Abstract
AIM: To assess the sensitivity and specificity of polymerase chain reaction (PCR) in detecting Helicobacter pylori (H pylori) infection in patients with bleeding peptic ulcers, and to compare its diagnostic efficacy with other invasive and non-invasive tests.
METHODS: From April to September 2002, H pylori status in 60 patients who consecutively presented with gastroduodenal ulcer bleeding was examined by rapid urease tests (RUT), histology, culture, PCR, serology and urea breath tests (UBT).
RESULTS: The sensitivity of PCR was significantly higher than that of RUT, histology and culture (91% vs 66%, 43% and 37%, respectively; P = 0.01, < 0.001, < 0.001, respectively), but similar to that of serology (94%) and UBT (94%). Additionally, PCR exhibited a greater specificity than serology (100% vs 65%, P < 0.01). However, the specificity of PCR did not differ from that of other tests. Further analysis revealed significant differences in the sensitivities of RUT, culture, histology and PCR between the patients with and those without blood in the stomach (P < 0.01, P = 0.09, P < 0.05, and P < 0.05, respectively).
CONCLUSION: PCR is the most accurate method among the biopsy-based tests to detect H pylori infection in patients with bleeding peptic ulcers. Blood may reduce the sensitivities of all biopsy-based tests.
Keywords: Polymerase chain reaction, Helicobacter pylori, Bleeding peptic ulcers
INTRODUCTION
Bleeding is a common and serious complication of peptic ulcer diseases. It is estimated that peptic ulcer bleeding accounts for approximately 150 000 hospitalizations per year in the USA[1,2]. The prevalence of Helicobacter pylori (H pylori) in bleeding peptic ulcers has not been definitely determined, but it is estimated to be 70%[3-5]. The accurate diagnosis of H pylori infection is crucial in the short-term and long-term management of patients with bleeding peptic ulcers[6]. If a patient with a bleeding ulcer requires surgical intervention, knowledge of his or her H pylori status may guide the selection of procedures for the patient (i.e., a simple closure vs full-blown ulcer surgery)[6]. Patients whose bleeding episodes cease in the short term, one-third of those who do not receive maintenance therapy, surgery or anti-H pylori therapy will experience recurrent bleeding within the next 1-2 years[7]. However, numerous studies have demonstrated that eradicating H pylori can drastically reduce the incidence of rebleeding in patients with bleeding peptic ulcers, preventing the need for long-term antisecretory therapy or surgical intervention[8-10]. Therefore, the H pylori status in a patient with bleeding peptic ulcers must be documented.
Currently, H pylori infection can be diagnosed by invasive assays, i.e., those requiring esophagogastroduodenoscopy (EGD), or by non-invasive assays in which EGD is not necessary. Invasive diagnostic tests include culture, histology, rapid urease test (RUT) and polymerase chain reaction (PCR). Non-invasive tests comprise serology, stool antigen test and urea breath test (UBT). The choice of a diagnostic test should depend on the clinical circumstances, sensitivity and specificity of the tests, and the cost effectiveness of the testing strategy. Because of its simplicity, accuracy and rapid determination of H pylori status, RUT is generally considered to be the initial endoscopic test of choice for uncomplicated peptic ulcers[11]. However, many studies have demonstrated that RUT lacks sensitivity in H pylori diagnosis when peptic ulcer diseases are presented with bleeding[5,12]. Moreover, a recent study by Colin et al[13] indicated that all direct tests on H pylori including RUT, culture and histology reduced the sensitivity in the setting of ulcer bleeding. The sensitivities of aforementioned three tests were 31%, 25% and 26%, respectively.
PCR can diagnose H pylori infection under non-bleeding conditions much more accurately than histology or culture[14,15]. The level of sensitivity of this test is extremely high and has a threshold of 10 to 100 H pylori strains per specimen[14-16]. An accurate diagnosis of H pylori at the time of bleeding episode is essential, but few studies have addressed the application of PCR to bleeding ulcers. We performed this prospective study to evaluate the sensitivity, specificity and accuracy of PCR assay for detecting H pylori infection in patients with bleeding peptic ulcers and to compare its diagnostic efficacy with that of other invasive and non-invasive tests.
MATERIALS AND METHODS
Patients
From April to September 2002, 60 consecutive patients with hematemesis, melena, or both due to gastroduodenal ulcer bleeding, who underwent an EGD, were enrolled in this study. Exclusion criteria included: age < 15 or > 80 years; history of coagulopathy or other disorders contraindicated for EGD or biopsy sampling; previous history of anti-H pylori therapy. Data regarding age, sex, medical history, drug history, presenting symptoms, gastroduodenal lesions and presence or absence of blood in the stomach were recorded. Written informed consent was obtained from each subject. This study was approved by the Human Medical Research Committee of the Kaohsiung Veterans General Hospital, Kaohsiung, Taiwan.
Endoscopy and biopsy sampling
During endoscopy, gastric biopsy specimens were taken from the lesser curvature of the antrum and corpus for RUT (one antrum biopsy specimen), histology (one antrum and one corpus biopsy specimen), culture (one antrum biopsy specimen) and PCR (one antrum biopsy specimen). Endoscopes were cleaned by a mechanical wash and then washed in an Olympus washing machine. They were then air-dried and cleaned with 70% ethanol.
RUT
RUT was performed according to our previous study[17,18]. A biopsy specimen from antrum was immediately placed in 1 mL of a 10% solution of urea in deionized water (pH 6.8) to which two drops of 1% phenol red solution was added and incubated at 37°C for up to 24 h. If the yellowish color around the area of inserted specimen changed to bright pink within the 24-h limit, the urease test was considered positive. In our laboratory, the sensitivity and specificity of RUT were 96% and 91%, respectively[17].
Histological examination
Biopsy specimens were fixed in 10% buffered formalin, embedded in paraffin, and sectioned. One 4-µm-thick section was cut and stained with hematoxylin-eosin to observe the presence of curved rod shaped bacteria on the mucosal surface[19,20]. The specimens were interpreted by a histopathologist (H-H Tseng) blinded to the patient status and the results of other laboratory tests.
Culture
The specimen for culture was transferred with brain heart infusion on ice for microbiological examination and inoculated onto the CDC anaerobic blood agar (Becton Dickinson Microbiology System, Cockeysville, MD) according to our previous studies[21,22]. The agar was incubated at 35°C for two days in a micro-aerophilic gas mixture containing 5% O2, 100 mL/L CO2, and 85% N2. Culture-positive patients were those with bacterial colonies grown in culture within 7 d. The organisms were identified as H pylori by Gram staining, colony morphology and positive oxidase, catalase and urease reaction.
PCR amplification
DNA extraction was performed using a commercially available kit (QIAamp Tissue kit, QIAGEN Inc., Valencia, CA) according to the manufacturer’s instructions[23]. The primers used were derived from the internal 411-bp fragment of the urease A gene as described by Clayton et al[24]: HPU1 (5’GCCAATGGTAAATTAGTT3’) and HPU2 (5’CTCCT-TAATTGTTTTTAC3’). Reactions were performed in a 25 µL volume in a thermal cycler 480 (Perkin Elmer Applied Biosystems, Foster City, CA). A reaction mixture contained 2.5 µL of extracted DNA, 0.5 µmol/L of each primer, 2.5 mmol/L of MgCl2, 2.5 µL of 10× PCR buffer, 1 U of AmpliTaq DNA polymerase (Perkin-Elmer Corp., Foster City, CA) and 100 µmol/L of each of dATP, dCTP, dGTP and dTTP. The amplification cycle consisted of an initial denaturation at 94°C for 1 min, primer annealing at 45°C for 1 min, and extension for 5 min at 72°C to ensure a full extension of the products. Samples were amplified in 35 consecutive cycles. The final cycle included a 7-min extension step to ensure a full extension of the PCR products. PCR products were analyzed on a 2% agarose electrophoresis gel stained with ethidium bromide.
UBT
UBT was performed according to our previous studies[21,25] within 1 d of EGD. The patients were fasted for at least 6 h. Fresh milk (1 000 mL) was taken to delay gastric emptying. The test consisted of a baseline breath sample and a second breath sample collected 15 min after oral administration of 100 mg of 13C-labeled urea (INER-Hp C-tester, Taiwan) dissolved in 50 mL sterile water. Values were expressed as an excess δ13CO2‰ excretion. The δ13CO2 was the ratio of 13C to 12C in the sample compared to the Pee Dee Belemnite (PDB) standard. The equation was given as: δ13CO2 = (Rsamp-Rstd)/Rstd × 1 000. Rsamp and Rstd represented the ratio of 13C to 12C in samples and standard, respectively. If the value of δ13CO2 was more than 4.8‰, this was considered as a positive result.
Serology
Blood samples for serological evaluation were obtained before EGD. A serological assay for IgG antibodies against H pylori was performed by an indirect solid-phase immunochromatographic assay using the ASSURETM H pylori rapid test kit (Genelabs Diagnostics, Cavendish Singapore Science Park, Singapore). The sensitivity and specificity of the assay were 96% and 92%, respectively according to the manufacturer’s instructions.
Gold standard definition
A patient was classified as being H pylori-positive on the basis of either a positive culture or a negative culture, at least three positive results of RUT, histology, UBT and serological tests.
Statistical analysis
Statistical tests were performed using the SPSS system. Sensitivity, specificity, accuracy, predictive values of positive and negative results were calculated in accordance with standard methods. χ2 test and 95%CI were used to compare the sensitivity, specificity and accuracy of different diagnostic methods. Two-sample t-tests were used to compare the excess δ13CO2 values of UBT between the true-positive and false-negative groups of various invasive tests. P < 0.05 was considered statistically significant.
RESULTS
Of the 60 patients initially enrolled in this study, five did not complete all the tests and were excluded from the statistical analysis. Table 1 presents the demographic data of the remaining 55 patients (37 males, 18 females; mean age: 62.2 ± 14.5 years) who finished all of the invasive and non-invasive assays. EGD of these subjects showed a gastric ulcer in 19 patients (35%), a duodenal ulcer in 13 (24%) and both gastric and duodenal ulcers in 23 (42%). According to the gold standard definition, 35 (63.6%) were H pylori-positive and 20 (36.4%) were H pylori-negative.
Table 1.
Baseline characteristics of patients with bleeding peptic ulcers (n, %)
| Number of patients (n= 55) | |
| Age (mean±SD) | 62.2 (14.5) |
| Sex | |
| Male | 37 (67) |
| Female | 18 (33) |
| Smoking | 13 (24) |
| Alcohol consumption | 4 (7) |
| Coffee consumption | 5 (9) |
| Ingestion of tea | 18 (33) |
| Ingestion of NSAID1 | 26 (47) |
| Sites of ulcer | |
| Stomach | 19 (35) |
| Duodenum | 13 (24) |
| Stomach and duodenum | 23 (42) |
| Ulcer lesions | |
| Bleeding visible vessel | 2 (4) |
| Non-bleeding visible vessel | 3 (6) |
| Adherent clot | 15 (27) |
| Red or black spot | 21 (38) |
| Clean base | 14 (26) |
NSAID: non-steroidal anti-inflammatory drugs.
Comparison of various tests for detecting H pylori infection in bleeding peptic ulcers
Table 2 presents the sensitivity, specificity and accuracy of various tests in diagnosing H pylori infection. The sensitivities of RUT, histology and culture were low (66%, 43% and 37%, respectively) although these assays exhibited a high specificity (95-100%). Their overall accuracies were 76%, 62% and 60%, respectively. Among the four biopsy-based methods, only PCR exhibited the satisfactory sensitivity (91%), specificity (100%), and accuracy (94%). Its sensitivity and accuracy were significantly higher than those of RUT, histology and culture (sensitivity: P < 0.05, P < 0.001 and P < 0.001, respectively; accuracy: P < 0.05, P < 0.001, P < 0.001, respectively).
Table 2.
Sensitivity, specificity, and accuracy of various tests for diagnosis of H pylori infection in bleeding peptic ulcers (n = 55) [%(95%CI)]
| Sensitivity | Specificity | Accuracy | PPV | NPV | |
| RUT | 66 (49 – 82)b | 95 (84 – 100) | 76 (64 – 88)b | 96 (87 – 100) | 61 (43 – 79)a |
| Histology | 43 (26 – 60)d | 95 (84 – 100) | 62 (48 – 75)d | 100 | 48 (31 – 64)b |
| Culture | 37 (20 – 54)d | 100 | 60 (46 – 73)d | 100 | 48 (32 – 63)b |
| PCR | 91 (82 – 100) | 100 | 95 (88 – 100) | 100 | 87 (72 – 100) |
| Serology | 94 (86 – 100) | 65 (42 – 88)b | 84 (73 – 93) | 83 (70 – 95)a | 87 (67 – 100) |
| UBT | 94 (86 – 100) | 85 (68 – 100) | 91 (83 – 98) | 92 (82 – 100) | 90 (74 – 100) |
P < 0.05 vs PCR;
P < 0.01 vs PCR;
P < 0.001 vs PCR.
Of the non-invasive assays, the serological test had a high sensitivity (95%) but its specificity (65%) was lower than that of PCR (P < 0.05). The sensitivity (94%), specificity (85%) and accuracy (91%) of UBT were similar to those of PCR. Among all of the tests investigated herein, PCR and UBT were the most accurate methods for diagnosing H pylori infection in patients with bleeding peptic ulcers.
Relationship between intragastric blood and sensitivity of various tests in diagnosis of H pylori infection
The patients were divided into two groups according to the presence or absence of blood in the stomach, to investigate the relationships between intragastric blood and sensitivities of biopsy-based tests for H pylori infection. The sensitivities of RUT, histology, culture, PCR, serology and UBT were 29%, 21%, 21%, 79%, 93% and 100% in the patients with intragastric blood, respectively, and 90%, 57%, 48%, 100%, 95% and 91% in the patients without intragastric blood, respectively. There were statistically significant differences in the sensitivities of RUT, histology and PCR between the patients with and without blood in the stomach (Figure 1; P < 0.01, P < 0.05 and P < 0.05, respectively). Additionally, there was a trend towards decreased sensitivity of culture in the patients with blood in the stomach (P = 0.09).
Figure 1.
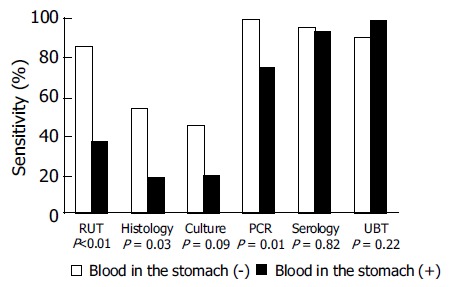
Sensitivities of various tests for detecting H pylori infection between the bleeding ulcer patients with and without blood in the stomach.
Comparison of bacterial loads between true-positive and false-negative groups of biopsy-based tests
Since the quantitative data obtained by UBT could reflect the intragastric bacterial load of H pylori, we further compared the excess δ13CO2 values of UBT between the true-positive and false-negative groups in biopsy-based tests. The excess δ13CO2 values of RUT, histology, culture and PCR were 26.7 ± 18.1, 31.5 ± 21.4, 33.7 ± 21.8 and 26.3 ± 19.4 in the group with true-positive results respectively, and 20.5 ± 16.3, 19.5 ± 16.3, 19.3 ± 15.9 and 7.2 ± 2.6 in the group with false-negative results respectively. The excess δ13CO2 values in the false-negative group were significantly lower than that in the true-positive group of culture test (Figure 2; P = 0.03). In addition, there was also a trend toward decreased δ13CO2 values in the false-negative group of histological examination and PCR assay (both P = 0.07).
Figure 2.
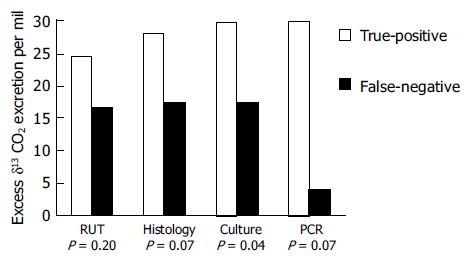
Comparison of the excess δ13CO2 values of UBT between the true-positive and false-negative groups in the biopsy-based methods.
DISCUSSION
The H pylori status in a patient presenting with a bleeding ulcer must be documented to determine the method of further management. However, many studies have disclosed that biopsy-based tests including RUT, histology and culture have low sensitivities in detecting H pylori in bleeding peptic ulcers[5,12,13]. Additionally, two recent reports[26,27] also revealed a lack of accuracy in H pylori stool antigen (HpSA) tests in patients with ulcer bleeding. In this study, we also demonstrated that the sensitivities of RUT, histology and culture in bleeding peptic ulcer were only 66%, 43% and 37%, respectively. However, PCR could reach a diagnostic sensitivity of 91%, much higher than that of RUT, histology and culture. Additionally, the test had 100% specificity in diagnosing H pylori infection under bleeding conditions. This, therefore, is the most accurate biopsy-based method for determining H pylori status in bleeding peptic ulcers.
UBT is one of the non-invasive methods for diagnosing H pylori infection. Both its sensitivity and specificity are greater than 90% in patients with bleeding peptic ulcers. Its accuracy is comparable to that of PCR. Although the serological test has a high sensitivity in patients with bleeding peptic ulcers, it may not be a good choice for diagnosing H pylori infection in patients with a complicated ulcer because antibody tests lack a good specificity (only 65%). The presence of anti-H pylori IgG antibody implies prior exposure to the organisms, but does not imply the presence of a current infection. If a patient presenting with ulcer bleeding has a false-positive antibody test and is treated by eradication therapy, the physician may mistakenly believe that the cause of bleeding has been removed and will then not provide further preventive therapy to the patient. In such a case, the patient would have a high risk of recurrent bleeding[6]. Therefore, establishing the H pylori status with certainty at the time of bleeding episode is quite important.
Following this work, we recommend that an endoscopist may initially perform a RUT to detect H pylori infection in a patient with bleeding peptic ulcer because of its simplicity, low cost, moderate sensitivity and excellent specificity. If the RUT is negative, either PCR or UBT can be used for the definite diagnosis of H pylori status. The choice of the final diagnostic modality may depend on the availability of tests in the hospital. Nonetheless, the aim of PCR is to detect specific DNA sequences rather than the whole viable bacterium. No special requirements pertain to the treatment, transport, or storage of the biopsy specimens for PCR[28,29]. Several laboratories have reported the successful detection of H pylori by PCR from a biopsy specimen placed and transported by mail in the RUT[16,30,31]. This capability is particularly useful for a gastroenterologist who does not have access to laboratory facilities and requires a confirmation of the RUT[16].
In this study, the sensitivities of RUT, histology, culture and PCR were found to be 29%, 21%, 21% and 79% in patients with intragastric blood, and 90%, 57%, 48% and 100% in patients without intragastric blood. These data imply that blood may reduce the diagnostic yield of all endoscopic biopsy tests in patients with bleeding peptic ulcers. Additionally, we also demonstrated that the bacterial load in the false-negative group of culture was significantly lower than that in the true-positive group. Furthermore, there was also a trend toward decreased bacterial load in the false-negative group of histological examination and PCR assay. The decreased bacterial density therefore, may be the major cause for decreased sensitivities of all biopsy-based assays. There are several possible reasons for the aforementioned findings. The decreased bacterial load in bleeding ulcer patients may be related to a direct suppression effect of intraluminal blood on H pylori, the administration of antisecretory drugs, or the removal of some H pylori from the gastric epithelium or mucus by gastric lavage before EGD[32]. Recently, Leung et al[33] reported that a false-negative result of RUT in bleeding ulcer might be caused by the buffered effects of blood. An in vitro study[34] also showed that sheep’s blood inhibited the growth of H pylori in broth media. The exact reason concerning the association between bleeding ulcer and intragastric bacterial density merits further investigations.
In conclusion, PCR is the most accurate biopsy-based method for determining H pylori status in patients with bleeding peptic ulcers. RUT, histology and culture have a poor sensitivity under bleeding conditions. A decline in the intragastric bacterial density during bleeding ulcer may be a major cause of the reduced sensitivity of biopsy-based assays.
ACKNOWLEDGMENTS
The authors express their deep appreciation to Dr. Wei-Lun Tsai, Dr. Wen-Chi Chen, Dr. Lung-Chih Cheng, Dr. Hsien-Chung Yu, Dr. Chung-Jen Wu, Miss Min-Rong Huang and Miss Pei-Min Tsai for their assistance in the clinical follow up of the patients.
Footnotes
Supported by the Research Foundation of Kaohsiung Veterans General Hospital, No. VGHKS-91-35 and No. VTY88-G3-2, VGH-NYMU Joint Research Program, Taiwan, China
Science Editor Wang XL and Li WZ Language Editor Elsevier HK
References
- 1.Kurata JH, Corboy ED. Current peptic ulcer time trends. An epidemiological profile. J Clin Gastroenterol. 1988;10:259–268. doi: 10.1097/00004836-198806000-00007. [DOI] [PubMed] [Google Scholar]
- 2.Vaira D, Menegatti M, Miglioli M. What is the role of Helicobacter pylori in complicated ulcer disease? Gastroenterology. 1997;113:S78–S84. doi: 10.1016/s0016-5085(97)80017-0. [DOI] [PubMed] [Google Scholar]
- 3.Hosking SW, Yung MY, Chung SC, Li AKC. Differing preva-lence of Helicobacter pylori in bleeding and non-bleeding ulcers. Gastroenterology. 1992;102:A85. [Google Scholar]
- 4.Jensen DM, You S, Pelayo E, Jensen ME. The prevalence of Helicobacter pylori and NSAID use in patients with severe UGI hemorrhage and their potential role in recurrence of ulcer bleeding. Gastroenterology. 1992;102:A85. [Google Scholar]
- 5.Lee JM, Breslin NP, Fallon C, O'Morain CA. Rapid urease tests lack sensitivity in Helicobacter pylori diagnosis when peptic ulcer disease presents with bleeding. Am J Gastroenterol. 2000;95:1166–1170. doi: 10.1111/j.1572-0241.2000.02004.x. [DOI] [PubMed] [Google Scholar]
- 6.Laine L, Cohen H. Helicobacter pylori: drowning in a pool of blood? Gastrointest Endosc. 1999;49:398–402. doi: 10.1016/s0016-5107(99)70024-6. [DOI] [PubMed] [Google Scholar]
- 7.Laine LA. Helicobacter pylori and complicated ulcer disease. Am J Med. 1996;100:52S–57S; discussion 57S-59S. doi: 10.1016/s0002-9343(96)80229-4. [DOI] [PubMed] [Google Scholar]
- 8.Rokkas T, Karameris A, Mavrogeorgis A, Rallis E, Giannikos N. Eradication of Helicobacter pylori reduces the possibility of rebleeding in peptic ulcer disease. Gastrointest Endosc. 1995;41:1–4. doi: 10.1016/s0016-5107(95)70266-0. [DOI] [PubMed] [Google Scholar]
- 9.Graham DY, Hepps KS, Ramirez FC, Lew GM, Saeed ZA. Treatment of Helicobacter pylori reduces the rate of rebleeding in peptic ulcer disease. Scand J Gastroenterol. 1993;28:939–942. doi: 10.3109/00365529309098288. [DOI] [PubMed] [Google Scholar]
- 10.Labenz J, Börsch G. Role of Helicobacter pylori eradication in the prevention of peptic ulcer bleeding relapse. Digestion. 1994;55:19–23. doi: 10.1159/000201117. [DOI] [PubMed] [Google Scholar]
- 11.Cutler AF, Havstad S, Ma CK, Blaser MJ, Perez-Perez GI, Schubert TT. Accuracy of invasive and noninvasive tests to diagnose Helicobacter pylori infection. Gastroenterology. 1995;109:136–141. doi: 10.1016/0016-5085(95)90278-3. [DOI] [PubMed] [Google Scholar]
- 12.Tu TC, Lee CL, Wu CH, Chen TK, Chan CC, Huang SH, Lee MS SC. Comparison of invasive and noninvasive tests for detecting Helicobacter pylori infection in bleeding peptic ulcers. Gastrointest Endosc. 1999;49:302–306. doi: 10.1016/s0016-5107(99)70005-2. [DOI] [PubMed] [Google Scholar]
- 13.Colin R, Czernichow P, Baty V, Touzé I, Brazier F, Bretagne JF, Berkelmans I, Barthélémy P, Hemet J. Low sensitivity of invasive tests for the detection of Helicobacter pylori infection in patients with bleeding ulcer. Gastroenterol Clin Biol. 2000;24:31–35. [PubMed] [Google Scholar]
- 14.Fabre R, Sobhani I, Laurent-Puig P, Hedef N, Yazigi N, Vissuzaine C, Rodde I, Potet F, Mignon M, Etienne JP. Polymerase chain reaction assay for the detection of Helicobacter pylori in gastric biopsy specimens: comparison with culture, rapid urease test, and histopathological tests. Gut. 1994;35:905–908. doi: 10.1136/gut.35.7.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin SY, Jeng YS, Wang CK, Ko FT, Lin KY, Wang CS, Liu JD, Chen PH, Chang JG. Polymerase chain reaction diagnosis of Helicobacter pylori in gastroduodenal diseases: comparison with culture and histopathological examinations. J Gastroenterol Hepatol. 1996;11:286–289. doi: 10.1111/j.1440-1746.1996.tb00077.x. [DOI] [PubMed] [Google Scholar]
- 16.Ho GY, Windsor HM. Accurate diagnosis of Helicobacter pylori. Polymerase chain reaction tests. Gastroenterol Clin North Am. 2000;29:903–915. doi: 10.1016/s0889-8553(05)70158-8. [DOI] [PubMed] [Google Scholar]
- 17.Hsu PI, Lai KH, Tseng HH, Liu YC, Yen MY, Lin CK, Lo GH, Huang RL, Huang JS, Cheng JS, et al. Correlation of serum immunoglobulin G Helicobacter pylori antibody titers with histologic and endoscopic findings in patients with dyspepsia. J Clin Gastroenterol. 1997;25:587–591. doi: 10.1097/00004836-199712000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Hsu PI, Lai KH, Chien EJ, Lin CK, Lo GH, Jou HS, Cheng JS, Chan HH, Hsu JH, Ger LP, et al. Impact of bacterial eradication on the cell proliferation and p53 protein accumulation in Helicobacter pylori-associated gastritis. Anticancer Res. 2000;20:1221–1228. [PubMed] [Google Scholar]
- 19.Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161–1181. doi: 10.1097/00000478-199610000-00001. [DOI] [PubMed] [Google Scholar]
- 20.Hsu PI, Lai KH, Lo GH, Tseng HH, Lo CC, Chen HC, Tsai WL, Jou HS, Peng NJ, Chien CH, et al. Risk factors for ulcer development in patients with non-ulcer dyspepsia: a prospective two year follow up study of 209 patients. Gut. 2002;51:15–20. doi: 10.1136/gut.51.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peng NJ, Hsu PI, Lee SC, Tseng HH, Huang WK, Tsay DG, Ger LP, Lo GH, Lin CK, Tsai CC, et al. A 15-minute [13C]-urea breath test for the diagnosis of Helicobacter pylori infection in patients with non-ulcer dyspepsia. J Gastroenterol Hepatol. 2000;15:284–289. doi: 10.1046/j.1440-1746.2000.02159.x. [DOI] [PubMed] [Google Scholar]
- 22.Peng NJ, Lai KH, Liu RS, Lee SC, Tsay DG, Lo CC, Tseng HH, Huang WK, Lo GH, Hsu PI. Clinical significance of oral urease in diagnosis of Helicobacter pylori infection by [13C]urea breath test. Dig Dis Sci. 2001;46:1772–1778. doi: 10.1023/a:1010626225949. [DOI] [PubMed] [Google Scholar]
- 23.Hsu PI, Hwang IR, Cittelly D, Lai KH, El-Zimaity HM, Gutierrez O, Kim JG, Osato MS, Graham DY, Yamaoka Y. Clinical presentation in relation to diversity within the Helicobacter pylori cag pathogenicity island. Am J Gastroenterol. 2002;97:2231–2238. doi: 10.1111/j.1572-0241.2002.05977.x. [DOI] [PubMed] [Google Scholar]
- 24.Clayton CL, Kleanthous H, Coates PJ, Morgan DD, Tabaqchali S. Sensitive detection of Helicobacter pylori by using polymerase chain reaction. J Clin Microbiol. 1992;30:192–200. doi: 10.1128/jcm.30.1.192-200.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peng NJ, Lai KH, Liu RS, Lee SC, Tsay DG, Lo CC, Tseng HH, Huang WK, Lo GH, Hsu PI. Endoscopic 13C-urea breath test for the diagnosis of Helicobacter pylori infection. Dig Liver Dis. 2003;35:73–77. doi: 10.1016/s1590-8658(03)00014-8. [DOI] [PubMed] [Google Scholar]
- 26.van Leerdam ME, van der Ende A, ten Kate FJ, Rauws EA, Tytgat GN. Lack of accuracy of the noninvasive Helicobacter pylori stool antigen test in patients with gastroduodenal ulcer bleeding. Am J Gastroenterol. 2003;98:798–801. doi: 10.1111/j.1572-0241.2003.07387.x. [DOI] [PubMed] [Google Scholar]
- 27.Peitz U, Leodolter A, Kahl S, Agha-Amiri K, Wex T, Wolle K, Günther T, Steinbrink B, Malfertheiner P. Antigen stool test for assessment of Helicobacter pylori infection in patients with upper gastrointestinal bleeding. Aliment Pharmacol Ther. 2003;17:1075–1084. doi: 10.1046/j.1365-2036.2003.01548.x. [DOI] [PubMed] [Google Scholar]
- 28.Ho SA, Hoyle JA, Lewis FA, Secker AD, Cross D, Mapstone NP, Dixon MF, Wyatt JI, Tompkins DS, Taylor GR. Direct polymerase chain reaction test for detection of Helicobacter pylori in humans and animals. J Clin Microbiol. 1991;29:2543–2549. doi: 10.1128/jcm.29.11.2543-2549.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Zwet AA, Thijs JC, Kooistra-Smid AM, Schirm J, Snijder JA. Sensitivity of culture compared with that of polymerase chain reaction for detection of Helicobacter pylori from antral biopsy samples. J Clin Microbiol. 1993;31:1918–1920. doi: 10.1128/jcm.31.7.1918-1920.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hua J, Roux D, de Mascarel A, Megraud F. PCR for Helicobacter pylori on biopsy samples in CLO test sent by mail [abstract]. Gastroenterology 1994; 106: A97 34 Coudron PE, Stratton CW. Factors affecting growth and sus-ceptibility testing of Helicobacter pylori in liquid media. J Clin Microbiol. 1995;33:1028–1030. doi: 10.1128/jcm.33.4.1028-1030.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin TT, Yeh CT, Yang E, Chen PC. Detection of Helicobacter pylori by polymerase chain reaction assay using gastric biopsy specimens taken for CLOtest. J Gastroenterol. 1996;31:329–332. doi: 10.1007/BF02355020. [DOI] [PubMed] [Google Scholar]
- 32.Hsu PI, Lai KH, Tseng HH, Lin CK, Lo GH, Cheng JS, Chan HH, Chen GC, Jou HS, Peng NJ, et al. Risk factors for presentation with bleeding in patients with Helicobacter pylori-related peptic ulcer diseases. J Clin Gastroenterol. 2000;30:386–391. doi: 10.1097/00004836-200006000-00007. [DOI] [PubMed] [Google Scholar]
- 33.Leung WK, Sung JJ, Siu KL, Chan FK, Ling TK, Cheng AF. False-negative biopsy urease test in bleeding ulcers caused by the buffering effects of blood. Am J Gastroenterol. 1998;93:1914–1918. doi: 10.1111/j.1572-0241.1998.00457.x. [DOI] [PubMed] [Google Scholar]
- 34.Coudron PE, Stratton CW. Factors affecting growth and susceptibility testing of Helicobacter pylori in liquid media. J Clin Microbiol. 1995;33:1028–1030. doi: 10.1128/jcm.33.4.1028-1030.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]


