Abstract
The pyloric antral hormone gastrin plays a role in remodeling of the gastric epithelium, but the specific targets of gastrin that mediate these effects are poorly understood. Glandular epithelial cells of the gastric corpus express matrix metalloproteinase (MMP)-1, which is a potential determinant of tissue remodeling; some of these cells express the CCK-2 receptor at which gastrin acts. We have now examined the hypothesis that gastrin stimulates expression of MMP-1 in the stomach. We determined MMP-1 transcript abundance in gastric mucosal biopsies from Helicobacter pylori negative human subjects with normal gastric mucosal histology, who had a range of serum gastrin concentrations due in part to treatment with proton pump inhibitors (PPI). The effects of gastrin were studied on gastric epithelial AGS-GR cells using Western blot and migration assays. In human subjects with increased serum gastrin due to PPI usage, MMP-1 transcript abundance was increased 2-fold; there was also increased MMP-7 transcript abundance but not MMP-3. In Western blots, gastrin increased proMMP-1 abundance, as well that of a minor band corresponding to active MMP-1, in the media of AGS-GR cells, and the response was mediated by protein kinase C and p42/44 MAP kinase. There was also increased MMP-1 enzyme activity. Gastrin-stimulated AGS-GR cell migration in both scratch wound and Boyden chamber assays was inhibited by MMP-1 immunoneutralization. We conclude that MMP-1 expression is a target of gastrin implicated in mucosal remodeling.
Keywords: gastrin, MMP-1, gastric epithelium
the pyloric antral hormone gastrin is a primary regulator of the gastric luminal environment by virtue of its action in stimulating acid secretion from parietal cells (11). Although these cells express the CCK-2 receptor at which gastrin acts, it is generally thought that gastrin increases acid secretion largely via indirect effects mediated by histamine release from enterochromaffin-like (ECL) cells which also express CCK-2 receptors (4, 13). Thus gastrin stimulates histamine release from these cells, which in turn stimulates acid secretion, and it also increases histamine synthesis and storage via induction of histamine decarboxylase (HDC) and vesicular monoamine transporter-2 (6–8). In addition, it is now well recognized that gastrin plays a role in regulating the organization of the gastric mucosa (29, 36). These effects include increased ECL cell numbers (19), stimulation of cell proliferation, mucosal thickness (25), epithelial cell migration (15, 23), and branching morphogenesis (26). The actions of gastrin in regulating mucosal organization are mediated, at least partly, by induction of genes encoding proteins involved in remodeling of the extracellular matrix, including both proteases, notably matrix metalloproteinases (MMPs), and urokinase plasminogen activator, and their inhibitors, notably tissue inhibitors of MMPs (TIMPs) and plasminogen activator inhibitor-1 and -2 (PAI-1, PAI-2) (5, 32, 33, 40).
The MMPs are a family of ∼25 members that degrade extracellular matrix and other extracellular proteins (22, 30). They may be either soluble or membrane bound and are typically produced as inactive precursors that are converted to their active form after delivery to the cell surface. The expression of MMPs in stromal cells is common, and with the exception of MMP-7, they are less commonly expressed in normal epithelial cells. However, gastric mucosa may be an exception in this regard since there is reported to be expression of MMP-1, -2, and -9 in parietal cells (31). In addition, there is evidence that MMP-1, -2, and -9 are expressed in gastric cancer in both tumor cells and stroma (14, 21), and infection with the oncogenic bacterium H. pylori is associated with induction of MMP-1 (17, 27, 41). In contrast, rather less is known of the factors that might regulate MMP-1 expression in normal gastric mucosa in the absence of H. pylori, inflammation, or cancer. In view of the expression in parietal cells of both MMP-1 and the CCK-2 receptor, we have now examined the hypothesis that gastrin regulates gastric MMP-1 expression. We report here an association between serum gastrin concentrations and MMP-1 transcript abundance in the gastric corpus mucosa of healthy subjects, and we show that gastrin increases MMP-1 expression in gastric epithelial AGS-GR cells via protein kinase C (PKC) and p42/44 MAP kinase together with a potential role in epithelial cell migration.
MATERIALS AND METHODS
Cells and reagents.
AGS-GR cells were maintained as previously described (35). HGT-1 cells were kindly donated by Dr. C. Laboisse (INSERM U239, Hôpital Bichat, Paris, France), (18) and RGM-1 cells were obtained from Riken BioResource Centre (Tsukuba, Ibaraki, Japan) (16); both had been transfected with the CCK-2 receptor and subcloned to yield HGT1-GR and RGM1-GR cells, as described (38). Unsulfated human heptadecapeptide gastrin (hG17ns) was obtained from Bachem (St Helens, Merseyside, UK). Phorbol 12-myristate 13-acetate (PMA) was obtained from Calbiochem (Darmstadt, Germany); brefeldin A (BFA) was obtained from Cambio (Dry Drayton, Cambs, UK); 125I-G17 was purchased from Perkin Elmer (Cambridge, Cambs, UK).
Patients.
Subjects were selected from a cohort of ∼1,400 patients, aged 18 and over, who had clinical indications for undergoing diagnostic upper gastrointestinal endoscopy. Subjects were selected for the current investigation if they were H. pylori negative and showed no endoscopic or histological evidence of upper gastrointestinal neoplasia or preneoplastic pathology (atrophic gastritis, gastric intestinal metaplasia, or Barrett's esophagus). Further exclusion criteria included diabetes mellitus, coma or hemodynamic instability, being moribund or having terminal malignancy, cirrhosis (Child B or C), abnormal clotting or bleeding diasthesis, inability to give informed consent, contraindication to endoscopy, pregnancy, HIV, hepatitis B or C infections. Subjects underwent diagnostic gastroscopy in the Gastroenterology Unit at the Royal Liverpool University Hospital. Endoscopic pinch biopsies of gastric corpus and antrum (2–4 of each) were obtained for histology; H. pylori status was determined on the basis of serology, antral urease test (Pronto Dry; Medical Instrument, Solothurn, Switzerland), and antral and corpus histology. An additional 8 corpus biopsies were taken for RNA extraction and real-time PCR analysis. The study groups consisted of controls and patients taking PPIs (n = 33, omeprazole 20–40 mg; n = 4, esomeprazole 20–40 mg; n = 41, lansoprazole 15–30 mg; n = 2, pantoprazole 20 mg; n = 4, rabeprazole 20 mg). The study was approved by the Liverpool Local Research Ethics Committee and by the Royal Liverpool and Broadgreen University Hospitals NHS Trust, and all patients gave written, informed consent.
INS-gas mice.
INS-Gas mice or FYB/N wild-type controls were maintained in an appropriately controlled environment with a 12:12-h light/dark cycle and were fed a commercial pellet diet with water ad libitum as previously described (37). Animals were killed by increasing CO2 concentration. Gastric corpus extracts were prepared from unfasted animals in RIPA buffer as previously described (20). All animal experiments were approved by the University of Liverpool Animal Welfare Committee, and were conducted in compliance with Home Office requirements and the UK Animals (Scientific Procedures) Act 1986.
Real-time PCR.
Corpus biopsies were collected in RNA Later (Life Technologies LTD, Paisley, Scotland, UK) and RNA extracted in 1.0 ml Tri-Reagent (Sigma, Dorset, UK) according to the manufacturer's instructions. RNA pellets were resuspended in 30 μl of nuclease free water and 2 μg of RNA reverse transcribed with avian myeloblastosis virus reverse transcriptase and oligo(dT) primers (Promega, Southampton, Hampshire, UK). Real-time PCR was carried out using an ABI7500 platform (Applied Biosystems, Warrington, Lancashire, UK) using TaqMan primer/probe sets (human MMP-1, MMP-3, MMP-7, GAPDH), Precision 2x real time PCR master mix (Primer Design, Southampton, UK), and 5′-FAM, 3′-TAMRA double dye probes (Eurogentec, Southampton, Hampshire, UK). All values were standardized to GAPDH. Assays included a no template control (NTC) and 3 quality controls and were only accepted if they met the following criteria: the quality controls within 15% of their anticipated mean quantity, PCR amplification efficiency between 90–110%, and the correlation coefficient of the slope of the standard curve greater than 0.97. Primers and probes were designed using Primer Express v3.0 (Applied Biosystems) and were purchased from Eurogentec (Seraing, Belgium). Probes for detection of human MMP-1, MMP-3, MMP-7, and GAPDH cDNA were intron-spanning and were as follows: MMP-1, 5′-TTG CAG CTC ATG AAC TCG GCC ATT C-3′ (probe), 5′-CCA ACA ATT TCA GAG AGT ACA ACT TAC AT-3′ (forward), 5′-TGA AGG TGT AGC TAG GGT ACA TCA AA-3′ (reverse); MMP-3, 5′-TTG CTG CTC ATG AAA TTG GCC ACT CC-3′ (probe), 5′-ACA AAG GAT ACA GGG ACC AA-3′ (forward), 5′-TAG AGT GGG TAC ATC AAA GCT TCA GT-3′ (reverse); MMP-7, 5′-CCT GTA TGC AAC TCA TGA ACT TGG C-3′ (probe), 5′-GGA TGG TAG CAG TCT AGG GAT TAA CT-3′ (forward), 5′-GGA ATG TCC CAT ACC CAA AGA A-3′ (reverse); GAPDH, 5′-CGT CGC CAG CCG AGC CAC A-3′ (probe), 5′- GCT CCT GTT CGA CAG TCA-3′ (forward), 5′- ACC TTC CCC ATG GTG TCT GA-3′ (reverse).
Gastrin radioimmunoassay.
Serum samples were assayed for total amidated gastrin concentrations by radioimmunoassay using antibody L2 (which reacts with G-17 and G-34 but not progastrin or Gly-gastrins) and 125I-G-17 as previously described (12). The upper limit of the reference range for fasting serum gastrin in this assay is 30 pM.
Immunohistochemistry.
Tissue sections from gastric corpus biopsies fixed in 10% neutral-buffered formalin and paraffin-embedded were processed for immunohistochemical detection of MMP-1 using mouse monoclonal antibody to MMP-1 (R and D Systems, Minneapolis, MN), which reacts with both pro- and active MMP-1, and En Vision FLEX/HRP (Dako, Carpinteria, CA) as secondary antibody; antigen retrieval was performed by incubating at pH 9.0, 930C, for 15 min as previously described (33).
AGS-GR cell secretion of MMP-1.
AGS-GR cells that express the CCK-2 receptor (35) were incubated in serum-free medium with or without hG17ns (1–10 nM) for up to 24 h. In different experiments, cells were treated with PMA (100 nM), Ro320432 (2 μM), U0126 (10 μM), or BFA (10 μg/ml). Media was concentrated using Strataclean resins (Agilent Technologies, Santa Clara, CA), and cell extracts prepared in RIPA buffer containing protease and phosphatase inhibitors (Calbiochem) were resolved by SDS-PAGE electrophoresis.
Western blots.
Proteins were resolved by SDS-PAGE electrophoresis, transferred to nitrocellulose (Amersham Pharmacia Biotech, Buckinghamshire, UK), and incubated with antibodies to MMP-1 (R and D Systems) followed by horseradish peroxidase-conjugated secondary antibody and detection by incubation with Clarity Western ECL Blotting Substrate (Bio-Rad Laboratories, Hertfordshire, UK) and exposure to HyperFilm (Amersham Pharmacia Biotech). Samples of cell extracts were reprobed for GAPDH (Biodesign, Saco, ME) to normalize for protein loading.
Enzyme assays.
Fluorogenic assays for enzyme activity were performed using the MMP-1 selective substrate DNP-Pro-Cha-Abu-Cys(Me)-His-Ala-Lys(N-Me-Abz)-NH2 (Cha, β-cyclohexylalanyl; Abu, l-α-aminobutyryl; Abz, 2-aminobenzoyl) (Calbiochem) at a concentration of 12 μM with equal volumes of assay buffer and media from 3 × 106 AGS-GR cells as previously described (14).
Migration assays.
The action of neutralizing MMP-1 antibodies (2.5 μg/ml; MAB901, R and D Systems) on the stimulation of AGS-GR cell migration by hG17ns (10 nM) was studied using scratch-wound assays and Boyden chambers as previously described (23, 24).
Statistics.
Results are expressed as means ± SE. The association between MMP-1 and serum gastrin was examined using Spearman rank correlation. Other comparisons were made using t-tests or ANOVA as appropriate.
RESULTS
Increased corpus MMP1 mRNA is associated with elevated serum gastrin.
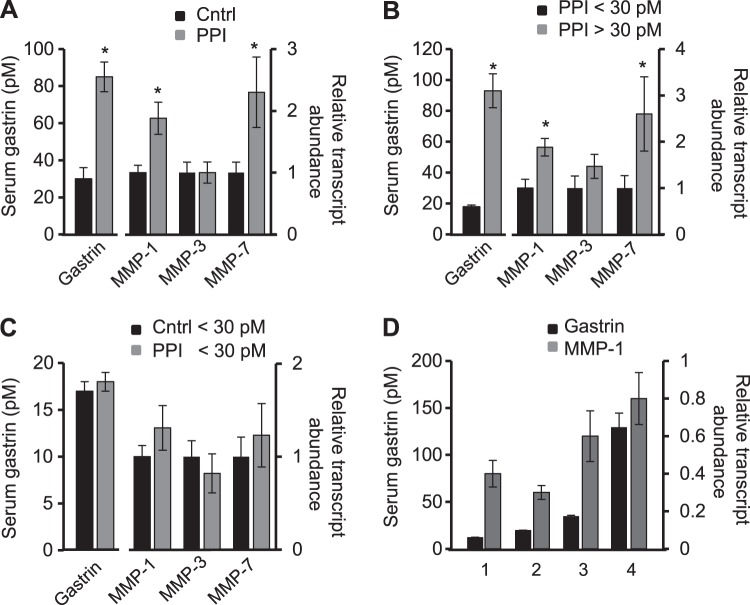
In human subjects with normal gastric histology and negative for H. pylori, the relative abundance of proMMP-1 transcripts in corpus biopsies was ∼2-fold higher in those receiving PPIs compared with those not (P < 0.05) (Fig. 1A). As expected there was also a significant difference in serum gastrin concentrations. To dissociate differences associated with PPI usage per se from those associated with serum gastrin, we first compared proMMP-1 transcript abundance in patients on PPIs divided into normal (<30 pM) and elevated (>30 pM) serum gastrin concentrations. There was again an ∼2-fold difference in MMP-1 transcripts in the two groups (P < 0.05) (Fig. 1B). We then compared proMMP-1 transcript abundance in control subjects and those PPI-treated subjects in whom serum gastrin concentrations were within the reference range (<30 pM): in this case there was no significant difference in transcript abundance (Fig. 1C). When the patients as a whole were divided into quartiles based on serum gastrin, it was clear that the increase in MMP-1 was associated with the third and fourth quartiles of serum gastrin (Fig. 1D). The correlation between serum gastrin and MMP-1 transcript abundance was statistically significant (Spearman rho, 0.255; degrees of freedom, 160; P = 0.00105). There was also a significant correlation between serum gastrin and MMP-1 transcript abundance in the PPI group alone (Spearman rho, 0.257; degrees of freedom, 83; P = 0.0175), but there was no significant correlation in the control group. The data therefore point to an association between serum gastrin in concentrations above those in normal fasting individuals and MMP-1 transcript abundance.
Fig. 1.
Association between serum gastrin and gastric matrix metalloproteinase (MMP)-1 transcript abundance. A: serum gastrin concentrations (left y-axis, pM) and abundance of MMP-1, MMP-3, and MMP-7 transcripts relative to GAPDH (right y-axis) in subjects on proton pump inhibitors (PPIs) (gray bars, n = 85) normalized to control (Cntrl, black bars, n = 77). B: similar data for subjects on PPIs divided into two groups on the basis of serum gastrin below (n = 27) or above (n = 58) the upper limit of the reference range (30 pM). C: similar data for those control (n = 65) and PPI-treated subjects (n = 27) in whom serum gastrin was below the upper limit of the reference range. D: MMP-1 transcript abundance relative to GAPDH expressed for each quartile of serum gastrin concentration and the mean gastrin concentration within each quartile. Data are means ± SE. *P < 0.05.
The specificity of the association between circulating gastrin and increased gastric mucosal proMMP-1 transcript abundance is indicated by the fact that proMMP-3 transcripts did not differ significantly in subjects with high and low serum gastrin concentrations (Fig. 1, A and B). However, previous studies have shown stimulation of MMP-7 expression by gastrin (33), and consistent with this we found a 2.3-fold elevated proMMP-7 transcript abundance in subjects on PPIs compared with controls (P < 0.05), and a 2.6-fold elevation in patients on PPIs with serum gastrin above the reference range compared with normal serum gastrin (P < 0.05) (Fig. 1, A and B); there was no significant difference between control subjects and those on PPIs in whom serum gastrin concentrations were within the reference range (Fig. 1C).
Immunohistochemical localization of MMP-1 in hypergastrinemia.
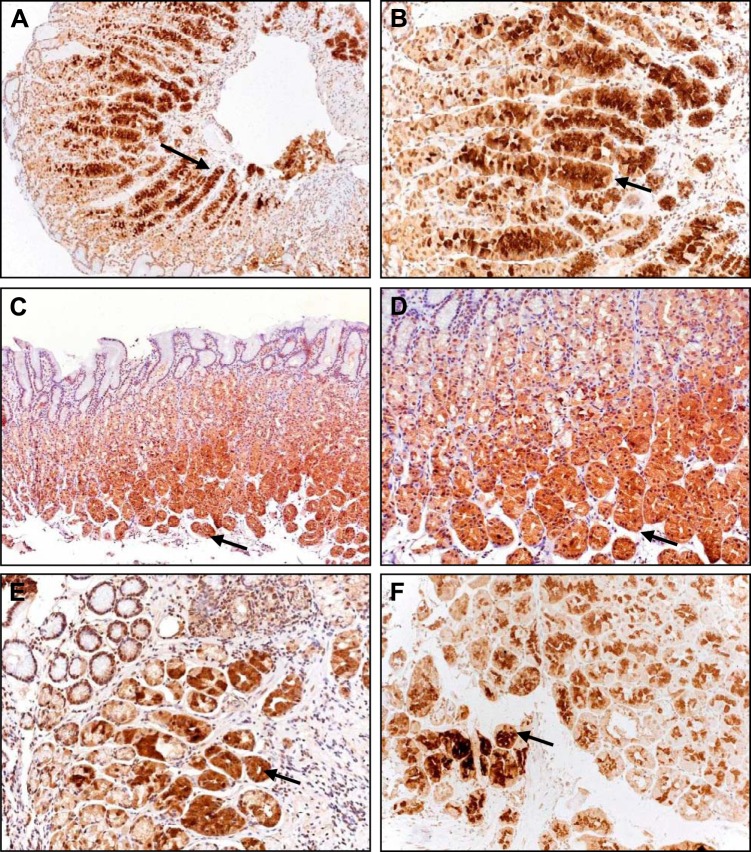
Immunohistochemistry of normal corpus mucosal biopsies revealed MMP-1 immunoreactivity in glandular epithelial cells (Fig. 2). The localization was consistent with expression in both parietal and chief cells. Generally mucus neck and surface epithelial cells were either lightly stained or were negative. Staining in the stroma was also either very light or negative. There was no obvious difference in intensity of staining between patients in the different groups (Fig. 2).
Fig. 2.
Immunohistochemical localization of MMP-1 in gastric epithelial cells. A and B: subject treated with PPIs and with normal serum gastrin. C and D: control subject with normal serum gastrin. E and F: two different subjects with elevated serum gastrin (>200 pM), both on PPIs. Note staining in glandular cells (arrows), but light staining in stroma, mucus neck, and surface epithelial cells. A and C: ×10; other panels: ×20.
Western blot analysis of MMP-1 in corpus mucosa.
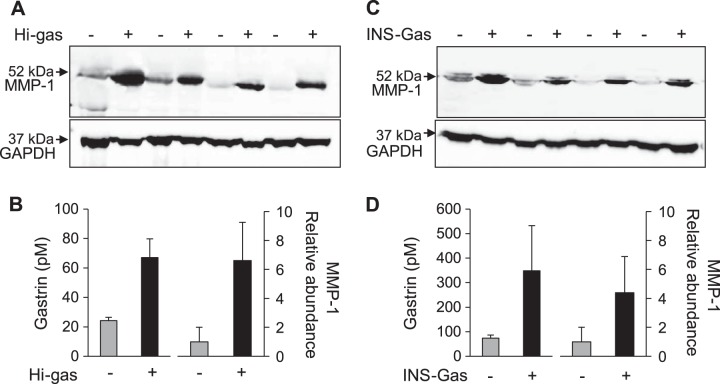
Gastric corpus biopsies selected from individuals with either low or high gastrin were processed for detection of MMP-1 by Western blot. In both cases, there was a major band corresponding to proMMP-1 (Fig. 3A). There was clear evidence of an association between proMMP-1 abundance and serum gastrin (P < 0.05) (Fig. 3, A and B). We then examined MMP-1 by Western blot of extracts of gastric corpus from an animal model of hypergastrinemia, the INS-Gas mouse (37). Again, there was a major band corresponding to proMMP-1 and a significant association between proMMP-1 abundance and serum gastrin (P < 0.05) (Fig. 3, C and D).
Fig. 3.
Increased proMMP-1 in corpus biopsies from hypergastrinemic humans and mice, detected by Western blot. A: Western blot showing proMMP-1 in gastric corpus biopsies from four subjects with serum gastrin < 30 pm (Hi-gas, −) and four subjects with hypergastrinemia (Hi-gas, +). B: means ± SE of serum gastrin and abundance of MMP-1 estimated by densitometry of Western blots of the data shown in A. C: Western blot showing proMMP-1 in gastric corpus biopsies from four wild-type mice and four INS-Gas hypergastrinemic mice. D: means ± SE of serum gastrin and abundance of MMP-1 estimated by densitometry of Western blots of data shown in C.
Stimulation of MMP-1 expression in AGS-GR cells: role of CCK-2 receptors, PKC, and p42/44 MAP kinase.
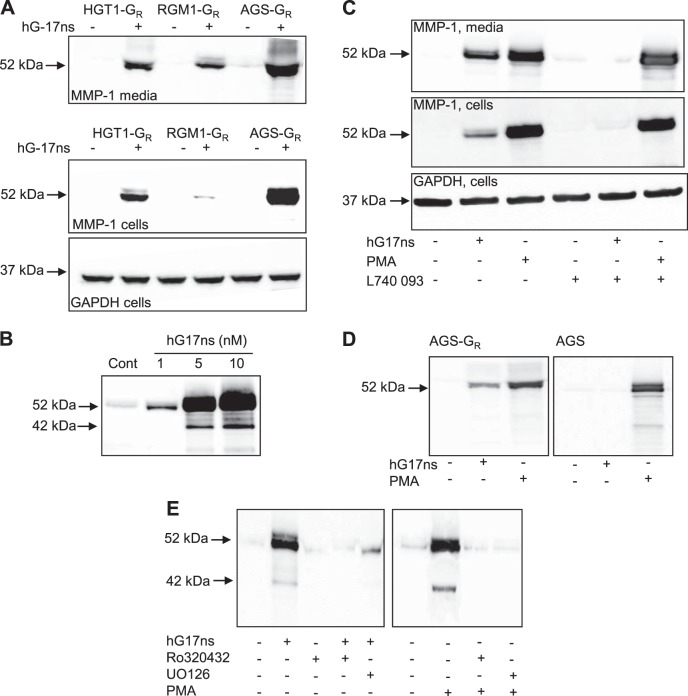
To study the cellular action of gastrin on MMP-1 expression we examined three different cell lines expressing CCK-2 receptors. In Western blots, MMP-1 immunoreactivity was virtually undetectable in unstimulated cells but in response to 10 nM hG17ns there was stimulation in media and cell extracts of all three cell lines of a band of ∼52 kDa corresponding to proMMP-1 (Fig. 4A). The response was greater in AGS-GR cells than either RGM1-GR or HGT1-GR; moreover AGS-GR cells have been intensively used for studies of the effects of gastrin in vitro, thereby justifying their use for more detailed studies. Western blots of media revealed a concentration-related increase of proMMP-1 over the range 1–10 nM hG17ns in AGS-GR cells (Fig. 4B); there was also a faint band of ∼42 kDa corresponding to the active form. The action of G17 was mediated by CCK-2 receptors because 1) a CCK-2 receptor antagonist, L740,093, inhibited the effect of G17 in AGS-GR cells but had no effect on PMA used as a positive control (Fig. 4C), and 2) G17 had no effect on MMP-1 abundance in the media of AGS cells which do not express the receptor while again PMA was a good stimulant (Fig. 4D).
Fig. 4.
Gastrin stimulates proMMP-1 expression by AGS-GR cells revealed by Western blot. A: Western blots of HGT1-GR, RMG1-GR, and AGS-GR cells reveal stimulation of proMMP-1 by hG17ns (10 nM) in media (top) and in cell extracts (middle) while there is no difference in cellular GAPDH (bottom). B: concentration-dependent increases in proMMP-1 (52 kDa) in response to hG17ns (1–10 nM). C: stimulation of proMMP-1 by hG17ns (10 nM) is inhibited by the CCK-2 receptor antagonist L740,093 (0.2 μg/ml), while responses to PMA (100 nM) are not; the changes in proMMP-1 can be seen both in media and cell extracts but there is no change in GAPDH abundance in cell extracts. D: hG17ns and PMA increase proMMP-1 abundance in AGS-GR media, but only PMA also stimulates AGS cells which lack the CCK-2 receptor. E: the effects of both gastrin and PMA on proMMP-1 in media are inhibited by a PKC inhibitor, Ro320432 (2.0 μM), and an inhibitor of activation of p42/44 MAP kinase, U0126 (10 μM).
Stimulation of proMMP-1 expression by G17 was reversed by the PKC inhibitor, Ro320432. Moreover, as expected, PMA-stimulated proMMP-1 expression was also inhibited by Ro320432. Evidence that PKC acts via a mechanism involving activation of p42/44 MAP kinase was provided by the observation that the effects of both G17 and PMA were inhibited by the MEK inhibitor, U0126 (Fig. 4E).
Activation of proMMP-1 by AGS-GR cells.
Although the primary secretory product of AGS-GR cells treated with G17 was proMMP-1, at high exposures Western blots revealed minor bands corresponding to the active enzyme. To determine the capacity of AGS-GR cells for proMMP-1 activation, we performed stop-flow experiments making use of the ability of BFA to arrest secretion by blocking transport through the early secretory pathway. In cells treated with BFA, proMMP-1 accumulated in cell extracts and was virtually undetectable in media (Fig. 5A). By treating AGS-GR cells with G17 followed by delayed addition of BFA after 16 h, we were able to examine the metabolism in media of the previously secreted protein. In these experiments, there was little further conversion over the period of 16–24 h of proMMP-1 to smaller bands; moreover, relatively abundant proMMP-1 was found in media even after 8 h of BFA treatment so that the precursor is relatively stable in AGS-GR cell media. Although Western blots indicated that bands corresponding to the active enzyme were minor compared with proMMP-1, enzyme activity assays for MMP-1 using the selective substrate DNP-Pro-Cha-Abu-Cys(Me)-His-Ala-Lys(N-Me-Abz)-NH2 (Cha, β-cyclohexylalanyl; Abu, l-α-aminobutyryl; Abz, 2-aminobenzoyl) suggested that gastrin increased enzyme activity ∼2-fold (Fig. 5B); BFA inhibited secretion of the active enzyme but in delayed addition experiments had no effect on the activity of previously secreted enzyme (Fig. 5B), again confirming that in the bulk phase of the cell media there is little or no conversion of proMMP-1 to active enzyme.
Fig. 5.
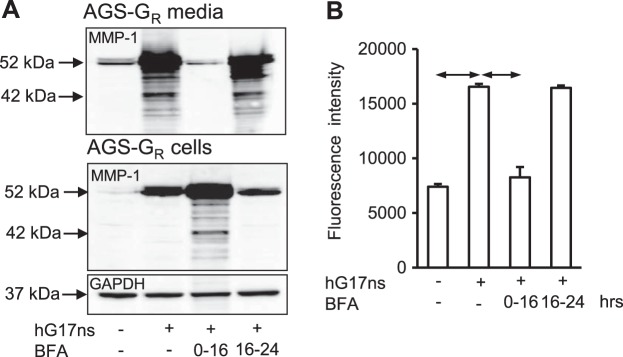
Brefeldin A (BFA) inhibits proMMP-1 secretion. A: BFA (10 μg/ml) inhibits the increase in proMMP-1 in media of AGS-GR cells in response to hG17ns treatment for 16 h by arresting secretion and causing accumulation in the cells; when BFA is added to cells already treated with hG17ns for 16 h the pattern of bands in media after a further 8 h (i.e., at 24 h) is similar to that at 16 h suggesting a low capacity for proMMP-1 activation or degradation. B: enzyme activity of MMP-1 determined using a specific substrate [DNP-Pro-Cha-Abu-Cys(Me)-His-Ala-Lys(N-Me-Abz)-NH2 (Cha, β-cyclohexylalanyl; Abu, l-α-aminobutyryl; Abz, 2-aminobenzoyl)] is increased in hG17ns stimulated AGS-GR media (16 h) and is inhibited by treatment with BFA; no further increase in MMP-1 activity was detected in AGS-GR media, after treatment with hG17ns and addition of BFA for a further 8 h. Data are means ± SE, n = 3; horizontal arrows, P < 0.05.
Stimulation of cell migration by G17 is partially mediated by MMP-1.
Gastrin stimulates AGS-GR cell migration (23) and to determine the role of MMP-1 we examined the effect of immunoneutralization of MMP-1 in scratch-wound migration assays (Fig. 6, A and B) and in Boyden chamber chemotaxis assays (Fig. 6C). As expected, gastrin stimulated AGS-GR cell migration in both types of assay. Immunoneutralization reduced the response by about 35% (P < 0.05) in both cases (Fig. 6, A–C).
Fig. 6.
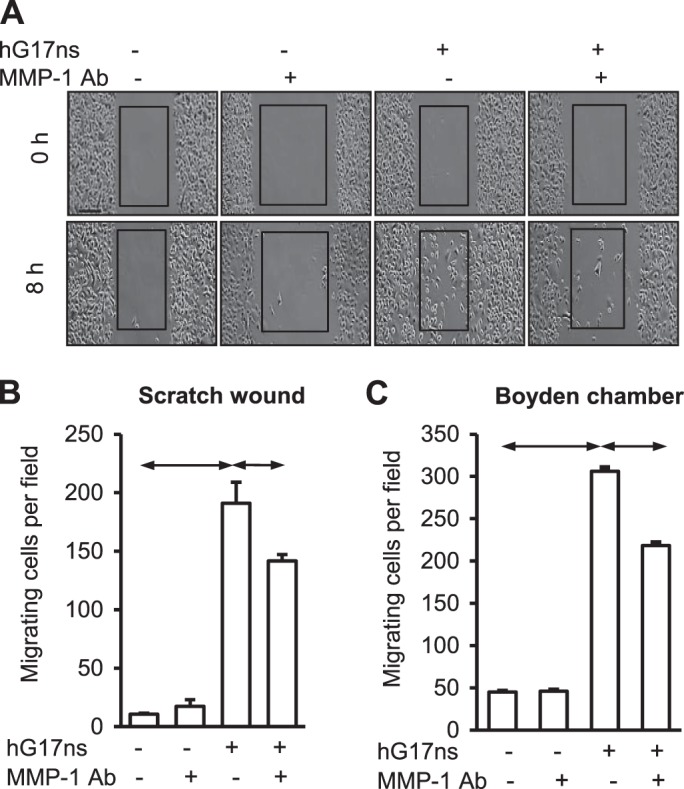
Gastrin increases AGS-GR cell migration in part via MMP-1. A: images of a scratch wound assay showing control AGS-GR cells, and cells treated with MMP-1 antibody (MMP-1 Ab, 2.5 μg/ml), gastrin (hG17ns, 10 nM, 8 h) and the combination of hG17ns and MMP-1 Ab. Gastrin stimulated AGS-GR cell migration is inhibited by neutralizing antibody to MMP-1. B: mean data ± SE, for three scratch wound assays. C: Boyden chamber migration assays using AGS-GR cells and treatments with gastrin, MMP-1 antibody alone, and in combination with gastrin. Data are means ± SE, n = 3; horizontal bars, P < 0.05.
DISCUSSION
The present data show an association between serum gastrin concentrations and the abundance of MMP-1 transcripts in biopsies from normal human stomach. In gastric epithelial cells expressing CCK-2 receptors, gastrin stimulates proMMP-1 expression through a mechanism involving PKC and p42/44 MAP kinase, and increases MMP-1 enzyme activity in the media. Moreover, MMP-1 is at least partly involved in mediating gastrin-stimulated cell migration. Thus MMP-1 is a candidate for mediating the actions of gastrin in the remodeling of gastric epithelia in both health and disease.
The observation that serum gastrin concentrations are linked to increased abundance of MMP-1 transcripts in corpus biopsies was made in subjects negative for H. pylori and excluding patients with neoplastic and preneoplastic histopathology. Thus although there is evidence that gastric MMP-1 expression might be increased in H. pylori infection and in cancer (17, 21, 27), the present data raise the prospect that there is also hormonal regulation in the healthy stomach. On average, fasting serum gastrin was increased 2- to 3-fold in subjects on PPIs; however, in ∼30% of these subjects serum gastrin was in the normal range and in 30% the concentration was >2-fold above the upper limit of normal. Since serum gastrin typically increases 3- to 4-fold on feeding, the association in the present study suggests that serum concentrations just above the physiological range are associated with gastrin MMP-1 transcript abundance.
Immunohistochemistry of selected biopsies from patients with normal or elevated serum gastrin, either on PPIs or not, all indicated that MMP-1 expression was found in glandular epithelial cells. There was no obvious difference in intensity of staining in subjects in these different groups. This may not be surprising given the magnitude of the differences in transcript abundance, and the fact that immunohistochemistry is qualitative rather than quantitative over rather limited ranges of expression. Nevertheless, immunochemical evidence of elevated proMMP-1 expression in gastric corpus was provided by Western blot of corpus extracts from patients with elevated serum gastrin due to PPI use, and also in a mouse model of hypergastrinemia (ING-Gas mice). The observations indicate that expression occurs in most cells at the base of gastric glands, i.e., both parietal and chief cells. In other (unpublished) studies, we have found increased MMP-1 transcript abundance in patients with elevated serum gastrin and chronic atrophic gastritis i.e., lacking parietal cells. Although caution should be exercised in extrapolating from these findings to those in normal subjects, nevertheless it seems possible that gastrin increases MMP-1 expression in both parietal and nonparietal cell populations. Gastrin is known to activate paracrine mechanisms, mediated for example by prostaglandins, HB-EGF, or IL-8, and it remains to be seen whether similar mechanisms are implicated in MMP-1 expression (34).
To study the action of gastrin in more detail, we examined two human (AGS-GR, HGT1-GR) and one rat (RGM1-GR) gastric epithelial cell lines expressing the CCK-2 receptor. Gastrin stimulated proMMP-1 expression in all three, with the strongest response in AGS-GR cells. We made use of the latter for more detailed studies since they are a relatively well studied model of gastrin-stimulated gene expression (1, 32, 33, 39) that has also been used for studies of H. pylori-induced MMP-1 expression (17, 27). The data suggest that gastrin increases proMMP-1 appearance in the media of these cells via stimulation of CCK-2 receptors. Other studies have previously reported that Gly-extended gastrins (which have low or no affinity for CCK-2 receptors) may increase MMP-1 expression in LoVo and HT29 colon cancer cells (3). If so, however, this is likely to be by a different mechanism to that reported here. Our data indicate that gastrin increases MMP-1 via activation of PKC and of p42/44 MAP kinase. Previously, a promoter element in the MMP-1 gene required for induction by PMA has been defined (2). Moreover, in AGS cells there are p42/44 MAP kinase-mediated increases in MMP-1 stimulated by inflammatory signals and H. pylori (27, 28). The present data suggest that a noninflammatory endocrine stimulus, gastrin, also activates this pathway via CCK-2 and PKC. In the same cells, gastrin increases PAI-1 and PAI-2 gene expression by PKC and p42/44 MAP kinase activation. Interestingly PAI-1 and PAI-2 are inhibitors of extracellular proteases and suppress migration. Thus activation of a common signaling pathway leads not just to release of proinvasive MMP-1 but also to putative restraining mechanisms.
Enzyme assays confirmed increases in MMP-1 activity in response to gastrin. Western blots of media from gastrin-stimulated cells showed a major band corresponding to proMMP-1 and a relatively minor band corresponding to the active form. Thus AGS-GR cells have some capacity to activate MMP-1, but this remains relatively modest, and following secretion proMMP-1 appears to be relatively stable in the media of these cells. A range of proteases may activate MMP-1, and it seems possible that in vivo activation is achieved by sub-epithelial cells. In this context it is worth noting that proteomic studies have demonstrated increased MMP-1 activation by myofibroblasts derived from gastric tumors compared with myofibroblasts from adjacent control tissue (14). While epithelial expression of proMMP-1 is regulated by gastrin, therefore, the functional significance of this depends on activation, which in turn reflects the cellular microenvironment which differs in normal, inflammatory, premalignant, and malignant conditions.
A role for extracellular proteases in mediating cell migration is well recognized (30). These are involved in facilitating the progressive remodeling of cell-matrix interactions at both leading and trailing edges of migrating cells. Migration of gastric epithelial cells normally occurs over a period of days as cells move from the proliferative zone to the base of gastric glands (15), but there is also migration in response to wound healing, and in cancer migration may reflect an epithelial-mesenchymal transition. In scratch-wound assays in vitro, there is migration of individual AGS-GR cells, rather than an advancing sheet of cells, and both in this model and in Boyden chamber migration experiments gastrin is a good stimulant of AGS-GR cell migration. There is presently a need for the development of effective specific inhibitors of MMP-1, but immunoneutralization provides an alternative for present purposes (14). The present study revealed a role for MMP-1 in mediating the effects of gastrin migration. Multiple proteases are likely to be involved in the remodeling of cell-matrix attachments in migration, which may well account for the fact that there was only partial inhibition of the effects of gastrin by neutralizing antibody.
It is now clear that gastrin stimulates expression of a number of genes involved in mucosal organization and protection (9, 10). Although MMP-1 expression is not normally associated with epithelial cells, the epithelial cells of the acid-secreting mucosa of the stomach are an exception. In addition to possible roles for gastric epithelial MMP-1 in response to inflammation and infection, our data suggest that it is now appropriate to consider a role for this enzyme in mediating the effects of gastrin in the healthy stomach. Moreover, while chromogranin A and HDC are useful biomarkers of gastrin effects on ECL cells, we suggest that MMP-1 may prove to be a useful biomarker of gastrin actions on other glandular cells.
GRANTS
This study was supported by grants from the National Institute for Health Research, The Wellcome Trust, and North West Cancer Research Fund.
DISCLOSURES
G. J. Dockray is a consultant for Astrimmune Ltd. A. Varro, D. M. Pritchard, and A. R. Moore have received financial support from Trio Medicines.
AUTHOR CONTRIBUTIONS
Author contributions: J.D.K., I.S., A.R.M., S.V.M., Z.R.J., V.V., D.M.P., R.D., L.T., A.V., and G.J.D. conception and design of research; J.D.K., I.S., A.R.M., S.V.M., Z.R.J., V.V., D.M.P., and L.T. performed experiments; J.D.K., I.S., Z.R.J., V.V., D.M.P., R.D., L.T., A.V., and G.J.D. analyzed data; J.D.K., I.S., S.V.M., D.M.P., R.D., L.T., A.V., and G.J.D. interpreted results of experiments; J.D.K. and G.J.D. prepared figures; J.D.K., I.S., A.R.M., Z.R.J., V.V., D.M.P., R.D., A.V., and G.J.D. edited and revised manuscript; J.D.K., I.S., A.R.M., Z.R.J., V.V., D.M.P., R.D., L.T., A.V., and G.J.D. approved final version of manuscript; G.J.D. drafted manuscript.
ACKNOWLEDGMENTS
We are grateful to J. Kozma and B. Balogh for help with immunohistochemistry, to A. Alqahtani for help with Western blotting, and to the staff of the endoscopy suite of the Gastrointestinal Unit, Royal Liverpool University Hospital.
Present address of Z. Rakonczay, Jr.: 1st Dept. of Medicine, Univ. of Szeged, Szeged, Hungary.
Present address of V. Venglovecz: Dept. of Pharmacology and Pharmacotherapy, Univ. of Szeged, Szeged, Hungary.
REFERENCES
- 1.Almeida-Vega S, Catlow K, Kenny S, Dimaline R, Varro A. Gastrin activates paracrine networks leading to induction of PAI-2 via MAZ and ASC-1. Am J Physiol Gastrointest Liver Physiol 296: G414–G423, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angel P, Baumann I, Stein B, Delius H, Rahmsdorf HJ, Herrlich P. 12-O-Tetradecanoyl-phorbol-13-acetate induction of the human collagenase gene is mediated by an inducible enhancer element located in the 5′-flanking region. Mol Cell Biol 7: 2256–2266, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baba M, Itoh K, Tatsuta M. Glycine-extended gastrin induces matrix metalloproteinase-1- and -3-mediated invasion of human colon cancer cells through type I collagen gel and Matrigel. Int J Cancer 111: 23–31, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Black JW, Shankley NP. How does gastrin act to stimulate oxyntic cell secretion. Trends Pharmacol Sci 8: 486–490, 1987. [Google Scholar]
- 5.Bodger K, Ahmed S, Pazmany L, Pritchard DM, Micheal A, Khan AL, Dimaline R, Dockray GJ, Varro A. Altered gastric corpus expression of tissue inhibitors of metalloproteinases in human and murine Helicobacter infection. J Clin Pathol 61: 72–78, 2008. [DOI] [PubMed] [Google Scholar]
- 6.Dimaline R, Evans D, Forster ER, Sandvik AK, Dockray GJ. Control of gastric corpus chromogranin A messenger RNA abundance in the rat. Am J Physiol Gastrointest Liver Physiol 264: G583–G588, 1993. [DOI] [PubMed] [Google Scholar]
- 7.Dimaline R, Sandvik AK. Histidine decarboxylase gene expression in rat fundus is regulated by gastrin. FEBS Lett 281: 20–22, 1991. [DOI] [PubMed] [Google Scholar]
- 8.Dimaline R, Struthers J. Expression and regulation of a vesicular monoamine transporter in rat stomach: a putative histamine transporter. J Physiol 490: 249–256, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dimaline R, Varro A. Novel roles of gastrin. J Physiol 592: 2951–2958, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dockray G, Dimaline R, Varro A. Gastrin: old hormone, new functions. Pflügers Arch 449: 344–355, 2005. [DOI] [PubMed] [Google Scholar]
- 11.Dockray GJ. Clinical endocrinology and metabolism. Gastrin. Best Pract Res Clin Endocrinol Metab 18: 555–568, 2004. [DOI] [PubMed] [Google Scholar]
- 12.Dockray GJ, Hamer C, Evans D, Varro A, Dimaline R. The secretory kinetics of the G cell in omeprazole-treated rats. Gastroenterology 100: 1187–1194, 1991. [PubMed] [Google Scholar]
- 13.Hakanson R, Ding XQ, Norlen P, Lindstrom E. CCK2 receptor antagonists: pharmacological tools to study the gastrin-ECL cell-parietal cell axis. Regul Pept 80: 1–12, 1999. [DOI] [PubMed] [Google Scholar]
- 14.Holmberg C, Ghesquiere B, Impens F, Gevaert K, Kumar JD, Cash N, Kandola S, Hegyi P, Wang TC, Dockray GJ, Varro A. Mapping proteolytic processing in the secretome of gastric cancer-associated myofibroblasts reveals activation of MMP-1, MMP-2, and MMP-3. J Proteome Res 12: 3413–3422, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kirton CM, Wang T, Dockray GJ. Regulation of parietal cell migration by gastrin in the mouse. Am J Physiol Gastrointest Liver Physiol 283: G787–G793, 2002. [DOI] [PubMed] [Google Scholar]
- 16.Kobayashi I, Kawano S, Tsuji S, Matsui H, Nakama A, Sawaoka H, Masuda E, Takei Y, Nagano K, Fusamoto H, Ohno T, Fukutomi H, Kamada T. RGM1, a cell line derived from normal gastric mucosa of rat. In Vitro Cell Dev Biol Anim 32: 259–261, 1996. [DOI] [PubMed] [Google Scholar]
- 17.Krueger S, Hundertmark T, Kalinski T, Peitz U, Wex T, Malfertheiner P, Naumann M, Roessner A. Helicobacter pylori encoding the pathogenicity island activates matrix metalloproteinase 1 in gastric epithelial cells via JNK and ERK. J Biol Chem 281: 2868–2875, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Laboisse CL, Augeron C, Couturier-Turpin MH, Gespach C, Cheret AM, Potet F. Characterization of a newly established human gastric cancer cell line HGT-1 bearing histamine H2-receptors. Cancer Res 42: 1541–1548, 1982. [PubMed] [Google Scholar]
- 19.Larsson H, Carlsson E, Mattsson H, Lundell L, Sundler F, Sundell G, Wallmark B, Watanabe T, Hakanson R. Plasma gastrin and gastric enterochromaffinlike cell activation and proliferation. Studies with omeprazole and ranitidine in intact and antrectomized rats. Gastroenterology 90: 391–399, 1986. [DOI] [PubMed] [Google Scholar]
- 20.McCaig C, Duval C, Hemers E, Steele I, Pritchard DM, Przemeck S, Dimaline R, Ahmed S, Bodger K, Kerrigan DD, Wang TC, Dockray GJ, Varro A. The role of matrix metalloproteinase-7 in redefining the gastric microenvironment in response to Helicobacter pylori. Gastroenterology 130: 1754–1763, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Murray GI, Duncan ME, Arbuckle E, Melvin WT, Fothergill JE. Matrix metalloproteinases and their inhibitors in gastric cancer. Gut 43: 791–797, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagase H, Woessner JF Jr. Matrix metalloproteinases. J Biol Chem 274: 21491–21494, 1999. [DOI] [PubMed] [Google Scholar]
- 23.Noble PJ, Wilde G, White MR, Pennington SR, Dockray GJ, Varro A. Stimulation of gastrin-CCKB receptor promotes migration of gastric AGS cells via multiple paracrine pathways. Am J Physiol Gastrointest Liver Physiol 284: G75–G84, 2003. [DOI] [PubMed] [Google Scholar]
- 24.Norsett KG, Steele I, Duval C, Sammut SJ, Murugesan SV, Kenny S, Rainbow L, Dimaline R, Dockray GJ, Pritchard DM, Varro A. Gastrin stimulates expression of plasminogen activator inhibitor-1 in gastric epithelial cells. Am J Physiol Gastrointest Liver Physiol 301: G446–G453, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohning GV, Wong HC, Lloyd KC, Walsh JH. Gastrin mediates the gastric mucosal proliferative response to feeding. Am J Physiol Gastrointest Liver Physiol 271: G470–G476, 1996. [DOI] [PubMed] [Google Scholar]
- 26.Pagliocca A, Wroblewski LE, Ashcroft FJ, Noble PJ, Dockray GJ, Varro A. Stimulation of the gastrin-cholecystokinin(B) receptor promotes branching morphogenesis in gastric AGS cells. Am J Physiol Gastrointest Liver Physiol 283: G292–G299, 2002. [DOI] [PubMed] [Google Scholar]
- 27.Pillinger MH, Marjanovic N, Kim SY, Lee YC, Scher JU, Roper J, Abeles AM, Izmirly PI, Axelrod M, Pillinger MY, Tolani S, Dinsell V, Abramson SB, Blaser MJ. Helicobacter pylori stimulates gastric epithelial cell MMP-1 secretion via CagA-dependent and -independent ERK activation. J Biol Chem 282: 18722–18731, 2007. [DOI] [PubMed] [Google Scholar]
- 28.Pillinger MH, Marjanovic N, Kim SY, Scher JU, Izmirly P, Tolani S, Dinsell V, Lee YC, Blaser MJ, Abramson SB. Matrix metalloproteinase secretion by gastric epithelial cells is regulated by E prostaglandins and MAPKs. J Biol Chem 280: 9973–9979, 2005. [DOI] [PubMed] [Google Scholar]
- 29.Samuelson LC, Hinkle KL. Insights into the regulation of gastric acid secretion through analysis of genetically engineered mice. Annu Rev Physiol 65: 383–400, 2003. [DOI] [PubMed] [Google Scholar]
- 30.Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol 17: 463–516, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tatsuguchi A, Fukuda Y, Ishizaki M, Yamanaka N. Localization of matrix metalloproteinases and tissue inhibitor of metalloproteinases-2 in normal human and rabbit stomachs. Digestion 60: 246–254, 1999. [DOI] [PubMed] [Google Scholar]
- 32.Varro A, Hemers E, Archer D, Pagliocca A, Haigh C, Ahmed S, Dimaline R, Dockray GJ. Identification of plasminogen activator inhibitor-2 as a gastrin- regulated gene: role of Rho GTPase and menin. Gastroenterology 123: 271–280, 2002. [DOI] [PubMed] [Google Scholar]
- 33.Varro A, Kenny S, Hemers E, McCaig C, Przemeck S, Wang TC, Bodger K, Pritchard DM. Increased gastric expression of MMP-7 in hypergastrinemia and significance for epithelial-mesenchymal signaling. Am J Physiol Gastrointest Liver Physiol 292: G1133–G1140, 2007. [DOI] [PubMed] [Google Scholar]
- 34.Varro A, Noble PJ, Pritchard DM, Kennedy S, Hart CA, Dimaline R, Dockray GJ. Helicobacter pylori induces plasminogen activator inhibitor 2 in gastric epithelial cells through nuclear factor-kappaB and RhoA: implications for invasion and apoptosis. Cancer Res 64: 1695–1702, 2004. [DOI] [PubMed] [Google Scholar]
- 35.Varro A, Noble PJ, Wroblewski LE, Bishop L, Dockray GJ. Gastrin-cholecystokinin(B) receptor expression in AGS cells is associated with direct inhibition and indirect stimulation of cell proliferation via paracrine activation of the epidermal growth factor receptor. Gut 50: 827–833, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang TC, Dockray GJ. Lessons from genetically engineered animal models. I. Physiological studies with gastrin in transgenic mice. Am J Physiol Gastrointest Liver Physiol 277: G6–G11, 1999. [DOI] [PubMed] [Google Scholar]
- 37.Wang TC, Koh TJ, Varro A, Cahill RJ, Dangler CA, Fox JG, Dockray GJ. Processing and proliferative effects of human progastrin in transgenic mice. J Clin Invest 98: 1918–1929, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Watson F, Kiernan RS, Deavall DG, Varro A, Dimaline R. Transcriptional activation of the rat vesicular monoamine transporter 2 promoter in gastric epithelial cells: regulation by gastrin. J Biol Chem 276: 7661–7671, 2001. [DOI] [PubMed] [Google Scholar]
- 39.Wroblewski LE, Noble PJ, Pagliocca A, Pritchard DM, Hart CA, Campbell F, Dodson AR, Dockray GJ, Varro A. Stimulation of MMP-7 (matrilysin) by Helicobacter pylori in human gastric epithelial cells: role in epithelial cell migration. J Cell Sci 116: 3017–3026, 2003. [DOI] [PubMed] [Google Scholar]
- 40.Wroblewski LE, Pritchard DM, Carter S, Varro A. Gastrin-stimulated gastric epithelial cell invasion: the role and mechanism of increased matrix metalloproteinase 9 expression. Biochem J 365: 873–879, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu JY, Lu H, Sun Y, Graham DY, Cheung HS, Yamaoka Y. Balance between polyoma enhancing activator 3 and activator protein 1 regulates Helicobacter pylori-stimulated matrix metalloproteinase 1 expression. Cancer Res 66: 5111–5120, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]