acute myocardial infarction (MI) is a major cause of morbidity and mortality worldwide. MI occurs when a portion of the heart is deprived of oxygen due to blockage of a coronary artery; this injures heart muscle, leading to impaired cardiac function. In patients with MI, the treatment of choice for reducing acute myocardial ischemic injury and limiting MI size is the timely and effective restoration of blood perfusion. However, the process of reperfusion itself can paradoxically induce myocardial injury, known as myocardial ischemia-reperfusion (I/R) injury, for which there is still no effective therapy (2). Experimental studies have identified several critical factors that act in concert to induce the detrimental effects of myocardial reperfusion injury. These factors are cytosolic and mitochondrial oxidative stress, intracellular Ca2+ overload, mitochondrial permeability transition pore (MPTP) opening, and inflammation. Of these identified factors, reactive oxygen species (ROS) may be the most important, due to their functions in 1) inducing the opening of the MPTP; 2) mediating dysfunction of the sarcoplasmic reticulum, which contributes to Ca2+ overload; and 3) acting as neutrophil chemoattractant.
In a previous study, Zhang and Cai (10) reported that netrin-1 functions as a potent cardioprotective agent. Netrin-1 reduces I/R-induced myocardial injury via an antioxidative mechanism triggered by the netrin-1 receptor-mediated endothelial nitric oxide synthase (eNOS)/nitric oxide (NO) pathway (Fig. 1). Netrins are a family of secreted proteins that were first identified as guidance cues, directing cell and axon migration during neural development (4). In mammals, three secreted netrins, netrin 1, 3, and 4, and two membrane-tethered glycophosphatidylinositol-linked netrins, netrin G1 and G2, have been identified (4, 8). Netrins function through interactions with their canonical receptors. Netrin-1's axonal functions have been linked to two classes of receptors, the DCC (deleted in colon cancer) family, including DCC and neogenin, and the Unc5 family (Unc5A through D). Besides their originally identified function in neural development, netrins have since been shown to play important roles in the development of various tissues and diseases, including morphogenesis of the vascular system, angiogenesis, atherosclerosis, and myocardial reperfusion injury.
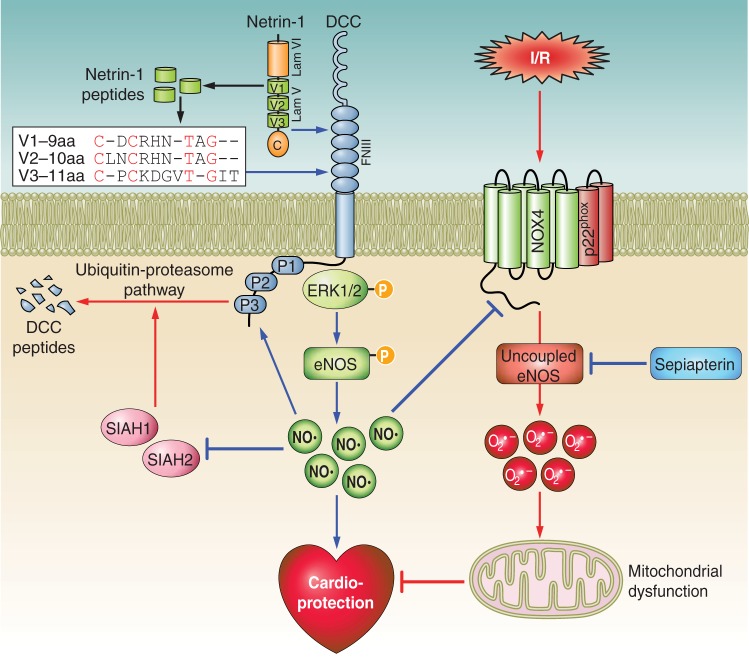
Fig. 1.
Molecular mechanisms of cardioprotection provoked by netrin-1 and netrin-1-derived small peptides. Netrin-1 or netrin-1-derived small peptides interact with their receptor DCC, leading to activation of ERK1/2 and eNOS, which increases production of NO, resulting in cardioprotection. NO downregulates SIAH, an E3 ligase for DCC, leading to reduced degradation of DCC by inhibition of the ubiquitin-proteasome pathway. This positive feedback mechanism augments cardioprotective signaling of netrin-1 and netrin-1-derived peptides by accumulation of the DCC receptor. At the same time, NO induced by netrin-1 or netrin-1-derived peptides also attenuates I/R injury by downregulation of NOX4, resulting in prevention of NOX4-dependent eNOS uncoupling and preservation of mitochondrial function, all of which contribute to cardioprotection. Cardioprotective pathways are highlighted in blue and myocardial ischemia-stress pathways in red.
Netrin-1's protection of myocardial I/R injury relies on the production of NO, which upregulates the accumulation of netrin-1's receptor DCC and can further amplify netrin-1's function (10) (Fig. 1). Therefore netrin-1-induced NO may be the key molecule underlying the marked reduction in infarct size and cardiac cell death. Netrin-1 reduction of infarct size was significantly attenuated in DCC+/− mice, indicating a key role of DCC in cardioprotection. Upon netrin-1 perfusion, its receptor DCC is activated, resulting in ERK1/2 activation, which leads to eNOSs1177 phosphorylation; activated eNOS then produces NO that mediates DCC upregulation, forming a positive feedback loop to amplify DCC activity and NO production. In three recent studies investigating roles and mechanisms of netrin-1 in cardioprotection, Cai's group demonstrated that netrin-1 improves postinjury cardiac function in vivo via DCC/NO-dependent preservation of mitochondrial integrity, while attenuating autophagy (1). The authors demonstrated that netrin-1 preserves mitochondrial integrity via NO-dependent inhibition of NADPH oxidase 4 (NOX4) activity, recoupling of NOS, augmented NO bioavailability, and reduction of oxidative stress—ultimately preserving mitochondrial function (9). While investigating the mechanism underlying netrin-1-induced DCC accumulation, they discovered that NO upregulates DCC by inhibition of E3 ubiquitin ligase seven in absentia homolog (SIAH), which regulates DCC protein abundance through an ubiquitin-proteasome pathway (6). RNAi inhibition of SIAH proved to be beneficial in reducing infarct size and improving cardiac function by accumulation of DCC. Thus, the novel NO/SIAH/DCC signaling pathway is important for cardioprotection. In addition to posttranslational regulation, DCC may also be regulated by netrin-1 via transcriptional/posttranscriptional mechanisms (10).
All the data above strongly support a novel role of netrin-1 in cardioprotection and its potential therapeutic application. However, the potential clinical application of netrin-1 is difficult to estimate because large protein molecules are generally unstable, difficult to purify, and costly to produce. Therefore, it is important to identify the minimal functional peptide that could preserve netrin-1 function in activating the receptor DCC and stimulating downstream signaling events. In this issue of the American Journal of Physiology-Cell Physiology, Cai's group extend their previous finding and identify a 9-amino acid (aa) core sequence in the laminin V region of netrin-1 to be highly effective in cardioprotection (5).
Structurally, netrins are laminin-related proteins. Netrin-1 has a laminin VI-like (Lam VI) domain, three cysteine-rich repeats similar to those of domain V of laminin subunits (Lam V, including V1, V2, and V3), and a COOH-terminal domain (C345C). Previous results have shown that the Lam VI and Lam V domains were involved in the association with receptor DCC (3). Lam V1, V2, and V3 domains were required primarily for the dorsal axon guidance activities of netrin-1 homolog UNC-6 in Caenorhabditis elegans (7). Therefore, these three domains in Lam V might contain the core element required for netrin-1 induced cardioprotection. Using synthesized peptides V1 (54 aa, residues 285-338), V2 (61 aa, residues 341–401), and V3 (48 aa, residues 404–451), the authors found that treatment of primary endothelial cells with the same molar concentration of peptide V1, V2, or V3 could activate ERK1/2 to the same extent as netrin-1. Interestingly, these peptides also significantly induced phosphorylation of eNOSs1179. These data strongly suggest that V1, V2, or V3 peptide can activate netrin-1 responses and its downstream signaling pathway, required for cardioprotection. Significantly, in preclinical studies, these synthetic peptides V1, V2, and V3 were observed to induce similar cardioprotection against I/R injury when compared with netrin-1 when either preperfused or simultaneously perfused to the heart. The data demonstrate that these peptides are consistently beneficial whenever they are delivered to the heart and have the potential to be developed as therapies for myocardial infarction (5).
In pursuing a minimal core functional sequence, Cai's group found the following areas sharing high sequence identity: V1-9 aa, residues 304–312; V2-10 aa, residues 368–377; and V3-11 aa, residues 423–433. Intriguingly, these shortened core peptides worked well as postconditioning agents to protect the heart from I/R injury. These smaller core peptides have a sequence resembling Cx(1–2)Cx(3–4)Tx(0–1)G, where x is any amino acid residue (Fig. 1). These core sequence peptides may function to bind and activate the membrane receptor DCC and trigger its downstream ERK1/2 signaling pathway, leading to NO production as identified for netrin-1. Another interesting finding was the augmented effects of combining smaller peptides in stimulating endothelial cell NO production. This was observed when V1-9 aa was added to V2-10 aa. These results identify the important cardioprotective abilities of the minimal peptide sequences, which could be used as a template for the development of novel peptide drugs for the treatment of I/R injury (5). Molecular mechanisms of cardioprotection provoked by netrin-1 and netrin-1-derived small peptides are illustrated in Fig. 1.
In summary, the identification of the core sequence (a 9-aa peptide) is valuable for the development of novel peptide drugs for treatment of myocardial infarction. Future studies should address how these core sequences interact with the DCC receptor and whether other receptors are also involved. In light of the ligand and receptor binding relationship between Lam VI and DCC, an effort to evaluate the Lam VI region of netrin-1 in myocardial protection may be rewarding. The finding that preperfusion and reperfusion of the smaller peptides are effective for cardioprotection implies a prevention/protection benefit of these short (9–11 aa) peptides in addition to alleviating postacute reperfusion damage. Future, substantial preclinical and clinical studies are necessary to evaluate the toxicity and side effects of these potential and promising short peptides and their modified forms. In light of the advantages of developing peptide rather than protein drugs, these findings might facilitate novel peptide-based therapeutic options for myocardial infarction.
GRANTS
This research work in M.-Z. Cui's laboratory is supported by grants from the National Institutes of Health (National Heart, Lung, and Blood Institute Grant HL-107466) and the University of Tennessee Center of Excellence in Livestock Diseases and Human Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author.
AUTHOR CONTRIBUTIONS
M.-Z.C. prepared figure; drafted manuscript; edited and revised manuscript; approved final version of manuscript.
REFERENCES
- 1.Bouhidel JO, Wang P, Siu KL, Li H, Youn JY, Cai H. Netrin-1 improves post-injury cardiac function in vivo via DCC/NO-dependent preservation of mitochondrial integrity, while attenuating autophagy. Biochim Biophys Acta 1852: 277–289, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hausenloy DJ, Yellon DM. Myocardial ischemia-reperfusion injury: a neglected therapeutic target. J Clin Invest 123: 92–100, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kruger RP, Lee J, Li W, Guan KL. Mapping netrin receptor binding reveals domains of Unc5 regulating its tyrosine phosphorylation. J Neurosci 24: 10826–10834, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lai Wing Sun K, Correia JP, Kennedy TE. Netrins: versatile extracellular cues with diverse functions. Development 138: 2153–2169, 2011. [DOI] [PubMed] [Google Scholar]
- 5.Li Q, Cai H. Induction of cardioprotection by small netrin-1 derived peptides. Am J Physiol Cell Physiol (April 29, 2015). doi: 10.1152/ajpcell.00332.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Q, Wang P, Ye K, Cai H. Central role of SIAH inhibition in DCC-dependent cardioprotection provoked by netrin-1/NO. Proc Natl Acad Sci USA 112: 899–904, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lim YS, Wadsworth WG. Identification of domains of netrin UNC-6 that mediate attractive and repulsive guidance and responses from cells and growth cones. J Neurosci 22: 7080–7087, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Serafini T, Colamarino SA, Leonardo ED, Wang H, Beddington R, Skarnes WC, Tessier-Lavigne M. Netrin-1 is required for commissural axon guidance in the developing vertebrate nervous system. Cell 87: 1001–1014, 1996. [DOI] [PubMed] [Google Scholar]
- 9.Siu KL, Lotz C, Ping P, Cai H. Netrin-1 abrogates ischemia/reperfusion-induced cardiac mitochondrial dysfunction via nitric oxide-dependent attenuation of NOX4 activation and recoupling of NOS. J Mol Cell Cardiol 78: 174–185, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang J, Cai H. Netrin-1 prevents ischemia/reperfusion-induced myocardial infarction via a DCC/ERK1/2/eNOS s1177/NO/DCC feed-forward mechanism. J Mol Cell Cardiol 48: 1060–1070, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]