Abstract
AIM: To investigate the uptake of 99mTc-HYNIC-Tyr3-octreotide (99mTc-HYNIC-TOC) in human hepatocellular carcinoma (HCC), which can provide the localizable diagnosis in hepatic carcinoma.
METHODS: The expression of somatostatin receptor 2 (SSTR2) messenger RNA (mRNA) in human HCC cell line HepG2 was examined by reverse transcriptase-polymerase chain reaction (RT-PCR). Uptake of 99mTc-HYNIC-TOC was evaluated in the human HCC implanted into BALB/c nude mice. ANMIS2000 nuclear medicine analysis system was used to calculate the ratio of 99mTc uptake between tumor tissue and vital organs.
RESULTS: We demonstrated the expression of SSTR2 mRNA in human HCC cell line HepG2 by RT-PCR. The size of the RT-PCR products was 364 bp detected by sequence analysis of the human SSTR2 mRNA. Scintigraphy proved that 99mTc-HYNIC-TOC was uptaken in the tumor tissue, liver and kidney of the tumor-bearing mice.
CONCLUSION: Based on expression of the SSTR2 mRNA in human HCC, 99mTc-HYNIC-TOC can markedly bind with and be uptaken by human HCC tissues as compared with normal liver tissue. The significant retention of radionuclide in kidney and bladder is probably related to non-specific peptide uptake in the tubulus cells of kidney and possibly due to excretion by kidney. Our results show that localizable diagnosis and targeting radiotherapy with radionuclide-labeled somatostatin analog for HCC are of great value to be further studied.
Keywords: Hepatocellular carcinoma, 99mTc-HYNIC-Tyr3-octreotide, Somatostatin receptor 2
INTRODUCTION
The role of somatostatin (SS) analogs in the tumor diagnostic and therapeutic applications has attracted the concern of the people. It has been widely reported about the growth suppressing effects of SS and its analogs on many tumors[1-4]. To date, it is known that their suppressing effect on tumor cell proliferation is mediated by the somatostatin receptors (SSTRs) presented in the tumor constitution[5]. Like most neuroendocrine tumors, adenocarcinomas originating in the breast, colon, or pancreatic tumor, as well as meningiomas, express SSTRs, and the majority of these tumors express the somatostatin receptor subtype 2 (SSTR2)[6,7]. However, all five receptor subtypes (SSTR1-5) bind native SS with a high affinity, while octreotide, an SS analog, binds with a very high affinity only to subtype 2 (SSTR2) and shows a moderately high affinity for SSTR5[8]. Overexpression of the SSTR2 in some tumors has made it possible to use SS-receptor scintigraphy with indium111 or technetium 99m-labeled octreotide for the visualization of SS receptor-positive cancers[9,10]. In addition to tumor scintigraphy, a new application of these radiolabeled peptides is peptide receptor radionuclide therapy[11,12].
It has been known that hepatocellular carcinoma (HCC) is a leading cause of cancer-related death. At present, surgical resection of malignant liver lesions offers the best outcome and the only hope of cure. The 5-year survival rate for selected patients undergoing surgical resection of primary HCC was 30%, with a median survival of 30 mo. However, approximately 90% of patients presenting with primary HCC have inoperable disease[13]. These patients must rely largely on various forms of chemotherapy and radiotherapy[14,15]. These treatments have at times shown promising response rates, symptom palliation and have occasionally down-staged hepatic tumors to allow surgical resection, but these treatment modalities have not improved 5-year survival rates, which remain in the order of 1%[16]. The persisting poor survival among the vast majority of patients presenting with liver cancer has led to renewed interest in developing targeted radiotherapy with radiolabeled SS analogs as a possible treatment option for patients with non-resectable liver cancer. However, it has not been known that HCC can uptake radiolabeled SS analogs. The aim of this study was to scintigraphically identify the localization of HCC in order to reveal a possible role of radiolabeled SS analogs in the treatment of HCC, and determine the target cell of receptor subtype selective radiolabeled SS analogs for targeting radiotherapy of HCC.
MATERIALS AND METHODS
Materials
All reagents and solvents were obtained from commercial sources and were used without further purification except HYNIC-[Tyr3]-octreotide (HYNIC-TOC). We applied hydrazinonicotinic acid (HYNIC) as the ligand for 99mTc. Labeling with 99mTc was performed using a co-ligand required to stabilize 99mTc bound to the hydrazino residue of the peptide conjugate. HYNIC-TOC was synthesized at the Medicine Isotope Research Center of Peking University. Na99mTcO4 was obtained from commercial 99Mo/99mTc generator. The methods of HYNIC-TOC synthesis and 99mTc labeling were employed as previously described[17,18]. Reaction solutions were tested for radiochemical purity by the C18-SepPak column of high-performance liquid chromatography immediately and up to 24 h after preparation. 99mTc of radioactive purity > 95% was bound to HYNIC-TOC.
RT-PCR and sequencing of products
The human HCC cell line HepG2 was assessed for the presence of somatostatin receptor subtypes (SSTR1-5) messenger RNA (mRNA) to confirm the presence of the target receptor subtype for 99mTc-HYNIC-Tyr3-octreotide (99mTc-HYNIC-TOC), especially SSTR2. The following primer pairs (Boya Co., Shanghai, China) were applied: for human SSTR1, sense (1 543-1 652) 5’-TCATCCTCGGCT-ATGCCAAC-3’ and antisense (1 789-1 898) 5’-GCAGGTG-CCATTACGGAAGA-3’; for SSTR2, sense (359-378) 5’-CTGTGGATGGCATCAATCAG-3’ and antisense (723-741) 5’-TCGGATTCCAGAGGACTTCA-3’; for SSTR3, sense (1 193-1 212) 5’-GCCTCTGCTACCTGCTCATC-3’ and antisense (1 618-1 637) 5’-CCATCCTCCTCCTCC-TCATC-3’; for SSTR4, sense (480-499) 5’-CAGCGTGGC-CAAGCTCATCA-3’ and antisense (962-981) 5’-GATCGG-CGGAAGTTGTCGGA-3’; for SSTR5, sense (205-224) 5’-GCCAAGATGAAGACCGTCAC-3’ and antisense (668-887) 5’-AGCAGGTAGCACAGGCAGAT-3’; for β-actin, sense 5’-ACGTTATGGATGATGTATCGC-3’ and antisense 5’-CTTAATGTCACGCACGATTTCC-3’. RNA extraction and reverse transcription were performed according to the manufacturer’s instructions. cDNA was amplified in a reaction mixture (total volume 20 µL) comprising cDNA (transcribed from 12 µg of total RNA), 2 µL of 10 × PCR buffer, 0.4 µL of 10 mmol/L dNTP, and 1.5 µL of 25 mmol/L MgCl2, 10 pmol/L of each of sense and antisense primers, and 2.5 U of Taq DNA polymerase. Following an initial denaturing step at 94°C for 5 min, the amplification program of 30 cycles, each cycle consisting of denaturation at 94°C for 30 s, annealing at 60°C for 20 s, and extension at 72°C for 30 s, was carried out by a Gene Amplification PCR system 2 400 (PE Corp., USA). The amplification was terminated with the final extension step at 72°C for 10 min. The amplified products, subsequently, were electrophoresed and photographed on 15 g/L agarose gel stained with ethidium bromide. The final products were verified by sequencing in Boya Co. (Shanghai, China). β-actin specific primers were used to amplify the cDNA fragment as an internal standard.
Tumor cell inoculation and tumor growth assessment
The human HCC cell line HepG2 was from Clinical Medical Institute of Sir Run Run Shaw Hospital. The cells were grown in RPMI1640 (Gibco Co., USA) supplemented with 100 mL/L fetal bovine serum (Hangzhou Sijiqing Co., China) at 37°C in a humidified atmosphere containing 50 mL/L CO2 and 950 mL/L O2. Subculturing was executed every 2-3 d and the cells grew well along the walls of culture tube. Then, the cells (2×106 cells/mouse) at logarithmic growth period were subcutaneously inoculated into right flank of BALB/c nude mice, aged 6-8 wk, weighing 18-20 g (purchased from Animal Center of Academy of Science, Shanghai, China). The total tumor load per mouse was approximately 1 cm3 at about 28 d post-injection. We took out the whole tumors, then, immediately put into saline containing 100 U/mL penicillin and streptomycin. Tumor pieces with a size 1-2 mm3 were made from the margins of the whole mass after winkling the connective tissue around the mass. The mice were randomly divided into three groups in accordance with subcutaneous re-transplantation of tumor into right flanks (subcutaneous group) or in situ liver (intra-liver group), or without re-transplantation (control group). Each group consisted of three mice. The experiments were started at 21 d post re-implantation, when the size of tumor per mice was approximately 1 cm3.
In vivo imaging with 99mTc-HYNIC-TOC
Each mouse of tumor-bearing groups (subcutaneous group and intra-liver group) and control mice group received 100 µCi of 99mTc-HYNIC-TOC injected into the caudal vein under soluble pentobarbitone anesthesia. The mice were imaged at 2 and 4.5 h, respectively, after injection of 99mTc-HYNIC-TOC with mini-radioisotope gamma camera equipped with a pinhole collimator (Bingsong Corp., China). During imaging, the mice were maintained with a fixed band and positioned on dorsal recumbency with the legs extending from the body. The imaging analyses of 99mTc uptake in tumor tissue and vital organs were conducted using ANMIS 2000 nuclear medicine analysis system (Bingsong Corp.). Animals of the intra-liver group were killed after the second imaging session (4.5 h after injection of 99mTc-HYNIC-TOC) for examination of intra-liver tumor growth.
RESULTS
Expression and PCR products sequencing of SSTR2 mRNA in HCC cell line HepG2
PCR products of SSTRs were obtained for HepG2 cells. The size of the products corresponded to the predicted length of the synthesized cDNA fragment based on the position of the PCR primer. SSTR1-5 mRNAs were variably expressed in HepG2 cells. Expressions of SSTR1, SSTR2, and SSTR4 mRNA were found in HepG2 cells, respectively (Figure 1), but those of SSTR3 and SSTR5 mRNAs were not observed. We identified that the product of SSTR2 mRNA was the specific fragment that we needed after performing sequencing and comparison with SSTRs mRNA rank of the GenBank[19], it turned out to be a perfect coincidence with the fragment rank (Figure 2). The result showed that the amplification we executed was specific.
Figure 1.
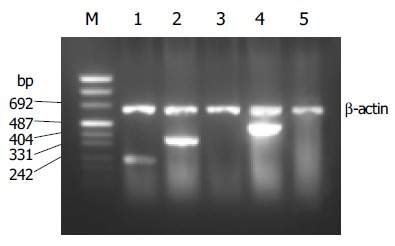
RT-PCR analysis for SSTR1-5 mRNA expression in HepG2. Lane m: marker; lane 1: SSTR1 (246 bp); lane 2: SSTR2 (384 bp); lane 3: SSTR3; lane 4: SSTR4 (502 bp); lane 5: SSTR5.
Figure 2.
Sequencing of SSTR2 mRNA PCR products. The products were specifically amplified after the sequencing fragment was compared with human SSTR2 of GenBank, and was a perfect coincidence with the fragment rank of human SSTR2 mRNA.
In vivo imaging with 99mTc-HYNIC-TOC
The gamma camera imagings of a representative mouse from the subcutaneous, intra-liver and control groups at 2 and 4.5 h after being injected with 99mTc- HYNIC-TOC are shown in Figure 3. These imagings showed visual accumulation of 99mTc-HYNIC-TOC detected in the tumor xenografts of mice (including subcutaneous group and intra-liver group). The retention of radioactivity in the kidney and excretion through the bladder were observed. The high-level uptake of radioactivity in the liver of tumor-bearing mice was also observed and compared with the control group. This tumor uptake was important to validate the dosing regimen that was used in therapy studies of liver cancer.
Figure 3.
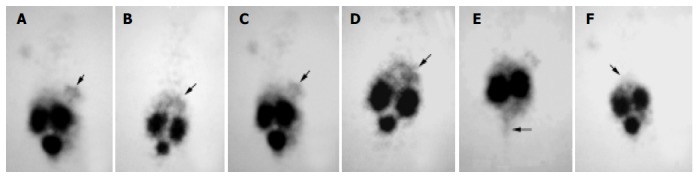
Scintigrams of mice at different times after injection of 99mTc-HYNIC-TOC. A and C: subcutaneous group at 2 and 3 h, respectively; B and D: intra-liver group at 2 and 4 h, respectively; E: subcutaneous group at 4.5 h, when the bladder was emptied; F: control group at 2 h. The marked decrease in the liver (arrows) uptake was apparent in the control group mouse.
DISCUSSION
Radiolabeled SS analogs have brought new prospects to nuclear oncology for diagnosis and therapy of tumor. Due to their low molecular weight and high affinity to SSTRs, good tissue penetration properties, and being internalized into the tumor cells after receptor binding[20-22], they provide promising strategies for diagnosis and internal radionuclide therapy for various cancer types. Before radiolabeled, octreotide was employed in HCC, it has been studied with 125I-[Tyr3]-octreotide for tumor diagnosis[11]. However, some studies demonstrated that the SSTRs density in HCC was considerably lower than the receptor density found[23,24], thus it has rarely been studied in the liver cancer. As radiotherapy of SS receptor-positive tumors with compounds such as 90Y-labeled ones was applied, it is necessary to develop a new strategy for the treatment of liver cancer.
In this study, we evaluated the expression of SS receptor in human HCC cell line HepG2. The results showed that SSTR1, SSTR2 and SSTR4 were frequently expressed in human HCC, which was similar to previous reports[25,26]. As the normal human liver does not express SSTR2 (predominant receptor subtype was SSTR1[27]), the presence of SSTRs in HCC may be regarded as an overexpression of the receptors in this tumor. Like previous therapeutic experiments[28], unlabeled octreotide was shown to bind with a high affinity to SSTR2, suggesting that the predominant receptor subtype was SSTR2. As to radiolabeled octreotide targeting therapy for HCC, the expression of SSTR2 in HCC is of high significance.
In scintigraphy experiment with 99mTc-HYNIC-TOC, the significant uptake of radionuclide by tumor xenografts in nude mice was found in both subcutaneous group and intra-liver group. And it was demonstrated that the hepatocarcinoma tissues had a fairly steady rate in uptaking 99mTc-HYNIC-TOC by the fact that there was still obvious radionuclide gathering at the tumor tissues at 4 h after administration. The results showed that the intensity of the signal was not as strong as for neuroendocrine tumor metastases. As a therapeutic tool, there are certainly potential clinical implications linked with the SS receptor expression in HCC.
The expression of some SS receptor subtypes was found in the liver and kidney as well[24,29]. However, the physiological uptake of 99mTc-HYNIC-TOC in these organs was probably mainly due to some loss of technetium out of chelation (liver) and non-specific peptide uptake in the tubulus cells (kidney). Thus, in our receptor scintigram, the retention of radionuclide prominently in both kidney and bladder as well as the less uptake in liver, which was similar to previous reports[30,31], was shown after injection of 99mTc-HYNIC-TOC. Because of the absence of SSTR2, the retention of technetium in the bladder, which was possibly due to the kidney excretion, almost vanished when bladder emptied (Figure 3E). Meanwhile, based on recent studies[32-34], most of the radiolabeled octreotide, like our results, would accumulate in the kidney after glomerular filtration, followed by re-absorption into renal cells. It is necessary to take into consideration the induction of the kidney damage while performing targeting radiotherapy with radiolabeled octreotide.
In conclusion, HCC cell line HepG2 expresses SSTR2 mRNA. 99mTc-HYNIC-TOC, relying on the receptor mediation, can markedly bind with and be uptaken by human HCC tissues as compared to the normal liver tissue, which is probably mainly due to some loss of technetium out of chelation. The significant retention of radionuclide in kidney and bladder is probably related to non-specific peptide uptake in the tubulus cells of kidney and possibly due to the excretion by kidney. This phenomenon has clued to potential radiation nephrotoxicity of the agents, which needs to be solved before a widespread application of targeting radiotherapy.
Footnotes
Science Editor Kumar M and Zhu LH Language Editor Elsevier HK
References
- 1.Lamberts SW, de Herder WW, Hofland LJ. Somatostatin analogs in the diagnosis and treatment of cancer. Trends Endocrinol Metab. 2002;13:451–457. doi: 10.1016/s1043-2760(02)00667-7. [DOI] [PubMed] [Google Scholar]
- 2.Froidevaux S, Eberle AN. Somatostatin analogs and radiopeptides in cancer therapy. Biopolymers. 2002;66:161–183. doi: 10.1002/bip.10256. [DOI] [PubMed] [Google Scholar]
- 3.Celinski SA, Fisher WE, Amaya F, Wu YQ, Yao Q, Youker KA, Li M. Somatostatin receptor gene transfer inhibits established pancreatic cancer xenografts. J Surg Res. 2003;115:41–47. doi: 10.1016/s0022-4804(03)00276-2. [DOI] [PubMed] [Google Scholar]
- 4.Kumar M, Liu ZR, Thapa L, Wang DY, Tian R, Qin RY. Mechanisms of inhibition of growth of human pancreatic carcinoma implanted in nude mice by somatostatin receptor subtype 2. Pancreas. 2004;29:141–151. doi: 10.1097/00006676-200408000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Pollak MN, Schally AV. Mechanisms of antineoplastic action of somatostatin analogs. Proc Soc Exp Biol Med. 1998;217:143–152. doi: 10.3181/00379727-217-44216. [DOI] [PubMed] [Google Scholar]
- 6.Reubi JC, Kvols L, Krenning E, Lamberts SW. Distribution of somatostatin receptors in normal and tumor tissue. Metabolism. 1990;39:78–81. doi: 10.1016/0026-0495(90)90217-z. [DOI] [PubMed] [Google Scholar]
- 7.Lamberts SW, Krenning EP, Reubi JC. The role of somatostatin and its analogs in the diagnosis and treatment of tumors. Endocr Rev. 1991;12:450–482. doi: 10.1210/edrv-12-4-450. [DOI] [PubMed] [Google Scholar]
- 8.Lamberts SW, van der Lely AJ, de Herder WW, Hofland LJ. Octreotide. N Engl J Med. 1996;334:246–254. doi: 10.1056/NEJM199601253340408. [DOI] [PubMed] [Google Scholar]
- 9.Weiner RE, Thakur ML. Radiolabeled peptides in the diagnosis and therapy of oncological diseases. Appl Radiat Isot. 2002;57:749–763. doi: 10.1016/s0969-8043(02)00192-6. [DOI] [PubMed] [Google Scholar]
- 10.Decristoforo C, Mather SJ. Technetium-99m somatostatin analogues: effect of labelling methods and peptide sequence. Eur J Nucl Med. 1999;26:869–876. doi: 10.1007/s002590050461. [DOI] [PubMed] [Google Scholar]
- 11.Towu E, Boxer G, Begent R, Zweit J, Spitz L, Hobbs K, Winslet M. In-vitro uptake of radioactive lipiodol I-131 and I-125 by hepatoblastoma: implications for targeted radiotherapy. Pediatr Surg Int. 2001;17:609–613. doi: 10.1007/s003830100004. [DOI] [PubMed] [Google Scholar]
- 12.Heppeler A, Froidevaux S, Eberle AN, Maecke HR. Receptor targeting for tumor localisation and therapy with radiopeptides. Curr Med Chem. 2000;7:971–994. doi: 10.2174/0929867003374516. [DOI] [PubMed] [Google Scholar]
- 13.Hussain SA, Ferry DR, El-Gazzaz G, Mirza DF, James ND, McMaster P, Kerr DJ. Hepatocellular carcinoma. Ann Oncol. 2001;12:161–172. doi: 10.1023/a:1008370324827. [DOI] [PubMed] [Google Scholar]
- 14.Rougier P, Mitry E, Clavero-Fabri MC. Chemotherapy and medical treatment of hepatocellular carcinoma (HCC) Hepatogastroenterology. 1998;45 Suppl 3:1264–1266. [PubMed] [Google Scholar]
- 15.Perez CA, Brady LW. Lippincott Company; 1987. Principles and Practice of Radiation Oncology: Pancreatic and Hepatobiliary Cancer; p. P810. [Google Scholar]
- 16.Liu CL, Fan ST. Nonresectional therapies for hepatocellular carcinoma. Am J Surg. 1997;173:358–365. doi: 10.1016/S0002-9610(96)00384-4. [DOI] [PubMed] [Google Scholar]
- 17.Decristoforo C, Mather SJ, Cholewinski W, Donnemiller E, Riccabona G, Moncayo R. 99mTc-EDDA/HYNIC-TOC: a new 99mTc-labelled radiopharmaceutical for imaging somatostatin receptor-positive tumours; first clinical results and intra-patient comparison with 111In-labelled octreotide derivatives. Eur J Nucl Med. 2000;27:1318–1325. doi: 10.1007/s002590000289. [DOI] [PubMed] [Google Scholar]
- 18.von Guggenberg E, Sarg B, Lindner H, Alafort LM, Mather SJ, Moncayo R, Decristoforo C. Preparation via coligand ex-change and characterization of [99mTc-EDDA-HYNICD-Phe1, Tyr3] Octreotide (99mTc–EDDA/HYNIC–TOC) J Label Compd Radiopharm. 2003;46:307–318. [Google Scholar]
- 19. Available from: http: //www.ncbi.nlm.nih.gov/blast.
- 20.Krenning EP, de Jong M, Kooij PP, Breeman WA, Bakker WH, de Herder WW, van Eijck CH, Kwekkeboom DJ, Jamar F, Pauwels S, et al. Radiolabelled somatostatin analogue(s) for peptide receptor scintigraphy and radionuclide therapy. Ann Oncol. 1999;10 Suppl 2:S23–S29. doi: 10.1093/annonc/10.suppl_2.s23. [DOI] [PubMed] [Google Scholar]
- 21.Weckbecker G, Raulf F, Stolz B, Bruns C. Somatostatin analogs for diagnosis and treatment of cancer. Pharmacol Ther. 1993;60:245–264. doi: 10.1016/0163-7258(93)90009-3. [DOI] [PubMed] [Google Scholar]
- 22.Paganelli G, Zoboli S, Cremonesi M, Bodei L, Ferrari M, Grana C, Bartolomei M, Orsi F, De Cicco C, Mäcke HR, et al. Receptor-mediated radiotherapy with 90Y-DOTA-D-Phe1-Tyr3-octreotide. Eur J Nucl Med. 2001;28:426–434. doi: 10.1007/s002590100490. [DOI] [PubMed] [Google Scholar]
- 23.Reubi JC, Zimmermann A, Jonas S, Waser B, Neuhaus P, Läderach U, Wiedenmann B. Regulatory peptide receptors in human hepatocellular carcinomas. Gut. 1999;45:766–774. doi: 10.1136/gut.45.5.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deng-Guo YAN, Qing-Jia OU. Somatostatin receptor sub-type SSTR2 and SSTR3 mRNA expression in primary hepatic Carcinoma. Aizheng. 2001;20:152–155. [Google Scholar]
- 25.Hofland LJ, Lamberts SW. Somatostatin receptor subtype expression in human tumors. Ann Oncol. 2001;12 Suppl 2:S31–S36. doi: 10.1093/annonc/12.suppl_2.s31. [DOI] [PubMed] [Google Scholar]
- 26.Rohrer L, Raulf F, Bruns C, Buettner R, Hofstaedter F, Schüle R. Cloning and characterization of a fourth human somatostatin receptor. Proc Natl Acad Sci USA. 1993;90:4196–4200. doi: 10.1073/pnas.90.9.4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kouroumalis E, Skordilis P, Thermos K, Vasilaki A, Moschandrea J, Manousos ON. Treatment of hepatocellular carcinoma with octreotide: a randomised controlled study. Gut. 1998;42:442–447. doi: 10.1136/gut.42.3.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hofland LJ, Lamberts SW. Somatostatin receptors and disease: role of receptor subtypes. Baillieres Clin Endocrinol Metab. 1996;10:163–176. doi: 10.1016/s0950-351x(96)80362-4. [DOI] [PubMed] [Google Scholar]
- 29.Patel YC. Somatostatin and its receptor family. Front Neuroendocrinol. 1999;20:157–198. doi: 10.1006/frne.1999.0183. [DOI] [PubMed] [Google Scholar]
- 30.Reubi JC, Horisberger U, Studer UE, Waser B, Laissue JA. Human kidney as target for somatostatin: high affinity receptors in tubules and vasa recta. J Clin Endocrinol Metab. 1993;77:1323–1328. doi: 10.1210/jcem.77.5.7915721. [DOI] [PubMed] [Google Scholar]
- 31.Bass LA, Lanahan MV, Duncan JR, Erion JL, Srinivasan A, Schmidt MA, Anderson CJ. Identification of the soluble in vivo metabolites of indium-111-diethylenetriaminepentaacetic acid-D-Phe1-octreotide. Bioconjug Chem. 1998;9:192–200. doi: 10.1021/bc970158h. [DOI] [PubMed] [Google Scholar]
- 32.Duncan JR, Stephenson MT, Wu HP, Anderson CJ. Indium-111-diethylenetriaminepentaacetic acid-octreotide is delivered in vivo to pancreatic, tumor cell, renal, and hepatocyte lysosomes. Cancer Res. 1997;57:659–671. [PubMed] [Google Scholar]
- 33.Akizawa H, Arano Y, Uezono T, Ono M, Fujioka Y, Uehara T, Yokoyama A, Akaji K, Kiso Y, Koizumi M, et al. Renal metabolism of 111In-DTPA-D-Phe1-octreotide in vivo. Bioconjug Chem. 1998;9:662–670. doi: 10.1021/bc9702258. [DOI] [PubMed] [Google Scholar]
- 34.Akizawa H, Arano Y, Mifune M, Iwado A, Saito Y, Mukai T, Uehara T, Ono M, Fujioka Y, Ogawa K, et al. Effect of molecular charges on renal uptake of 111In-DTPA-conjugated peptides. Nucl Med Biol. 2001;28:761–768. doi: 10.1016/s0969-8051(01)00241-4. [DOI] [PubMed] [Google Scholar]


