Abstract
We have shown that netrin-1 induces potent cardioprotection via extracellular signal-regulated kinases 1 and 2 (ERK1/2)-dependent endothelial nitric oxide synthase (eNOS)/NO activation. The present study investigated cardioprotective effects of small netrin-1-derived peptides. We synthesized three laminin (Lam) V peptides and found those time dependently increased phosphorylation of ERK1/2 and eNOSs1179 in endothelial cells at the same molar concentration used for netrin-1. Preperfusion with Lam V peptides induced a substantial reduction in infarct size (control 39.3 ± 0.2% vs. 14.6 ± 2.3%, 23.0 ± 2.8%, and 18.8 ± 0.8% for V1, V2, and V3, respectively). Furthermore, reperfusion with all three also induced potent cardioprotection (control 37.6 ± 1.3% vs. 17.6 ± 3.2%, 20.6 ± 1.7%, and 15.8 ± 2.0% for V1, V2, and V3, respectively), implicating that these peptides are consistently beneficial whenever they are delivered to the heart. Based on the sequence alignment, we found a region of high sequence homology and synthesized smaller peptides [V1-9 amino acid (aa), V2-10aa, and V3-11aa]. These smaller peptides markedly reduced infarct size during reperfusion (V1-9aa 16.8 ± 2.2%, V2-10aa 18.6 ± 1.7%, and V3-11aa 16.7 ± 3.0% vs. control 37.6 ± 1.3%). A negative control V3-16aa with no sequence homology failed to protect the heart. Of note, the core area has the characteristic sequence of: Cx(1–2)Cx(3–4)Tx(0–1)G. Furthermore, all three smaller peptides induced NO production in endothelial cells that could in turn diffuse to cardiomyocytes to promote survival. Combined applications of V1-9aa and V2-10aa synergistically induced more NO production. Taken together, these data strongly suggest that small netrin-1-derived peptides are highly effective in protecting the heart against myocardial ischemia-reperfusion injury and has the potential to be developed into peptide drugs directly applicable to the treatment of myocardial infarction.
Keywords: netrin-1-derived small peptides, cardioprotection, ischemia-reperfusion injury, I/R, ERK1/2, endothelial nitric oxide synthase, eNOS
myocardial infarction (MI) occurs when blood cannot flow to part of the heart because of occlusion of coronary arteries, and the heart muscle is injured as a result, which leads to impaired cardiac function (13). The conventional therapy for MI is to restore blood perfusion as soon as possible to salvage some of the myocardium (8). However, reperfusion itself can paradoxically cause myocardial injury, at least in part mediated by increased oxidative stress (3, 9). Until now, no pharmacological agents have been proven clinically effective in reducing reperfusion injury. We have previously shown that a novel protein, netrin-1, can function as a potent cardioprotective agent (5, 20, 21, 26). It induces cardioprotection against ischemia-reperfusion (I/R) injury both ex vivo and in vivo (5, 6, 26), which is mediated by deleted in colorectal cancer (DCC)/extracellular signal-regulated kinases 1 and 2 (ERK1/2)/endothelial nitric oxide synthase (eNOS)/NO-dependent protection of cardiomyocytes from necrosis and apoptosis, through attenuation of oxidative stress and preservation of mitochondrial function (6, 21). Furthermore, netrin-1 can also increase the protein abundance of its receptor DCC through a NO/seven in absentia homolog (SIAH)/DCC feedforward pathway, resulting in augmented cardioprotection (16).
Although we have fully defined its role and mechanisms in cardioprotection (5, 6, 16, 21, 26), netrin-1 is a large protein of ∼68 kDa, which has some potential drawbacks for its utilization. For example, it is more difficult to purify a large protein while preserving its activity, and the cost of production is usually higher for longer proteins or peptides. The complexity in production of a larger protein or peptide may also result in impurities that can cause off-target effects. To solve these problems, we investigated small netrin-1-derived peptides for their efficacies in cardioprotection.
In the present study, we synthesized peptides based on netrin-1 laminin (Lam) V1, V2, and V3 domains and confirmed that they activate the ERK1/2/eNOS signaling pathway as netrin-1 does in endothelial cells. With the use of a Langendorff system to perform global I/R of isolated mouse heart, perfusion of these peptides dramatically reduced infarct size given pre- or postconditionally. Through protein sequence analysis, we further identified the minimal cardioprotective core sequence of nine amino acids: Cx(1–2)Cx(3–4)Tx(0–1)G. Indeed, the smaller peptides containing the core sequence significantly stimulated NO production in endothelial cells, which could in turn diffuse to cardiomyocytes to promote survival. Of note, combined applications of V1-9 amino acid (aa) and V2-10aa smaller peptides synergistically produced more NO. These observations clearly reveal a minimal functional sequence of netrin-1 that is required for cardioprotection, and this core sequence may be used for development of novel peptide drugs for the treatment of MI.1
MATERIALS AND METHODS
Materials.
Purified mouse netrin-1 was purchased from R&D Systems (Minneapolis, MN). Peptide V1 (285–338 amino acid of human netrin-1), V2 (341–401 aa), V3 (404–451 aa), V1-9aa (304–312 aa), V2-10aa (368–377 aa), V3-16aa (407–422 aa), and V3-11aa (423–433 aa) were synthesized by GenicBio Limited (Shanghai, China). Polyclonal antibodies specific for phosphorylated ERK1/2, ERK1/2, and eNOSs1179 were obtained from Cell Signaling Technology (Danvers, MA). Monoclonal antibody for eNOS was purchased from BD Biosciences (San Jose, CA).
Cell culture.
Bovine aortic endothelial cells (BAECs; Cell Systems, Kirkland, WA) were cultured in media 199 containing 10% fetal bovine serum (FBS) as previously described (10, 20). One day postconfluent cells were starved in media containing 5% FBS overnight, stimulated with netrin-1 or different peptides, and harvested at different time points.
Western blotting.
For Western blotting, ∼20–40 μg of proteins were separated by 10% SDS-PAGE, transferred to nitrocellulose membranes, and probed with phosphorylated ERK1/2, ERK1/2, eNOS, and eNOSs1179 (1:1,000) antibodies following standard Western blotting procedure (12).
Langendorff perfusion.
Male C57BL/6 mice (8–12 wk old) were obtained from Charles River Laboratories (Wilmington, MA). After intraperitoneal pentobarbitone (60 mg/kg) anesthesia, mouse hearts were harvested immediately, and the aortas were cannulated with a 20-gauge stainless steel blunt needle, transferred to the Langendorff rig, and perfused retrograde instantly with modified Krebs-Henseleit buffer (KHB) for 30 min as previously described (5, 26). Next, hearts were preperfused for 45 min with or without netrin-1 (100 ng/ml) or different peptides at the same molar concentration of 1.47 nmol/l as netrin-1, before being subjected to I/R injury (20 min global ischemia followed by 60 min reperfusion with or without netrin-1 or different peptides). Hearts were then harvested for analyses of infarct size. For postconditioning treatment with peptides, hearts underwent 40 min of KHB perfusion, 20 min of global ischemia, and 60 min of reperfusion with different peptides. The use of animals and experimental procedures were approved by the Institutional Animal Care and Usage Committee at the University of California Los Angeles.
Infarct size analysis.
At the end of the I/R protocol, hearts were sliced perpendicular to the long axis of the heart at 1-mm intervals and stained with 1% triphenyl tetrazolium chloride (TTC) in PBS for 10 min at room temperature. After being washed with PBS one time, sections of the hearts were fixed in 10% formalin overnight. The heart slices were then digitally photographed for planimetry using National Institutes of Health Image 1.62. Infarct size is expressed as an infarct-to-risk zone ratio (the risk zone is the whole ventricular volume in this global ischemic model).
Electron spin resonance detection of NO radical.
Bioavailable NO radical in endothelial cells was detected by using electron spin resonance (ESR) as previously described (20). In brief, endothelial cells were rinsed with modified Krebs/HEPES buffer and incubated with freshly prepared NO-specific spin trap Fe2+(DETC)2 colloid (0.5 mmol/l) for 60 min. Gently collected cell suspensions were snap-frozen in liquid nitrogen and loaded in a finger Dewar for analysis with an ESR spectrophotometer (eScan; Bruker) at the following settings: Bio-field, 3,267; field sweep, 100 G; microwave frequency, 9.78 GHz; microwave power, 40 mW; modulation amplitude, 10 G; 4,096 points resolution; and receiver gain, 900.
Statistical analysis.
Densitometric data of Western blotting were obtained by Image J software. Grouped data were analyzed by Gradpad Prism 6 software. All values are expressed as means ± SE. Comparisons of more than two groups were performed using a one-way ANOVA with Newman-Keuls test as a post hoc test. Statistical significance is set as P < 0.05.
RESULTS
Netrin-1 induces phosphorylation of ERK1/2 and eNOSs1179.
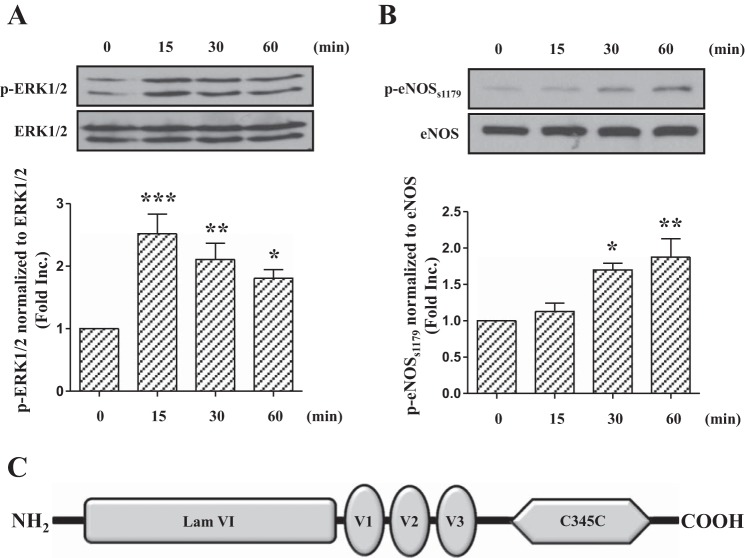
We have previously shown that netrin-1 functions as a potent angiogenic stimulator via DCC-dependent activation of the ERK1/2-eNOS pathway (20). In addition, we have also characterized netrin-1 as a robust cardioprotective agent that attenuates I/R-induced MI via a DCC/ERK1/2/eNOS/NO/DCC feedforward signaling mechanism (26). To identify the minimal functional peptide that could simulate netrin-1 to activate its receptor DCC in endothelial cells to activate downstream signaling events such as eNOS phosphorylation, we first examined the effect of netrin-1 on phosphorylation of ERK1/2 and eNOSs1179 (s1179 for bovine) in endothelial cells. As shown in Fig. 1A, exposure of BAECs to netrin-1 (100 ng/ml) resulted in a consistent increase in ERK1/2 phosphorylation that maximized at 15 min (2.52 ± 0.31-fold vs. 0 min, P < 0.001). As shown in Fig. 1B, eNOSs1179 phosphorylation was time dependently upregulated by netrin-1, maximizing at 30 (1.70 ± 0.09-fold vs. 0 min, P < 0.05) and 60 (1.88 ± 0.25-fold vs. 0 min, P < 0.01) min. These data implicate that netrin-1 can induce ERK1/2 and eNOSs1179 phosphorylation in endothelial cells.
Fig. 1.
Netrin-1 induces phosphorylation of extracellular signal-regulated kinases 1 and 2 (ERK1/2) and endothelial nitric oxide synthase at Ser1179(eNOSs1179). Bovine aortic endothelial cells were stimulated by netrin-1 (100 ng/ml, molar concentration 1.47 nmol/l) and harvested at different time points (0, 15, 30, and 60 min). Western blot analysis was performed to detect phosphorylated ERK1/2, ERK1/2, phosphorylated eNOS at Ser1179, and eNOS levels. A: representative Western blots and grouped densitometric data of ERK1/2 phosphorylation (means ± SE, n = 5). B: representative Western blots and grouped densitometric data of eNOSs1179 phosphorylation (means ± SE, n = 4). ***P < 0.001, **P < 0.01, and *P < 0.05 vs. 0 min. C: structural composition of the netrin-1 protein, including a laminin VI-like domain, three repeats of laminin V-like domains (V1, V2, and V3), and a COOH-terminal C345C domain.
Peptide V1, V2, or V3 induces phosphorylation of ERK1/2 and eNOSs1179.
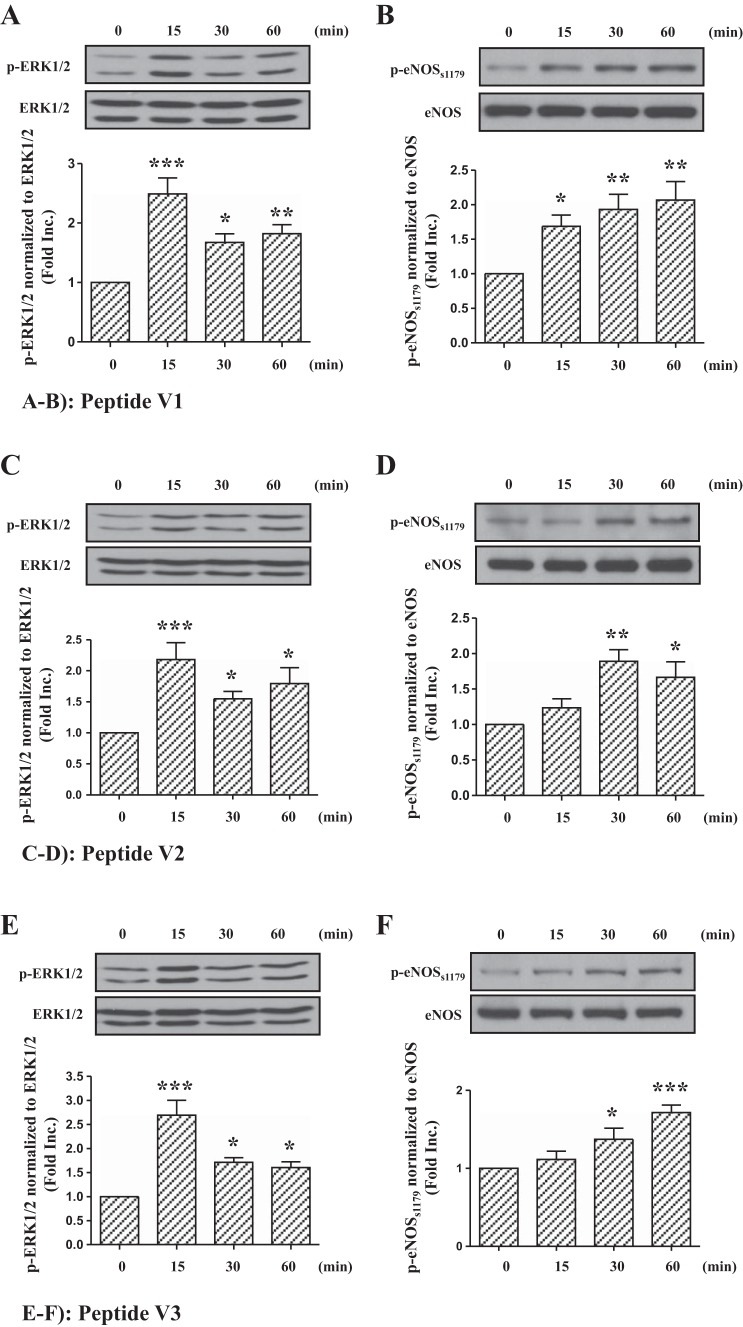
As shown in Fig. 1C, netrin-1 has a laminin VI like (named Lam VI) domain, three cysteine-rich repeats similar to those of domain V of laminin subunits (named Lam V, including V1, V2, and V3), and a COOH-terminal domain (named C345C) with homology to complement factors C3, C4, and C5 (15, 17). Previous results have shown that Lam VI and V domains were involved in the association with its receptor DCC (15). Moreover, another group confirmed that Lam VI, V2, and V3 domains were required primarily for the dorsal axon guidance activities of netrin-1 homolog UNC-6 in Caenorhabditis elegans (17). Therefore, these three domains might contain the core element required for netrin-1-induced cardioprotection. We synthesized peptides V1 (residues 285–338), V2 (residues 341–401), and V3 (residues 404–451) and treated BAECs at the same molar concentration (1.47 nmol/l) as used for netrin-1 (100 ng/ml). As shown in Fig. 2, peptide V1, V2, or V3 could activate the phosphorylation of ERK1/2 in endothelial cells, and the response maximized at 15 min (V1: 2.47 ± 0.27-fold vs. 0 min, P < 0.001; V2: 2.18 ± 0.27-fold vs. 0 min, P < 0.001; and V3: 2.69 ± 0.31-fold vs. 0 min, P < 0.001), which was similar to the response to netrin-1 (Fig. 2, A, C, and E). Furthermore, these peptides also significantly induced phosphorylation of eNOSs1179 at 30 (V1 1.93 ± 0.22-fold vs. 0 min, P < 0.01; V2 1.89 ± 0.16-fold vs. 0 min, P < 0.01; and V3 1.37 ± 0.14-fold vs. 0 min, P < 0.05) and 60 (V1 2.07 ± 0.24-fold vs. 0 min, P < 0.01; V2 1.67 ± 0.22-fold vs. 0 min, P < 0.05; and V3 1.72 ± 0.10-fold vs. 0 min, P < 0.001) (Fig. 2, B, D, and F) min. Taken together, these data strongly suggest that V1, V2, or V3 peptide can activate the netrin-1 responsive, downstream signaling pathway that is required for cardioprotection.
Fig. 2.
Netrin-1 peptide V1, V2, or V3 induces phosphorylation of ERK1/2 and eNOSs1179. Bovine aortic endothelial cells were stimulated by netrin-1 peptide V1, V2, or V3 at the same molar concentration of 1.47 nmol/l and harvested at different time points (0, 15, 30, and 60 min). Western blot analysis was performed to detect phosphorylated ERK1/2, ERK1/2, phosphorylated eNOS at Ser1179, and eNOS levels. A: representative Western blots and grouped densitometric data of ERK1/2 phosphorylation induced by peptide V1 (means ± SE, n = 5). B: representative Western blots and grouped densitometric data of eNOSs1179 phosphorylation induced by peptide V1 (means ± SE, n = 5). C: representative Western blots and grouped densitometric data of ERK1/2 phosphorylation induced by peptide V2 (means ± SE, n = 6). D: representative Western blots and grouped densitometric data of eNOSs1179 phosphorylation induced by peptide V2 (means ± SE, n = 6). E: representative Western blots and grouped densitometric data of ERK1/2 phosphorylation induced by peptide V3 (means ± SE, n = 6). F: representative Western blots and grouped densitometric data of eNOSs1179 phosphorylation induced by peptide V3 (means ± SE, n = 6). ***P < 0.001, **P < 0.01, and *P < 0.05 vs. 0 min.
Peptides V1, V2, or V3 induce cardioprotection against I/R injury.
We next determined whether small netrin-1 derived peptides can induce cardioprotection similar to netrin-1. Given as a preconditioning mimetic, V1, V2, or V3 peptide-perfused hearts had a substantial reduction in infarct size (control I/R 39.3 ± 0.3% vs. I/R w. V1 14.6 ± 2.3% vs. I/R w. V2 23.0 ± 2.8% vs. I/R w. V3 18.8 ± 0.8%, P < 0.001, Fig. 3), implicating that these peptides are highly effective in inducing cardioprotection against I/R injury at the efficacy that is equivalent to netrin-1 (26). Furthermore, given as a postconditioning agent, all three peptides also induced potent cardioprotection against I/R injury (control I/R 37.6 ± 1.3% vs. I/R w. V1 17.6 ± 3.2% vs. I/R w. V2 20.6 ± 1.7% vs. I/R w. V3 15.8 ± 2.0%, P < 0.001, Fig. 4). These data demonstrate that these peptides are consistently beneficial whenever they are delivered to the heart and have the potential to be developed as therapies for MI.
Fig. 3.
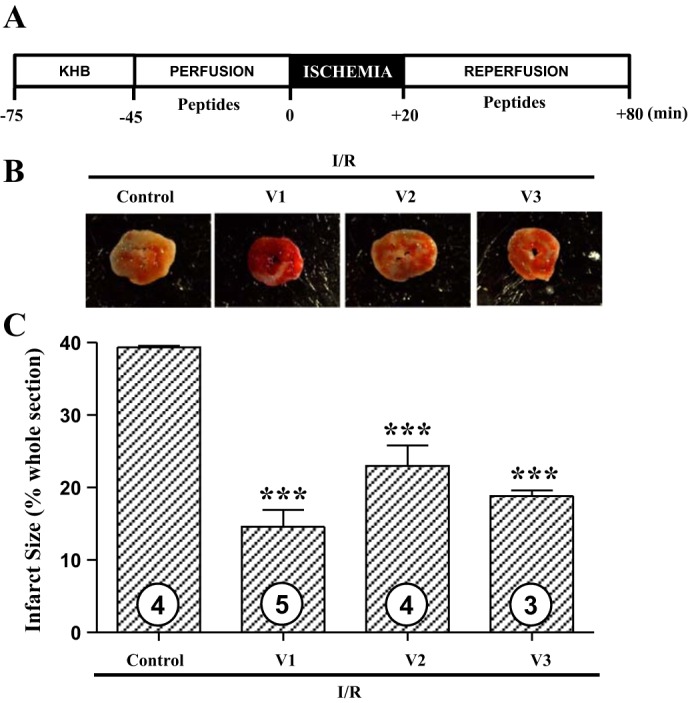
Netrin-1 peptide V1, V2, or V3 induces potent cardioprotection against ischemia-reperfusion (I/R) injury. A: hearts of C57BL/6 mice were freshly isolated and subjected to I/R injury using a Langendorff perfusion system. Hearts were preperfused with Krebs-Henseleit buffer for 30 min, followed by a 45-min perfusion with netrin-1 peptide V1, V2, or V3 at the same molar concentration of 1.47 nmol/l. Next, I/R injury was consistently produced by subjecting the hearts to 20 min of normothermic ischemia followed by reperfusion for 60 min with or without corresponding peptides. Sections of the hearts were stained with 2,3,5-triphenyl tetrazolium chloride (TTC), and infarct area was calculated as % of risk area. B: representative TTC staining of I/R-injured mouse hearts. C: quantitative grouped data of B; control I/R (n = 4), I/R w. V1 (n = 5), I/R w. V2 (n = 4), and I/R w. V3 (n = 3), means ± SE. ***P < 0.001 vs. control I/R.
Fig. 4.
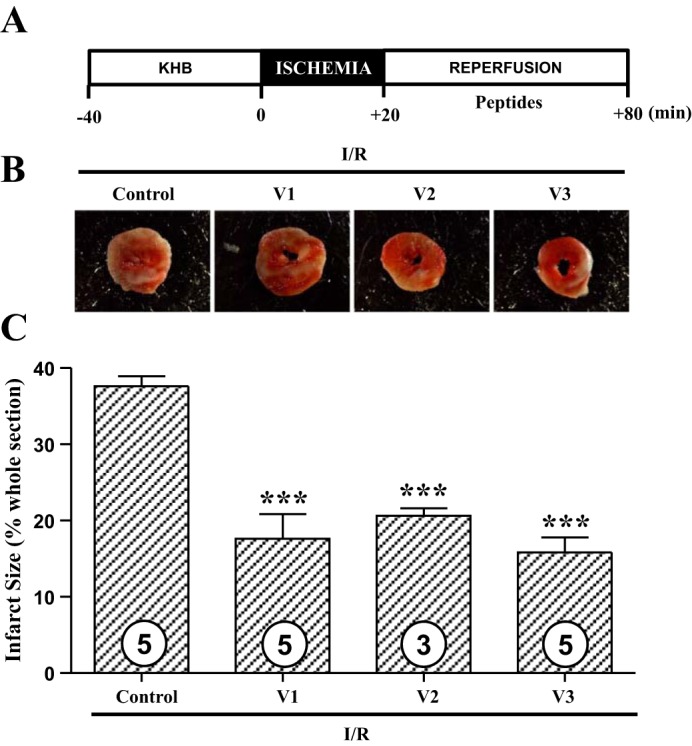
Netrin-1 peptide V1, V2, or V3 induces potent cardioprotection against I/R injury given postconditionally. A: hearts of C57BL/6 mice were freshly isolated and subject to I/R injury using a Langendorff perfusion system. Hearts were preperfused with Krebs-Henseleit buffer for 40 min, and then I/R injury was consistently produced by subjecting the hearts to 20 min of normothermic ischemia, followed by reperfusion for 60 min with or without peptide V1, V2, or V3 at the same molar concentration of 1.47 nmol/l. Sections of hearts were stained with 2,3,5-TTC, and infarct area was calculated as % of risk area. B: representative TTC staining of I/R-injured mice hearts. C: quantitative grouped data of B; control I/R (n = 5), I/R w. V1 (n = 5), I/R w. V2 (n = 3), and I/R w. V3 (n = 5), means ± SE. ***P < 0.001 vs. control I/R.
Smaller truncated peptides effective in cardioprotection: Identification of core sequences.
The above-described peptides remain to be slightly long (48–61 aa). To find the minimal core functional sequence, the peptides were aligned and analyzed by ClustalW. We synthesized the area sharing high sequence identity (V1-9aa: residues 304–312; V2-10aa: residues 368-377aa; and V3-11aa: residues 423–433) and also a random negative control in the V3 domain (V3-16aa: residues 407-422aa). It turns out that the shortened core peptides worked well as postconditioning agents to protect the heart from I/R injury. As shown in Fig. 5, A and B, V1-9aa, V2-10aa, and V3-11aa peptides significantly reduced infarct size compared with the control group (control I/R 37.6 ± 1.3% vs. I/R w. V1-9aa 16.8 ± 2.2%; I/R w. V2-10aa 18.6 ± 1.7%; and I/R w. V3-11aa 16.7 ± 3.0%, P < 0.001). However, the negative control V3-16aa showed no protection, and the infarct size was 37.9 ± 2.3%. These smaller core peptides have a sequence resembling Cx(1–2)Cx(3–4)Tx(0–1)G, where x is any residue (Fig. 5C). These results reveal the minimal functional sequences important for cardioprotection that could be used as template for development of novel peptide drugs for the treatment of MI.
Fig. 5.
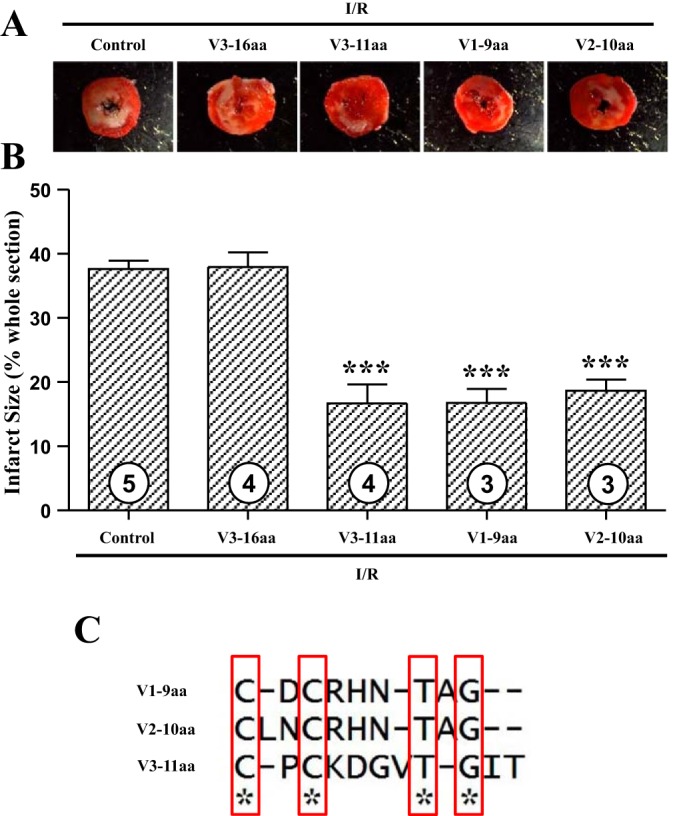
Smaller peptides containing core sequences of netrin-1 laminin V domain induce potent cardioprotection against I/R injury postconditionally. Postconditioning I/R experiments were performed as shown in Fig. 4. Different peptides V1-9 amino acid (aa), V2-10aa, V3-16aa, and V3-11aa were used at the same molar concentration of 1.47 nmol/l. Sections of hearts were stained with 2,3,5-TTC, and infarct area was calculated as % of risk area. A: representative TTC staining of I/R-injured mouse hearts. B: quantitative grouped data of A; control I/R (n = 5), I/R w. V1-9aa (n = 3), I/R w. V2-10aa (n = 3), I/R w. V3-16aa (n = 4), and I/R w. V3-11aa (n = 4), means ± SE. ***P < 0.001 vs. control I/R. C: alignment of the three working smaller peptides revealed minimal core sequences necessary to provoke cardioprotection.
Smaller peptides stimulate endothelial cell NO production.
To explore the cardioprotective mechanisms of these smaller peptides, we examined their effects on endothelial production of NO, which is anticipated to diffuse to cardiomyocytes to activate survival signaling. As shown in Fig. 6, exposure of endothelial cells to V1-9aa, V2-10aa, and V3-11aa, at the same molar concentration used for netrin-1 perfusion, resulted in significantly elevated NO production (V1-9aa 1.50 ± 0.09-fold, V2-10aa 1.44 ± 0.08-fold, and V3-11aa 1.45 ± 0.11-fold, P < 0.001). These data indicate that the smaller, core sequence-bearing peptides likely exert their potent cardioprotective effect via NO-dependent mechanisms, as we previously demonstrated for netrin-1.
Fig. 6.
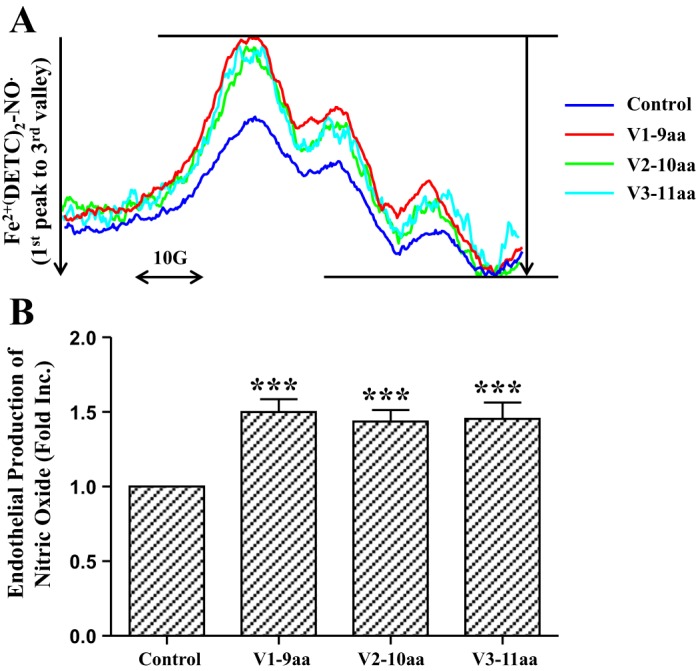
Smaller peptides containing core sequences of netrin-1 laminin V domain stimulate endothelial cell NO production. Confluent endothelial cells were incubated with different peptides V1-9aa, V2-10aa, and V3-11aa at the same molar concentration of 1.47 nmol/l at 37°C for 60 min in modified Krebs/HEPES buffer containing the NO-specific spin trap Fe2+(DETC)2. Cells were then gently collected for analysis of NO production by using electron spin resonance (ESR). A: representative ESR spectra. B: quantitative grouped data; n = 5–6, means ± SE. ***P < 0.001 vs. control.
Combinatory effects of smaller peptides on stimulating endothelial cell NO production.
To further identify the synergistic effects of smaller peptides, we treated endothelial cells with different combinations, including the V1-9aa + V2-10aa group, V1-9aa + V3-11aa group, and V2-10aa + V3-11aa group; each smaller peptide accounts for 50% and the final molar concentration still 1.47 nmol/l. The control and V1-9aa groups (1.47 nmol/l) were used to compare with these combination groups. The results, shown in Fig. 7, indicate that the V1-9aa + V2-10aa group produced more NO than the V1-9aa group (V1-9aa + V2-10aa 1.90 ± 0.50-fold vs. V1-9aa 1.42 ± 0.18-fold, P < 0.05). However, the V1-9aa + V3-11aa and V2-10aa + V3-11aa groups did not show this augmented effect. These data suggest that V1-9aa and V2-10aa smaller peptides can synergistically induce more NO production.
Fig. 7.
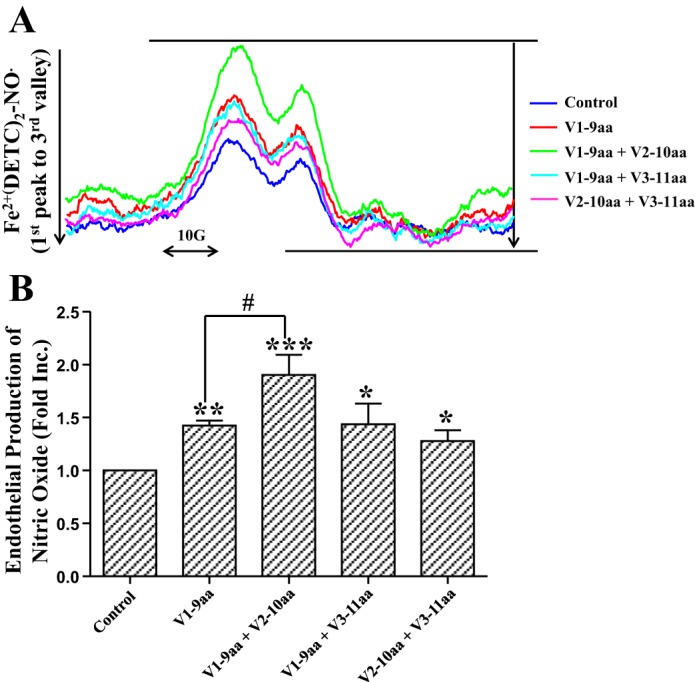
Combination effects of smaller peptides on stimulating endothelial cell NO production. Confluent endothelial cells were incubated with different combination of peptides V1-9aa alone, V1-9aa + V2-10aa (1:1), V1-9aa + V3-11aa (1:1), and V2-10aa + V3-11aa (1:1) at the same total molar concentration of 1.47 nmol/l at 37°C for 60 min in modified Krebs/HEPES buffer containing the NO-specific spin trap Fe2+(DETC)2. Cells were then gently collected for analysis of NO production by using ESR. A: representative ESR spectra. B: quantitative grouped data; n = 5–14, means ± SE. ***P < 0.001, **P < 0.01, and *P < 0.05 vs. control. #P < 0.05 vs. V1-9aa group.
DISCUSSION
The current study identified a core peptide sequence of netrin-1 that is sufficient and equally robust in inducing cardioprotection. By activating the ERK1/2-eNOS axis to produce NO, small netrin-1-derived peptides provoke cardioprotection given at the time of reperfusion, which has been considered as a simple, clinically applicable procedure to improve outcome of MI (25).
None of the proposed regiments have been approved by the Food and Drug Administration to treat cardiac I/R injury in the past two decades, including some peptides that have been shown to be beneficial in animal studies (4, 7, 14). There is still an urgent need for development of an effective cardioprotective agent that can be easily translated into clinical practice. We have previously established that netrin-1 perfusion induces potent cardioprotection against I/R injury (26). Our present data identified novel, small netrin-1-derived peptides that are equally robust in inducing cardioprotection at the same molar concentration of netrin-1. These short peptides have the potential to be directly employed, or further modified, into peptide drugs for the treatment of MI.
Exogenously applied netrin-1 plays an essential role in cardioprotection mainly through a DCC/ERK1/2/eNOS/NO/DCC feedforward mechanism (5, 16, 26) that is believed to be activated in vascular endothelial cells to promote survival of cardiomyocytes (Fig. 1) (20). The activation of this signaling pathway attenuates oxidative stress and preserves mitochondrial function, which protect the heart from necrosis and apoptosis (5, 6, 16, 21, 26). As discussed earlier, netrin-1 contains Lam VI, three Lam V, and C345C domains (15, 17) that are conserved from C. elegans to Homo sapiens. Although both Lam VI and Lam V domains took part in the interaction with its receptor DCC and in maintaining dorsal axon guidance activities in C. elegans (15, 17), we prefer to use the much shorter Lam V domain, which is only 48–61 amino acids long. As expected, all three Lam V domains time dependently activated phosphorylation of ERK1/2 and eNOSs1179 at the same molar concentration as used for netrin-1 in endothelial cells, which clearly confirmed functional similarity of Lam V domain with netrin-1 (Fig. 2). Several previous studies have shown that activated ERK1/2 mediates protection against I/R injury (11, 18). Moreover, mice expressing an unphosphorylatable (S1179A) form of eNOS show more severe infarction than wild-type mice (1). Because our ex vivo Langendorff system data and in vivo coronary artery occlusion model data have confirmed netrin-1's cardioprotection role (6, 26), it is believed that Lam V domain peptides might also induce similar cardioprotection. Indeed, these peptides are consistently beneficial to the heart with both pre- and postconditioning perfusion (Figs. 3 and 4).
Both Lam V and III domains belong to laminin epidermal growth factor-like domain (LE domain), which was first discovered in the protein named laminin (2). Laminin protein is an important and biologically active part of the basal lamina, influencing cell differentiation, migration, and adhesion, as well as phenotype and survival (24). The seventh LE domain of mouse laminin γ1-chain took part in its interaction with nidogen (19) and the binding sites located on the surface within the loops Cys1-Cys3 (loop a) and Cys5-Cys6 (loop c) (22, 23). The core sequences we concluded by ClustalW alignment analysis are also located in loop c. Of note, the core sequence peptides effectively protected the heart against I/R injury as robust as netrin-1 when given at the same molar concentration (Fig. 5). Our finding provided a template of Cx(1–2)Cx(3–4)Tx(0–1)G, which may simulate netrin-1's key binding sites to activate DCC and its downstream protective signaling. Indeed, the small core peptides also activate NO production through the ERK1/2/eNOS pathway, as previously identified for netrin-1. Whereas all three core peptides of V1-9aa, V2-10aa, and V3-11aa proved to be highly effective in inducing cardioprotection, combined applications of V1-9aa and V2-10aa synergistically generated more NO from endothelial cells. While further investigation may be necessary to modify the template to make it more feasible for pharmaceutical applications, identification of the core sequence is essentially valuable for the development of novel peptide drugs for treatment of MI.
In summary, our findings have identified small netrin-1-derived peptides that are as equally robust in provoking cardioprotection as netrin-1. These peptides activate the ERK1/2/eNOSs1179 pathway and increase NO production, again similarly to netrin-1. A nine-amino acid core sequence has also been identified. Perfusion of I/R-injured heart with these peptides resulted in substantial reduction in infarct size. In view of the well-recognized advantages in developing peptide rather than protein drugs, these findings may facilitate novel peptide-based therapeutic options for MI.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants HL-077440 (H. Cai), HL-088975 (H. Cai), HL-119968 (H. Cai), and HL-108701 (H. Cai), an American Heart Association Established Investigator Award (Grant 12EIA8990025 to H. Cai), and an American Heart Association Postdoctoral Fellowship Award (Grant 14POST20380966 to Q. Li)].
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Q.L. performed experiments; Q.L. and H.C. analyzed data; Q.L. and H.C. interpreted results of experiments; Q.L. and H.C. prepared figures; Q.L. and H.C. drafted manuscript; Q.L. and H.C. edited and revised manuscript; Q.L. and H.C. approved final version of manuscript; H.C. conception and design of research.
Footnotes
This article is the topic of an Editorial Focus by Mei-Zhen Cui (10a).
REFERENCES
- 1.Atochin DN, Wang A, Liu VW, Critchlow JD, Dantas AP, Looft-Wilson R, Murata T, Salomone S, Shin HK, Ayata C, Moskowitz MA, Michel T, Sessa WC, Huang PL. The phosphorylation state of eNOS modulates vascular reactivity and outcome of cerebral ischemia in vivo. J Clin Invest 117: 1961–1967, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beck K, Hunter I, Engel J. Structure and function of laminin: anatomy of a multidomain glycoprotein. FASEB J 4: 148–160, 1990. [DOI] [PubMed] [Google Scholar]
- 3.Bolli R, Jeroudi MO, Patel BS, DuBose CM, Lai EK, Roberts R, McCay PB. Direct evidence that oxygen-derived free radicals contribute to postischemic myocardial dysfunction in the intact dog. Proc Natl Acad Sci USA 86: 4695–4699, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bose AK, Mocanu MM, Carr RD, Brand CL, Yellon DM. Glucagon-like peptide 1 can directly protect the heart against ischemia/reperfusion injury. Diabetes 54: 146–151, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Bouhidel JO, Wang P, Li Q, Cai H. Pharmacological Postconditioning Treatment of Myocardial Infarction with Netrin-1. Front Biosci (Landmark Ed) 19: 566–570, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bouhidel JO, Wang P, Siu KL, Li H, Youn JY, Cai H. Netrin-1 improves post-injury cardiac function in vivo via DCC/NO-dependent preservation of mitochondrial damage, while attenuating autophagy. BBA-Mol Basis Dis 1852: 277–289, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burley DS, Hamid SA, Baxter GF. Cardioprotective actions of peptide hormones in myocardial ischemia. Heart Fail Rev 12: 279–291, 2007. [DOI] [PubMed] [Google Scholar]
- 8.Calvert JW, Lefer DJ. Clinical translation of nitrite therapy for cardiovascular diseases. Nitric Oxide 22: 91–97, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cannon RO., 3rd Mechanisms, management and future directions for reperfusion injury after acute myocardial infarction. Nat Clin Pract Cardiovasc Med 2: 88–94, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Chalupsky K, Cai H. Endothelial dihydrofolate reductase: critical for nitric oxide bioavailability and role in angiotensin II uncoupling of endothelial nitric oxide synthase. Proc Natl Acad Sci USA 102: 9056–9061, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10a.Cui MZ. Potential therapeutics for myocardial ischemia-reperfusion injury. Focus on “Induction of cardioprotection by small netrin-1-derived peptides.” Am J Physiol Cell Physiol (June 3, 2015) 10.1152/ajpcell.00150.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Das A, Salloum FN, Xi L, Rao YJ, Kukreja RC. ERK phosphorylation mediates sildenafil-induced myocardial protection against ischemia-reperfusion injury in mice. Am J Physiol Heart Circ Physiol 296: H1236–H1243, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao L, Chalupsky K, Stefani E, Cai H. Mechanistic insights into folic acid-dependent vascular protection: Dihydrofolate reductase (DHFR)-mediated reduction in oxidant stress in endothelial cells and angiotensin II-infused mice: a novel HPLC-based fluorescent assay for DHFR activity. J Mol Cell Cardiol 47: 752–760, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER 3rd, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics–2014 update: a report from the American Heart Association. Circulation 129: e28–e292, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kloner RA, Hale SL, Dai W, Gorman RC, Shuto T, Koomalsingh KJ, Gorman JH 3rd, Sloan RC, Frasier CR, Watson CA, Bostian PA, Kypson AP, Brown DA. Reduction of ischemia/reperfusion injury with bendavia, a mitochondria-targeting cytoprotective peptide. J Am Heart Assoc 1: e001644, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kruger RP, Lee J, Li W, Guan KL. Mapping netrin receptor binding reveals domains of Unc5 regulating its tyrosine phosphorylation. J Neurosci 24: 10826–10834, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Q, Wang P, Ye K, Cai H. Central role of SIAH inhibition in DCC-dependent cardioprotection provoked by netrin-1/NO. Proc Natl Acad Sci USA 112: 899–904, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lim YS, Wadsworth WG. Identification of domains of netrin UNC-6 that mediate attractive and repulsive guidance and responses from cells and growth cones. J Neurosci 22: 7080–7087, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lips DJ, Bueno OF, Wilkins BJ, Purcell NH, Kaiser RA, Lorenz JN, Voisin L, Saba-El-Leil MK, Meloche S, Pouyssegur J, Pages G, De Windt LJ, Doevendans PA, Molkentin JD. MEK1-ERK2 signaling pathway protects myocardium from ischemic injury in vivo. Circulation 109: 1938–1941, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Mayer U, Poschl E, Gerecke DR, Wagman DW, Burgeson RE, Timpl R. Low nidogen affinity of laminin-5 can be attributed to two serine residues in EGF-like motif gamma 2III4. FEBS Lett 365: 129–132, 1995. [DOI] [PubMed] [Google Scholar]
- 20.Nguyen A, Cai H. Netrin-1 induces angiogenesis via a DCC-dependent ERK1/2-eNOS feed-forward mechanism. Proc Natl Acad Sci USA 103: 6530–6535, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siu KL, Lotz C, Ping PP, Cai H. Netrin-1 abrogates ischemia reperfusion-induced cardiac mitochondrial dysfunction via nitric oxide-mediated attenuation of NOX4 activation and recoupling of NOS. J Mol Cell Cardiol 78: 174–185, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stetefeld J, Mayer U, Timpl R, Huber R. Crystal structure of three consecutive laminin-type epidermal growth factor-like (LE) modules of laminin gamma1 chain harboring the nidogen binding site. J Mol Biol 257: 644–657, 1996. [DOI] [PubMed] [Google Scholar]
- 23.Stetefeld J, Mayer U, Timpl R, Huber R. Crystal structure of three consecutive laminin-type epidermal growth factor-like (LE) modules of laminin gamma 1 chain harboring the nidogen binding site. J Mol Biol 257: 644–657, 1996. [DOI] [PubMed] [Google Scholar]
- 24.Timpl R, Rohde H, Robey PG, Rennard SI, Foidart JM, Martin GR. Laminin–a glycoprotein from basement membranes. J Biol Chem 254: 9933–9937, 1979. [PubMed] [Google Scholar]
- 25.Vinten-Johansen J, Yellon DM, Opie LH. Postconditioning: a simple, clinically applicable procedure to improve revascularization in acute myocardial infarction. Circulation 112: 2085–2088, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Zhang J, Cai H. Netrin-1 prevents ischemia/reperfusion-induced myocardial infarction via a DCC/ERK1/2/eNOS s1177/NO/DCC feed-forward mechanism. J Mol Cell Cardiol 48: 1060–1070, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]