Abstract
Testosterone (TES) and other androgens exert a direct vasorelaxing action on the vasculature in vitro that is structurally specific and independent of cytosolic androgen receptor (AR). The effects of intravenous androgen infusions on mean arterial blood pressure (BP) and heart rate (HR) were determined in conscious, unrestrained, chronically catheterized, ganglionically blocked (hexamethonium, HEX; 30 mg/kg ip) male Sprague-Dawley (SD) and testicular-feminized male (Tfm; AR-deficient) rats, 16–20 wk of age. BP and HR were recorded at baseline and with increasing doses of androgens (0.375–6.00 μmol·kg−1·min−1 iv; 10 min/dose). Data are expressed as means ± SE (n = 5–8 rats/group). In SD rats, baseline BP and HR averaged 103 ± 4 mmHg and 353 ± 12 beats/min (bpm). TES produced a dose-dependent reduction in BP to a low of 87 ± 4 mmHg (Δ16%), while HR was unchanged (354 ± 14 bpm). Neither BP (109 ± 3 mmHg) nor HR (395 ± 13 bpm) were altered by vehicle (10% EtOH in 0.9% saline; 0.15 ml·kg−1·min−1, iv). In Tfm, TES produced a similar reduction in BP (99 ± 3 to 86 ± 3 mmHg, Δ13%); HR was unchanged (369 ± 18 bpm). In SD, 5β-dihydrotestosterone (genomically inactive metabolite) produced a greater reduction in BP than TES (102 ± 2 to 79 ± 2 mmHg, Δ23%); HR was unchanged (361 ± 9). A 20-μg iv bolus of sodium nitroprusside in both SD and Tfm rats reduced BP 30–40 mmHg, while HR was unchanged, confirming blockade by HEX. Pretreatment of SD rats with neuronal nitric oxide synthase (nNOS) inhibitor (S-methyl-thiocitrulline, SMTC; 20 μg·kg−1·min−1 × 30 min) abolished the hypotensive effects of TES infusion on BP (104 ± 2 vs. 101 ± 2 mmHg) and HR (326 ± 11 vs. 324 ± 8 bpm). These data suggest the systemic hypotensive effect of TES and other androgens involves a direct vasodilatory action on the peripheral vasculature which, like the effect observed in isolated arteries, is structurally specific and AR-independent, and involves activation of nNOS.
Keywords: 5β-DHT, testosterone, vasodilation, neuronal NOS, blood pressure
the well-established clinical observations that hypertension and coronary artery disease occur more frequently in men than in premenopausal women (13, 14, 15, 16, 18, 22, 51) have led to the dogmatic concept that testosterone (TES) has deleterious effects on the heart and vasculature and exacerbates the development of cardiovascular disease (CVD) in males (10, 21, 36). However, more recent clinical and epidemiological studies on the role of TES in CVD are at best controversial, and in-depth reviews and analyses of the role of androgens in CVD reveal that there is little sound evidence from either animal or human studies that TES, or other androgens, shorten men's lives (18, 51). Further, available human studies reveal both acute and chronic beneficial effects of TES on CVD (18, 51). Indeed, there is increasing evidence that TES replacement therapy in aging hypogonadal men significantly improves cardiovascular and metabolic functions, including a reduction in diastolic blood pressure (9, 19). Further, serum TES levels are reduced in both hypertensive men and women (11, 15, 34, 44).
In parallel, accumulating evidence from recent animal studies, using isolated blood vessels in vitro, suggests that TES and other androgen metabolites exert beneficial effects by inducing relaxation of vascular smooth muscle (VSM) through rapid, nongenomic mechanisms (for recent reviews, see Refs. 26 and 31). Although this acute effect of TES and other androgens has been observed at micromolar concentrations in several large arteries (e.g., aorta, coronary, and umbilical arteries) from a variety of species, more recent studies have reported relaxation of smaller resistance arteries at nanomolar (physiological) concentrations (for recent reviews, see Refs. 26 and 31). The key mechanism underlying this effect of TES on VSM appears to be activation of voltage-operated (Kv) and calcium-dependent K+ channels (BKCa) via TES-induced activation of neuronal nitric oxide synthase (nNOS) and/or inactivation of L-type voltage-operated Ca2+ channels (Ref. 31). Studies employing TES analogs and metabolites reveal that androgen-induced vasorelaxation is a structurally specific, nongenomic effect, with efficacies and potencies fundamentally different from those for the genomic effects of androgens on reproductive targets (7, 29, 31, 33, 53).
Although the mechanism of TES-induced vasorelaxation has been established in vitro, little is known about the acute systemic vascular effects of androgens in vivo. Thus, in the present investigation, the acute effects of intravenous androgen infusions, and of nNOS inhibition, on arterial blood pressure (BP), and systemic hemodynamics were determined in conscious male Sprague-Dawley (SD) and testicular-feminized male [androgen receptor (AR)-deficient; Tfm] rats. The hypothesis tested was that TES exerts a systemic hypotensive effect on BP by activation of a direct, nNOS-dependent mechanism. The results reveal that TES and other androgens exert systemic hypotensive effects on BP that appear to involve a direct, nongenomic vasodilatory action on the peripheral vasculature which, like the effect observed in isolated arteries in vitro, is structurally specific, AR-independent, and nNOS-dependent.
MATERIALS AND METHODS
Animals
Age-matched (16–20-wk old) male SD rats [428 ± 8.4 g body wt (n = 26); Harlan, Houston, TX] and testicular-feminized male rats [Tfm; 403 ± 19.7 g (n = 7), bred in-house] were used in all studies. The Tfm rat exhibits an X-linked, recessive defect in AR function in affected males (40, 43, 52), similar to the human testicular feminization syndrome. Although genotypically male (XY), these rats develop phenotypically as female and, thus, serve as a natural “knockout” model to study AR-mediated effects on vascular function (5, 42). Several studies on the acute vascular effects of TES reported very similar vasorelaxing effects in female, as well as male blood vessels in vitro (5, 53) and in vivo (3). In the present study, the first to examine the hypotensive effects of TES on systemic BP in vivo, it was important to focus on the vascular mechanism of action of TES in males (where the mechanisms are likely to be the most clear-cut), and pursue other related issues such as male vs. female differences in the acute effects of TES in subsequent studies.
The rats were housed in vivarium facilities at the Texas A&M University (TAMU) Laboratory Animal Resources and Research facility with controlled temperature (22–26°C), relative humidity (∼50%), and lighting (12:12-h light-dark cycle). The animals were housed in pairs in standard plastic laboratory rat cages, and tap water and an alfalfa- and soy oil-free laboratory rat chow (Harlan Tek-Lad, Indianapolis, IN) were provided ad libitum. This diet is free of phytoestrogens, which are often present in commonly used laboratory rat chows (38, 39) and have been reported to confound studies on sex differences in vascular function (17). All experiments were reviewed and approved by the TAMU Institutional Animal Care and Use Committee.
Animal Surgical Preparation
Under isoflurane inhalation anesthesia, rats were implanted with chronic, indwelling intravascular catheters in the left carotid artery and left jugular vein (0.033 in OD × 0.014 in ID Microrenathane tubing, Braintree Scientific, Braintree, MA), exteriorized at the neck. Following the surgery, the animals were given 15,000 U procaine penicillin G (prophylactic antibiotic) and 2.5 mg/kg flunixin (analgesic) and were fitted with polyester cloth vests with Velcro closures that surrounded the chest and neck to protect the catheters. The rats were allowed 5 to 7 days of recovery. Food and water consumption was monitored, and maintenance of a stable body weight was required before being used in any experimentation.
Blood Pressure and Heart Rate Recording
Before and after surgery, the animals were trained to lie in small, opaque Plexiglas boxes (17.5 cm long × 7.0 cm wide × 7.5 cm high) that allowed them freedom to move backward and forward, but restricted turning and up and down movement. A lengthwise slot along the top of the boxes allowed free movement of the arterial and venous catheters. On the day of the experiment, rats were allowed to acclimate to the boxes in a quiet laboratory for at least 30 min before the experiment was initiated and baseline BP and heart rate (HR) were recorded. BP and HR were recorded from the carotid artery catheter by a Statham p23ID transducer (Statham, Oxnard, CA) connected to a PowerLab Data Acquisition System (PowerLab, Colorado Springs, CO), and the data were analyzed using Lab Chart Software (PowerLab) on an Apple Macintosh Computer (Apple, Cupertino, CA).
Experimental Protocols
Dose-response effects of androgens on systemic hemodynamics.
At the beginning of the experiment, conscious male SD or Tfm rats were given hexamethonium bromide (HEX; 30 mg/kg ip) to produce an autonomic ganglionic blockade, and then acclimated in the Plexiglas restraining box for 30 min to achieve stable hemodynamic conditions. After on-line recording of baseline BP and HR for 5 min, rats received five successive 10-min infusions of TES (0.375–6.00 μmol·kg−1·min−1 iv) or vehicle (10% EtOH in 0.9% NaCl) via the jugular catheter at 0.15 ml·kg−1·min−1. BP and HR were recorded continuously via the carotid artery catheter and were averaged over the final minute of each 10-min infusion period to determine the BP and HR response to each dose of TES. Rats were given supplemental doses of HEX (15 mg/kg iv) every 20 min to ensure continued ganglionic blockade. After the final TES infusion, rats were given a bolus injection of sodium nitroprusside (SNP; 20 μg/kg iv), and BP and HR were monitored for 1 min to evaluate efficacy of the ganglionic blockade.
Additional dose-response experiments were performed using 5β-dihydrotestosterone (5β-DHT; 0.375–6.00 μmol·kg−1·min−1 iv), a genomically inactive metabolite of TES and the stereoisomer of 5α-DHT, the genomically active metabolite of TES in reproductive tissues (31). Identical ganglionic blockade, 10-min infusion periods, BP and HR recording, and ganglionic blockade verification with SNP were used.
Effects of nNOS inhibition on TES-induced systemic hemodynamics.
To determine the role of nNOS in the hypotensive effects of TES, male SD rats were prepared for study as above, including ganglionic blockade with HEX, and baseline BP and HR were recorded for 5 min. Then, the animals were pretreated with an infusion of the nNOS-selective inhibitor S-methyl-thiocitrulline (SMTC) (20 μg·kg−1·min−1 iv) for 30 min (1), and new baseline BP and HR were recorded for 5 min. Following the post-SMTC baseline recording, an identical series of TES infusions and BP and HR recording were performed, as above, with the continued infusion of SMTC.
Chemical Reagents and Drugs
The following drugs and reagents were used: TES (17β-hydroxy-4-androsten-3-one) and 5β-DHT (17β-hydroxy-5β-androstan-3-one; Steraloids, Newport, RI), sodium nitroprusside (Hospira, Lake Forest, IL), hexamethonium bromide (Sigma Chemical; St. Louis, MO), and s-methyl-thiocitrulline (Cayman Chemical, Ann Arbor, MI). Working dilutions of androgens were prepared daily from 10-mM stock solutions of TES and 5β-DHT, dissolved in 50% EtOH, and stored at 4°C. SNP was diluted daily from pharmaceutical stock solution, and working solutions of HEX and SMTC were prepared fresh daily from powdered stock.
Data Analysis
All data are expressed as means ± SE; n indicates the number of animals studied. Baseline mean arterial BP and HR values were obtained by averaging 5-min recordings at the beginning of each experiment, prior to experimental intervention. BP and HR responses to androgens were obtained by averaging recordings of the final minute of each 10-min androgen infusion period. In preliminary experiments, it was determined that BP and HR responses to androgen infusions exhibited clear plateaus during that time. The dose of TES or 5β-DHT that produced 50% of the maximal BP response (EC50) was calculated individually from the log dose-response curve for each animal, and these values are reported as the means ± SE for each experimental group. Dose-response curves for BP and HR for each group (e.g., TES and 5β-DHT) were analyzed by one-way ANOVA with repeated measures. For mean BP data, ANOVA tests were followed by Dunnett's modification of the t-test for post hoc detection of significant differences between any two means of the data groups (e.g., TES vs. vehicle, TES vs. 5β-DHT, TES [Sprague-Dawley] vs. TES [Tfm]). Paired comparisons were made by paired t-test (e.g., baseline BP or HR in control vs. HEX or HR in pre-SNP vs. post-SNP). Differences between any two means were accepted as significant if P < 0.05.
RESULTS
Dose-Response Effects of Androgens on Systemic Hemodynamics
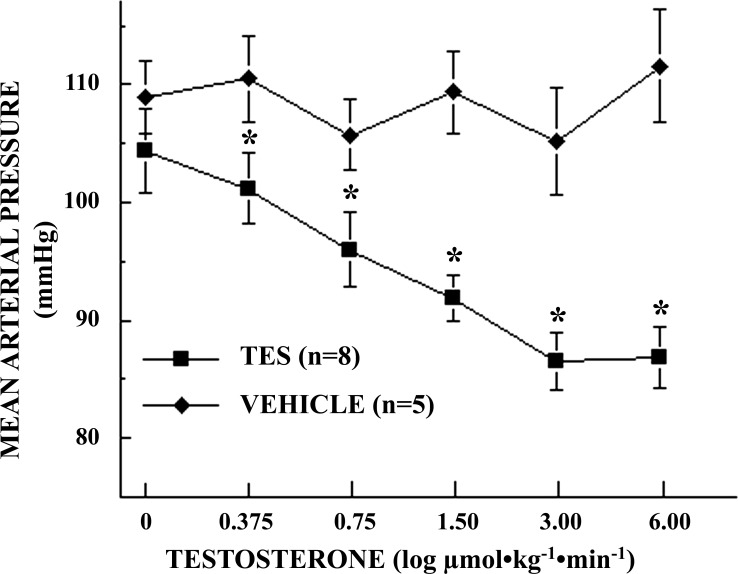
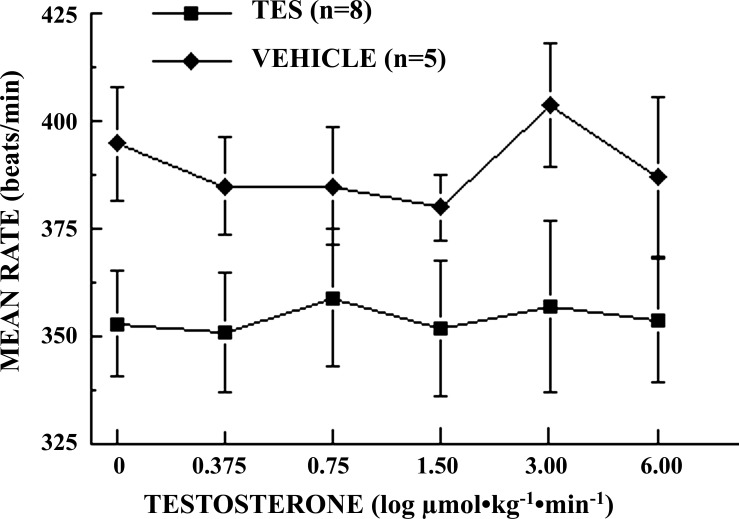
In preliminary experiments, infusions of TES failed to consistently alter BP in male SD rats, although significant variability in both BP and HR were observed during the 50-min infusion period. It was suspected that baroreflex-mediated changes in sympathetic outflow could be masking the vasodilatory effects of TES widely observed in vitro. Pretreatment of rats with HEX resulted in small, but significant, reductions in both BP (113 ± 2.7 vs. 104 ± 3.8 mmHg; P = 0.045) and HR [391 ± 12.1 vs. 353 ± 14.2 beats per min (bpm); P = 0.0290] and eliminated the observed variability in BP and HR. Thus, pretreatment with HEX resulted in progressive and significant (P = 0.0009), dose-dependent reductions in BP in response to TES, from 103 ± 4.3 mmHg at baseline to 87 ± 3.9 mmHg at the highest dose of TES (Fig. 1), while HR remained nearly constant and did not vary significantly from baseline (353 ± 12.4 bpm) over the course of the experiment (P = 0.9994; Fig. 2). In contrast, in vehicle-control rats, neither BP (109 ± 3.1 mmHg; P = 0.893; Fig. 1) nor HR (395 ± 13.2 bpm; P = 0.971; Fig. 2) varied significantly from baseline over the course of the infusion experiments.
Fig. 1.
Log dose-response effects of testosterone (TES) infusion on mean arterial blood pressure (MAP) in conscious, unrestrained, chronically catheterized, ganglion-blocked male Sprague-Dawley rats [0.375–6.00 μmol·kg−1·min−1 iv (0.15 ml·kg−1·min−1); n = 8 rats]. Vehicle-control rats (VEHICLE) received identical volumes of the vehicle (10% EtOH in normal saline, 0.15 ml·kg−1·min−1; n = 5 rats). Data points are means ± SE. MAP differed significantly with dose of TES (P = 0.0009) but not in VEHICLE (P = 0.893). *0.0005 ≤ P ≤ 0.047 TES vs. VEHICLE MAP at each dose.
Fig. 2.
Log dose-response effects of testosterone (TES) infusion on heart rate (HR) in conscious, unrestrained, chronically catheterized, ganglion-blocked male Sprague-Dawley rats [0.375–6.00 μmol·kg−1·min−1 iv (0.15 ml·kg−1·min−1); n = 8 rats]. Vehicle-control rats (VEHICLE) received identical volumes of the vehicle (10% EtOH in normal saline, 0.15 ml·kg−1·min−1; n = 5 rats). Data points are means ± SE. HR did not differ significantly with dose of TES (P = 0.9994) or in VEHICLE (P = 0.9713) groups.
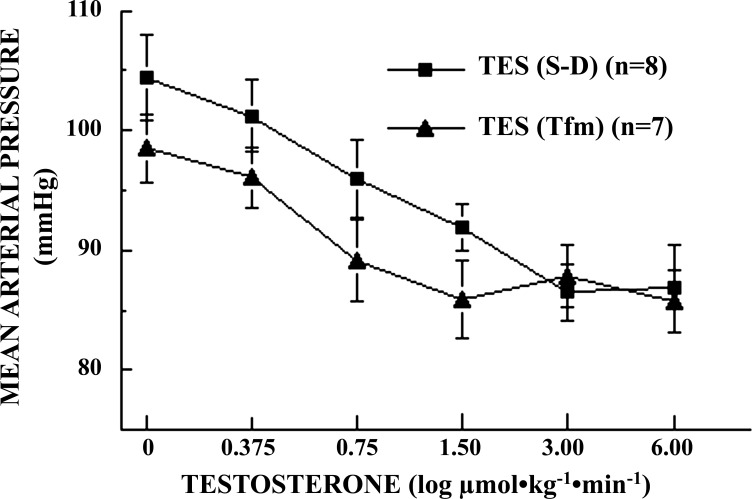
In HEX-pretreated Tfm rats, infusions of TES resulted in significant (P = 0.0178), dose-dependent reductions in BP, qualitatively and quantitatively similar to those observed in SD male rats. BP declined from 99 ± 2.8 mmHg at baseline to 86 ± 2.6 mmHg at the highest dose of TES (Fig. 3), while HR remained nearly constant and did not vary significantly from baseline (369 ± 17.8 bpm) over the course of the experiment (P = 0.9676; data not shown). BP responses to TES did not differ significantly between Tfm and SD male rats at baseline or at any dose of TES (P > 0.05). Neither the maximal BP response (86 ± 2.6 vs. 87 ± 3.6 mmHg; P = 0.4138) nor the potency (EC50) of TES infusions (0.73 ± 0.11 μmol·kg−1·min−1 vs. 1.06 ± 0.14 μmol·kg−1·min−1; P = 0.2780) differed significantly in Tfm vs. SD male rats, respectively.
Fig. 3.
Log dose-response effects of testosterone (TES) infusion on mean arterial blood pressure (MAP) in conscious, unrestrained, chronically catheterized, ganglion-blocked testicular-feminized (Tfm, n = 7) and Sprague-Dawley (S-D; n = 8) male rats (0.375–6.00 μmol·kg−1·min−1 iv; 0.15 ml·kg−1·min−1). Data points are means ± SE. MAP differed significantly with a dose of TES in both Tfm (P = 0.018) and SD (P = 0.0009) rats. Effects of TES on MAP did not differ between SD and Tfm rats at all doses (0.054 ≤ P ≤ 0.486).
Following infusions of TES in both SD male and Tfm rats, bolus injections of SNP were given to confirm the efficacy of ganglionic blockade. SNP produced rapid, short-lived reductions in BP of ∼40–50 mmHg; however, HR did not change significantly from pre-SNP HR in either SD male rats (337 ± 8.8 vs. 351 ± 11.6 bpm; P = 0.1646) or Tfm rats (375 ± 26.7 vs. 395 ± 22.5 bpm; P = 0.2847).
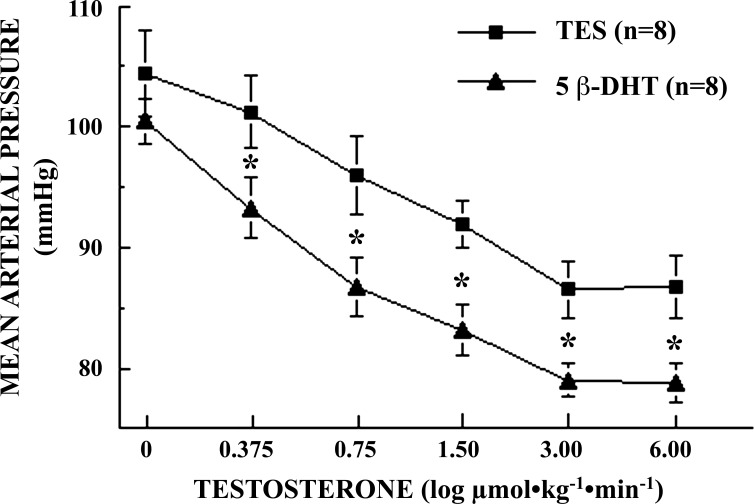
In HEX-pretreated SD male rats, infusions of the 5β-reduced metabolite of TES, 5β-DHT, resulted in significant (P = 0.0001), dose-dependent reductions in BP, qualitatively similar, but quantitatively greater, than those observed with TES, from 102 ± 1.6 mmHg at baseline to 79 ± 1.7 mmHg at the highest dose of 5β-DHT (Fig. 4). HR remained nearly constant and did not vary significantly from baseline (361 ± 8.9 bpm) over the course of the experiment (P = 0.9222; data not shown). BP responses differed significantly between 5β-DHT and TES at all doses (0.0084 ≤ P ≤ 0.0350; Fig. 4). The maximal BP response differed significantly (P = 0.0395) between 5β-DHT (79 ± 1.7 mmHg) and TES (87 ± 3.6 mmHg), while the potency (EC50) was nearly identical (P = 0.2780) for 5β-DHT (0.98 ± 0.17 μmol·kg−1·min−1) and TES (1.06 ± 0.14 μmol·kg−1·min−1).
Fig. 4.
Log dose-response effects of 5β-dihydrotestosterone (5β-DHT; n = 8) vs. testosterone (TES, n = 8) infusion on mean arterial blood pressure (MAP) in conscious, unrestrained, chronically catheterized, ganglion-blocked male Sprague-Dawley rats (0.375–6.00 μmol·kg−1·min−1 iv; 0.15 ml·kg−1·min−1). Data points are means ± SE. MAP differed significantly with a dose of 5β-DHT (P = 0.0001) and a dose of TES (P = 0.0009). Effects of 5β-DHT on MAP were greater than TES at all doses (*0.0084 ≤ P ≤ 0.0395).
Effects of nNOS Inhibition on TES-Induced Systemic Hemodynamics
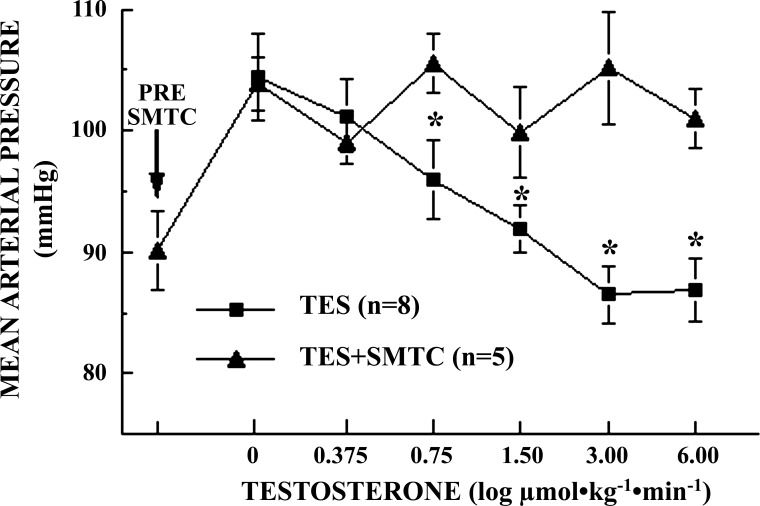
Pretreatment of male SD rats with SMTC (30-min infusion) increased baseline BP from 90 ± 3.3 mmHg to 104 ± 2.2 mmHg (P = 0.0018) and eliminated the dose-dependent hypotensive effects of TES infusion (Fig. 5). During TES infusion, BP exhibited some slight oscillations over time (approximately ± 5–8 mmHg) but neither BP (P = 0.0930) nor HR (P = 0.3657) varied significantly during TES infusion in the presence of SMTC. BP in TES-infused rats (control) differed from that of SMTC + TES-infused rats at all but the lowest dose of TES (0.0059 ≤ P ≤ 0.0357; Fig. 5).
Fig. 5.
Effects of nNOS inhibition with S-methyl thiocitrulline (SMTC) on log dose-response effects of testosterone (TES) infusions on mean arterial blood pressure (MAP) in conscious, unrestrained, chronically catheterized, ganglion-blocked male Sprague-Dawley rats (0.375–6.00 μmol·kg−1·min−1 iv; 0.15 ml·kg−1·min−1), pretreated with SMTC (TES+SMTC, 20 μg·kg−1·min−1; n = 5 rats) or not (TES; n = 8 rats). Rats were pretreated with SMTC for 30 min before and then during TES infusions. Data points are means ± SE. MAP differed significantly with dose of TES (P = 0.0009) but not with dose of TES+SMTC (P = 0.0930). Effects of TES on MAP differed between TES and TES+SMTC at each dose (*0.0027 ≤ P ≤ 0.038).
DISCUSSION
In the present investigation, the systemic vascular effects of intravenous infusions of androgens on BP and HR were determined in male SD and Tfm rats. The results reveal that TES and its 5β-reduced metabolite, 5β-DHT, exert a systemic hypotensive effect, which appears to involve a direct vasodilatory action on the peripheral vasculature, and like the vasorelaxing effect observed in isolated arteries in vitro, is structurally specific and AR-independent, and involves activation of nNOS.
Dose-Response Effects of Androgens on Systemic Hemodynamics
In the presence of autonomic ganglionic blockade, TES exerted a dose-dependent systemic hypotensive effect in both SD and Tfm rats. Interestingly, the genomically inactive TES metabolite, 5β-DHT, produced a greater hypotensive effect, which strongly suggests that TES and other androgens exert systemic hypotensive effects that involve a direct, nongenomic vasodilatory action on the peripheral vasculature. These effects, like those observed in a variety of arterial vessels in vitro, are structurally specific and AR-independent. The abundance of previous in vitro studies of TES-induced vasorelaxation (6, 26, 31, 46, 47, 49, 50), together with more limited in vivo studies of TES-induced regional vasodilation (3, 23, 48), provides clear and consistent support for the idea that the hypotensive effects of TES on systemic BP observed in vivo in the present study result from a systemic vasodilatory action, which is nongenomic and independent of the AR.
In the present study, it should be noted that preliminary experiments with intravenous infusions of TES failed to consistently alter BP in male SD rats. The significant variability in BP and HR observed during TES infusions suggested that baroreflex-mediated changes in sympathetic outflow to the heart and vasculature could be masking systemic vasodilatory effects of TES, if they were present. Indeed, pretreatment with the ganglionic blocker, HEX, eliminated this variability in BP and HR in response to TES, resulting in a clear dose-dependent hypotensive effect, with virtually no changes in HR. Pretreatment of rats with HEX (supplemented during the experiment) resulted in significant reductions in baseline BP and HR. Although the depressor effects of HEX on baseline BP and HR in the present study were relatively small compared with those reported in previous studies (25, 27), the rats were trained extensively prior to the experiments and were well acclimated to the restraining boxes. Thus, the rats were quite relaxed and quiet during the TES infusion experiments, and resting sympathetic tone was likely low in these animals; the relatively low initial BP and HR recorded prior to HEX administration support this suggestion and likely explain the relatively small effect of HEX on baseline BP and HR. The intravenous administration of SNP at the end of each experiment transiently lowered BP by 40–50 mmHg but failed to reflexively increase HR, verifying the efficacy of autonomic blockade by HEX in these experiments.
Although numerous studies have clearly established the rapid, nongenomic vasorelaxing effects of TES and other androgens in vitro, the evidence for TES producing coronary or systemic vasodilation in vivo at physiological concentrations (100 pM to 100 nM; Ref. 31) is rare. Indeed, intra-arterial infusion of TES produces coronary vasodilation in anesthetized dogs (3), pigs (23), and humans (48), and regional vasodilation of mesenteric, renal, and skeletal muscle vascular beds in anesthetized pigs (23). All of these studies used intra-arterial infusions of TES that were estimated to produce physiological plasma concentrations, and most resulted in strikingly similar levels of vasodilation (8–15%). Interestingly, the intravenous infusions of TES in conscious SD rats in the present study reduced BP by a maximum of 16%, strikingly similar and fully consistent with the local vasodilatory action of TES in previous studies. The results of the present study extend those findings and are the first to establish that TES and other androgens can exert hypotensive effects on systemic BP through a direct systemic vasodilatory action in vivo, consistent with their vasorelaxing effects observed in isolated arteries in vitro.
The Tfm rat exhibits an x-linked, recessive defect in AR function in affected males (40, 43, 52). Although these animals synthesize adequate, indeed higher than normal, amounts of TES compared with normal male SD rats, the inability of reproductive target tissues to respond to this hormone results in the development of a female phenotype in the reproductive tract and in overall morphology. Some of the TES in these animals is aromatized to estradiol, so that plasma levels of both TES and estradiol are elevated in the Tfm rat (42). Similarly, the absence of a functional AR in these animals results in clear differences in vascular reactivity and endothelial function compared with normal male SD rats (42), but similar to the changes in vascular function observed in castrated male SD rats (41). Infusion of TES in HEX-pretreated Tfm rats in the present study produced a dose-dependent systemic hypotension nearly identical to that observed in normal male SD rats. These findings are consistent with those of previous studies in the rat aorta, which revealed identical vasorelaxing effects of TES in both SD and Tfm rats (5), clearly eliminating a role for the AR receptor and genomic mechanisms in the vasorelaxing action of androgens in vitro.
Since TES and estradiol share the same biosynthetic pathway, it has been suggested that TES-induced vasorelaxation in vitro or vasodilation in vivo might be an indirect effect mediated by the local conversion of TES to 17β-estradiol by vascular P-450 aromatase. However, this possibility has been excluded by extensive in vitro and in vivo evidence that 1) inhibition of P-450 aromatase does not prevent TES-induced vasorelaxation; 2) estrogen receptor antagonism does not alter vasorelaxing effects of TES; and 3) nonaromatizable metabolites of TES (e.g., DHT) cause vasorelaxation (for review, see Ref. 31). Therefore, it seems likely that the systemic hypotensive effect of TES observed in vivo in the present study results from the direct vasodilatory effect of TES on the peripheral vasculature, although an indirect vasodilatory effect of TES by aromatization to estradiol cannot be categorically excluded.
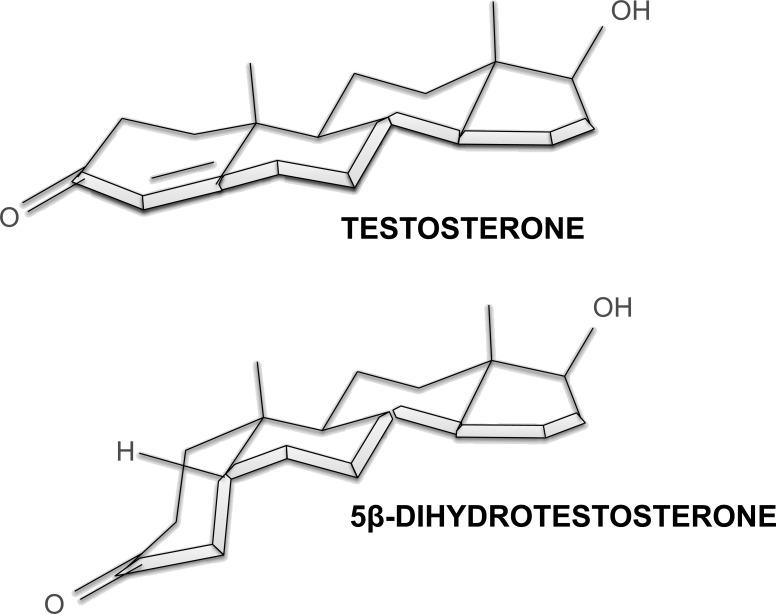
In the present study, infusion of the genomically inactive TES metabolite, 5β-DHT, produced dose-dependent reductions in BP in male SD rats, qualitatively similar in nature, but quantitatively greater than those observed with TES. The hypotensive effects of 5β-DHT were consistently greater than those of TES, although the potency of the two androgens was quite similar. These findings are entirely consistent with previous in vitro studies of the rat aorta, as well as the human umbilical artery and myometrium, which all reported that 5β-DHT exhibits significantly greater efficacy and/or potency than TES in the relaxation of both vascular and uterine smooth muscle (29–32). It is interesting to note that the bioconversion of TES into 5β-DHT results in a dramatic change in its molecular structure: The A ring bends 90° relative to the steroid nucleus when the C5 hydrogen is β/cis-oriented (see structural conformations in Fig. 6). These findings are of particular interest for two reasons. First, they reveal that androgen-induced vasorelaxation is a structurally specific, AR-independent nongenomic effect of the androgen molecule, with efficacies and potencies fundamentally different from those for the genomic effects of androgens in reproductive tissues. Second, these findings indicate that the structure-function relationship of the acute vascular action of androgens is consistent in both isolated arteries in vitro (7, 33, 53) and intact animals in vivo.
Fig. 6.
The three-dimensional conformation of the androgen molecules: Δ4,3-keto structure (TES; top) and the 5β/cis-conformation of the 5β-reduced metabolite, 5β-DHT (bottom). These molecular conformations reveal that minor changes in the orientation of C5 in the A-ring of the steroid nucleus can result in major changes in the potency and efficacy of nongenomic vascular effects of the androgen molecule (e.g., TES vs. 5β-DHT; see text for details).
Effects of nNOS Inhibition on TES-Induced Systemic Hemodynamics
In the present study, pretreatment of male SD rats with the nNOS-selective inhibitor SMTC eliminated the dose-dependent hypotensive effects of TES infusions, suggesting that these effects are nNOS-dependent. These findings are fully consistent with earlier in vivo studies employing nonselective NOS inhibitors in canine and porcine coronary arteries, and in porcine regional systemic arteries (3, 23) and in vitro studies in rat aorta (5) that identified a role for NO in the vasodilatory effects of TES, and more recent preliminary in vitro studies (49, 50) that suggested a role for nNOS in the vasodilatory action of TES in the microvasculature at low physiological concentrations (1–100 nM). Although the findings of the SMTC experiments clearly suggest involvement of nNOS in TES-induced vasodilation, they do not fully preclude a possible role of endothelial nitric oxide synthase (eNOS), because the dose of SMTC employed is sufficient to at least partially inhibit eNOS (Ki ∼11 nM), as well as nNOS (Ki ∼1 nM). Given that the kidney is also a key regulator of BP, an abundant source of nNOS, and sensitive to the effects of HEX, a role of the kidney in the systemic hypotensive effects of TES also cannot be excluded in the present study.
Physiological Relevance of the Systemic Hypotensive Effects of Androgens
That TES-induced vasodilation does occur at physiological concentrations in both in vitro and in vivo human and animal models strongly suggests a physiological role for TES and other androgens in cardiovascular regulation; indeed, there are several lines of evidence from human clinical studies that support this concept. First, strong inverse relationships exist between serum TES and diastolic and/or systolic BP in elderly normotensive and hypertensive men (9); second, TES replacement therapy in aging, hypogonadal men reduces diastolic BP (19); third, serum levels of TES are reduced in both hypertensive men and women, compared with their normotensive counterparts (11, 15, 44); fourth, serum concentrations of TES and its components decline as adult men age, and are consistently lower in men with CVD (as reviewed in Refs. 18, 51); and finally, TES replacement therapy in middle-aged hypogonadal men not only reduces diastolic BP, but also improves body composition and serum lipid profiles (19, 20). Despite these suggestive findings, a recent meta-analysis of clinical trials involving TES therapy in adult men failed to detect any significant effects of TES on mortality, prostate, or cardiovascular outcomes (8). Nevertheless, the endogenous TES metabolite 5β-DHT, which is genomically inactive at both the AR and estrogen receptors, is a potent and efficacious vasodilator. Interestingly, tissue levels of 5β-reductase, the enzyme that catalyzes the conversion of TES to 5β-DHT, are significantly lower in patients with essential hypertension compared with their normotensive controls (12). This suggests that 5β-DHT may play an important role in the regulation of BP by reducing vascular tone, as consistently observed in previous animal studies in vitro (24, 28, 29) and in the present study in vivo. Finally, 5β-DHT, which is produced in the materno-fetoplacental unit (2), is a potent and efficacious dilator of the human umbilical artery (29). Thus, 5β-DHT may play a role in the maintenance of fetoplacental blood flow, which is an important rate-limiting factor for normal fetal growth. It is possible then, that an insufficiency of 5β-DHT and other androgens produced by the materno-fetoplacental unit and/or impaired responsiveness to these hormones, could contribute, at least in part, to the development of preeclampsia/eclampsia, and that exogenous 5β-DHT may be therapeutic in the treatment of gestational hypertension. In conclusion, the results of the present study suggest that androgen deficiencies in both males and females may be responsible, at least in part, for various forms of hypertension, and that in the future, androgen replacement therapy may be employed to treat or prevent hypertension.
Perspectives and Significance
The present study is the first to establish that TES, and its genomically inactive metabolite, 5β-DHT, produce significant hypotensive effects on the systemic vasculature in conscious male SD rats. These effects of the androgens appear to involve a direct vasodilatory action on the peripheral vasculature, which is androgen structure-specific and AR-independent and which appears to be nNOS-dependent. These acute vascular actions of the androgens may play physiologically relevant roles in cardiovascular health and disease. Androgen replacement therapy with vasoselective androgens such as 5β-DHT, which exert little or no effect on the AR, may be an emerging therapeutic option for the treatment of vascular dysfunctions, such as hypertension in aging men or preeclampsia in pregnant women. Indeed, the development of selective AR modulators may allow for the selective treatment of CVD, while avoiding androgenic side effects in the clinical applications of androgen therapy.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: M.P. and J.N.S. conception and design of research; M.P., C.D.G., L.M.P., and J.N.S. analyzed data; M.P. and J.N.S. interpreted results of experiments; M.P., L.M.P., and J.N.S. edited and revised manuscript; M.P., C.D.G., L.M.P., and J.N.S. approved final version of manuscript; L.M.P. and J.N.S. performed experiments; J.N.S. prepared figures; J.N.S. drafted manuscript.
ACKNOWLEDGMENTS
This study was supported by the National Heart, Lung, and Blood Institute (NHLBI) grant, HL-080402, to J. N. Stallone, and by the Programa de Apoyo a Proyectos de Investigacion y Inovacion Tecnologica/Direccion General de Asuntos del Personal Academico Grant IN203815 to M. Perusquía. M. Perusquía was on sabbatical leave from the Departamento de Biología Celular y Fisiología, Instituto de Investigaciones Biomédicas, Universidad Nacional Autónoma de México.
REFERENCES
- 1.Bagnall NM, Dent PC, Walkowska A, Sadowski J, Johns EJ. Nitric oxide inhibition and the impact on renal nerve-mediated antinatriuresis and antidiuresis in the anesthetized rat. J Physiol 569: 849–856, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benagiano G, Mancuso S, Mancuso FP, Wiqvist N, Diczfalusy E. Studies on the metabolism of C-19 steroids in the human foeto-placental unit. 3. Dehydrogenation and reduction products formed by previable foetuses with androstenedione and testosterone. Acta Endocrinol 57: 187–207, 1968. [DOI] [PubMed] [Google Scholar]
- 3.Chou TM, Sudhir K, Hutchison SJ, Ko E, Amidon TM, Collins P, Chatterjee K. Testosterone induces dilation of canine coronary conductance and resistance arteries in vivo. Circulation 94: 2614–2619, 1996. [DOI] [PubMed] [Google Scholar]
- 4.Corona G, Mannucci E, Lotti F, Fisher AD, Bandini E, Balercia G, Forti G, Maggi M. Pulse pressure, an index of arterial stiffness, is associated with androgen deficiency and impaired penile blood flow in men with ED. J Sex Med 6: 285–293, 2009. [DOI] [PubMed] [Google Scholar]
- 5.Costarella CE, Stallone JN, Rutecki GW, Whittier FC. Testosterone causes direct relaxation of rat thoracic aorta. J Pharmacol Exp Ther 277: 34–39, 1996. [PubMed] [Google Scholar]
- 6.Deenadayalu VP, White RE, Stallone JN, Gao X, Garcia AJ. Testosterone relaxes coronary arteries by opening the large-conductance, calcium-activated potassium channel. Am J Physiol Heart Circ Physiol 281: H1720–H1727, 2001. [DOI] [PubMed] [Google Scholar]
- 7.Ding AQ, Stallone JN. Testosterone-induced relaxation of rat aorta is androgen structure-specific and involves K+ channel activation. J Appl Physiol 91: 2742–2750, 2001. [DOI] [PubMed] [Google Scholar]
- 8.Fernandez-Balsells MM, Murad MH, Lane M, Lampropulos JF, Albuquerque F, Mullan RJ, Agrwal N, Elamin MB, Gallegos-Orozco JF, Wang AT, Erwin PJ, Bhasin S, Montori VM. Adverse effects of testosterone therapy in adult men: A systematic review and meta-analysis. J Clin Endocrinol Metab 95: 2560–2575, 2010. [DOI] [PubMed] [Google Scholar]
- 9.Fogari R, Preti P, Zoppi A, Fogari E, Rinaldi A, Corradi L, Mugellini A. Serum testosterone levels and arterial blood pressure in the elderly. Hypertens Res 28: 625–630, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Herman SM, Robinson JT, McCredie RJ, Adams MR, Boyer MJ, Celermajer DS. Androgen deprivation is associated with enhanced endothelium-dependent dilatation in adult men. Arterioscler Thromb Vasc Biol 17: 2004–2009, 1997. [DOI] [PubMed] [Google Scholar]
- 11.Hughes GS, Mathur RS, Margolius HS. Sex steroid hormones are altered in essential hypertension. J Hypertens 7: 181–187, 1989. [PubMed] [Google Scholar]
- 12.Iki K, Miyamori I, Hatakeyama H, Yoneda T, Takeda Y, Takeda R, Dai QL. The activities of 5 beta-reductase and 11 β-hydroxysteroid dehydrogenase in essential hypertension. Steroids 59: 656–660, 1994. [DOI] [PubMed] [Google Scholar]
- 13.Kalin MF, Zumoff B. Sex hormones and coronary disease: a review of the clinical studies. Steroids 55: 330–352, 1990. [DOI] [PubMed] [Google Scholar]
- 14.Kannel WB, Thom TJ. Incidence, prevalence and mortality and cardiovascular disease. In: The Heart, edited by Schlant RC, Alexander RW, New York: McGraw-Hill, 1994. [Google Scholar]
- 15.Khaw KT, Barrett-Connor E. Blood pressure and endogenous testosterone in men: an inverse relationship. J Hypertens 6: 329–332, 1988. [PubMed] [Google Scholar]
- 16.Levy D, Kannel WB. Cardiovascular risks: new insights from Framingham. Am Heart J 116: 266–272, 1988. [DOI] [PubMed] [Google Scholar]
- 17.Li M, Stallone JN. Estrogen potentiates vasopressin-induced contraction of female rat aorta by enhancing cyclooxygenase-2 and thromboxane function. Am J Physiol Heart Circ Physiol 289: H1542–H1550, 2005. [DOI] [PubMed] [Google Scholar]
- 18.Liu PY, Death AK, Handelsman DJ. Androgens and cardiovascular disease. Endocr Rev 24: 313–340, 2003. [DOI] [PubMed] [Google Scholar]
- 19.Mårin P, Holmäng S, Jönsson L, Sjöström L, Kvist H, Holm G, Lindstedt G, Bjorntoröp P. The effects of testosterone treatment on body composition and metabolism in middle-aged obese men. Int J Obes Relat Metab Disord 16: 991–997, 1992. [PubMed] [Google Scholar]
- 20.Mårin P, Lönn L, Andersson B, Odén B, Olbe L, Bengtsson BA, Björntorp P. Assimilation of triglycerides in subcutaneous and intraabdominal adipose tissues in vivo in men: effects of testosterone. J Clin Endocrinol Metab 81: 1018–1022, 1996. [DOI] [PubMed] [Google Scholar]
- 21.Mendoza SG, Zerpa A, Carrasco H, Colmenares O, Rangel A, Gartside PS, Kashyap ML. Estradiol, testosterone, apolipoproteins, lipoprotein cholesterol, and lipolytic enzymes in men with premature myocardial infarction and angiographically assessed coronary occlusion. Artery 12: 1–23, 1983. [PubMed] [Google Scholar]
- 22.Messerli FH, Garavaglia GE, Schmieder RE, Sundgaard-Riise K, Nunez BD, Amodeo C. Disparate cardiovascular findings in men and women with essential hypertension. Ann Intern Med 107: 158–161, 1987. [DOI] [PubMed] [Google Scholar]
- 23.Molinari C, Battaglia A, Grossini E, Mary DA, Vassanelli C, Vacca G. The effect of testosterone on regional blood flow in prepubertal anaesthetized pigs. J Physiol 543: 365–372, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Montaño LM, Calixto E, Figueroa A, Flores-Soto E, Carbajal V, Perusquía M. Relaxation of androgens on rat thoracic aorta: testosterone concentration dependent agonist/antagonist L-type Ca2+ channel activity, and 5β-dihydrotestosterone restricted to L-type Ca2+ channel blockade. Endocrinology 149: 2517–2526, 2008. [DOI] [PubMed] [Google Scholar]
- 25.Nagahama S, Chen YF, Lindheimer MD, Oparil S. Mechanism of the pressor action of LY171555, a specific dopamine D2 receptor agonist, in the conscious rat. J Pharmacol Exp Ther 236: 735–742, 1986. [PubMed] [Google Scholar]
- 26.Nettleship JE, Jones RD, Channer KS, Jones TH. Testosterone and coronary artery disease. Front Horm Res 37: 91–107, 2009. [DOI] [PubMed] [Google Scholar]
- 27.Pegoraro AA, Carretero OA, Sigmon DH, Beierwaltes WH. Sympathetic modulation of endothelium-derived relaxing factor. Hypertension 19: 643–647, 1992. [DOI] [PubMed] [Google Scholar]
- 28.Perusquía M, Hernández R, Morales MA, Campos MG, Villalón CM. Role of endothelium in the vasodilating effect of progestins and androgens on the rat thoracic aorta. Gen Pharmacol 27: 181–185, 1996. [DOI] [PubMed] [Google Scholar]
- 29.Perusquía M, Navarrete E, Gonzalez L, Villalón CM. The modulatory role of androgens and progestins in the induction of vasorelaxation in human umbilical artery. Life Sci 81: 993–1002, 2007. [DOI] [PubMed] [Google Scholar]
- 30.Perusquía M, Navarrete E, Jasso-Kamel J, Montaño LM. Androgens induce relaxation of contractile activity in pregnant human myometrium at term: a nongenomic action on L-type calcium channels. Biol Reprod 73: 214–221, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Perusquía M, Stallone JN. Do androgens play a beneficial role in the regulation of vascular tone? Nongenomic vascular effects of testosterone metabolites. Am J Physiol Heart Circ Physiol 298: H1301–H1307, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perusquía M, Villalón CM. Possible role of Ca2+ channels in the vasodilating effect of 5β-dihydrotestosterone in rat aorta. Eur J Pharmacol 371: 169–178, 1999. [DOI] [PubMed] [Google Scholar]
- 33.Perusquía M, Villalón CM. The vasodepressor effect of androgens in pithed rats: potential role of calcium channels. Steroids 67: 1021–1028, 2002. [DOI] [PubMed] [Google Scholar]
- 34.Phillips GB, Jing TY, Resnick LM, Barbagallo M, Laragh JH, Sealey JE. Sex hormones and hemostatic risk factors for coronary heart disease in men with hypertension. J Hypertens 11: 699–702, 1993. [DOI] [PubMed] [Google Scholar]
- 35.Phillips GB, Pinkernell BH, Jing TY. The association of hypotestosteronemia with coronary artery disease in men. Arterioscler Thromb 14: 701–706, 1994. [DOI] [PubMed] [Google Scholar]
- 36.Reckelhoff JF, Zhang H, Granger JP. Testosterone exacerbates hypertension and reduces pressure-natriuresis in male spontaneously hypertensive rats. Hypertension 31: 435–439, 1998. [DOI] [PubMed] [Google Scholar]
- 37.Rowell KO, Hall J, Pugh PJ, Jones TH, Channer KS, Jones RD. Testosterone acts as an efficacious vasodilator in isolated human pulmonary arteries and veins: evidence for a biphasic effect at physiological and supra-physiological concentrations. J Endocrinol Invest 32: 718–723, 2009. [DOI] [PubMed] [Google Scholar]
- 38.Setchell KDR. Phytoestrogens: the biochemistry, physiology, and implications for human health of soy isoflavones. Am J Clin Nutr 68 Suppl: 1333S–1334S, 1998. [DOI] [PubMed] [Google Scholar]
- 39.Setchell KDR, Cassidy A. Dietary isoflavones: biological effects and relevance to human health. Am J Nutr 129. Suppl: 758S–767S, 1999. [DOI] [PubMed] [Google Scholar]
- 40.Shapiro BH, Levine DC, Adler NT. The testicular feminized rat: a naturally occurring model of androgen independent brain masculinization. Science 209: 418–420, 1980. [DOI] [PubMed] [Google Scholar]
- 41.Stallone JN. Sex differences in nitric oxide-mediated attenuation of vascular reactivity to vasopressin are abolished by gonadectomy. Eur J Pharmacol 259: 273–283, 1994. [DOI] [PubMed] [Google Scholar]
- 42.Stallone JN, Salisbury RL, Fulton CT. Androgen-receptor defect abolishes sex differences in nitric oxide and reactivity to vasopressin in rat aorta. J Appl Physiol 91: 2602–2610, 2001. [DOI] [PubMed] [Google Scholar]
- 43.Stanley AJ, Gumbreck LG, Allison JE, Easley RB. Part I. Male pseudohermaphroditism in the laboratory Norway rat. Recent Prog Horm Res 29: 43–64, 1973. [DOI] [PubMed] [Google Scholar]
- 44.Svartberg J, von Mühlen D, Schirmer H, Barrett-Connor E, Sundfjord J, Jorde R. Association of endogenous testosterone with blood pressure and left ventricular mass in men. The Tromso Study. Eur J Endocrinol 150: 65–71, 2004. [DOI] [PubMed] [Google Scholar]
- 45.Tenover JS. Effects of testosterone supplementation in the aging male. J Clin Endocrinol Metab 75: 1092–1098, 1992. [DOI] [PubMed] [Google Scholar]
- 46.Tep-areenan P, Kendall DA, Randall MD. Testosterone-induced vasorelaxation in the rat mesenteric arterial bed is mediated predominantly via potassium channels. Br J Pharmacol 135: 735–740, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Traish AM, Guay A, Feeley R, Saad F. The dark side of testosterone deficiency. I. Metabolic syndrome and erectile dysfunction. J Androl 30: 10–22, 2009. [DOI] [PubMed] [Google Scholar]
- 48.Webb CM, McNeill JG, Hayward CS, de Zeigler D, Collins P. Effects of testosterone on coronary vasomotor regulation in men with coronary heart disease. Circulation 100: 1690–1696, 1999. [DOI] [PubMed] [Google Scholar]
- 49.White RE, Owen MP, Stallone JN. Testosterone-induced vasorelaxation of rat mesenteric microvasculature is K+ channel- and nitric oxide-dependent but estrogen-independent. FASEB J 21: 972–10., 2007. [Google Scholar]
- 50.White RE, Sellers MM, Stallone JN. Testosterone-induced vasorelaxation of rat mesenteric microvasculature is neuronal nitric oxide synthase-dependent but androgen receptor-independent. FASEB J 22: 941–13, 2008. [Google Scholar]
- 51.Wu FCW, von Eckardstein A. Androgens and coronary artery disease. Endocr Rev 24: 183–217, 2003. [DOI] [PubMed] [Google Scholar]
- 52.Yarbrough WG, Quarmby VE, Simental JE, Joseph DR, Sar M, Lubahn DB, Olsen KL, French FS, Wilson EM. A single base mutation in the androgen receptor gene causes androgen insensitivity in the testicular feminized rat. J Biol Chem 265: 8893–8900, 1990. [PubMed] [Google Scholar]
- 53.Yue P, Chatterjee K, Beale C, Poole-Wilson PA, Collins P. Testosterone relaxes rabbit coronary arteries and aorta. Circulation 91: 1154–1160, 1995. [DOI] [PubMed] [Google Scholar]