Our findings indicate that muscle blood flow and oxygen consumption are increased during exercise 2 h after ingestion of ascorbic acid in older adults, and this is due to local vasodilation. This may represent a strategy to improve skeletal muscle perfusion and exercise tolerance in older healthy and diseased humans.
Keywords: vitamin C, aging, sympathetic vasoconstriction
Abstract
Human aging is associated with reduced skeletal muscle perfusion during exercise, which may be a result of impaired endothelium-dependent dilation and/or attenuated ability to blunt sympathetically mediated vasoconstriction. Intra-arterial infusion of ascorbic acid (AA) increases nitric oxide-mediated vasodilation and forearm blood flow (FBF) during handgrip exercise in older adults, yet it remains unknown whether an acute oral dose can similarly improve FBF or enhance the ability to blunt sympathetic vasoconstriction during exercise. We hypothesized that 1) acute oral AA would improve FBF (Doppler ultrasound) and oxygen consumption (V̇o2) via local vasodilation during graded rhythmic handgrip exercise in older adults (protocol 1), and 2) AA ingestion would not enhance sympatholysis in older adults during handgrip exercise (protocol 2). In protocol 1 (n = 8; 65 ± 3 yr), AA did not influence FBF or V̇o2 during rest or 5% maximal voluntary contraction (MVC) exercise, but increased FBF (199 ± 13 vs. 248 ± 16 ml/min and 343 ± 24 vs. 403 ± 33 ml/min; P < 0.05) and V̇o2 (26 ± 2 vs. 34 ± 3 ml/min and 43 ± 4 vs. 50 ± 5 ml/min; P < 0.05) at both 15 and 25% MVC, respectively. The increased FBF was due to elevations in forearm vascular conductance (FVC). In protocol 2 (n = 10; 63 ± 2 yr), following AA, FBF was similarly elevated during 15% MVC (∼20%); however, vasoconstriction to reflex increases in sympathetic activity during −40 mmHg lower-body negative pressure at rest (ΔFVC: −16 ± 3 vs. −16 ± 2%) or during 15% MVC (ΔFVC: −12 ± 2 vs. −11 ± 4%) was unchanged. Our collective results indicate that acute oral ingestion of AA improves muscle blood flow and V̇o2 during exercise in older adults via local vasodilation.
NEW & NOTEWORTHY
Our findings indicate that muscle blood flow and oxygen consumption are increased during exercise 2 h after ingestion of ascorbic acid in older adults, and this is due to local vasodilation. This may represent a strategy to improve skeletal muscle perfusion and exercise tolerance in older healthy and diseased humans.
during exercise, an increase in metabolic demand drives an elevation in blood flow to working muscle as a means to adequately deliver oxygen and nutrients to the active skeletal muscle (1, 6, 7, 37). The net blood flow response to a given exercise intensity is determined by mechanical factors associated with skeletal muscle contraction, locally released metabolic and endothelium-derived substances, substances within the blood, and the sympathetic nervous system (49). A number of local and endothelial-derived factors have been implicated in governing increases in blood flow, including but not limited to nitric oxide (NO), prostaglandins, K+, adenosine, adenosine triphosphate, and lactate (14). However, to date, no single factor is considered entirely responsible for augmenting muscle blood flow; rather, there appears to be redundancy such that blood flow is maintained following the elimination of one dilator (14, 27). Furthermore, as exercise intensity increases, unidentified local factors are capable of blunting sympathetically mediated vasoconstriction (functional sympatholysis), a phenomenon believed to ensure adequate blood flow and oxygen delivery to exercising muscles as the sympathetic nervous system is activated (8, 44, 53, 55, 57).
Human aging is typically associated with reduced skeletal muscle perfusion in response to single muscle contractions (10, 11, 13, 31) as well as during continuous exercise (28, 31–33, 40, 41, 46) although the mechanism(s) underlying this age-associated impairment may differ depending on the stimuli (31). With respect to “steady-state” exercise, the reduction in muscle blood flow observed with age has been attributed, in part, to impaired endothelial-mediated vasodilation (51, 52) and, more specifically, reduced NO bioavailability (50). Recent work also indicates that greater endothelin-mediated vasoconstriction with age may also limit blood flow to active muscle in older adults (3). Furthermore, older adults have a decreased ability to blunt sympathetically mediated vasoconstriction during exercise (20, 29, 39, 60), which could potentially exacerbate any age-related impairment in muscle perfusion as exercise intensity and/or the amount of muscle mass engaged increases.
Our laboratory previously demonstrated that intra-arterial ascorbic acid (AA) significantly improves endothelium-dependent vasodilation and skeletal muscle perfusion during exercise in older adults (31). We further demonstrated that this ability of AA to improve muscle blood flow during exercise in older adults is abolished by NO synthase inhibition (16). Although these experiments were designed to understand the potential roles of endothelial dysfunction, oxidative stress, and reduced NO bioavailability in the impaired muscle blood flow during exercise with advancing age, intra-arterial (and iv) AA infusions yield supraphysiological circulating concentrations. Thus, it is presently unknown whether an acute oral dose of AA is capable of improving muscle blood flow and oxygen delivery in otherwise healthy older adults during exercise and, if so, whether this results in greater oxygen utilization by the working tissue. Additionally, whether oral AA enhances the ability to blunt sympathetically mediated vasoconstriction during exercise is also unknown. Although NO is an important vasodilator, experimental evidence in humans indicates that it may not be involved in functional sympatholysis (18, 48), and therefore augmenting NO bioavailability via AA may improve local blood flow control but may not improve sympatholysis in older adults.
Accordingly, the purpose of this study was twofold: 1) to determine whether acute ingestion of AA improves forearm blood flow (FBF) and oxygen consumption (V̇o2) via increases in vascular conductance during mild and moderate-intensity rhythmic forearm exercise (protocol 1), and 2) to determine whether acute ingestion of AA improves the ability to blunt a sympathetically mediated vasoconstrictor stimulus during exercise (sympatholysis) in healthy older adults (protocol 2). We hypothesized that acute oral AA ingestion would improve exercising FBF and that elevated FBF would be associated with greater V̇o2. Furthermore, we hypothesized that oral AA ingestion would not influence the ability to blunt sympathetically mediated vasoconstriction during handgrip exercise in older adults.
METHODS
Subjects
With Institutional Review Board approval and following written informed consent, a total of 8 older healthy male subjects participated in protocol 1, and 10 older healthy adults (6 males and 4 females) participated in protocol 2. We chose to include women in protocol 2 in an effort to study a more heterogeneous participant population. All participants were sedentary to moderately active, nonsmokers, nonobese, normotensive, and not taking any medications, including over the counter supplements. Female participants were postmenopausal (2+ yr) and not taking hormone supplements. All participants were evaluated for clinical evidence of cardiopulmonary disease with a physical examination and resting and maximal exercise electrocardiograms. Studies were performed after a 12-h fast with the subjects in the supine position. The experimental arm of the subject was slightly elevated above heart level to minimize any potential influence of the muscle pump on forearm hemodynamics (54). All studies were performed according to the Declaration of Helsinki.
Venous Catheterization, Blood Gas Measurements, and V̇o2: Protocol 1
In protocol 1, an 18-gauge catheter (3.8 cm) was inserted in retrograde fashion in an antecubital vein of the experimental arm for deep venous blood samples. Saline was continuously infused through the catheter at a rate of ∼3 ml/min for the duration of the study to keep it patent (15). Venous blood samples were immediately analyzed with a clinical blood gas analyzer (Siemens Rapid Point 405 Automatic Blood Gas System, Los Angeles, CA) for partial pressures of venous oxygen and carbon dioxide, venous oxygen content, pH, and oxygen saturation. Forearm V̇o2 was calculated as: FBF × (arterial − venous O2 content). Arterial oxygen content was assumed to be 20 ml/dl (25, 47), similar to values reported by our laboratory ∼5,000 feet above sea level (15). Importantly, several studies have shown that arterial oxygen content does not change during mild to moderate forearm (handgrip) exercise in humans (12, 15).
Heart Rate and Mean Arterial Pressure: Protocols 1 and 2
Heart rate (HR) was monitored with a three-lead electrocardiogram. Mean arterial pressure (MAP) was measured by placing a finger pressure cuff around the middle phalanx of the middle finger on the nonexperimental arm (Finometer; Finapres Medical Systems, Amsterdam, The Netherlands). Resting arterial blood pressure was measured over the brachial artery following 30 min of supine rest, and just before each exercise trial (Cardiocap 5; Datex Ohmeda, Louisville, CO), and resting Finometer MAP was corrected for differences between the two readings (30).
FBF and Vascular Conductance: Protocols 1 and 2
A 12-MHz linear-array ultrasound probe (Vivid 7; General Electric, Milwaukee, WI) was used to determine brachial artery mean blood velocity and brachial artery diameter. Foam tape was used to mark the outline of the probe for consistent placement and measurement over the course of the experiments. For blood velocity measurements, the probe insonation angle was maintained at <60 degrees, and the frequency used was 5 MHz. The Doppler shift frequency spectrum was analyzed via a Multigon 500M TCD (Multigon Industries, Mt. Vernon, NY) spectral analyzer from which mean velocity was determined as a weighted mean of the spectrum of Doppler shift frequencies. Brachial artery diameter measurements were made in duplex mode at end diastole at rest and between contractions (in triplicate) during steady-state conditions. FBF was calculated as: FBF = mean blood velocity × π (brachial artery diameter/2)2 × 60, where the FBF is in milliliters per minute, the mean blood velocity is in centimeters per second, the brachial diameter is in centimeters, and 60 was used to convert from milliliters per second to milliliters per minute. A fan was directed toward the experimental arm to minimize the potential contribution of skin blood flow to forearm hemodynamics. As an index of vascular tone, forearm vascular conductance (FVC) was calculated as: (FBF/MAP) × 100 and expressed as (ml/min/100 mmHg) (16, 45).
Handgrip Exercise: Protocols 1 and 2
Maximum voluntary contraction (MVC) was determined for each subject as the average of at least three maximal squeezes of a handgrip dynamometer (Stoelting, Chicago, IL) that were within 3% of each other. Subjects lifted a weight corresponding to their %MVC over a 4- to 5-cm distance using a pulley. Both audio and visual cues were used to ensure correct timing of contraction (1 s) and relaxation (2 s) (18, 19).
Sympathetic Nervous System Activation via Lower-Body Negative Pressure: Protocol 2
To elicit sympathetically mediated vasoconstriction during rest and exercise, subjects were supine and the lower extremities were sealed in an airtight chamber at the level of the iliac crest (30). Lower-body negative pressure (LBNP) was administered at −40 mmHg for 2 min to unload baroreceptors and reflexively increase muscle sympathetic nervous activity (17). Previous studies in our laboratory indicate that, when separated by 15 min of rest, the forearm vasoconstrictor response to LBNP under resting conditions is repeatable over time (30).
Plasma Measures: Protocol 1
In study 1, plasma samples were collected to determine AA (High Performance Liquid Chromatography; ARUP Laboratories, Salt Lake City, UT) and oxidized low-density lipoprotein (LDL) (ELISA; University of Colorado Hospital CTRC Core Laboratory, Aurora, CO) concentrations before and 2 h following ingestion of 2 g of AA.
Experimental Protocols
In protocol 1, each exercise trial consisted of 2 min of resting baseline followed by continuous incremental handgrip exercise, 4 min each at 5, 15, and 25% MVC. These workloads translate to ∼15, 40, and 70% of maximum work rate (46). Following the first bout of exercise (trial 1), participants consumed 2 g of AA (C-1000; NOW Foods, Bloomingdale, IL) and rested quietly for 2 h before repeating the exercise bout (trial 2). This oral dose has been shown to reduce augmentation index, a measure of arterial stiffness in healthy adult males (58), and improve flow-mediated dilation in smokers (42), coronary artery disease patients (34), and renal allograft patients (59). Furthermore, when this same dose is administered intravenously, flow-mediated vasodilation significantly increased in healthy adults (2) and chronic heart failure patients (23). Two hours was chosen as the optimal time to increase plasma concentrations of ascorbate following oral consumption (35, 38). In protocol 1, four male subjects returned to the laboratory on a separate day and performed two bouts of graded handgrip exercise separated by 2 h and placebo consumption. This group served as a control to account for the influence of time on measured hemodynamic variables. During both visits, subjects were blinded to whether they were receiving AA or placebo. Trial 1 served as the control condition to which trial 2 (AA or placebo) was compared.
In protocol 2, LBNP (−40 mmHg) was administered for 2 min during rest. After the determination of resting forearm vasoconstrictor responses (15 min), subjects then performed 4 min of rhythmic handgrip exercise at 15% of MVC, and LBNP (−40 mmHg) was applied for the last 2 min of exercise (6 min of exercise total). Following trial 1 (control) subjects ingested 2 g of AA (C-1000; NOW Foods), rested quietly for 2 h, and then repeated the trial. The degree of vasoconstriction that occurred during LBNP administration was calculated as [(FVC end LBNP − FVC pre LBNP)/(FVC pre LBNP)] × 100.
Statistics
Data are presented as means ± SE. Within each protocol, differences between trials were determined via two-way repeated-measures analysis of variance. Post hoc comparisons were made with the Holm-Sidak test. Significance was set at P < 0.05.
RESULTS
Subject Characteristics
Eight older healthy males [age: 65 ± 3 yr; body mass index (BMI): 28.6 ± 1.4 kg/m2] participated in the AA trial in protocol 1, whereas 10 older healthy adults (6 males and 4 females; age: 63 ± 2 yr; BMI 25.1 ± 1.6 kg/m2) participated in protocol 2. All participants in both protocols had normal cholesterol (total, high-density lipoprotein, and LDL) levels, and there were no significant differences with respect to body fat (31 ± 3 vs. 29 ± 1% body fat in protocols 1 and 2, respectively), forearm volume (1,148 ± 53 vs. 971 ± 53 ml), or handgrip MVC (44 ± 3 vs. 40 ± 1 kg).
Protocol 1: Influence of Acute AA Ingestion on FBF and V̇o2 during Graded Handgrip Exercise in Older Adults
Hemodynamics.
There were no significant differences in resting forearm (FBF and FVC) or systemic hemodynamics (MAP and HR) between the trials performed before and following AA (Table 1). Compared with control conditions (trial 1), there were no significant differences in any hemodynamic variable at the lowest workload (5% MVC) following AA consumption. Oral AA consumption (trial 2) significantly increased FBF at 15% MVC (248 ± 16 vs. 198 ± 13 ml/min; P < 0.05) and 25% MVC (403 ± 33 vs. 342 ± 24 ml/min; P < 0.05) (Fig. 1A). FVC was also significantly greater at both 15% (245 ± 19 vs. 199 ± 16 ml·min−1·mmHg−1; P < 0.05) and 25% (387 ± 38 vs. 325 ± 27 ml·min−1·mmHg−1; P < 0.05) MVC following AA (Table 1). In addition, V̇o2 was significantly greater following AA during both 15% (34 ± 3 vs. 26 ± 2 ml/min; P < 0.05) and 25% (50 ± 6 vs. 43 ± 4 ml/min; P < 0.05) MVC exercise (Fig. 1B). Although FBF, FVC, and V̇o2 increased following AA at 15 and 25% exercise, AA had no effect on HR or MAP (Table 1). Within the time control visit, there were no significant differences in any variable between trial 1 (control) and trial 2 (placebo) at rest or during exercise (Table 1).
Table 1.
Hemodynamics during protocol 1 (graded handgrip exercise) and protocol 2 (exercise + LBNP) at rest and during exercise
| Rest |
5% MVC |
15% MVC |
25% MVC |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Condition | HR, beats/min | MAP, mmHg | FBF, ml/min | FVC, ml · min−1 · mmHg−1 | HR, beats/min | MAP, mmHg | FBF, ml/min | FVC, ml · min−1 · mmHg−1 | HR, beats/min | MAP, mmHg | FBF, ml/min | FVC, ml · min−1 · mmHg−1 | HR, beats/min | MAP, mmHg | FBF, ml/min | FVC, ml · min−1 · mmHg−1 |
| Protocol 1: Graded Handgrip Exercise | ||||||||||||||||
| Control (n = 8) | 57 ± 2 | 93.9 ± 2.8 | 35.8 ± 5.1 | 38 ± 5.2 | 63 ± 2 | 100.6 ± 3.7 | 96.5 ± 9.0 | 97 ± 10.2 | 64 ± 2 | 101.0 ± 3.1 | 198.6 ± 12.5 | 199 ± 16.3 | 68 ± 2 | 107.2 ± 4.8 | 342.4 ± 24.0 | 325.5 ± 27.2 |
| Ascorbic acid (n = 8) | 57 ± 2 | 95.3 ± 2.9 | 30.2 ± 3.5 | 31 ± 3.5 | 59 ± 2 | 99.6 ± 4.0 | 93.0 ± 5.0 | 94 ± 3.4 | 64 ± 3 | 105.0 ± 4.1 | 248.3 ± 16.1* | 245 ± 4.8* | 68 ± 2 | 105.8 ± 4.0 | 402.8 ± 33.1* | 387.6 ± 38.1* |
| Protocol 1: Placebo Trial | ||||||||||||||||
| Placebo control (n = 4) | 60 ± 2 | 90.6 ± 3.5 | 33.3 ± 7.3 | 37 ± 7.4 | 63 ± 2 | 96.9 ± 4.3 | 84.8 ± 7.6 | 88 ± 9.2 | 65 ± 3 | 99.2 ± 3.4 | 197.9 ± 17.1 | 201 ± 20.2 | 70 ± 5 | 112.1 ± 6.9 | 309.3 ± 31.8 | 277.8 ± 27.4 |
| Placebo (n = 4) | 54 ± 3 | 96.6 ± 1.1 | 28.4 ± 5.0 | 29 ± 5.6 | 61 ± 1 | 98.3 ± 0.9 | 85.8 ± 5.4 | 87 ± 6.4 | 62 ± 1 | 103.7 ± 1.0 | 194.3 ± 9.2 | 188 ± 18.1 | 67 ± 6 | 115.2 ± 6.3 | 283.2 ± 17.4 | 247.6 ± 22.1 |
| Rest |
LBNP |
15% MVC |
15% MVC + LBNP |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Condition | HR, beats/min | MAP, mmHg | FBF, ml/min | FVC, ml · min−1 · mmHg−1 | HR, beats/min | MAP, mmHg | FBF, ml/min | FVC, ml · min−1 · mmHg−1 | HR, beats/min | MAP, mmHg | FBF, ml/min | FVC, ml · min−1 · mmHg−1 | HR, beats/min | MAP, mmHg | FBF, ml/min | FVC, ml · min−1 · mmHg−1 |
| Protocol 2: 15% MVC + LBNP | ||||||||||||||||
| Control (n = 9) | 60 ± 3 | 97.5 ± 3.0 | 26.7 ± 2.3 | 27.3 ± 2.0 | 64 ± 3 | 93.9 ± 2.7 | 21.7 ± 2.2 | 22.9 ± 2.4 | 64 ± 3 | 102.6 ± 3.5 | 185.4 ± 15.2 | 183.4 ± 17.0 | 71 ± 3 | 103.7 ± 2.9 | 164.8 ± 14 | 161.0 ± 15.3 |
| Ascorbic acid (n = 9) | 57 ± 3 | 101.3 ± 4.2 | 26.7 ± 2.0 | 26.5 ± 1.6 | 64 ± 3 | 97.9 ± 3.2 | 22.0 ± 1.8 | 22.4 ± 1.6 | 64 ± 3 | 105.2 ± 5.8 | 212.2 ± 19.6* | 208.9 ± 23.8* | 67 ± 3 | 99.9 ± 4.3 | 179.8 ± 18.1 | 185.9 ± 22.7* |
Values are means ± SE; n, no. of subjects. MVC, maximal voluntary contraction; HR, heart rate; MAP, mean arterial blood pressure; FBF, forearm blood flow; FVC, forearm vascular conductance; LBNP, lower-body negative pressure.
P < 0.05 vs. control in respective study.
Fig. 1.
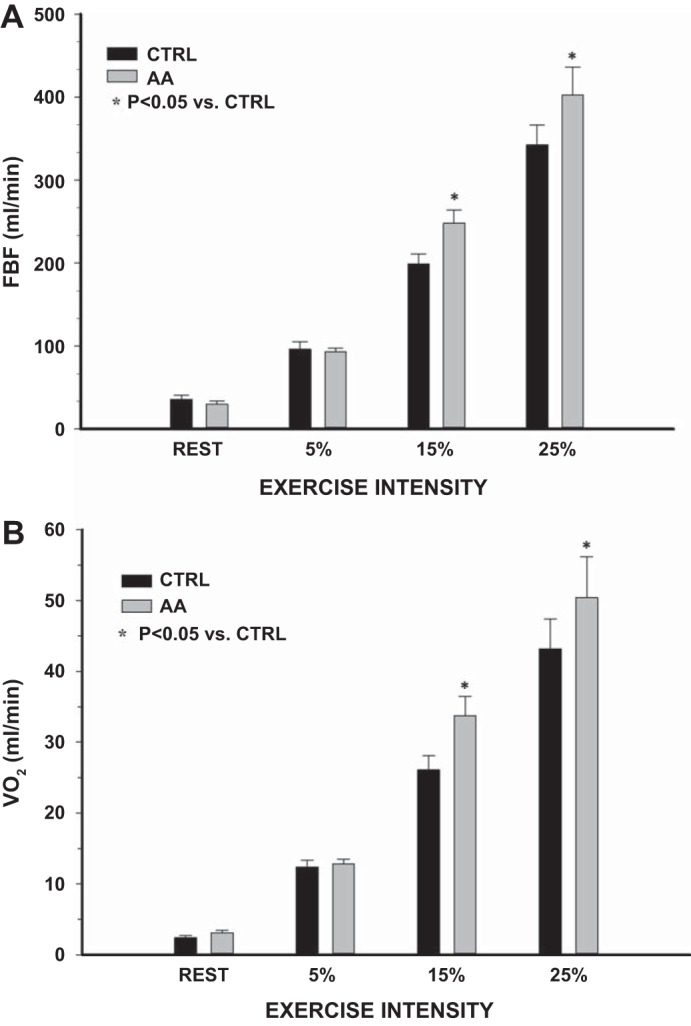
Protocol 1: rest and exercise forearm blood flow (FBF, A) and oxygen consumption (V̇o2; B) during control (trial 1) and 2 h post-ascorbic acid (AA) consumption (trial 2). AA significantly increased FBF and V̇o2 at 15 and 25% of maximum voluntary contraction (MVC). *P < 0.05 vs. control (CTRL).
Deep venous blood gases.
There were no significant differences in any blood gas variables between trial 1 (control) or trial 2 (AA or placebo) at rest or during exercise (Table 2).
Table 2.
Blood gas data for protocol 1 (graded handgrip exercise)
| Rest |
5% MVC |
15% MVC |
25% MVC |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Condition | pH | Pco2, mmHg | Po2, mmHg | So2, % | ctO2, ml/dl | pH | Pco2, mmHg | Po2, mmHg | So2, % | ctO2, ml/dl | pH | Pco2, mmHg | Po2, mmHg | So2, % | ctO2, ml/dl | pH | Pco2, mmHg | Po2, mmHg | So2, % | ctO2, ml/dl |
| Control (n = 8) | 7.36 ± 0.0 | 46.8 ± 1.8 | 30.3 ± 2.0 | 54.2 ± 4.7 | 11.4 ± 1.0 | 7.34 ± 0.0 | 51.0 ± 1.5 | 20.8 ± 1.4 | 30.9 ± 3.6 | 6.3 ± 0.8 | 7.31 ± 0.0 | 53.0 ± 5.4 | 22.3 ± 1.0 | 33.2 ± 2.2 | 6.7 ± 0.5 | 7.28 ± 0.0 | 59.0 ± 2.9 | 24.8 ± 0.9 | 36.7 ± 2.5 | 7.7 ± 0.6 |
| Ascorbic acid (n = 8) | 7.36 ± 0.0 | 46.5 ± 1.5 | 28.5 ± 2.3 | 49.0 ± 5.0 | 10.2 ± 1.1 | 7.34 ± 0.0 | 52.9 ± 1.6 | 21.3 ± 1.1 | 31.5 ± 2.8 | 6.5 ± 0.6 | 7.31 ± 0.0 | 55.7 ± 0.9 | 21.9 ± 1.0 | 31.7 ± 2.4 | 6.6 ± 0.5 | 7.30 ± 0.0 | 55.2 ± 1.7 | 23.9 ± 1.2 | 36.0 ± 3.3 | 7.6 ± 0.7 |
| Placebo trial | ||||||||||||||||||||
| Time control (n = 4) | 7.37 ± 0.0 | 49.7 ± 1.6 | 26.6 ± 4.5 | 45.2 ± 8.5 | 9.4 ± 1.9 | 7.35 ± 0.0 | 55.6 ± 2.1 | 19.7 ± 2.4 | 28.8 ± 6.6 | 5.9 ± 1.4 | 7.29 ± 0.0 | 62.9 ± 3.3 | 21.3 ± 1.4 | 31.6 ± 4.3 | 6.5 ± 0.9 | 7.25 ± 0.2 | 68.8 ± 4.5 | 25.0 ± 1.1 | 35.0 ± 3.5 | 7.4 ± 0.7 |
| Placebo (n = 4) | 7.36 ± 0.0 | 51.5 ± 1.9 | 24.7 ± 3.1 | 39.9 ± 7.6 | 8.3 ± 1.6 | 7.34 ± 0.0 | 52.5 ± 1.8 | 19.9 ± 1.9 | 28.3 ± 3.9 | 5.8 ± 0.8 | 7.29 ± 0.0 | 59.7 ± 2.3 | 21.9 ± 1.4 | 30.9 ± 3.4 | 6.4 ± 0.8 | 7.27 ± 0.0 | 60.6 ± 3.3 | 26.6 ± 0.6 | 39.8 ± 1.8 | 8.4 ± 0.3 |
Values are means ± SE; n, no. of subjects. Pco2, partial pressure of carbon dioxide; Po2, partial pressure of oxygen; So2, oxyhemoglobin saturation; ctO2, venous oxygen content.
P < 0.05 vs. control.
Plasma measures.
Following oral ingestion of 2 g of AA, plasma concentrations of AA at 2 h were significantly greater than control (73 ± 7 vs. 38 ± 5 μmol/l; P < 0.001) (Fig. 2A). Oxidized LDL did not change following consumption of AA (76 ± 8 vs.73 ± 5 U/l) (Fig. 2B).
Fig. 2.
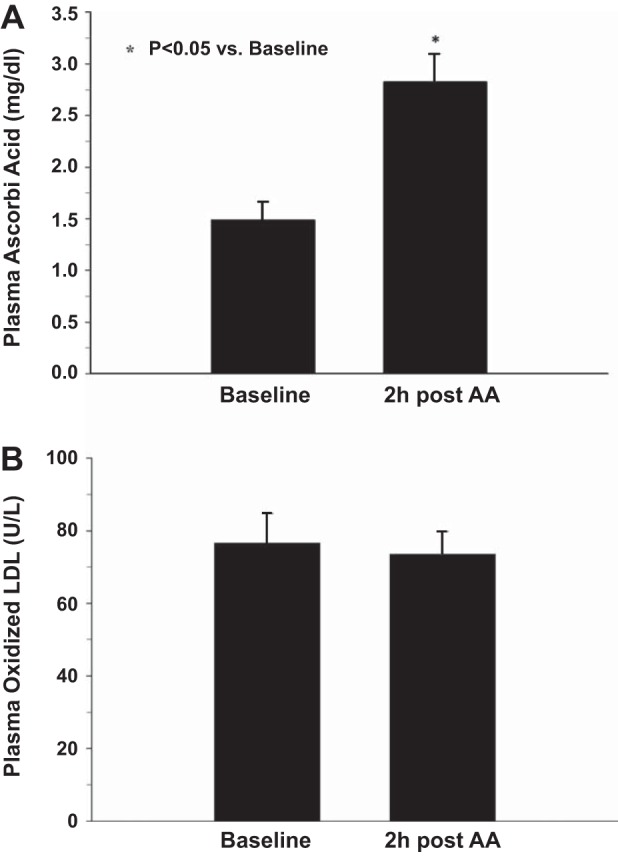
Baseline and 2 h post-AA consumption plasma concentrations of AA (A) and oxidized low-density lipoprotein (LDL, B). *P < 0.05 vs. baseline.
Protocol 2: Influence of Acute AA Ingestion on Sympathetically Mediated Vasoconstriction during 15% MVC Handgrip Exercise in Older Adults
Hemodynamics.
There were no significant differences in resting forearm or systemic hemodynamics between the control and AA trial (Table 1). The degree of vasoconstriction that occurred in response to LBNP at rest in trial 1 (control) and trial 2 (AA) was not different (ΔFVC −16 ± 2 vs. −16 ± 2%; Fig. 3). During handgrip exercise at 15% MVC, FBF was significantly greater following AA ingestion (212 ± 20 vs. 185 ± 15 ml/min; P < 0.05) (Table 1). However, the degree of vasoconstriction to LBNP during exercise was not significantly different before or after AA ingestion (ΔFVC −12 ± 2 vs. −11 ± 4%) (Fig. 3). Furthermore, within each condition (control and AA), the vasoconstrictor responses to LBNP during 15% handgrip exercise were not significantly different from that observed at rest.
Fig. 3.
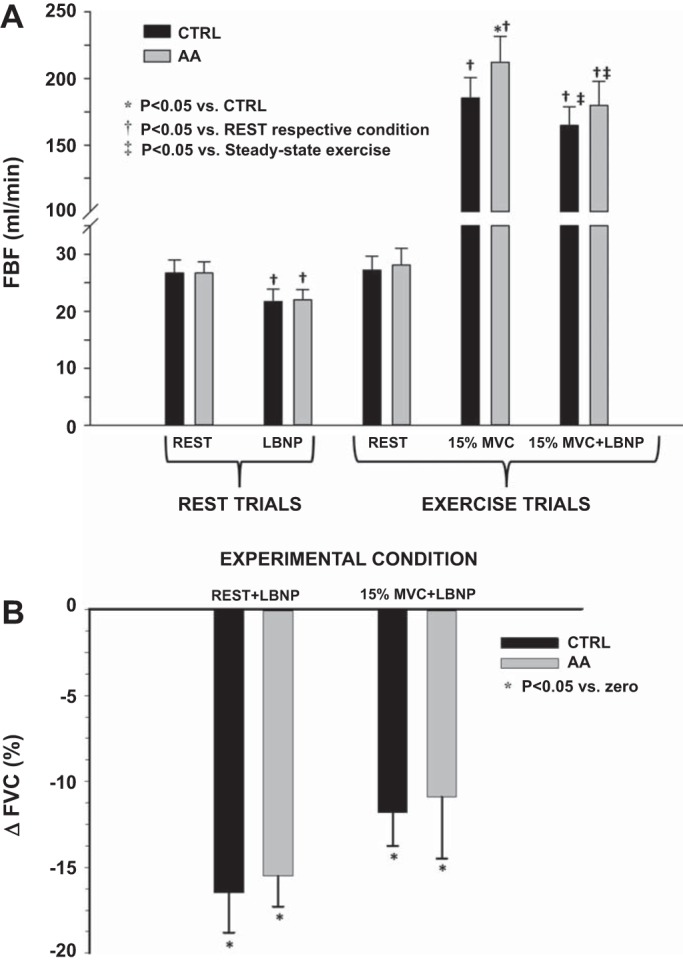
Protocol 2. A: FBF at rest and exercise and during superimposed lower-body negative pressure (LBNP, −40 mmHg) before and after AA consumption. *P < 0.05 vs. control. †P < 0.05 vs. rest within respective condition. ‡P < 0.05 vs. steady-state exercise. B: forearm vasoconstrictor responses (ΔFVC%) to −40 mmHg LBNP at rest and during handgrip exercise (15% MVC) before and after AA consumption. *P < 0.05 vs. zero.
DISCUSSION
The primary findings of the present study are as follows. First, an acute oral dose of AA significantly improves FBF and V̇o2 during moderate-intensity (15 and 25% MVC) handgrip exercise in older healthy adults (protocol 1). Second, this improvement in skeletal muscle perfusion was not due to elevations in HR or MAP (i.e., perfusion pressure); rather this was due to elevations in FVC (protocols 1 and 2). Third, despite the ability of AA to improve local blood flow and vascular conductance during exercise, it did not enhance the ability of older individuals to blunt a sympathetically mediated vasoconstrictor stimulus in resting or contracting skeletal muscle (protocol 2). Collectively, these findings indicate that acute oral AA can augment exercising muscle blood flow and V̇o2 (∼20%) in older humans, and this is due to improvements in local vasodilator signaling.
AA and Skeletal Muscle Blood Flow during Exercise in Older Adults
Our laboratory and others have demonstrated impaired skeletal muscle blood flow in response to both single and sustained muscle contractions in aging humans (10, 13, 28, 31, 41, 46, 60). Previously, we demonstrated that acute intra-arterial infusion of AA improves muscle blood flow during mild steady-state handgrip exercise in older healthy adults, and this was associated with improved endothelial function as assessed via local vasodilator response to acetylcholine (31). In a follow-up study, we found that the improvement in exercising muscle blood flow in older adults during intra-arterial AA infusion was abolished by NO synthase inhibition, indicating that the improvement in blood flow was via elevated NO-mediated vasodilation (16). Given that these studies used supraphysiological local doses of AA, it was not clear whether similar improvements in skeletal muscle hemodynamics would be observed in response to a physiological oral dose of AA.
In protocol 1 of the present study, we directly determined whether ingestion of 2 g of AA improved muscle blood flow during exercise in older adults. Our data indicate that, although AA did not improve FBF at rest (16, 31) or during mild intensity exercise, it did significantly improve blood flow during moderate- to high-intensity handgrip exercise. This improvement was not due to changes in systemic hemodynamics, but was due to elevations in local vasodilator signaling as reflected by parallel increases in FVC. In addition to a significant improvement in blood flow during 15 and 25% MVC exercise, we also observed a significant increase in forearm V̇o2 at these exercise intensities. The increase in oxygen utilization of the tissue following AA suggests that blood flow impairments in older individuals may be limiting oxygen utilization by contracting skeletal muscle.
AA and Sympathetic Vasoconstriction during Exercise in Older Adults
In young healthy humans, sympathetic vasoconstrictor responses are blunted in contracting muscle in a graded fashion with exercise intensity, a phenomenon believed to ensure optimal tissue blood flow and oxygen delivery during exercise (8, 44, 49). This is particularly important during larger muscle mass and high-intensity exercise, since sympathetic vasoconstrictor activity is increased to restrain peripheral vasodilation and thus maintain arterial blood pressure (49). The ability of contracting muscle to blunt sympathetically mediated vasoconstriction has been termed “functional sympatholysis” (44), and, compared with young, older adults demonstrate impaired functional sympatholysis during exercise (20, 29, 39). It is plausible that this impairment further limits blood flow and oxygen delivery to active skeletal muscle of aging humans during activation of the sympathetic nervous system.
In the present study, we hypothesized that acute ingestion of AA would not augment the ability to blunt sympathetic vasoconstriction in contracting muscle of older adults. We reasoned if AA exerts its positive vascular influence by increasing NO bioavailability, a number of studies from our laboratory and others indicate that NO may not be sympatholytic (9, 18, 19, 48). In protocol 2, we found that muscle blood flow and vascular conductance were augmented during 15% MVC handgrip exercise after AA, consistent with observations in protocol 1. However, despite elevations in steady-state forearm hemodynamics during exercise, the ability of muscle contractions to blunt baroreflex-mediated increases in sympathetic vasoconstriction evoked via LBNP was not different compared with the control condition. Furthermore, our findings of similar vasoconstriction to LBNP at rest and during exercise are consistent with impaired functional sympatholysis with age independent of AA ingestion. Taken together, acute oral AA appears to improve muscle blood flow via local vasodilation in older adults without influencing the ability to blunt sympathetic vasoconstriction during acute sympathoexcitation.
Potential Mechanisms
One mechanism by which AA may have improved skeletal muscle perfusion during exercise is via increased NO bioavailability. AA is an antioxidant that could indirectly increase NO signaling by reducing superoxide (26, 36), a primary source of oxidative stress in the vasculature that inactivates NO and is elevated with age (43, 56). This mechanism is consistent with what we have demonstrated experimentally in prior studies whereby local intra-arterial infusion of AA led to significant improvements in skeletal muscle blood flow during exercise (31) that were abolished via NO synthase inhibition (16). In the present study, a significant increase in plasma AA concentration was observed 2 h following consumption, yet there was no change in oxidized LDL concentrations (a surrogate marker of oxidative stress). The lack of a decrease in oxidized LDL following AA is in agreement with other studies (4), and, although it may be unlikely that 2 h was sufficient to observe an influence of AA on oxidized LDL, this does not preclude the ability of AA to act as an antioxidant and improve NO bioavailability (16, 31).
Experimental Considerations
In the present study, we used forearm (handgrip) exercise to examine the effect of oral AA ingestion on skeletal muscle blood flow in older adults. This type of exercise engages a small muscle mass, and therefore the increase in skeletal muscle perfusion during exercise is not limited centrally by cardiac output. Additionally, activation of this small muscle mass during mild-moderate handgrip exercise may limit any reflex-mediated activation of the sympathetic nervous system. In this context, our laboratory recently reported that acute blockade of sympathetic α-adrenergic vasoconstriction did not impact the change in skeletal muscle blood flow and vascular conductance from rest to graded handgrip exercise of similar intensities in young or older adults, indicating that the age-related impairment in skeletal muscle perfusion in this model of exercise is not due to greater sympathetic vasoconstriction with age but rather due to impaired local vasodilator signaling (46).
Our findings associated with improvements in exercising muscle blood flow during handgrip exercise may not be extrapolated to the vasculature within larger muscle masses such as the lower limbs. At rest, unlike the upper limbs, the lower limbs of older adults exhibit a greater degree of basal sympathetic vasoconstrictor tone (21, 22), and it is unknown whether there is further sympathetically mediated restraint of skeletal muscle blood flow during lower limb exercise in older adults. Given that AA did not improve sympatholysis in the forearm model, it is unknown whether AA would lead to improvements in exercising blood flow in the leg of older adults if there is a greater role for sympathetically mediated vasoconstrictor tone both at rest and during exercise. This awaits further study.
In the second protocol we determined whether acute ingestion of AA would improve the ability to blunt a sympathetically mediated vasoconstrictor stimulus by applying LBNP for 2 min at the end of exercise. Despite a significantly greater blood flow during muscle contractions following AA ingestion, there was no difference in the degree of vasoconstriction observed during exercise, indicating that AA does not improve functional sympatholysis in older adults. Given that previous studies have reported that intravenous infusion of AA does not alter sympathetic outflow as assessed by microneurography (muscle sympathetic nerve activity) in both young and older adults (5), we do not believe that acute ingestion of AA influenced sympathetic nervous system activity in our subjects. Although it remains uncertain whether AA influenced the transduction of sympathetic nerve activity to vasoconstrictor responses, the finding that resting hemodynamics were unaffected by AA with presumably similar levels of sympathetic outflow would argue against this.
Perspectives and Conclusions
In the present study, we observed a significant increase in exercising FBF and V̇o2 in older adults following acute ingestion of AA. The improvement in exercising blood flow in the present study is similar to that previously observed following intra-arterial infusion of AA (∼20%), and thus indicates improved skeletal muscle perfusion during exercise 2 h after acute ingestion of AA that was facilitated by greater local vasodilation. These findings are of potential clinical significance in that improving muscle blood flow to active muscle may improve exercise tolerance in older healthy and diseased populations characterized by reduced muscle perfusion (e.g., heart failure, peripheral arterial disease). Whether chronic supplementation with oral AA continues to elicit improvements in muscle blood flow 2 h after the last ingestion remains to be determined.
Recently, there has been growing interest in understanding how antioxidant supplementation in conjunction with an aerobic exercise training program may impact cardiovascular adaptations in humans. In the present study, we acutely administered a dose of AA and observed a significant increase in exercising muscle blood flow 2 h after ingestion when blood concentrations were anticipated to be at their peak. However, a recent study reported that, compared with placebo, 8 wk of daily resveratrol (believed to increase endogenous antioxidant activity) supplementation resulted in smaller increases in exercise capacity (i.e., V̇o2max) at the end of the training program in older adults (24). These findings suggest that there was no added benefit of including resveratrol to an exercise training program and that it may actually limit some positive adaptations. However, it is unknown if the same results would be observed with chronic ingestion of AA in older populations participating in an exercise training program, particularly if the exercise training bout was performed within a similar time frame as the peak increases in circulating AA. It is feasible that repeatedly ingesting AA 2 h before the start of an exercise program could lead to greater gains in aerobic performance or peripheral vascular function. If blood flow and oxygen delivery were maximized following AA ingestion, then perhaps aerobic capacity would increase to a greater extent compared with a placebo condition. This warrants further study.
In conclusion, acute ingestion of AA significantly increased skeletal muscle blood flow and V̇o2 during moderate-intensity handgrip exercise in healthy older adults. Furthermore, despite greater exercise hyperemia associated with AA ingestion, this did not impact the ability to modulate sympathetic vasoconstriction in contracting muscle. It is unknown whether the enhanced vasodilation observed in the forearm would translate to improvements in skeletal muscle blood flow during leg or whole body exercise, since these likely engage the sympathetic nervous system, whereas the forearm model does not. Finally, additional studies are warranted to determine whether repeated ingestion of an oral dose of AA 2 h before exercise would impact any cardiovascular or skeletal muscle adaptations to regular exercise training particularly in clinical populations such as heart failure patients where augmented oxygen delivery to the skeletal muscle during exercise may improve exercise tolerance.
GRANTS
This research was supported by National Heart, Lung, and Blood Institute Grant HL-095573 and HL-119337 (F. A. Dinenno).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: J.C.R., A.R.C., D.G.L., and F.A.D. conception and design of research; J.C.R. and A.R.C. performed experiments; J.C.R. and F.A.D. analyzed data; J.C.R., A.R.C., D.G.L., and F.A.D. interpreted results of experiments; J.C.R. prepared figures; J.C.R. and F.A.D. drafted manuscript; J.C.R., A.R.C., and F.A.D. edited and revised manuscript; J.C.R., A.R.C., and D.G.L. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the subjects who volunteered to participate and Leora Garcia, Hannah Scott, and Devin Dinenno for assistance in conducting these studies.
REFERENCES
- 1.Anrep GV, von Saalfeld E. The blood flow through the skeletal muscle in relation to its contraction. J Physiol 85: 375–399, 1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arcaro G, Cretti A, Balzano S, Lechi A, Muggeo M, Bonora E, Bonadonna RC. Insulin causes endothelial dysfunction in humans: sites and mechanisms. Circulation 105: 576–582, 2002. [DOI] [PubMed] [Google Scholar]
- 3.Barrett-O'Keefe Z, Ives SJ, Trinity JD, Morgan G, Rossman MJ, Donato AJ, Runnels S, Morgan DE, Gmelch BS, Bledsoe AD, Richardson RS, Wray DW. Endothelin-A-mediated vasoconstriction during exercise with advancing age. J Gerontol A Biol Sci Med Sci 70: 554–565, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bell C, Carson JM, Motte NW, Seals DR. Ascorbic acid does not affect the age-associated reduction in maximal cardiac output and oxygen consumption in healthy adults. J Appl Physiol 98: 845–849, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Bell C, Jones PP, Seals DR. Oxidative stress does not modulate metabolic rate or skeletal muscle sympathetic activity with primary aging in adult humans. J Clin Endocrinol Metab 88: 4950–4954, 2003. [DOI] [PubMed] [Google Scholar]
- 6.Bockman EL. Blood flow and oxygen consumption in active soleus and gracilis muscles in cats. Am J Physiol Heart Circ Physiol 244: H546–H551, 1983. [DOI] [PubMed] [Google Scholar]
- 7.Bockman EL, McKenzie JE, Ferguson JL. Resting blood flow and oxygen consumption in soleus and gracilis muscles of cats. Am J Physiol Heart Circ Physiol 239: H516–H524, 1980. [DOI] [PubMed] [Google Scholar]
- 8.Buckwalter JB, Clifford PS. The paradox of sympathetic vasoconstriction in exercising skeletal muscle. Exerc Sport Sci Rev 29: 159–163, 2001. [DOI] [PubMed] [Google Scholar]
- 9.Buckwalter JB, Taylor JC, Hamann JJ, Clifford PS. Role of nitric oxide in exercise sympatholysis. J Appl Physiol 97: 417–423, 2004. [DOI] [PubMed] [Google Scholar]
- 10.Carlson RE, Kirby BS, Voyles WF, Dinenno FA. Evidence for impaired skeletal muscle contraction-induced rapid vasodilation in aging humans. Am J Physiol Heart Circ Physiol 294: H1963–H1970, 2008. [DOI] [PubMed] [Google Scholar]
- 11.Casey DP, Joyner MJ. Influence of alpha-adrenergic vasoconstriction on the blunted skeletal muscle contraction-induced rapid vasodilation with aging. J Appl Physiol 113: 1201–1212, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Casey DP, Madery BD, Curry TB, Eisenach JH, Wilkins BW, Joyner MJ. Nitric oxide contributes to the augmented vasodilatation during hypoxic exercise. J Physiol 588: 373–385, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Casey DP, Walker BG, Ranadive SM, Taylor JL, Joyner MJ. Contribution of nitric oxide in the contraction-induced rapid vasodilation in young and older adults. J Appl Physiol 115: 446–455, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clifford PS, Hellsten Y. Vasodilatory mechanisms in contracting skeletal muscle. J Appl Physiol 97: 393–403, 2004. [DOI] [PubMed] [Google Scholar]
- 15.Crecelius AR, Kirby BS, Voyles WF, Dinenno FA. Augmented skeletal muscle hyperaemia during hypoxic exercise in humans is blunted by combined inhibition of nitric oxide and vasodilating prostaglandins. J Physiol 589: 3671–3683, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crecelius AR, Kirby BS, Voyles WF, Dinenno FA. Nitric oxide, but not vasodilating prostaglandins, contributes to the improvement of exercise hyperemia via ascorbic acid in healthy older adults. Am J Physiol Heart Circ Physiol 299: H1633–H1641, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davy KP, Seals DR, Tanaka H. Augmented cardiopulmonary and integrative sympathetic baroreflexes but attenuated peripheral vasoconstriction with age. Hypertension 32: 298–304, 1998. [DOI] [PubMed] [Google Scholar]
- 18.Dinenno FA, Joyner MJ. Blunted sympathetic vasoconstriction in contracting skeletal muscle of healthy humans: is nitric oxide obligatory? J Physiol 553: 281–292, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dinenno FA, Joyner MJ. Combined NO and PG inhibition augments alpha-adrenergic vasoconstriction in contracting human skeletal muscle. Am J Physiol Heart Circ Physiol 287: H2576–H2584, 2004. [DOI] [PubMed] [Google Scholar]
- 20.Dinenno FA, Masuki S, Joyner MJ. Impaired modulation of sympathetic alpha-adrenergic vasoconstriction in contracting forearm muscle of ageing men. J Physiol 567: 311–321, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dinenno FA, Seals DR, DeSouza CA, Tanaka H. Age-related decreases in basal limb blood flow in humans: time course, determinants and habitual exercise effects. J Physiol 531: 573–579, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dinenno FA, Tanaka H, Stauffer BL, Seals DR. Reductions in basal limb blood flow and vascular conductance with human ageing: role for augmented alpha-adrenergic vasoconstriction. J Physiol 536: 977–983, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ellis GR, Anderson RA, Chirkov YY, Morris-Thurgood J, Jackson SK, Lewis MJ, Horowitz JD, Frenneaux MP. Acute effects of vitamin C on platelet responsiveness to nitric oxide donors and endothelial function in patients with chronic heart failure. J Cardiovasc Pharmacol 37: 564–570, 2001. [DOI] [PubMed] [Google Scholar]
- 24.Gliemann L, Schmidt JF, Olesen J, Bienso RS, Peronard SL, Grandjean SU, Mortensen SP, Nyberg M, Bangsbo J, Pilegaard H, Hellsten Y. Resveratrol blunts the positive effects of exercise training on cardiovascular health in aged men. J Physiol 591: 5047–5059, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holmgren A, Linderholm H. Oxygen and carbon dioxide tensions of arterial blood during heavy and exhaustive exercise. Acta Physiol Scand 44: 203–215, 1958. [DOI] [PubMed] [Google Scholar]
- 26.Jackson TS, Xu A, Vita JA, Keaney JF Jr. Ascorbate prevents the interaction of superoxide and nitric oxide only at very high physiological concentrations. Circ Res 83: 916–922, 1998. [DOI] [PubMed] [Google Scholar]
- 27.Joyner MJ, Wilkins BW. Exercise hyperaemia: is anything obligatory but the hyperaemia? J Physiol 583: 855–860, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kirby BS, Crecelius AR, Voyles WF, Dinenno FA. Impaired skeletal muscle blood flow control with advancing age in humans: attenuated ATP release and local vasodilation during erythrocyte deoxygenation. Circ Res 111: 220–230, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kirby BS, Crecelius AR, Voyles WF, Dinenno FA. Modulation of postjunctional alpha-adrenergic vasoconstriction during exercise and exogenous ATP infusions in ageing humans. J Physiol 589: 2641–2653, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kirby BS, Markwald RR, Smith EG, Dinenno FA. Mechanical effects of muscle contraction do not blunt sympathetic vasoconstriction in humans. Am J Physiol Heart Circ Physiol 289: H1610–H1617, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Kirby BS, Voyles WF, Simpson CB, Carlson RE, Schrage WG, Dinenno FA. Endothelium-dependent vasodilatation and exercise hyperaemia in ageing humans: impact of acute ascorbic acid administration. J Physiol 587: 1989–2003, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lawrenson L, Hoff J, Richardson RS. Aging attenuates vascular and metabolic plasticity but does not limit improvement in muscle V̇o2max. Am J Physiol Heart Circ Physiol 286: H1565–H1572, 2004. [DOI] [PubMed] [Google Scholar]
- 33.Lawrenson L, Poole JG, Kim J, Brown C, Patel P, Richardson RS. Vascular and metabolic response to isolated small muscle mass exercise: effect of age. Am J Physiol Heart Circ Physiol 285: H1023–H1031, 2003. [DOI] [PubMed] [Google Scholar]
- 34.Levine GN, Frei B, Koulouris SN, Gerhard MD, Keaney JF Jr, Vita JA. Ascorbic acid reverses endothelial vasomotor dysfunction in patients with coronary artery disease. Circulation 93: 1107–1113, 1996. [DOI] [PubMed] [Google Scholar]
- 35.Levine M, Conry-Cantilena C, Wang Y, Welch RW, Washko PW, Dhariwal KR, Park JB, Lazarev A, Graumlich JF, King J, Cantilena LR. Vitamin C pharmacokinetics in healthy volunteers: evidence for a recommended dietary allowance. Proc Natl Acad Sci USA 93: 3704–3709, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.May JM. How does ascorbic acid prevent endothelial dysfunction? Free Radic Biol Med 28: 1421–1429, 2000. [DOI] [PubMed] [Google Scholar]
- 37.Mohrman DE, Regal RR. Relation of blood flow to endothelin-A-mediated vasoconstriction during exercise V̇o2, Po2, and Pco2 in dog gastrocnemius muscle. Am J Physiol Heart Circ Physiol 255: H1004–H1010, 1988. [DOI] [PubMed] [Google Scholar]
- 38.Padayatty SJ, Sun H, Wang Y, Riordan HD, Hewitt SM, Katz A, Wesley RA, Levine M. Vitamin C pharmacokinetics: implications for oral and intravenous use. Ann Intern Med 140: 533–537, 2004. [DOI] [PubMed] [Google Scholar]
- 39.Parker BA, Smithmyer SL, Jarvis SS, Ridout SJ, Pawelczyk JA, Proctor DN. Evidence for reduced sympatholysis in leg resistance vasculature of healthy older women. Am J Physiol Heart Circ Physiol 292: H1148–H1156, 2007. [DOI] [PubMed] [Google Scholar]
- 40.Poole JG, Lawrenson L, Kim J, Brown C, Richardson RS. Vascular and metabolic response to cycle exercise in sedentary humans: effect of age. Am J Physiol Heart Circ Physiol 284: H1251–H1259, 2003. [DOI] [PubMed] [Google Scholar]
- 41.Proctor DN, Shen PH, Dietz NM, Eickhoff TJ, Lawler LA, Ebersold EJ, Loeffler DL, Joyner MJ. Reduced leg blood flow during dynamic exercise in older endurance-trained men. J Appl Physiol 85: 68–75, 1998. [DOI] [PubMed] [Google Scholar]
- 42.Raitakari OT, Adams MR, McCredie RJ, Griffiths KA, Stocker R, Celermajer DS. Oral vitamin C and endothelial function in smokers: short-term improvement, but no sustained beneficial effect. J Am Coll Cardiol 35: 1616–1621, 2000. [DOI] [PubMed] [Google Scholar]
- 43.Raju SV, Barouch LA, Hare JM. Nitric oxide and oxidative stress in cardiovascular aging (Abstract). Sci Aging Knowledge Environ 25: re4, 2005. [DOI] [PubMed] [Google Scholar]
- 44.Remensnyder JP, Mitchell JH, Sarnoff SJ. Functional sympatholysis during muscular activity. Observations on influence of carotid sinus on oxygen uptake. Circ Res 11: 370–380, 1962. [DOI] [PubMed] [Google Scholar]
- 45.Richards JC, Crecelius AR, Kirby BS, Larson DG, Dinenno FA. Muscle contraction duration and fibre recruitment influence blood flow and oxygen consumption independent of contractile work during steady-state exercise in humans. Exp Physiol 97: 750–761, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Richards JC, Luckasen GJ, Larson DG, Dinenno FA. Role of alpha-adrenergic vasoconstriction in regulating skeletal muscle blood flow and vascular conductance during forearm exercise in ageing humans. J Physiol 592: 4775–4788, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roach RC, Koskolou MD, Calbet JA, Saltin B. Arterial O2 content and tension in regulation of cardiac output and leg blood flow during exercise in humans. Am J Physiol Heart Circ Physiol 276: H438–H445, 1999. [DOI] [PubMed] [Google Scholar]
- 48.Rosenmeier JB, Fritzlar SJ, Dinenno FA, Joyner MJ. Exogenous NO administration and alpha-adrenergic vasoconstriction in human limbs. J Appl Physiol 95: 2370–2374, 2003. [DOI] [PubMed] [Google Scholar]
- 49.Saltin B, Radegran G, Koskolou MD, Roach RC. Skeletal muscle blood flow in humans and its regulation during exercise. Acta Physiol Scand 162: 421–436, 1998. [DOI] [PubMed] [Google Scholar]
- 50.Schrage WG, Eisenach JH, Joyner MJ. Ageing reduces nitric-oxide- and prostaglandin-mediated vasodilatation in exercising humans. J Physiol 579: 227–236, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Taddei S, Virdis A, Ghiadoni L, Salvetti G, Bernini G, Magagna A, Salvetti A. Age-related reduction of NO availability and oxidative stress in humans. Hypertension 38: 274–279, 2001. [DOI] [PubMed] [Google Scholar]
- 52.Taddei S, Virdis A, Mattei P, Ghiadoni L, Gennari A, Fasolo CB, Sudano I, Salvetti A. Aging and endothelial function in normotensive subjects and patients with essential hypertension. Circulation 91: 1981–1987, 1995. [DOI] [PubMed] [Google Scholar]
- 53.Tschakovsky ME, Hughson RL. Rapid blunting of sympathetic vasoconstriction in the human forearm at the onset of exercise. J Appl Physiol 94: 1785–1792, 2003. [DOI] [PubMed] [Google Scholar]
- 54.Tschakovsky ME, Shoemaker JK, Hughson RL. Vasodilation and muscle pump contribution to immediate exercise hyperemia. Am J Physiol Heart Circ Physiol 271: H1697–H1701, 1996. [DOI] [PubMed] [Google Scholar]
- 55.Tschakovsky ME, Sujirattanawimol K, Ruble SB, Valic Z, Joyner MJ. Is sympathetic neural vasoconstriction blunted in the vascular bed of exercising human muscle? J Physiol 541: 623–635, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Walker AE, Kaplon RE, Lucking SM, Russell-Nowlan MJ, Eckel RH, Seals DR. Fenofibrate improves vascular endothelial function by reducing oxidative stress while increasing endothelial nitric oxide synthase in healthy normolipidemic older adults. Hypertension 60: 1517–1523, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Watanabe H, Watanabe K, Wadazumi T, Yoneyama F. Effect of exercise intensity on mild rhythmic-handgrip-exercise-induced functional sympatholysis. J Physiol Anthropol 26: 593–597, 2007. [DOI] [PubMed] [Google Scholar]
- 58.Wilkinson IB, Megson IL, MacCallum H, Sogo N, Cockcroft JR, Webb DJ. Oral vitamin C reduces arterial stiffness and platelet aggregation in humans. J Cardiovasc Pharmacol 34: 690–693, 1999. [DOI] [PubMed] [Google Scholar]
- 59.Williams MJ, Sutherland WH, McCormick MP, de Jong SA, McDonald JR, Walker RJ. Vitamin C improves endothelial dysfunction in renal allograft recipients. Nephrol Dial Transplant 16: 1251–1255, 2001. [DOI] [PubMed] [Google Scholar]
- 60.Wray DW, Nishiyama SK, Richardson RS. Role of α1-adrenergic vasoconstriction in the regulation of skeletal muscle blood flow with advancing age. Am J Physiol Heart Circ Physiol 296: H497–H504, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]


