Abstract
Context and Objective:
The histrelin implant has proven to be an effective method of delivering GnRH analog (GnRHa) therapy to children with central precocious puberty (CPP), yet there are limited data available regarding hormonal suppression and auxological changes during an extended course of therapy.
Design:
This was a phase 3, prospective, open-label study.
Setting and Participants:
Thirty-six children with CPP who participated in a phase 3, open-label study and required further GnRHa therapy were eligible to continue treatment receiving a new implant upon removal of the prior 12-month histrelin implant during a long-term extension phase.
Outcome Measures:
Hormone levels and auxologic parameters were measured periodically for up to 6 years of treatment and up to 1 year of posttreatment follow-up.
Results:
Hormonal suppression was maintained throughout the study in patients who had prior GnRHa therapy (n = 16) and in treatment-naive patients (n = 20). Bone age to chronological age ratio decreased from 1.417 (n = 20) at baseline to 1.18 (n = 8) at 48 months in treatment-naive children (P < .01). Predicted adult height in girls increased from 151.9 cm at baseline to 166.5 cm at month 60 (n = 6; P < .05), with a 10.7-cm height gain observed among treatment-naive children (n = 5). No adverse effect on growth or recovery of the hypothalamic-pituitary-gonadal axis was observed with hormonal suppression. The histrelin implant was generally well tolerated during long-term therapy.
Conclusions:
Long-term histrelin implant therapy provided sustained gonadotropin suppression safely and effectively and improved predicted adult height in children with CPP.
Physical and hormonal changes associated with central precocious puberty (CPP) result from premature reactivation of the hypothalamic-pituitary-gonadal (HPG) axis, which is normally dormant during childhood. If left untreated, CPP can have a demonstrable effect on children. The advancement in bone age relative to chronological age induced by CPP results in early epiphyseal fusion, thereby limiting statural growth and reducing full adult-height potential. The effect of CPP on adult height is one of the key reasons for initiating medical therapy (1). Another factor in the decision to treat is the potential psychosocial ramifications that accompany advancement through puberty earlier than peers, including social anxiety, early sexual activity, and increased risk for substance abuse that have been seen in epidemiological studies in the normal population (1–3). However, there are only limited data on the psychosocial effects of CPP and the benefits of treatment (4, 5). The epidemiology of CPP has not been well characterized, but the prevalence is generally estimated to be in the range of 1 in 5000–10 000 children, with a higher prevalence in girls (6).
For more than 20 years, treatment with GnRH analogs (GnRHa) has been the cornerstone of CPP management, suppressing pubertal development and slowing bone age advancement to improve adult height attainment and better align physical development with chronological age and emotional maturity. Depot formulations are preferred over short-acting formulations on the basis of improved patient adherence with therapy (1) and because of improved suppression of the HPG axis by the continuous presence of GnRHa in depot formulations (7). Depot preparations of the GnRHa, leuprolide, triptorelin, and goserelin, may be administered every 1–3 months (8); however, leuprolide for im injection (Lupron Depot; AbbVie Inc) is the only depot GnRHa indicated for the treatment of CPP in the United States. Adherence rates between once-monthly and multimonthly GnRHa administration for CPP management have not been compared. Depot formulations are generally well tolerated, although injections may be painful and site reactions (occasionally manifesting as sterile abscesses) have been reported in approximately 10%–15% of patients (8–10). Another long-acting formulation for GnRHa therapy is the once-yearly sc histrelin 50-mg implant (Supprelin LA; Endo Pharmaceuticals Inc). A minor surgical procedure is required for sc implantation, and implantation site reactions have been reported as the most common adverse event (AE). There is also risk for infection and, rarely, spontaneous implant extrusion (11). The histrelin implant delivers a therapeutic dose for at least 12 months (12–15), with one study suggesting a duration of action of at least 2 years (16), allowing for flexibility in scheduling office visits, and effectively suppressing the HPG axis in patients with CPP throughout the treatment interval.
Children with CPP may require extended therapy, often for 3 or more years, to reach an age at which the patient, family, and practitioner agree that puberty should resume (15, 17, 18). Although multiple studies have evaluated long-term HPG axis suppression during treatment with a 1-month depot leuprolide formulation (15, 18–21), there is a paucity of data assessing the ability of multimonth depot formulations to maintain pubertal suppression over multiyear therapy. To date, there are no published reports involving the 3-month depot leuprolide formulation beyond 2–3 years (22). Clinical data regarding long-term therapy with the histrelin implant are also limited (23–25). Results from a phase 3, open-label study of the histrelin implant demonstrated continuous suppression of the HPG axis for 1 year (13), and an extension period of that trial demonstrated continuous suppression through 2 years of therapy, after an annual explantation and implantation (14). However, there have been no detailed reports on the suppression of the HPG axis with once-yearly histrelin therapy beyond 2 years. In addition, there have been only three reports on the recovery of the HPG axis after once-yearly histrelin therapy was discontinued, two of which were in the same population of girls (26–28). Herein we provide full presentation on an open-label phase 3 multicenter histrelin study, including an extended access phase of up to 6 years and a posttreatment follow-up phase of up to 12 months.
Materials and Methods
Patients
This study included girls aged 2–8 years and boys aged 2–9 years who had evidence of CPP and who had not previously received GnRHa therapy and girls aged 2–10 years and boys aged 2–11 years who had received prior treatment with a standard GnRHa regimen for at least 6 months. Participants were required to have pretreatment bone-age advancement of 2 or more SDs based on their chronological age and demonstrate a pubertal luteinizing hormone (LH) response to GnRHa challenge (peak serum LH concentration of > 7 mIU/mL after gonadarelin or > 10 mIU/mL after leuprolide acetate) at screening. Breast development rated as Tanner stage 2 or greater was necessary for enrollment of girls and testicular volume of 4 cc or greater was obligatory for boys. Children for whom discontinuation of GnRHa therapy was planned within 1 year or who had comorbid conditions that would interfere with study participation were not eligible for enrollment. Written informed consent was provided by the patient's parent or guardian before any procedures were performed, and written assent was obtained from any patient 7 years of age or older. Data from year 1 and year 2 of this study have been previously published (13, 14).
Study design and treatment
The data presented herein are from a long-term extension of a phase 3, prospective, open-label study in which all patients received a single histrelin 50-mg sc implant and were monitored over the course of 12 months. Patients for whom continued treatment was deemed clinically appropriate were eligible to enter the study extension; the previous implant was removed after 12 months and a new implant was placed, during the same procedure, sc in the medial aspect of the upper arm, for up to 6 years. Each implant was designed to provide controlled delivery of histrelin at an average of 65 μg/d over 12 months. Details regarding the histrelin implant and the implantation procedure have been previously described in the year 1 and year 2 reports for this study (13, 14).
Prior to commencement, the study protocol received approval from the institutional review board or independent ethics committee at each study site. Study procedures were conducted in accordance with the Declaration of Helsinki and the principles of good clinical practice.
Assessments
The primary endpoint of LH suppression was measured in response to GnRHa stimulation. Blood samples for LH measurement were collected before sc injection of leuprolide acetate 20 μg/kg and at 30 and 60 minutes after administration at screening and months 1, 3, 6, 9, 12, 13, 18, and 24; at 40 minutes after administration at months 36, 42, 48, 54, 60, 66, and 72; and at 1 and 6 months after the removal of the last implant. Gonadotropin levels were determined via an immunochemiluminescent assay with a lower limit of quantification of 0.02 mIU/mL (13). Secondary end point measurements included FSH, which was assessed in the same manner as LH, and suppression of serum testosterone (T) and estradiol. Serum estradiol levels for girls and serum T levels for boys were evaluated using baseline blood samples before GnRHa stimulation. Intraassay and interassay coefficients of variation ranged from 3.2% to 16% and 6.7% to 9.0%, respectively, for FSH and from 3.4% to 4.7% and 3.8% to 10.7%, respectively, for LH. Estradiol was initially measured using a radioimmunoassay (RIA) but was converted to liquid chromatography-tandem mass spectrometry (LC/MSMS) assessment at approximately the 36-month time point and for all measures thereafter. For consistency between assays, any value less than 18.36 pmol/L detected by LC/MSMS was imputed as 18.36 pmol/L, the lower limit of quantification for RIA. For RIA measures, the intraassay and interassay coefficients of variation for estradiol ranged from 3.0% to 13.1% and 9.2% to 14%, respectively. The intraassay and interassay values for the LC/MSMS assessments ranged from 3.7% to 8.9% and from 6.6% to 11.9%, respectively. T measurement was performed via RIA throughout the study; the lower limit of quantification was 0.1041 nmol/L (13). Intraassay and interassay coefficients of variation for T ranged from 2.9% to 18.8% and 8% to 15%, respectively. All hormone measurements were performed at a central laboratory (Esoterix Clinical Trial Services).
Additional secondary end points included growth velocity and bone advancement measurements. Radiographs of the left hand and wrist for the determination of bone age were obtained every 12 months and were evaluated by a single facility by the same radiologist over the entire study period (Lifespan Health Research Center, Kettering, Ohio). The radiologist was aware that patients had CPP. Bone age assessments were completed using the Fels method (29). Predicted adult height (PAH; determined using the Bayley-Pinneau method average tables) was calculated for each time point. SD scores (SDSs) for height and body mass index (BMI) were calculated using data derived from the 2000 Centers for Disease Control and Prevention growth charts (30). Growth velocity SDSs were calculated using the Growth Calculator, version 2.01, from Children's Hospital in Boston (31).
Observational endpoints included pubertal development, which was routinely assessed during the initial and extension phases of the study using the Tanner stages of development for pubic hair, breast (girls), and genital (boys) development. Absence or presence of menses was recorded as well. Safety end points included occurrence of AEs, results from clinical laboratory test measurements, and findings from physical examinations. Physical examinations complete with height, weight, and vital sign (eg, resting pulse, blood pressure) measurements were conducted at each study visit (screening, the initial implant, and at months 1, 3, 6, 9, 12, 13, 15, 18, 21, 24, 36, 42, 48, 54, 60, 66, and 72 after implantation, and at 1, 6, and 12 months after the explant).
Statistics
Descriptive statistics (eg, mean, median, range, frequency) were calculated for demographic, anthropomorphic, hormonal, and auxological variables. The paired Student t test or Wilcoxon signed rank test (if data were not normally distributed) were used to assess significant differences vs baseline in study parameters. All statistical analyses were performed using SAS version 9.1.3 statistical software (SAS Institute).
Results
Patients
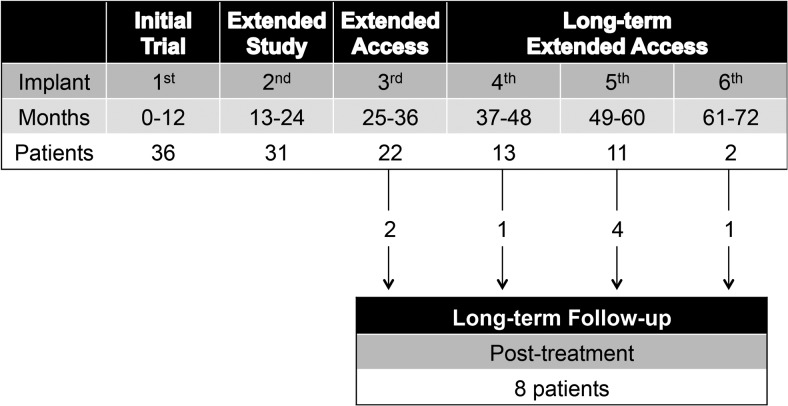
A total of 36 children including 33 girls and three boys were enrolled in the initial trial; 16 patients had received prior GnRHa therapy and 20 patients were naive to GnRHa therapy. Baseline demographic and anthropomorphic characteristics of the children are presented in Table 1. As expected, the mean age at baseline was higher among patients who had received prior GnRHa treatment (8.9 vs 7.1 y). Patient disposition throughout the initial treatment, extension treatment, and posttreatment follow-up phases of the trial are depicted in Figure 1. Patients who completed a phase of treatment and were considered to have completed the full course of therapy (by the individual investigator) were listed as completed. Following the initial end-of-treatment period, 31 patients continued into the initial extension phase, of whom 22 (71%) received at least three implants, and nine patients did not complete the third year due to age inappropriateness and progression into puberty or parental decision. Only one of the three boys enrolled in the study received more than two implants. After removal of their final implant, additional data were collected on eight of nine patients who entered an optional posttreatment follow-up.
Table 1.
Baseline Demographics and Anthropometric Characteristics
| Parameter | Prior GnRHa Treatment (n = 16) | GnRHa Treatment-naïve (n = 20) | All Patients (n = 36) |
|---|---|---|---|
| Age, y | |||
| Mean (SD) | 8.9 (1.47) | 7.1 (1.37) | 7.9 (1.66) |
| Median (range) | 9.1 (5.6–11.6) | 7.5 (4.5–9.1) | 8.1 (4.5–11.6) |
| Sex, n, % | |||
| Female | 13 (81.3) | 20 (100) | 33 (91.7) |
| Male | 3 (18.8) | 0 | 3 (8.3) |
| Height, cm | |||
| Mean (SD) | 143.4 (13.89) | 129.6 (12.9) | 135.7 (14.87) |
| Median (range) | 144.3 (117.3–178.3) | 133.3 (105.9–153.4) | 137.3 (105.9–178.3) |
| Mean (SD) height z-score | 1.66 (1.14) | 1.30 (1.50) | 1.48 (1.36) |
| BMI, kg/m2 | |||
| Mean (SD) | 22.0 (3.31) | 19.5 (3.38) | 20.6 (3.54) |
| Median (range) | 21.2 (16.2–27.0) | 19.1 (13.3–25.2) | 20.3 (13.3–27.0) |
| Mean (SD) BMI percentile | 91 (10) | 85 (18) | 88 (15) |
| Pubic hair Tanner stage (girls) | |||
| Mean | 2.9 | 2.3 | 2.5 |
| Median (range) | 3.0 (1–4) | 2.0 (1–4) | 3.0 (1–4) |
| Breast Tanner stage (girls) | |||
| Mean | 2.9 | 3.3 | 3.1 |
| Median (range) | 3.0 (2–5) | 3.0 (2–4) | 3.0 (2–5) |
| Pubic hair Tanner stage (boys) | |||
| Mean | 2.3 | – | 2.3 |
| Median (range) | 2.0 (2–3) | 2.0 (2–3) | |
| Genital Tanner stage (boys) | |||
| Mean | 2.3 | – | 2.3 |
| Median (range) | 2.0 (2–3) | 2.0 (2–3) |
Figure 1.
Patient disposition throughout the extension treatment phases and posttreatment follow-up phase of the trial. Only patients for whom continued therapy was considered clinically appropriate entered each stage subsequent to the initial treatment phase. Most patients who discontinued after entering a subsequent stage did so because of age appropriateness for entry into puberty or parental decision. One patient did not continue due to weight gain, one patient died, and one patient was lost to follow-up due to study site closing.
Hormone assessments
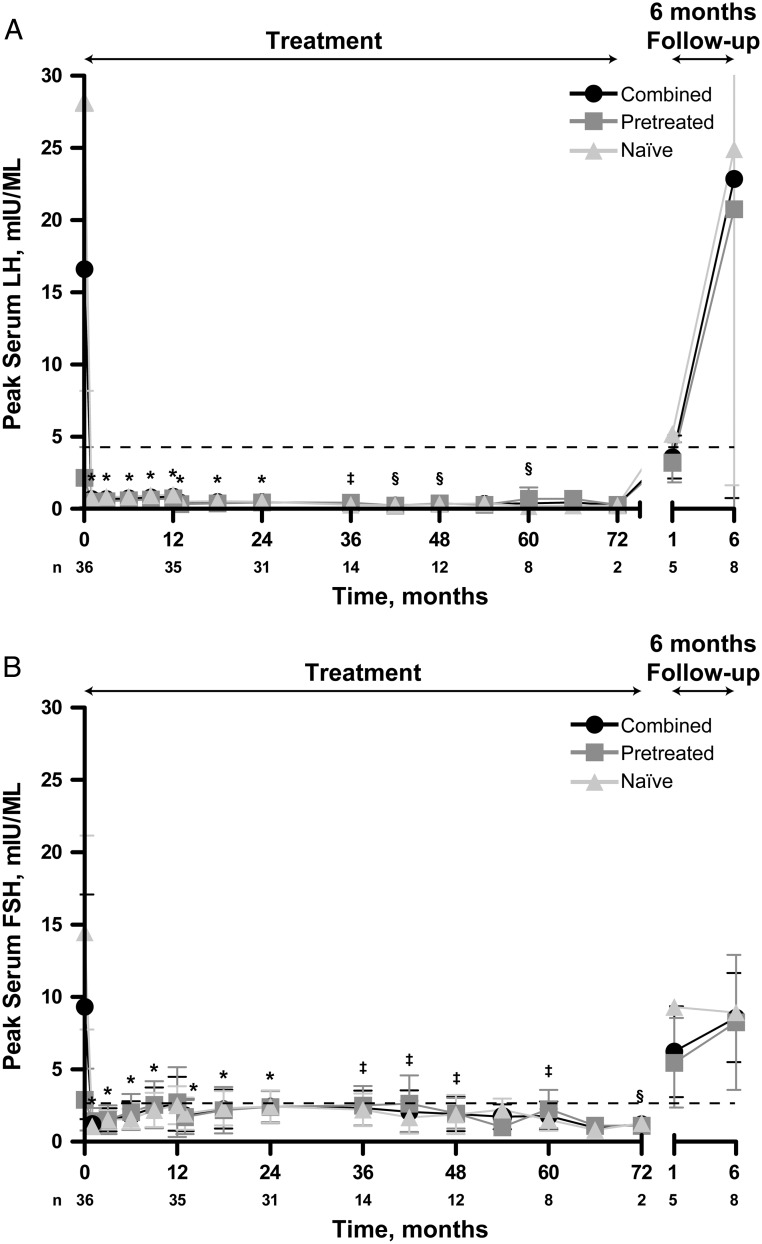
Within 1 month of treatment initiation, all peak stimulated serum LH levels were below the predetermined peak pubertal response of 4 mIU/mL, indicative of HPG axis suppression; stimulated LH remained consistently suppressed throughout the 72-month evaluation period (Figure 2A). During the study, no individual patient had an on-therapy stimulated LH measurement that exceeded 2.3 mIU/mL. Mean changes in peak LH from baseline preimplant levels were statistically significant at each time point from month 1 through month 48 and at month 60 (P < .05). By 6 months after the last histrelin implant was removed, mean peak serum LH concentrations increased to pubertal levels, with similar mean LH values between patients who had received prior GnRHa therapy and patients who were treatment naive.
Figure 2.
Peak stimulated serum LH and FSH levels. LH (A) and FSH (B) levels are mean ± SD. Dashed line represents predetermined pubertal response for peak LH (4 mIU/mL) or peak FSH (2.5 mIU/mL) levels. Patients' hormone levels had to be below this level to be considered suppressed. Histrelin treatment period (treatment) as well as posttreatment follow-up (6-month follow-up) is indicated. Pretreated and naive patients, as well as the two groups combined, are indicated. *, P ≤ .0001; †, P < .001; ‡, P < .01; §, P < .05 combined patient group vs baseline.
Significant reductions from baseline in mean peak stimulated serum FSH levels were evident throughout the extension study (P < .05; Figure 2B). Mean peak FSH concentrations were comparable in the previously treated and treatment-naive subgroups. By 6 months after the final explant, mean peak serum FSH concentrations increased to 8.59 ± 1.09 mIU/mL (n = 8).
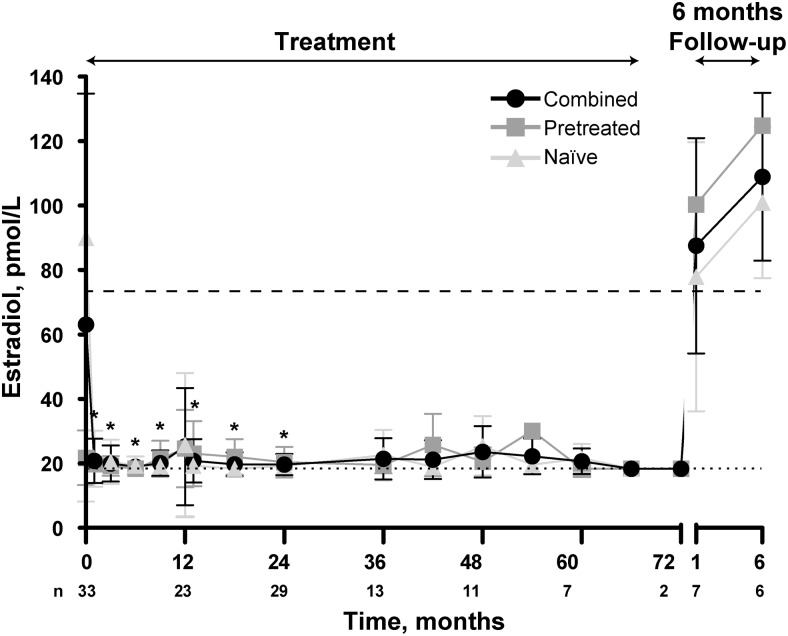
Suppression of mean estradiol concentrations below the predetermined threshold (73.42 pmol/L) was consistent throughout the extension study, regardless of treatment history (Figure 3 and data not shown). Overall, mean estradiol concentrations were 20.67 ± 1.51 pmol/L at month 60 (n = 7) and 18.36 pmol/L for the two remaining patients at month 72. Of note, 79% of the estradiol levels analyzed by RIA and LC/MSMS from month 1 through month 72 were at or below the 18.36 pmol/L threshold of detection for RIA. Estradiol levels analyzed by LC/MSMS (months 36–72) had a mean of 16.56 ± 10.02 pmol/L. By 12 months after explant, mean estradiol levels increased to 109.03 ± 10.65 pmol/L (n = 6). In the follow-up period, the menarche and menses diary data were available for two patients. One girl had her first menstrual cycle of 7 days' duration 9 months after last explant, and her 1-month peak LH was 2.7 mIU/mL. The second girl (treatment naive upon entering the study) reported spotting for 2 days 2 months after the last explant. She had a peak stimulated LH value of 31 mIU/mL at screening. Her peak stimulated LH value 1 month after explant was 5.2 mIU/mL. She reported a 2-day period 13.7 months after her first episode of spotting, with no bleeding in between. In the three boys, T levels were maintained at or below 0.521 nmol/L during treatment, half of the predetermined suppression level of 1.041 nmol/L, as described previously (13, 14). Values of 0.253 nmol/L at month 48 and 0.243 nmol/L at month 60 were measured in the one boy who continued treatment beyond 24 months. At 12 months after explant, T levels for this boy increased to 4.511 nmol/L.
Figure 3.
Serum estradiol in girls. Data are mean ± SD. Dashed line represents predetermined suppression levels of 73.42 pmol/L. Patients' hormone levels had to be below these to be considered suppressed. Histrelin treatment period (treatment) as well as posttreatment follow-up (6- and 12-month follow-up) is indicated. Pretreated and naive patients, as well as the two groups combined, are indicated. Dotted lines represent the lower level of quantification (18.36 pmol/L) of the RIA. *, P < .01, combined patient group vs baseline.
Puberty assessments
Overall, physical evidence of continued pubertal development while patients were undergoing therapy was minimal. Median Tanner breast stage was similar at month 60 compared with baseline in treatment-naive female patients [3.0 (range 1–3) at month 60 compared with 3.0 (range, 2–4) at baseline] but lower in previously treated female patients [2.0 (range 1–3) at month 60 compared with 3.0 (range, 2–5) at baseline]. Median pubic hair Tanner stage increased from 2.0 (range 1–4) at baseline to 3.5 (range 1–4) at 60 months among girls who were treatment naive at baseline. Little evidence of further maturation was observed for girls who had been previously treated with GnRHa therapy (median pubic hair Tanner stage of 3.0 at baseline and 2.5 at month 60). Changes from baseline in Tanner pubic hair and testes staging were minimal for the one boy who continued histrelin implant therapy for 60 months. Dehydroepiandrosterone values increased or remained steady throughout the study.
Auxology assessments
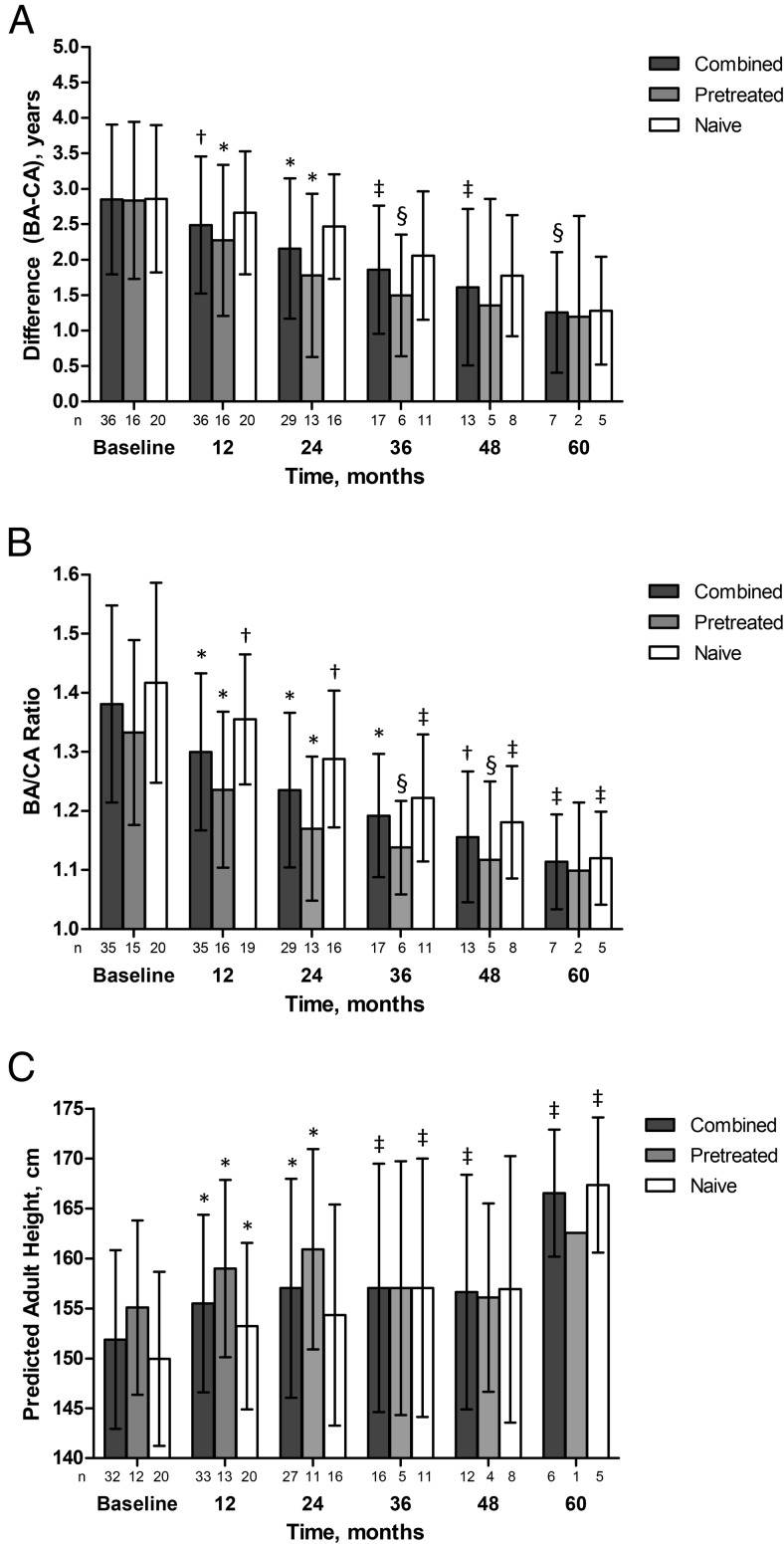
Mean values for bone age minus chronological age decreased throughout the study (Figure 4A), decreasing from approximately 2.85 years at baseline across all groups to 1.28, 1.20, and 1.26, respectively in the treatment-naive, pretreated, and combined groups at month 60. Changes from baseline were significant from month 12 through 36 in pretreated patients (P < .02). Although changes from baseline were not significant for treatment-naïve patients, there was a trend (P < .10) at 36–60 months that may not have reached significance due to the small number of patients. For the combined group, changes were significant from month 12 through 60 (P < .05) Mean bone age to chronological age ratio also decreased throughout the study (Figure 4B). Changes were statistically significant (P < .05) in all groups when compared with baseline at months 12, 24, 36, and 48. In addition, there were several significant decreases in the bone age/chronological age ratio between years of treatment in the group combining both pretreated and treatment-naive patients, including month 12 compared with baseline (P < .0001), the change from month 12 to month 24 (P < .0001), the change from month 24 to month 36 (P < .001), and the change from month 36 to month 48 (P < .01).
Figure 4.
Bone age minus chronological age, bone age to chronological age ratio, and predicted adult height. A, Bone age minus chronological age measurements, and B, Bone age to chronological age ratio measurements. Data are mean ± SD. Pretreated patients, treatment-naive patients, and the two groups combined are indicated. *, P ≤ .0001; †, P < .001; ‡, P < .01; §, P < .05 vs baseline. Baseline bone age was missing for one patient. C, Predicted adult height for girls only. Data are mean ± SD. Histrelin treatment period (treatment) as well as posttreatment follow-up (6 months' follow-up) is indicated. Pretreated patients, treatment-naive patients, and the two groups combined are indicated. *, P < .001; ‡, P < .02; §, P < .05 combined patient group vs baseline. Baseline bone age was missing for one patient.
Mean PAH was determined by excluding boys from the analysis and was significantly greater (P < .05; Figure 4C) compared with baseline at all visits through 60 months of therapy in the combined group. At 60 months, overall mean PAH increased by 10.7 cm compared with baseline among treatment-naive children (n = 5); only one pretreated girl was still on study at month 60. At month 48, mean PAH increased by 7.87 cm from baseline (n = 3) in girls who had received prior GnRHa therapy. Improvements in PAH were maintained in the postexplant follow-up period. PAH for the one pretreated boy who stayed in the study beyond 2 years improved from 169.4 cm at baseline to 173.2 cm at month 60.
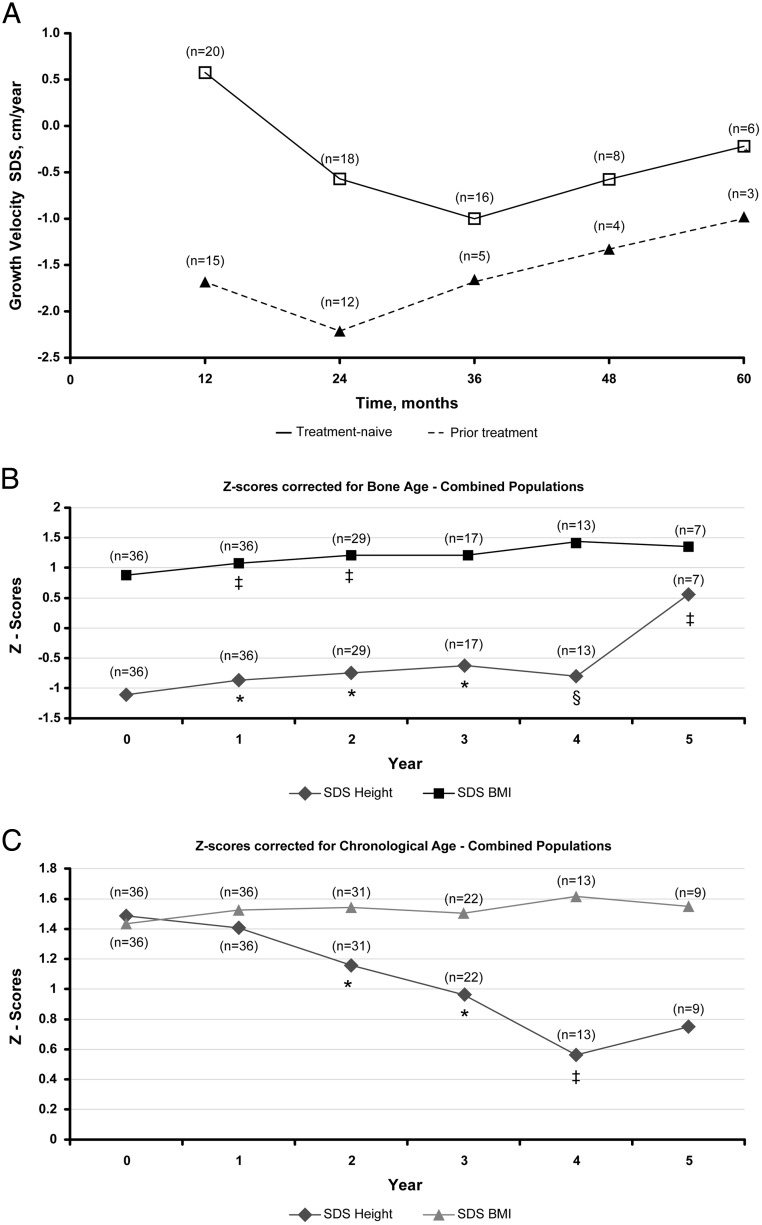
The growth velocity SDSs were higher in the treatment-naive patients at baseline. The growth velocity SDS decreased to normal levels in 2 and 3 years for pretreated and treatment-naive patients, respectively, before trending upward each subsequent year (Figure 5A).
Figure 5.
A, Growth velocity SDSs from baseline up to 5 years. *, P ≤ .0001; ‡, P < .01; §, P < .05 vs baseline. Growth velocity SDSs were based on the Growth Calculator, version 2.01, from Children's Hospital in Boston. B and C, Mean height and BMI SDSs in the combined population adjusted for bone age (B) or chronological age (C). *, P ≤ .01; ‡, P < .02; §, P < .05 vs baseline.
Height and BMI SDS were assessed in the combined population using bone age- or chronological age-corrected scores (Figure 5B and C). In the chronological age-corrected analysis, height SDS generally decreased with significant changes from baseline for years 2–4. For the chronological age-corrected BMI SDS, the minor increases observed did not significantly change from baseline. In the bone age-corrected analysis, changes from baseline generally increased and were significant through year 5 for height SDS; for BMI SDS, changes from baseline gradually increased and were significant in years 1 and 2. Among patients who received prior GnRHa therapy, height SDS corrected for bone age was significantly increased from SDS −0.7 at baseline (23rd percentile) to SDS −0.52 (30th percentile; P < .01) after 24 months of histrelin therapy and increased to SDS 0.16 (56th percentile; P > .05) by month 60. Height SDS corrected for bone age increased significantly from SDS −1.4 (ninth percentile) at baseline to SDS −0.68 (75th percentile) at 60 months in treatment-naive patients. Furthermore, there was a linear correlation between end-of-treatment height SDS with baseline height SDS, regardless of correction for chronological age (R2 = 0.7411) or bone age (R2 = 0.7051), as well as the number of implants each patient received.
Safety
Among all patients, the most frequently occurring treatment-emergent AEs were implant site reactions (ie, pain or discomfort), which were reported by 19 patients (52.8%) over 72 months. The most common implant site reactions that occurred over 72 months included bruising at the implant site (18 reports; 16%), incisional pain (18 reports; 16%), and discomfort at the incision site (11 reports; 10%) as well as other reactions (four or fewer reports; < 4%) including aching, extrusion of suture, soreness, irritation, redness, swelling, scarring, keloids, and a tingling or transient icy feeling. In all cases, implant site reactions were mild to moderate in severity and resolved without sequelae. Throughout the study, implant breakage on implant removal was noted during 25 of 113 explants (22.1%). Implant breakage was noted at least once at every site. Fifteen patients experienced at least one breakage, five of whom had multiple breakages. The most common difficulty noted was encapsulation of implant or presence of scar tissue. Complete removal of implant during the same procedure was achieved in all but two instances. No child experienced extrusion of the implant or infection at the implant site during the 6 years implants were in place.
A few serious AEs were reported throughout the study. During the first year of treatment, a patient experienced amblyopia that was unlikely related to the treatment, and another patient had a benign pituitary tumor, reported as possibly related to the treatment by the investigator. During the extension phase of the study (years 2–6), three patients had at least one serious AE during the course of treatment, all of which were considered to be not related to the study medication: one patient (with a history of bipolar disorder) had depression, two patients experienced aggression, and one patient had apnea and acute bronchopneumonia. One patient died during the extension safety period from acute bronchopneumonia and Dravet syndrome (ie, severe myoclonic epilepsy of infancy). This death was not considered to be related to the study medication.
The majority of patients who chose to not receive a subsequent year of histrelin therapy (92%) were considered age appropriate for discontinuation of therapy and progression into puberty or completed the study. One patient was no longer considered to be age appropriate for the study and was subsequently lost to follow-up after explant due to the study site closing, one patient did not continue because of weight gain, and one patient died, as already described. No other patients required premature discontinuation of therapy.
Discussion
Over the 72-month treatment phase, sequential histrelin implant therapy provided sustained gonadotropin suppression in children with CPP. Suppressed (prepubertal) stimulated serum LH levels were observed within 1 month of treatment initiation and were maintained below the predetermined threshold (<4 mIU/mL) for suppression throughout the entire study duration in all patients. Treatment with the histrelin implant also significantly reduced peak stimulated FSH concentrations from baseline. Six months after final explant, peak LH and FSH levels had increased to pubertal levels in all patients evaluated, demonstrating recovery of the HPG axis after long-term gonadotropin suppression. Estradiol and T were consistently in the prepubertal range over the course of long-term histrelin implant therapy and increased above suppression thresholds in the follow-up period.
Notably, in our study, all patients achieved and maintained prepubertal stimulated LH levels below 2.5 mIU/L within 1 month of treatment. An analysis of the baseline (nonstimulated or random) and GnRHa-stimulated LH levels performed throughout this study revealed that nonstimulated LH values frequently failed to demonstrate suppression to prepubertal values during GnRHa therapy for CPP, despite suppression of 100% of GnRHa-stimulated LH values and otherwise apparent pubertal suppression (32). This finding is consistent with a previous report indicating that random LH levels are not reliable for therapeutic monitoring in children being treated with a histrelin implant for CPP (33).
Auxological results in our study demonstrated a significant increase in mean PAH and a sustained decrease in bone age to chronological age ratio, which indicates movement toward normalization of skeletal maturity relative to patient age, which is consistent with previous reports of depot GnRHa treatment in patients with CPP (10, 19, 34). Gains in mean PAH for the overall population of girls were consistent with previous reports of depot GnRHa treatment in patients with CPP (19–21, 35). Increases were observed in patients who were treatment-naive as well as in those who had received prior GnRHa therapy. As expected, the increase from baseline in PAH was greater for patients who had not received prior therapy compared with those who had a history of GnRHa use because these patients likely already derived some benefit from the 6 months or more of prior GnRHa therapy. However, adult height may be significantly less than PAH at the end of treatment (36). Additionally, naive patients were younger at the start of the study, which correlates with gains in PAH or final height as has been previously described (36). Changes in height SDS in treatment-naive patients followed patterns consistent with other studies of GnRHa therapy in children with CPP (35, 37). Given overall improvements in PAH, sustained decreases in growth velocity SDS relative to baseline indicate a return to, but not below, prepubertal growth velocity norms, rather than an adverse effect on growth velocity from potent HPG axis suppression.
Advantages of using an extended-duration GnRHa formulation include continuous suppression of the HPG axis and flexibility around scheduling medical appointments. Potential disadvantages include the need for repeated implantation, the possibility of device breakage, and the difficulty in removing the device. Emerging data on the extended-duration GnRHa formulations (ie, 3-month depot agents or the histrelin implant) have thus far indicated no adverse consequences of long-term therapy. A recent study did not note any adverse consequences in 11 girls during 6 years of posttreatment follow-up after histrelin therapy and reported an average time to menarche of 9.3 months after the implant removal compared with 16.1 months after depot GnRHa treatment (27). However, at least some of this difference is attributable to rapid systemic clearance of histrelin after explant vs slow clearance after the last injection of a slowly released GnRHa depot preparation. Moreover, a recent retrospective analysis that included 36 girls found an average time to menarche after histrelin explantation (12.75 months) comparable with that observed after the depot GnRHa formulations and taking into account the difference in clearance (28).
Before this report, there have been no published reports of data on once-yearly histrelin implants beyond 2 years of therapy. These results indicate that treatment with up to 6 yearly cycles of the histrelin implant may be an option to arrest the progression of CPP. Moreover, as a once-yearly treatment, the histrelin implant offers an alternative to conventional depot formulations. Because implants were replaced at 12-month intervals in this study, we did not collect any data demonstrating duration of action beyond 1 year. In the initial clinical trial of the implant, some patients (n = 6) were treated for 15–18 months and remained suppressed, but the number was deemed too small to comment about extended use (12). However, others have demonstrated duration of action of at least 2 years (16). Use of a single implant for 2 years has the potential to further decrease costs and numbers of surgical procedures in children being treated with this modality. There are no data examining the ease of explant/implant or incidence of implant breakage for use longer than 12 months.
There are several limitations that must be considered when interpreting the results of this study. In terms of study design, the trial was open label and conducted without a comparator arm. Ethical considerations preclude the conduct of placebo-controlled trials in the CPP population, given the established benefit of approved therapies. Second, because a high number of patients discontinued therapy to resume normal pubertal development at different time points during the study, the number of patients decreased over time, thereby reducing the power to detect statistically significant changes from baseline at later time points. Pretreatment in half of the patients and lack of final height data limit the auxological analysis in this study. Additionally, future studies would benefit from more follow-up on the posttreatment time to menses, adult height, and fertility.
Conclusion
Continuous histrelin implant therapy leads to safe and effective suppression of the HPG axis in patients with CPP. Hormonal and physical evaluations confirmed that ongoing pubertal development was halted during treatment, whereas statural growth continued and significant improvement was made in the PAH and bone age to chronological age ratio. Within 6 months of cessation of the histrelin implant therapy, LH and FSH levels increased to pubertal levels, indicating the ability of the HPG axis to recover after long-term gonadotropin suppression. Long-term treatment with histrelin implant therapy was well tolerated. Sequential therapy with once-yearly histrelin implants is a viable option for patients with CPP who are candidates for continuous long-term GnRHa therapy.
Acknowledgments
We thank Lamara D. Shrode, PhD (International Society for Medical Publication Professionals Certified Medical Publication Professional) and Kelly M. Cameron, PhD (The J. B. Ashtin Group, Inc) for assistance in preparing this manuscript for publication based on the authors' input and direction.
We verify that we have met all of the journal's requirements for authorship. The authors received no financial compensation for the development of this manuscript.
This study was registered at ClinicalTrials.gov with the identifier of NCT00779103.
This work was supported by Endo Pharmaceuticals Inc (Malvern, Pennsylvania). Editorial support for the preparation of this manuscript was also provided by Endo Pharmaceuticals Inc.
Disclosure Summary: L.A.S. has received grant/research support from Pfizer and Endo Pharmaceuticals Inc; served as a consultant for Pfizer, AbbVie, and Endo Pharmaceuticals Inc; and has participated in a speakers bureau for Endo Pharmaceuticals Inc. E.K.N. has received grant/research support from Abbott and Endo Pharmaceuticals Inc and has served as a consultant for Endo Pharmaceuticals Inc and Abbott. G.B.K. has received grant/research support funding from Eli Lilly and Endo Pharmaceuticals Inc; served as a consultant for Endo Pharmaceuticals Inc; and has participated in a speakers bureau for Abbott and Endo Pharmaceuticals Inc. S.C. and O.T. are full-time employees of Endo Pharmaceuticals Inc. E.A.E. has received grant/research support funding from Endo Pharmaceuticals Inc and Abbott. K.L. has nothing to declare.
Footnotes
- AE
- adverse event
- BMI
- body mass index
- CPP
- central precocious puberty
- GnRHa
- GnRH analog
- HPG
- hypothalamic-pituitary-gonadal
- LC/MSMS
- liquid chromatography-tandem mass spectrometry
- PAH
- predicted adult height
- SDS
- SD score.
References
- 1. Carel JC, Eugster EA, Rogol A, et al. Consensus statement on the use of gonadotropin-releasing hormone analogs in children. Pediatrics. 2009;123:e752–e762. [DOI] [PubMed] [Google Scholar]
- 2. Downing J, Bellis MA. Early pubertal onset and its relationship with sexual risk taking, substance use and anti-social behaviour: a preliminary cross-sectional study. BMC Public Health. 2009;9:446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Blumenthal H, Leen-Feldner EW, Babson KA, Gahr JL, Trainor CD, Frala JL. Elevated social anxiety among early maturing girls. Dev Psychol. 2011;47:1133–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mul D, Versluis-den Bieman HJ, Slijper FM, Oostdijk W, Waelkens JJ, Drop SL. Psychological assessments before and after treatment of early puberty in adopted children. Acta Paediatr. 2001;90:965–971. [DOI] [PubMed] [Google Scholar]
- 5. Xhrouet-Heinrichs D, Lagrou K, Heinrichs C, et al. Longitudinal study of behavioral and affective patterns in girls with central precocious puberty during long-acting triptorelin therapy. Acta Paediatr. 1997;86:808–815. [DOI] [PubMed] [Google Scholar]
- 6. Partsch CJ, Sippell WG. Treatment of central precocious puberty. Best Pract Res Clin Endocrinol Metab. 2002;16:165–189. [DOI] [PubMed] [Google Scholar]
- 7. Mul D, Hughes IA. The use of GnRH agonists in precocious puberty. Eur J Endocrinol. 2008;159(suppl1):S3–S8. [DOI] [PubMed] [Google Scholar]
- 8. Carel JC, Lahlou N, Jaramillo O, et al. Treatment of central precocious puberty by subcutaneous injections of leuprorelin 3-month depot (11.25 mg). J Clin Endocrinol Metab. 2002;87:4111–4116. [DOI] [PubMed] [Google Scholar]
- 9. Manasco PK, Pescovitz OH, Blizzard RM. Local reactions to depot leuprolide therapy for central precocious puberty. J Pediatr. 1993;123:334–335. [DOI] [PubMed] [Google Scholar]
- 10. Neely EK, Hintz RL, Parker B, et al. Two-year results of treatment with depot leuprolide acetate for central precocious puberty. J Pediatr. 1992;121:634–640. [DOI] [PubMed] [Google Scholar]
- 11. Lewis KA, Eugster EA. Experience with the once-yearly histrelin (GnRHa) subcutaneous implant in the treatment of central precocious puberty. Drug Des Devel Ther. 2009;3:1–5. [PMC free article] [PubMed] [Google Scholar]
- 12. Hirsch HJ, Gillis D, Strich D, et al. The histrelin implant: a novel treatment for central precocious puberty. Pediatrics. 2005;116:e798–e802. [DOI] [PubMed] [Google Scholar]
- 13. Eugster EA, Clarke W, Kletter GB, et al. Efficacy and safety of histrelin subdermal implant in children with central precocious puberty: a multicenter trial. J Clin Endocrinol Metab. 2007;92:1697–1704. [DOI] [PubMed] [Google Scholar]
- 14. Rahhal S, Clarke WL, Kletter GB, et al. Results of a second year of therapy with the 12-month histrelin implant for the treatment of central precocious puberty. Int J Pediatr Endocrinol. 2009;2009:812517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fuqua JS. Treatment and outcomes of precocious puberty: an update. J Clin Endocrinol Metab. 2013;98:2198–2207. [DOI] [PubMed] [Google Scholar]
- 16. Lewis KA, Goldyn AK, West KW, Eugster EA. A single histrelin implant is effective for 2 years for treatment of central precocious puberty. J Pediatr. 2013;163:1214–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Carel JC, Leger J. Clinical practice. Precocious puberty. N Engl J Med. 2008;358:2366–2377. [DOI] [PubMed] [Google Scholar]
- 18. Neely EK, Lee PA, Bloch CA, et al. Leuprolide acetate 1-month depot for central precocious puberty: hormonal suppression and recovery. Int J Pediatr Endocrinol. 2010;2010:398639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Clemons RD, Kappy MS, Stuart TE, Perelman AH, Hoekstra FT. Long-term effectiveness of depot gonadotropin-releasing hormone analogue in the treatment of children with central precocious puberty. Am J Dis Child. 1993;147:653–657. [DOI] [PubMed] [Google Scholar]
- 20. Lee PA, Neely EK, Fuqua J, et al. Efficacy of leuprolide acetate 1-month depot for central precocious puberty (CPP): growth outcomes during a prospective, longitudinal study. Int J Pediatr Endocrinol. 2011;2011:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tanaka T, Niimi H, Matsuo N, et al. Results of long-term follow-up after treatment of central precocious puberty with leuprorelin acetate: evaluation of effectiveness of treatment and recovery of gonadal function. The TAP-144-SR Japanese Study Group on Central Precocious Puberty. J Clin Endocrinol Metab. 2005;90:1371–1376. [DOI] [PubMed] [Google Scholar]
- 22. Fuld K, Chi C, Neely EK. A randomized trial of 1- and 3-month depot leuprolide doses in the treatment of central precocious puberty. J Pediatr. 2011;159:982–987.e981. [DOI] [PubMed] [Google Scholar]
- 23. Liang Y, Wei H, Zhang J, Hou L, Luo X. Efficacy of subcutaneous administration of gonadotropin-releasing hormone agonist on idiopathic central precocious puberty. J Huazhong Univ Sci Technolog Med Sci. 2006;26:558–561. [DOI] [PubMed] [Google Scholar]
- 24. Pucarelli I, Segni M, Ortore M, Arcadi E, Pasquino AM. Effects of combined gonadotropin-releasing hormone agonist and growth hormone therapy on adult height in precocious puberty: a further contribution. J Pediatr Endocrinol Metab. 2003;16:1005–1010. [DOI] [PubMed] [Google Scholar]
- 25. Lee PA, Klein K, Mauras N, Lev-Vaisler T, Bacher P. 36-month treatment experience of two doses of leuprolide acetate 3-month depot for children with central precocious puberty. J Clin Endocrinol Metab. 2014;99:3153–3159. [DOI] [PubMed] [Google Scholar]
- 26. Hirsch HJ, Lahlou N, Gillis D, et al. Free α-subunit is the most sensitive marker of gonadotropin recovery after treatment of central precocious puberty with the histrelin implant. J Clin Endocrinol Metab. 2010;95:2841–2844. [DOI] [PubMed] [Google Scholar]
- 27. Gillis D, Karavani G, Hirsch HJ, Strich D. Time to menarche and final height after histrelin implant treatment for central precocious puberty. J Pediatr. 2013;163:532–536. [DOI] [PubMed] [Google Scholar]
- 28. Fisher MM, Lemay D, Eugster EA. Resumption of puberty in girls and boys following removal of the histrelin implant. J Pediatr. 2014;164:912–916 e911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Roche AF, Chumlea WC, Thissen D. Assessing the Skeletal Maturity of the Hand Wrist: Fels Method. Springfield, IL: Charles C. Thomas Publishing Ltd; 1988. [Google Scholar]
- 30. National Center for Health Statistics. 2000. CDC growth charts: United States. http://www.cdc.gov/growthcharts Accessed December 11, 2014.
- 31. Children's Hospital. Growth Calculator, version 2.01, Children's Hospital, Boston, MA: http://growthcalc.chip.org Accessed December 11, 2014. [Google Scholar]
- 32. Neely EK, Silverman LA, Geffner ME, Danoff TM, Gould E, Thornton PS. Random unstimulated pediatric luteinizing hormone levels are not reliable in the assessment of pubertal suppression during histrelin implant therapy. Int J Pediatr Endocrinol. 2013;2013:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lewis KA, Eugster EA. Random luteinizing hormone often remains pubertal in children treated with the histrelin implant for central precocious puberty. J Pediatr. 2013;162:562–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lee PA, Klein K, Mauras N, et al. Efficacy and safety of leuprolide acetate 3-month depot 11.25 milligrams or 30 milligrams for the treatment of central precocious puberty. J Clin Endocrinol Metab. 2012;97:1572–1580. [DOI] [PubMed] [Google Scholar]
- 35. Lee SJ, Yang EM, Seo JY, Kim CJ. Effects of gonadotropin-releasing hormone agonist therapy on body mass index and height in girls with central precocious puberty. Chonnam Med J. 2012;48:27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Carel JC, Lahlou N, Roger M, Chaussain JL. Precocious puberty and statural growth. Hum Reprod Update. 2004;10:135–147. [DOI] [PubMed] [Google Scholar]
- 37. Poomthavorn P, Suphasit R, Mahachoklertwattana P. Adult height, body mass index and time of menarche of girls with idiopathic central precocious puberty after gonadotropin-releasing hormone analogue treatment. Gynecol Endocrinol. 2011;27:524–528. [DOI] [PubMed] [Google Scholar]