This research article shows for the first time that exercise training attenuates chemoreflex-mediated tonic and hypoxia-evoked reductions in renal blood flow in an animal model of heart failure.
Keywords: heart failure, exercise training, renal blood flow, carotid chemoreflex
Abstract
In chronic heart failure (CHF), carotid body chemoreceptor (CBC) activity is increased and contributes to increased tonic and hypoxia-evoked elevation in renal sympathetic nerve activity (RSNA). Elevated RSNA and reduced renal perfusion may contribute to development of the cardio-renal syndrome in CHF. Exercise training (EXT) has been shown to abrogate CBC-mediated increases in RSNA in experimental heart failure; however, the effect of EXT on CBC control of renal blood flow (RBF) is undetermined. We hypothesized that CBCs contribute to tonic reductions in RBF in CHF, that stimulation of the CBC with hypoxia would result in exaggerated reductions in RBF, and that these responses would be attenuated with EXT. RBF was measured in CHF-sedentary (SED), CHF-EXT, CHF-carotid body denervation (CBD), and CHF-renal denervation (RDNX) groups. We measured RBF at rest and in response to hypoxia (FiO2 10%). All animals exhibited similar reductions in ejection fraction and fractional shortening as well as increases in ventricular systolic and diastolic volumes. Resting RBF was lower in CHF-SED (29 ± 2 ml/min) than in CHF-EXT animals (46 ± 2 ml/min, P < 0.05) or in CHF-CBD animals (42 ± 6 ml/min, P < 0.05). In CHF-SED, RBF decreased during hypoxia, and this was prevented in CHF-EXT animals. Both CBD and RDNX abolished the RBF response to hypoxia in CHF. Mean arterial pressure increased in response to hypoxia in CHF-SED, but was prevented by EXT, CBD, and RDNX. EXT is effective in attenuating chemoreflex-mediated tonic and hypoxia-evoked reductions in RBF in CHF.
NEW & NOTEWORTHY
This research article shows for the first time that exercise training attenuates chemoreflex-mediated tonic and hypoxia-evoked reductions in renal blood flow in an animal model of heart failure.
reduced renal function is common in patients with chronic heart failure (CHF) and is associated with poor prognosis and increased mortality rate (2, 4, 8, 15). Cardio-renal syndrome (CRS) is a general term that refers to a positive feedback loop between cardiac and renal function in which dysfunction in one of these organs precipitates or exacerbates dysfunction in the other. The mechanisms underlying development of CRS are incompletely understood; however, numerous studies indicate that renal hypoperfusion, increased renal sympathetic nerve activity (RSNA), activation of the renin-angiotensin-aldosterone system (RAAS), and alterations in renal redox state play important roles in the pathogenesis of CRS (2, 15). Thus therapies aimed at reducing sympathetic activation and improving renal perfusion may prove to be beneficial to patient populations at risk for CRS (15, 19). Accumulating evidence suggests that nonpharmacological therapies such as exercise training (EXT) may result in improved prognosis (1, 21) and that this improvement in part may be attributed to reductions in sympathetic activation (3, 28, 30, 31).
Previous work from our laboratory and others indicates that carotid body chemoreceptors (CBC) play a significant role in contributing to increased tonic sympathetic outflow to the heart (35) and kidneys in CHF (33, 27). In addition to these tonic increases in resting SNA, we have previously shown that hypoxic chemoreflex activation results in exaggerated increases in RSNA superimposed on the higher tonic levels of RSNA already present in CHF (33, 27). We have previously shown that EXT decreases hypoxia-evoked afferent activity from CBCs and decreases hypoxia-evoked increases in RSNA (25). The extent to which enhanced chemoreflex control of RSNA contributes to alterations in renal perfusion is unknown; however, renal denervation (RDNX) improves renal blood flow (RBF) in CHF, indicating the importance of RSNA to control of RBF in CHF (5).
Thus the focus of the present studies was to determine the effect of increased tonic CBC activation and augmented chemoreflex sensitivity on RBF under normoxic (baseline) and hypoxic conditions. Furthermore, we sought to determine the therapeutic potential of EXT to improve renal perfusion. We hypothesized that enhanced tonic CBC activation and hypoxic-sensitivity contributes to lower resting RBF as well as greater decreases in RBF during hypoxia-evoked CBC activation, and that these alterations are attenuated in EXT animals.
METHODS
Ethical approval.
The experimental protocols were approved by the University of Nebraska Medical Center Institutional Animal Care and Use Committee and were carried out in accordance with the National Institutes of Health (NIH Publication No. 85-23, revised 1996) and the American Physiological Society's Guide for the Care and Use of Laboratory Animals.
Experimental groups.
We used a rapid-ventricular pacing model to induce CHF. Animals were randomly assigned to one of four experimental groups: 1) CHF sedentary (CHF-SED, n = 10), 2) CHF exercise training (CHF-EXT, n = 10), 3) CHF carotid body denervation (CHF-CBD, n = 6), and CHF renal denervation (CHF-RDNX, n = 6). All baseline and experimental measurements were obtained from paced animals with the pace maker turned off for at least 30 min before measurement.
General surgical preparation.
Adult male New Zealand White rabbits weighing 3.5–4.0 kg were housed in individual cages under controlled temperature and humidity and a 12-h:12-h dark/light cycle and fed standard rabbit chow with water available ad libitum.
Animals were surgically implanted with cardiac pacing electrodes as previously described (32). Briefly, rabbits were anesthetized with a cocktail of 5.8 mg/kg xylazine, 35 mg/kg ketamine, and 0.01 mg/kg atropine given intramuscularly, intubated, and connected to a small animal anesthesia respiration unit using 2.0–5.0% inhalation isoflurane with oxygen for the duration of the surgery. With use of the sterile technique, a pressure telemetry unit was implanted into a branch of the femoral artery and advanced into the iliac artery or into the abdominal aorta. A left thoracotomy was performed to implant an electrode on the base of the left ventricle for pacing. The leads of the pacing electrodes were tunneled subcutaneously exiting the skin in the mid-scapular region and fixed on the back. Postoperative analgesia was provided with buprenorphine (Reckitt Benckiser Healthcare, Hull, England, UK; 0.07 mg sc) for the first 24 h and with a cutaneous fentanyl patch for the next 3 days (Apotex, Weston, FL; 25 μg/h). Antibiotic therapy was administered over the next 5 days (Baytril; Bayer Health Care, Shawnee Mission, KS; 22.7 mg/day).
Placement of renal flow probe.
After ∼2 wk of recovery a second surgery was performed as previously described (5) to implant a 2-mm ultrasonic flow probe transducer (Transonics, Ithaca, NY; Model 2PSB) on the left renal artery. The flow probe cable was also tunneled beneath the skin and exited in the midscapular region.
Selective renal denervation.
At the time of flow probe placement, a group of randomly assigned rabbits underwent unilateral RDNX performed on the left kidney as previously described (5). All visible neural structures were stripped from the area surrounding the renal artery and vein. Both vessels were cleaned down to the adventitia and up to the hilum of the kidney. Postoperative analgesia was provided with buprenorphine (Reckitt Benckiser Healthcare; 0.07 mg sc) for the first 24 h and with a cutaneous fentanyl patch for the next 3 days (Apotex; 25 μg/h). Antibiotic therapy was administered over the next 5 days (Baytril; 22.7 mg/day). Using renal norepinephrine content as an index of RDNX efficacy, we have previously shown that this technique results in reductions in renal norepinephrine content to near zero (5). For illustrative purposes, in one animal, flow probes were placed on both the right and left renal artery, and RDNX was performed only on the left renal artery.
Experimental measurements.
After a 2-wk recovery period from the last surgery, all rabbits went through the experimental protocol for prepace measurements. Before and after induction of CHF, resting mean arterial pressure (MAP), heart rate (HR), and RBF were recorded with the rabbit contained in a Plexiglass chamber in a quiet and dimly lit laboratory. The animal was allowed to acclimate to the environment for ∼20 min after which time a 60-min segment of data was obtained for calculation of resting values. After resting data was recorded, the gas mixture flowing through the chamber was changed to an equal mix of oxygen and nitrogen with a small amount of CO2 to elicit a rapid reduction in chamber FiO2 to 10%. The flow rate was maintained at the same level as during the baseline recording, and the exposure was maintained for 5 min, after which time the chamber was returned to the normoxic condition (21% O2).
Induction of chronic heart failure.
We used a rapid ventricular pacing model to induce CHF. Using a pacemaker of our own design, we initially began pacing at a rate of 340 beats/min, which was held for 7 days, and then the rate was gradually increased to 380 beats/min, with an increment of 20 beats/min each week, as described in our prior studies (27, 32). Each rabbit was checked daily to ensure chronic pacing. Rabbits were paced continually for 3 to 4 wk. The progression of CHF was monitored by weekly echocardiograms (Siemens/Acuson Sequoia 512C with a 4-MHz probe; Siemens Medical Solutions, Malvern, PA), with the pacemaker turned off for at least 30 min before the recordings were started. This pacing protocol results in decreases in cardiac contractility and ejection fraction (EF%) and increases in cardiac volume, resting heart rate, and central venous pressure (32). CHF was characterized by >35% reduction in EF% and fractional shortening (FS%).
Exercise training regimen.
Before the beginning of the EXT regimen, rabbits were exercise tested on a treadmill at a rate of 8 m/min for 5 min and subsequently at a rate of 13 m/min until they were unable to continue. Time to exhaustion was assessed again after the completion of the training program. Exercise training was initiated at the time ventricular pacing began. The EXT protocol was similar to that described previously (10, 25, 26). In brief, rabbits ran for a 5-min warm-up period at a rate of 8 m/min followed by 20 min at a rate of 13 m/min and a subsequent cool down period of 8 meters/min for 5 min. This was carried out 5 days/wk.
Selective denervation of carotid body chemoreceptors.
After 3 to 4 wk of pacing, a subgroup of sedentary rabbits underwent carotid body denervation (CBD) as described previously (27). Anesthesia was induced with an anesthetic cocktail and maintained with inhalation isoflurane (see thoracotomy above). With the use of the sterile techniques, the carotid sinus regions were exposed and the CBC region visually identified. The CBCs were cryogenically destroyed using a fine-tipped forceps cooled in liquid nitrogen. After surgery, all animals were placed on an antibiotic regimen consisting of 5 mg/kg sc Baytril for 5 days. Rabbits were allowed to recover for 24 h postsurgery before cardiac pacing resumed. All animals were allowed to recover for 3 days postsurgery before any experiments were performed. CBD was confirmed before study by the abolition of an increase in ventilation in response to 10% oxygen inhalation.
Data analysis.
All parameters were recorded with a Powerlab data acquisition and analysis system (ADInstruments, Colorado Springs, CO) using LabChart 7 Pro. HR and renal vascular conductance (RVC = RBF/MAP) were calculated and displayed continuously by LabChart from the RBF and MAP signals. Resting and chemoreflex-evoked RBF, RVC, and MAP responses were measured longitudinally through the course of pacing. Resting values for RBF, MAP, HR, and RVC were quantified by the average of 60 min of data as described in Experimental Measurements.
The change in RBF in response to acute hypoxia was quantified from the mean of 10 s of stable baseline data taken during the 60 s before hypoxia exposure and 10 s of data centered on the nadir value during hypoxia. All records were inspected visually to ensure that aberrant points or artifacts were not identified as the nadir for determination of the change during hypoxia. Values for change in MAP and RVC were taken from the same time point as the nadir value for RBF. All parameters were analyzed by a two-way ANOVA for repeated measures. The student Newman-Keuls procedure was used as the multiple comparison test to determine the significance between groups. All data are expressed as means ± SE. By convention, statistical significance was accepted when the two-tailed critical value of P was less than 0.05.
RESULTS
Effects of EXT, CBD, or RDNX on cardiac function and time to exhaustion.
Mean data for cardiac function and ventricular volumes are displayed in Table 1. No significant differences were noted between groups for EF%, FS%, left ventricular systolic (LVs Vol), and left ventricular diastolic (LVd Vol) before pacing. After 3 to 4 wk of pacing EF% and FS% were reduced to similar levels in all groups (Table 1). In addition, we observed increased LVs Vol and LVd Vol dimensions in all groups (Table 1). None of the interventions (EXT, CBD, or RDNX) affected the degree to which cardiac function declined or ventricular volumes increased with pacing (Table 1). The running time to exhaustion was increased from 18 ± 1 min before EXT to 36 ± 3 min in the CHF-EXT rabbits (P < 0.05).
Table 1.
Echocardiographic measurements and body weight
| Prepace | CHF | |
|---|---|---|
| SED | ||
| Body weight, kg | 3.5 ± 0.2 | 3.7 ± 0.2 |
| HR, beats/min | 220 ± 7 | 236 ± 8* |
| Volume, ml | ||
| End diastolic | 6 ± 1 | 9 ± 1* |
| End systolic | 2 ± 0 | 5 ± 1* |
| Ejection fraction, % | 66 ± 3 | 41 ± 2* |
| Fractional shortening, % | 33 ± 2 | 18 ± 0* |
| EXT | ||
| Body weight, kg | 3.5 ± 0.2 | 3.6 ± 0.2 |
| HR, beats/min | 220 ± 9 | 208 ± 9 |
| Volume, ml | ||
| End diastolic | 6 ± 1 | 9 ± 1* |
| End systolic | 2 ± 0 | 6 ± 1* |
| Ejection fraction, % | 67 ± 1 | 38 ± 1* |
| Fractional shortening, % | 35 ± 1 | 17 ± 1* |
| CBD | ||
| Body weight, kg | 3.6 ± 0.3 | 3.6 ± 0.2 |
| HR, beats/min | 225 ± 6 | 230 ± 10 |
| Volume, ml | ||
| End diastolic | 6 ± 1 | 10 ± 1* |
| End systolic | 2.2 ± 0 | 6 ± 1* |
| Ejection fraction, % | 68 ± 3 | 38 ± 2* |
| Fractional shortening, % | 33 ± 2 | 16 ± 1* |
| RDNX | ||
| Body weight, kg | 3.6 ± 0.3 | 3.6 ± 0.2 |
| HR, beats/min | 238 ± 6 | 246 ± 9 |
| Volume, ml | ||
| End diastolic | 7 ± 1 | 10 ± 1* |
| End systolic | 2 ± 0 | 6 ± 1* |
| Ejection fraction, % | 64 ± 2 | 40 ± 1* |
| Fractional shortening, % | 32 ± 2 | 18 ± 1* |
Values are means ± SE; n = 10 congestive heart failure (CHF)-sedentary (SED) and CHF-exercise training (EXT) and n = 6 CHF-carotid body denervation (CBD) and CHF-renal denervation (RDNX). HR, heart rate.
P < 0.05 compared with prepace.
Effects of EXT, CBD, or RDNX on resting renal hemodynamics.
As illustrated in Fig. 1, resting RBF and RVC were similar among groups before pacing. After pacing, resting RBF and RVC were reduced in the CHF-SED group (P < 0.05). In stark contrast, EXT prevented the reduction in resting RBF and RVC after pacing. CBD performed after the development of CHF attenuated the reduction in resting RBF and RVC but did not completely normalize it (CHF-SED vs. CHF-CBD, P < 0.05). RDNX prevented the reduction in RVC with pacing, but had less of an effect on attenuating the reduction of RBF with pacing (CHF-RDNX vs. prepace, P < 0.05).
Fig. 1.
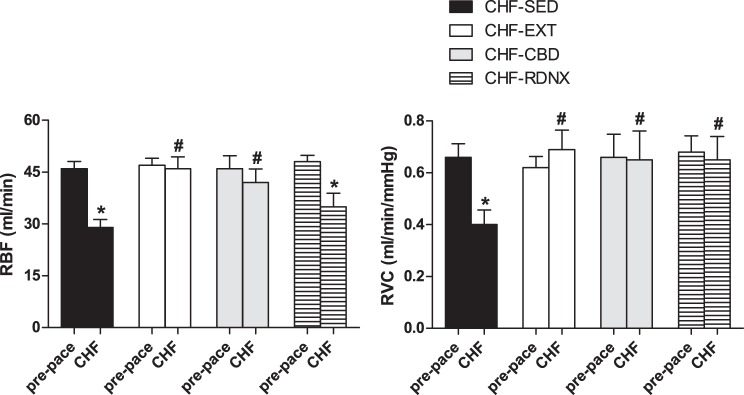
Exercise training prevented reductions in resting renal blood flow (RBF) associated with congestive heart failure (CHF). Mean values for resting RBF and renal vascular conductance (RVC) before and after CHF are shown at left and right, respectively. Baseline RBF and RVC were significantly reduced in CHF-sedentary (SED) animals, and this effect was attenuated by exercise training (EXT). Reductions in RBF and RVC associated with CHF were attenuated after carotid body denervation (CBD). *P < 0.05 vs. prepace; #P < 0.05 vs. CHF-SED. RDNX, renal denervation.
MAP was not significantly different among groups before pacing (72 ± 4 mmHg CHF-SED, 77 ± 4 mmHg CHF-EXT, 72 ± 5 mmHg CHF-CBD, 81 ± 3 mmHg CHF-RDNX). After pacing, MAP was not different from prepace values in CHF-SED, CHF-EXT, or CHF-CBD groups, but was lower in the CHF-RDNX group relative to baseline (74 ± 5 mmHg CHF-SED, 71 ± 5 mmHg CHF-EXT, 69 ± 6 mmHg CHF-CBD, 67 ± 5 mmHg CHF-RDNX; P < 0.05 for RDNX only). Thus the reduction in MAP after pacing in the CHF-RDNX group likely was responsible for the decrease in RBF but not RVC in that group.
Heat rate was not different among groups before pacing (220 ± 7 beats/min CHF-SED, 220 ± 9 beats/min CHF-EXT, 225 ± 6 beats/min CHF-CBD, 238 ± 6 mmHg CHF-RDNX). In the CHF-SED animals, HR (measured at rest with the pacer turned off for 30 min before measurement) increased after pacing (236 ± 8 beat/min CHF-SED vs. 220 ± 7 beats/min prepace, P < 0.05). In the CHF-EXT animals there was a trend for a reduction in resting HR after pacing; however, this difference did not reach statistical significance (208 ± 9 beats/min CHF-EXT vs. 220 ± 9 beats/min prepace, P = 0.14). HR did not increase in either CHF-CBD (230 ± 10 beats/min CHF-CBD vs. 225 ± 6 beats/min prepace) or CHF-RDNX (246 ± 9 beats/min CHF-RDNX vs. 238 ± 6 beats/min prepace) groups after pacing.
Effects of EXT, CBD, or RDNX on renal hemodynamic response to hypoxia.
Representative records illustrating the renal hemodynamic response to hypoxia before and after pacing are shown in Fig. 2. The mean baseline and mean nadir/peak hypoxia values for RBF, MAP, HR, and RVC are shown in Table 2, and the mean maximal changes in RBF, MAP, and RVC in response to hypoxia are illustrated in Fig. 3. Before pacing, there was no significant change in RBF, MAP, RVC, or HR in response to hypoxia (FiO2 10%) in any of the groups (Fig. 3).
Fig. 2.
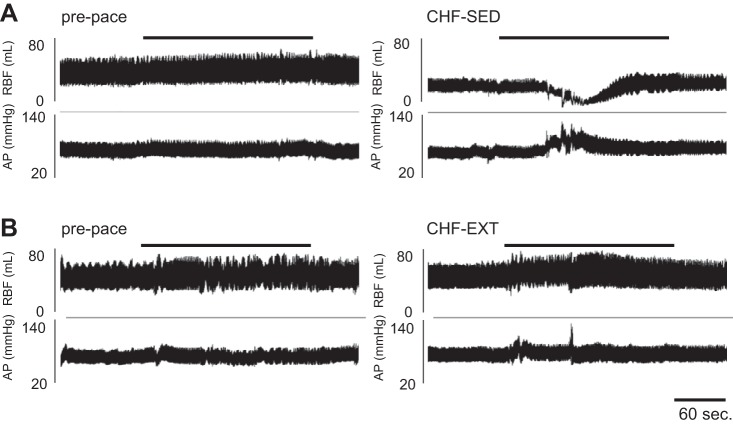
Exercise training prevented RBF response to chemoreflex activation. A: representative RBF and arterial pressure (AP) traces in 1 animal before pacing and after pacing-induced CHF. B: representative tracings from 1 animal, illustrating that reductions in RBF, which occurred during hypoxic chemoreflex activation, were prevented with EXT. Black bar indicates hypoxia exposure. (FiO2 10%, SaO2 80%).
Table 2.
Values for RBF, MAP, and RVC at baseline (FiO2 21%) and during hypoxia FiO2 10%)
| CHF-SED, % |
CHF-EXT, % |
CHF-CBD, % |
CHF-RDNX, % |
|||||
|---|---|---|---|---|---|---|---|---|
| FiO2 | 21 | 10 | 21 | 10 | 21 | 10 | 21 | 10 |
| RBF, ml/min | ||||||||
| Prepace | 43 ± 3 | 41 ± 3 | 47 ± 2 | 40 ± 3 | 46 ± 3 | 42 ± 3 | 50 ± 3 | 49 ± 3 |
| CHF | 32 ± 3# | 17 ± 3#* | 45 ± 3 | 43 ± 3 | 42 ± 4 | 41 ± 4 | 34 ± 3# | 34 ± 3# |
| MAP, mmHg | ||||||||
| Prepace | 68 ± 3 | 72 ± 3 | 77 ± 4 | 83 ± 5 | 71 ± 5 | 73 ± 5 | 81 ± 2 | 85 ± 3 |
| CHF | 72 ± 6 | 85 ± 7 | 70 ± 6 | 75 ± 5 | 69 ± 4 | 66 ± 5 | 68 ± 6# | 75 ± 7 |
| RVC, ml·min−1· mmHg−1 | ||||||||
| Prepace | 0.66 ± 0.04 | 0.58 ± 0.04 | 0.62 ± 0.05 | 0.51 ± 0.05 | 0.65 ± 0.09 | 0.55 ± 0.08 | 0.69 ± 0.05 | 0.68 ± 0.06 |
| CHF | 0.47 ± 0.08# | 0.30 ± 0.06#* | 0.69 ± 0.07 | 0.60 ± 0.06 | 0.56 ± 0.05 | 0.58 ± 0.04 | 0.61 ± 0.09 | 0.57 ± 0.09 |
| HR, beats/min | ||||||||
| Prepace | 222 ± 6 | 214 ± 7 | 211 ± 10 | 200 ± 14 | 218 ± 6 | 226 ± 5 | 229 ± 5 | 229 ± 6 |
| CHF | 230 ± 10 | 243 ± 14 | 197 ± 8 | 194 ± 11 | 223 ± 13 | 234 ± 11 | 241 ± 9 | 244 ± 13 |
Values are means ± SE. RBF, renal blood flow; MAP, mean arterial pressure; RVC, renal vascular conductance.
P < 0.05 compared with 21%;
P < 0.05 compared with prepace.
Fig. 3.
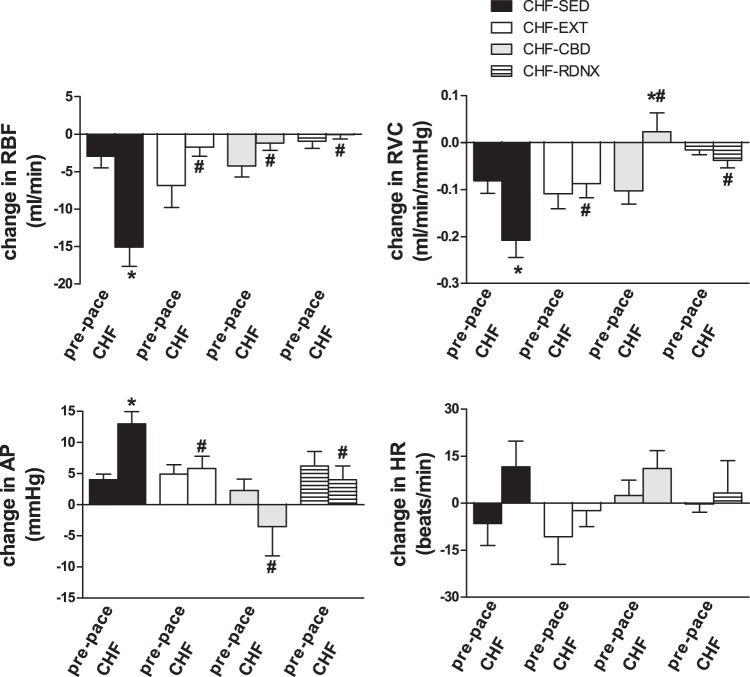
Exercise training prevented exaggerated hemodynamic responses to chemoreflex activation in CHF. Mean values for peak change in RBF, RVC, AP, and heart rate (HR) from baseline during exposure to hypoxia (FiO2 10%) are shown. The changes in RBF, RVC, AP, and HR were significantly enhanced after pacing in CHF-SED animals; however, this effect was attenuated by EXT and carotid body denervation (CBD) (FiO2 10%, SaO2 80%). *P < 0.05 vs. prepace; #P < 0.05 vs. CHF SED.
In the CHF-SED animals, RBF decreased and MAP increased progressively as chamber FiO2 began to fall (Fig. 2), and the nadir value for RBF typically occurred within the first 2 min of exposure (although we did not specifically quantify the time course). The peak reductions in RBF and RVC in response to hypoxia in CHF-SED animals were larger than those in the prepace condition (Fig. 3, P < 0.05). The increase in MAP evoked by hypoxia was also larger in CHF-SED than at prepace (Fig. 3, P < 0.05). Conversely, in the CHF-EXT animals, the reduction in RBF in response to hypoxia (Fig. 2) was not significantly different from prepace (Fig. 3, P < 0.05). Similarly, in the CHF EXT animals, there were no significant differences in the RVC and MAP responses to hypoxia with pacing.
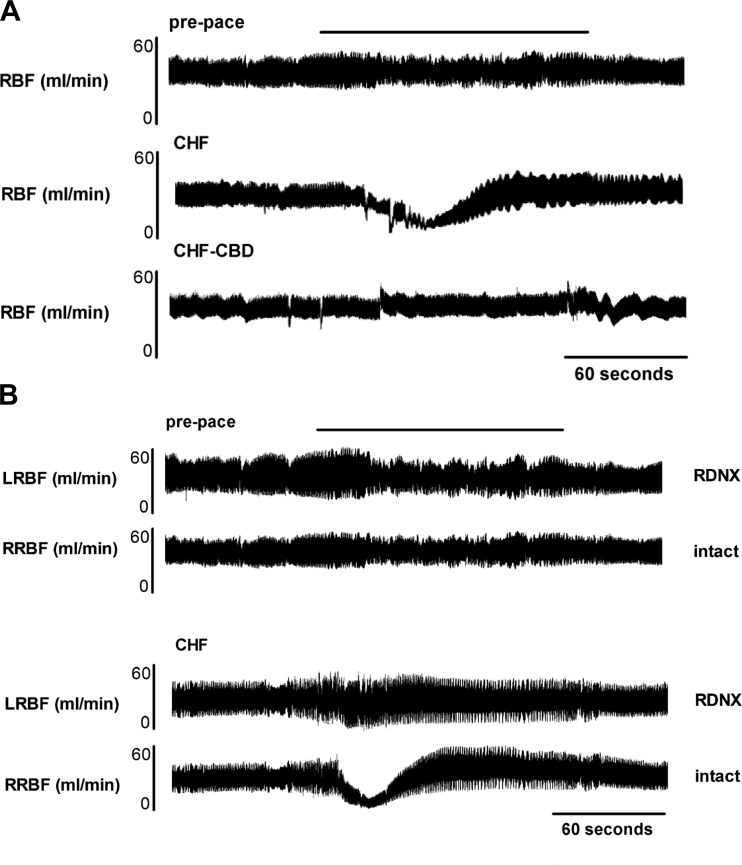
CBD in CHF animals abolished the decrease in RBF and RVC and the increase in MAP associated with hypoxia exposure in CHF (Fig. 3 and Fig. 4A). Similarly, RDNX in CHF animals abolished the decrease in RBF and RVC and the increase in MAP associated with hypoxia exposure in CHF (Fig. 3). For illustrative purposes, in one animal, flow probes were placed on both the right and left renal artery, and RDNX was performed only on the left renal artery (Fig. 4B). This example illustrates the effect of CHF on the renal hemodynamic response to hypoxia and the role that the renal nerves play in this response. When groups in the CHF state are compared, the reduction in RBF and RVC and the increase in MAP in response to hypoxia were significantly attenuated in the CHF- EXT, CHF- CBD, and CHF-RDNX groups in comparison with the CHF-SED group after pacing (Fig. 3, P < 0.05).
Fig. 4.
CBD and RDNX prevented RBF response to chemoreflex activation. A: representative RBF traces before pacing, after pacing-induced CHF, and after CBD in the CHF-SED state in the same animal. B: representative tracings from a different rabbit in the prepace and CHF-SED state instrumented with bilateral renal flow probes and with RDNX on the left side. Reductions in RBF, which occurred during hypoxic chemoreflex activation in CHF, were mediated by renal nerves. Black bar indicates hypoxia exposure (FiO2 10%, SaO2 80%). LRBF, left renal blood flow; RRBF, right renal blood flow.
DISCUSSION
The major findings of this study are that EXT prevents tonic and hypoxia-evoked reductions in RBF in CHF rabbits. To confirm that this phenomenon was mediated by changes in CBC control of renal nerves we performed RDNX and CBD in two different subsets of animals that did not undergo EXT. We found that either CBD or RDNX was sufficient to abolish the renal hemodynamic response to hypoxia and the potentiation of that response associated with CHF. These findings underscore the functional significance of our previous studies linking the CBC to control of tonic and hypoxia-evoked RSNA in CHF and the salutary effect of EXT.
Chemoreflex control of renal hemodynamics.
Numerous studies in both conscious and anesthetized animals demonstrate that efferent renal sympathetic nerves play an important role in modulating RBF (5, 9, 12, 13, 17, 20, 23, 24). Electrical stimulation of renal sympathetic nerves produces frequency and amplitude-dependent decreases in total RBF and renal cortical perfusion (17). Physiological stimuli that elicit activation of renal sympathetic nerves have similar hemodynamic effects. In normal healthy animals, activation of the CBC with moderate hypoxia (10% O2) results in increases in RSNA and modest reductions in total RBF and renal cortical perfusion, with little effect on MAP or HR (9, 20, 24).
Our previous work has shown that afferent activity from CBC is increased in CHF (32) and that activity contributes to tonic elevations in resting RSNA to the renal vasculature (27). In addition, we have shown that carotid body afferent responses to hypoxia are exaggerated in CHF and result in exaggerated increases in efferent RSNA (32, 33). These exaggerated responses are attributable to changes in CBC sensitivity rather than changes in stimulus intensity since we have previously shown that for a given FiO2 there are no significant differences in PaO2 between CHF and control animals (7, 25). The effect of altered CBC function on control of RBF in CHF has not previously been addressed.
Renal blood flow in heart failure.
Clinical studies suggest that RBF is reduced in patients with CHF and that the renal resistance index is an independent predictor of patient outcome and may be useful in stratifying patient's prognosis (4, 8, 15). Evidence from animal studies supports the notion that RBF is chronically reduced in CHF and shows that RDNX can preserve RBF, inhibit RAAS activation, and preserve renal function (5). The results of the current study confirm the role of renal sympathetic nerves in mediating reductions in resting RVC observed in CHF and further indicate that these decreases are mediated in part by the CBC, because the reductions in resting RVC were attenuated after CBD. Numerous previous studies have characterized the relationship between RSNA and the control of RBF during chemoreceptor stimulation in healthy animals (9, 20, 24). We have shown that the RSNA response to hypoxia is enhanced in CHF (27, 33). In this article we show for the first time to our knowledge that the RBF response to hypoxia is exaggerated in CHF. The finding that enhanced chemoreflex sensitivity contributes to tonic and hypoxia-evoked reductions in RBF is of potential clinical significance, because patients with CHF frequently have comorbid sleep disordered breathing of both obstructive and central origin (34, 36). Our results suggest that the CBC can have a deleterious impact on chronic reductions in RBF with further superimposed periodic reductions in RBF associated with hypoxic apneic events and/or oscillatory breathing. This notion is supported by clinical evidence that preventing sleep disordered breathing in patients with CHF using adaptive servo-ventilation results in improvements in several indexes of renal function (36).
Chronic activation of renal sympathetic nerves, RAAS activation, and renal hypoperfusion have been theorized to be important contributing factors in the development of the cardio-renal syndrome (2). Our study did not specifically address the role of a reduction in RBF on RAAS activation in CHF; however, a previous study showed that CHF is associated with decreased RBF, increased ANG II type 1 receptor (AT1R) expression and decreased ANG II Type 2 receptor expression in the kidney and that reducing sympathetic stimulation of the kidney by RDNX prevents these changes (5). Thus our current and previous (5, 25) findings suggest that CBC activation in CHF may contribute to RAAS activation and development of cardio-renal syndrome by increasing RSNA and decreasing RBF.
Exercise training in heart failure.
Exercise training has been shown to be efficacious in improving survival and quality of life in patients with CHF (1). Clinical studies in patient populations as well as animal studies indicate that EXT has profound effects on autonomic function as well as modulating afferent feedback from peripheral cardiovascular reflexes (3, 10, 18, 21, 25, 26, 28, 29, 30, 31). Previous work from our laboratory indicates that EXT specifically reduces sympathetic outflow to the kidneys in CHF (10, 25). Furthermore, we have shown that EXT specifically prevents increases in CBC sensitivity that occur with the development of CHF and the attendant chemoreflex-mediated increases in RSNA (25). Our current results suggest that the effects of EXT on CBC function translate to functional changes in regulation of RBF. Increased CBC control of tonic RBF and hypoxia-evoked changes in RBF in CHF are likely related to blood flow-dependent changes in glomus cell excitability in the carotid bodies (7).
We have previously shown that reducing carotid body blood flow using vascular occluders is sufficient by itself (in the absence of CHF) to elicit increases in carotid body sensitivity and exaggerated hypoxia-evoked increases in RSNA (7). In these studies, chronic reduction of blood flow with vascular occluders resulted in increased AT1R expression and decreased neuronal nitric oxide synthase (nNOS) expression in the CB, a molecular phenotype that is similar to what has been previously observed in animal models of CHF (11, 26, 32, 37). It is likely that the beneficial effects of EXT on chemoreflex sensitivity and by extension chemoreflex control of RBF are mediated by the intermittent increases in blood flow that occur during EXT. In support of this notion, numerous studies have shown that EXT prevents increases in AT1R expression and decreases in nNOS expression associated with CHF in the central nervous system (26, 37), skeletal muscle (11), and the carotid bodies (25, 37). Further studies are needed to determine exactly how EXT normalizes CBC sensitivity.
Limitations.
These findings provide important insight into regulation of RBF in CHF and suggest that EXT may play an important role in mitigating some of the deleterious changes that occur in CBC control of RBF. There are a few important caveats that should be considered in interpreting these findings. First, in this set of studies, we used an experimental design in which EXT was performed during pacing to CHF. Exercise training in clinical populations is typically not initiated until after suffering a myocardial infarction or the development of heart failure, and thus direct comparisons between our findings and clinical populations are not possible. Future studies will address the potentially beneficial effects of EXT when initiated after the development of CHF.
Second, in this study we did not directly address the effects of preventing the reduction in resting and hypoxia-evoked RBF on renal function. Nonetheless, our work elucidates important mechanisms underlying changes in control of RBF and suggests that EXT has preventive and possibly therapeutic potential. Renal hypoperfusion, increased RSNA, and RAAS activation are all hypothesized to play important roles in the pathogenesis of cardio-renal syndrome, and our work has collectively shown that all of these parameters are improved with EXT. In addition, other investigators have shown that reductions in renal perfusion with chemoreflex activation result in alterations in kidney function, specifically a reduction in glomerular filtration rate, and alterations in sodium metabolism (9). The extent to which interventions that alter chemoreflex sensitivity affect renal function in CHF is undetermined and requires further study.
Third, previous evidence indicates that EXT reduces chemoreflex-mediated RSNA (25), and in this study we have shown that this is associated with attenuated reductions in RBF during chemoreflex activation. It is possible that the effect we observed is mediated in part by the effects of EXT on vascular function in the renal artery. Previous work shows that EXT enhances functional sympatholysis via nitric oxide-dependent mechanisms (18), thus the blunted vasoconstrictor responses we observed in these studies may represent altered vascular responsiveness to sympathetic stimulation in addition to the reductions in RSNA previously observed with EXT (25).
Summary
In this study we have shown that alterations in chemoreflex function contribute to reductions in resting RBF as well as exaggerated hypoxia-evoked vasoconstrictor responses, and that EXT prevents these potentially deleterious changes. Both renal denervation (16) and carotid body denervation (6, 27) have generated recent interest as potential therapeutic applications for treatment of autonomic dysfunction in heart failure. In addition to the mitigation of sympathetic overactivity, our results show that either of these procedures have the potential to reduce the impact of tonic and hypoxia-evoked carotid body activation on RBF. More importantly, we demonstrate that a noninvasive intervention, EXT, which prevents or attenuates changes in chemoreflex function, may help to mitigate some of the processes associated with development and progression of the cardio-renal syndrome.
GRANTS
This study was supported by Program Project Grant PO1-HL-62222 and by Ruth L. Kirschstein National Research Service Award 5F32HL108592 (to N. J. Marcus) from the National Heart, Lung, and Blood Institute of the NIH.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: N.J.M., C.P., A.M.S., R.D.R., I.H.Z., and H.D.S. conception and design of research; N.J.M., C.P., J.K.M., and A.M.S. performed experiments; N.J.M., C.P., J.K.M., and A.M.S. analyzed data; N.J.M., C.P., J.K.M., A.M.S., R.D.R., I.H.Z., and H.D.S. interpreted results of experiments; N.J.M. prepared figures; N.J.M. drafted manuscript; N.J.M., R.D.R., I.H.Z., and H.D.S. edited and revised manuscript; N.J.M., C.P., J.K.M., A.M.S., R.D.R., I.H.Z., and H.D.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Kaye Talbitzer for surgical assistance and management of the heart failure animal core at University of Nebraska Medical Center and Mary Ann Zink for technical support.
REFERENCES
- 1.Belardinelli R, Georgiou D, Cianci G, Purcaro A. Randomized, controlled trial of long-term moderate exercise training in chronic heart failure: effects on functional capacity, quality of life, and clinical outcome. Circulation 99: 1173–1182, 1999. [DOI] [PubMed] [Google Scholar]
- 2.Bock JS, Gottlieb SS. Cardiorenal syndrome: new perspectives. Circulation 121: 2592–2600, 2010. [DOI] [PubMed] [Google Scholar]
- 3.Braith RW, Welsch MS, Feigenbawm MS, Kluess HA, Pepine CJ. Neuroendocrine activation in heart failure is modified by endurance exercise training. J Am Coll Cardiol 34: 1170–1175, 1999. [DOI] [PubMed] [Google Scholar]
- 4.Ciccone MM, Iacoviello M, Gesualdo L, Puzzovivo A, Antoncecchi V, Doronzo A, Monitillo F, Citarelli G, Paradies V, Favale S. The renal arterial resistance index: a marker of renal function with an independent and incremental role in predicting heart failure progression. Eur J Heart Fail 16: 210–216, 2014. [DOI] [PubMed] [Google Scholar]
- 5.Clayton SC, Haack KK, Zucker IH. Renal denervation modulates angiotensin receptor expression in the renal cortex of rabbits with chronic heart failure. Am J Physiol Renal Physiol 300: F31–F39, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Del Rio R, Marcus NJ, Schultz HD. Carotid chemoreceptor ablation improves survival in heart failure: rescuing autonomic control of cardiorespiratory function. J Am Coll Cardiol 62: 2422–2430, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ding Y, Li YL, Schultz HD. Role of blood flow in carotid body chemoreflex function in heart failure. J Physiol 589: 245–258, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ennezat PV, Maréchaux S, Six-Carpentier M, Pinçon C, Sediri I, Delsart P, Gras M, Mounier-Véhier C, Gautier C, Montaigne D, Jude B, Asseman P, Le Jemtel TH. Renal resistance index and its prognostic significance in patients with heart failure with preserved ejection fraction. Nephrol Dial Transplant 26: 3908–3913, 2011. [DOI] [PubMed] [Google Scholar]
- 9.Evans RG, Burke SL, Lambert GW, Head GA. Renal responses to acute reflex activation of renal sympathetic nerve activity and renal denervation in secondary hypertension. Am J Physiol Regul Integr Comp Physiol 293: R1247–R1256, 2007. [DOI] [PubMed] [Google Scholar]
- 10.Gao L, Wang W, Liu D, Zucker IH. Exercise training normalizes sympathetic outflow by central antioxidant mechanisms in rabbits with pacing-induced chronic heart failure. Circulation 115: 3095–3102, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Gomes-Santos IL, Fernandes T, Couto GK, Ferreira-Filho JC, Salemi VM, Fernandes FB, Casarini DE, Brum PC, Rossoni LV, de Oliveira EM, Negrao CE. Effects of exercise training on circulating and skeletal muscle renin-angiotensin system in chronic heart failure rats. PLoS One 9: e98012, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guild SJ, Eppel GA, Malpas SC, Rajapakse NW, Stewart A, Evans RG. Regional responsiveness of renal perfusion to activation of the renal nerves. Am J Physiol Regul Integr Comp Physiol 283: R1177–R1186, 2002. [DOI] [PubMed] [Google Scholar]
- 13.Guild SJ, Malpas SC, Eppel GA, Nguang SK, Evans RG. Effect of renal perfusion pressure on responses of intrarenal blood flow to renal nerve stimulation in rabbits. Clin Exp Pharmacol Physiol 31: 35–45, 2004. [DOI] [PubMed] [Google Scholar]
- 14.Hering D, Zdrojewski Z, Król E, Kara T, Kucharska W, Somers VK, Rutkowski B, Narkiewicz K. Tonic chemoreflex activation contributes to the elevated muscle sympathetic nerve activity in patients with chronic renal failure. J Hypertens 25: 157–161, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Hillege HL, Girbes ARJ, Kam de PJ, Boomsma F, Zeeuw de D, Charlesworth A, Hampton JR, van Veldhuisen DJ. Renal function, neurohormonal activation, and survival in patients with chronic heart failure. Circulation 102: 203–210, 2000. [DOI] [PubMed] [Google Scholar]
- 16.Hu J, Li Y, Cheng W, Yang Z, Wang F, Lv P, Niu C, Hou Y, Yan Y, Ge J. A comparison of the efficacy of surgical renal denervation and pharmacologic therapies in post-myocardial infarction heart failure. PLoS One 9: e96996, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Janssen BJ, Malpas SC, Burke SL, Head GA. Frequency-dependent modulation of renal blood flow by renal nerve activity in conscious rabbits. Am J Physiol Regul Integr Comp Physiol 273: R597–R608, 1997. [DOI] [PubMed] [Google Scholar]
- 18.Jendzjowsky NG, Delorey DS. Short-term exercise training enhances functional sympatholysis through a nitric oxide-dependent mechanism. J Physiol 591: 1535–1549, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Judson WE, Hollander W, Hatcher JD, Halperin MH. The effects of exercise on cardiovascular and renal function in cardiac patients with and without heart failure. J Clin Invest 34: 1546–1558, 1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karim F, Poucher SM, Summerhill RA. The effects of stimulating carotid chemoreceptors on renal haemodynamics and function in dogs. J Physiol 392: 451–462, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kitzman DW, Brubaker PH, Morgan TM, Stewart KP, Little WC. Exercise training in older patients with heart failure and preserved ejection fraction: a randomized, controlled, single-blind trial. Circ Heart Fail 3: 659–667, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koyama T, Watanabe H, Terada S, Makabe S, Igarashi G, Nobori K, Ito H. Adaptive servo-ventilation improves renal function in patients with heart failure. Respir Med 105: 1946–1953, 2011. [DOI] [PubMed] [Google Scholar]
- 23.Leonard BL, Evans RG, Navakatikyan MA, Malpas SC. Differential neural control of intrarenal blood flow. Am J Physiol Regul Integr Comp Physiol 279: R907–R916, 2000. [DOI] [PubMed] [Google Scholar]
- 24.Leonard BL, Malpas SC, Denton KM, Madden AC, Evans RG. Differential control of intrarenal blood flow during reflex increases in sympathetic nerve activity. Am J Physiol Regul Integr Comp Physiol 280: R62–R68, 2001. [DOI] [PubMed] [Google Scholar]
- 25.Li Y, Ding Y, Agnew C, Schultz HD. Exercise training improves peripheral chemoreflex function in heart failure rabbits. J Appl Physiol 105: 782–790, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu J, Irvine S, Reid IA, Patel KP, Zucker IH. Chronic exercise reduces sympathetic nerve activity in rabbits with pacing-induced heart failure: a role for angiotensin II. Circulation 102: 1854–1862, 2000. [DOI] [PubMed] [Google Scholar]
- 27.Marcus NJ, Del Rio R, Schultz EP, Xia XH, Schultz HD. Carotid body denervation improves autonomic and cardiac function in congestive heart failure. J Physiol 592: 391–408, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martinez DG, Nicolau JC, Lage RL, Toschi-Dias E, de Matos LD, Alves MJ, Trombetta IC, Dias da Silva VJ, Middlekauff HR, Negrão CE, Rondon MU. Effects of long-term exercise training on autonomic control in myocardial infarction patients. Hypertension 58: 1049–1056, 2011. [DOI] [PubMed] [Google Scholar]
- 29.Mortensen SP, Nyber M, Gliemann L, Thaning P, Saltin B, Hellsten Y. Exercise training modulates functional sympatholysis and α-adrenergic vasoconstrictor responsiveness in hypertensive and normotensive individuals. J Physiol 592: 3063–3073, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Negrão CE, Middlekauff HR. Exercise training in heart failure: reduction in angiotensin II, sympathetic nerve activity, and baroreflex control. J Appl Physiol 104: 577–578, 2008. [DOI] [PubMed] [Google Scholar]
- 31.Roveda F, Middlekauff HR, Rondon MUP, Reis SF, Souza M, Nastari L, Barretto ACP, Krieger EM, Negrão CE. The effects of exercise training on sympathetic neural activation in advanced heart failure. J Am Coll Cardiol 42: 854–860, 2003. [DOI] [PubMed] [Google Scholar]
- 32.Sun SY, Wang W, Zucker IH, Schultz HD. Enhanced activity of carotid body chemoreceptors in rabbits with heart failure: role of nitric oxide. J Appl Physiol 86: 1273–1282, 1999. [DOI] [PubMed] [Google Scholar]
- 33.Sun SY, Wang W, Zucker IH, Schultz HD. Enhanced peripheral chemoreflex function in conscious rabbits with pacing-induced heart failure. J Appl Physiol 86: 1264–1272, 1999. [DOI] [PubMed] [Google Scholar]
- 34.van de Borne P, Oren R, Abouassaly C, Anderson E, Somers VK. Effect of Cheyne-Stokes respiration on muscle sympathetic nerve activity in severe congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol 81: 432–436, 1998. [DOI] [PubMed] [Google Scholar]
- 35.Xing DT, May CN, Booth LC, Ramchandra R. Tonic arterial chemoreceptor activity contributes to cardiac sympathetic activation in mild ovine heart failure. Exp Physiol 99: 1031–1041, 2014. [DOI] [PubMed] [Google Scholar]
- 36.Yoshihisa A, Shimizu T, Owada T, Nakamura Y, Iwaya S, Yamauchi H, Miyata M, Hoshino Y, Sato T, Suzuki S, Sugimoto K, Yamaki T, Kunii H, Nakazato K, Suzuki H, Saitoh S, Takeishi Y. Adaptive servo-ventilation improves cardiac dysfunction and prognosis in chronic heart failure patients with Cheyne-Stokes respiration. Int Heart J 52: 218–223, 2011. [DOI] [PubMed] [Google Scholar]
- 37.Zucker IH, Schultz HD, Patel KP, Wang HJ. Modulation of angiotensin II signaling following exercise training in heart failure. Am J Physiol Heart Circ Physiol. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]