Abstract
Colorectal cancer (CRC) is one of the most common human malignancies and a leading cause of cancer-related deaths in developed countries. Identifying effective preventive strategies aimed at inhibiting the development and progression of CRC is critical for reducing the incidence and mortality of this malignancy. The prevention of carcinogenesis by anti-inflammatory agents including nonsteroidal anti-inflammatory drugs (NSAIDs), selective cyclooxygenase-2 (COX-2) inhibitors, and natural products is an area of considerable interest and research. Numerous anti-inflammatory agents have been identified as potential CRC chemopreventive agents but vary in their mechanism of action. This review will discuss the molecular mechanisms being studied for the CRC chemopreventive activity of NSAIDs (i.e., aspirin, sulindac, and ibuprofen), COX-2 inhibitors (i.e., celecoxib), natural products (i.e., curcumin, resveratrol, EGCG, genistein, and baicalein), and metformin. A deeper understanding of how these anti-inflammatory agents inhibit CRC will provide insight into the development of potentially safer and more effective chemopreventive drugs.
Keywords: NSAIDS, natural products, colorectal cancer, chemoprevention, anti-inflammatory agents
chemoprevention strategies utilizing natural products or synthetic drugs provide opportunities to prevent or delay the development and reverse the progression of cancer. Carcinogenesis is a multistage process often occurring over decades that involves cumulative molecular and cellular aberrations. The stages consist of initiation, promotion, and progression in which each stage involves a disruption of a multitude of signaling cascades (126). This multistage sequence of biological processes provides opportunities to target specific biochemical targets by small molecules for cancer intervention (99). Cancer chemoprevention strategies can be divided into three types: 1) primary prevention, a strategy aimed to prevent cancer in all individuals; 2) secondary prevention, a strategy to prevent cancer in individuals with premalignant conditions; and 3) tertiary prevention, a strategy to prevent further disease progression (i.e., metastasis) after an initial diagnosis of malignant disease (47, 56). The use of nonsteroidal anti-inflammatory drugs (NSAIDs) and certain natural products has been shown to prevent colorectal cancer (CRC) during each stage of carcinogenesis. The preventive activities of both NSAIDs and natural products have been a major focus of research involving in vitro and in vivo experimental models, epidemiological studies, and clinical trials (38, 47, 99). Chronic inflammation is associated with the development of cancer and promoting progression into a malignant phenotype (25). Because of this association, the use of anti-inflammatory agents to disrupt and retard carcinogenesis is considered to provide a highly effective strategy for cancer chemoprevention.
Colorectal Cancer
CRC is the third leading cause of cancer-related deaths in men and women in the United States (6). CRC is the second most commonly diagnosed cancer in women and the third in men worldwide (52). CRC rate trends are higher in developed countries compared with developing countries, which is supported by evidence from ecological, migrant, and secular trend studies, suggesting that environmental, dietary, and lifestyle factors may be important causes of CRC (9, 52). This trend may be due to the variations in risk factors such as diet, red meat consumption, cigarette smoking, and alcohol consumption (52). In Western countries, especially the United States, there is a recent decline in CRC incidence rates that may be due to increased screening (i.e., colonoscopy and fecal occult blood test). Increased screening allows for the early detection and removal of precancerous polyps (52, 107). Indeed, the 5-year survival rate of CRC if diagnosed at an early and localized stage is 90% (6, 99). Unfortunately, greater than 50% of patients are diagnosed with advanced disease when distant metastasis has already occurred, in which the 5-year survival rate precipitously drops to 10% (6, 99, 128). The use of anti-inflammatory agents could substantially reduce the incidence and mortality of CRC (50), although safety and efficacy are important considerations for developing such approaches.
Sporadic CRC.
CRC can be divided into two classes: 1) sporadic where there is no (or very little) prior family history of CRC, and 2) familial or hereditary in which inherited gene mutations predispose an individual to develop CRC. Only about 5–10% of CRCs are due to inherited gene defects, whereas the majority of CRC cases are sporadic (2). Although age, sex, and ethnicity are risk factors for CRC, there are key lifestyle risk factors that can be moderated including smoking, physical inactivity, obesity, heavy alcohol consumption, and diets high in saturated fats (52, 101, 125). Diets high in red and processed meat intake are also associated with increased risk of CRC development (101, 125). Red and processed meats have been shown to be high in DNA-damaging N-nitroso compounds as well as meats cooked at high temperatures (i.e., grilled, charbroiled, or blackened) that contain high levels of mutagenic heterocyclic amines (125).
In 1990, Fearon and Vogelstein (31) described colon tumorigenesis as a multistep process in which the accumulation of multiple genetic mutations is required for cancer initiation, promotion, and progression. The colorectal mucosa consists of a large number of invaginations called the crypts of Lieberkühn. Epithelial cell renewal occurs in these crypts through a series of events involving proliferation, differentiation, and migration (124). The progression of CRC is though stages that range from single crypt lesions (e.g., aberrant crypt foci) to adenomatous polyps to malignant carcinomas (77). The progression of these stages is believed to require mutations in multiple oncogenes and tumor suppressor genes (124). The progression of precancerous cells into an invasive phenotype can span over a decade (42). A key molecular step in colorectal tumorigenesis is the loss of genomic stability. Genomic instability is the hallmark of CRC and creates an environment suitable for alterations in tumor suppressor genes and oncogenes (35, 50). Identification of anti-inflammatory agents that suppress and/or prevent the consequences of genetic mutations that give rise to CRC is therefore of critical importance.
Hereditary CRC.
There are multiple inherited conditions that predispose an individual to CRC. The two major hereditary CRC syndromes are hereditary nonpolyposis CRC (HNPCC) and familial adenomatous polyposis (FAP). HNPCC, or Lynch syndrome, is the most commonly inherited CRC syndrome (15). Patients with HNPCC have an 80% chance of developing CRC (128). HNPCC is a distinct autosomal dominant inheritable condition caused by germline mutations in DNA mismatch repair (MMR) genes. The loss of MMR function leads to mutations in microsatellites (repetitive sequences) and microsatellite instability (MSI) thus causing a mutable phenotype (15, 66). The primary MMR enzymes associated with this MSI are MLH1 and MSH2 (69, 128). It is estimated that HNPCC accounts for 1–5% of CRC cases. FAP is rare autosomal dominant disorder due to germline mutations in the adenomatous polyposis coli (APC) gene, resulting in loss of function of the APC protein, which has tumor suppressor function (128). FAP is characterized by the occurrence of hundreds to thousands of colorectal polyps, which if not removed can develop into adenocarcinoma (15, 66).
The APC protein plays a key regulatory role in suppressing the oncogenic Wnt/β-catenin pathway (104). In FAP, mutations in the APC gene by insertions, deletions, or nonsense mutations result in a truncated APC protein. The truncated APC protein is associated with loss of β-catenin binding sites, thus allowing for the constitutive activation of β-catenin signaling (73, 104). In colonocytes in the absence of Wnt signaling, cytoplasmic β-catenin is normally degraded by the Axin complex (73). This complex consists of APC, the scaffold protein Axin, casein kinase 1 (CK1), and glycogen synthase kinase 3β (GSK3β). CK1 and GSK3β phosphorylate β-catenin, resulting in the ubiquitination and proteasomal degradation of β-catenin (71, 73). The degradation of β-catenin prevents its availability to act as a nuclear coactivator of the transcription factor family, T cell factor/lymphoid enhancer factor (TCF/LEF), thus inhibiting Wnt target gene expression such as c-Myc, cyclin D1, survivin, and PPAR (44, 72, 73, 95).
In unstimulated cells free β-catenin levels are regulated by ubiquitination and proteasomal degradation through the promotion of phosphorylation by the APC/Axin/GSK3β complex (72). The Wnt/β-catenin pathway is activated when Wnt ligands (e.g., WNT3A), cysteine-rich proteins consisting of 350–400 amino acids and containing a NH2-terminal secretion peptide, bind the Frizzled receptor and its coreceptor low-density lipoprotein receptor-related protein 6 (LRP6) or LRP5 (73). This complex along with the scaffold protein Dishevelled (Dvl) results in the recruitment of the APC/Axin/GSK3β complex, thus preventing β-catenin degradation, leading to its translocation into the nucleus and binding of TCF/LEF to stimulate transcription of proliferative and oncogenic genes (i.e., c-myc and cyclin D1) (71, 73, 127). In both inherited and sporadic CRC, mutations in the APC gene result in the deregulation of β-catenin degradation and thus lead to increased stability and activation of β-catenin transcriptional activity (Fig. 1) (8). Disruption of the APC pathway is one of the most prevalent genetic events identified in CRC that is altered in 95% of colorectal tumors (77). Therefore, identifying inhibitors that target this pathway either directly or indirectly can delay or prevent the development and/or progression of CRC.
Fig. 1.
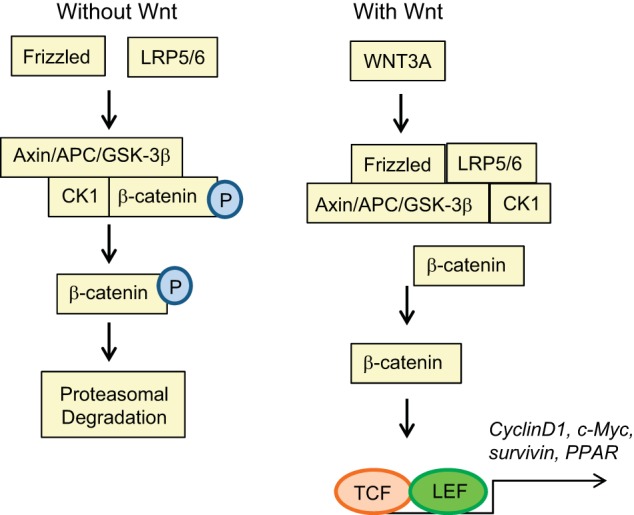
Simplified overview of the WNT/β-catenin signal transduction cascade. TCF, T cell factor; LEF, lymphoid enhancer factor.
NSAIDs and COX-2 Inhibitors
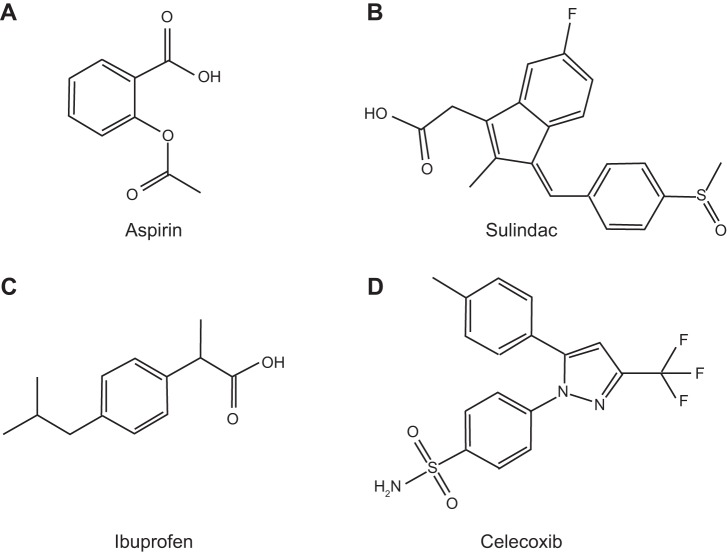
NSAIDs and cyclooxygenase (COX)-2 inhibitors (coxibs) have been proven to be promising agents for chemoprevention of CRC. The suppressive effect of these agents on tumorigenesis has been supported by a number of epidemiological studies, clinical trials, and animal studies. NSAID and coxib inhibition of tumorigenesis is believed to be mediated through multiple cellular processes including apoptosis, cell-cycle arrest, and inhibition of angiogenesis (40, 50). For the purposes of this review, the NSAIDs and coxibs discussed are aspirin, sulindac, ibuprofen, and celecoxib (Fig. 2). The non-COX inhibitory sulfone metabolite of sulindac, which is not a NSAID, is also discussed. A commonality of both NSAIDs and coxibs is the inhibition of COX enzyme activity, although they vary in their potency and selectivity to inhibit COX-1 and/or COX-2.
Fig. 2.
Selected nonsteroidal anti-inflammatory drug (NSAID) chemical structures. Aspirin (A), sulindac (B), ibuprofen (C), and celecoxib (D) are shown.
There are three isoforms of COX enzymes. COX-1 is widely distributed, is constitutively expressed, regulates renal blood flow, and protects intestinal mucosal integrity (115). COX-2 is highly inducible and has been shown to play a role in tumor progression, whereas COX-3 is a splice variant form of COX-1 (42, 116). COX-1 and COX-2 catalyze the rate-limiting step in metabolic conversion arachidonic acid (AA) to prostaglandins (PGs) and thromboxanes (131). The COX-2 gene is an early response gene transcriptionally regulated by neoplastic and inflammatory stimuli (i.e., cytokines, growth factors, and mitogens) and plays an important role in tumorigenesis (42, 116). COX-2 levels have been demonstrated to be elevated in premalignant and malignant tissues (116). In 85–90% of CRC and 40–50% of adenomas, COX-2 expression is overexpressed and associated with lower survival of patients with CRC (116, 128). Both COX enzymes catalyze the conversion of AA into prostaglandin H2 (PGH2), which is the PG precursor (Fig. 3) (40). PGs, specifically PGE2, have been shown to be an important mediator of the proinflammatory and tumor-promoting effects of COX-2 (66). PGE2 induces cell growth and numerous processes associated with inflammation (51). The degradation of PGE2 is catalyzed by the tumor suppressor 15-prostaglandin dehydrogenase (15-PGDH), which has been shown to be downregulated in FAP patients and is a target of β-catenin gene repression (110, 116). The increased production of PGE2 is correlated with lipid peroxidation and the formation of DNA adducts to accelerate colon carcinogenesis (85).
Fig. 3.
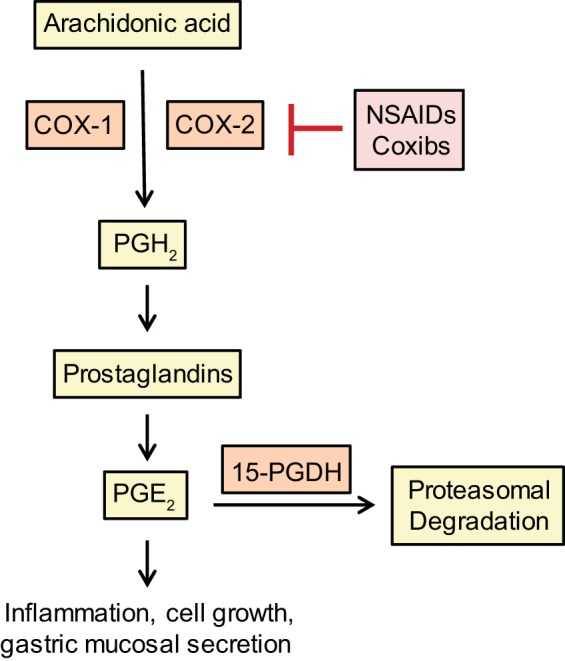
Simplified overview of the NSAIDs and coxibs, inhibition of COX signal transduction cascade.
The inhibition of COX and PG synthesis may not be the only mechanisms of action of NSAIDs. Although the initial mechanism thought to be responsible for the chemopreventive activity of NSAIDs was attributed to the inhibition of COX, it is now recognized that the chemopreventive activity may involve COX-independent mechanisms (3, 39, 90, 93, 106).
Aspirin.
NSAIDs are a chemically diverse group of compounds that inhibit COX-1 and COX-2 and share similar side effects (32). The oldest and most common NSAID is aspirin (acetylsalicylic acid), which irreversibly inhibits both COX-1 and COX-2, by acetylation of serine residues Ser529 and Ser516 in the catalytic domain (40, 66). However, the inhibition of COX-1 and COX-2 is concentration dependent. At low concentrations aspirin preferentially inhibits COX-1, whereas higher concentrations will inhibit both COX-1 and COX-2 (123). The irreversible inhibition of COX due to acetylation is a unique property of aspirin that is not shared by other NSAIDs. Aspirin inhibits PG synthesis in tumors, but the effect is not complete, which may explain its modest efficacy or suggest that other mechanisms are involved (104).
Because of its widespread use for preventing heart attacks and strokes, aspirin is the most investigated NSAID for CRC prevention in epidemiological studies (Fig. 2A) (19). An inverse association between aspirin use and CRC risk has been observed in numerous cohort and case-control studies (19, 119). In the Cancer Prevention Study II, men and women showed that aspirin use at least 16 times per month over 6-year period was associated with a 40% reduced risk of colon cancer mortality. The U.S. Health Professionals and U.S. Nurses' Health studies showed similar findings that regular aspirin use (>2×/wk) was associated with a reduced risk of CRC with 18–20 years follow-up (19). A consistent observation of these studies is that the benefits of aspirin use increase with longer duration of use (19). Although aspirin has been shown to be an effective chemopreventive agent in sporadic CRC, it has shown little efficacy in clinical trials of hereditary CRC. In the Colorectal Adenoma/Carcinoma Prevention Programme 1 (CAPP1) and CAPP2 studies, aspirin use did not significantly inhibit polyp formation in patients with FAP or Lynch syndrome (19).
A COX-independent mechanism for the chemopreventive activity of aspirin may involve the inhibition of the transcription factors, specificity proteins (Sp) Sp1-4. Sp proteins regulate the expression of several critical genes involved in cell proliferation, cell survival, angiogenesis, and inflammation pathways (21). Aspirin and its major metabolite, sodium salicylate, inhibit the expression of Sp1, Sp3, and Sp4 through caspase-dependent proteolysis in colon cancer cells (88). The COX-2 inhibitor, celecoxib, can downregulate Sp1 and Sp4 proteins in several colon cancer cells (1). Aspirin has been reported to decrease colon tumor growth and Sp1, Sp3, and Sp4 expressions in a xenograft model (88). The inhibition of expressions and activities of Sp1, Sp3, and Sp4 by aspirin may be a potential explanation for the anti-tumorigenic activity of aspirin that involves a COX-independent mechanism.
Sulindac.
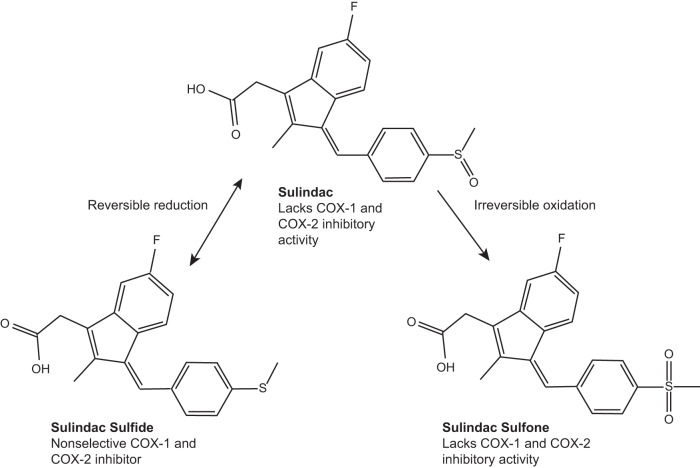
The NSAID sulindac is a prodrug with a sulfoxide moiety that requires in vivo reduction to the pharmacologically active sulindac sulfide (SS) by colonic bacteria (18). Sulindac and SS are irreversibly oxidized to a sulfone metabolite following oral administration (Fig. 4). SS is known to be a potent inhibitor of COX-1 and COX-2 enzyme activity compared with the sulindac sulfone metabolite, which does not inhibit COX-1 or COX-2 (91, 92). In clinical trials, FAP patients had reduced size and number of polyps after 6–9 mo of treatment with sulindac (33). Sulindac was also shown to reduce and/or regress adenomatous polyps in sporadic CRC patients (76).
Fig. 4.
Sulindac and metabolites. Prodrug sulidac, which lacks cyclooxygenase (COX) inhibitory activity, undergoes a reversible reduction into the active COX inhibitory sulindac sulfide form. Sulindac can also be irreversibly oxidized into the sulindac sulfone form, which lacks COX inhibitory activity. Figure adapted from Ref. 40.
Sulindac sulfone (exisulind) demonstrates an anti-tumor effect that is independent of COX inhibition in multiple rodent models of cancer including colon, mammary, lung, bladder, and prostate (75, 81, 90, 94, 102, 117). In colon cancer cells, sulindac sulfone inhibits tumor cell growth by inducing apoptosis and causing cell cycle arrest (93). Sulindac sulfone was later demonstrated to reduce the number of colonic neoplasms in azoxymethane (AOM)-treated rats compared with controls (90) and with modest activity in patients with sporadic polyposis (7), but it never received FDA approval because of hepatotoxicity.
Mechanistic studies showed that sulindac sulfone can suppress oncogenic β-catenin signaling by inhibiting cyclic guanosine monophosphate phosphodiesterase (cGMP PDE) (118). Cyclic nucleotide PDEs are an important enzyme superfamily responsible for regulating second messenger signaling by hydrolyzing the 3′,5′-phosphodiester bond in cGMP or cAMP to suppress cyclic nucleotide signaling. The inhibition of cGMP PDEs lead to the increased levels of cGMP and activation of protein kinase G (PKG) (17). Activation of PKG has been shown to induce apoptosis in colon cancer cells (28). Although sulindac sulfone inhibits cGMP PDE activity at concentrations equivalent to those required for apoptosis, its inhibitory activity did not appear to be selective for any specific PDE isozyme. SS, however, showed greater potency and selectivity to inhibit specific cGMP PDEs (i.e., PDE2, 3, 5 and 10), consistent with its improved potency to inhibit colon tumor cell growth (40). The selective inhibition of the cGMP-specific PDE5 isozyme by SS was reported to be critical for the induction of apoptosis and inhibition of the Wnt/β-catenin signaling pathway in human colon cancer cells, although the involvement of additional or alternative cGMP PDE isozymes could not be ruled out (120).
SS and other NSAIDs also induce the expression of NSAID-activated gene 1 (NAG-1). Studies investigating the proapoptotic and antitumorigenic activity of NAG-1 suggest that it acts as a tumor suppressor gene (11, 12). Increased NAG-1 expression inhibited tumor growth and development in mouse models of CRC (12). Colon cancer cells treated with SS showed an increase at both the mRNA and protein levels of NAG-1 (10). The mechanism of SS regulation of NAG-1 appears to be associated with the transcription factor, early growth response-1 (EGR-1) (10). The inhibition of the Wnt/β-catenin pathway and induction of NAG-1 provide a more detailed understanding of the COX-independent mechanisms responsible for the chemopreventive activity of sulindac.
Ibuprofen.
The NSAID ibuprofen is a nonspecific COX-1 and COX-2 inhibitor that can also inhibit the Wnt/β-catenin pathway through the increased phosphorylation and degradation of β-catenin (Fig. 2C) (36). Additionally, ibuprofen can inhibit activation of the nuclear factor-κB (NF-κB) pathway (36). NF-κB is an inducible transcription factor that regulates immune responses, cell proliferation, and survival and is critical in the activation of the proinflammatory pathway in CRC (2, 132). NF-κB is a master transcription factor that promotes transcription of target genes through the recruitment of coactivators and corepressors (132). Several CRC risk factors have been shown to activate NF-κB, including saturated fatty acids, fried foods, grilled meat, and environmental pollutants (2). In unstimulated cells, NF-κB is sequestered in the cytoplasm bound to inhibitory IκB proteins. Upon stimulation, activation events of the IκB kinase (IKK) complex results in the proteasomal degradation of the IκB proteins and release of NF-κB. The release of NF-κB allows for nuclear translocation and transcriptional activity to be activated (82). NF-κB regulates several genes ranging in their cellular activity from immune and inflammatory [e.g., inducible nitric oxide synthase (iNOS)] gene expression, cell proliferation (e.g., c-myc, cyclin D1, and COX-2) and apoptosis. NF-κB also regulates genes that mediate invasion (e.g., MMP-9) and angiogenesis (e.g., VEGF and TNF) (2, 64, 132). Constitutive activation of the NF-κB pathway has been demonstrated in CRC (2). The ability of NSAIDs to suppress these COX-independent mechanisms provides alternative targets for the development of potentially safer and more efficacious drugs for CRC chemoprevention.
Celecoxib.
The long-term use of NSAIDs for CRC chemoprevention is not recommended because of potentially serious side effects, including gastrointestinal ulceration and perforation that have been attributed to the inhibition of COX-1. In an effort to avoid these side effects, selective COX-2 inhibitors such as celecoxib and rofecoxib were developed to spare COX-1 (37). Clinical studies have shown that coxibs have anti-inflammatory activities similar to traditional NSAIDs, but with less gastrointestinal toxicity (37). COX-2 inhibitors, however, are associated with renal toxicity (136) and increased risk of cardiovascular side effects including increased blood pressure, stroke, and myocardial infarction (37, 79). However, clinical trials with celecoxib have demonstrated contradicting results in which cardiovascular side effects were associated with long-term use or no cardiovascular side effects were found (Fig. 2D) (37). Nonetheless, adverse cardiovascular effects associated with specific COX-2 inhibitors have limited their development for CRC chemoprevention because high dosages are necessary. Clinical trials have demonstrated the efficacy of celecoxib in reducing mean size and number of colorectal polyps in FAP patients (19). In 1999, celecoxib was approved by the FDA for the adjuvant treatment of FAP patients (37), but its use was suspended in 2004 by the manufacturer, Pfizer (122).
Celecoxib and SS inhibit the expression and activity of survivin in human colon cancer cells (97, 121). Survivin is a bifunctional member of the inhibitor of apoptosis (IAP) family and is an essential regulator of cell proliferation and survival (4, 61). Under normal conditions, survivin is primarily present during embryonic development but is undetectable in most adult tissues. Survivin is highly tumor specific and is overexpressed in CRC and almost all other human cancers (4, 61). Survivin expression is used as a diagnostic biomarker of tumor onset and recurrence, unfavorable disease outcome, and a target for cancer drug discovery (4).
Natural Products/Dietary Phytochemicals
Resveratrol, curcumin, EGCG, genistein, baicalein.
RESVERATROL.
Resveratrol (3,5,4′-trihydroxy-trans-stilbene) is a phytoalexin, present in grapes, red wine, peanuts, mulberries, and other plants (Fig. 5A) (87, 108, 114). It is an antioxidant because of its aromatic structure, which acts as a free radical scavenger (16). Resveratrol has several mechanisms of action, which demonstrate its potent anti-inflammatory and anti-carcinogenic effects. One of the antioxidant mechanisms includes increased expression of the enzyme manganese superoxide dismutase (MnSOD), which converts the highly reactive and damaging free radical, superoxide, into molecular oxygen and hydrogen peroxide (16). The anticancer activity of resveratrol in colon adenocarcinoma cells appears to involve the inhibition of matrix metalloproteinase 9 (MMP-9) and vascular endothelial growth factor (VEGF) signaling. Inhibition of MMP-9 activation by resveratrol can inhibit metastasis and VEGF-VEGFR to inhibit angiogenesis (16, 63). In addition, resveratrol induces apoptosis and can reduce the constitutive activation of NF-κB in colon cancer cells (2).
Fig. 5.
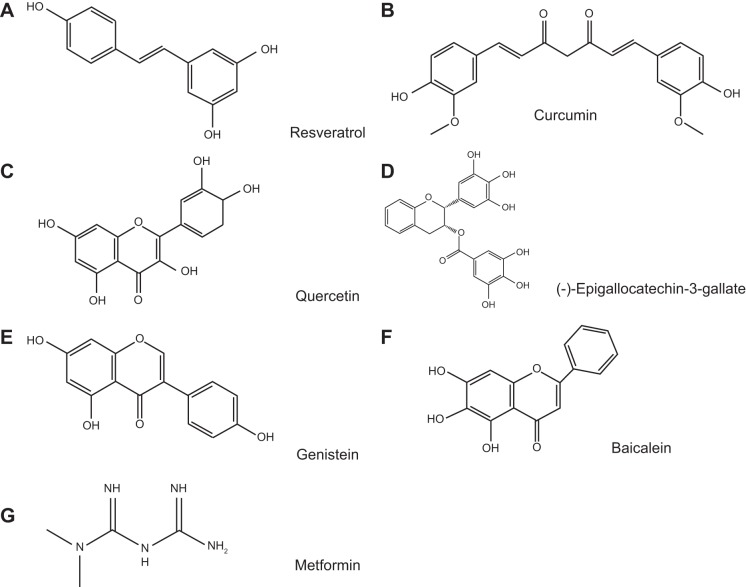
Selected natural products and synthetic derivative chemical structures. Resveratrol (A), curcumin (B), quercetin (C), EGCG (D), genistein (E), baicalein (F), and metformin (G) are shown.
Resveratrol has also been reported to inhibit iNOS expression in colon cancer cells (83). Nitric oxide (NO) is synthesized by three NO synthase isozymes, two of which are calcium/calmodulin dependent and constitutively expressed, whereas iNOS is inducible by cytokines and proinflammatory agents and is calcium independent (22). The production of NO can exert beneficial effects (i.e., antimicrobial and antiapoptotic); however, overproduction can result in cellular injury and inflammation (64). NO plays an important role in colon tumorigenesis, and increased expression and activity of iNOS has been demonstrated in human CRC tissue and animal models (85). Although the mechanism by which resveratrol acts is promising, it is rapidly metabolized following oral administration, thus preventing its development as a chemopreventive agent (14).
A number of studies have shown the ability of resveratrol to induce apoptosis and inhibit cell cycle progression of colon cancer cells (87). The induction of apoptosis by resveratrol has been attributed the clustering of Fas receptors, resulting in the formation of the death-inducing signaling complex, induction of endoplasmic reticulum stress, activation of caspases, and release of cytochrome c (87). In vivo studies in which resveratrol was orally administered showed that it can prevent the development of intestinal tumors in the APCMin mouse model, an animal model of human CRC. Mice treated with resveratrol showed a 70% reduction of intestinal tumors compared with the control group. Furthermore, resveratrol inhibited the expression of cell cycle progression and cell proliferation genes but increased expression of genes involved in immune response and inhibition of tumor promoting pathways (103).
CURCUMIN.
Curcumin [diferuloylmethane; 1,7-bis-(4-hydroxy-3-methoxyphenyl)−1,6-heptadiene-3,5-dione] is the major yellow pigment in turmeric that is isolated from the roots (rhizomes) of Curcuma longa Lim (Fig. 5B). Curcumin has been shown to have anti-inflammatory and antioxidant properties and to induce apoptosis (38, 101). Curcumin's mechanism of inducing apoptotic cell death appears to vary depending on cancer type, cell type, and experimental conditions. In murine models, curcumin reduced the formation of early preneoplastic lesions in the colon and significantly inhibited the incidence and multiplicity adenocarcinomas in the AOM-induced rat model of colon carcinogenesis (101). A Phase I clinical study with orally administered curcumin reported that it is a safe agent with no observed toxicity, but it has low systemic bioavailability (105). Although curcumin has a low bioavailability, it has been shown in multiple human cancer cell lines to inhibit several key pathways involved in colon tumorigenesis (2).
One of curcumin's mechanisms of action is the inhibition of the NF-κB pathway. Curcumin suppressed NF-κB activation by known inducers: TNF-α, phorbol ester, and hydrogen peroxide (64, 109). In addition to inhibition of the NF-κB pathway, curcumin has been shown to inhibit the phosphatidylinositol-3-kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) (PI3K/Akt/mTOR) pathway in CRC (16). The activation of the PI3K/Akt pathway is associated with colon tumorigenesis (23). Akt is a proto-oncogene, which is activated upon phosphorylation by PI3K. Phosphatase and tensin homolog (PTEN) is a tumor suppressor gene and negative regulator of Akt. PTEN inhibits the phosphorylation of Akt though its inhibition of PI3K (23). Impaired expression of PTEN by mutations or loss has been reported in 60–70% of human CRC (84). Activation of the NF-κB pathway has been shown to be regulated by the downstream signaling of activated Akt (70). In addition to inhibition of the NF-κB pathway, curcumin also induces p53-independent apoptosis and inhibits the WNT/β-catenin pathway in human colon cancer cells (64).
In combination studies involving human cancer cell lines, curcumin has synergistic activity with other natural products including resveratrol (87), catechins (64), genistein, and quercetin (38). Quercetin (3,3′,4′,5,7-pentahydroxyflavone), a flavonoid with antioxidant activity found in various fruits, vegetables, tea, and wine, has been reported to induce apoptosis in human colon cancer cells (Fig. 5C) (60). In a study of five patients with FAP, the combination of curcumin and quercetin effectively decreased the number (64%) and size (50.9%) of adenomatous polyps (26). The combination of resveratrol and curcumin synergistically inhibited the growth of p53-positive and p53-negative colon cancer cells in vitro and p53-positive cells in vivo (74). Curcumin in combination with chemotherapy drugs also enhances their anticancer efficacy in various cancer types (38). The combination of curcumin with 5-fluorouracil (5-FU) significantly inhibited cell growth compared with 5-FU or curcumin alone in human gastric carcinoma cells (67). In an orthotopic pancreatic cancer model curcumin enhanced the anticancer activity of gemcitabine (68).
EGCG.
Epigallocatechingallate (EGCG) is polyphenol found in green tea (Camellia sinensis L.) that has antioxidant, anti-inflammatory, and chemopreventive activities (Fig. 5D) (30, 114). The green tea catechins consist of (−)-epicatechin (EC), (−)-epigallocatechin (EGC), (−)-epicatechingallate (ECG), and (−)-epigallocatechin-3-gallate (EGCG), which are strong antioxidants (30). Of these catechins, EGCG has been found to be the most effective chemopreventive agent and has been studied in several cancer models (20). Oxidative stress and the production of reactive oxygen species (ROS) play an important role in cancer development and progression. ROS generation performs a critical role in cancer initiation and the metabolic activation of procarcinogens (20). During oxidative stress or other toxic insult, normal cells maintain homeostasis through the activation of the transcription factor nuclear factor-erythroid 2p45-related factor-2 (NRF-2). NRF-2 regulates the expression of several antioxidant genes and protective enzymes (55). During oxidative stress NRF-1 and NRF-2 heterodimerize and bind the antioxidant responsive element (ARE) sequence to activate transcription (114). Under normal conditions NRF-2 is sequestered in the cytoplasm by the negative regulator Kelch-like ECH-associated protein 1 (KEAP-1). NRF-2 target genes include glutathione S-transferase, NAD(P)H quinone oxidoreductase (NQO1), and other Phase II detoxifying and antioxidant enzymes. EGCG has been reported to enhance the activity and expression of NRF2 and its downstream gene uridine 5′-diphosphate-glucuronosyltranferases (UGT) 1A in colon cancer cells and BALB/c mice (135). The transcriptional activation of NRF-2 by EGCG has been reported in multiple human cancer types including colon, gastric, hepatocellular, and pancreatic carcinoma cells (38, 114). The antioxidant activity of EGCG is an important mechanism of its anticancer activity but additional mechanisms have been reported.
Alternative mechanisms of EGCG's anticancer activity are the inhibition of lipoxygenase (LOX) and COX activity (46) as well as the inhibition of VEGF and basic fibroblast growth factor (bFGF) expression (113). EGCG and other catechins inhibit COX-dependent AA metabolism in both human colon tumor and normal mucosal microsomes (46). EGCG inhibited protein expression of proangiogenic factors (i.e., VEGF and bFGF) in human CRC cells. In APCMin mice, animals treated with EGCG showed significant reduction of polyp formation and tumor load compared with controls. The EGCG-treated animals also showed a significant reduction of bFGF levels in tumors (113). Human colon cancer cells and xenograft studies have shown that EGCG can inhibit the Erk-1 and Erk-2 signaling pathway, which leads to the increased expression of VEGF mRNA (54). Inhibition of Notch2 expression and its target protein, HES1, has been suggested to be an alternative mechanism by which EGCG inhibits CRC cell proliferation and induces apoptosis. Notch signaling plays a critical role in the renewal and differentiation of CRC stem cells (53). These studies suggest potential mechanisms of EGCG that may be explored further for strategies in the prevention of CRC.
GENISTEIN AND BAICALEIN.
Flavonoids are a group of polyphenolic compounds, which are plant secondary metabolites and present in many fruits and vegetables and all vascular plants (13, 80). The classification of flavonoids is based on their chemical structure and the positions of substitutes present on the parent molecule. Flavonoids are made up of flavonols, flavonones, flavanols, flavan-3-ols, and isoflavones (80). The different classes of flavonoids have been studied for their pharmacological activities and biological properties (13, 80). The activity of selective flavonoids as potential CRC chemopreventive agents has been the focus of several studies; however, their mechanism of action is still under investigation. In this review we have briefly discussed the flavonol quercetin and its combinatorial effects with curcumin. For this section we will focus primarily on the isoflavone genistein and briefly discuss the activity of the flavone, baicalein, and their potential mechanism(s) as colorectal chemopreventive agents.
Genistein (4′,5,7-trihydroxy-isoflavone) is one of three major soy isoflavone aglycones; the other two are daidzein and glycitein (100). Genistein is a phytoestrogen and considered to be the primary anticancer component of soybeans (Fig. 5E) (86). Several epidemiological studies have supported that diets high in soy intake contribute to the lower incidence of several cancer types (i.e., breast, endometrium, prostate, and CRC) (24, 86). In breast and prostate cancer, the preventive activity of genistein is believed to be through its antiestrogenic activity (5). However, genistein has been shown to inhibit growth of both hormone-dependent and hormone-independent cancer cells. The chemopreventive activity of genistein has been further contributed to its modulation of cell cycle, induction of apoptosis, antioxidant activity, and anti-inflammatory activity (5).
In the AOM-induced colon cancer rat model, rats fed a diet rich in soy had ∼76% lower colon tumor incidence compared with controls (41). In multiple cancer types, genistein has been shown to be an inhibitor of protein kinase C (PKC), topoisomerase II and ribosomal S6 kinase (86). PKC is a family of serine/threonine protein kinases that play central roles in cell proliferation, differentiation, and apoptosis. The PKC isozyme PKCβII has been shown to be highly induced in the preneoplastic lesions and colon tumors of AOM-treated animals compared with the normal colonic epithelium (34). Transgenic animals overexpressing PKCβII in the colonic epithelium were more susceptible to colon tumor formation after exposure to AOM (34). PKCβII has been further shown to induce COX-2 gene expression through stabilization in colon cancer cells (133), suggesting that the inhibitory effect of genistein on tumorigenesis may involve the suppression of COX-2 expression.
Genistein has also been reported to inhibit cell growth, induce apoptosis, and stimulate G2/M phase cell cycle arrest in a time- and dose-dependent manner through the activation of ataxia telangiectasia mutated/p53-p21 regulatory network in multiple human CRC cell lines (134). Genistein has also been shown to inhibit cell proliferation and induce apoptosis through the inhibition of IGF-1 and the PI3K/AKT pathway (59). Another antiproliferative mechanism of genistein has been demonstrated through its inhibition of epidermal growth factor (EGF) and activation of forkhead box O3 (FOXO3) (98). Active FOXO3 negatively regulates proliferation and is critical for EGF-mediated proliferation (98). An additional mechanism of genistein activity in colon cancer cell lines is the regulation of gene transcription through epigenetic modifications. The activity of a demethylation agent (130) and inducer of histone modifications have both been demonstrated by genistein (129). Genistein inhibits the WNT/β-catenin pathway through demethylation of multiple promoters and histone acetylation (129).
The flavonoid baicalein has been studied for its potential as CRC chemopreventive agent (Fig. 5F). Baicalein (5,6,7-trihydroxyflavone) is extracted from the root of Scutellaria baicalensis Georgi (57). Baicalein has been reported to have anti-inflammatory and antitumorigenic activities in various cancer models (58). In CRC tumor cell models, treatment with baicalein has been shown to inhibit cell proliferation, induce apoptosis, and inhibit selective pathways (i.e., PI3K/AKT and NF-κB). In a study performed to determine a potential mechanism of inhibition by baicalein in HT29 colon cancer cells, baicalein induced apoptosis in a p53-dependent manner. In HT-29 xenografts baicalein inhibited tumor growth and activation of the PI3K/AKT pathway (62). An additional study demonstrated that treatment with baicalein inhibited cell growth, induced apoptosis, and inhibited NF-κB activation in a HCT116 colon cancer cell model (57). This study also demonstrated the inhibitory effects of baicalein on colon tumor growth in an AOM and proinflammatory, dextran sulfate sodium (DSS), colitis-associated colon cancer mouse model (57). Baicalein was further shown to induce expression of the Pregnane X Receptor (PXR) in human colon cancer cells (i.e., HCT116 and LS174T) and in vivo through a caudal type homeobox 2 (Cdx2)-dependent mechanism (29). The PXR is a nuclear receptor that transcriptionally regulates several drug metabolism and transporter genes (29, 78). Treatment of colon cancer cells with baicalein induced Cdx2, an intestinal cell differentiation factor, transcriptional regulation of PXR expression (29). In a DSS model of murine colitis, a model of human inflammatory bowel disease, baicalein treatment demonstrated anti-inflammatory activities and protected from crypt loss (29). The mechanism(s) by which baicalein exerts its anti-inflammatory activity through PXR was shown in DSS-treated wild-type and Pxr-null mice. Baicalein significantly reduced TNF-α and IL-6 mRNA expression in DSS-treated wild-type mice but not Pxr-null mice (29). Baicalein has been shown to reduce the production of ROS by the upregulation of peroxiredoxin-6 (PRDX6) in CRC cells (49). In these studies knockdown of PRDX6 by siRNA resulted in an increased in ROS production and cell proliferation after treatment with baicalein (49). These studies provide a better understanding of the potential antitumorigenic and anti-inflammatory mechanisms of baicalein in CRC, which can lead to the further development of chemopreventive strategies.
Natural products synthetic derivative.
METFORMIN.
The antidiabetic drug metformin (1,1dimethylbiguanide), mainly used to treat Type 2 diabetes mellitus, has been shown to decrease the risk of CRC (Fig. 5G) (116). Metformin is derived from the herbaceous plant goat's rue (Galega officinalis). Metformin directly inhibits gluconeogenesis and glycogenolysis in the liver, increases anaerobic glycolysis in peripheral tissues, and increases GLUT4 glucose transporters and insulin receptors (89). Metformin has been shown to decrease the risk of colon cancer by targeting the insulin growth factor (IGF) pathway, which plays an important role in cell proliferation, growth, and differentiation. This pathway consists of three ligands (insulin, IGF-1 and IGF-2), six receptors [insulin receptor (IR) alpha (fetal), IR beta (adult), IGF-1 receptor (IGF-1R), IGF-2R, hybrid IGF-1R/IR alpha, hybrid IGF-1R/IR beta], and up to seven binding proteins (IGFBP1-7) (112). IGF-1 and IGF-2 are able to bind and activate IGF-R1 signaling and have been shown to be upregulated in cancer (116). Upon activation, IGF-R1 undergoes autophosphorylation and recruits and phosphorylates insulin receptor substrate-1 (IRS-1), IRS-2, and src homology/collagen (Shc), which are involved in the oncogenic processes (116). The downstream effects of IRS-1 and IRS-2 phosphorylation results in activation of PI3K leading to activation of the AKT pathway and Bcl-2 and inhibition of p27 and BAD. The RAS/MAPK pathway can also be activated by the binding of Shc to IGF-R1 (96, 116). Increased IGF activation and IGFR activation of PI3K pathway can be due to insulin resistance and hyperinsulinemia (116). Hyperinsulinemia can increase the risk of pancreas, breast, and bladder cancer. A potential mechanism of action of metformin is an inhibitory effect on insulin and the suppression of protein synthesis through the activation of the adenosine monophosphate-activated protein kinase (AMPK) (16).
The increased risk of breast, colon, and pancreatic cancers associated with Type 2 diabetes, obesity, and insulin resistance may be due the influence of the insulin-IGF signaling pathway (27). However, it is still unclear whether differences of circulating insulin levels influence cancer progression in diabetic patients receiving treatment (i.e., metformin). The observational studies investigating metformin use and the decreased incidence of CRC report conflicting findings (111). In a retrospective study more than 62,000 diabetic patients were taking metformin, sulfonylurea, or insulin. Patients on metformin therapy had the lowest risk of developing colorectal or pancreatic cancer (27). However, in a population study the use of metformin was not associated with a decreased risk of CRC incidence (111). Although discrepancies can be explained by limitations of sample size, duration of treatment, environmental differences, and additional confounding factors. The potential CRC chemopreventive mechanism of metformin through the inhibition of the insulin/IGF signaling pathway merits further exploration.
AMPK is a highly conserved heterotrimeric kinase, which coordinates several enzymes to regulate ATP conservation and synthesis (43). In a study evaluating the downstream mechanisms of metformin activation of AMPK in human endothelial cells, metformin suppressed TNF-α-induced NF-κB activity (43). The suppression of NF-κB activity was attenuated by knockdown of AMPK in these cells (43) and these observations were independently supported (48). The anti-inflammatory activity of metformin through the inhibition of NF-κB as a potential chemopreventive mechanism is still relatively new. This activity of metformin is supported by its inhibition of the NF-κB pathway in intestinal inflammation, colitis-associated colon cancer (65), and breast cancer (45). The results from these studies indicate that the anti-tumor activity of metformin may be through activity as an anti-inflammatory agent in certain cancer types through inhibition of the NF-κB signaling pathway. However, there is limited research to support this novel role of metformin, which warrants further investigation.
Summary and Conclusion
Increased understanding of the molecular events involved in the colorectal adenoma-carcinoma sequence provides the opportunity to develop targeted drugs for chemoprevention. The development of CRC is strongly influenced by genetics, dietary, and lifestyle risk factors. Inflammation plays a critical role in the promotion and development of cancer into a malignant phenotype. The progression into a malignant phenotype requires activation of key cellular mechanisms including uncontrolled growth, evasion of apoptotic pathways, angiogenesis, and metastasis, to name a few. Early inhibition of the inflammatory state can prevent the activation of several of these pathways and thus prevent the carcinogenesis process. Although there are limitations due to side effects attributed to the COX inhibitory activity of celecoxib and other NSAIDs (i.e., aspirin and SS), their promising chemopreventive activity have led investigators to study COX-independent mechanisms for the purpose of developing derivatives that lack COX inhibitory activity but retain or have improved anticancer activity. The identification of the specific therapeutic targets and an understanding of the tumorigenic pathways dependent on those targets ideally allows for the inhibition or reversal of the malignant phenotype. The multiple pharmacological agents and natural products as discussed in this review have shown promising results for CRC chemoprevention but also limitations in both safety and efficacy. The rich diversity of the chemical structures and biological mechanism(s) of action of these agents provides opportunities to develop safer and more efficacious CRC preventive agents by chemical optimization of existing agents to improve target selectivity or the identification of new agents.
GRANTS
This research was supported by National Cancer Institute Grants 1R01CA148817 and 1R01CA148817-03S1.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
A.M.F. prepared figures; A.M.F. drafted manuscript; A.M.F. and G.A.P. edited and revised manuscript; A.M.F. and G.A.P. approved final version of manuscript.
REFERENCES
- 1.Abdelrahim M, Safe S. Cyclooxygenase-2 inhibitors decrease vascular endothelial growth factor expression in colon cancer cells by enhanced degradation of Sp1 and Sp4 proteins. Mol Pharmacol 68: 317–329, 2005. [DOI] [PubMed] [Google Scholar]
- 2.Aggarwal B, Prasad S, Sung B, Krishnan S, Guha S. Prevention and treatment of colorectal cancer by natural agents from mother nature. Curr Colorectal Cancer Rep 9: 37–56, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alberts DS, Hixson L, Ahnen D, Bogert C, Einspahr J, Paranka N, Brendel K, Gross PH, Pamukcu R, Burt RW. Do NSAIDs exert their colon cancer chemoprevention activities through the inhibition of mucosal prostaglandin synthetase? J Cell Biol Suppl 22: 18–23, 1995. [DOI] [PubMed] [Google Scholar]
- 4.Altieri DC. Survivin, cancer networks and pathway-directed drug discovery. Nat Rev Cancer 8: 61–70, 2008. [DOI] [PubMed] [Google Scholar]
- 5.Amado NG, Fonseca BF, Cerqueira DM, Neto VM, Abreu JG. Flavonoids: potential Wnt/beta-catenin signaling modulators in cancer. Life Sci 89: 545–554, 2011. [DOI] [PubMed] [Google Scholar]
- 6.American Cancer Society. Colorectal Cancer Detailed Guide. American Cancer Society, 2013. http://www.cancer.org/acs/groups/cid/documents/webcontent/003096-pdf.pdf [Google Scholar]
- 7.Arber N, Kuwada S, Leshno M, Sjodahl R, Hultcrantz R, Rex D. Sporadic adenomatous polyp regression with exisulind is effective but toxic: a randomised, double blind, placebo controlled, dose-response study. Gut 55: 367–373, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Armaghany T, Wilson JD, Chu Q, Mills G. Genetic alterations in colorectal cancer. Gastrointest Cancer Res 5: 19–27, 2012. [PMC free article] [PubMed] [Google Scholar]
- 9.Aune D, Chan DS, Lau R, Vieira R, Greenwood DC, Kampman E, Norat T. Dietary fibre, whole grains, and risk of colorectal cancer: systematic review and dose-response meta-analysis of prospective studies. BMJ 343: d6617, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baek SJ, Kim JS, Moore SM, Lee SH, Martinez J, Eling TE. Cyclooxygenase inhibitors induce the expression of the tumor suppressor gene EGR-1, which results in the up-regulation of NAG-1, an antitumorigenic protein. Mol Pharmacol 67: 356–364, 2005. [DOI] [PubMed] [Google Scholar]
- 11.Baek SJ, Kim KS, Nixon JB, Wilson LC, Eling TE. Cyclooxygenase inhibitors regulate the expression of a TGF-beta superfamily member that has proapoptotic and antitumorigenic activities. Mol Pharmacol 59: 901–908, 2001. [PubMed] [Google Scholar]
- 12.Baek SJ, Okazaki R, Lee SH, Martinez J, Kim JS, Yamaguchi K, Mishina Y, Martin DW, Shoieb A, McEntee MF, Eling TE. Nonsteroidal anti-inflammatory drug-activated gene-1 over expression in transgenic mice suppresses intestinal neoplasia. Gastroenterology 131: 1553–1560, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Birt DF, Hendrich S, Wang W. Dietary agents in cancer prevention: flavonoids and isoflavonoids. Pharmacol Ther 90: 157–177, 2001. [DOI] [PubMed] [Google Scholar]
- 14.Bishayee A. Cancer prevention and treatment with resveratrol: from rodent studies to clinical trials. Cancer Prev Res (Phila) 2: 409–418, 2009. [DOI] [PubMed] [Google Scholar]
- 15.Bogaert J, Prenen H. Molecular genetics of colorectal cancer. Ann Gastroenterol 27: 1–6, 2014. [PMC free article] [PubMed] [Google Scholar]
- 16.Boghossian S, Hawash A. Chemoprevention in colorectal cancer—where we stand and what we have learned from twenty year's experience. Surgeon 10: 43–52, 2012. [DOI] [PubMed] [Google Scholar]
- 17.Browning DD. Protein kinase G as a therapeutic target for the treatment of metastatic colorectal cancer. Expert Opin Ther Targets 12: 367–376, 2008. [DOI] [PubMed] [Google Scholar]
- 18.Brunell D, Sagher D, Kesaraju S, Brot N, Weissbach H. Studies on the metabolism and biological activity of the epimers of sulindac. Drug Metab Dispos 39: 1014–1021, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan AT, Arber N, Burn J, Chia WK, Elwood P, Hull MA, Logan RF, Rothwell PM, Schror K, Baron JA. Aspirin in the chemoprevention of colorectal neoplasia: an overview. Cancer Prev Res (Phila) 5: 164–178, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen L, Zhang HY. Cancer preventive mechanisms of the green tea polyphenol (−)-epigallocatechin-3-gallate. Molecules 12: 946–957, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chintharlapalli S, Papineni S, Lei P, Pathi S, Safe S. Betulinic acid inhibits colon cancer cell and tumor growth and induces proteasome-dependent and -independent downregulation of specificity proteins (Sp) transcription factors. BMC Cancer 11: 371, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cianchi F, Cortesini C, Fantappie O, Messerini L, Schiavone N, Vannacci A, Nistri S, Sardi I, Baroni G, Marzocca C, Perna F, Mazzanti R, Bechi P, Masini E. Inducible nitric oxide synthase expression in human colorectal cancer: correlation with tumor angiogenesis. Am J Pathol 162: 793–801, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Colakoglu T, Yildirim S, Kayaselcuk F, Nursal TZ, Ezer A, Noyan T, Karakayali H, Haberal M. Clinicopathological significance of PTEN loss and the phosphoinositide 3-kinase/Akt pathway in sporadic colorectal neoplasms: is PTEN loss predictor of local recurrence? Am J Surg 195: 719–725, 2008. [DOI] [PubMed] [Google Scholar]
- 24.Cotterchio M, Boucher BA, Manno M, Gallinger S, Okey A, Harper P. Dietary phytoestrogen intake is associated with reduced colorectal cancer risk. J Nutr 136: 3046–3053, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coussens LM, Werb Z. Inflammation and cancer. Nature 420: 860–867, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cruz-Correa M, Shoskes DA, Sanchez P, Zhao R, Hylind LM, Wexner SD, Giardiello FM. Combination treatment with curcumin and quercetin of adenomas in familial adenomatous polyposis. Clin Gastroenterol Hepatol 4: 1035–1038, 2006. [DOI] [PubMed] [Google Scholar]
- 27.Currie CJ, Poole CD, Gale EA. The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia 52: 1766–1777, 2009. [DOI] [PubMed] [Google Scholar]
- 28.Deguchi A, Thompson WJ, Weinstein IB. Activation of protein kinase G is sufficient to induce apoptosis and inhibit cell migration in colon cancer cells. Cancer Res 64: 3966–3973, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Dou W, Mukherjee S, Li H, Venkatesh M, Wang H, Kortagere S, Peleg A, Chilimuri SS, Wang ZT, Feng Y, Fearon ER, Mani S. Alleviation of gut inflammation by Cdx2/Pxr pathway in a mouse model of chemical colitis. PloS One 7: e36075, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Du GJ, Zhang Z, Wen XD, Yu C, Calway T, Yuan CS, Wang CZ. Epigallocatechin gallate (EGCG) is the most effective cancer chemopreventive polyphenol in green tea. Nutrients 4: 1679–1691, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell 61: 759–767, 1990. [DOI] [PubMed] [Google Scholar]
- 32.Fischer SM, Hawk ET, Lubet RA. Coxibs and other nonsteroidal anti-inflammatory drugs in animal models of cancer chemoprevention. Cancer Prev Res (Phila) 4: 1728–1735, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Giardiello FM, Hamilton SR, Krush AJ, Piantadosi S, Hylind LM, Celano P, Booker SV, Robinson CR, Offerhaus GJ. Treatment of colonic and rectal adenomas with sulindac in familial adenomatous polyposis. N Engl J Med 328: 1313–1316, 1993. [DOI] [PubMed] [Google Scholar]
- 34.Gokmen-Polar Y, Murray NR, Velasco MA, Gatalica Z, Fields AP. Elevated protein kinase C betaII is an early promotive event in colon carcinogenesis. Cancer Res 61: 1375–1381, 2001. [PubMed] [Google Scholar]
- 35.Grady WM. Genomic instability and colon cancer. Cancer Metastasis Rev 23: 11–27, 2004. [DOI] [PubMed] [Google Scholar]
- 36.Greenspan EJ, Madigan JP, Boardman LA, Rosenberg DW. Ibuprofen inhibits activation of nuclear β-catenin in human colon adenomas and induces the phosphorylation of GSK-3β. Cancer Prev Res (Phila) 4: 161–171, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grosch S, Maier TJ, Schiffmann S, Geisslinger G. Cyclooxygenase-2 (COX-2)-independent anticarcinogenic effects of selective COX-2 inhibitors. J Natl Cancer Inst 98: 736–747, 2006. [DOI] [PubMed] [Google Scholar]
- 38.Gullett NP, Ruhul Amin AR, Bayraktar S, Pezzuto JM, Shin DM, Khuri FR, Aggarwal BB, Surh YJ, Kucuk O. Cancer prevention with natural compounds. Semin Oncol 37: 258–281, 2010. [DOI] [PubMed] [Google Scholar]
- 39.Gurpinar E, Grizzle WE, Piazza GA. COX-independent mechanisms of cancer chemoprevention by anti-inflammatory drugs. Front Oncol 3: 181, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gurpinar E, Grizzle WE, Piazza GA. NSAIDs inhibit tumorigenesis, but how? Clin Cancer Res 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hakkak R, Korourian S, Ronis MJ, Johnston JM, Badger TM. Soy protein isolate consumption protects against azoxymethane-induced colon tumors in male rats. Cancer Lett 166: 27–32, 2001. [DOI] [PubMed] [Google Scholar]
- 42.Half E, Arber N. Chemoprevention of gastrointestinal neoplasia. Curr Gastroenterol Rep 15: 320, 2013. [DOI] [PubMed] [Google Scholar]
- 43.Hattori Y, Suzuki K, Hattori S, Kasai K. Metformin inhibits cytokine-induced nuclear factor kappaB activation via AMP-activated protein kinase activation in vascular endothelial cells. Hypertension 47: 1183–1188, 2006. [DOI] [PubMed] [Google Scholar]
- 44.He X, Semenov M, Tamai K, Zeng X. LDL receptor-related proteins 5 and 6 in Wnt/beta-catenin signaling: arrows point the way. Development 131: 1663–1677, 2004. [DOI] [PubMed] [Google Scholar]
- 45.Hirsch HA, Iliopoulos D, Struhl K. Metformin inhibits the inflammatory response associated with cellular transformation and cancer stem cell growth. Proc Natl Acad Sci USA 110: 972–977, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hong J, Smith TJ, Ho CT, August DA, Yang CS. Effects of purified green and black tea polyphenols on cyclooxygenase- and lipoxygenase-dependent metabolism of arachidonic acid in human colon mucosa and colon tumor tissues. Biochem Pharmacol 62: 1175–1183, 2001. [DOI] [PubMed] [Google Scholar]
- 47.Hong WK, Spitz MR, Lippman SM. Cancer chemoprevention in the 21st century: genetics, risk modeling, and molecular targets. J Clin Oncol 18: 9S–18S, 2000. [PubMed] [Google Scholar]
- 48.Huang NL, Chiang SH, Hsueh CH, Liang YJ, Chen YJ, Lai LP. Metformin inhibits TNF-alpha-induced IkappaB kinase phosphorylation, IkappaB-alpha degradation and IL-6 production in endothelial cells through PI3K-dependent AMPK phosphorylation. Int J Cardiol 134: 169–175, 2009. [DOI] [PubMed] [Google Scholar]
- 49.Huang WS, Kuo YH, Chin CC, Wang JY, Yu HR, Sheen JM, Tung SY, Shen CH, Chen TC, Sung ML, Liang HF, Kuo HC. Proteomic analysis of the effects of baicalein on colorectal cancer cells. Proteomics 12: 810–819, 2012. [DOI] [PubMed] [Google Scholar]
- 50.Huls G, Koornstra JJ, Kleibeuker JH. Non-steroidal anti-inflammatory drugs and molecular carcinogenesis of colorectal carcinomas. Lancet 362: 230–232, 2003. [DOI] [PubMed] [Google Scholar]
- 51.Iwama T. NSAIDs and colorectal cancer prevention. J Gastroenterol 44, Suppl 19: 72–76, 2009. [DOI] [PubMed] [Google Scholar]
- 52.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 61: 69–90, 2011. [DOI] [PubMed] [Google Scholar]
- 53.Jin H, Gong W, Zhang C, Wang S. Epigallocatechin gallate inhibits the proliferation of colorectal cancer cells by regulating Notch signaling. Onco Targets Ther 6: 145–153, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jung YD, Kim MS, Shin BA, Chay KO, Ahn BW, Liu W, Bucana CD, Gallick GE, Ellis LM. EGCG, a major component of green tea, inhibits tumour growth by inhibiting VEGF induction in human colon carcinoma cells. Br J Cancer 84: 844–850, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kang KW, Lee SJ, Kim SG. Molecular mechanism of nrf2 activation by oxidative stress. Antioxid Redox Signal 7: 1664–1673, 2005. [DOI] [PubMed] [Google Scholar]
- 56.Kauh J, Umbreit J. Colorectal cancer prevention. Curr Probl Cancer 28: 240–264, 2004. [DOI] [PubMed] [Google Scholar]
- 57.Kim DH, Hossain MA, Kang YJ, Jang JY, Lee YJ, Im E, Yoon JH, Kim HS, Chung HY, Kim ND. Baicalein, an active component of Scutellaria baicalensis Georgi, induces apoptosis in human colon cancer cells and prevents AOM/DSS-induced colon cancer in mice. Int J Oncol 43: 1652–1658, 2013. [DOI] [PubMed] [Google Scholar]
- 58.Kim DH, Sung B, Chung HY, Kim ND. Modulation of colitis-associated colon tumorigenesis by baicalein and betaine. J Cancer Prev 19: 153–160, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim EJ, Shin HK, Park JH. Genistein inhibits insulin-like growth factor-I receptor signaling in HT-29 human colon cancer cells: a possible mechanism of the growth inhibitory effect of genistein. J Med Food 8: 431–438, 2005. [DOI] [PubMed] [Google Scholar]
- 60.Kim HJ, Kim SK, Kim BS, Lee SH, Park YS, Park BK, Kim SJ, Kim J, Choi C, Kim JS, Cho SD, Jung JW, Roh KH, Kang KS, Jung JY. Apoptotic effect of quercetin on HT-29 colon cancer cells via the AMPK signaling pathway. J Agric Food Chem 58: 8643–8650, 2010. [DOI] [PubMed] [Google Scholar]
- 61.Kim PJ, Plescia J, Clevers H, Fearon ER, Altieri DC. Survivin and molecular pathogenesis of colorectal cancer. Lancet 362: 205–209, 2003. [DOI] [PubMed] [Google Scholar]
- 62.Kim SJ, Kim HJ, Kim HR, Lee SH, Cho SD, Choi CS, Nam JS, Jung JY. Antitumor actions of baicalein and wogonin in HT-29 human colorectal cancer cells. Mol Med Rep 6: 1443–1449, 2012. [DOI] [PubMed] [Google Scholar]
- 63.Kimura Y, Sumiyoshi M, Baba K. Antitumor activities of synthetic and natural stilbenes through antiangiogenic action. Cancer Sci 99: 2083–2096, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ko JK, Auyeung KK. Target-oriented mechanisms of novel herbal therapeutics in the chemotherapy of gastrointestinal cancer and inflammation. Curr Pharm Des 19: 48–66, 2013. [DOI] [PubMed] [Google Scholar]
- 65.Koh SJ, Kim JM, Kim IK, Ko SH, Kim JS. Anti-inflammatory mechanism of metformin and its effects in intestinal inflammation and colitis-associated colon cancer. J Gastroenterol Hepatol 29: 502–510, 2014. [DOI] [PubMed] [Google Scholar]
- 66.Komiya M, Fujii G, Takahashi M, Iigo M, Mutoh M. Prevention and intervention trials for colorectal cancer. Jpn J Clin Oncol 43: 685–694, 2013. [DOI] [PubMed] [Google Scholar]
- 67.Koo JY, Kim HJ, Jung KO, Park KY. Curcumin inhibits the growth of AGS human gastric carcinoma cells in vitro and shows synergism with 5-fluorouracil. J Med Food 7: 117–121, 2004. [DOI] [PubMed] [Google Scholar]
- 68.Kunnumakkara AB, Guha S, Krishnan S, Diagaradjane P, Gelovani J, Aggarwal BB. Curcumin potentiates antitumor activity of gemcitabine in an orthotopic model of pancreatic cancer through suppression of proliferation, angiogenesis, and inhibition of nuclear factor-kappaB-regulated gene products. Cancer Res 67: 3853–3861, 2007. [DOI] [PubMed] [Google Scholar]
- 69.Lawes DA, Pearson T, Sengupta S, Boulos PB. The role of MLH1, MSH2 and MSH6 in the development of multiple colorectal cancers. Br J Cancer 93: 472–477, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li F, Sethi G. Targeting transcription factor NF-kappaB to overcome chemoresistance and radioresistance in cancer therapy. Biochim Biophys Acta 1805: 167–180, 2010. [DOI] [PubMed] [Google Scholar]
- 71.Li H, Pamukcu R, Thompson WJ. β-Catenin signaling: therapeutic strategies in oncology. Cancer Biol Ther 1: 621–625, 2002. [DOI] [PubMed] [Google Scholar]
- 72.Luo J, Chen J, Deng ZL, Luo X, Song WX, Sharff KA, Tang N, Haydon RC, Luu HH, He TC. Wnt signaling and human diseases: what are the therapeutic implications? Lab Invest 87: 97–103, 2007. [DOI] [PubMed] [Google Scholar]
- 73.MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell 17: 9–26, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Majumdar AP, Banerjee S, Nautiyal J, Patel BB, Patel V, Du J, Yu Y, Elliott AA, Levi E, Sarkar FH. Curcumin synergizes with resveratrol to inhibit colon cancer. Nutr Cancer 61: 544–553, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Malkinson AM, Koski KM, Dwyer-Nield LD, Rice PL, Rioux N, Castonguay A, Ahnen DJ, Thompson H, Pamukcu R, Piazza GA. Inhibition of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced mouse lung tumor formation by FGN-1 (sulindac sulfone). Carcinogenesis 19: 1353–1356, 1998. [DOI] [PubMed] [Google Scholar]
- 76.Matsuhashi N, Nakajima A, Fukushima Y, Yazaki Y, Oka T. Effects of sulindac on sporadic colorectal adenomatous polyps. Gut 40: 344–349, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Michor F, Iwasa Y, Lengauer C, Nowak MA. Dynamics of colorectal cancer. Semin Cancer Biol 15: 484–493, 2005. [DOI] [PubMed] [Google Scholar]
- 78.Miura K, Fujibuchi W, Unno M. Splice isoforms as therapeutic targets for colorectal cancer. Carcinogenesis 33: 2311–2319, 2012. [DOI] [PubMed] [Google Scholar]
- 79.Mukherjee D, Nissen SE, Topol EJ. Risk of cardiovascular events associated with selective COX-2 inhibitors. JAMA 286: 954–959, 2001. [DOI] [PubMed] [Google Scholar]
- 80.Narayana KR, Reddy MS, Chaluvadi MR, Krishna DR. Bioflavonoids classification, pharmacological, biochemical effects and therapeutic potential. Indian J Pharmacol 33: 2–16, 2001. [Google Scholar]
- 81.Narayanan BA, Reddy BS, Bosland MC, Nargi D, Horton L, Randolph C, Narayanan NK. Exisulind in combination with celecoxib modulates epidermal growth factor receptor, cyclooxygenase-2, and cyclin D1 against prostate carcinogenesis: in vivo evidence. Clin Cancer Res 13: 5965–5973, 2007. [DOI] [PubMed] [Google Scholar]
- 82.Oeckinghaus A, Hayden MS, Ghosh S. Crosstalk in NF-kappaB signaling pathways. Nat Immunol 12: 695–708, 2011. [DOI] [PubMed] [Google Scholar]
- 83.Panaro MA, Carofiglio V, Acquafredda A, Cavallo P, Cianciulli A. Anti-inflammatory effects of resveratrol occur via inhibition of lipopolysaccharide-induced NF-kappaB activation in Caco-2 and SW480 human colon cancer cells. Br J Nutr 108: 1623–1632, 2012. [DOI] [PubMed] [Google Scholar]
- 84.Pandurangan AK. Potential targets for prevention of colorectal cancer: a focus on PI3K/Akt/mTOR and Wnt pathways. Asian Pac J Cancer Prev 14: 2201–2205, 2013. [DOI] [PubMed] [Google Scholar]
- 85.Pandurangan AK, Esa NM. Dietary non-nutritive factors in targeting of regulatory molecules in colorectal cancer: an update. Asian Pac J Cancer Prev 14: 5543–5552, 2013. [DOI] [PubMed] [Google Scholar]
- 86.Park OJ, Surh YJ. Chemopreventive potential of epigallocatechin gallate and genistein: evidence from epidemiological and laboratory studies. Toxicol Lett 150: 43–56, 2004. [DOI] [PubMed] [Google Scholar]
- 87.Patel VB, Misra S, Patel BB, Majumdar AP. Colorectal cancer: chemopreventive role of curcumin and resveratrol. Nutr Cancer 62: 958–967, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pathi S, Jutooru I, Chadalapaka G, Nair V, Lee SO, Safe S. Aspirin inhibits colon cancer cell and tumor growth and downregulates specificity protein (Sp) transcription factors. PloS One 7: e48208, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pawalowska M, Markowska A. The influence of metformin in the etiology of selected cancers. Contemp Oncol (Pozn) 16: 223–229, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Piazza GA, Alberts DS, Hixson LJ, Paranka NS, Li H, Finn T, Bogert C, Guillen JM, Brendel K, Gross PH, Sperl G, Ritchie J, Burt RW, Ellsworth L, Ahnen DJ, Pamukcu R. Sulindac sulfone inhibits azoxymethane-induced colon carcinogenesis in rats without reducing prostaglandin levels. Cancer Res 57: 2909–2915, 1997. [PubMed] [Google Scholar]
- 91.Piazza GA, Keeton AB, Tinsley HN, Gary BD, Whitt JD, Mathew B, Thaiparambil J, Coward L, Gorman G, Li Y, Sani B, Hobrath JV, Maxuitenko YY, Reynolds RC. A novel sulindac derivative that does not inhibit cyclooxygenases but potently inhibits colon tumor cell growth and induces apoptosis with antitumor activity. Cancer Prev Res (Phila) 2: 572–580, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Piazza GA, Rahm AK, Finn TS, Fryer BH, Li H, Stoumen AL, Pamukcu R, Ahnen DJ. Apoptosis primarily accounts for the growth-inhibitory properties of sulindac metabolites and involves a mechanism that is independent of cyclooxygenase inhibition, cell cycle arrest, and p53 induction. Cancer Res 57: 2452–2459, 1997. [PubMed] [Google Scholar]
- 93.Piazza GA, Rahm AL, Krutzsch M, Sperl G, Paranka NS, Gross PH, Brendel K, Burt RW, Alberts DS, Pamukcu R, Ahnen DJ. Antineoplastic drugs sulindac sulfide and sulfone inhibit cell growth by inducing apoptosis. Cancer Res 55: 3110–3116, 1995. [PubMed] [Google Scholar]
- 94.Piazza GA, Thompson WJ, Pamukcu R, Alila HW, Whitehead CM, Liu L, Fetter JR, Gresh WE Jr, Klein-Szanto AJ, Farnell DR, Eto I, Grubbs CJ. Exisulind, a novel proapoptotic drug, inhibits rat urinary bladder tumorigenesis. Cancer Res 61: 3961–3968, 2001. [PubMed] [Google Scholar]
- 95.Polakis P. Wnt signaling and cancer. Genes Dev 14: 1837–1851, 2000. [PubMed] [Google Scholar]
- 96.Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer 8: 915–928, 2008. [DOI] [PubMed] [Google Scholar]
- 97.Pyrko P, Soriano N, Kardosh A, Liu YT, Uddin J, Petasis NA, Hofman FM, Chen CS, Chen TC, Schonthal AH. Downregulation of survivin expression and concomitant induction of apoptosis by celecoxib and its non-cyclooxygenase-2-inhibitory analog, dimethyl-celecoxib (DMC), in tumor cells in vitro and in vivo. Mol Cancer 5: 19, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Qi W, Weber CR, Wasland K, Savkovic SD. Genistein inhibits proliferation of colon cancer cells by attenuating a negative effect of epidermal growth factor on tumor suppressor FOXO3 activity. BMC Cancer 11: 219, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rajamanickam S, Agarwal R. Natural products and colon cancer: current status and future prospects. Drug Dev Res 69: 460–471, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Raju J, Bielecki A, Caldwell D, Lok E, Taylor M, Kapal K, Curran I, Cooke GM, Bird RP, Mehta R. Soy isoflavones modulate azoxymethane-induced rat colon carcinogenesis exposed pre- and postnatally and inhibit growth of DLD-1 human colon adenocarcinoma cells by increasing the expression of estrogen receptor-beta. J Nutr 139: 474–481, 2009. [DOI] [PubMed] [Google Scholar]
- 101.Reddy BS. The Fourth DeWitt S. Goodman lecture. Novel approaches to the prevention of colon cancer by nutritional manipulation and chemoprevention. Cancer Epidemiol Biomarkers Prev 9: 239–247, 2000. [PubMed] [Google Scholar]
- 102.Reddy BS, Kawamori T, Lubet RA, Steele VE, Kelloff GJ, Rao CV. Chemopreventive efficacy of sulindac sulfone against colon cancer depends on time of administration during carcinogenic process. Cancer Res 59: 3387–3391, 1999. [PubMed] [Google Scholar]
- 103.Schneider Y, Duranton B, Gosse F, Schleiffer R, Seiler N, Raul F. Resveratrol inhibits intestinal tumorigenesis and modulates host-defense-related gene expression in an animal model of human familial adenomatous polyposis. Nutr Cancer 39: 102–107, 2001. [DOI] [PubMed] [Google Scholar]
- 104.Schror K. Pharmacology and cellular/molecular mechanisms of action of aspirin and non-aspirin NSAIDs in colorectal cancer. Best Pract Res Clin Gastroenterol 25: 473–484, 2011. [DOI] [PubMed] [Google Scholar]
- 105.Sharma RA, McLelland HR, Hill KA, Ireson CR, Euden SA, Manson MM, Pirmohamed M, Marnett LJ, Gescher AJ, Steward WP. Pharmacodynamic and pharmacokinetic study of oral Curcuma extract in patients with colorectal cancer. Clin Cancer Res 7: 1894–1900, 2001. [PubMed] [Google Scholar]
- 106.Shiff SJ, Rigas B. The role of cyclooxygenase inhibition in the antineoplastic effects of nonsteroidal antiinflammatory drugs (NSAIDs). J Exp Med 190: 445–450, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Siegel R, Naishadham D, Jemal A. Cancer statistics 2013. CA Cancer J Clin 63: 11–30, 2013. [DOI] [PubMed] [Google Scholar]
- 108.Singh CK, George J, Ahmad N. Resveratrol-based combinatorial strategies for cancer management. Ann NY Acad Sci 1290: 113–121, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Singh S, Aggarwal BB. Activation of transcription factor NF-kappa B is suppressed by curcumin (diferuloylmethane) [corrected]. J Biol Chem 270: 24995–25000, 1995. [DOI] [PubMed] [Google Scholar]
- 110.Smartt HJ, Greenhough A, Ordonez-Moran P, Talero E, Cherry CA, Wallam CA, Parry L, Al Kharusi M, Roberts HR, Mariadason JM, Clarke AR, Huelsken J, Williams AC, Paraskeva C. β-Catenin represses expression of the tumour suppressor 15-prostaglandin dehydrogenase in the normal intestinal epithelium and colorectal tumour cells. Gut 61: 1306–1314, 2012. [DOI] [PubMed] [Google Scholar]
- 111.Smiechowski B, Azoulay L, Yin H, Pollak MN, Suissa S. The use of metformin and colorectal cancer incidence in patients with type II diabetes mellitus. Cancer Epidemiol Biomarkers Prev 22: 1877–1883, 2013. [DOI] [PubMed] [Google Scholar]
- 112.Sridhar SS, Goodwin PJ. Insulin-insulin-like growth factor axis and colon cancer. J Clin Oncol 27: 165–167, 2009. [DOI] [PubMed] [Google Scholar]
- 113.Sukhthankar M, Yamaguchi K, Lee SH, McEntee MF, Eling TE, Hara Y, Baek SJ. A green tea component suppresses posttranslational expression of basic fibroblast growth factor in colorectal cancer. Gastroenterology 134: 1972–1980, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer 3: 768–780, 2003. [DOI] [PubMed] [Google Scholar]
- 115.Tegeder I, Pfeilschifter J, Geisslinger G. Cyclooxygenase-independent actions of cyclooxygenase inhibitors. FASEB J 15: 2057–2072, 2001. [DOI] [PubMed] [Google Scholar]
- 116.Temraz S, Mukherji D, Shamseddine A. Potential targets for colorectal cancer prevention. Int J Mol Sci 14: 17279–17303, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Thompson HJ, Jiang C, Lu J, Mehta RG, Piazza GA, Paranka NS, Pamukcu R, Ahnen DJ. Sulfone metabolite of sulindac inhibits mammary carcinogenesis. Cancer Res 57: 267–271, 1997. [PubMed] [Google Scholar]
- 118.Thompson WJ, Piazza GA, Li H, Liu L, Fetter J, Zhu B, Sperl G, Ahnen D, Pamukcu R. Exisulind induction of apoptosis involves guanosine 3′,5′-cyclic monophosphate phosphodiesterase inhibition, protein kinase G activation, and attenuated beta-catenin. Cancer Res 60: 3338–3342, 2000. [PubMed] [Google Scholar]
- 119.Thun MJ, Henley SJ, Patrono C. Nonsteroidal anti-inflammatory drugs as anticancer agents: mechanistic, pharmacologic, and clinical issues. J Natl Cancer Inst 94: 252–266, 2002. [DOI] [PubMed] [Google Scholar]
- 120.Tinsley HN, Gary BD, Keeton AB, Lu W, Li Y, Piazza GA. Inhibition of PDE5 by sulindac sulfide selectively induces apoptosis and attenuates oncogenic Wnt/beta-catenin-mediated transcription in human breast tumor cells. Cancer Prev Res (Phila) 4: 1275–1284, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tinsley HN, Gary BD, Thaiparambil J, Li N, Lu W, Li Y, Maxuitenko YY, Keeton AB, Piazza GA. Colon tumor cell growth-inhibitory activity of sulindac sulfide and other nonsteroidal anti-inflammatory drugs is associated with phosphodiesterase 5 inhibition. Cancer Prev Res (Phila) 3: 1303–1313, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.US Department of Health and Human Services. NIH halts use of COX-2 inhibitor in large cancer prevention trial. NIH News, December 17 2004. [Google Scholar]
- 123.Ulrich CM, Bigler J, Potter JD. Non-steroidal anti-inflammatory drugs for cancer prevention: promise, perils and pharmacogenetics. Nat Rev Cancer 6: 130–140, 2006. [DOI] [PubMed] [Google Scholar]
- 124.van de Wetering M, Sancho E, Verweij C, de Lau W, Oving I, Hurlstone A, van der Horn K, Batlle E, Coudreuse D, Haramis AP, Tjon-Pon-Fong M, Moerer P, van den Born M, Soete G, Pals S, Eilers M, Medema R, Clevers H. The beta-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell 111: 241–250, 2002. [DOI] [PubMed] [Google Scholar]
- 125.Vargas AJ, Thompson PA. Diet and nutrient factors in colorectal cancer risk. Nutr Clin Pract 27: 613–623, 2012. [DOI] [PubMed] [Google Scholar]
- 126.Vogelstein B, Kinzler KW. Cancer genes and the pathways they control. Nat Med 10: 789–799, 2004. [DOI] [PubMed] [Google Scholar]
- 127.Waltzer L, Bienz M. The control of beta-catenin and TCF during embryonic development and cancer. Cancer Metastasis Rev 18: 231–246, 1999. [DOI] [PubMed] [Google Scholar]
- 128.Wang D, DuBois RN. The role of anti-inflammatory drugs in colorectal cancer. Annu Rev Med 64: 131–144, 2013. [DOI] [PubMed] [Google Scholar]
- 129.Wang H, Li Q, Chen H. Genistein affects histone modifications on Dickkopf-related protein 1 (DKK1) gene in SW480 human colon cancer cell line. PloS One 7: e40955, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang Z, Chen H. Genistein increases gene expression by demethylation of WNT5a promoter in colon cancer cell line SW1116. Anticancer Res 30: 4537–4545, 2010. [PubMed] [Google Scholar]
- 131.Williams CS, Mann M, DuBois RN. The role of cyclooxygenases in inflammation, cancer, and development. Oncogene 18: 7908–7916, 1999. [DOI] [PubMed] [Google Scholar]
- 132.Yamamoto Y, Gaynor RB. Therapeutic potential of inhibition of the NF-kappaB pathway in the treatment of inflammation and cancer. J Clin Invest 107: 135–142, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yu W, Murray NR, Weems C, Chen L, Guo H, Ethridge R, Ceci JD, Evers BM, Thompson EA, Fields AP. Role of cyclooxygenase 2 in protein kinase C beta II-mediated colon carcinogenesis. J Biol Chem 278: 11167–11174, 2003. [DOI] [PubMed] [Google Scholar]
- 134.Zhang Z, Wang CZ, Du GJ, Qi LW, Calway T, He TC, Du W, Yuan CS. Genistein induces G2/M cell cycle arrest and apoptosis via ATM/p53-dependent pathway in human colon cancer cells. Int J Oncol 43: 289–296, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhang ZM, Yang XY, Yuan JH, Sun ZY, Li YQ. Modulation of NRF2 and UGT1A expression by epigallocatechin-3-gallate in colon cancer cells and BALB/c mice. Chin Med J (Engl) 122: 1660–1665, 2009. [PubMed] [Google Scholar]
- 136.Zhao SZ, Reynolds MW, Lejkowith J, Whelton A, Arellano FM. A comparison of renal-related adverse drug reactions between rofecoxib and celecoxib, based on the World Health Organization/Uppsala Monitoring Centre safety database. Clin Ther 23: 1478–1491, 2001. [DOI] [PubMed] [Google Scholar]