Abstract
Impaired nitric oxide (NO), soluble guanylyl cyclase (sGC), and cyclic guanosine monophosphate (cGMP) signaling (NO-sGC-cGMP) has been implicated in the pathogenesis of diabetic vascular dysfunction. Efforts to directly target this signaling have led to the development of sGC agonists that activate the heme group of sGC (stimulators) or preferentially activate sGC when the heme is oxidized (activators). In this study, we hypothesized that resistance arteries from female rats with spontaneous type 2 diabetes (Goto-Kakizaki rats, GK) would have reduced vasodilatory responses to heme-dependent sGC activation and increased responses to heme-independent sGC activation compared with control rats (Wistar). Endothelium-dependent and -independent relaxation was assessed in isolated segments from mesenteric resistance arteries (MA) mounted in a wire myograph. GK MA had reduced responses to acetylcholine (pEC50: 7.96 ± 0.06 vs. 7.66 ± 0.05, P < 0.05) and sodium nitroprusside (pEC50: 8.34 ± 0.05 vs. 7.77 ± 0.04, P < 0.05). There were no group differences in 8-bromoguanosine cGMP-induced relaxation and protein kinase G1 expression (P > 0.05). GK MA had attenuated responses to BAY 41–2272 (heme-dependent sGC stimulator; pEC50: 7.56 ± 0.05 vs. 6.93 ± 0.06, P < 0.05) and BAY 58–2667 (heme-independent sGC activator; pEC50: 10.82 ± 0.07 vs. 10.27 ± 0.08, P < 0.05) and increased sensitivity to sildenafil [phosphodiesterase 5 (PDE5) inhibitor; pEC50: 7.89 ± 0.14 vs. 8.25 ± 0.13, P < 0.05]. Isolated resistance arteries from female rats of reproductive age that spontaneously develop type 2 diabetes have increased sensitivity to PDE5 inhibition and reduced responsiveness to sGC activators and stimulators.
Keywords: vasorelaxation, type 2 diabetes, vascular smooth muscle, soluble guanylyl cycle agonists, sildenafil
macrovascular and microvascular complications are distinctive features of type 2 diabetes and contribute to an increased prevalence of hypertension and risk of cardiovascular disease-associated mortality in diabetic patients (28). Dysfunction of the endothelial layer in the vascular wall contributes to diabetic vascular complications (12, 15); however, emerging evidence suggests that the smooth muscle is also functionally impaired in diabetic vessels and may be a novel therapeutic target of diabetes-associated vascular disease (21, 26).
Diabetic patients and rodents with experimental diabetes have reduced responsiveness of vascular smooth muscle to nitric oxide (NO) (11, 20, 39, 41), suggesting that the signaling pathway downstream of NO is impaired. Soluble guanylyl cyclase (sGC) is the intracellular receptor of NO (4). Upon its release in endothelial cells, NO reaches the vascular smooth muscle cells by diffusion and binds to sGC, leading to accumulation of intracellular cyclic guanosine monophosphate (cGMP) levels (22). Increased levels of cGMP mainly activate cGMP-dependent protein kinase (PKG) and lead to vasodilation resulting from various events, including reduction of intracellular Ca2+ levels and membrane hyperpolarization (17, 22, 40). To terminate the NO-sGC-cGMP signaling, intracellular cGMP is hydrolyzed by phosphodiesterase 5 (PDE5) (24, 29).
The NO-sGC-cGMP pathway is impaired in diabetes, contributing to reduced endothelium-dependent and -independent relaxation. Various components of this pathway have been used as pharmacological targets to promote vasodilation in disease states (i.e., organic nitrates, NO donors, and PDE5 inhibitors). Most recently, efforts have focused on the development of pharmacological compounds that can directly activate sGC (35). These compounds are divided into two categories, sGC stimulators and activators (23). sGC stimulators enhance the sensitivity of sGC to NO by stabilizing the nitrosyl-heme complex or by increasing sGC activity in the absence of NO (32, 35). The actions of sGC stimulators depend on the presence of a reduced (ferrous) prosthetic heme. Therefore, they are not effective when heme is oxidized or lost, such as in disease states of oxidative stress. On the contrary, sGC activators preferentially activate sGC when the enzyme is at the oxidized or heme-free state (36). Accordingly, selective targeting of oxidized heme with sGC activators has been proposed as an effective pharmacological tool to promote vasodilation in diseased vessels (37). However, the impact of sGC stimulators and activators in vascular dysfunction in type 2 diabetes is unclear.
Most of the studies in the area of diabetes-associated vascular dysfunction have been conducted in older individuals with long-lasting diabetes (37). Studies in experimental diabetes have used animals with extreme and uncontrolled glycemia, which more accurately represent the human condition in its later stage or under no medication. In addition, although it is known that the burden of cardiovascular disease is greater in women with diabetes compared with diabetic men (14), previous studies have primarily included diabetic men or male animal models of diabetes. In the present study, we investigated the function of sGC and its downstream signaling in resistance mesenteric arteries from young female rats with spontaneous type 2 diabetes. The Goto-Kakizaki rat was produced by selective inbreeding of glucose-intolerant Wistar rats (10). Goto-Kakizaki rats present with mild hyperglycemia, glucose intolerance, vascular dysfunction, and high blood pressure and are not obese, allowing the investigation of molecular mechanisms independent of the confounding effects of obesity (10, 11, 27, 30). In this study, we hypothesized that resistance arteries from Goto-Kakizaki rats would have reduced vasodilatory responses to NO-independent/heme-dependent sGC activation and increased responses to NO- and heme-independent sGC activation.
MATERIALS AND METHODS
Animals and experimental design.
Female Goto-Kakizaki rats (in-house bred, derived from the Tampa colony) and age-matched Wistar rats (Charles River Laboratories International, Wilmington, MA) were housed in a temperature- and humidity-controlled environment under 12-h:12-h light/dark cycles and fed with standard laboratory rodent chow and water. Rats were studied at 20–22 wk of age at the diestrus stage of their estrus cycle determined by microscopic examination of cellular changes in vaginal smears (11). All animal care and experimental procedures were performed in accordance with the Guiding Principles in the Care and Use of Animals and were approved by the Georgia Regents University Committee on the Use of Animals in Research and Education.
Blood glucose and blood pressure measurements.
Whole blood glucose was determined from tail blood samples (FreeStyle Lite, Alemeda, CA) before vascular reactivity studies. Rats were anesthetized with isoflurane via a nose cone for surgical procedures (initially with 5% and then maintained at 2.0% in 100% oxygen). A P-10 catheter was inserted into the right femoral artery, and blood pressure was recorded via an attached pressure transducer. After a stable recording of blood pressure for 10 min, rats were killed by removal of their hearts.
Tissue preparation.
Following death, the mesenteric arcade was quickly removed and placed in ice-cold physiological salt solution (PSS) of the following composition (in mM): 130 NaCl, 4.7 KCl, 14.9 NaHCO3, 5.5 dextrose, 1.18 KH2PO4, 1.17 MgSO4, 1.6 CaCl2, and 0.026 EDTA. Second- and third-order mesenteric arteries (internal diameter <200 μm) were isolated by dissection of fat and connective tissue and cut into 2-mm rings. In all experiments, arteries with intact endothelium were used.
Isometric tension recording.
Arteries were suspended in a multiwire myograph system (Danish Myo Technology, Aarhus, Denmark) for in vitro monitoring of smooth muscle function. Each arterial segment was mounted on a tissue bath using two stainless wires (40 μm each). Each tissue chamber was filled with 5 ml PSS and aerated with 95% O2-5% CO2 at 37°C. Arteries were allowed to equilibrate for ∼45 min before they were stretched to an optimal tension [1.8 mN; determined in preliminary experiments (data not shown)] for an additional 45 min. They were then exposed twice to 120 mM KCl to assess arterial integrity and obtain a reference contraction. Vascular responses to acetylcholine (ACh, 3 × 10−6 M) following incubation with phenylephrine (PE, 3 × 10−6 M) were recorded to assess endothelial integrity. Endothelium-dependent relaxation was assessed by performing concentration-response curves to ACh (10−10–10−6 M) in the presence or absence of an NO synthase inhibitor (NG-nitro-l-arginine, l-NNA, 10−4 M). Endothelium-independent relaxation was examined by conducting concentration-response curves to 1) an NO donor, sodium nitroprusside (SNP, 10−11–10−6 M), in the presence or absence of 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ) (sGC inhibitor, 10−6 M); 2) a cGMP analog, 8-bromoguanosine cGMP (8-Br-cGMP, 10−8–10−3 M); 3) an NO-independent, heme-dependent sGC stimulator, 5-cyclopropyl-2-[1-[(2-fluorophenyl)methyl]-1H-pyrazolo[3,4-b]pyridin-3-yl]-4-pyrimidinamine (BAY 41–2272, 10−10–10−6 M), in the presence and absence of ODQ (10−6 M); and 4) an NO- and heme-independent sGC activator, 4-((4-carboxybutyl)(2-((4-phenethylbenzol)-oxy)phenethyl)amino)methyl(benzoic) acid (BAY 58–2667, 10−13–10−7 M), in the presence or absence of ODQ (10−6 M). Concentration-response curves to a PDE5 inhibitor, sildenafil (10−9-10−6 M), were also performed. All concentration-response curves to various vasodilators were performed following constriction to PE that corresponded to 60% of maximum contractile response to KCl (120 mM). Arteries were incubated with vehicle (distilled water) and inhibitors for 30 min before the concentration-response curves.
Protein extraction and Western blotting.
Mesenteric arteries were dissected, snap frozen in liquid nitrogen, and stored at −80°C. Tissues were homogenized in ice-cold lysis buffer containing T-Per tissue protein extraction solution (Thermo Scientific, Rockford, IL), 100 mM sodium orthovanadate (Na3VO4), 100 mM PMSF, and 1% proteinase inhibitor cocktail (Sigma, St. Louis, MO). Homogenates were centrifuged at 10,000 g for 15 min at 4°C, the supernatant was collected, and the proteins were solubilized in Laemmli buffer containing mercaptoethanol. Protein concentration was measured by bicinchoninic acid assay (Thermo Scientific). Protein (10–30 μg protein/lane) was loaded onto 10% SDS-PAGE gels and then transferred to nitrocellulose membranes. Membranes were blocked in Tris-buffered saline-Tween 20 with 5% skim dry milk and subsequently probed with rabbit anti-sGC-α1 (77–82 kDa, 1:1,000), rabbit anti-sGC-β1 (70 kDa; 1:1,000), rabbit anti-PDE5A (105 kDa; 1:500), rabbit anti-PKG-1 (78 kDa; 1:1,000), and mouse anti-β-actin (42 kDa; 1:15,000) overnight at 4°C. The immunostaining was detected using horseradish peroxidase-conjugated anti-rabbit immunoglobulin G (IgG) (GE Healthcare, Little Chalfont, Buckinghamshire, UK) or anti-mouse IgG (GE Healthcare) for 1 h at room temperature. Results were normalized by β-actin expression. Immunoreactive bands were revealed by an enhanced chemiluminescence detection system and quantified using UN-SCAN-IT gel analysis software (v. 6.1; Silk Scientific, Orem, UT).
Drugs.
PE, ACh, SNP, ODQ, sildenafil citrate salt, l-NNA, 8-Br-cGMP, and antibody against β-actin were obtained from Sigma Chemical. Antibodies against sGC-α1 and -β1 subunits and BAY 41–2272 were purchased from Cayman Chemical (Ann Arbor, MI). Antibody against PDE5A was obtained from Abcam (Cambridge, MA). Antibody against PKG-1 was purchased from Cell Signaling (Beverly, MA). BAY 58–2667.HCl was obtained from AdipoGen (San Diego, CA). Stock solutions were prepared in distilled water.
Data analysis.
Sigmoidal curve fitting was performed on concentration-response curve data using GraphPad Prism software (v. 6.0; GraphPad Software, San Diego, CA). From this analysis, the maximal effect generated by the agonist (maximum vasodilatation) and the molar concentration of agonist producing 50% of the maximum response (EC50) were determined and presented as Emax and pEC50 (negative logarithm to base 10 of the EC50), respectively. Emax was expressed relative to the maximal changes from the contraction produced by PE in each segment, which was determined as 0% relaxation. The baseline tension before addition of PE was considered 100% relaxation.
Statistical analysis.
Values are presented as means ± SE, and n represents the number of animals used in the experiments. Group differences (Wistar vs. Goto-Kakizaki) in Emax, pEC50, and protein expression were determined using Student's t-tests for unpaired observations. Repeated-measures two-way ANOVA [factor 1: group (Goto-Kakizaki vs. Wistar) or drug (drug alone vs. drug plus inhibitor), factor 2: drug concentrations] followed by Bonferroni's or Tukey's post hoc tests was used to make comparisons in concentration-response curves. Two-way ANOVA [factor 1: group (Wistar vs. Goto-Kakizaki), factor 2: drug (drug alone vs. drug plus inhibitor)] followed by Bonferroni's post hoc test was used to determine group differences in pEC50. GraphPad Prism (v. 6.0; GraphPad Software) was used for all statistical analyses. The significance level of all tests was set at α = 0.05.
RESULTS
Increased arterial blood pressure in Goto-Kakizaki rats.
Goto-Kakizaki rats weighed less compared with age-matched Wistar controls (246.9 ± 6.0 g vs. 281.8 ± 5.0 g, P < 0.05) and had greater blood glucose levels (133.3 ± 6.8 mg/dl vs. 95.4 ± 2.6 mg/dl, P < 0.05) and higher mean arterial pressure (112 ± 3 mmHg vs. 88 ± 1 mmHg, P < 0.05).
Reduced NO-dependent relaxation in mesenteric arteries from female Goto-Kakizaki rats.
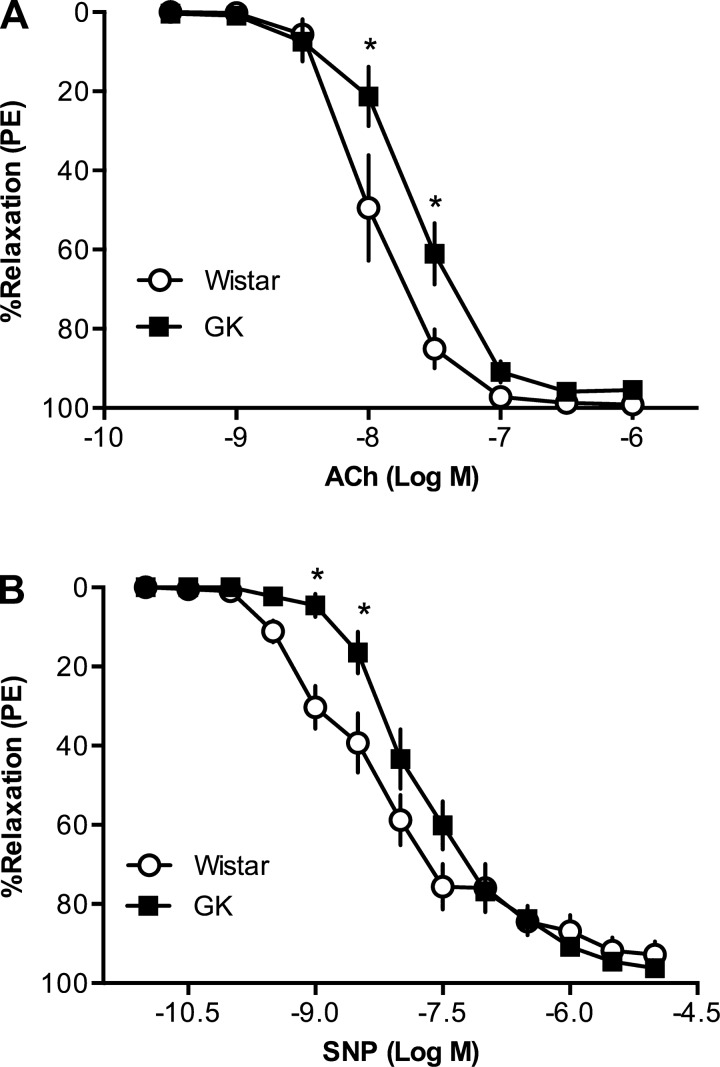
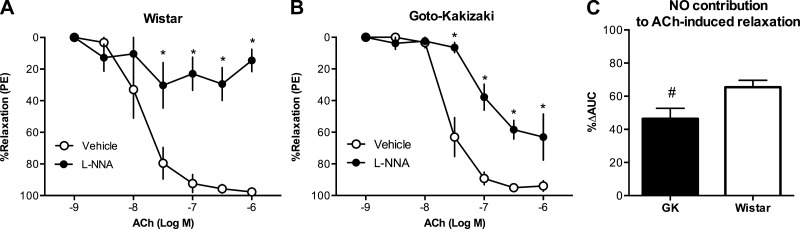
Endothelium-intact mesenteric arteries from Goto-Kakizaki rats had reduced relaxation responses to ACh [pEC50: Wistar (n = 7): 7.96 ± 0.06 vs. Goto-Kakizaki (n = 13): 7.66 ± 0.05, P < 0.05] and SNP [pEC50: Wistar (n = 12): 8.34 ± 0.05 vs. Goto-Kakizaki (n = 14): 7.77 ± 0.04, P < 0.05] compared with arteries from Wistar rats (Fig. 1, A and B). NOS inhibition reduced relaxation responses to ACh (Fig. 2, A and B), and this effect was smaller in arteries from Goto-Kakizaki compared with Wistar rats [Emax (%relaxation to PE): Wistar (n = 6), vehicle: 96.2 ± 1.8, l-NNA: 46.6 ± 8.0 vs. Goto-Kakizaki (n = 8), vehicle: 93.9 ± 3.3, l-NNA: 71.0 ± 9.2; P < 0.05]. We calculated the magnitude of reduction in ACh-induced relaxation in the presence of l-NNA and used it as an index of NO contribution to ACh-induced relaxation (34). The relative contribution of NO to ACh-induced relaxation was diminished in mesenteric arteries from Goto-Kakizaki rats compared with controls [%Δarea under the curve, Wistar (n = 6): 65.5 ± 4.2 vs. Goto-Kakizaki (n = 8): 46.4 ± 6.4, P < 0.05; Fig. 2C]. sGC inhibition by ODQ abolished relaxation responses to SNP in both groups (data not shown).
Fig. 1.
Concentration-response curves to acetylcholine (ACh) and sodium nitroprusside (SNP) in resistance mesenteric arteries from female Goto-Kakizaki (GK) and Wistar rats. A: arteries from GK type 2 diabetic rats (n = 13) had reduced responses to ACh compared with arteries from Wistar rats (n = 7) (*P < 0.05, Wistar vs. GK). B: arteries from GK type 2 diabetic rats (n = 14) had reduced responses to SNP compared with arteries from Wistar rats (n = 12) (*P < 0.05, Wistar vs. GK).
Fig. 2.
Nitric oxide (NO) contribution to ACh-induced relaxation in mesenteric arteries from female Wistar and GK rats. Inhibition of NO synthase with NG-nitro-l-arginine (l-NNA) (10−4 M) reduced relaxation responses to ACh in mesenteric arteries from Wistar (n = 6) (A) and GK (n = 8) rats (B) (*P < 0.05, ACh plus vehicle vs. ACh plus l-NNA). PE, phenylephrine. C: relative contribution of NO to ACh-induced relaxation was reduced in mesenteric arteries from GK rats [#P < 0.05, Wistar (n = 6) vs. GK (n = 8)]. AUC, area under the curve.
Increased expression of sGC but reduced relaxation responses to sGC activation in small mesenteric arteries from Goto-Kakizaki rats.
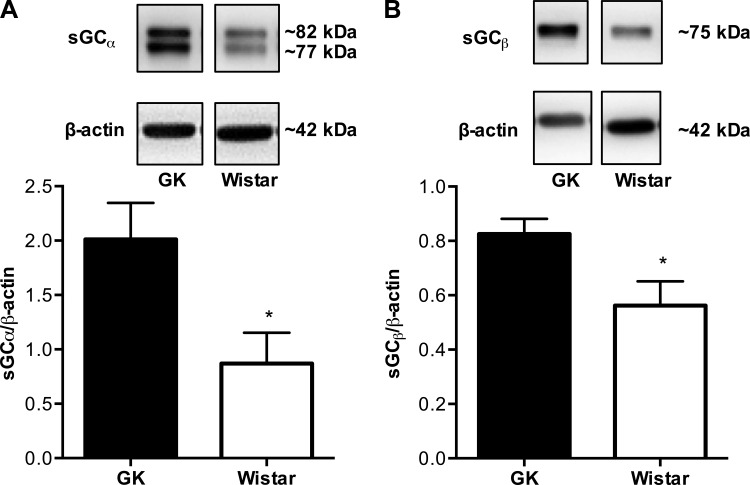
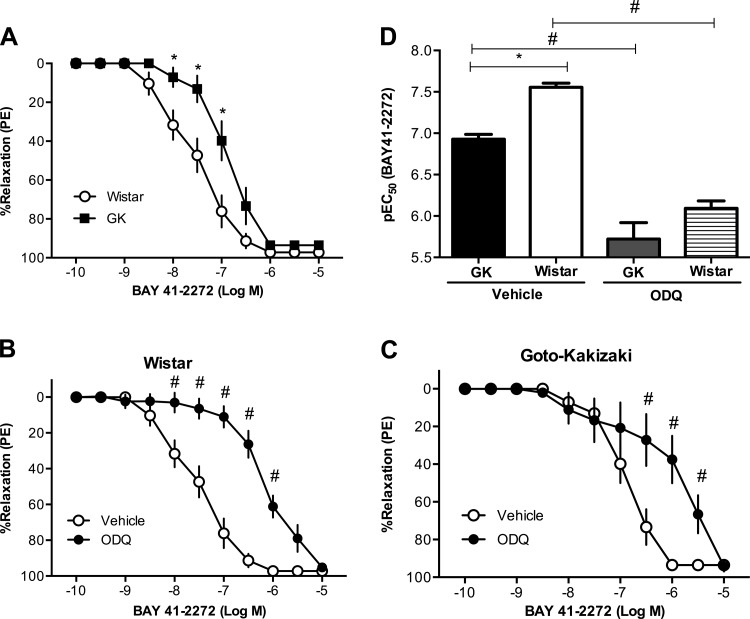
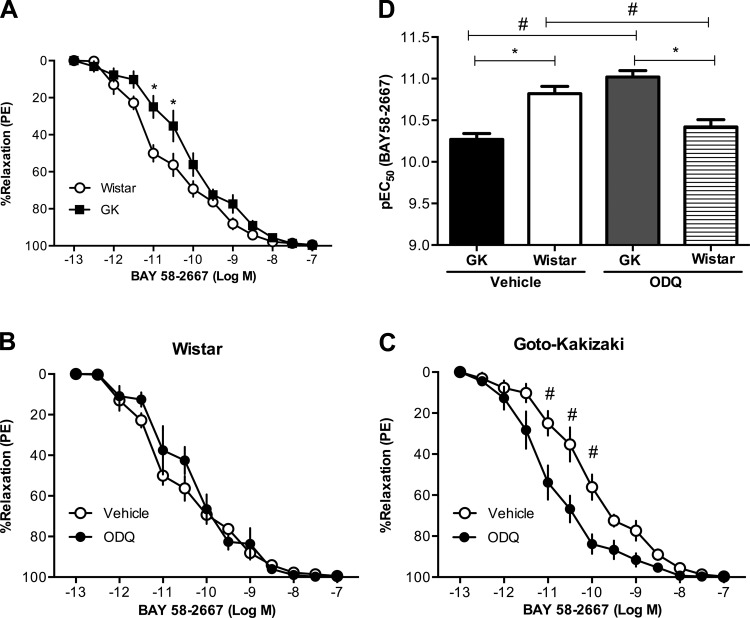
Mesenteric arteries from Goto-Kakizaki rats (n = 5) had increased expression of sGC (both subunits α and β) compared with arteries from Wistar rats (n = 5) (Fig. 3, A and B). BAY 41–2272 induced relaxation responses in a concentration-response manner in arteries from Goto-Kakizaki and Wistar rats, but these responses were reduced in Goto-Kakizaki arteries [pEC50, Wistar (n = 8): 7.56 ± 0.05 vs. Goto-Kakizaki (n = 10): 6.93 ± 0.06, P < 0.05; Fig. 4, A and D]. Treatment with ODQ, an inhibitor of sGC, caused a rightward shift of the concentration-response curve to BAY 41–2272 in both groups (n = 6/group; Fig. 4, B and C) and abolished the group differences (Fig. 4D). BAY 58–2667 induced relaxation responses in arteries from both groups, but these responses were diminished in arteries from Goto-Kakizaki rats [pEC50, Wistar (n = 7): 10.82 ± 0.07 vs. Goto-Kakizaki (n = 9): 10.27 ± 0.06, P < 0.05; Fig. 5, A and D]. ODQ improved mesenteric artery relaxation responses to BAY 58–2667 in Goto-Kakizaki but had no effect on BAY 58-2667-induced relaxation responses in arteries from Wistar rats (n = 6/group; Fig. 5, B–D).
Fig. 3.
Protein expression levels of soluble guanylyl cyclase-α (sGCα) (A) and sGCβ (B) in mesenteric resistance arteries from female Wistar and GK rats. Protein levels of sGCα and sGCβ were increased in mesenteric resistance arteries from GK (n = 5) compared with Wistar (n = 5) rats. Values are means ± SE. *P < 0.05, Wistar vs. GK.
Fig. 4.
Relaxation responses to BAY 41–2272 (an sGC stimulator) in mesenteric resistance arteries from Wistar and GK rats. A: mesenteric resistance arteries from female GK rats (n = 10) had reduced relaxation responses to BAY 41–2272 compared with arteries from Wistar rats (n = 8) (*P < 0.05, Wistar vs. GK). Inhibition of sGC by 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ, 10−6 M) caused a rightward shift in the concentration response curves in arteries from Wistar (B) and GK (C) rats (#P < 0.05, BAY 41–2272 plus vehicle vs. BAY 41–2272 plus ODQ; n = 6 rats/group). D: BAY 41–2272 pEC50 was reduced in arteries from GK rats, and inhibition of sGC by ODQ abolished these group differences (*P < 0.05, Wistar vs. GK; #P < 0.05, BAY 41–2272 plus vehicle vs. BAY 41–2272 plus ODQ; n = 6 rats/group).
Fig. 5.
Relaxation responses to BAY 58–2667 (an sGC activator) in mesenteric resistance arteries from Wistar and GK rats. A: mesenteric resistance arteries from female GK rats (n = 9) had reduced relaxation responses to BAY 58–2667 compared with arteries from Wistar rats (n = 7) (*P < 0.05, Wistar vs. GK). Inhibition of sGC by ODQ (10−6 M) caused a rightward shift in the BAY 58–2667 concentration-response curves in arteries from Wistar (n = 6) rats (B) and a leftward shift in arteries from GK rats (n = 6) (C) (#P < 0.05, BAY 58–2667 plus vehicle vs. BAY 58–2667 plus ODQ). D: BAY 58–2667 pEC50 was reduced in control arteries (vehicle treated) from GK rats (n = 9) compared with vehicle-treated Wistar arteries (n = 7). Inhibition of sGC by ODQ had differential effects on GK (n = 6) and Wistar (n = 6) arteries such as that ODQ treatment increased pEC50 in GK arteries and increased pEC50 in Wistar arteries (*P < 0.05, Wistar vs. GK; #P < 0.05, BAY 58–2667 plus vehicle vs. BAY 58–2667 plus ODQ).
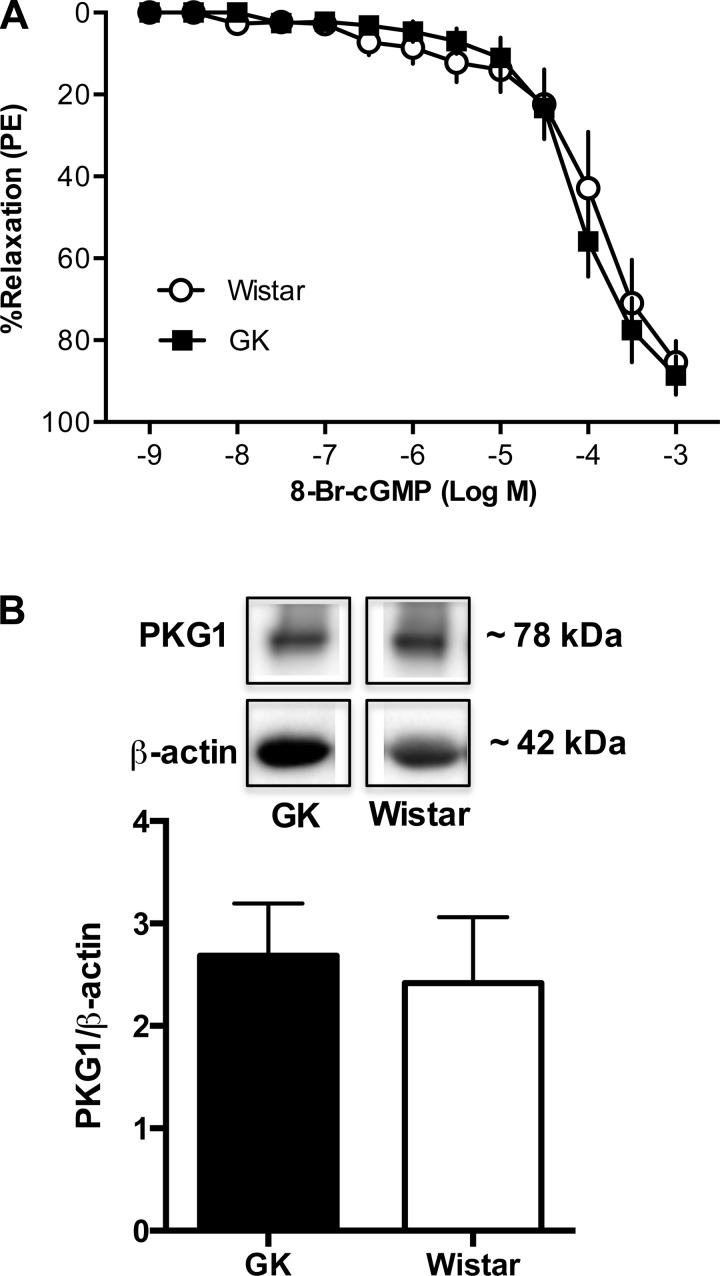
No differences in relaxation responses to cGMP and PKG activation between mesenteric arteries from Goto-Kakizaki and Wistar rats.
To assess the integrity of the sGC-cGMP-PKG signaling downstream of sGC, we performed concentration-response curves to a mimetic of cGMP, 8-Br-cGMP, and measured expression of PKG (PKG-1, including both α and β isoforms). 8-Br-cGMP induced relaxation responses in a concentration-response manner, but there were no differences between groups (Wistar, n = 7; Goto-Kakizaki, n = 11; Fig. 6A). Furthermore, there were no group differences in PKG expression (n = 6/group; Fig. 6B).
Fig. 6.
Concentration-response curves to a cyclic guanosine monophosphate (cGMP) mimetic and protein levels of protein kinase G (PKG) in mesenteric arteries from female Wistar and GK rats. A: there were no differences between mesenteric arteries from GK (n = 7) and Wistar rats (n = 11) in 8-bromoguanosine cGMP (8-Br-cGMP) concentration-response curves (P > 0.05, Wistar vs. GK). B: protein levels of PKG were not different between Wistar and GK mesenteric arteries (P > 0.05, Wistar vs. GK; n = 6/group).
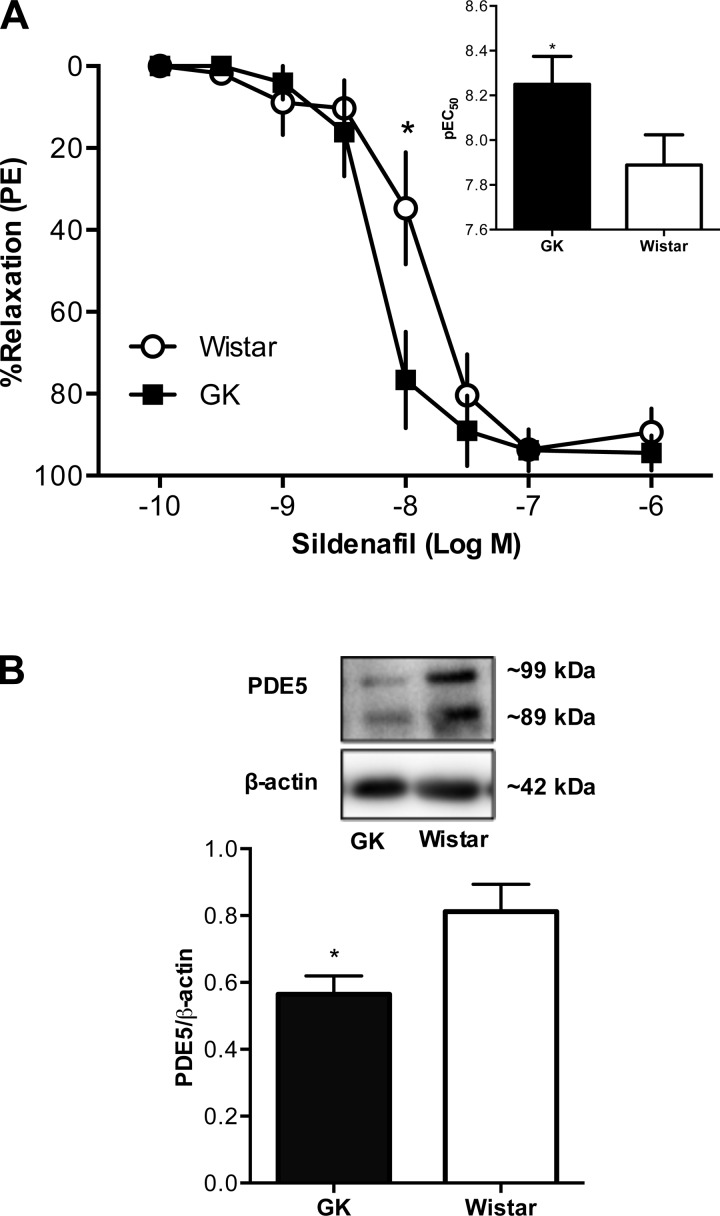
Increased relaxation responses to PDE5 inhibition in mesenteric arteries from Goto-Kakizaki rats.
Sildenafil, a potent and selective inhibitor of PDE5, caused concentration-dependent relaxation in mesenteric arteries (Fig. 7A). Arteries from Goto-Kakizaki rats had increased sensitivity to sildenafil [pEC50, Wistar (n = 6): 7.89 ± 0.14 vs. Goto-Kakizaki (n = 8): 8.25 ± 0.13, P < 0.05] but reduced expression of PDE5 compared with Wistar controls (n = 6/group; Fig. 7B).
Fig. 7.
Concentration-response curves to sildenafil [a phosphodiesterase 5 (PDE5) inhibitor] and protein levels of PDE5A in mesenteric resistance arteries from female Wistar and GK rats. A: mesenteric arteries from GK rats (n = 8) had greater relaxation responses to sildenafil compared with arteries from Wistar rats (n = 6). Inset: group comparisons for sildenafil pEC50. (*P < 0.05, Wistar vs. GK). B: protein levels of PDE5 were lower in mesenteric arteries from GK rats (*P < 0.05, Wistar vs. GK; n = 6/group).
DISCUSSION
The main finding of this study was that resistance mesenteric arteries from female rats with type 2 diabetes had reduced responses to NO-dependent and -independent activation of sGC but greater sensitivity to inhibition of PDE5 compared with nondiabetic rats.
There is a strong association between hypertension and diabetes, with hypertension affecting >50% of diabetic patients and contributing to their increased risk for cardiovascular disease (16). Women with type 2 diabetes have greater risk for cardiovascular-related mortality (13), but the mechanisms underlying this risk are not clear. In this study, we used the Goto-Kakizaki rat, a genetic model of type 2 diabetes with mild hyperglycemia, elevated blood pressure, and vascular dysfunction (11, 30). Resistance mesenteric arteries from young female Goto-Kakizaki rats had reduced responses to ACh, an indication of endothelial dysfunction. Our results are in agreement with previously published data in patients and other animal models with diabetes (15, 38) and support the overall consensus that endothelial dysfunction is a hallmark of diabetic vasculopathy. In previous studies, male Goto-Kakizaki rats of the same age but with greater blood glucose levels did not show reduced mesenteric artery responses to ACh unless they were fed with a high-fat diet (30), indicating potential sex differences in the vascular phenotype of this animal model. Recently, we reported that, in addition to endothelial dysfunction, uterine arteries from Goto-Kakizaki rats had impaired vascular smooth muscle relaxation responses (11). In the present study, resistance arteries from a nonreproductive vascular bed showed a similar defect, suggesting that dysfunction of the smooth muscle layer is a feature of vascular dysfunction in the Goto-Kakizaki animal model of type 2 diabetes. This finding and our previous studies are in support of earlier reports in conduit arteries from male Goto-Kakizaki rats (42) and studies in humans showing reduced endothelium-independent relaxation in patients with type 2 diabetes (20, 39, 41).
The protein expression of sGC, the intracellular receptor of NO, was increased (both α1 and β1 subunits) in resistance vessels of Goto-Kakizaki rats. Furthermore, the contribution of NO to endothelium-dependent relaxation was attenuated in resistance arteries from diabetic rats. Thus the increased sGC expression may be a compensatory mechanism to counteract the reduced vasodilatory stimulus. In previous studies, basal levels of sGC protein did not differ between Goto-Kakizaki and Wistar rats in uterine arteries (11) and aortae (42), suggesting that the adaptations of the NO-sGC-cGMP pathway are vascular bed specific. These adaptations may also be modulated by sex and age, as the studies in aortae of Goto-Kakizaki rats were conducted in older male animals (42). The use of a cGMP analog elicited similar relaxation responses in Wistar and Goto-Kakizaki rats. In addition, the protein levels of PKG did not differ in mesenteric arteries from diabetic rats compared with control animals. Collectively, these data suggest that the reduced relaxation responses seen in the present study are attributed to defects at the level of sGC and beyond, including reduced production/bioavailability of NO and reduced sensitivity of sGC.
We used various agonists to activate sGC and induce smooth muscle relaxation and found that the arteries from the diabetic group had reduced dilatory responses regardless of the agonist used (i.e., NO donor vs. heme-dependent vs. heme-independent sGC activation). On the basis of previous findings demonstrating that sGC activators (i.e., BAY 58–2667) preferentially activate sGC when the enzyme is in the oxidized state (36) and evidence showing that vascular damage in diabetes is often due to hyperglycemia-induced generation of reactive oxygen species (5), we had initially speculated that mesenteric arteries from female diabetic rats would have reduced vascular relaxation responses to a sGC stimulator but enhanced responses to a sGC activator. This hypothesis was further supported by previous reports demonstrating that male rats with type 2 diabetes had preserved responses to the heme-independent activator protoporphyrin-IX (42) and that isolated vessels from patients with type 2 diabetes had enhanced relaxation responses to BAY 58–2667 (37). In contrast to our hypothesis and these previous findings, we found reduced responses to BAY 58–2667 in female Goto-Kakizaki compared with Wistar rats. It is noteworthy, however, that the study by Witte et al. (42) examined the function of sGC in aorta, a conduit artery, compared with our investigation in mesenteric resistance arteries, and they used older male animals. Furthermore, studies in isolated vessels from diabetic patients were performed in older (50–75 yr) adults, and their sex and medication regimens, which could be potential confounding factors, were not reported (37).
Vascular reactivity studies in animal models of diabetes have revealed contradictory results with variable responses to vasoconstrictors and vasodilators (1). The duration of diabetes plays an important role in this variability because previous reports showed enhanced responses to ACh 1 day after the induction of diabetes, unchanged responses at 1–2 wk, and reduced responses at 8 wk after diabetes induction (25). Similar trends were seen in vasoconstrictor responses (43). Time-dependent alterations of endothelial function have been previously studied in animal models of diabetes (1, 25), but the temporal changes in vascular oxidative stress and smooth muscle dilatory responses have not been extensively investigated. Interestingly, the presence of ODQ, which inhibits sGC by oxidation of the ferrous iron in the heme cofactor (7), potentiated the responses to BAY 58–2667 in diabetic but not in control arteries. These data suggest that vascular sGC from female Goto-Kakizaki rats may be more susceptible to oxidation. Therefore, in the presence of the same amount of ODQ, the levels of heme oxidation may be greater in the diabetic rats, favoring greater responses to BAY 58–2667. An alternative interpretation of these results is that ODQ potentiates the vasodilatory effects of BAY 58–2667, and thus a pharmacological approach combining the two compounds may promote better dilatory responses in arteries from animals with mild hyperglycemia.
Resistance mesenteric arteries from Goto-Kakizaki rats had reduced responses to sGC agonists but increased sensitivity to sildenafil, a PDE5 inhibitor. PDE5 is a metallo hydrolase that catalyzes the breakdown of cGMP into the inactive 5′-GMP (18). The binding of cGMP to GAF allosteric binding site in PDE5 promotes PKG-mediated phosphorylation and activation of the enzyme (6, 19). PDE5 is highly expressed in vascular cells, and its physiological importance in regulating vascular tone has been highlighted by the use of its specific inhibitors (i.e., sildenafil), which promote vasodilation (18). Currently, PDE inhibitors are approved by the US Food and Drug Administration for the treatment of erectile dysfunction, pulmonary hypertension, and benign prostatic hyperplasia. Studies support the beneficial effects of sildenafil in diabetes, as they have shown that chronic treatment with PDE inhibitors, including sildenafil, improves cardiomyopathy in diabetic patients (9). A recent meta-analysis, however, pointed out the lack of data in sex differences in the efficacy of sildenafil in cardiovascular disease (8). Denardo et al. (3) reported that PDE5 inhibition acutely improves microvascular dysfunction in women with symptoms and signs of cardiac ischemia. On the other hand, studies in female mice have shown that the cardioprotective effects of chronic treatment with sildenafil were dependent on the presence of estrogen, as sildenafil failed to induce antiremodeling effects after ovariectomy, whereas estrogen replacement restored the beneficial effects of sildenafil (31). Others have demonstrated that estrogen withdrawal is a risk factor for flushing induced by PDE5 inhibition (33). Of note, we have previously reported that estradiol levels are greater in Goto-Kakizaki rats compared with Wistar rats (2). Together, this evidence and our results suggest that PDE5 inhibition may have beneficial effects on cardiovascular function of women with type 2 diabetes during their reproductive age, when estrogen is at normal levels. Whether hormonal variations in premenopausal diabetic women contribute to greater sensitivity to PDE5 inhibition is unknown.
In this study, we used lean rats with type 2 diabetes. This approach increased the internal validity of our experimental design because it allowed the investigation of vasodilatory mechanisms in the absence of the confounding effects of obesity. In the human population, however, type 2 diabetes is often accompanied by an obese phenotype. Thus future studies should examine the contribution of increased adiposity vs. hyperglycemia and insulin resistance on the vascular phenotype seen in the present study. Another limitation of our study is the lack of direct evidence of reduced ability of the diabetic vessels to produce cGMP. Because of the small size of resistance mesenteric vessels, we were unable to measure production of cGMP in response to sGC activation. Previous reports, however, showed reduced levels of cGMP in response to sGC activation in aortae of female GK rats.
The effects of sGC agonists have been primarily studied in males with established diabetes of several years. The recommendation for clinical use of sGC activators and stimulators also relies on studies primarily conducted in men with diabetes or male animal models of diabetes. The rats used in the present study were young, female, and had mild hyperglycemia, representing a female patient at the initial stages of diabetes. The effects of sex on the efficacy of sGC agonists should be addressed before they are used in clinical practice. Previous studies in experimental diabetes have shown that the vascular adaptations to diabetes differ at the early compared with established stages (1, 25). Thus the duration of diabetes and other factors such as levels of glycemia and status of oxidative stress should also been considered. Here, we report, for first time, increased sensitivity of diabetic mesenteric arteries to sildenafil, a PDE5 inhibitor. Similar results have been previously reported in uterine arteries from diabetic rats (11). Future studies should address the hypothesis that PDE5 inhibition may be preferable over sGC agonists in female subjects at the beginning stages of type 2 diabetes.
GRANTS
This study was supported in part by the National Institutes of Health (Grants: R01HL071138, R01DK083685, and R01NS083559), the Society for Women's Health Research, and the American Heart Association (13SDG17050056).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: S.G., J.L.H., T.M., and R.C.W. conception and design of research; S.G., J.L.H., and S.O. performed experiments; S.G. and S.O. analyzed data; S.G., J.L.H., T.M., A.E., and R.C.W. interpreted results of experiments; S.G. prepared figures; S.G. drafted manuscript; S.G., J.L.H., T.M., S.O., A.E., and R.C.W. edited and revised manuscript; S.G., J.L.H., T.M., S.O., A.E., and R.C.W. approved final version of manuscript.
ACKNOWLEDGMENTS
Present address for S. Goulopoulou: Department of Integrative Physiology and Anatomy, University of North Texas Health Science Center, 3500 Camp Bowie Boulevard, Fort Worth, TX.
REFERENCES
- 1.Abboud K, Bassila JC, Ghali-Ghoul R, Sabra R. Temporal changes in vascular reactivity in early diabetes mellitus in rats: role of changes in endothelial factors and in phosphodiesterase activity. Am J Physiol Heart Circ Physiol 297: H836–H845, 2009. [DOI] [PubMed] [Google Scholar]
- 2.Allahdadi KJ, Hannan JL, Ergul A, Tostes RC, Webb RC. Internal pudendal artery from type 2 diabetic female rats demonstrate elevated endothelin-1-mediated constriction. J Sex Med 8: 2472–2483, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Denardo SJ, Wen X, Handberg EM, Bairey Merz CN, Sopko GS, Cooper-Dehoff RM, Pepine CJ. Effect of phosphodiesterase type 5 inhibition on microvascular coronary dysfunction in women: a Women's Ischemia Syndrome Evaluation (WISE) ancillary study. Clin Cardiol 34: 483–487, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Derbyshire ER, Marletta MA. Structure and regulation of soluble guanylate cyclase. Annu Rev Biochem 81: 533–559, 2012. [DOI] [PubMed] [Google Scholar]
- 5.Fiorentino TV, Prioletta A, Zuo P, Folli F. Hyperglycemia-induced oxidative stress and its role in diabetes mellitus related cardiovascular diseases. Curr Pharm Des 19: 5695–5703, 2013. [DOI] [PubMed] [Google Scholar]
- 6.Francis SH, Blount MA, Corbin JD. Mammalian cyclic nucleotide phosphodiesterases: molecular mechanisms and physiological functions. Physiol Rev 91: 651–690, 2011. [DOI] [PubMed] [Google Scholar]
- 7.Garthwaite J, Southam E, Boulton CL, Nielsen EB, Schmidt K, Mayer B. Potent and selective inhibition of nitric oxide-sensitive guanylyl cyclase by 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one. Mol Pharmacol 48: 184–188, 1995. [PubMed] [Google Scholar]
- 8.Giannetta E, Feola T, Gianfrilli D, Pofi R, Dall'Armi V, Badagliacca R, Barbagallo F, Lenzi A, Isidori AM. Is chronic inhibition of phosphodiesterase type 5 cardioprotective and safe? A meta-analysis of randomized controlled trials. BMC Med 12: 185, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giannetta E, Isidori AM, Galea N, Carbone I, Mandosi E, Vizza CD, Naro F, Morano S, Fedele F, Lenzi A. Chronic inhibition of cGMP phosphodiesterase 5A improves diabetic cardiomyopathy: a randomized, controlled clinical trial using magnetic resonance imaging with myocardial tagging. Circulation 125: 2323–2333, 2012. [DOI] [PubMed] [Google Scholar]
- 10.Goto Y, Kakizaki M, Masaki N. Production of spontaneous diabetic rats by repetition of selective breeding. Tohoku J Exp Med 119: 85–90, 1976. [DOI] [PubMed] [Google Scholar]
- 11.Goulopoulou S, Hannan JL, Matsumoto T, Ergul A, Webb RC. Augmented dilation to nitric oxide in uterine arteries from rats with type 2 diabetes: implications for vascular adaptations to pregnancy. Am J Physiol Heart Circ Physiol 306: H610–H618, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hadi HA, Suwaidi JA. Endothelial dysfunction in diabetes mellitus. Vasc Health Risk Manag 3: 853–876, 2007. [PMC free article] [PubMed] [Google Scholar]
- 13.Hu G. Gender difference in all-cause and cardiovascular mortality related to hyperglycaemia and newly-diagnosed diabetes. Diabetologia 46: 608–617, 2003. [DOI] [PubMed] [Google Scholar]
- 14.Juutilainen A, Kortelainen S, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Gender difference in the impact of type 2 diabetes on coronary heart disease risk. Diabetes Care 27: 2898–2904, 2004. [DOI] [PubMed] [Google Scholar]
- 15.Kim JA, Montagnani M, Koh KK, Quon MJ. Reciprocal relationships between insulin resistance and endothelial dysfunction: molecular and pathophysiological mechanisms. Circulation 113: 1888–1904, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Lastra G, Syed S, Kurukulasuriya LR, Manrique C, Sowers JR. Type 2 diabetes mellitus and hypertension: an update. Endocrinol Metab Clin North Am 43: 103–122, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lincoln TM, Komalavilas P, Cornwell TL. Pleiotropic regulation of vascular smooth muscle tone by cyclic GMP-dependent protein kinase. Hypertension 23: 1141–1147, 1994. [DOI] [PubMed] [Google Scholar]
- 18.Matsumoto T, Kobayashi T, Kamata K. Phosphodiesterases in the vascular system. J Smooth Muscle Res 39: 67–86, 2003. [DOI] [PubMed] [Google Scholar]
- 19.Maurice DH, Ke H, Ahmad F, Wang Y, Chung J, Manganiello VC. Advances in targeting cyclic nucleotide phosphodiesterases. Nat Rev Drug Discov 13: 290–314, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McVeigh GE, Brennan GM, Johnston GD, McDermott BJ, McGrath LT, Henry WR, Andrews JW, Hayes JR. Impaired endothelium-dependent and independent vasodilation in patients with type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia 35: 771–776, 1992. [DOI] [PubMed] [Google Scholar]
- 21.Montero D, Walther G, Perez-Martin A, Vicente-Salar N, Roche E, Vinet A. Vascular smooth muscle function in type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetologia 56: 2122–2133, 2013. [DOI] [PubMed] [Google Scholar]
- 22.Morgado M, Cairrao E, Santos-Silva AJ, Verde I. Cyclic nucleotide-dependent relaxation pathways in vascular smooth muscle. Cell Mol Life Sci 69: 247–266, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nossaman B, Pankey E, Kadowitz P. Stimulators and activators of soluble guanylate cyclase: review and potential therapeutic indications. Crit Care Res Pract 2012: 290805, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Omori K, Kotera J. Overview of PDEs and their regulation. Circ Res 100: 309–327, 2007. [DOI] [PubMed] [Google Scholar]
- 25.Pieper GM. Enhanced, unaltered and impaired nitric oxide-mediated endothelium-dependent relaxation in experimental diabetes mellitus: importance of disease duration. Diabetologia 42: 204–213, 1999. [DOI] [PubMed] [Google Scholar]
- 26.Porter KE, Riches K. The vascular smooth muscle cell: a therapeutic target in Type 2 diabetes? Clin Sci (Lond) 125: 167–182, 2013. [DOI] [PubMed] [Google Scholar]
- 27.Portha B, Lacraz G, Kergoat M, Homo-Delarche F, Giroix MH, Bailbe D, Gangnerau MN, Dolz M, Tourrel-Cuzin C, Movassat J. The GK rat beta-cell: a prototype for the diseased human beta-cell in type 2 diabetes? Mol Cell Endocrinol 297: 73–85, 2009. [DOI] [PubMed] [Google Scholar]
- 28.Rahman S, Rahman T, Ismail AA, Rashid AR. Diabetes-associated macrovasculopathy: pathophysiology and pathogenesis. Diabetes Obes Metab 9: 767–780, 2007. [DOI] [PubMed] [Google Scholar]
- 29.Rybalkin SD, Rybalkina IG, Shimizu-Albergine M, Tang XB, Beavo JA. PDE5 is converted to an activated state upon cGMP binding to the GAF A domain. EMBO J 22: 469–478, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sachidanandam K, Harris A, Hutchinson J, Ergul A. Microvascular vs. macrovascular dysfunction in type 2 diabetes: differences in contractile responses to endothelin-1. Exp Biol Med (Maywood) 231: 1016–1021, 2006. [PubMed] [Google Scholar]
- 31.Sasaki H, Nagayama T, Blanton RM, Seo K, Zhang M, Zhu G, Lee DI, Bedja D, Hsu S, Tsukamoto O, Takashima S, Kitakaze M, Mendelsohn ME, Karas RH, Kass DA, Takimoto E. PDE5 inhibitor efficacy is estrogen dependent in female heart disease. J Clin Invest 124: 2464–2471, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmidt P, Schramm M, Schroder H, Stasch JP. Mechanisms of nitric oxide independent activation of soluble guanylyl cyclase. Eur J Pharmacol 468: 167–174, 2003. [DOI] [PubMed] [Google Scholar]
- 33.Sejima H, Tominaga K, Egawa T, Ikeda M, Shibuya K, Kameyama N, Yamauchi A, Shuto H, Kataoka Y. Gender differences in tail-skin flushing induced by nitrates and phosphodiesterase type 5 inhibitors in a climacteric mouse model. Eur J Pharmacol 624: 66–70, 2009. [DOI] [PubMed] [Google Scholar]
- 34.Stanley JL, Ashton N, Taggart MJ, Davidge ST, Baker PN. Uterine artery function in a mouse model of pregnancy complicated by diabetes. Vascul Pharmacol 50: 8–13, 2009. [DOI] [PubMed] [Google Scholar]
- 35.Stasch JP, Pacher P, Evgenov OV. Soluble guanylate cyclase as an emerging therapeutic target in cardiopulmonary disease. Circulation 123: 2263–2273, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stasch JP, Schmidt P, Alonso-Alija C, Apeler H, Dembowsky K, Haerter M, Heil M, Minuth T, Perzborn E, Pleiss U, Schramm M, Schroeder W, Schroder H, Stahl E, Steinke W, Wunder F. NO- and haem-independent activation of soluble guanylyl cyclase: molecular basis and cardiovascular implications of a new pharmacological principle. Br J Pharmacol 136: 773–783, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stasch JP, Schmidt PM, Nedvetsky PI, Nedvetskaya TY, H S AK, Meurer S, Deile M, Taye A, Knorr A, Lapp H, Muller H, Turgay Y, Rothkegel C, Tersteegen A, Kemp-Harper B, Muller-Esterl W, Schmidt HH. Targeting the heme-oxidized nitric oxide receptor for selective vasodilatation of diseased blood vessels. J Clin Invest 116: 2552–2561, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taguchi K, Matsumoto T, Kamata K, Kobayashi T. Akt/eNOS pathway activation in endothelium-dependent relaxation is preserved in aortas from female, but not from male, type 2 diabetic mice. Pharmacol Res 65: 56–65, 2012. [DOI] [PubMed] [Google Scholar]
- 39.Watts GF, O'Brien SF, Silvester W, Millar JA. Impaired endothelium-dependent and independent dilatation of forearm resistance arteries in men with diet-treated non-insulin-dependent diabetes: role of dyslipidaemia. Clin Sci (Lond) 91: 567–573, 1996. [DOI] [PubMed] [Google Scholar]
- 40.Weber S, Bernhard D, Lukowski R, Weinmeister P, Worner R, Wegener JW, Valtcheva N, Feil S, Schlossmann J, Hofmann F, Feil R. Rescue of cGMP kinase I knockout mice by smooth muscle specific expression of either isozyme. Circ Res 101: 1096–1103, 2007. [DOI] [PubMed] [Google Scholar]
- 41.Williams SB, Cusco JA, Roddy MA, Johnstone MT, Creager MA. Impaired nitric oxide-mediated vasodilation in patients with non-insulin-dependent diabetes mellitus. J Am Coll Cardiol 27: 567–574, 1996. [DOI] [PubMed] [Google Scholar]
- 42.Witte K, Jacke K, Stahrenberg R, Arlt G, Reitenbach I, Schilling L, Lemmer B. Dysfunction of soluble guanylyl cyclase in aorta and kidney of Goto-Kakizaki rats: influence of age and diabetic state. Nitric Oxide 6: 85–95, 2002. [DOI] [PubMed] [Google Scholar]
- 43.Zhu BH, Guan YY, Min J, He H. Contractile responses of diabetic rat aorta to phenylephrine at different stages of diabetic duration. Acta Pharmacol Sin 22: 445–449, 2001. [PubMed] [Google Scholar]