Key Points
HDCA plus CY/TBI improved overall survival relative to CY/TBI in CBT for myeloid malignancy.
HDCA suppressed relapse but did not increase the incidence of severe adverse events or nonrelapse mortality.
Abstract
Cord blood transplantation (CBT) is an effective therapeutic option for adults with acute myelogenous leukemia (AML) and myelodysplastic syndrome (MDS) after the conventional cyclophosphamide and total body irradiation (CY/TBI) regimen, but posttransplant relapse is still of high importance. High-dose cytarabine (HDCA) can be added to CY/TBI for an intensified regimen; however, its additional effects have not yet been completely elucidated. Therefore, we conducted a cohort study to compare the prognosis of HDCA/CY/TBI (n = 617) and CY/TBI (n = 312) in CBT for AML/MDS, using a Japanese transplant registry database. The median age was 40 years, and 86.2% of the patients had AML; high-risk disease was observed in 56.2% of the patients. The median follow-up period after CBT was approximately 3.5 years. Overall survival was significantly superior in the HDCA/CY/TBI group (adjusted hazard ratio [HR], 0.56; 95% confidence interval [CI], 0.45-0.69; P < .01), and tumor-related mortality was lower (HR, 0.50; P < .01). The incidence of grade II to IV acute graft-vs-host disease (aGVHD) and chronic GVHD was significantly higher in the HDCA/CY/TBI group (HR, 1.33 and 2.30, respectively), but not grade III to IV aGVHD. Incidence of infectious episodes showed no significant difference. Nonrelapse mortality was not increased by the addition of HDCA. Higher-dose CA (12 rather than 8 g/m2) was more effective, particularly in patients at high-risk for disease. This study is the first to show the superiority of HDCA/CY/TBI to CY/TBI in CBT for AML/MDS. A large-scale prospective study is warranted to establish new conditioning regimens including HDCA administration.
Introduction
Allogeneic hematopoietic cell transplantation (HCT) is an effective, and therefore indispensable, therapy for myeloid malignancies, including acute myelogenous leukemia (AML) and myelodysplastic syndrome (MDS).1 Cord blood transplantation (CBT), which was first performed approximately 25 years ago,2 has been technically improved all over the world and is now as safe and effective as bone marrow or mobilized peripheral blood stem cell transplantation.3 CBT has been increasingly used as a stem-cell source for allogeneic HCT for myeloid malignancies.2
Meanwhile, various conditioning regimens have been developed. The combination of cyclophosphamide (CY; 60 mg/kg for 2 days) with total body irradiation (TBI; 10-12 Gy divided into 4-6 fractions) (CY/TBI regimen), and intravenous busulfan (BU) with CY (BU/CY regimen) are the conventional myeloablative regimens in CBT,2,4 as well as in bone marrow or peripheral blood stem cell transplantation.5,6 Moreover, regimens harboring stronger antileukemic effects without increased adverse effects have been requested, especially for those with recurrent or refractory AML/MDS, to reduce posttransplant relapse.2
Among these intensified regimens, addition of high-dose cytarabine (HDCA; total dose of 6-12 g/m2) to the conventional CY/TBI regimen can be a promising strategy because cytarabine (CA) has long been used as an effective agent during induction and consolidation therapy of AML/MDS.7 In previous smaller studies including CBT for leukemia, the HDCA/CY/TBI regimen was reportedly related have to a low incidence of relapse and a fair prognosis,3,8,9 whereas comparison with CY/TBI has not been fully reported. In contrast, in studies of bone marrow transplantation, this regimen resulted in increased nonrelapse mortality (NRM) and inferior overall survival (OS) compared with that of the conventional CY/TBI regimen.10,11 With respect to the rationale for adding HDCA in CBT, there is only limited evidence to inform decisions on whether to add HDCA or not.
Therefore, we performed a cohort study to compare the prognosis in patients who underwent CBT after the HDCA/CY/TBI regimen with that in patients receiving the CY/TBI regimen using the Japanese transplant registry database, with respect to OS, NRM, incidence of relapse, and CBT-related adverse effects such as engraftment failure, infection, graft-vs-host disease (GVHD) or specific HDCA-related adverse effects. Moreover, the relationship between the dose of CA and prognosis was also determined.
Patients and methods
Inclusion and exclusion criteria
Data for 957 adult patients (aged 16 years or older) with AML and MDS who underwent a single-unit CBT as a first HCT after the myeloablative conditioning regimens of CY/TBI (CY, 60 mg/kg for 2 days; TBI, 10-12 Gy divided into 4-6 fractions) or CY/TBI plus HDCA (HDCA/CY/TBI) between January 1, 2000, and December 31, 2012, were obtained from the Transplant Registry Unified Management Program in Japan.12 HDCA was defined as CA administration of 2 to 3 g/m2 twice a day for 2 to 3 days (total dose, 6-12 g/m2) before CBT. The choice of conditioning regimen was made according to the decision of each attending physician. The CB unit selection was based primarily on the total nucleated cell number among 4/6 to 6/6 human leukocyte antigen (HLA)-A, HLA-B, and DR antigen-matched units.4 Cases receiving double-unit CBT were excluded because this is not yet considered the standard therapy in Japan. Patients who lacked data on disease risk, data pertaining to diagnosis, mismatch in HLA or ABO blood type, or GVHD prophylaxis were excluded (n = 28); 929 patients were included in this study. Our protocol complied with the Declaration of Helsinki, and it was approved by the Transplant Registry Unified Management Program Data Management Committee and by the Ethics Committee of Kyoto University, where the study was performed. Written informed consent was obtained from each patient at each institution.
Data collection and definition of each covariate
From the registry database, we extracted data on basic pretransplant characteristics and posttransplant clinical courses. Patients were categorized into 2 groups with respect to age (younger than 50 years vs age 50 years or older), performance status (PS; 0-1 vs 2-4), and hematopoietic cell transplant commodity index (0-2 vs 3-29).13 With respect to disease risk, the standard-risk was defined as AML in complete remission or MDS in the phase of refractory anemia, ringed sideroblasts, or refractory cytopenia with multilineage dysplasia on the day of transplantation, according to the criteria modified from that in the previous study14; other disease types were treated as high-risk disease. The pretransplant therapy period (duration between the initial diagnosis of AML or MDS and the day of CBT) and the year of CBT was categorized into 2 groups: longer vs shorter or older vs newer than the median. HLA disparity in HLA-A, HLA-B, and DR antigens was determined at the serologic level; a 6/6 match was categorized as a HLA-matched group, and 5/6 and 4/6 as a mismatched group.4
With respect to posttransplant clinical courses, engraftment of neutrophils and platelets was defined as the first day of 3 consecutive days during which neutrophil and platelet counts were at least 500/μL and 2.0 or 5.0 × 104/μL without transfusion support, respectively. Diagnosis and classification of aGVHD were performed on the basis of traditional criteria by attending physicians at each center.15,16 Chronic GVHD (cGVHD) was diagnosed according to the criteria determined at the 2005 National Institutes of Health consensus conference.17 The cumulative incidence of infection including any bacteria, fungi (such as Candia spp. and Aspergillus spp.), and viruses (such as cytomegalovirus [CMV] and human herpes virus-6) was compared between the CY/TBI and the HDCA/CY/TBI groups. Standard prevention strategies for infection were adopted in accordance with the guideline from the Japanese Society for Hematopoietic Cell Transplantation,18 which includes protective environment, prophylactic administration of antibiotics (normally fluoroquinolone, fluconazole, and acyclovir), and intravenous immunoglobulin replacement for hypogammaglobulinemia. Data on long-term follow-up for survivors such as systemic organ function, immunosuppressive status, or quality of life19 were not included in our dataset.
Statistical analyses
Differences in pretransplant patient characteristics and the cause of NRM between the CY/TBI and the HDCA/CY/TBI groups were analyzed using the χ2-test or Student t test. OS was calculated with the Kaplan-Meier method and compared using log-rank tests for each covariant related to pretransplant patient characteristics. Factors with significance or borderline significance (P < .1) in the univariate analysis were subjected to a multivariate analysis using the Cox proportional hazards model. Tumor-related mortality was defined as death without remission or after relapse and was calculated using Gray’s method, considering therapy-related death as a competing risk.20 NRM was analyzed considering relapse as a competing risk. The Fine-Gray proportional hazards model was used in multivariate analyses for tumor-related mortality and NRM.21 Statistical analyses were performed using Stata (version 13.1; Stata Corp LP, College Station, TX). The α level of all tests and the P value was set at .05.
Results
Patient characteristics
We evaluated 929 patients aged 16 to 66 years (median, 40 years) who underwent CBT with CY/TBI (n = 312) or HDCA/CY/TBI (n = 617) (Table 1). The median follow-up period for survivors was 1276.5 days (range, 37-4911 days) after CBT. Variables regarding pretransplant patient characteristics are shown in Table 1 and supplemental Tables 1 and 2, available on the Blood Web site; patients with good PS or high-risk disease (particularly those with AML) were conditioned more frequently with HDCA/CY/TBI. GVHD prophylaxis was composed of cyclosporine- and tacrolimus-based regimens, and both were coupled with short-term methotrexate (95.6% and 81.9%, respectively) or mycophenolate mofetil administration (0.9% and 8.1%, respectively). Proportion of cyclosporine-based GVHD prophylaxis was significantly higher in the HDCA/CY/TBI group. Antithymocyte globulin was not used in our cohort. Other variables such as sex, age, hematopoietic cell transplant commodity index, disease type (AML or MDS), pretransplant therapy period, sex mismatch, nuclear cell count of grafts, and year of CBT (before or after 2007) showed no significant differences between these 2 groups.
Table 1.
Patient characteristics
| Variables | Total | CY/TBI | HDCA/CY/TBI | P | |||
|---|---|---|---|---|---|---|---|
| n = 929 | % | n = 312 | % | n = 617 | % | ||
| Sex | |||||||
| Male | 505 | 54.4 | 171 | 54.8 | 334 | 54.1 | |
| Female | 424 | 45.6 | 141 | 45.2 | 283 | 45.9 | .85 |
| Age | |||||||
| Median, years (range) | 40 (16-66) | 40.5 (16-66) | 40 (16-64) | .58 | |||
| <50 | 736 | 79.2 | 238 | 76.3 | 498 | 80.7 | |
| ≥50 | 193 | 20.8 | 74 | 23.7 | 119 | 19.3 | .12 |
| PS | |||||||
| 0-1 | 774 | 83.3 | 243 | 77.8 | 531 | 86.0 | |
| 2-4 | 80 | 8.6 | 32 | 10.3 | 48 | 7.8 | |
| Unknown | 75 | 8.1 | 37 | 11.9 | 38 | 6.2 | <.01* |
| Hematopoietic cell transplant commodity index | |||||||
| 0-2 | 513 | 55.2 | 165 | 52.8 | 348 | 56.4 | |
| 3-29 | 64 | 6.9 | 17 | 5.5 | 47 | 7.6 | |
| Unknown | 352 | 37.9 | 130 | 41.7 | 222 | 36.0 | .16 |
| CMV serostatus | |||||||
| Negative | 157 | 16.9 | 59 | 18.9 | 98 | 15.9 | |
| Positive | 689 | 74.2 | 213 | 68.3 | 476 | 77.1 | |
| Unknown | 83 | 8.9 | 40 | 12.8 | 43 | 7.0 | <.01* |
| Disease | |||||||
| AML | 801 | 86.2 | 277 | 88.8 | 524 | 84.9 | |
| MDS | 128 | 13.8 | 35 | 11.2 | 93 | 15.1 | .11 |
| Disease risk | |||||||
| Standard | 407 | 43.8 | 157 | 50.3 | 250 | 40.5 | |
| High | 522 | 56.2 | 155 | 49.7 | 367 | 59.5 | <.01* |
| In AML | |||||||
| Standard | 371 | 46.3 | 148 | 53.4 | 223 | 42.6 | |
| High | 430 | 53.7 | 129 | 46.6 | 301 | 57.4 | <.01* |
| In MDS | |||||||
| Standard | 36 | 28.1 | 9 | 25.7 | 27 | 29.0 | |
| High | 92 | 71.9 | 26 | 74.3 | 66 | 71.0 | .71 |
| Pretransplant therapy period | |||||||
| Median, days | 218 | 202.5 | 231 | .12 | |||
| ≤200 | 423 | 45.5 | 155 | 49.7 | 268 | 43.4 | |
| ≥201 | 506 | 54.5 | 157 | 50.3 | 349 | 56.6 | .07 |
| HLA mismatch | |||||||
| Matched | 37 | 4.0 | 18 | 5.8 | 19 | 3.1 | |
| Mismatched | 892 | 96.0 | 294 | 94.2 | 598 | 96.9 | .05* |
| Sex mismatch | |||||||
| Matched | 353 | 38.0 | 127 | 40.7 | 226 | 36.6 | |
| Male to female | 163 | 17.6 | 50 | 16.0 | 113 | 18.3 | |
| Female to male | 181 | 19.5 | 64 | 20.5 | 117 | 19.0 | |
| Unknown | 232 | 24.9 | 71 | 22.8 | 161 | 26.1 | .44 |
| ABO mismatch | |||||||
| Matched | 312 | 33.6 | 87 | 27.9 | 225 | 36.4 | |
| Minor | 254 | 27.3 | 102 | 32.6 | 152 | 24.6 | |
| Major | 212 | 22.8 | 71 | 22.8 | 141 | 22.9 | |
| Both | 151 | 16.3 | 52 | 16.7 | 99 | 16.1 | .02* |
| NCC, 107 cells/kg | |||||||
| Median | 3.03 | 3.00 | 3.10 | .50 | |||
| GVHD prophylaxis | |||||||
| CyA based | 548 | 59.0 | 159 | 51.0 | 389 | 63.0 | |
| Tac based | 381 | 41.0 | 153 | 49.0 | 228 | 37.0 | <.01* |
| Year of CBT | |||||||
| 2000-2007 | 424 | 45.6 | 154 | 49.4 | 270 | 43.8 | |
| 2008-2012 | 505 | 54.4 | 158 | 50.6 | 347 | 56.2 | .11 |
| Follow-up period | |||||||
| Median (range) | 1276.5 (37-4,911) | 1084 (82-4007) | 1328 (37-4911) | .09 | |||
NCC, nuclear cell count; CyA, cyclosporine; Tac, tacrolimus.
Statistically significant.
HDCA improves OS and reduces relapse without augmentation of NRM
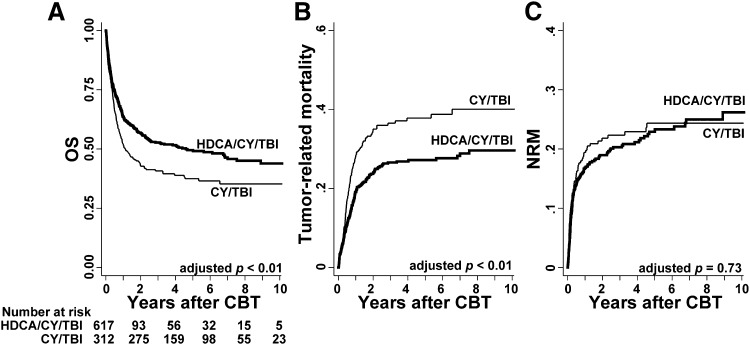
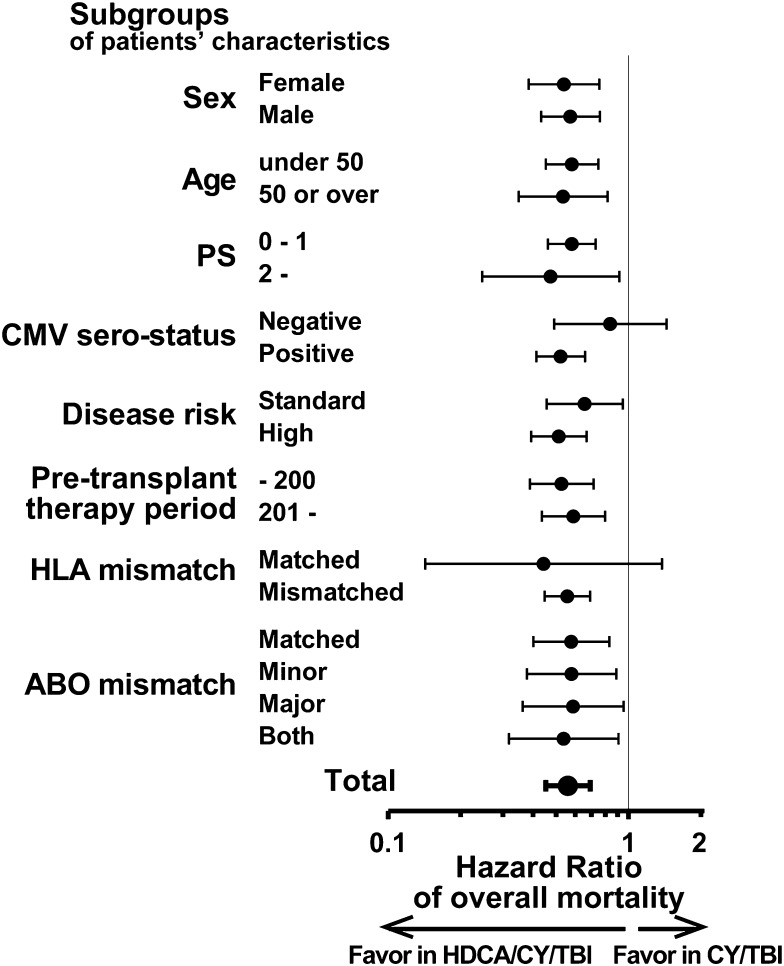
OS of the HDCA/CY/TBI group was superior to that of the CY/TBI group (Figure 1A; 63.6% vs 51.9% at 1 year; 53.0% vs 41.3% at 3 years after CBT). This difference was significant in the univariate analysis (supplemental Table 3; hazard ratio [HR] of overall mortality in the HDCA/CY/TBI group compared with the CY/TBI group, 0.72; 95% confidence interval [CI], 0.60-0.86; P < .01). Among other variables, male patients, older age (aged 50 years or older), poor PS (2 or more), CMV seropositivity, and high-risk disease were associated with poorer survival (P < .05), whereas a longer pretransplant therapy period (more than 200 days), HLA mismatch, and ABO minor mismatch were associated with better survival with borderline significance in univariate analyses (P < .1; supplemental Table 3). In the multivariate analysis including these factors, the CA/CY/TBI group showed a significantly lower overall mortality than the CY/TBI group (HR, 0.56; 95% CI, 0.45-0.69; P < .01; Table 2). This superiority of OS in the HDCA/CY/TBI group was observed in each subgroup according to patient characteristics, with unadjusted HRs being less than 1 in almost all subgroups (Figure 2).
Figure 1.
Prognosis after CBT in each group of the conditioning regimen. (A) HDCA/CY/TBI showed significantly better OS than CY/TBI after being adjusted for confounding factors such as patient sex, age, PS, CMV serostatus, disease risk, pretransplant therapy period, HLA mismatch, and ABO mismatch (P < .01). (B) Tumor-related mortality, defined as death without remission or after relapse, was significantly higher in the CY/TBI group (P < .01 adjusted for the above-mentioned confounding factors). (C) NRM showed no significant differences between the 2 groups.
Table 2.
Multivariate analysis of prognosis in patients with HDCA/CY/TBI compared with CY/TBI
| Variables | Overall mortality | Tumor-related mortality | NRM | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95%CI) | P | HR (95%CI) | P | |
| Conditioning | ||||||
| CY/TBI | Reference | Reference | Reference | |||
| HDCA/CY/TBI | 0.56 (0.45-0.69) | <.01* | 0.50 (0.38-0.67) | <.01* | 0.94 (0.67-1.33) | .73 |
| Sex | ||||||
| Female | Reference | Reference | Reference | |||
| Male | 1.35 (1.10-1.65) | <.01* | 1.18 (0.90-1.55) | .22 | 1.32 (0.96-1.82) | .08 |
| Age, years | ||||||
| <50 | Reference | Reference | Reference | |||
| ≥50 | 1.46 (1.15-1.84) | <.01* | 1.16 (0.84-1.61) | .37 | 1.50 (1.05-2.15) | .03* |
| PS | ||||||
| 0-1 | Reference | Reference | Reference | |||
| 2-4 | 1.80 (1.33-2.43) | <.01* | 1.57 (1.03-2.38) | .03* | 1.23 (0.72-2.11) | .44 |
| CMV serostatus | ||||||
| Negative | Reference | Reference | Reference | |||
| Positive | 1.22 (0.93-1.61) | .15 | 1.43 (0.97-2.11) | .07 | 1.00 (0.67-1.49) | 1.00 |
| Disease risk | ||||||
| Standard | Reference | Reference | Reference | |||
| High | 2.23 (1.78-2.79) | <.01* | 3.94 (2.81-5.52) | <.01* | 0.83 (0.60-1.14) | .25 |
| Pretransplant therapy period, days | ||||||
| ≤200 | Reference | Reference | Reference | |||
| ≥201 | 1.04 (0.85-1.28) | .71 | 0.98 (0.74-1.29) | .88 | 1.11 (0.80-1.53) | .53 |
| HLA mismatch | ||||||
| Matched | Reference | Reference | Reference | |||
| Mismatched | 0.75 (0.46-1.21) | .23 | 0.84 (0.44-1.62) | .61 | 0.84 (0.39-1.83) | .67 |
| ABO mismatch | ||||||
| Matched | Reference | Reference | Reference | |||
| Minor | 0.67 (0.51-0.87) | <.01* | 0.93 (0.66-1.31) | .67 | 0.58 (0.38-0.90) | .02* |
| Major | 0.70 (0.54-0.92) | .01* | 0.77 (0.53-1.12) | .17 | 0.90 (0.61-1.35) | .62 |
| Both | 0.94 (0.70-1.25) | .65 | 1.02 (0.68-1.52) | .93 | 1.01 (0.65-1.57) | .95 |
Statistically significant.
Figure 2.
Subgroup analyses of OS in each group of the conditioning regimen. Superiority in OS of HDCA/CY/TBI (shown in Table 2) was analyzed in detail by each subgroup with respect to patient characteristics. Compared with the CY/TBI group, the unadjusted HRs of overall mortality in the HDCA/CY/TBI group were significantly lower than 1 (ie, HDCA/CY/TBI is prognostically advantageous) in almost all the subgroups. HRs are shown by black dots, and 95% CI ranges are indicated by black bars.
Relapse, tumor-related mortality, and NRM were calculated in the same model; relapse was significantly reduced in the HDCA/CY/TBI group (HR, 0.49; 95% CI, 0.30-0.80; P < .01), resulting in lower tumor-related mortality in this group (Figure 1B; Table 2; HR, 0.50; 95% CI, 0.38-0.67; P < .01), regardless of disease risk (high-risk disease: HR, 0.51; 95% CI, 0.37-0.70; P < .01; standard-risk disease: HR, 0.50; 95% CI, 0.29-0.89; P = .02). HDCA/CY/TBI did not increase NRM in the whole cohort (Figure 1C; Table 2; HR, 0.94; 95% CI, 0.67-1.33; P = .73) or in the subgroups confined to each disease risk (high-risk disease: HR, 0.94; 95% CI, 0.56-1.60; P = .83; standard-risk disease: HR, 0.92; 95% CI, 0.57-1.50; P = .75).
Effects of HDCA on post-CBT clinical course
To compare the clinical courses that lead to the differences in survival and relapse, we focused on engraftment, GVHD, and infection in each group.
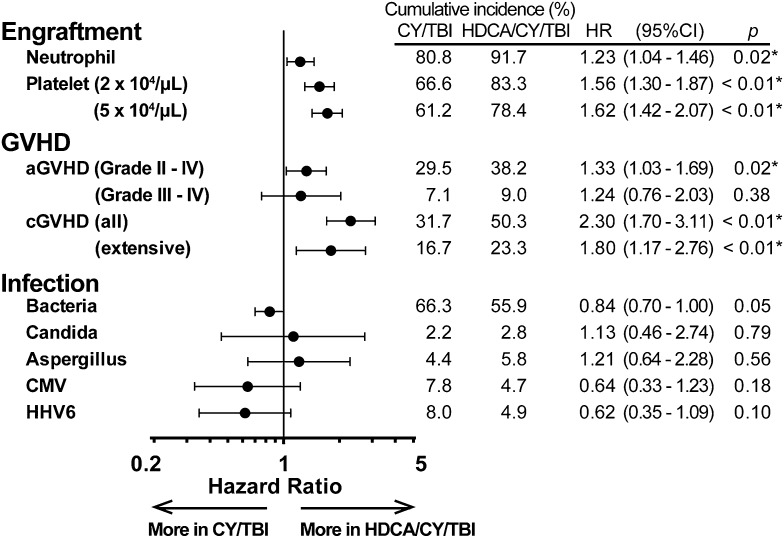
Granulocyte- or macrophage-colony-stimulating factors (G-CSF or M-CSF) were used in most of the patients (95.5% in CY/TBI and 97.1% in HDCA/CY/TBI) after CBT, and the HDCA/CY/TBI group showed a significantly higher proportion of neutrophil and platelet engraftment after CBT (Figure 3). Complete chimerism was achieved in 83.9% (CY/TBI) and 95.3% (HDCA/CY/TBI; P < .01) of those who lived longer than 100 days after CBT. Grade II to IV aGVHD was observed significantly more often in the HDCA/CY/TBI group, whereas grade III to IV aGVHD differed without significance. The incidence of cGVHD was significantly higher in the HDCA/CY/TBI group (Figure 3). This trend of a higher incidence of GVHD in HDCA/CY/TBI was also confirmed in subgroup analyses for GVHD prophylaxis (cyclosporine- and tacrolimus-based) and HLA mismatch (matched and mismatched) (data not shown). With respect to infectious episodes, no significant differences were observed between the 2 groups (Figure 3).
Figure 3.
Clinical courses after CBT in each group of CY/TBI and HDCA/CY/TBI. The cumulative incidence of major events after CBT, such as engraftment, GVHD, and infection, are summarized. In each event, HRs in the HDCA/CY/TBI group were analyzed in comparison with the CY/TBI group after being adjusted for confounding factors. HRs are shown by black dots, and 95% CI ranges are indicated by black bars. Engraftment of neutrophils and platelets was in favor of HDCA/CY/TBI. The incidence of grade II to IV aGVHD and cGVHD was significantly higher in the HDCA/CY/TBI group, but not grade III to IV aGVHD. Addition of HDCA did not cause any increase in the incidence of infection episodes (including bacteria, fungi such as Candia spp. and Aspergillus spp., and viruses such as CMV and human herpes virus-6) compared with the conventional CY/TBI regimen.
The causes of NRM were also compared (Table 3). The major causes of NRM included infection, GVHD, and organ failure in both groups, indicating that the HDCA/CY/TBI regimen did not induce any particular complications leading to post-CBT NRM.
Table 3.
Causes of NRM
| CY/TBI | HDCA/CY/TBI | ||||
|---|---|---|---|---|---|
| Cause of death | N | % | N | % | P |
| Infection | 20 | 29.0 | 34 | 25.7 | .62 |
| Bacteria | 14 | 18 | .22 | ||
| Virus | 3 | 8 | .61 | ||
| Fungi | 2 | 6 | .57 | ||
| GVHD | 5 | 7.3 | 15 | 11.4 | .35 |
| Acute GVHD | 3 | 8 | .61 | ||
| Chronic GVHD | 2 | 7 | .43 | ||
| Rejection/engraftment failure | 6 | 8.7 | 7 | 5.3 | .35 |
| Hemorrhage | 10 | 14.5 | 7 | 5.3 | .03 |
| Interstitial pneumonia | 9 | 13.1 | 14 | 10.6 | .61 |
| VOD/TMA | 4 | 5.8 | 14 | 10.6 | .26 |
| Organ failure | 6 | 8.7 | 15 | 11.4 | .56 |
| Others | 9 | 12.9 | 26 | 19.7 | |
| Total | 69 | 100.0 | 132 | 100.0 | |
TMA, thrombotic microangiopathy; VOD, veno-occlusive disease.
Higher CA dose related to better OS within the HDCA/CY/TBI group
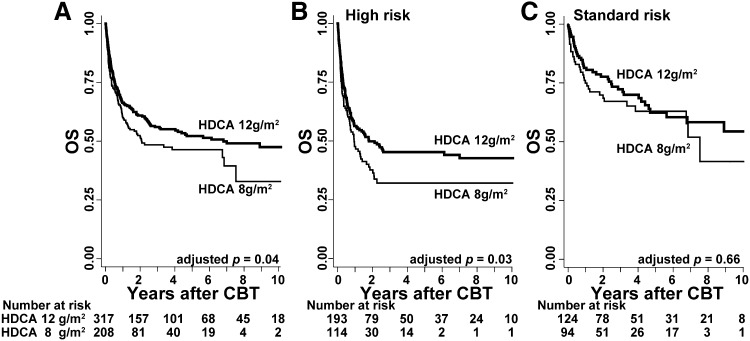
We analyzed the effects of HDCA according to the administrated dosages. In the HDCA/CY/TBI group (n = 617), the majority of the total CA dosage was composed of 12 g/m2 (3 g/m2 per infusion 4 times; n = 317) and 8 g/m2 (2 g/m2 per infusion 4 times; n = 208). Patient characteristics are shown in detail in supplemental Table 4. Comparison of these 2 dosages with respect to OS indicated that OS in the higher-dose (12 g/m2) group was significantly superior to that in the lower-dose (8 g/m2) group (Figure 4A; 68.3% vs 61.6% at 1 year; 58.3% vs 50.5% at 3 years; P = .04) after being adjusted for confounding factors. This improvement in OS was mainly observed for those with high-risk disease (Figure 4B; HR, 0.67; 95% CI, 0.47-0.95; P = .03) compared with those with standard-risk disease (Figure 4C; HR, 0.81; 95% CI, 0.47-1.40; P = .45).
Figure 4.
OS with respect to the dose of CA in the HDCA/CY/TBI group. OS was calculated using the Kaplan-Meier method, and prognosis was compared between the 2 major dosages of HDCA (ie, 12 vs 8 g/m2). (A) OS was higher in the higher-dose HDCA group (12 g/m2) than in the lower-dose group (8 g/m2) (68.3% vs 61.6% at 1 year; 58.3% vs 50.5% at 3 years); this difference was significant (P = .04) after being adjusted for other confounding factors such as patient sex, age, PS, CMV serostatus, disease risk, pretransplant therapy period, HLA mismatch, and ABO mismatch. In the subgroup analyses according to disease risk, (B) higher-dose HDCA significantly showed the better OS in the high-risk group, but (C) not in the standard-risk group.
This difference in OS was mainly a result of lower tumor-related mortality (HR, 0.64; 95% CI, 0.43-0.96; P = .03), and NRM was almost the same between these groups (HR, 0.97; 95% CI, 0.63-1.50; P = .90).
Discussion
The present cohort study regarding addition of HDCA to the conventional CY/TBI regimen in myeloablative CBT for AML/MDS revealed 3 major findings: the HDCA/CY/TBI regimen improved OS mainly through a reduction in relapse and tumor-related mortality, addition of HDCA did not lead to an increase in the incidence of severe adverse events after CBT or NRM, and a higher dose of CA (total 12 vs 8 g/m2) particularly improved the prognosis for patients with high-risk disease.
First, we succeeded in showing the superiority of HDCA/CY/TBI with respect to OS and relapse compared with CY/TBI. Lower relapse rate and tumor-related mortality, as a result, are partially a result of the antileukemia effects of HDCA. CA can eradicate remaining leukemia cells as a result of active uptake into target cells and the subsequent metabolism of CA into its active metabolite (cytarabine triphosphate).22 HDCA can exert stronger effects than normal doses of CA because of the longer contacting time between CA triphosphate and target cells.22 Moreover, administration of HDCA can maintain therapeutic concentrations of CA even in the cerebrospinal fluid.23 These pharmacokinetic and pharmacodynamic features of HDCA may explain the reduction of relapse and the prognostic improvement in our study.
In addition to these antitumor effects, the higher incidence of acute and chronic GVHD in the HDCA/CY/TBI group appears to induce stronger graft-vs-leukemia effects and a lower incidence of relapse. Complications of aGVHD (grade II-IV) and limited cGVHD were related to significantly longer OS and lower tumor-related deaths in the HDCA/CY/TBI group when treating aGVHD or cGVHD as a time-dependent covariant (OS, HR, 0.68 [95% CI, 0.53-0.88; P < .01] and tumor-related death, HR, 0.64 [95% CI, 0.45-0.91; P = .01] in aGVHD; and OS, HR, 0.43 [95% CI, 0.31-0.59; P < .01], and tumor-related death, HR, 0.59 [95% CI, 0.40-0.86; P < .01] in cGVHD), which indicates graft-vs-leukemia effects. A higher incidence of GVHD in the HDCA/CY/TBI group was also observed in the subgroup analysis of GVHD prophylaxis (cyclosporine or tacrolimus) and HLA disparity (mismatched or matched) (data not shown). Larger amounts of proinflammatory cytokine release resulting from more severe tissue injury by HDCA may possibly be involved in a higher incidence of GVHD, similar to that in the case of TBI.24,25 To the best of our knowledge, however, there are no other reports revealing a relationship between HDCA and the incidence of GVHD.
Two effects of HDCA, such as the antileukemia effect and stronger graft-vs-tumor effect, may synergistically reduce the risk for relapse and account for the superiority in HDCA/CY/TBI after CBT. This regimen should be compared not only with CY/TBI but also with other myeloablative regimens in CBT. Several retrospective registry-based studies compared 2 major conventional myeloablative regimens in patients with AML (CY/TBI vs BU/CY), with some conflicting results on outcome and toxicity.5,6 Addition of HDCA to CY/TBI may possibly overwhelm the BU/CY regimen. In addition to these conventional regimens, newly developed myeloablative protocols including BU or treosulfan, a prodrug of bifunctional alkylating agent, have widely been known. Recent data from Eurocord showed that CBT with thiotepa/BU/fludarabine led significantly better results compared with CY/TBI and BU/CY.26 The combination of treosulfan and fludarabine was associated with limited NRM and favorable OS,27 and the addition of low-dose TBI to treosulfan/fludarabine in a phase 2 trial provided low relapse incidence without increased NRM.28 These regimens should be compared with HDCA/CY/TBI in future randomized controlled studies.
Second, we clearly showed that the adverse events in HDCA/CY/TBI are similar to those for CY/TBI with respect to type and incidence. Whether the NRM increases or not by the addition of HDCA has long been a matter of debate; controversial reports8,10,11,29-35 have been published, including various donor sources. Our study, for the first time, revealed the same incidence of NRM after CBT, which can be strong evidence justifying CBT for AML/MDS with the conditioning regimen of HDCA/CY/TBI.
The causes of NRM were compared between 2 groups, and no lethal adverse events specific to HDCA/CY/TBI were found. Moreover, infectious episodes were analyzed in detail, and we found that no infections resulting from bacteria, fungi, or virus increased even after the HDCA/CY/TBI regimen (Figure 3). These results are different from previous reports showing a higher incidence of infection and NRM.10,11 The discrepancy between studies may be attributed partly to the progress in supportive therapies, such as antibiotics, during this decade. A higher incidence of engraftment in the HDCA/CY/TBI group may also contribute to a reduction in severe infections, particularly in the acute phase after CBT. With respect to the other adverse effects unique to HDCA, such as encephalopathy, retinitis, mucositis, or hepatotoxicity,22 our database unfortunately lacks these types of data. Other reports showed that these symptoms were commonly observed in the HDCA/CY/TBI regimen, although they were self-limiting and well-managed,30,33 which supports the feasibility of this regimen in myeloablative transplantation.
In terms of the adverse events, statistical analyses showed that male patient and HLA match are associated with poor prognosis (Table 2; supplemental Table 3). Higher overall mortality and NRM in male patients were found in the whole cohort and in each group of the conditioning regimens (data not shown). These data can be explained by the significantly higher proportion of males in the older patients (P < .01), or the slightly higher mortality in CBT from female donor to male recipient (supplemental Table 3), which may be attributed to the existence of human minor histocompatibility antigens encoded by the Y chromosome.36 In contrast, we showed that mismatched HLA was related to better OS with borderline significance in the univariate analysis, but not in the multivariate model. Regarding the association with HLA mismatch and prognosis, some controversial data have been published. Relationship between greater HLA discrepancy and higher NRM or poorer OS in CBT was shown from the National Cord Blood Program of the New York Blood Center37 or the Center for International Blood and Marrow Transplant Research and Eurocord.38 In contrast, pooled analysis data from Center for International Blood and Marrow Transplant Research, New York Blood Center, and Eurocord-Netcord registry39 and another report from Japan4 indicate there was no significant relationship between HLA disparity and the prognosis when focusing only on adult patients. In our cohort, as few as 37 CBTs (4%) were HLA-matched, and further recruitment of HLA-matched CBT cases is necessary to confirm the association between them and to resolve this controversy.
The relationship between dosage (“high-dose” CA) and prognosis is also a novel finding, comparing the use of 8 and 12 g/m2 in our cohort. Dosage selection was subject to each attending physician, and 12 g/m2 was often used for patients with high-risk disease, younger patients, or patients with a better PS, although the difference was not statistically significant (supplemental Table 4).
This study, for the first time, showed better OS, lower relapse or tumor-related mortality, and comparable NRM, particularly in the high-risk disease group with 12 g/m2 of CA compared with 8 g/m2; these results may possibly provide a rationale for higher-dose CA usage in the patients with high-risk AML/MDS. An even higher dose of CA (>12 g/m2) was used in some previous reports but was not selected for use in our cohort.
The combination of G-CSF with HDCA is another important topic: It is hypothesized that G-CSF increases the susceptibility of myeloid leukemic cells to HDCA.40 In a previous report, CBT for myeloid malignancies with G-CSF-combined HDCA/CY/TBI provided low NRM (15% at 3 years) and high OS (51% at 3 years) in a multicenter prospective analysis.9 This regimen may reduce posttransplant relapse in patients with AML/MDS, particularly those transplanted in nonremission status.8 In our cohort, patients with G-CSF-combined HDCA/CY/TBI regimen (n = 146) revealed relatively promising prognosis (OS; 79.2% at 1 year, and 69.1% at 3 years after CBT). However, we have data on only a part of the cohort concerning G-CSF administration during conditioning regimens, and statistical comparisons to other groups were not carried out. Further analysis will be necessary, comparing HDCA/CY/TBI with and without G-CSF combination.
In summary, we found the superiority of HDCA/CY/TBI regimen relative to the conventional CY/TBI regimen in myeloablative CBT for AML/MDS with respect to a lower incidence of relapse and better OS. Adverse effects resulting from HDCA can be properly managed. Our data should be validated internationally because outcomes of CBT in Japan have been better than those in other countries, partly as a result of genetic homogeneity in the Japanese population.41 Moreover, large-scale prospective studies, which also involve systemic screening of late-onset complications for long-term survivors,19 are necessary to establish new conditioning regimens including HDCA administration.
Acknowledgments
We thank all the physicians and data managers at the centers, who contributed valuable data on transplantation to the Japan Society for Hematopoietic Cell Transplantation (JSHCT), the Japan Cord Blood Bank Network (JCBBN), and the Transplant Registry Unified Management Program (TRUMP). We also thank the members of the Data Management Committees of JSHCT, JCBBN, and TRUMP for their assistance and Dr Kazuya Okada (Kurashiki Central Hospital), Dr Tomoaki Ueda (Osaka University), and Dr Tomoyasu Jo (Kyoto University) for their critical discussion on this study.
This study was supported by research funding from the Ministry of Education, Science, Sports, and Culture in Japan to T.K.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: Y. Arai designed the study, reviewed and analyzed data, and wrote the paper; J.T., K.A, T.K., and A.T. interpreted data and revised the manuscript; and S.T., Y. Onishi, Y. Ozawa, N.A., Y.K., H.N., S.O., C.N., H.Y., K.K., and Y. Atsuta contributed to the data collection and critiqued the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Tadakazu Kondo, Department of Hematology and Oncology, Graduate School of Medicine, Kyoto University, 54, Shogoin Kawahara-cho, Sakyo-ku, Kyoto, 606-8507, Japan; e-mail: tadakazu@kuhp.kyoto-u.ac.jp.
References
- 1.Duval M, Klein JP, He W, et al. Hematopoietic stem-cell transplantation for acute leukemia in relapse or primary induction failure. J Clin Oncol. 2010;28(23):3730–3738. doi: 10.1200/JCO.2010.28.8852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ballen KK, Gluckman E, Broxmeyer HE. Umbilical cord blood transplantation: the first 25 years and beyond. Blood. 2013;122(4):491–498. doi: 10.1182/blood-2013-02-453175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takahashi S, Ooi J, Tomonari A, et al. Comparative single-institute analysis of cord blood transplantation from unrelated donors with bone marrow or peripheral blood stem-cell transplants from related donors in adult patients with hematologic malignancies after myeloablative conditioning regimen. Blood. 2007;109(3):1322–1330. doi: 10.1182/blood-2006-04-020172. [DOI] [PubMed] [Google Scholar]
- 4.Atsuta Y, Kanda J, Takanashi M, et al. HLA Working Group of the Japan Society for Hematopoietic Cell Transplantation. Different effects of HLA disparity on transplant outcomes after single-unit cord blood transplantation between pediatric and adult patients with leukemia. Haematologica. 2013;98(5):814–822. doi: 10.3324/haematol.2012.076042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nagler A, Rocha V, Labopin M, et al. Allogeneic hematopoietic stem-cell transplantation for acute myeloid leukemia in remission: comparison of intravenous busulfan plus cyclophosphamide (Cy) versus total-body irradiation plus Cy as conditioning regimen—a report from the acute leukemia working party of the European group for blood and marrow transplantation. J Clin Oncol. 2013;31(28):3549–3556. doi: 10.1200/JCO.2013.48.8114. [DOI] [PubMed] [Google Scholar]
- 6.Copelan EA, Hamilton BK, Avalos B, et al. Better leukemia-free and overall survival in AML in first remission following cyclophosphamide in combination with busulfan compared with TBI. Blood. 2013;122(24):3863–3870. doi: 10.1182/blood-2013-07-514448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hiddemann W. Cytosine arabinoside in the treatment of acute myeloid leukemia: the role and place of high-dose regimens. Ann Hematol. 1991;62(4):119–128. doi: 10.1007/BF01702925. [DOI] [PubMed] [Google Scholar]
- 8.Konuma T, Kato S, Ooi J, et al. Single-unit cord blood transplantation after granulocyte colony-stimulating factor-combined myeloablative conditioning for myeloid malignancies not in remission. Biol Blood Marrow Transplant. 2014;20(3):396–401. doi: 10.1016/j.bbmt.2013.12.555. [DOI] [PubMed] [Google Scholar]
- 9.Mori T, Tanaka M, Kobayashi T, et al. Kanto Study Group for Cell Therapy. Prospective multicenter study of single-unit cord blood transplantation with myeloablative conditioning for adult patients with high-risk hematologic malignancies. Biol Blood Marrow Transplant. 2013;19(3):486–491. doi: 10.1016/j.bbmt.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 10.Inamoto Y, Nishida T, Suzuki R, et al. Significance of additional high-dose cytarabine in combination with cyclophosphamide plus total body irradiation regimen for allogeneic stem cell transplantation. Bone Marrow Transplant. 2007;39(1):25–30. doi: 10.1038/sj.bmt.1705543. [DOI] [PubMed] [Google Scholar]
- 11.Kanda Y, Sakamaki H, Sao H, et al. Japan Marrow Donor Program. Effect of conditioning regimen on the outcome of bone marrow transplantation from an unrelated donor. Biol Blood Marrow Transplant. 2005;11(11):881–889. doi: 10.1016/j.bbmt.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 12.Atsuta Y, Suzuki R, Yoshimi A, et al. Unification of hematopoietic stem cell transplantation registries in Japan and establishment of the TRUMP System. Int J Hematol. 2007;86(3):269–274. doi: 10.1532/IJH97.06239. [DOI] [PubMed] [Google Scholar]
- 13.Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106(8):2912–2919. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ho VT, Kim HT, Aldridge J, et al. Use of matched unrelated donors compared with matched related donors is associated with lower relapse and superior progression-free survival after reduced-intensity conditioning hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2011;17(8):1196–1204. doi: 10.1016/j.bbmt.2010.12.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glucksberg H, Storb R, Fefer A, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18(4):295–304. doi: 10.1097/00007890-197410000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15(6):825–828. [PubMed] [Google Scholar]
- 17.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11(12):945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 18.Okamoto S, Kondo S, Yano K, et al. Tokyo, Japan: Japanese Society for Hematopoietic Cell Transplantation; 2012. Guideline for hematopoietic cell transplantation - Infection control in early-phase after transplantation. pp. 5–25. [Google Scholar]
- 19.Majhail NS, Rizzo JD, Lee SJ, et al. Center for International Blood and Marrow Transplant Research (CIBMTR); American Society for Blood and Marrow Transplantation (ASBMT); European Group for Blood and Marrow Transplantation (EBMT); Asia-Pacific Blood and Marrow Transplantation Group (APBMT); Bone Marrow Transplant Society of Australia and New Zealand (BMTSANZ); East Mediterranean Blood and Marrow Transplantation Group (EMBMT); Sociedade Brasileira de Transplante de Medula Ossea (SBTMO) Recommended screening and preventive practices for long-term survivors after hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2012;18(3):348–371. doi: 10.1016/j.bbmt.2011.12.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gray RJ. A class of κ-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16(3):1141–1154. [Google Scholar]
- 21.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509. [Google Scholar]
- 22.Reese ND, Schiller GJ. High-dose cytarabine (HD araC) in the treatment of leukemias: a review. Curr Hematol Malig Rep. 2013;8(2):141–148. doi: 10.1007/s11899-013-0156-3. [DOI] [PubMed] [Google Scholar]
- 23.Frick J, Ritch PS, Hansen RM, Anderson T. Successful treatment of meningeal leukemia using systemic high-dose cytosine arabinoside. J Clin Oncol. 1984;2(5):365–368. doi: 10.1200/JCO.1984.2.5.365. [DOI] [PubMed] [Google Scholar]
- 24.Clift RA, Buckner CD, Appelbaum FR, et al. Allogeneic marrow transplantation in patients with chronic myeloid leukemia in the chronic phase: a randomized trial of two irradiation regimens. Blood. 1991;77(8):1660–1665. [PubMed] [Google Scholar]
- 25.Deeg HJ, Spitzer TR, Cottler-Fox M, Cahill R, Pickle LW. Conditioning-related toxicity and acute graft-versus-host disease in patients given methotrexate/cyclosporine prophylaxis. Bone Marrow Transplant. 1991;7(3):193–198. [PubMed] [Google Scholar]
- 26.Ruggeri A, Sanz G, Bittencourt H, et al. Eurocord and Acute Leukemia Working Party of European Blood and Marrow Transplant Group. Comparison of outcomes after single or double cord blood transplantation in adults with acute leukemia using different types of myeloablative conditioning regimen, a retrospective study on behalf of Eurocord and the Acute Leukemia Working Party of EBMT. Leukemia. 2014;28(4):779–786. doi: 10.1038/leu.2013.259. [DOI] [PubMed] [Google Scholar]
- 27.Danylesko I, Shimoni A, Nagler A. Treosulfan-based conditioning before hematopoietic SCT: more than a BU look-alike. Bone Marrow Transplant. 2012;47(1):5–14. doi: 10.1038/bmt.2011.88. [DOI] [PubMed] [Google Scholar]
- 28.Gyurkocza B, Gutman J, Nemecek ER, et al. Treosulfan, fludarabine, and 2-Gy total body irradiation followed by allogeneic hematopoietic cell transplantation in patients with myelodysplastic syndrome and acute myeloid leukemia. Biol Blood Marrow Transplant. 2014;20(4):549–555. doi: 10.1016/j.bbmt.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Riddell S, Appelbaum FR, Buckner CD, et al. High-dose cytarabine and total body irradiation with or without cyclophosphamide as a preparative regimen for marrow transplantation for acute leukemia. J Clin Oncol. 1988;6(4):576–582. doi: 10.1200/JCO.1988.6.4.576. [DOI] [PubMed] [Google Scholar]
- 30.Jillella AP, Doria R, Khan K, et al. Cyclophosphamide, cytosine arabinoside and TBI as a conditioning regimen for allogeneic bone marrow transplantation in patients with leukemia. Bone Marrow Transplant. 1999;23(11):1095–1100. doi: 10.1038/sj.bmt.1701786. [DOI] [PubMed] [Google Scholar]
- 31.Mineishi S, Longo WL, Atkinson ME, et al. Addition of high-dose Ara-C to the BMT conditioning regimen reduces leukemia relapse without an increase in toxicity. Bone Marrow Transplant. 1999;23(12):1217–1222. doi: 10.1038/sj.bmt.1701793. [DOI] [PubMed] [Google Scholar]
- 32.Takahashi S, Iseki T, Ooi J, et al. Single-institute comparative analysis of unrelated bone marrow transplantation and cord blood transplantation for adult patients with hematologic malignancies. Blood. 2004;104(12):3813–3820. doi: 10.1182/blood-2004-03-1001. [DOI] [PubMed] [Google Scholar]
- 33.Mori T, Aisa Y, Kato J, et al. Safety and efficacy of total body irradiation, cyclophosphamide, and cytarabine as a conditioning regimen for allogeneic hematopoietic stem cell transplantation in patients with acute lymphoblastic leukemia. Am J Hematol. 2012;87(4):349–353. doi: 10.1002/ajh.23109. [DOI] [PubMed] [Google Scholar]
- 34.Petersen FB, Appelbaum FR, Buckner CD, et al. Simultaneous infusion of high-dose cytosine arabinoside with cyclophosphamide followed by total body irradiation and marrow infusion for the treatment of patients with advanced hematological malignancy. Bone Marrow Transplant. 1988;3(6):619–624. [PubMed] [Google Scholar]
- 35.Woods WG, Ramsay NK, Weisdorf DJ, et al. Bone marrow transplantation for acute lymphocytic leukemia utilizing total body irradiation followed by high doses of cytosine arabinoside: lack of superiority over cyclophosphamide-containing conditioning regimens. Bone Marrow Transplant. 1990;6(1):9–16. [PubMed] [Google Scholar]
- 36.Ringdén O, Labopin M, Solders M, et al. Acute Leukaemia Working Party of the European Group for Blood and Marrow Transplantation. Who is the best hematopoietic stem-cell donor for a male patient with acute leukemia? Transplantation. 2014;98(5):569–577. doi: 10.1097/TP.0000000000000102. [DOI] [PubMed] [Google Scholar]
- 37.Barker JN, Scaradavou A, Stevens CE. Combined effect of total nucleated cell dose and HLA match on transplantation outcome in 1061 cord blood recipients with hematologic malignancies. Blood. 2010;115(9):1843–1849. doi: 10.1182/blood-2009-07-231068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eapen M, Klein JP, Ruggeri A, et al. Center for International Blood and Marrow Transplant Research, Netcord, Eurocord, and the European Group for Blood and Marrow Transplantation. Impact of allele-level HLA matching on outcomes after myeloablative single unit umbilical cord blood transplantation for hematologic malignancy. Blood. 2014;123(1):133–140. doi: 10.1182/blood-2013-05-506253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cohen YC, Scaradavou A, Stevens CE, et al. Factors affecting mortality following myeloablative cord blood transplantation in adults: a pooled analysis of three international registries. Bone Marrow Transplant. 2011;46(1):70–76. doi: 10.1038/bmt.2010.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Löwenberg B, van Putten W, Theobald M, et al. Dutch-Belgian Hemato-Oncology Cooperative Group; Swiss Group for Clinical Cancer Research. Effect of priming with granulocyte colony-stimulating factor on the outcome of chemotherapy for acute myeloid leukemia. N Engl J Med. 2003;349(8):743–752. doi: 10.1056/NEJMoa025406. [DOI] [PubMed] [Google Scholar]
- 41.Ballen KK, Barker JN. Has umbilical cord blood transplantation for AML become mainstream? Curr Opin Hematol. 2013;20(2):144–149. doi: 10.1097/MOH.0b013e32835dd936. [DOI] [PubMed] [Google Scholar]