Abstract
Objective
Oxidative metabolism is impaired in several medical conditions including psychiatric disorders, and this imbalance may be involved in the etiology of these diseases. The present study evaluated oxidative balance in pediatric and adolescent patients with attention deficit hyperactivity disorder (ADHD).
Methods
The study included 48 children and adolescents (34 male, 14 female) with ADHD who had no neurological, systemic, or comorbid psychiatric disorders, with the exception of oppositional defiant disorder (ODD), and 24 sex- and age-matched healthy controls (17 male and seven female).
Results
TAS was significantly lower, and TOS and OSI were significantly higher in patients with ADHD than in healthy controls. Total antioxidant levels were lower in patients with comorbid ODD than in those with no comorbidity. No difference was found in TOS or OSI among the ADHD subtypes; however, TAS was higher in the attention-deficient subtype.
Conclusion
Our findings demonstrated that oxidative balance is impaired and oxidative stress is increased in children and adolescents with ADHD. This results are consistent with those of previous studies.
Keywords: Attention deficit hyperactivity disorder, Oxidative stress, Total oxidant status, Total antioxidant status, Oxidative imbalance, Child and adolescent
INTRODUCTION
Oxidation-reduction reactions in the body produce waste material referred to as "oxidants". The body has antioxidant defense systems that prevent the formation of oxidants and their harmful effects. The term "oxidative stress" describes the biological damage that arises from the imbalance of oxidants and antioxidants. Oxidants damage the protein structure of cell membranes. It is thought that oxidants inhibit uptake of enzymes and/or neurotransmitters involved in the physiological functioning of cells, which may be a predisposing factor for disease.1,2,3,4,5
Adult ADHD (A-ADHD) is a common, childhood-onset, chronic neuropsychiatric disorder characterized by symptoms of inattention, hyperactivity, and impulsivity. Its worldwide prevalence in children and adolescents is between 5% and 10%. The exact causes of A-ADHD are still unknown. In addition to neurochemical and neuroanatomic disorders, genetic and environmental factors are considered in its etiology.5,6 Either genetic or environmental risk factors could increase the likelihood of oxidative stress; several authors have studied peripheral measures of oxidative stress in patients with ADHD.7,8,9,10,11 Some of these studies concluded that measures of oxidative stress were elevated among ADHD patients but others could not confirm that finding.6,12
Despite considerable research, the neurobiological foundations of attention deficit hyperactivity disorder (ADHD) are not well understood. A growing body of evidence supports the theory that ADHD involves various brain structures and functions, rather than a single region or specific biological mechanism. Accordingly, one area of intense investigation is oxidative metabolism. The present study investigated oxidative metabolism in children and adolescents with ADHD using a more valid and reliable method that measures total oxidant status (TOS) and total antioxidant status (TAS) to identify changes in oxidant and antioxidant parameters that may contribute to the etiopathogenesis of the disease.
METHODS
Study design and patients
The study used a controlled, cross-sectional design and was approved by the 3rd Ankara Clinical Studies Ethics Committee. Written consent was obtained from all participants and their families. The study population included 48 patients, who had been referred to the polyclinics in the Department of Child and Adolescent Psychiatry at Hacettepe University and received a first-time diagnosis of ADHD according to DSM-IV criteria, and 24 healthy control subjects with no psychopathology.
Patient inclusion criteria included ADHD diagnosis based on DSM-IV criteria, absence of concomitant psychiatric comorbidities with the exception of oppositional defiant disorder (ODD), and age 7-18 years. Exclusion criteria were any form of intellectual disability, chronic neurological and/or metabolic disorder, evidence of infection or drug use within the past week, current smoker, and present and/or past evidence of substance addiction. Inclusion criteria for healthy controls were absence of psychopathology according to a psychiatric evaluation based on DSM-IV criteria and age 7-18 years. Exclusion criteria were any form of intellectual disability, chronic neurological and/or metabolic disorder, evidence of infection or drug use within the past week, current smoker, and present and/or past evidence of substance addiction.
Patients socioeconomic status was calculated based on a standard Hollingshead and Redlich formula that weighted, then combined father's occupation and education; first, the occupation score is weighted ×5 and education is weighted ×3. The Hollingshead and Redlich system defines occupational categories ranging from 0 (not working); 1, 2, 3, 4 (menial labor; unskilled labor; semiskilled labor, skilled labor) to 9 (major professional).13
Psychiatric investigation of the participants
Children and adolescents who were referred to the polyclinics of the Department of Child and Adolescent Psychiatry at Hacettepe University were evaluated using the Schedule for Affective Disorders and Schizophrenia for School Age Children (K-SADS-PL),14 a semi-structured interview. Turkish adaptation of this form was made by Gokler and colleagues.15
Parents were asked to complete the Conners' Parent Rating Scale and The Conners' Teacher Form16 was given to the parents to be completed by the class teachers. Turkish adaptation of thise form was made by Dereboy et al.17 Turgay DSM-IV-based Disruptive Behavior Disorders Child and Adolescent Rating & Screening Scale (T-DSM-IV-S)18 was obtained from all parents. The validity and reliability study was performed in Turkey by Ercan et al.19 The Wechsler Intelligence Scale for Children-Revised (WISC-R) test was administered to all participants.20
The control group of the study included healthy participants who were admitted to our hospital for routine clinical health examinations and vaccinations, and were assessed for psychopathology using psychiatric interviews and K-SADS-PL and WISC-R test scores. The control group was free of usage of any medication for at least 6 weeks prior to blood sampling. None of the control participants had chronic diseases and none of them consumed alcohol or had ever taken psychotropic drugs. They had no personal or family history of psychiatric disorders.
After including the study participants the TAS, TOS, and oxidative stress index (OSI) were compared between groups.
Measurement of the total oxidant status
Venous blood samples (5 cc) were drawn from each patient and healthy control to measure TAS, TOS, and OSI. The blood samples were centrifuged for 10 min at 3000 rpm and then stored at -80℃. TOS of plasma was determined using a novel automated measurement method, developed by Erel et al.21,22 Oxidants present in the sample oxidize the ferrous ion-o-dianisidine complex to ferric ion. The oxidation reaction is enhanced by glycerol molecules, which are abundantly present in the reaction medium. The ferric ion makes a colored complex with xylenol orange in an acidic medium. The color intensity, which can be measured spectrophotometrically, is related to the total amount of oxidant molecules present in the sample. The assay is calibrated with hydrogen peroxide and the results were expressed in terms of micromolar hydrogen peroxide equivalent per liter (µmol H2O2 Eq/L).
Measurement of the total antioxidant status
TAS of plasma was determined using a novel automated measurement method, developed by Erel et al.21,22 In this method, the most potent biological radical, hydroxyl radical, is produced. In the assay, ferrous ion solution, which is present in reagent 1 [o-dianisidine (10 mM), ferrous ion (45 AM) in the Clark and Lubs solution (75 mM, pH 1.8)] is mixed with hydrogen peroxide, which is present in reagent 2 [H2O2 (7.5 mM) in the Clark and Lubs solution]. The sequentially produced radicals such as brown colored dianisidinyl radical cation, produced by the hydroxyl radical, are also potent radicals. Using this method, the antioxidative effect of the sample against the potent free radical reactions that is initiated by the produced hydroxyl radical, is measured. The assay has excellent precision values of lower than 3%. The results were expressed as micromoles Trolox Equiv/L (µmol TE/L).
Oxidative stress index
OSI was defined as percentage rate of TOS values to TAS values. Before the calculation the TAS test mmol unit value was translated to micromol units as in the TOS test. The results were expressed as Arbitrary units, calculated by the following formula: OSI (arbitrary unit)=TOS (µmol H2O2 equivalent/L)/TAS (mmol Trolox equivalent/L)×100.21,22
Statistical analysis and sample size calculation
The Statistical Package for the Social Sciences version 11.5 (SPSS Inc., Chicago, IL, USA) was used to conduct the statistical tests. We performed a post-hoc power analysis (G*Power, Version 2.0 program written by Franz Faul, Universität Kiel, Kiel, Germany) using the OSI levels and found the power of the study is 0.99 (α=0.05, sigma=2.2, and effect size=1.15). The chi-squared test was used to evaluate continuous data. Between-group comparisons were made using independent-sample t-tests, and multiple categorical data were assessed using a one-way analysis of variance (ANOVA) with the Bonferroni correction. Pearson's correlation analysis was used to evaluate correlations between the scales and variables. All analyses were conducted as two-tailed tests. p-values <0.05 were deemed statistically significant.
RESULTS
The study consisted of 48 patients with ADHD and 24 age-, sex-, and socioeconomic level -matched healthy controls. Demographic variables were not significantly different between groups (p>0.05 for all) (Table 1). Compared to the controls; TOS and OSI values were significantly higher, and TAS was significantly lower in the patient group (p<0.05 for all) (Table 2). The comparison of ADHD subtypes revealed no difference between TOS and OSI values (p=0.81, p=0.42, respectively); whereas TAS values were higher in the patients with ADHD-AD than in the other subtypes (p=0.04) (Table 3).
Table 1. Demographic characteristic of patients with attention deficit hyperactivity disorder and control subjects.
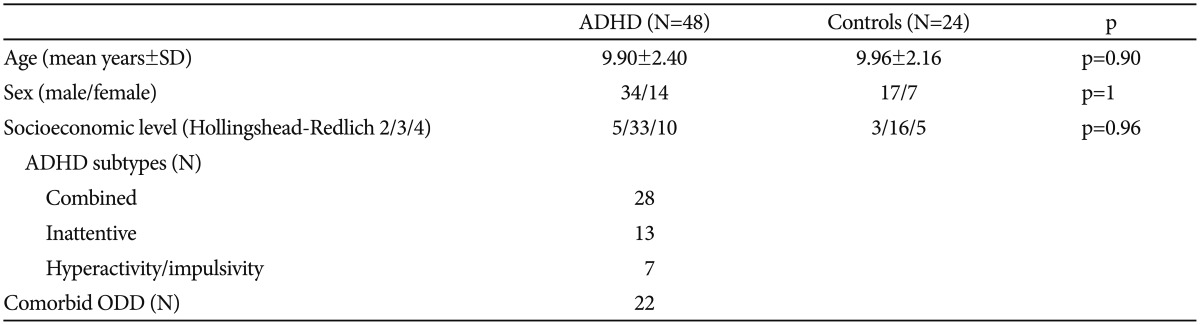
Variables were expressed as mean±standard deviations, and numbers. ADHD: attention deficit hyperactivity disorder, SD: standard deviation, Hollingshead-Redlich 2/3/4: The Hollingshead and Redlich system defines occupational categories 0 (not working), 1, 2, 3, 4 (menial labor, unskilled labor; semiskilled labor, skilled labor), ODD: oppositional defiant disorder
Table 2. Comparison of the oxidative stress parameters among groups.
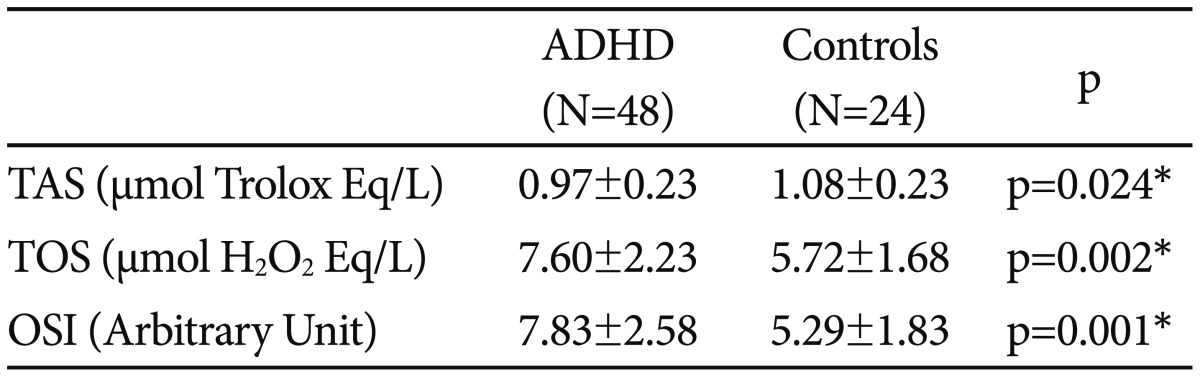
Variables were expressed as mean±standard deviations. *statistically significant, p<0.05. ADHD: attention deficit hyperactivity disorder, TAS: total antioxidant status, TOS: total oxidant status, OSI: oxidative stress index
Table 3. Comparison of the oxidative stress parameters among ADHD subtypes.

Variables were expressed as mean±standard deviations. *p<0.05 after Bonferroni correction. TAS: total antioxidant status, TOS: total oxidant status, OSI: oxidative stress index
Patients diagnoses according to ADHD subtype were ADHD-combined (ADHD-C, 58.3%; n=28), ADHD-inattentive (ADHD-IA; 27.1%; n=13), and ADHD-hyperactive and impulsive (ADHD-H/I; 14.6%; n=7). Comorbid ODD was present in 45.8% (n=22), and no comorbidity was present in the remaining (54.2%; n=26) patients. The oxidative values of patients with comorbid ODD (n=22) were compared with those of non-comorbid patients (n=26). TOS levels (F=0.27, p=0.60) and OSI values (F=0.96, p=0.30) were not significantly different between groups; however, TAS was significantly lower in the ADHD group with comorbid ODD than in non-comorbid patients (F=4.46, p=0.04).
DISCUSSION
Measuring different oxidant and antioxidant molecules is impractical, and oxidant and antioxidant effects are additive. Because there are numerous oxidants (e.g., malondialdehyde, lipid hydroperoxide) and antioxidants (e.g., superoxide dismutase, catalase) in the body, measuring total oxidant-antioxidant status is more valid and reliable. When only a few parameters are measured, levels may remain unchanged or decrease, even though the actual oxidant status increased or vice versa.21,22,23 In the light of this information, we used TOS and TAS levels in our study, and this study yielded in three major findings.
First, TOS levels were significantly higher in the ADHD group than in the control group. Few studies have investigated oxidative metabolism in ADHD. Ceylan et al.9 found that nitric oxide, a potent oxidant, and malondialdehyde were higher in pediatric and adolescent patients with ADHD than in healthy subjects. Furthermore, previous studies in patients with adult ADHD (A-ADHD) found higher nitric oxide24 and malondialdehyde8 levels in patients compared with controls. Moreover, Selek et al.11 found that patients with A-ADHD had higher TOS levels than did the control group. Thus, our finding of higher TOS in the ADHD group compared with controls is consistent with previous reports.
Previous investigations of the effect of oxidative stress on cerebral structures found that free radicals exerted adverse effects on brain development by interfering with neuronal cell migration and by inducing mutations in genes critical for brain development.25,26 Moreover, previous studies demonstrated an association between increased oxidative stress and DNA damage in bipolar disorder.27,28 Increased oxidative stress may impair various genes that regulate neurotransmitters, thereby predisposing the brain to ADHD.
Destructive changes in neuronal cell membranes, which are the target of oxidants, may weaken the binding affinity of neurotransmitters such as serotonin, norepinephrine, the opiates, and dopamine and the neurotransmission regulatory mechanism.29,30,31 Increased oxidative stress may interfere with the biological effectiveness of enzymes by causing destructive changes in relevant proteins.25,26 Furthermore, an increase in oxidant levels and impaired oxidative balance may alter the structure and function of neurotransmitters, such as dopamine, contributing to the development of ADHD.10,32
Second, we found that TAS levels in the ADHD group were significantly lower than those of the control group. Several investigations of oxidative metabolism in ADHD have examined molecules with potent antioxidant effects such as glutathion peroxidase, catalase, and superoxide dismutase (SOD). Ceylan et al.9 found decreased glutathion peroxidase enzyme activity in pediatric and adolescent ADHD patients compared with the control group; however, no difference was detected in catalase or SOD enzyme activity. Two studies showed that plasma levels of trace elements such as copper and zinc, which play a role in the antioxidant defense mechanism, were lower in children with ADHD compared with a control group.33,34 Furthermore, Selek et al.24 found that SOD activity in patients with A-ADHD was lower than that in the control group. Thus, our finding that TAS was significantly lower in patients than in controls is consistent with previous studies. However, Selek et al.11 found that TAS values were higher in patients with A-ADHD than in the control group. The authors suggested that the high TAS levels were secondary to an increase in TOS values. The disparity between our results and those of Selek et al. may be explained by disease duration. When differences in patient age and disease duration are taken into account, it appears that an increase in TOS over time may be a significant factor. The low TAS levels observed in the ADHD group may have contributed to the development of the disorder by enhancing the mechanisms that trigger an increase in oxidant levels.
Our third major finding was that the OSI in the ADHD group was significantly higher than that in the controls. OSI is the ratio of TOS to TAS and is a general indicator of oxidative stress. Previous studies in A-ADHD patients with comorbid bipolar disorder found that OSI was significantly higher in the patient compared with the control group.11,35
A comparison of our patients with and without comorbid ODD revealed no intergroup differences in TOS and OSI values. However, TAS levels in the patients with comorbid ODD were lower than those in patients with no comorbidities. This finding indicates that the total oxidant increase in patients with ADHD is independent of comorbid ODD, despite its adverse impact on the antioxidant defense mechanism. Additionally, our study compared oxidative parameters among the ADHD subtypes. TOS levels were similar among all subtypes. TAS levels were similar in the ADHD-C and ADHD-H/I subtypes but were higher in the ADHD-IA subtype. Similarly, OSI values were not significantly different between the ADHD-C and ADHD-H/I subtypes. OSI values were lower in the ADHD-IA subtype compared with the other subtypes. In conclusion, the ADHD-C and ADHD-H/I subtypes did not show excessive oxidant levels, and the oxidant levels were similar across ADHD subtypes. Additionally, it is noteworthy that the TAS levels in ADHD-C and ADHD-H/I subtypes were lower than those in the ADHD-IA subtype. This finding is novel; we could not compare our results with those of previous studies, because in literature comparison of oxidative stress parameters among ADHD subtypes have not been analyzed yet. Although not definitively; several factors such as environmental, biological variables, or unknown factors may contribute to development of this condition. Nonetheless, impairment of the antioxidant defense mechanisms in the ADHD-H/I compared with the ADHD-IA subtype should be considered in prospective studies.
The generalizability of our findings is limited by the fact that a cross-sectional design, and was conducted in a tertiary healthcare institution with a relative small sample size. However, the strengths include use of the K-SADS-PL to exclude patients with comorbidities other than ODD, WISC-R assessments to exclude patients with intellectual disabilities, and the fact that participants were not drug users.
The results of the present study reveal an increase in oxidant and a decrease in antioxidant levels in pediatric and adolescent patients with ADHD. These findings indicate that oxidative balance is disrupted in patients with ADHD, causing increased oxidative stress. Although our focus was to identify mechanisms underlying the etiology of the ADHD, our findings may be useful for the development of novel ADHD therapies. Future studies with larger sample sizes are needed to confirm our results and identify the molecules that mediate impaired oxidative metabolism.
References
- 1.Lee SY, Lee SJ, Han C, Patkar AA, Masand PS, Pae CU. Oxidative/nitrosative stress and antidepressants: targets for novel antidepressants. Prog Neuropsychopharmacol Biol Psychiatry. 2013;46:224–235. doi: 10.1016/j.pnpbp.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 2.Pae CU, Paik IH, Lee C, Lee SJ, Kim JJ, Lee CU. Decreased plasma antioxidants in schizophrenia. Neuropsychobiology. 2004;50:54–56. doi: 10.1159/000077942. [DOI] [PubMed] [Google Scholar]
- 3.Pae CU, Yu HS, Kim JJ, Lee CU, Lee SJ, Jun TY, et al. Quinone oxidoreductase (NQO1) gene polymorphism (609C/T) may be associated with tardive dyskinesia, but not with the development of schizophrenia. Int J Neuropsychopharmacol. 2004;7:495–500. doi: 10.1017/S1461145704004419. [DOI] [PubMed] [Google Scholar]
- 4.Haddad JJ. Oxygen sensing and oxidant/redox-related pathways. Biochem Biophys Res Commun. 2004;316:969–977. doi: 10.1016/j.bbrc.2004.02.162. [DOI] [PubMed] [Google Scholar]
- 5.Joseph N, Zhang-James Y, Perl A, Faraone SV. Oxidative stress and ADHD: a meta-analysis. J Atten Disord. 2013 doi: 10.1177/1087054713510354. Inpress. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karababa IF, Savas SN, Selek S, Cicek E, Cicek EI, Asoglu M, et al. Homocysteine Levels and Oxidative Stress Parameters in Patients With Adult ADHD. J Atten Disord. 2014 doi: 10.1177/1087054714538657. Inpress. [DOI] [PubMed] [Google Scholar]
- 7.Archana E, Pai P, Prabhu BK, Shenoy RP, Prabhu K, Rao A. Altered biochemical parameters in saliva of pediatric attention deficit hyperactivity disorder. Neurochem Res. 2012;37:330–334. doi: 10.1007/s11064-011-0616-x. [DOI] [PubMed] [Google Scholar]
- 8.Bulut M, Selek S, Gergerlioglu HS, Savas HA, Yilmaz HR, Yuce M, et al. Malondialdehyde levels in adult attention-deficit hyperactivity disorder. J Psychiatry Neurosci. 2007;32:435–438. [PMC free article] [PubMed] [Google Scholar]
- 9.Ceylan M, Sener S, Bayraktar AC, Kavutcu M. Oxidative imbalance in child and adolescent patients with attention-deficit/hyperactivity disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:1491–1494. doi: 10.1016/j.pnpbp.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 10.Ceylan MF, Sener S, Bayraktar AC, Kavutcu M. Changes in oxidative stress and cellular immunity serum markers in attention-deficit/hyperactivity disorder. Psychiatry Clin Neurosci. 2012;66:220–226. doi: 10.1111/j.1440-1819.2012.02330.x. [DOI] [PubMed] [Google Scholar]
- 11.Selek S, Bulut M, Ocak AR, Kalenderoğlu A, Savaş HA. Evaluation of total oxidative status in adult attention deficit hyperactivity disorder and its diagnostic implications. J Psychiatr Res. 2012;46:451–455. doi: 10.1016/j.jpsychires.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 12.Oztop D, Altun H, Baskol G, Ozsoy S. Oxidative stress in children with attention deficit hyperactivity disorder. Clin Biochem. 2012;45:745–748. doi: 10.1016/j.clinbiochem.2012.03.027. [DOI] [PubMed] [Google Scholar]
- 13.Hollingshead AB, Redlich FC. Social mobility and mental illness. Am J Psychiatry. 1955;112:179–185. doi: 10.1176/ajp.112.3.179. [DOI] [PubMed] [Google Scholar]
- 14.Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. Schedule for afectivedisorders and schizophrenia for school-age childrenpresent and lifetime version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 15.Gökler B, Ünal F, Pehlivantürk B, Kultur EC, Akdemir D, Taner Y. Schedule for affective disorders and schizophrenia for school-age childrenpresent and lifetime version-the validity and reliability of adaptation in Turkish. Turk J Child Adolesc Ment Health. 2004;11:109–116. [Google Scholar]
- 16.Goyette CH, Conners CK, Ulrich RF. Normative data on revised Conners Parent and Teacher Rating Scales. J Abnorm Child Psychol. 1978;6:221–236. doi: 10.1007/BF00919127. [DOI] [PubMed] [Google Scholar]
- 17.Dereboy C, Senol S, Sener S, Dereboy F. Validation of the Turkish versions of the short-form Conners' teacher and parent rating scales. Turk Psikiyatri Derg. 2007;18:48–58. [PubMed] [Google Scholar]
- 18.Turgay A. Disruptive Behavior Disorders Child and Adolescent Screening and Rating Scale for Children, Adolescents, Parents, and Teachers. West Blomfield, MI: Integrative Therapy Institute Publication; 1994. [Google Scholar]
- 19.Ercan ES, Amado S, Somer O, Cıkoglu S. Development of a test battery for the assessment of attention deficit hyperactivity disorder. Turk J Child Adolesc Ment Health. 2001;8:132–144. [Google Scholar]
- 20.Wechsler D. WISC-R, Manual for the Wechsler Intelligence Scale for Children-Revised. New York: Psychological Corporation; 1974. [Google Scholar]
- 21.Ulas T, Buyukhatipoglu H, Kirhan I, Dal MS, Ulas S, Demir ME, et al. Evaluation of oxidative stress parameters and metabolic activities of nurses working day and night shifts. Rev Esc Enferm USP. 2013;47:471–476. doi: 10.1590/s0080-62342013000200028. [DOI] [PubMed] [Google Scholar]
- 22.Ulas T, Buyukhatipoglu H, Kirhan I, Dal MS, Eren MA, Hazar A, et al. The effect of day and night shifts on oxidative stress and anxiety symptoms of the nurses. Eur Rev Med Pharmacol Sci. 2012;16:594–599. [PubMed] [Google Scholar]
- 23.Ulas T, Tursun I, Demir ME, Dal MS, Buyukhatipoglu H. Comment on: infusion of lin-/sca-1+ and endothelial progenitor cells improves proinflammatory and oxidative stress markers in atherosclerotic mice. Int J Cardiol. 2013;164:128. doi: 10.1016/j.ijcard.2012.06.077. [DOI] [PubMed] [Google Scholar]
- 24.Selek S, Savas HA, Gergerlioglu HS, Bulut M, Yılmaz HR. Oxidative imbalance in adult attention deficit/hyperactivity disorder. Biol Psychol. 2008;79:256–259. doi: 10.1016/j.biopsycho.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 25.Mahadik SP, Mukherjee S. Free radical pathology and antioxidant defense in schizophrenia:a review. Schizophr Res. 1996;19:1–17. doi: 10.1016/0920-9964(95)00049-6. [DOI] [PubMed] [Google Scholar]
- 26.Mukerjee S, Mahadik SP, Scheffer R, Correnti EE, Kelkar H. Impaired antioxidant defense at the onset of psychosis. Schizophr Res. 1996;19:19–26. doi: 10.1016/0920-9964(95)00048-8. [DOI] [PubMed] [Google Scholar]
- 27.Andreazza AC, Frey BN, Erdtmann B, Salvador M, Rombaldi F, Santin A, et al. DNA damage in bipolar disorder. Psychiatry Res. 2007;153:27–32. doi: 10.1016/j.psychres.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 28.Frey BN, Andreazza AC, Kunz M, Gomes FA, Quevedo J, Salvador M, et al. Increased oxidative stress and DNA damage in bipolar disorder: a twin-case report. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:283–285. doi: 10.1016/j.pnpbp.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 29.Reddy RD, Yao JK. Free radical pathology in schizophrenia: a review. Prostaglandins Leukot Essent Fatty Acids. 1996;55:33–43. doi: 10.1016/s0952-3278(96)90143-x. [DOI] [PubMed] [Google Scholar]
- 30.Yorbık O, Sayal A, Akay C, Akbıyık DI, Sohmen T. Investigation of antioxidant enzymes in children with autistic disorder. Prostaglandins Leukot Essent Fatty Acids. 2002;67:341–343. doi: 10.1054/plef.2002.0439. [DOI] [PubMed] [Google Scholar]
- 31.Kandemir H, Abuhandan M, Aksoy N, Savik E, Kaya C. Oxidative imbalance in child and adolescent patients with obsessive compulsive disorder. J Psychiatr Res. 2013;47:1831–1834. doi: 10.1016/j.jpsychires.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 32.Swanson JM, Kinsbourne M, Nigg J, Lanphear B, Stefanatos GA, Volkow N, et al. Etiologic subtypes of attention-deficit/hyperactivity disorder: Brain imaging, molecular genetic and environmental factors and the dopamine hypothesis. Neuropsychol Rev. 2007;17:39–59. doi: 10.1007/s11065-007-9019-9. [DOI] [PubMed] [Google Scholar]
- 33.Yorbık Ö, Olgun A, Kırmızıgül P, Akman Ş. Plasma copper and zinc levels in male childrens with Attention Deficit Hiperactivity Disorder. Turk J Psychiatry. 2004;7:80–84. [Google Scholar]
- 34.Starobrat-Hermelin B. The effect of deficiency of selected bioelements on hyperactivity in children with certain specified mental disorders. Ann Acad Med Stetin. 1998;44:297–314. [PubMed] [Google Scholar]
- 35.Yumru M, Savaş HA, Kalenderoğlu A, Bulut M, Çelik H, Erel O. Oxidative imbalance in bipolar disorder subtypes: a comparative study. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1070–1074. doi: 10.1016/j.pnpbp.2009.06.005. [DOI] [PubMed] [Google Scholar]


