Abstract
This study was designed to determine whether cyclic stretch induces a persistent pulmonary hypertension of the newborn (PPHN) phenotype of increased NADPH oxidase (Nox) 4 signaling in control pulmonary artery smooth muscle cells (PASMC), and to identify the signal transduction molecules involved. To achieve this, PPHN was induced in lambs by antenatal ligation of the ductus arteriosus at 128 days gestation. After 9 days, lungs and PASMC were isolated from control (twin) and PPHN lambs. Control PASMC were exposed to cyclic stretch at 1 Hz and 15% elongation for 24 h. Stretch-induced Nox4 expression was attenuated by inhibition of mitochondrial complex III and NF-κB, and stretch-induced protein thiol oxidation was attenuated by Nox4 small interfering RNA and complex III inhibition. NF-κB activity was increased by stretch in a complex III-dependent fashion, and stretch-induced cyclin D1 expression was attenuated by complex III inhibition and Nox4 small interfering RNA. This is the first study to show that cyclic stretch increases Nox4 expression via mitochondrial complex III-induced activation of NF-κB in fetal PASMC, resulting in ROS signaling and increased cyclin D1 expression. Targeting these signaling molecules may attenuate pulmonary vascular remodeling associated with PPHN.
Keywords: pulmonary hypertension, reactive oxygen species, NADPH oxidase
at birth, pulmonary vascular resistance must rapidly decrease, to allow pulmonary blood flow to increase 10-fold and establish the lung as the organ of gas exchange. This fetal-to-newborn transition is regulated by complex physiological and biochemical processes, which are necessary to promote pulmonary artery (PA) vasodilation. Abnormalities in the transition at birth produce persistent pulmonary hypertension of the newborn (PPHN), a life-threatening clinical disorder of newborn infants (41). PPHN is characterized by elevated pulmonary vascular resistance (PVR), right-to-left extrapulmonary shunting of deoxygenated blood, and life-threatening hypoxemia. Pathological findings include pulmonary vascular remodeling and smooth muscle hyperplasia (20), and the severity of the disease correlates with the extent of vascular remodeling. However, the abnormal in utero events that trigger the development of PPHN are poorly understood.
A lamb model involving antenatal ligation of the ductus arteriosus followed by delivery at near-term gestation has been used to simulate PPHN. These newborn lambs display pulmonary vascular remodeling, increased PA pressure, and other physiological changes consistent with clinical PPHN (33, 52). Ductal ligation initially induces a large increase in pulmonary blood flow followed by a return to baseline flow while PA pressure remains high (1). In vivo experiments suggest that the fetal pulmonary circulation exhibits a myogenic response, where pressure-induced vasoconstriction opposes the initial increase in pulmonary blood flow following ductal ligation (43). Although the underlying mechanisms are incompletely understood, they involve impaired nitric oxide (NO) synthase (NOS)-mediated vasodilation and increased levels of reactive oxygen species (ROS) (23, 43). PPHN lambs also exhibit elevated pulmonary vascular oxidant stress relative to control lambs (7, 50), and recent studies have demonstrated improved oxygenation in ventilated PPHN lambs that received intratracheal antioxidants at birth (13, 49, 50). ROS may promote vasoconstriction directly and by impairing NO-mediated vasodilation (6, 13, 39), suggesting that the sources of increased ROS generation in PPHN are potential therapeutic targets.
The NADPH oxidase (Nox) enzymes are major sources of vascular ROS. The Nox4 isoform generates vascular hydrogen peroxide (H2O2) and has been reported to mediate proliferation and migration in pulmonary vascular smooth muscle. The activity of the Nox4 isoform is regulated primarily at the level of expression of its subunits (4, 37), and its activation leads to increased H2O2 levels in vascular smooth muscle cells (SMCs) (9). We recently demonstrated increased expression of Nox4 in the lungs and PAs of fetal PPHN lambs, which was associated with increased PA H2O2 levels (48). Increased Nox4 expression was also evident in PA SMCs (PASMC) isolated from fetal PPHN lambs relative to controls. H2O2 generated by Nox4 potentially contributes to the development of PPHN by stimulating vascular remodeling and by promoting vasoconstriction. However, the in utero mechanisms that stimulate Nox4 expression in PPHN remain poorly understood.
We hypothesized that the myogenic response in fetal lambs following ductal ligation exposes PASMC to increased vascular stretch in utero, which upregulates ROS signaling, leading to Nox4 expression and subsequent ROS-mediated PA remodeling and vasoconstriction. Thus the aim of the present study was to subject PASMC from late gestation fetal lambs to cyclic stretch to mimic the mechanical forces that trigger PPHN, to identify upstream and downstream mediators of Nox4 signaling.
MATERIALS AND METHODS
Animals.
This protocol was approved by the Laboratory Animal Care committees at University at Buffalo and University of California Davis. Time-dated pregnant ewes were obtained from New Pasteur farms in Attica, NY. Fetal lambs underwent antenatal ligation of the ductus arteriosus at 128 days gestation (term 143–145 days) to induce PPHN, as previously described (33, 54). Lambs were delivered 9 days later and killed with an overdose of pentobarbital and exsanguination before their first breath. Twin lambs without ductal ligation were used as controls. Fifth generation PAs were collected from control and PPHN lambs for further analysis.
Cell culture.
Primary cultures of PASMCs from control and PPHN fetal lambs were isolated by the explant technique and maintained in culture, as described previously (47). The mitochondrial complex III inhibitors myxothiazol and 1 μM antimycin A (Sigma-Aldrich, St. Louis, MO) were used at a final concentration of 1 μM, and the NF-κB inhibitor helenalin (Sigma) was used at a final concentration of 5 μM.
Cyclic stretch.
Cells were maintained in DMEM containing 10% serum six-well BioFlex plates coated with collagen type IV (FlexCell) for 24 h, then subjected to biaxial cyclic stretch using the FlexCell 3000 Strain Unit. Plates were placed on a loading station and stretched by applying an oscillatory vacuum to the underside of the membranes. Cells were stretched at a frequency of 1 Hz with 15% amplitude for 24 h in accordance with a previous study (36).
DAPI staining.
PASMC were treated with vehicle or with 1 μM myxothiazol for 24 h, washed in PBS, and fixed in 4% paraformaldehyde (Santa Cruz Biotechnology, Dallas, TX). Fixed cells were permeabilized with 0.2% Triton X-100 (Sigma) for 5 min, washed in PBS, and stained with 2 μg/ml 4′,6-diamidino-2-phenylindole (DAPI; Sigma) for 5 min. Cells were washed in PBS, and DAPI-stained nuclei were visualized by fluorescence microscopy.
Western blot analysis.
Lysates were prepared from PASMC using lysis buffer (Millipore, Billerica, MA), and proteins were analyzed by Western blotting, as previously described (48). Expression within each Western blot was normalized to β-actin. Data are shown as fold relative to control lambs.
Quantitative RT real-time PCR.
RNA from PASMC was prepared and analyzed by real-time PCR, as described previously (48).
siRNA transfection.
An ovine Nox4-specific Silencer Select small interfering RNA (siRNA) (Invitrogen), 5′-GUA CUA UUC UUG AUG AUU ATT-3′ (sense), 5′-UAA UCA UCA AGA AUA GUA CCA-3′ (antisense), was used to knock down Nox4, and a scrambled siRNA was used as a control (Silencer Negative Control No. 1 siRNA, Invitrogen). After optimization studies, cells were transfected with 12.5 nM siRNA using lipofectamine RNAiMAX and Opti-MEM (Invitrogen), according to the manufacturer's instructions. Complete medium was added after 4 h, and after 24 h medium was aspirated and changed to complete medium. Analyses were performed 72 h after transfection.
Detection of ROS.
PASMC in serum-free DMEM without phenol red and with antibiotics and antimycotics were infected in 60-mm culture dishes with 100 plaque-forming units/cell of a reduction-oxidation-sensitive green fluorescent protein (roGFP) adenoviral construct that expresses roGFP protein in the cytosol or in the mitochondrial matrix. roGFP is a previously characterized ratiometric fluorescent probe sensitive to cellular oxidative stress (10, 19). The percent oxidation of roGFP in each sample was determined by flow cytometry, as described previously (48).
Plasmid DNA transfection and luciferase assays.
NF-κB and cyclin D1 promoter activities were determined by transfecting cells with plasmids carrying promoter-luciferase constructs, as described previously (48). Where appropriate, cells were transfected with siRNA as above or treated with 1 μM myxothiazol or 5 μM helenalin (Sigma) before luciferase assays.
Statistical analysis.
All data are expressed as the means ± SE. Results were analyzed by two-sided unpaired t-test or by ANOVA with Newman-Keuls post hoc testing using Prism software (GraphPad Software, San Diego, CA). Statistical significance was set at P < 0.05.
RESULTS
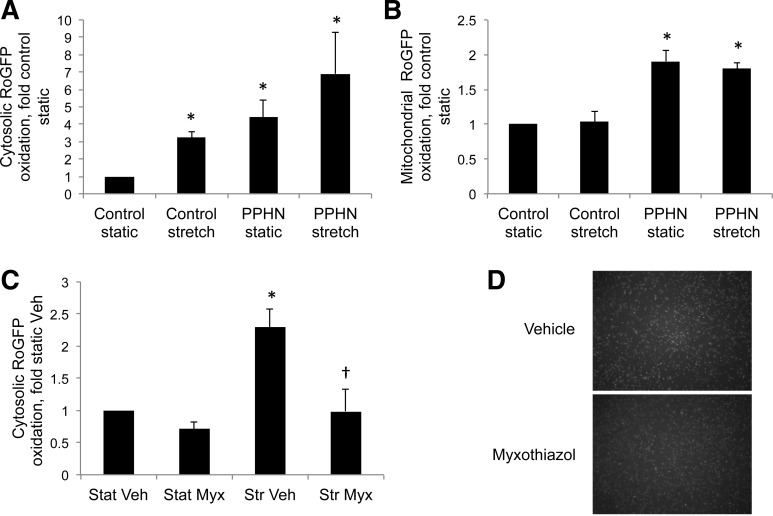
We previously reported an increase in PA ROS in fetal PPHN lambs relative to controls (48), and that PASMC isolated from fetal PPHN lambs exhibit over fivefold higher basal oxidation of the redox-sensitive sensor roGFP in the cytosol (48). Recently, we demonstrated that cyclic stretch at 20% elongation and 1 Hz for 24 h increased cytosolic roGFP oxidation almost twofold in control PASMC (38). We, therefore, hypothesized that cyclic stretch mimics the myogenic response and induces a PPHN phenotype of increased cytosolic oxidant stress in control PASMC. First, we determined that 24-h stretch at 5 and 10% elongation and 1 Hz failed to increase cytosolic roGFP oxidation in control PASMC (0.95 ± 0.17-fold and 0.83 ± 0.21-fold, respectively, relative to static cells), while 15% elongation for 24 h at 1 Hz increased cytosolic roGFP oxidation by 3.2-fold relative to static PASMC (Fig. 1A). Cytosolic roGFP oxidation was 4.4-fold higher in static PPHN PASMC and 6.9-fold higher in PPHN PASMC exposed to stretch relative to static control cells (Fig. 1A). We have reported that basal oxidation of roGFP targeted to the mitochondrial matrix is over fourfold higher in PPHN PASMC relative to control cells (11), and other studies using endothelial cells demonstrated that 25% stretch increases ROS via the mitochondria (2, 3). In this study, we found that basal oxidation of roGFP in the mitochondrial matrix was significantly higher in PPHN PASMC relative to control cells, while stretch did not alter the oxidation of this probe in either cell type (Fig. 1B). In the present study, we also found that the mitochondrial complex III inhibitor myxothiazol attenuated stretch-induced cytosolic roGFP oxidation (Fig. 1C). There are concerns that myxothiazol is cytotoxic at high concentrations, but exposure to the same concentration used in Fig. 1C did not reduce the number of PASMC, as detected by DAPI-stained nuclei after 24 h (Fig. 1D).
Fig. 1.
Cyclic stretch increases cytosolic protein thiol oxidation via mitochondrial complex III to induce a persistent pulmonary hypertension of the newborn (PPHN) pulmonary artery smooth muscle cell (PASMC) phenotype. Control and PPHN PASMC were infected with an adenovirus expressing reduction-oxidation-sensitive green fluorescent protein (roGFP) in the cytosol (A) or mitochondrial matrix (B). After 48 h, control cells were subjected to 24-h cyclic stretch at 1 Hz and 15% elongation. The extent of roGFP oxidation in lysates was determined by flow cytometry, and the percent oxidation determined by fully oxidizing and fully reducing the probe. Between 5,000 and 20,000 roGFP-positive cells were quantified for each sample. A and B: RoGFP oxidation in static and stretch PPHN PASMC is included for comparison. C: RoGFP-infected control PASMC were treated with vehicle (Veh) or 1 μM myxothiazol (Myx) to inhibit complex III and then exposed to 24-h stretch (Str), as above. Stat, static. D: PASMC were treated with Veh or 1 μM Myx for 24 h and then fixed and nuclei stained with DAPI. Values are means ± SE; n ≥ 3. *P < 0.05 vs. control static (A and B) or control Stat Veh (C). †P < 0.05 vs. Str Veh. UTD, ???.
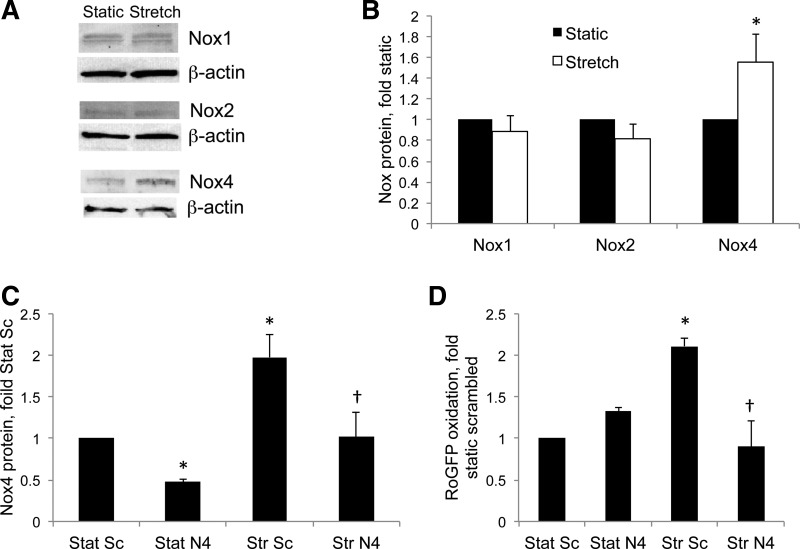
We then investigated the potential sources of increased cytosolic ROS in PASMC exposed to stretch. Several studies have indicated a role for members of the Nox family in ROS generation in PASMC, and we found that 24-h stretch increased protein levels of Nox4, but not Nox1 or Nox2, in control fetal PASMC (Fig. 2, A and B). Nox4 expression is increased in PPHN PA and PASMC relative to controls (48), and we recently reported that a Nox4-specific siRNA decreases Nox4 protein levels and attenuates cytosolic roGFP oxidation in PPHN PASMC (48). In the present study, we found that the same Nox4-specific siRNA decreased Nox4 protein in static and stretched control PASMC (Fig. 2C) and blocked stretch-induced cytosolic roGFP oxidation (Fig. 2D), indicating a major role for this Nox isoform in the ROS signaling pathway induced by stretch.
Fig. 2.
Stretch induces NADPH oxidase (Nox) 4 expression and increases cytosolic reactive oxygen species (ROS) in PASMC. A: control cells were subjected to 24-h cyclic stretch at 1 Hz and 15% elongation, and protein levels were analyzed by Western blotting. B: Nox isoform band intensities were normalized to β-actin and expressed relative to static controls. C: control PASMC were transfected with a scrambled (Sc) small interfering RNA (siRNA) or a siRNA specific to ovine Nox4 (N4). After 48 h, cells were subjected to 24-h cyclic stretch (Str) at 1 Hz and 15% elongation, and Nox4 protein levels were quantified by Western blotting as in A and B to determine the level of knockdown. D: control PASMC were transfected with a Sc siRNA (Sc) or a siRNA specific to ovine Nox4 (N4) and infected with an adenovirus expressing roGFP in the cytosol. After 48 h, cells were subjected to 24-h cyclic Str at 1 Hz and 15% elongation, and roGFP oxidation was determined by flow cytometry. Values are means ± SE; n ≥ 3. *P < 0.05 vs. static (B) or Stat Sc siRNA (C and D). †P < 0.05 vs. Str Sc siRNA (C and D).
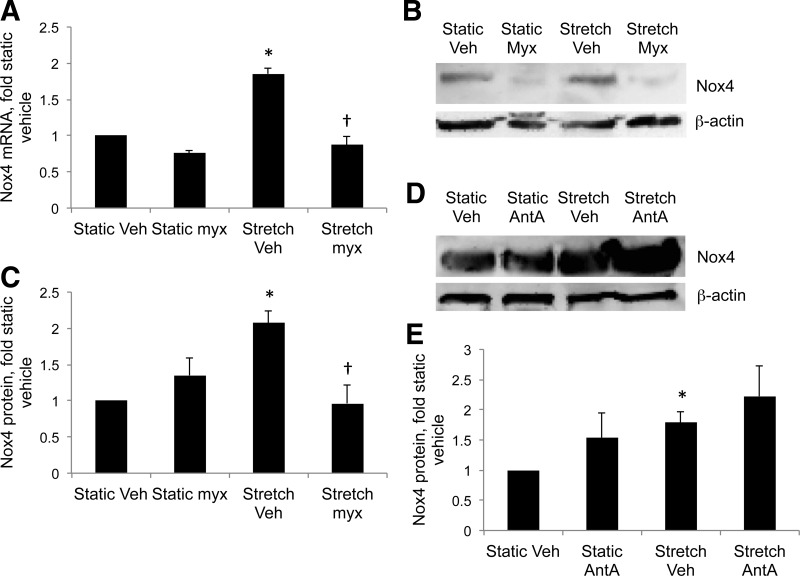
Twenty-four-hour stretch increased Nox4 mRNA and protein by 1.85-fold and 2.1-fold, respectively, in control PASMC (Fig. 3, A–C). Increasing evidence suggests cross talk between Nox enzymes and the mitochondria. Accordingly, myxothiazol blocked stretch-induced Nox4 expression in control PASMC (Fig. 3, A–C), indicating that mitochondrial complex III is upstream of Nox4 in stretch-induced cytosolic ROS signaling. Myxothiazol reduces superoxide generation at complex III, while antimycin A increases superoxide production (44). Antimycin A increased Nox4 expression in static and stretched PASMC, although the increases did not reach statistical significance (Fig. 3, D and E). These data suggest that ROS generated at complex III are able to upregulate Nox4 expression.
Fig. 3.
Inhibition of mitochondrial complex III attenuates stretch-induced Nox4 expression. Control PASMC were treated with Veh, 1 μM Myx, or 1 μM antimycin A (AntA) to inhibit complex III and subjected to 24-h cyclic stretch at 1 Hz and 15% elongation. Protein and mRNA were analyzed for Nox4 expression. A: real-time PCR using primers to ovine Nox4. Expression levels were normalized to β-actin mRNA levels and expressed relative to controls. B: representative Western blots for Nox4 from PASMC treated with Myx and stretched for 24 h. C: Nox4 band intensities in B were normalized to β-actin and expressed relative to static controls. D: representative Western blots for Nox4 from PASMC treated with AntA and stretched for 24 h. E: Nox4 band intensities in D were normalized to β-actin and expressed relative to static controls. Values are means ± SE; n ≥ 3. *P < 0.05 vs. static Veh. †P < 0.05 vs. stretch Veh.
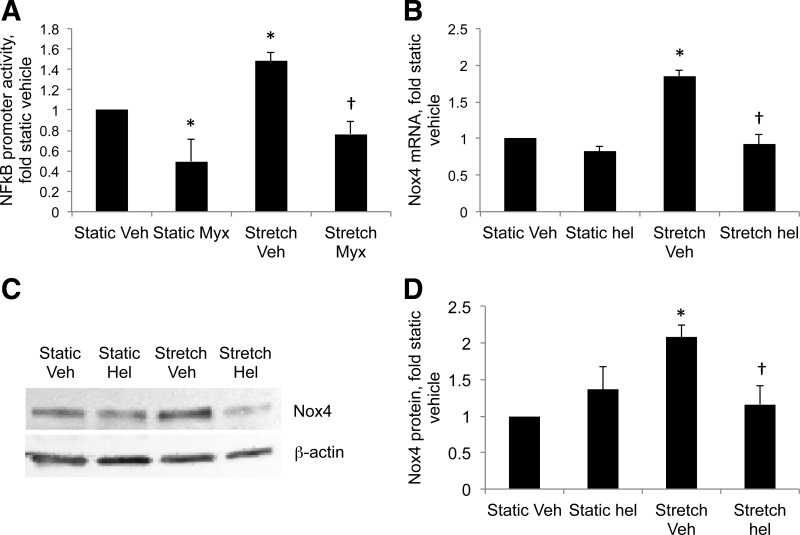
Next we investigated the mechanisms of stretch-induced Nox4 expression in control PASMC, and the increase in stretch-induced Nox4 mRNA (Fig. 3A) suggests that increased transcription may be involved. The transcription factor NF-κB controls the response to multiple genes related to inflammation and has been shown to upregulate Nox4 expression in aortic SMCs (31). We recently demonstrated an increase in basal NF-κB activity in PA isolated from PPHN lambs (48). Stretch increased NF-κB activity by 1.5-fold in fetal PASMC cells exposed to 24-h stretch, and myxothiazol blocked this increase (Fig. 4A). Stretch-induced increases in Nox4 mRNA and protein were attenuated in cells treated with the NF-κB inhibitor helenalin (Fig. 4, B–D).
Fig. 4.
Stretch activates NF-κB via mitochondrial complex III, resulting in increased expression of Nox4. Control PASMC were transfected with a plasmid containing 5 consensus κB sites in tandem upstream of a luciferase reporter, treated with Veh or 1 μM Myx and subjected to 24-h cyclic stretch at 1 Hz and 15% elongation. A: relative light units were determined in a luminometer and expressed relative to static Veh cells. Real-time PCR was performed on mRNA from PASMC treated with helenalin (hel) and stretched for 24 h using primers to ovine Nox4. B: expression levels were normalized to β-actin mRNA levels and expressed relative to static Veh cells. C: representative Western blots for Nox4 from PASMC treated with hel and stretched for 24 h. D: Nox4 band intensities in C were normalized to β-actin and expressed relative to static controls. Values are means ± SE; n ≥ 3. *P < 0.05 vs. static Veh. †P < 0.05 vs. stretch Veh.
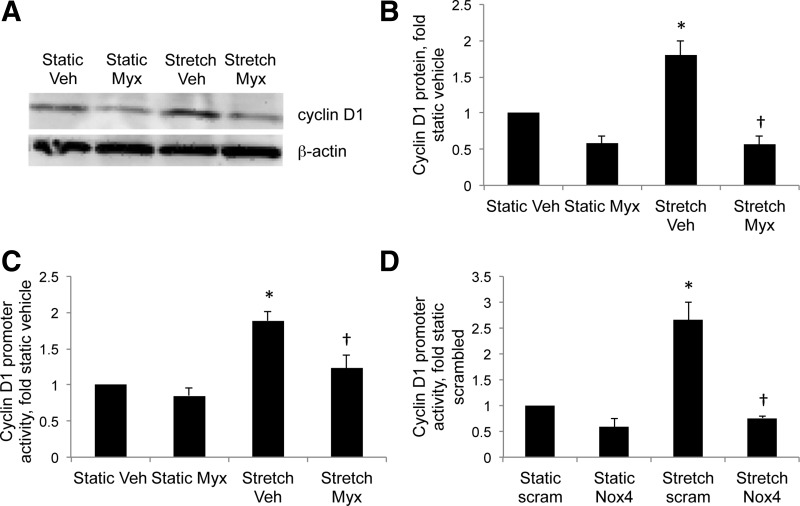
We previously observed an increase in cyclin D1 expression in PPHN lungs and PASMC, and Nox4 knockdown decreased cyclin D1 promoter activity in PPHN PASMC (48). In the present study, stretch increased cyclin D1 protein (Fig. 5, A and B) and promoter activity (Fig. 5, C and D) in fetal PASMC by 1.8-fold and 2-fold, respectively. Myxothiazol attenuated stretch-induced increases in cyclin D1 protein levels (Fig. 5, A and B), and myxothiazol (Fig. 5C) and Nox4 siRNA (Fig. 5D) attenuated stretch-induced cyclin D1 promoter activity.
Fig. 5.
Inhibition of Nox4 and mitochondrial complex III attenuates stretch-induced cyclin D1 expression in PASMC. A and B: PASMC were treated with Veh or with 1 μM Myx, and subjected to 24-h cyclic stretch at 1 Hz and 15% elongation. Protein was analyzed for Nox4 expression. A: representative Western blot for Nox4 from PASMC. B: Nox4 band intensities were normalized to β-actin and expressed relative to static controls. PASMC were transfected with a plasmid containing the human cyclin D1 promoter region, treated with Veh or 1 μM Myx (C), or with Sc (scram) or Nox4 siRNA (Nox4) and subjected to 24-h cyclic stretch at 1 Hz and 15% elongation (C and D). Relative light units were determined in a luminometer and expressed relative to static controls. Values are means ± SE; n ≥ 3. *P < 0.05 vs. static Veh or static Sc siRNA. †P < 0.05 vs. stretch Veh or stretch Sc siRNA.
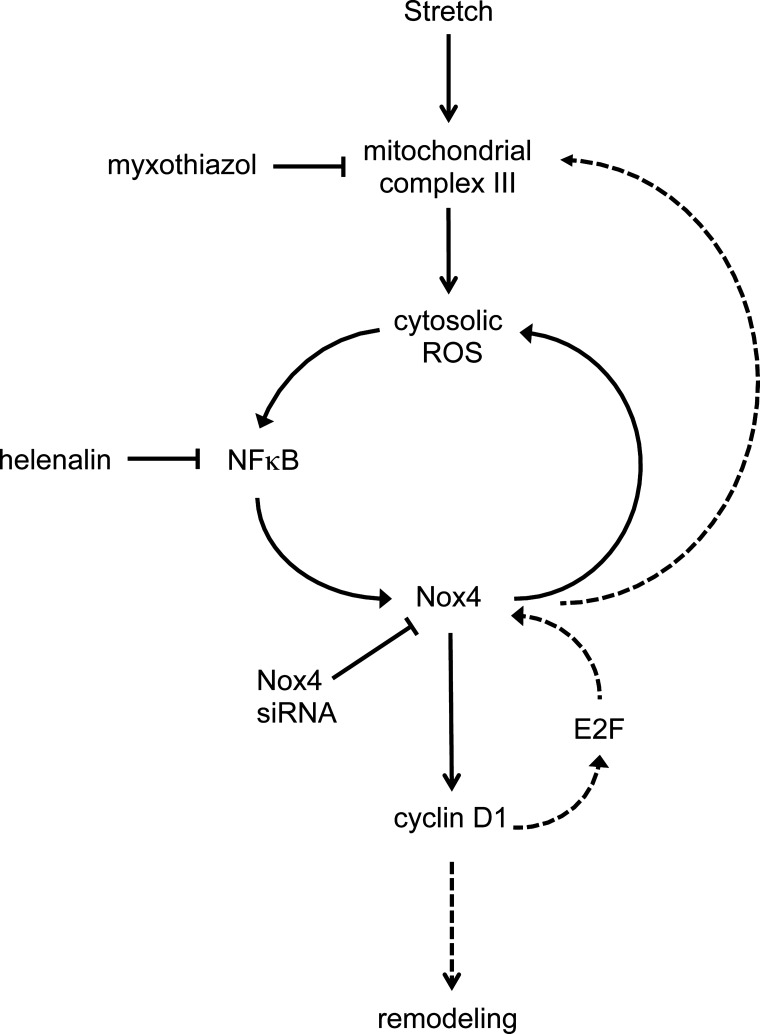
Based on the results detailed above, we present our proposed signal transduction pathway that mediates stretch-induced pulmonary vascular remodeling in PPHN in Fig. 6.
Fig. 6.
Proposed pathway based on data from the present study showing increased Nox4 expression in response to stretch. Stretch increases cytosolic ROS via mitochondrial complex III, triggering a feed-forward mechanism via NF-κB, resulting in increased Nox4 expression and a sustained increase in cytosolic ROS. Nox4 increases cyclin D1 expression, leading to PASMC proliferation and vascular remodeling. Dashed lines highlight other potential pathways, as evidenced by the literature, but not investigated in the present study. Increased cyclin D1 expression may contribute to the positive feedback mechanism via the release of E2F, which have been shown to upregulate Nox4 expression. There is increasing evidence of cross talk between Nox isoforms and mitochondrial ROS, and increased Nox4 expression may augment ROS generation at complex III.
DISCUSSION
PPHN is defined by a failure to decrease PVR normally at birth, resulting in pulmonary hypertension, right-to-left extrapulmonary shunting, and hypoxemia (42). Autopsy studies of fatal PPHN demonstrate severe hypertensive structural remodeling in newborns who die shortly after birth (15), suggesting that many cases of severe disease are associated with chronic intrauterine stress and antenatal vascular remodeling. The intrauterine events that alter pulmonary vascular reactivity and structure are poorly understood, yet critically important to improving treatment. One cause of idiopathic PPHN is constriction of the fetal ductus arteriosus in utero from exposure to nonsteroidal anti-inflammatory drugs during the third trimester (30, 45). Antenatal surgical closure of the ductus arteriosus in fetal lambs alters lung vascular reactivity and structure, causes failure of postnatal adaptation at delivery, and provides an important experimental model of PPHN (1, 28, 34). Marked right ventricular hypertrophy and structural remodeling of small PAs develops after 8 days of hypertension. After delivery, these lambs behave remarkably like human infants with PPHN and have persistent hypoxemia and elevation of PVR, despite mechanical ventilation with high oxygen concentrations.
Occlusion of the ductus arteriosus generates a myogenic response where stretch-induced vasoconstriction opposes the increase in pulmonary blood flow, resulting in an increase in PVR and a return to preconstriction flow, while PA pressure remains high (43). The underlying mechanisms that maintain high PA pressures include decreased NOS activity, increased circulating vasoconstrictors, and an increase in ROS (23, 43). We previously reported finding an increase in H2O2 signaling in the PA of PPHN lambs (48, 51), which may induce abnormal vascular tone through a variety of mechanisms (40, 55), thereby contributing importantly to the pathogenesis of PPHN (50). H2O2 attenuates NO-mediated vasorelaxation in PPHN via inhibition of endothelial NOS (26) and extracellular superoxide dismutase (50), downregulation of soluble guanylate cyclase (51), and stimulation of cGMP-specific phosphodiesterase (12). Catalase treatment normalized vasodilator responses to NO in isolated fetal PPHN PAs (51), while intratracheal catalase improved oxygenation and vasorelaxation in ventilated PPHN lambs (50). These improvements were associated with enhanced lung extracellular superoxide dismutase activity (50), increased PA cGMP levels, and decreased phosphodiesterase activity (11). However, antioxidant therapy has had surprisingly limited success in clinical trials, potentially due to the importance and complexity of ROS signaling in normal cell signaling. Targeting the sources of increased ROS that trigger abnormal pulmonary vascular responses may be more effective in treating diseases such as PPHN.
After antenatal ductus ligation, PA pressure and PVR progressively increase, but low flow persists and arterial Po2 remains unchanged (1). Increased PA pressure subjects vascular cells within the vessel walls to biomechanical forces of increased shear and cyclic stretch. In the systemic circulation, pulsatile distension of the vessel wall during systole is typically 9–12%, while the diameter of smaller resistance vessels may change by up to 60% (25). We previously demonstrated that PASMC from PPHN lambs display increased cytosolic protein thiol oxidation relative to controls, as determined by the redox-sensitive probe roGFP (48). Furthermore, PASMC isolated from control lambs increased the oxidation of cytosolic roGFP when exposed to 20% cyclic stretch at 1 Hz for 24 h (38). Cyclic stretch may, therefore, represent an in vitro model of ROS signaling in PPHN. In the present study, we found that 24-h cyclic stretch at 15% elongation and 1 Hz increased cytosolic roGFP oxidation by 3.2-fold in control PASMC (Fig. 1A). By comparison, cytosolic roGFP oxidation was 4.4-fold higher in PASMC isolated from PPHN lambs under static conditions relative to control cells and increased to 6.9-fold higher after 24-h stretch (Fig. 1A).
Our previous studies indicate that mitochondrial dysfunction may also occur during the development of PPHN, since PASMC isolated from PPHN lambs display elevated roGFP oxidation in the mitochondrial matrix relative to control cells (11). In the present study, the oxidation of roGFP targeted to the mitochondrial matrix was 1.9-fold higher in PPHN PASMC relative to control cells, while 24-h cyclic stretch had no effect on mitochondrial matrix roGFP oxidation in either cell type (Fig. 1B). These data suggest that stretch increases cytosolic ROS signaling in control cells, although the mechanisms that increase mitochondrial matrix oxidation in PPHN PASMC could not be reproduced by exposure to stretch alone. Stretch was previously shown to increase ROS via the mitochondria in human umbilical vein endothelial cells and bovine PA endothelial cells, leading to the activation of downstream signaling pathways (2, 3). Moreover, in PASMC from adult rats, hypoxia increased cytosolic ROS via complex III of the mitochondrial electron transport chain (18), which was accompanied by a decrease in ROS in the mitochondrial matrix (46). From these results, we investigated a potential role for complex III in stretch-induced increases in cytosolic ROS, and in the present study we found that inhibition of complex III abrogated stretch-induced cytosolic roGFP oxidation (Fig. 1C). Myxothiazol reduces ROS generation at complex III by competing with ubiquinol binding at the Qo site located near the outer surface of the inner mitochondrial membrane (44). We hypothesize that stretch increases the levels of ubisemiquinone generated at the Qo site of complex III in a myxothiazol-sensitive fashion, although the mechanisms involved are currently unknown. Molecular oxygen captures the electron from ubisemiquinone to yield superoxide, which is converted to H2O2 by copper zinc superoxide dismutase in the intermembrane space. Other components of the electron transport chain may also contribute to increased ROS in response to stretch. However, pharmacological inhibition upstream of complex III will disrupt electron transport and, therefore, prevent ROS generation at complex III, making their potential identities unknown. H2O2 generated in the intermembrane space is able to cross the outer membrane into the cytosol, where it oxidizes cytosolic roGFP. While other ROS in addition to H2O2 may also oxidize roGFP, this probe reflects how ROS-sensitive proteins in the cytosol ultimately respond to stretch. These proteins include transcription factors, and stretch may further increase cytosolic roGFP oxidation by increasing the expression of other ROS-generating systems.
The activity of the Nox4 isoform of Nox is regulated primarily at the level of expression of its subunits (4, 37), and its activation leads to increased H2O2 levels in vascular SMCs (9). We recently identified increased Nox4 expression as a potential contributor to increased H2O2 generation in PPHN PA and PASMC (48). In the present study, we found that cyclic stretch selectively increased Nox4 expression in control PASMC, and our siRNA data indicate that Nox4 is a major source of cytosolic ROS in response to stretch. While other studies have demonstrated that biomechanical forces regulate Nox isoform expression, inconsistencies in the results may reflect differences in the type of cells under investigation or the developmental age of the animal. In adult rat thoracic aortic SMC, cyclic stretch increased Nox1 expression only when cells were coincubated with angiotensin II (22), while stretch alone was sufficient to activate Nox2 via membrane translocation of p47phox in juvenile PASMC (32). In human umbilical vein endothelial cells, 12% cyclic stretch downregulated Nox4 expression (16), although a contrasting recent report found that 20% cyclic stretch at 0.5 Hz increased Nox4 expression in neonatal rat PASMC (8). Similar to this report in neonatal rats, our data indicate that, in PASMC isolated from late gestation fetal lambs, 15% stretch at 1 Hz increases Nox4 expression and ROS generation. Furthermore, our findings in stretched cells are strikingly similar to those observed in PAs and PASMC isolated from PPHN lambs (48). We previously reported that Nox1 levels were unchanged in the lungs of PPHN lambs relative to controls, similar to the stretch data presented here (48). Conversely, Nox2 protein was elevated in PPHN lungs (48), while stretch had no effect on Nox2 expression on control PASMC (Fig. 2B). However, lambs studied 2 days after ductus ligation did not show increased Nox2 protein in PAs of similar size to those used to isolate PASMC for the present study (7). Stretch did not induce all phenotypic features of PASMC isolated from PPHN lungs, which could be due to many factors, including the length of exposure and lack of exposure to endothelial cells. However, stretch may be a useful in vitro model to provide insight into the abnormal and/or early in utero events that induce the development of PPHN.
The present study suggests that mitochondrial complex III is involved in stretch-induced upregulation of Nox4 expression, adding to the evidence of cross talk between Nox isoforms and the mitochondria (35, 53). By contrast with myxothiazol, antimycin A prevents the oxidation of ubisemiquinone at the Qo site of complex III, resulting in a marked increase in superoxide production (44). Our data suggest that antimycin A augments stretch-induced Nox4 expression (Fig. 3, D and E). Interestingly, Nox4 protein has been reported to localize to the mitochondria in the right ventricles of a mouse model of pressure overload pulmonary hypertension (14) and in breast epithelial cells (17). These data suggest the possibility of a feed-forward mechanism involving cytosolic and mitochondrial ROS, although the mechanisms involved remain to be determined.
Nox4 transcription is upregulated in systemic vascular SMC by factors including NF-κB (31), and here we demonstrate that the NF-κB inhibitor helenalin blocked stretch-induced Nox4 expression in PASMC from fetal lambs. We previously showed that basal NF-κB activity was higher in PPHN PASMC relative to controls, while helenalin decreased Nox4 expression in PPHN PASMC (48). Stretch increased NF-κB activity in PASMC in agreement with other studies (27, 29), whereas myxothiazol attenuated activity, indicating that complex III lies upstream of NF-κB and Nox4 in the pathway. NF-κB is a ROS-sensitive transcription factor, providing further evidence of a feed-forward mechanism (Fig. 6).
Our previous studies demonstrated increased expression of the cell cycle regulator cyclin D1 in PPHN lungs and PASMC, which correlated with increased PA H2O2 levels, whereas intratracheal catalase decreased cyclin D1 expression in oxygen-ventilated PPHN lambs (48). Cyclin D1 regulates the transition from G0/G1 to S phase in the cell cycle, resulting in activation of genes necessary for cell cycle progression. Here we found that stretch increased cyclin D1 expression in PASMC via mechanisms involving Nox4 and mitochondrial complex III. Cyclin D1 expression is upregulated by NF-κB (21), whereas Nox4 transcription is potentially upregulated by members of the E2F family (56), factors activated downstream of cyclin D1 in the cell cycle. Together these data suggest that cyclic stretch may induce vascular remodeling via a feed-forward mechanism via Nox4- and ROS-mediated cell cycle progression and PASMC proliferation in PPHN (Fig. 6). In addition, other factors may also be involved in stretch-induced vascular remodeling. As discussed above, other components of the mitochondrial electron transport chain may be influenced by stretch, while other ROS generating systems not investigated in the present study may also contribute to stretch-induced ROS signaling. Factors other than NF-κB may augment stretch-induced Nox4 expression, whereas cytosolic ROS and ROS-sensitive transcription factors may influence cell cycle regulatory protein independent of Nox4. In vivo, stretch-mediated release of endothelial growth factors and/or increased expression of PASMC growth factor receptors may further compound vascular remodeling in PPHN.
Understanding the pathogenesis of pulmonary hypertension and remodeling may improve the efficacy of treatment and outcome for patients by providing the option of in utero intervention (for example, in patients with congenital diaphragmatic hernia). The stretch model of PPHN in vitro, together with PPHN lambs, may identify potential therapeutic targets in the prevention and/or treatment of PPHN. The continuing development of novel pharmacological inhibitors of mitochondrial complex III, such as terpestacin (24), and Nox4, such as GKT137831 (5), may prove to be beneficial in these studies.
GRANTS
This research was supported by National Heart, Lung, and Blood Institute Grant HL-54705 to R. H. Steinhorn.
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.W., S.L., P.T.S., and R.H.S. conception and design of research; S.W. performed experiments; S.W., S.L., P.T.S., and R.H.S. analyzed data; S.W., S.L., P.T.S., and R.H.S. interpreted results of experiments; S.W. prepared figures; S.W. and R.H.S. drafted manuscript; S.W., S.L., P.T.S., and R.H.S. edited and revised manuscript; S.W., S.L., P.T.S., and R.H.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Lyubov Czech at Northwestern University for assistance with real-time PCR analysis, and Sylvia Gugino at SUNY Buffalo for PASMC isolations.
REFERENCES
- 1.Abman SH, Accurso FJ. Acute effects of partial compression of ductus arteriosus on fetal pulmonary circulation. Am J Physiol Heart Circ Physiol 257: H626–H634, 1989. [DOI] [PubMed] [Google Scholar]
- 2.Ali M, Mungai P, Schumacker P. Stretch-induced phosphorylation of focal adhesion kinase in endothelial cells:role of mitochondrial oxidants. Am J Physiol Lung Cell Mol Physiol 291: L38–L45, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Ali M, Pearlstein D, Mathieu C, Schumacker P. Mitochondrial requirement for endothelial responses to cyclic strain: implications for mechanotransduction. Am J Physiol Lung Cell Mol Physiol 287: L486–L496, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Ambasta R, Kumar P, Griendling K, Schmidt H, Busse R, Brandes R. Direct interaction of the novel Nox proteins with p22phox is required for the formation of a functionally active NADPH oxidase. J Biol Chem 279: 45935–45941, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Aoyama T, Paik YH, Watanabe S, Laleu B, Gaggini F, Fioraso-Cartier L, Molango S, Heitz F, Merlot C, Szyndralewiez C, Page P, Brenner DA. Nicotinamide adenine dinucleotide phosphate oxidase in experimental liver fibrosis: GKT137831 as a novel potential therapeutic agent. Hepatology 56: 2316–2327, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Black SM, Johengen MJ, Soifer SJ. Coordinated regulation of genes of the nitric oxide and endothelin pathways during the development of pulmonary hypertension in fetal lambs. Pediatr Res 44: 821–830, 1998. [DOI] [PubMed] [Google Scholar]
- 7.Brennan LA, Steinhorn RH, Wedgwood S, Mata-Greenwood E, Roark EA, Russell JA, Black SM. Increased superoxide generation is associated with pulmonary hypertension in fetal lambs: a role for NADPH oxidase. Circ Res 92: 683–691, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Dick AS, Ivanovska J, Kantores C, Belcastro R, Keith Tanswell A, Jankov RP. Cyclic stretch stimulates nitric oxide synthase-1-dependent peroxynitrite formation by neonatal rat pulmonary artery smooth muscle. Free Radic Biol Med 61C: 310–319, 2013. [DOI] [PubMed] [Google Scholar]
- 9.Dikalov S, Dikalova A, Bikineyeva A, Schmidt H, Harrison D, Griendling K. Distinct roles of Nox1 and Nox4 in basal and angiotensin II-stimulated superoxide and hydrogen peroxide production. Free Radic Biol Med 45: 1340–1351, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dooley C, Dore T, Hanson G, Jackson W, Remington S, Tsien R. Imaging dynamic redox changes in mammalian cells with green fluorescent protein indicators. J Biol Chem 279: 22284–22293, 2004. [DOI] [PubMed] [Google Scholar]
- 11.Farrow K, Wedgwood S, Lee K, Czech L, Gugino S, Lakshminrusimha S, Schumacker P, Steinhorn R. Mitochondrial oxidant stress increases PDE5 activity in persistent pulmonary hypertension of the newborn. Respir Physiol Neurobiol 174: 272–281, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farrow KN, Groh BS, Schumacker PT, Lakshminrusimha S, Czech L, Gugino SF, Russell JA, Steinhorn RH. Hyperoxia increases phosphodiesterase 5 expression and activity in ovine fetal pulmonary artery smooth muscle cells. Circ Res 102: 226–233, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farrow KN, Lakshminrusimha S, Reda WJ, Wedgwood S, Czech L, Gugino SF, Davis JM, Russell JA, Steinhorn RH. Superoxide dismutase restores eNOS expression and function in resistance pulmonary arteries from neonatal lambs with persistent pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 295: L979–L987, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frazziano G, Al Ghouleh I, Baust J, Shiva S, Champion HC, Pagano PJ. Nox-derived ROS are acutely activated in pressure overload pulmonary hypertension: indications for a seminal role for mitochondrial Nox4. Am J Physiol Heart Circ Physiol 306: H197–H205, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geggel R, Reid LM. The structural basis for PPHN. Clin Perinatol 11: 525–549, 1984. [PubMed] [Google Scholar]
- 16.Goettsch C, Goettsch W, Arsov A, Hofbauer LC, Bornstein SR, Morawietz H. Long-term cyclic strain downregulates endothelial Nox4. Antioxid Redox Signal 11: 2385–2397, 2009. [DOI] [PubMed] [Google Scholar]
- 17.Graham K, Kulawiec M, Owens K, Li X, Desouki M, Chandra D, Singh K. NADPH oxidase 4 is an oncoprotein localized to mitochondria. Cancer Biol Ther 10: 223–231, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guzy R, Hoyos B, Robin E, Chen H, Liu L, Mansfield K, Simon M, Hammerling U, Schumacker P. Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing. Cell Metab 1: 401–408, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Hanson G, Aggeler R, Oglesbee D, Cannon M, Capaldi R, Tsien R, Remington S. Investigating mitochondrial redox potential with redox-sensitive green fluorescent protein indicators. J Biol Chem 279: 13044–13053, 2004. [DOI] [PubMed] [Google Scholar]
- 20.Haworth S. Pulmonary vascular remodeling in neonatal pulmonary hypertension. Chest 93: 133S–138S, 1988. [PubMed] [Google Scholar]
- 21.Hinz M, Krappmann D, Eichten A, Heder A, Scheidereit C, Strauss M. NF-kappaB function in growth control: regulation of cyclin D1 expression and G0/G1-to-S-phase transition. Mol Cell Biol 19: 2690–2698, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hitomi H, Fukui T, Moriwaki K, Matsubara K, Sun GP, Rahman M, Nishiyama A, Kiyomoto H, Kimura S, Ohmori K, Abe Y, Kohno M. Synergistic effect of mechanical stretch and angiotensin II on superoxide production via NADPH oxidase in vascular smooth muscle cells. J Hypertens 24: 1089–1095, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Hsu JH, Oishi P, Wiseman DA, Hou Y, Chikovani O, Datar S, Sajti E, Johengen MJ, Harmon C, Black SM, Fineman JR. Nitric oxide alterations following acute ductal constriction in the fetal lamb: a role for superoxide. Am J Physiol Lung Cell Mol Physiol 298: L880–L887, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jung HJ, Shim JS, Lee J, Song YM, Park KC, Choi SH, Kim ND, Yoon JH, Mungai PT, Schumacker PT, Kwon HJ. Terpestacin inhibits tumor angiogenesis by targeting UQCRB of mitochondrial complex III and suppressing hypoxia-induced reactive oxygen species production and cellular oxygen sensing. J Biol Chem 285: 11584–11595, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koller A. Signaling pathways of mechanotransduction in arteriolar endothelium and smooth muscle cells in hypertension. Microcirculation 9: 277–294, 2002. [DOI] [PubMed] [Google Scholar]
- 26.Kumar S, Sun X, Wedgwood S, Black SM. Hydrogen peroxide decreases endothelial nitric oxide synthase promoter activity through the inhibition of AP-1 activity. Am J Physiol Lung Cell Mol Physiol 295: L370–L377, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lemarie CA, Tharaux PL, Esposito B, Tedgui A, Lehoux S. Transforming growth factor-alpha mediates nuclear factor kappaB activation in strained arteries. Circ Res 99: 434–441, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Levin DL, Hyman AI, Heymann MA, Rudolph AM. Fetal hypertension and the development of increased pulmonary vascular smooth muscle: a possible mechanism for persistent pulmonary hypertension of the newborn infant. J Pediatr 92: 265–269, 1978. [DOI] [PubMed] [Google Scholar]
- 29.Leychenko A, Konorev E, Jijiwa M, Matter ML. Stretch-induced hypertrophy activates NFkB-mediated VEGF secretion in adult cardiomyocytes. PLoS One 6: e29055, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manchester D, Margolis HS, Sheldon RE. Possible association between maternal indomethacin therapy and primary pulmonary hypertension of the newborn. Am J Obstet Gynecol: 467–469, 1976. [DOI] [PubMed] [Google Scholar]
- 31.Manea A, Tanase L, Raicu M, Simionescu M. Transcriptional regulation of NADPH oxidase isoforms, Nox1 and Nox4, by nuclear factor-kappaB in human aortic smooth muscle cells. Biochem Biophys Res Commun 396: 901–907, 2010. [DOI] [PubMed] [Google Scholar]
- 32.Mata-Greenwood E, Grobe A, Kumar S, Noskina Y, Black SM. Cyclic stretch increases VEGF expression in pulmonary arterial smooth muscle cells via TGF-beta1 and reactive oxygen species:a requirement for NAD(P)H oxidase. Am J Physiol Lung Cell Mol Physiol 289: L288–L298, 2005. [DOI] [PubMed] [Google Scholar]
- 33.Morin FC., 3rd Ligating the ductus arteriosus before birth causes persistent pulmonary hypertension in the newborn lamb. Pediatr Res 25: 245–250, 1989. [DOI] [PubMed] [Google Scholar]
- 34.Morin FC III, Eagan EA. The effect of closing the ductus arteriosus on the pulmonary circulation of the fetal sheep. J Dev Physiol 11: 245–250, 1989. [PubMed] [Google Scholar]
- 35.Pendyala S, Natarajan V. Redox regulation of Nox proteins. Respir Physiol Neurobiol 174: 265–271, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Quinn TP, Schlueter M, Soifer SJ, Gutierrez JA. Cyclic mechanical stretch induces VEGF and FGF-2 expression in pulmonary vascular smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 282: L897–L903, 2002. [DOI] [PubMed] [Google Scholar]
- 37.Serrander L, Cartier L, Bedard K, Banfi B, Lardy B, Plastre O, Sienkiewicz A, Forro L, Schlegel W, Krause K. NOX4 activity is determined by mRNA levels and reveals a unique pattern of ROS generation. Biochem J 406: 105–114, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shah MR, Wedgwood S, Czech L, Kim GA, Lakshminrusimha S, Schumacker PT, Steinhorn RH, Farrow KN. Cyclic stretch induces inducible nitric oxide synthase and soluble guanylate cyclase in pulmonary artery smooth muscle cells. Int J Mol Sci 14: 4334–4348, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shaul PW, Yuhanna IS, German Z, Chen Z, Steinhorn RH, Morin FC III. Pulmonary endothelial NO synthase gene expression is decreased in fetal lambs with pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 272: L1005–L1012, 1997. [DOI] [PubMed] [Google Scholar]
- 40.Sheehan DW, Giese EC, Gugino SF, Russell JA. Characterization and mechanisms of H2O2-induced contractions of pulmonary arteries. Am J Physiol Heart Circ Physiol 264: H1542–H1547, 1993. [DOI] [PubMed] [Google Scholar]
- 41.Steinhorn RH. Neonatal pulmonary hypertension. Pediatr Crit Care Med 11: S79–S84, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Steinhorn RH, Millard SL, Morin F III. Persistent pulmonary hypertension of the newborn: role of nitric oxide and endothelin in pathophysiology and treatment. Clin Perinatol 22: 405–428, 1995. [PubMed] [Google Scholar]
- 43.Storme L, Rairigh RL, Parker TA, Kinsella JP, Abman SH. In vivo evidence for a myogenic response in the fetal pulmonary circulation. Pediatr Res 45: 425–431, 1999. [DOI] [PubMed] [Google Scholar]
- 44.Turrens JF, Alexandre A, Lehninger AL. Ubisemiquinone is the electron donor for superoxide formation by complex III of heart mitochondria. Arch Biochem Biophys 237: 408–414, 1985. [DOI] [PubMed] [Google Scholar]
- 45.Villaneuva ME, Zaher FM, Svinarich DM, Konduri GG. Decreased gene expression of endothelial nitric oxide synthase in newborns with persistent pulmonary hypertension. Pediatr Res 44: 338–343, 1998. [DOI] [PubMed] [Google Scholar]
- 46.Waypa G, Marks J, Guzy R, Mungai P, Schriewer J, Dokic D, Schumacker P. Hypoxia triggers subcellular compartmental redox signaling in vascular smooth muscle cells. Circ Res 106: 526–535, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wedgwood S, Dettman RW, Black SM. ET-1 stimulates pulmonary arterial smooth muscle cell proliferation via induction of reactive oxygen species. Am J Physiol Lung Cell Mol Physiol 281: L1058–L1067, 2001. [DOI] [PubMed] [Google Scholar]
- 48.Wedgwood S, Lakshminrusimha S, Czech L, Schumacker PT, Steinhorn RH. Increased p22phox/Nox4 expression triggers remodeling through hydrogen peroxide signaling in persistent pulmonary hypertension of the newborn. Antioxid Redox Signal 18: 1765–1776, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wedgwood S, Lakshminrusimha S, Farrow KN, Czech L, Gugino SF, Soares F, Russell JA, Steinhorn RH. Apocynin Improves Oxygenation and Increases eNOS in Persistent Pulmonary Hypertension of the Newborn. Am J Physiol Lung Cell Mol Physiol 302: L616–L626, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wedgwood S, Lakshminrusimha S, Fukai T, Russell J, Schumacker P, Steinhorn R. Hydrogen peroxide regulates extracellular superoxide dismutase activity and expression in neonatal pulmonary hypertension. Antioxid Redox Signal 15: 1497–1506, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wedgwood S, Steinhorn RH, Bunderson M, Wilham J, Lakshminrusimha S, Brennan LA, Black SM. Increased hydrogen peroxide downregulates soluble guanylate cyclase in the lungs of lambs with persistent pulmonary hypertension of the newborn. Am J Physiol Lung Cell Mol Physiol 289: L660–L666, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wild LM, Nickerson PA, Morin FC 3rd. Ligating the ductus arteriosus before birth remodels the pulmonary vasculature of the lamb. Pediatr Res 25: 251–257, 1989. [DOI] [PubMed] [Google Scholar]
- 53.Wosniak J Jr, Santos CX, Kowaltowski AJ, Laurindo FR. Cross-talk between mitochondria and NADPH oxidase: effects of mild mitochondrial dysfunction on angiotensin II-mediated increase in Nox isoform expression and activity in vascular smooth muscle cells. Antioxid Redox Signal 11: 1265–1278, 2009. [DOI] [PubMed] [Google Scholar]
- 54.Zayek M, Cleveland D, Morin FC 3rd. Treatment of persistent pulmonary hypertension in the newborn lamb by inhaled nitric oxide. J Pediatr 122: 743–750, 1993. [DOI] [PubMed] [Google Scholar]
- 55.Zhang J, Jin N, Liu Y, Rhoades RA. Hydrogen peroxide stimulates extracellular signal-regulated protein kinases in pulmonary arterial smooth muscle cells. Am J Respir Cell Mol Biol 19: 324–332, 1998. [DOI] [PubMed] [Google Scholar]
- 56.Zhang L, Sheppard OR, Shah AM, Brewer AC. Positive regulation of the NADPH oxidase NOX4 promoter in vascular smooth muscle cells by E2F. Free Radic Biol Med 45: 679–685, 2008. [DOI] [PubMed] [Google Scholar]