic fat), (2) lymph node involvement (lymph node short axis 1 cm or larger, or node less than 1 cm in diameter with obvious enhancement), and (3) distal and/or extensive disease (liver or lung metastases, direct extension into adjacent solid or hollow organs).
All patients with colorectal carcinoma were staged on CT according to the modified Dukes’ classification[7]: stage A, tumor limited to the colonic wall; stage B, tumor affecting the serosa or the pericolonic fat; stage C, lymph node involvement; and stage D, tumor infiltrating adjacent organs and/or with metastases. The modified Dukes’ classification was used because this system was currently used by surgeons at our institution.
Image interpretation
Two experienced radiologists, who were blind to the surgical and pathologic findings of each patient, interpreted the images as compared to above parameters, and any discrepant readings were solved by consensus. After a minimum of 4 wk, the same two radiologists reviewed the images for the second time. Intraobserver variability was evaluated by means of a weighed k-statistic[9].
RESULTS
The overall results showed good agreement between the two reviews by the two radiologists. The k-statistic for the data was 0.77, representing good intraobserver agreement.
Normal findings and primary tumor
All patients tolerated the spiral CT pneumocolon well with no significant discomfort, and had good bowel preparation and no fluid levels or residual fecal material. The distended colon lumen and normal colonic wall were well seen on spiral CT (Figure 1). Using this technique the normal colonic wall represented a single layer which was 1-2-mm thick.
Figure 1.
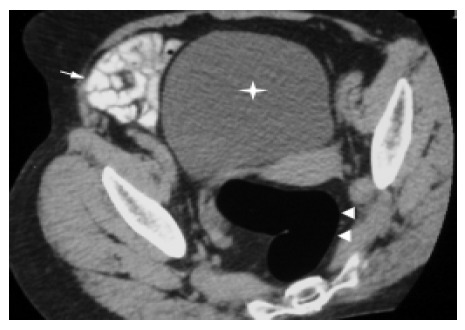
Optimal visualization of normal rectal wall (arrowheads), small intestine (arrow), and urinary bladder (star).
Spiral CT detected the tumor in all patients and the smallest mass was 0.7 cm × 1.0 cm. The lesion was shown as an eccentric focal mass with irregular segmental or circumferential wall ranging 0.7-4.5 cm in thickness, and their extension ranged 1.0-10.0 cm (Figures 2A-C). Most lesions had an uneven, lobulated configuration and large masses had patchy areas of necrosis. Different degrees of distal colonic stricture were presented. The majority of the mass showed moderate to obvious enhancement.
Figure 2.
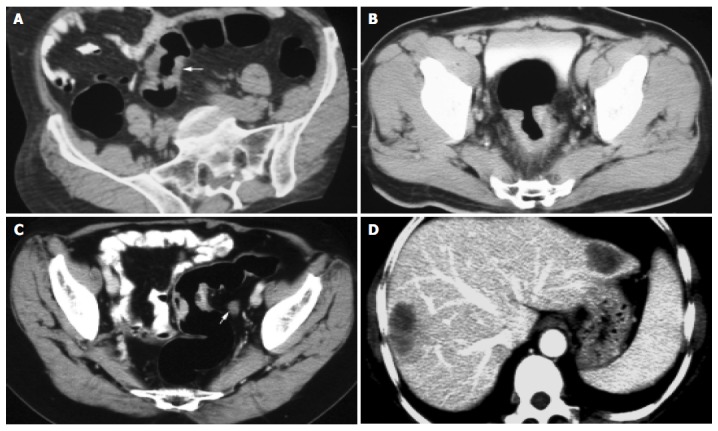
Correctly staged lesions. A: Dukes stage A carcinoma (arrow); B: Dukes stage B carcinoma; C: Dukes stage C carcinoma; D: Dukes stage D carcinoma.
Local invasion
Tumor invasion of serosa and/or pericolonic fat was correctly staged by spiral CT in 49 (76.6%) of 64 patients (Figures 2A and B). In the incorrectly staged group, spiral CT overstaged 12 patients (Figure 3A) and understaged 2 patients (Figure 3B). Spiral CT evaluation had a sensitivity of 95.2% and a specificity of 40.9%.
Figure 3.
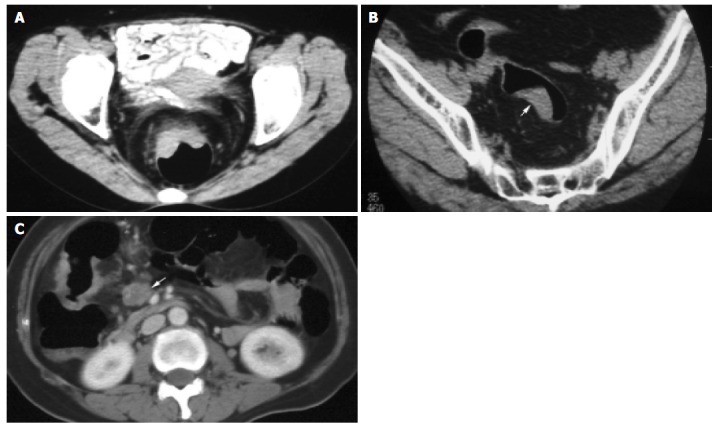
Incorrectly staged lesions. A: Dukes stage A carcinoma; B: Dukes stage B carcinoma (arrow); C: Dukes stage B carcinoma.
Lymph node involvement
Involvement of lymph nodes less than 5 mm in diameter was seen in 7 (35.0%) of 20 patients. Spiral CT correctly diagnosed lymph node metastasis in 15 of 20 patients (Figure 2C). In the correctly diagnosed group, seven patients showed nodal enhancement, and eight patients showed no enhancement of lymph nodes larger than 1 cm in diameter. Clusters of three or more smaller nodes (each less than 1 cm in diameter) were seen by CT in five patients, histology revealed no evidence of nodal involvement.
Nodal involvement was correctly staged by spiral CT in 55 (85.9%) of 64 patients. In the incorrectly staged group, spiral CT overstaged 3 (Figure 3C) and understaged 5 of 20 patients. Spiral CT evaluation had a sensitivity of 75.0% and a specificity of 90.9%.
Distal metastasis
Liver metastasis was presented in four patients (Figure 2D), lung metastasis in two patients, and abdominal wall metastasis in one patient. They were all correctly diagnosed by CT. Only one patient with peritoneal seeding was missed due to the small lesion.
Preoperative staging
Staging results are presented in Table 1. CT stage A was correct in 6 of 18, stage B in 18 of 23, stage C in 10 of 15, and stage D in 7 of 8 patients. Overall, the diagnostic accuracy was 64.1% (41/64).
Table 1.
CT findings and pathologic staging
| CT staging |
Pathologic staging |
||||
| A | B | C | D | Total | |
| A | 6 | 2 | 1 | 9 | |
| B | 12 | 18 | 4 | 34 | |
| C | 3 | 10 | 1 | 14 | |
| D | 7 | 7 | |||
| Total | 18 | 23 | 15 | 8 | 64 |
DISCUSSION
Colonoscopy and barium enema are the main methods for diagnosis of colorectal tumors. However, both modalities do not permit a precise preoperative prediction as to whether a tumor is limited to the colonic wall or has spread into surrounding tissues. Patients with severe colonic stricture or barium incontinence may be poor candidates for the two examinations. Our study showed that CT pneumocolon had the potential utility as an adjunctive imaging technique for patients with colorectal carcinoma.
Imaging with spiral CT pneumocolon could clearly show the lumen and wall of the colon and colonic lesions. Normal colonic wall thickness should not exceed 3 mm in a well-distended segment, and the thickness greater than 6 mm is considered abnormal[1]. In our study with spiral CT pneumocolon technique, the thickness of the normal colonic wall ranged 1-2 mm, and the thickness of the lesion was greater than 6 mm. We were unable to identify the mucosal lining nor the different anatomic layers of the colonic wall as reported by others who used water enema technique[6,7].
The sensitivity of CT detection depends mainly on the size of the lesion and on the quality of the CT examination. It varies from 68% if no special attempts are made to promote visualization of the colonic lumen to 95% when the colonic lumen is distended well. In this study, the overall detection rate was 100%, and masses with a diameter in 1 cm were identified. Our results corresponded favorably with previous reports[10,11]. This may be due to the adequate preparation of patients and CT pneumocolon technique. CT colonography generated from CT pneumocolon has emerged in recent years. This technique can detect lesions less than 5 mm in diameter, and its sensitivity is over 85% in detection of polyps 10 mm or greater in size, 70-80% of polyps 5-9 mm in size and 60% of polyps smaller than 5 mm[12,13]. It is a viable alternative for screening primary colorectal neoplasms and examining portions of the colon proximal to an obstructing lesion that cannot be traversed by colonoscopy or by barium[3,10,13].
In our experience, CT has a sensitivity of 95.2% and an accuracy of 76.6% in evaluating the local invasion. However, the specificity is only 40.9%. Harvey et al[8] reported that its sensitivity is 100% and specificity is 33%. Zhou et al[2] reported that its sensitivity is 92.9% and specificity is 50.0%. The reasons for the low specificity in local extension may be that CT is not possible to distinguish the single layers comprising the wall and there is no simple CT criterion to differentiate inflammation of the serosa from tumor invasion[2,8]. Matsuoka et al[14] reported that using sagittal or coronal sections improves diagnostic accuracy from 79.4% to 90.4% in assessing the depth of tumor invasion. Endoscopic ultraso-nography (EUS) is superior to CT in detecting the exact depth of parietal invasion, and its accuracy is 84.9%[15]. In our study, the accuracy of EUS was 87.5%, and its specificity was 100% in assessing the local extension.
Traditionally, CT detection of abnormal lymph nodes is based on imaging nodes greater than 1 cm in diameter or finding of clustered lymph nodes[5,8]. However, lymph node metastasis in colon cancer occurs frequently in lymph nodes measuring less than 5 mm. Herrera-Ornelas et al[16] have reported a 65% incidence of lymph node metastasis. Whereas enlarged lymph nodes may be infiltrated by inflammatory or neoplastic cells. A relatively low sensitivity of CT in detecting nodal metastases is anticipated. Harvey et al[8] reported that its sensitivity is 56% and specificity is 95%. Gazelle et al[5] reported that its sensitivity is 60% and specificity is 79%. Hundt et al[4] reported that its sensitivity is 84.3%, its specificity being 60% and accuracy being 81.0%. In this present study, its sensitivity, specificity, and accuracy in detecting lymph node involvement were 75.0%, 90.9%, and 85.9%, respectively. The disparity in these reports is probably due to the different criteria used. In our study, five patients with clustered lymph nodes (< 1 cm) had no pathologic evidence of nodal involvement, suggesting that this criterion is unreliable. Further investigation is needed.
The data in this series showed that compared to the Dukes classification, CT correctly staged 64.1% of all patients, which is consistent with previous reports[2,8]. CT staging accuracy, however, showed significant variations in different Dukes categories. It correctly staged 6 (33.3%) of 18 patients with Dukes A lesion, 18 (78.3%) of 23 patients with Dukes B lesion, 10 (66.7%) of 15 patients with Dukes C tumor, and 7 (87.5%) of 8 patients with Dukes D tumor.
Colon cancer is potentially curable and decision on treatment is based on the extent of tumor. If extensive local spread of tumor is shown by CT or MRI, the patients can be treated with radiation therapy alone or undergo tumor resection after radiation therapy. The success of subsequent chemotherapy and irradiation can be determined in patients by follow-up CT or MRI, which can be compared to the base-line study before treatment. Recent studies showed that endorectal surface coil MR imaging is valuable in patients with rectal carcinoma to assess involvement of the levator ani[1]. If involvement of the levator ani is demonstrated, an abdominoperineal resection is needed.
In conclusion, spiral CT pneumocolon is a quick and noninvasive method for detecting colorectal carcinoma, and can provide valuable information preoperatively. In addition, it may represent a useful adjunct to colonoscopy or barium enema in patients with colorectal carcinoma.
Footnotes
Supported by the Medical Science Foundation of Guangdong Province, No. A2002185
Science Editor Wang XL and Guo SY Language Editor Elsevier HK
References
- 1.Dobos N, Rubesin SE. Radiologic imaging modalities in the diagnosis and management of colorectal cancer. Hematol Oncol Clin North Am. 2002;16:875–895. doi: 10.1016/s0889-8588(02)00032-1. [DOI] [PubMed] [Google Scholar]
- 2.Zhou C, Li J, Zhao X. Spiral CT in the preoperative staging of colorectal carcinoma-radiologic-pathologic correlation. Zhonghua Zhongliu Zazhi. 2002;24:274–277. [PubMed] [Google Scholar]
- 3.Laghi A, Iannaccone R, Trenna S, Mangiapane F, Sinibaldi G, Piacentini F, Sammartino P, Stipa V, Passariello R. Multislice spiral CT colonography in the evaluation of colorectal neoplasms. Radiol Med. 2002;104:394–403. [PubMed] [Google Scholar]
- 4.Hundt W, Braunschweig R, Reiser M. Evaluation of spiral CT in staging of colon and rectum carcinoma. Eur Radiol. 1999;9:78–84. doi: 10.1007/s003300050632. [DOI] [PubMed] [Google Scholar]
- 5.Gazelle GS, Gaa J, Saini S, Shellito P. Staging of colon carcinoma using water enema CT. J Comput Assist Tomogr. 1995;19:87–91. doi: 10.1097/00004728-199501000-00017. [DOI] [PubMed] [Google Scholar]
- 6.Gossios KJ, Tsianos EV, Kontogiannis DS, Demou LL, Tatsis CK, Papakostas VP, Merkouropoulos MM, Tsimoyiannis EC. Water as contrast medium for computed tomography study of colonic wall lesions. Gastrointest Radiol. 1992;17:125–128. doi: 10.1007/BF01888526. [DOI] [PubMed] [Google Scholar]
- 7.Angelelli G, Macarini L, Lupo L, Caputi-Jambrenghi O, Pannarale O, Memeo V. Rectal carcinoma: CT staging with water as contrast medium. Radiology. 1990;177:511–514. doi: 10.1148/radiology.177.2.2217794. [DOI] [PubMed] [Google Scholar]
- 8.Harvey CJ, Amin Z, Hare CM, Gillams AR, Novelli MR, Boulos PB, Lees WR. Helical CT pneumocolon to assess colonic tumors: radiologic-pathologic correlation. AJR Am J Roentgenol. 1998;170:1439–1443. doi: 10.2214/ajr.170.6.9609150. [DOI] [PubMed] [Google Scholar]
- 9.Song JH, Francis IR, Platt JF, Cohan RH, Mohsin J, Kielb SJ, Korobkin M, Montie JE. Bladder tumor detection at virtual cystoscopy. Radiology. 2001;218:95–100. doi: 10.1148/radiology.218.1.r01ja4995. [DOI] [PubMed] [Google Scholar]
- 10.Britton I, Dover S, Vallance R. Immediate CT pneumocolon for failed colonoscopy; comparison with routine pneumocolon. Clin Radiol. 2001;56:89–93. doi: 10.1053/crad.2000.0559. [DOI] [PubMed] [Google Scholar]
- 11.Miao YM, Amin Z, Healy J, Burn P, Murugan N, Westaby D, Allen-Mersh TG. A prospective single centre study comparing computed tomography pneumocolon against colonoscopy in the detection of colorectal neoplasms. Gut. 2000;47:832–837. doi: 10.1136/gut.47.6.832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yee J, Kumar NN, Hung RK, Akerkar GA, Kumar PR, Wall SD. Comparison of supine and prone scanning separately and in combination at CT colonography. Radiology. 2003;226:653–661. doi: 10.1148/radiol.2263010701. [DOI] [PubMed] [Google Scholar]
- 13.Harvey CJ, Renfrew I, Taylor S, Gillams AR, Lees WR. Spiral CT pneumocolon: applications, status and limitations. Eur Radiol. 2001;11:1612–1625. doi: 10.1007/s003300100890. [DOI] [PubMed] [Google Scholar]
- 14.Matsuoka H, Nakamura A, Masaki T, Sugiyama M, Takahara T, Hachiya J, Atomi Y. Preoperative staging by multidetector-row computed tomography in patients with rectal carcinoma. Am J Surg. 2002;184:131–135. doi: 10.1016/s0002-9610(02)00911-x. [DOI] [PubMed] [Google Scholar]
- 15.Shimizu S, Tada M, Kawai K. Use of endoscopic ultrasonography for the diagnosis of colorectal tumors. Endoscopy. 1990;22:31–34. doi: 10.1055/s-2007-1012783. [DOI] [PubMed] [Google Scholar]
- 16.Herrera-Ornelas L, Justiniano J, Castillo N, Petrelli NJ, Stulc JP, Mittelman A. Metastases in small lymph nodes from colon cancer. Arch Surg. 1987;122:1253–1256. doi: 10.1001/archsurg.1987.01400230039006. [DOI] [PubMed] [Google Scholar]


