Abstract
Genetic data suggest that IL-6 trans-signaling may have a pathogenic role in the lung; however, the effects of IL-6 trans-signaling on lung effector cells have not been investigated. In this study, human airway smooth muscle (HASM) cells were treated with IL-6 (classical) or IL-6+sIL6R (trans-signaling) for 24 h and gene expression was measured by RNAseq. Intracellular signaling and transcription factor activation were assessed by Western blotting and luciferase assay, respectively. The functional effect of IL-6 trans-signaling was determined by proliferation assay. IL-6 trans-signaling had no effect on phosphoinositide-3 kinase and Erk MAP kinase pathways in HASM cells. Both classical and IL-6 trans-signaling in HASM involves activation of Stat3. However, the kinetics of Stat3 phosphorylation by IL-6 trans-signaling was different than classical IL-6 signaling. This was further reflected in the differential gene expression profile by IL-6 trans-signaling in HASM cells. Under IL-6 trans-signaling conditions 36 genes were upregulated, including PLA2G2A, IL13RA1, MUC1, and SOD2. Four genes, including CCL11, were downregulated at least twofold. The expression of 112 genes was divergent between IL-6 classical and trans-signaling, including the genes HILPDA, NNMT, DAB2, MUC1, WWC1, and VEGFA. Pathway analysis revealed that IL-6 trans-signaling induced expression of genes involved in regulation of airway remodeling, immune response, hypoxia, and glucose metabolism. Treatment of HASM cells with IL-6+sIL6R induced proliferation in a dose-dependent fashion, suggesting a role for IL-6 trans-signaling in asthma pathogenesis. These novel findings demonstrate differential effect of IL-6 trans-signaling on airway cells and identify IL-6 trans-signaling as a potential modifier of airway inflammation and remodeling.
Keywords: IL-6, IL-6 trans-signaling, human airway smooth muscle, gene expression, airway disease
interleukin 6 (IL-6), a proinflammatory cytokine, is implicated in a variety of inflammatory diseases (16). IL-6 binds the cognate IL-6 receptor (IL6R) that is in complex with the coreceptor glycoprotein 130 (gp130), resulting in activation of Jak/Stat-associated gene expression. The IL-6 receptor is membrane bound but also exists in a soluble form (sIL6R). Extracellular levels of sIL6R are increased through a proteolytic cleavage process termed “shedding.” Extracellular IL-6 can bind sIL6R, resulting in a complex that can regulate classical IL-6 signaling by adsorbing free IL-6. In addition, the IL-6/sIL6R complex can interact with the constitutively expressed membrane-bound gp130, resulting in altered Jak/Stat cascade activation in cells that do not express the IL-6 receptor. This extracellular process is termed IL-6 trans-signaling and is associated with a range of inflammatory diseases, including rheumatoid arthritis (54), inflammatory bowel disease (57), and cancer (60). Findings from genomic studies further revealed a potential pathological role of IL-6 trans-signaling in multiple diseases including in asthma.
The IL6R coding variation Asp358Ala (rs2228145) exists in all ethnicities tested (http://www.ncbi.nlm.nih.gov/SNP/), with minor allele frequencies ranging from 5 to 50%. This common coding change modifies the IL-6 receptor peptide structure adjacent to the exterior cell surface (18) and significantly enhances proteolytic cleavage of IL-6 receptor into the extracellular space, thus increasing the amount of sIL6R available for IL-6 buffering or IL-6 trans-signaling (60). We have recently shown that the IL6R 358Ala allele is associated with altered lung function in asthma and hence identified sIL6R as a novel genetic marker of asthma severity (23). Furthermore, we have shown that the IL-6 receptors are highly expressed on the lung epithelium and that sIL6R is present in bronchoalveolar lavage (BAL) fluids in subjects with asthma. Given these observations, we proposed that sIL6R has a pathogenic role in the lung, possibly by increasing IL-6 trans-signaling. However, the effect of IL-6 trans-signaling on resident airway cells and the molecular mechanism by which IL-6 trans-signaling may contribute to pathological changes in airway inflammation are not known.
Airway smooth muscle (ASM) cells are the regulators of airway tone and play a significant role in the pathogenesis of airway inflammation. ASM cells are one of the primary targets of inflammatory mediators (1, 9, 14, 15), and inflammatory cells can bind directly to ASM cells (28). ASM cells express receptors for a variety of cytokines, namely IL-13, IL-1β, and TNF-α (73), and exposure of ASM cells to inflammatory cytokines results in significant changes in the expression of genes and cell functions (contraction, relaxation, secretion, migration) (9). There are conflicting reports whether ASM cells express the IL-6 receptor (1, 39). Furthermore, the effects of classical IL-6 signaling and IL-6 trans-signaling in ASM have not been thoroughly investigated. In this study, we hypothesized that IL-6+sIL6R activates intracellular signaling pathways, induces gene expression, and thereby results in functional changes in human ASM (HASM) cells. The findings from a combination of high-throughput RNAseq analysis and biochemical and functional studies on HASM cells reveals that IL-6 trans-signaling induces differential gene expression in HASM cells compared with classical IL-6 signaling. Most importantly, IL-6 trans-signaling, but not IL-6 alone, induced HASM proliferation, suggesting a pathological role of IL-6 trans-signaling.
MATERIALS AND METHODS
Cell culture, cytokine treatment, and protein isolation.
HASM cells were obtained from deidentified human trachea from human lung transplant donors according to Human Subject Protection protocols at the University of Pennsylvania. HASM cells were cultured under normoxic conditions in DMEM-F-12 or SMGM (Lonza, for the Clonetics lines) until confluent and then starved in serum- and growth factor-free medium for 24 h. Cells were then treated with IL-6 (20 ng/ml) (Sigma, St. Louis, MO), or IL-6+sIL6R (20 ng/ml each) (Sigma) for the indicated times and harvested in lysis buffer [50 mM Tris·HCl, pH 7.5, 150 mM NaCl, 1% Nonidet P-40, 1 mM EDTA, and protease, and phosphatase inhibitors (Sigma)]. Protein extracts were generated by vortexing the samples for 30 s then centrifuging at 20,000 g for 20 min. Protein concentrations were determined by BCA protein assay (Pierce, Grand Island, NY).
Polyacrylamide gel electrophoresis and Western blot analysis.
Protein separation and Western blot analysis was carried out by using standard protocols. Briefly, equal amounts of protein were separated on a 10% SDS polyacrylamide (Bio-Rad, Hercules, CA) gel for 1 h at 150 V, 60 mA per gel and transferred to a polyvinylidene difluoride membrane (Millipore, Billerica, MA) for 1 h. Western blotting was performed using primary antibodies, pStat3, panStat3, pAkt, pan Akt, pErk, pan Erk, or GAPDH (Cell Signaling Technologies, Danvers, MA) diluted in TBS-T (Tris-buffered saline-0.1% Tween 20) with 5% BSA per the manufacturer's instructions. Secondary antibodies were used at 1:2,500 dilutions. The bands were visualized by using Super Signal Chemiluminescent West Pico Substrate (Pierce) on Kodak BioMax Light film for 15–60 s.
Luciferase and cell proliferation assays.
Human ASM cells stably expressing luciferase gene under the control of transcription factor Stat3 were generated by using lentiviral particles (SABiosciences, Qiagen, Valencia, CA) as described previously (45, 46). Stable cells were selected by using 200 μg/ml of G418 (Life Technologies, Grand Island, NY) for 2–3 wk. For luciferase assays, cells were plated on a 24-well plate and treated with IL-6 (20 ng/ml) or IL-6+sIL6R (20 ng/ml each) for 24 h, lysate was harvested in the lysis buffer, and luciferase activity was assessed by using a luciferase assay kit (Promega, Madison, WI) per manufacturer's protocol. In a select set of experiments, the cells were pretreated with WP1066 (Santa Cruz Biotechnology, Santa Cruz, CA), a Stat3 inhibitor, 10 min before adding IL-6 and IL-6+sIL6R. Luciferase activity was calculated by normalizing luminescence values (arbitrary units) to total protein loading.
Cell proliferation was determined by use of the Chemiluminescent BrdU Proliferation Assay (Roche, Nutley, NJ). Cells were plated at 500 cells/well in a 96-well plate for 24 h, serum starved for 24 h, and then treated with IL-6 (20, 40, and 80 ng/ml), sIL6R (20, 40, and 80 ng/ml), or IL-6+sIL6R (20, 40, and 80 ng/ml of each). After serum deprivation followed by drug treatment, cells were labeled with bromodeoxyuridine (BrdU) per the manufacturer's protocol for 24 h. The chemiluminescence was developed and measured on a Victor 3 plate reader (Perkin Elmer, Waltham, MA) with luminescence capability and a photomultiplier.
IL-6 and IL6R treatments, RNA preparation, and RNASeq.
HASM cells from six deidentified subjects without asthma were grown to confluence under normoxic conditions in medium containing serum and then maintained in serum-free medium for 24 h to allow for cell cycle synchronization. Cells were then provided fresh serum-free medium containing IL-6 (20 ng/ml), sIL6R (20 ng/ml), or IL-6+sIL6R (20 ng/ml each) and incubated at 37°C for 24 h. HASM cells were then harvested and total RNA was isolated by using TRIzol reagent (Invitrogen, Grand Island, NY) per manufacturer's instructions. The total RNA concentration, purity, and RNA integrity (RIN) were assessed using a 2100 Bioanalyzer (Agilent, Santa Clara, CA). All RNA samples analyzed had RIN values >8.5. Poly-A mRNA was enriched using the Dynabeads mRNA DIRECT Micro purification kit (Life Technologies, Foster City, CA). Barcoded RNAseq libraries were generated by using the SOLiD Total RNAseq kit (Life Technologies) on an automated SOLiD Library Builder (Life Technologies).
RNAseq data analysis.
The RNAseq libraries were sequenced on an ABI 5500XL W DNA sequencer (Life Technologies) generating >50 million reads per sample by use of single-end 75-bp sequencing. Data generated on the ABI SOLiD (.xseq) platform were analyzed by first pass using the LifeScope Genomic Analysis Software v 2.5 (Life Technologies). Binary Alignment/Map files generated against the reference human genome GRCh37/hg19 were used in the secondary analysis using the LifeScope software to generate expression values as RPKM (reads per kilobyte per million).
Statistical analysis.
RPKM values were converted to RPKM+1 for log2 conversion and then analyzed by linear modeling and empirical Bayes statistics, which was implemented in R package limma available from Bioconductor (64). The genes selected were those where expression passed thresholds of P < 0.05 and log2 ratio > or < 0.5. False discovery rate (FDR) was determined by Benjamini-Hochberg procedure (6). The Ingenuity Pathway Analysis (IPA) (Ingenuity, http://www.ingenuity.com) was used to identify functional networks for genes with significant increases or decreases in expression.
qPCR validation.
Quantitative (q)PCR was used to validate RNAseq for three genes. The premade TaqMan expression assays (Life Technologies) for the genes PLA2G2A (Hs00179898), MUC1 (Hs00159357), and CCL11 (Hs00237013) were used. The RNA was converted to cDNA by using the Reverse Transcription Reagents kit (Life Technologies, Grand Island, NY) using 1 μg of RNA and random hexamers. PCR was performed with TaqMan primers. Thermocycling conditions were those recommended: 25°C for 10 min, 48°C for 30 min, and 95°C for 5 min. qPCR was performed on an ABI 7300 HT real-time PCR machine. GAPDH was used as an internal control.
RESULTS
Kinetics of activation and functional effects of classical IL-6 signaling and IL-6 trans-signaling.
There has been a disagreement in the literature whether HASM cells express IL-6 receptors and whether HASM cells are responsive to IL-6 alone (1, 39). Expression of IL6R was first confirmed in all six cell lines of HASM cells by RNAseq at baseline and 24-h time points (data not shown). RPKM measures for IL6R expression (range 2.6–9.5) were consistent across all cell lines under all treatment conditions and remained relatively unchanged by classical (IL-6 alone) or trans-signaling (IL-6+sIL6R) treatments. Our data thus confirm the previous observation that IL6R is expressed in HASM.
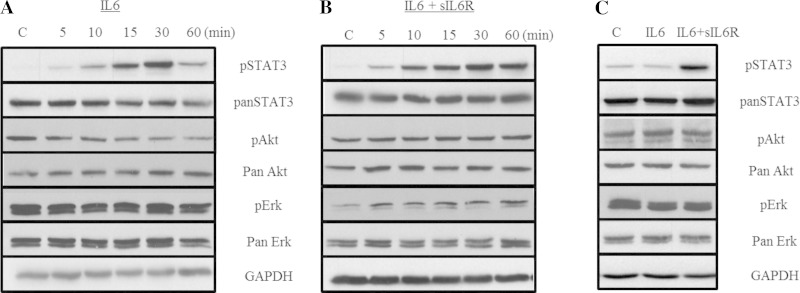
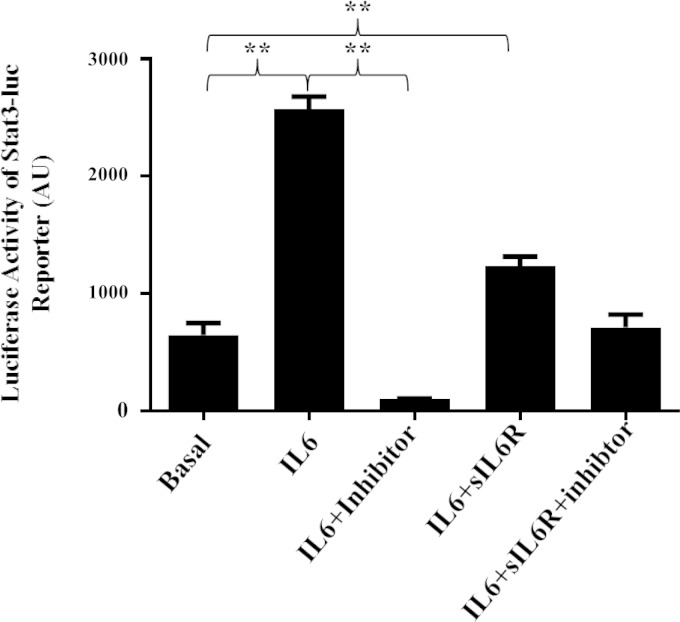
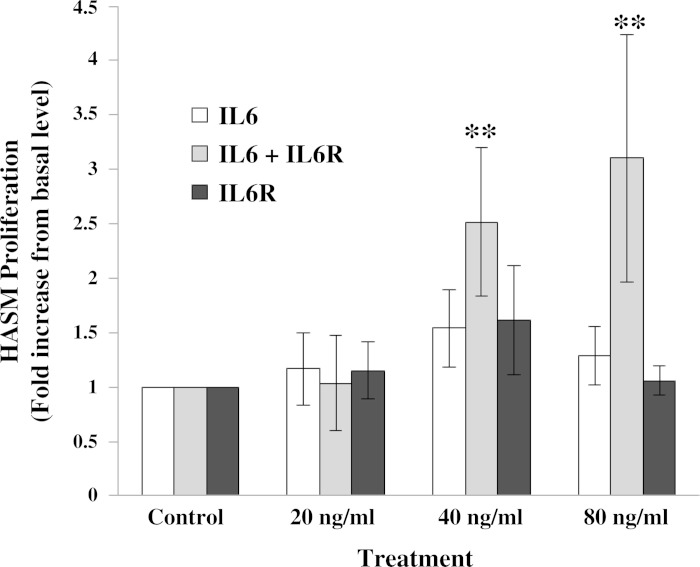
To confirm that HASM cells are responsive to both classical IL-6 signaling and IL-6 trans-signaling, HASM cells were treated with IL-6 alone or IL-6+sIL6R, and three downstream effectors of IL-6 signaling, Stat3, Erk, and Akt, were assessed for changes in activation by phosphorylation. Western blot analysis revealed that both classical IL-6 signaling and IL-6 trans-signaling induce Stat3 phosphorylation (Fig. 1). IL-6 alone rapidly induced Stat3 phosphorylation with attenuation of phosphorylation over a period of 60 min (Fig. 1A) demonstrating time-dependent loss of classical IL-6 signaling. In contrast, IL-6+sIL6R treatment resulted in rapid Stat3 phosphorylation at 60 min (Fig. 1B) and was sustained at 24 h (Fig. 1C). Neither classical IL-6 nor IL-6 trans-signaling treatment induced a change in phosphorylation of Erk or Akt at any time points. Treating HASM cells stably expressing Stat3-luc reporter with IL-6 or IL-6+sIL6R resulted in a significant (P < 0.05) increase in the luciferase activity (Fig. 2). Pretreating cells with 1 μM WP1066, a Stat3 inhibitor, abrogated luciferase expression induced by IL-6 and IL-6+sIL6R. These data suggest that signaling via classical and IL-6 trans-signaling in HASM cells primarily involves activation of the Stat3 transcription factor. Finally, to determine whether classical IL-6 signaling and IL-6 trans-signaling have functional effect on HASM, HASM cells were treated with increasing amounts of either IL-6, sIL6R, or IL-6+sIL6R for 24 h, and cell proliferation assessed by BrdU (Fig. 3). Under serum-free conditions, cells treated with IL-6+sIL6R showed a dose-dependent increase in BrdU incorporation (P = 0.048; Friedman's test). However, IL-6 alone did not have any effect on HASM proliferation. These findings suggest that IL-6 trans-signaling induces functional changes in HASM cells.
Fig. 1.
Classical and trans-IL-6 signaling in human airway smooth muscle (HASM) cells. HASM cells (n = 3) were treated with IL-6 (A) or IL-6 + soluble IL-6 receptor (sIL6R) (B), and activation of Akt, Erk, and Stat3 was assessed at 60 min and 24 h by Western blotting using pan- and phospho-antibodies. Shown are representative Western blot images. Both IL-6 and IL-6+sIL6R stimulation of HASM cells did not induce activation of phosphoinositide (PI)-3 kinase and mitogen-activated protein (MAP) kinase at both time points tested. Stat3 phosphorylation was evident under both classical and trans-IL-6 stimulation of HASM cells. Most notably, phosphorylation of Stat3 under IL-6+sIL6R, but not IL-6-only, treatment (C) was sustained for an extended period of time (24 h). These findings suggest differential kinetics of transcriptional activation by trans-IL-6 signaling in HASM cells. C, control.
Fig. 2.
Stat3-induced promoter activity in HASM cells. Transcription factor Stat3-induced promoter activity in HASM cells after stimulation of cells with IL-6 or IL-6+sIL6R was determined by luciferase assay using HASM cells stably expressing Stat3-luc. Stimulation of HASM cells with IL-6 or IL-6+sIL6R resulted in significant increase in luciferase activity (n = 3). Pretreating cells with WP1066, a Stat3 inhibitor, abrogated luciferase activity induced by IL-6 or IL-6+sIL6R, further confirming the involvement of Stat3 transcription factor in mediating classical and IL-6 trans-signaling in HASM cells (**P < 0.001). AU, arbitrary units.
Fig. 3.
Effect of IL-6 and IL-6+sIL6R on HASM cell proliferation. HASM were treated with serum-free media containing vehicle, 20, 40, and 80 ng/ml of IL-6; 20, 40, and 80 ng/ml each of IL-6+sIL6R; or 20, 40, and 80 ng/ml of sIL6R for 24 h. Bromodeoxyuridine (BrdU) incorporation was assessed as described in materials and methods. IL-6+sIL6R treatment resulted in a significant increase (**P < 0.05, Friedman test) in HASM proliferation compared with controls in a dose-dependent fashion. Data (means ± SE) are pooled from experiments using 5 individual cell lines.
Effects of classical IL-6 signaling and IL-6 trans-signaling on HASM cell gene expression.
Given the significant differences between the two IL-6 signaling paradigms in activation of Stat3 phosphorylation and ASM growth, we evaluated the effects of IL-6 and IL-6+sIL6R treatments on global gene expression profile in HASM cells from six deidentified subjects without asthma by use of RNAseq. RNA samples obtained from untreated HASM cells and HASM cells treated with sIL6R alone served as controls. Under IL-6 trans-signaling conditions, expression of 205 genes changed (decreased or increased) significantly (P ≤ 0.05) (Supplemental Table S1; Supplemental Material for this article is available online at the Journal website). None of these 205 genes were significantly upregulated or downregulated by sIL6R treatment alone. Of these 205 genes, expression of thirty-eight genes increased at least twofold (log 2 ≥ 1) over baseline in response to IL-6+sIL6R treatment (Table 1, columns 2 and 3). Expression of only two genes increased at least twofold over baseline under classical IL-6 signaling conditions (Table 1, columns 4 and 5). Under both classical and trans-signaling treatment conditions, the gene SOCS3 (suppressor of cytokine signaling 3) had the highest increase in expression. The top expressed gene other than SOCS3 under both treatment paradigms was PLA2G2A [type-II secretory phospholipase A2: sPLA2 (AII)], an enzyme associated with acute respiratory distress syndrome (ARDS) (35, 61, 74). PLA2G2A expression was higher under IL-6 trans-signaling conditions compared with classical IL-6 conditions (Table 2). Other upregulated genes with potential roles in airway diseases based on literature searches include SLC39A8 [solute carrier family 39 (zinc transporter), member 8] (8), IL6 (interleukin 6) (47, 51), MUC1 (mucin 1) (29–31, 36, 52), PTX3 (pentraxin 3) (3, 21, 22, 33, 75, 76), NNMT (nicotinamide N-methyltransferase) (58, 59), IL13RA1 (interleukin 13 receptor alpha 1) (5), and SOD2 (superoxide dismutase 2) (41). OSMR (oncostatin M receptor), a member of the IL-6 receptor family (complexes with coreceptor gp130) (15), was significantly elevated under both treatment conditions. The gene CCL11 (eotaxin 1) was the most downregulated gene under IL-6 trans-signaling conditions (Table 1).
Table 1.
Genes with ≥2-fold increase or decrease in expression under IL-6 trans-signaling vs. classical IL-6 signaling
| IL-6Trans-signaling |
Classical IL-6 Signaling |
|||||
|---|---|---|---|---|---|---|
| Gene | Log2 FC | P value | FDR P value | Log2 FC | P value | FDR P value |
| SOCS3 | 2.40 | 3.5 × 10−6 | 0.008 | 1.15 | 0.004 | * |
| PLA2G2A | 2.40 | 1.4 × 10−5 | 0.017 | 0.96 | 0.023 | * |
| STEAP2 | 1.74 | 0.00015 | 0.08 | 0.58 | * | |
| STEAP1 | 1.70 | 0.01 | * | 0.70 | * | |
| TEX29 | 1.70 | 7.6 × 10−5 | 0.06 | 0.54 | * | |
| AGT | 1.66 | 0.005 | * | 0.92 | * | |
| APLN | 1.56 | 0.0006 | * | 0.48 | * | |
| C10orf10 | 1.48 | 1.0 × 10−5 | 0.014 | 0.71 | 0.007 | * |
| SLC39A8 | 1.45 | 0.003 | * | 0.36 | * | |
| FAM167B | 1.39 | 2.5 × 10−6 | 0.007 | 0.73 | 0.0017 | * |
| SNED1 | 1.37 | 0.004 | * | 0.61 | * | |
| STEAP4 | 1.33 | 0.006 | * | 0.40 | * | |
| IL6 | 1.30 | 0.005 | * | 0.22 | * | |
| MUC1 | 1.30 | 3.3 × 10−8 | 0.0003 | 0.55 | 0.0006 | * |
| WWC1 | 1.29 | 1.9 × 10−6 | 0.006 | 0.36 | * | |
| BCL3 | 1.28 | 0.0001 | 0.07 | 0.70 | 0.012 | * |
| JUNB | 1.27 | 0.0003 | * | 0.68 | 0.025 | * |
| SOD2 | 1.27 | 0.006 | * | 0.42 | * | |
| PTX3 | 1.25 | 0.05 | * | 0.35 | * | |
| CA12 | 1.24 | 0.002 | * | 0.46 | * | |
| NNMT | 1.23 | 3.3 × 10−8 | 0.0003 | 0.34 | 0.013 | * |
| KIAA0226L | 1.23 | 0.0009 | * | 0.42 | * | |
| C3 | 1.22 | 0.004 | * | 0.52 | * | |
| NAMPT | 1.21 | 0.01 | * | 0.42 | * | |
| CEBPD | 1.20 | 2.8 × 10−5 | 0.03 | 0.43 | * | |
| SBNO2 | 1.17 | 6.5 × 10−5 | 0.05 | 0.59 | 0.016 | * |
| PCBP3 | 1.15 | 0.00055 | * | 0.47 | * | |
| ANGPTL4 | 1.13 | 0.006 | * | 0.34 | * | |
| IFI30 | 1.13 | 0.0005 | * | 0.43 | * | |
| KIAA1199 | 1.09 | 0.01 | * | 0.44 | * | |
| PDPN | 1.08 | 0.0002 | * | 0.35 | * | |
| GREM1 | 1.08 | 0.004 | * | 0.41 | * | |
| OSMR | 1.06 | 9.3 × 10−6 | 0.013 | 0.38 | 0.035 | * |
| TUBB3 | 1.03 | 0.0007 | * | 0.64 | 0.02 | * |
| RARRES3 | 1.02 | 5.2 × 10−6 | 0.009 | 0.47 | 0.007 | * |
| IL13RA1 | 1.01 | 0.00056 | * | 0.31 | * | |
| GDF10 | −1.02 | 0.0004 | * | −0.24 | * | |
| SCN7A | −1.16 | 0.007 | * | −0.71 | * | |
| SDPR | −1.32 | 0.05 | * | −0.50 | * | |
| CCL11 | −1.52 | 0.009 | * | −0.24 | * | |
False discovery rate (FDR) P value >0.09. FC, fold change.
Table 2.
Genes significantly differentially up- or downregulated by IL-6 (contrasting classical IL-6 signaling and IL-6 trans-signaling)
| Trans-signaling | Classical Signaling | Trans-signaling | Classical Signaling | Trans-signaling | Classical Signaling | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gene | Log2 FC | Log2 FC | P Value | Gene | Log2 FC | Log2 FC | P Value | Gene | Log2 FC | Log2 FC | P Value |
| HILPDA* | 0.78 | 0.05 | 4.2 × 10−7 | MAP3K8 | 0.84 | 0.27 | 0.006 | SLC39A8 | 1.45 | 0.36 | 0.02 |
| NNMT* | 1.23 | 0.34 | 2.2 × 10−6 | BHLHE40 | 0.78 | 0.22 | 0.007 | UGCG | 0.61 | 0.15 | 0.02 |
| DAB2 | 0.72 | 0.30 | 2.9 × 10−5 | GPR146 | 0.55 | 0.08 | 0.007 | ARHGAP31 | −0.50 | −0.18 | 0.03 |
| MUC1 | 1.30 | 0.55 | 3.0 × 10−5 | HK2 | 0.74 | 0.23 | 0.007 | BCL3 | 1.28 | 0.70 | 0.03 |
| WWC1 | 1.29 | 0.36 | 7.9 × 10−5 | S1PR3 | −0.57 | −0.20 | 0.007 | BNIP3 | 0.81 | 0.16 | 0.03 |
| VEGFA | 0.95 | 0.31 | 0.0001 | TNK1 | 0.51 | 0.14 | 0.007 | C1GALT1 | 0.70 | 0.17 | 0.03 |
| RHOJ | −0.94 | −0.28 | 0.0002 | LPCAT1 | 0.65 | 0.22 | 0.008 | C1S | 0.56 | 0.11 | 0.03 |
| GLIS3 | 0.72 | 0.26 | 0.0003 | ZNF395 | 0.56 | 0.13 | 0.008 | DENND2A | −0.69 | −0.22 | 0.03 |
| FAM115C | 0.59 | 0.10 | 0.0006 | APLN | 1.56 | 0.48 | 0.009 | DTX3L | 0.66 | 0.26 | 0.03 |
| PGK1 | 0.84 | 0.12 | 0.0006 | IL13RA1 | 1.01 | 0.31 | 0.009 | ENO2 | 0.72 | 0.20 | 0.03 |
| PARP9 | 0.72 | 0.21 | 0.0007 | ATF3 | 0.83 | 0.22 | 0.01 | EPSTI1 | 0.91 | 0.12 | 0.03 |
| AVIL | 0.56 | 0.04 | 0.0008 | BCL6 | 0.83 | 0.41 | 0.01 | GPI | 0.53 | 0.22 | 0.03 |
| OSMR | 1.06 | 0.38 | 0.0008 | C1RL | 0.82 | 0.38 | 0.01 | HIF1A | 0.61 | 0.15 | 0.03 |
| LPCAT3 | 0.71 | 0.32 | 0.0009 | ENO1 | 0.51 | 0.13 | 0.01 | LDHA | 0.68 | 0.16 | 0.03 |
| TNFSF13B | 0.66 | 0.04 | 0.0009 | ICAM1 | 0.97 | 0.46 | 0.01 | PDK1 | 0.78 | 0.16 | 0.03 |
| PFKFB4 | 0.60 | 0.11 | 0.001 | IFI6 | 0.53 | 0.06 | 0.01 | SH3D19 | −0.58 | −0.21 | 0.03 |
| TEX29 | 1.68 | 0.54 | 0.002 | PLOD2 | 0.62 | −0.04 | 0.01 | SLC2A1 | 0.76 | 0.28 | 0.03 |
| CEBPD | 1.20 | 0.43 | 0.002 | ZBTB46 | −0.55 | −0.17 | 0.01 | SLC2A3 | 0.87 | 0.19 | 0.03 |
| PLA2G2A | 2.40 | 0.96 | 0.002 | ADAMTS14 | −0.56 | 0.01 | 0.02 | TMEM2 | 0.64 | 0.18 | 0.03 |
| RARRES3 | 1.02 | 0.47 | 0.002 | AIF1L | −0.67 | −0.13 | 0.02 | ANGPTL4 | 1.13 | 0.34 | 0.04 |
| RUNX1 | 0.78 | 0.25 | 0.002 | APOL1 | 0.78 | 0.32 | 0.02 | CA12 | 1.24 | 0.46 | 0.04 |
| SLC2A5 | 0.69 | 0.11 | 0.002 | ASAP2 | −0.54 | −0.26 | 0.02 | CASS4 | −0.70 | −0.08 | 0.04 |
| SOCS3 | 2.41 | 1.15 | 0.002 | KIAA0226L | 1.23 | 0.42 | 0.02 | CNTNAP1 | 0.73 | 0.38 | 0.04 |
| KIAA1755 | −0.71 | −0.23 | 0.003 | C7orf63 | 0.62 | 0.11 | 0.02 | GLRX | 0.61 | 0.22 | 0.04 |
| SLC16A3 | 0.81 | 0.28 | 0.003 | CCL11 | −1.52 | −0.24 | 0.02 | GYS1 | 0.84 | 0.33 | 0.04 |
| ADM | 0.71 | 0.22 | 0.004 | CSRP2 | 0.41 | −0.11 | 0.02 | ID2 | 0.66 | 0.24 | 0.04 |
| AKAP2 | 0.88 | 0.35 | 0.004 | EGR2 | −0.97 | −0.33 | 0.02 | STEAP4 | 1.33 | 0.40 | 0.04 |
| C10orf10 | 1.48 | 0.71 | 0.004 | GRIK4 | −0.52 | −0.16 | 0.02 | AOX1 | 0.84 | 0.20 | 0.05 |
| ELL2 | 0.62 | 0.17 | 0.004 | IFI30 | 1.13 | 0.43 | 0.02 | C1R | 0.62 | 0.21 | 0.05 |
| FAM167B | 1.39 | 0.73 | 0.004 | IL16 | −0.72 | −0.20 | 0.02 | ENOX1 | 0.58 | 0.17 | 0.05 |
| GDF10 | −1.02 | −0.24 | 0.004 | IL6 | 1.30 | 0.22 | 0.02 | FGFR4 | −0.61 | −0.10 | 0.05 |
| GFPT2 | 0.52 | 0.16 | 0.004 | KIAA0247 | 0.60 | 0.14 | 0.02 | GREM1 | 1.08 | 0.41 | 0.05 |
| BASP1 | 0.55 | 0.18 | 0.005 | OBFC2A | 0.63 | 0.10 | 0.02 | JUNB | 1.27 | 0.68 | 0.05 |
| HRH1 | 0.87 | 0.34 | 0.005 | PCBP3 | 1.15 | 0.47 | 0.02 | NXPH3 | −0.51 | −0.20 | 0.05 |
| PALM2-AKAP2 | 0.88 | 0.34 | 0.005 | PIM1 | 0.91 | 0.22 | 0.02 | PTGER4 | −0.99 | −0.36 | 0.05 |
| PDPN | 1.08 | 0.35 | 0.005 | RASL11A | 0.54 | 0.15 | 0.02 | SOD2 | 1.27 | 0.42 | 0.05 |
| STEAP2 | 1.74 | 0.58 | 0.005 | SBNO2 | 1.17 | 0.59 | 0.02 | ||||
| TCF21 | −0.78 | −0.18 | 0.005 | SEL1L3 | 0.64 | 0.19 | 0.02 |
Two genes with significant FDR P values <0.05; HILPDA (P = 0.0096) and NNMT (P = 0.025). FC, fold change.
One hundred twelve genes were differentially expressed (P ≤ 0.05) when comparing the fold change in gene expression between two treatment paradigms (Table 2). In all 112 genes, the greatest change in expression occurred under IL-6 trans-signaling conditions, with 20 genes being significantly downregulated and 92 genes upregulated. A few notable genes that showed differential increase in expression include HILPDA (hypoxia-inducible lipid droplet-associated), NNMT (nicotinamide N-methyltransferase), DAB2 (mitogen-responsive phosphoprotein, homolog 2), MUC1 (cell surface mucin 1), WWC1 (WW and C2 domain containing 1), and VEGFA (vascular endothelial growth factor A). On the contrary, expression of following genes decreased with IL-6 treatment: RHOJ (ras homolog family member J), KIAA1755, GDF10 (growth differentiation factor 10), TCF21 (transcription factor 21), and S1PR3 (sphingosine-1-phosphate receptor 1).
Pathway analysis of differentially expressed genes.
The 205 genes with significant increase or decrease in expression under both treatment paradigms were analyzed by using the IPA program and were assessed based on the degree of upregulation or downregulation in each pathway. Under IL-6 trans-signaling conditions (Table 3), several pathways were enriched for gene expression (P ≤ 0.05 after FDR adjustment) (6), including positive regulation of cell proliferation, response to oxygen levels/hypoxia, immune response, and hexose/monosaccharide/glucose metabolism pathways. Under classical IL-6 conditions (Table 4), only two pathways were enriched after FDR adjustment: inflammatory response and response to external stimulus. Table 5 illustrates the role of some of the upregulated genes in lung function and lung disease.
Table 3.
Pathways upregulated by IL-6 trans-signaling
| Pathways | Genes | P Value | FDR P Value |
|---|---|---|---|
| Positive regulation of cell proliferation | NAMPT, FGFR4, IL6, CCL2, OSMR, ARNT2, SOX4, HES1, S1PR3, ATF3, HIF1A, OSR2, TNFSF13B, ADM, ID2, AGT, VEGFA, IL12A, PDGFRB, BCL6, IL13RA1, MYC | 2.3 × 10−7 | 3.9 × 10−5 |
| Response to oxygen levels/hypoxia | SLC8A1, CCL2, SOCS3, PDPN, ALDOC, ARNT2, BNIP3, SOD2, HIF1A, ADM, PLOD2, VEGFA, ANGPTL4 | 1.2 × 10−7 | 2.1 × 10−4 |
| Regulation of response to external stimulus | ZFP36, SBNO2, IL6, IL16, C3, OSMR, PDPN, GREM1, NPC1, AGT, VEGFA, PLA2G2A, BCL6, TUBB3 | 4.5 × 10−7 | 7.7 × 10−4 |
| Hexose/monosaccharide/glycolysis metabolic processes | PDK1, LDHA, PFKFB4, GMDS, ALDOC, HK2, GPI, ATF3, GFPT2, GYS1, ENO2, PGK1, MYC, ENO1 | 5.2 × 10−7 | 8.8 × 10−4 |
| Regulation of cell proliferation | RARRES3, NAMPT, FGFR4, CCL2, OSMR, ARNT2, SOX4, IFI30, S1PR3, OSR2, CDKN2B, AGT, BCL6, IL13RA1, MYC, IL6, SOD2, HES1, ATF3, HIF1A, ADAMTS8, TNFSF13B, ID2, ADM, VEGFA, IL12A, PLA2G2A, PDGFRB | 6.6 × 10−7 | 0.001 |
| Immune response | SBNO2, CCL2, IL16, C3, BNIP3, C1R, C1S, IL7R, IFI35, GCH1, BCL3, PTX3, APLN, ICAM1, IL6, PTGER4, CCL11, GPI, APOL1, IL18BP, TNFSF13B, VEGFA, C1RL, IL12A, IFI6 | 2.4 × 10−6 | 0.004 |
| Immune effector process | ICAM1, IL6, TNFSF13B, C3, C1RL, BCL3, BNIP3, C1R, C1S, IL7R, PTX3 | 4.7 × 10−6 | 0.008 |
| Positive regulation of cellular biosynthetic process | GLIS3, ICAM1, IL6, EGR2, NFE2, HSD3B7, ABLIM3, ARNT2, GLIS1, SOX4, AFAP1L2, JUNB, SOD2, HES1, TCF21, HRH1, HIF1A, AGT, VEGFA, TEAD4, BCL3, PTX3, RUNX1, MYC | 7.0 × 10−6 | 0.01 |
| Negative regulation of apoptosis | IL6, CCL2, SOCS3, ARNT2, PIM1, BNIP3, SOX4, SOD2, TNFSF13B, AGT, VEGFA, BCL3, BCL6, MYC, IFI6, ANGPTL4 | 2.3 × 10−5 | 0.04 |
| Positive regulation of nitrogen compound metabolic process | GLIS3, ICAM1, IL6, EGR2, NFE2, ABLIM3, ARNT2, GLIS1, SOX4, AFAP1L2, JUNB, SOD2, HES1, TCF21, HRH1, HIF1A, VEGFA, TEAD4, BCL3, PTX3, RUNX1, MYC | 2.8 × 10−5 | 0.048 |
Genes in bold with gene expression changes ≥2-fold.
Table 4.
Pathways upregulated by classical IL-6 signaling
| Pathways | Genes | P Value | FDR P Value |
|---|---|---|---|
| Regulation of inflammatory response | ZFP36, SBNO2, C3, AGT, PLA2G2A | 6.3 × 10−7 | 9.4 × 10−4 |
| Regulation of response to external stimulus | ZFP36, SBNO2, C3, AGT, PLA2G2A | 1.2 × 10−5 | 0.02 |
| Response to abiotic stimulus | SOCS3, AGT, BCL3, JUNB, TUBB3 | 3.2 × 10−4 | 0.5 |
| Positive regulation of inflammatory response | C3, AGT, PLA2G2A | 3.7 × 10−4 | 0.5 |
| Response to radiation | SOCS3, BCL3, JUNB, TUBB3 | 8.2 × 10−4 | 1.2 |
Genes in bold with gene expression changes ≥2-fold.
Table 5.
Lung disease genes upregulated by IL-6 trans-signaling
| Gene | Disease/Condition | References |
|---|---|---|
| PLAG2G2A | ARDS, lung cancer, sepsis | 35, 61, 72, 74 |
| SLC39A8 | COPD, lung cancer, cadmium toxicity | 4, 8, 49 |
| IL6 | Asthma, COPD, sepsis | 10, 23, 24, 47, 51, 67 |
| MUC1 | Asthma, COPD, lung cancer, pulmonary fibrosis | 29, 31, 36, 52 |
| PTX3 | Asthma, lung cancer, ARDS, cystic fibrosis, sepsis | 33, 69, 75, 76 |
| IL13RA1 | Asthma, COPD | 5 |
| SOD2 | Lung cancer | 41 |
| VEGFA | COPD, Asthma, lung cancer, hypoxia | 2, 17, 34, 38 |
| S1PR3 | ARDS/ALI | 65 |
| NNMT | COPD, lung cancer | 58, 59 |
| CCL11 | Asthma, COPD | 11, 32, 53, 72 |
| GREM1 | Pulmonary sarcoidosis, idiopathic pulmonary fibrosis | 25, 37 |
| TCF21 | Lung cancer | 56, 63 |
| NAMPT | ARDS | 66 |
| APLN | Hypoxia, lung cancer | 7, 13, 55 |
ARDS, acute respiratory distress syndrome; COPD, chronic obstructive pulmonary disease; ALI, acute lung injury.
qPCR validation of PLA2G2A, MUC1, and CCL11 expression.
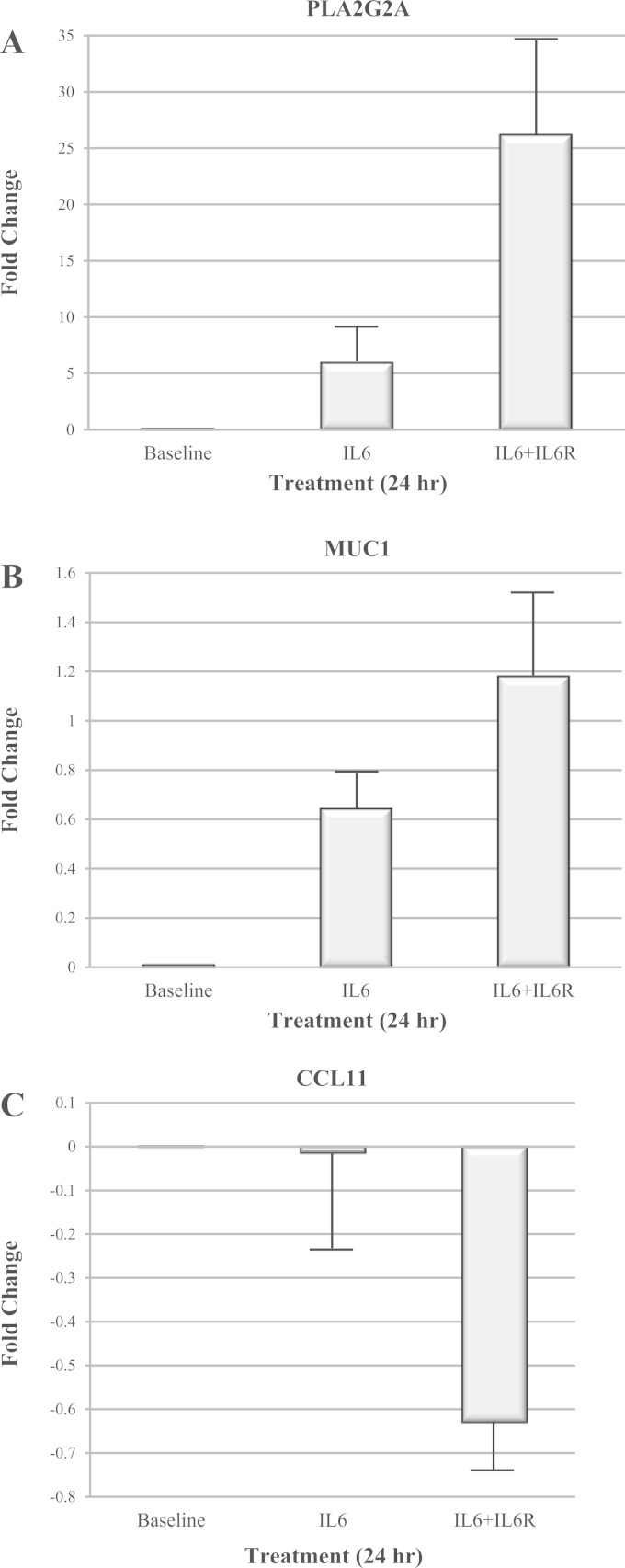
To validate the gene expression datasets, we selected two genes that were strongly upregulated (PLA2G2A and MUC1), and one gene that was strongly downregulated (CCL11) under IL-6 trans-signaling conditions, and determined gene expression by qPCR. Gene expression was again averaged across the six HASM cell lines. As observed in the RNAseq data, expression of PLA2G2A and MUC1 (Fig. 4, A and B, respectively) in HASM cells increased significantly (P < 0.05) upon treatment with IL-6 or IL-6+sIL6R compared with baseline. However, magnitude of increase in the expression of both the genes was higher under IL-6 trans-signaling conditions compared with classical IL-6 conditions. A similar pattern was observed for CCL11 (Fig. 4C), the gene whose expression decreased significantly (P < 0.05) in response to IL-6 treatment. Although the changes in expression levels of MUC1 and CCL11 were proportional to levels observed in the RNAseq analysis, the expression levels detected for PLA2G2A by using qPCR showed a more robust change in gene expression under IL-6 trans-signaling conditions by the qPCR method (∼26-fold increase) than observed in the RNAseq analysis (∼5.3-fold increase). A similar trend was also observed under classical IL-6 signaling with the qPCR showing a sixfold increase in PLA2G2A expression compared with the RNAseq analysis, which showed a twofold increase in PLA2G2A expression. This discrepancy is due to the very low baseline gene expression levels of PLA2G2A in the untreated cells and the conversion of RNAseq RPKM values to RPKM+1 elevating the values for the baseline expression levels. When changes in PLA2G2A expression were evaluated by using raw RPKM values, an average 6.1-fold change in PLA2G2A expression was observed under IL-6 treatment conditions, whereas a 29.2-fold change in PLA2G2A expression was observed under IL-6 trans-signaling conditions. These fold changes in PLA2G2A gene expression are nearly identical to the values observed with qPCR.
Fig. 4.
Validation of gene expression in HASM cells by quantitative (q)PCR. Expression of PLA2G2A (A), MUC1 (B), and CCL11 (C) in HASM cells after treating cells with IL-6 and IL-6+sIL6R for 24 h was determined by qPCR. Note a significant increase in the expression of PLA2G2A and MUC1 upon classical and IL-6 trans-signaling treatment. CCL11 expression was significantly decreased in cells treated with IL-6+sIL6R. These findings validated our RNAseq data.
DISCUSSION
We have previously shown that the IL-6 receptor is expressed on airway epithelial and airway smooth muscle (ASM) cells (23). Furthermore, we have measured sIL6R in BAL and have shown that the BAL sIL6R levels are correlated with lung function and with inheritance of the IL6R 358Ala variant. Thus a prime environment exists in the lung of subjects inheriting the IL6R 358Ala variant for IL-6 receptor shedding and associated activation of IL-6 trans-signaling under inflammatory conditions (i.e., elevated IL-6). The objective of this study was to determine whether IL-6 trans-signaling plays a role in modulating signaling and functions in lung effector cells by examining the effects of IL-6 trans-signaling on HASM cells and whether the signaling and functional effects of IL-6 trans-signaling are divergent from classical IL-6 signaling. The data from this study show that both classical IL-6 signaling and IL-6 trans-signaling primarily affect gene expression through activation of the transcription factor Stat3. However, only IL-6 trans-signaling resulted in sustained Stat3 activation at 24 h. Stat3 activation (luciferase assay) by IL-6 was robustly inhibited by a Stat3 inhibitor. However, Stat3 activation by IL-6 trans-signaling was less sensitive to inhibition by the same pharmacological inhibitor. Although this observation needs additional detailed investigation, such as measurement of phosphorylation at different amino acid residues in Stat3, it nevertheless provides hint at potential pathological role of IL-6 trans-signaling. Finally, analysis of gene expression by RNAseq showed that IL-6 trans-signaling results in a more robust upregulation of gene expression in HASM compared with classical IL-6 signaling, and the genes upregulated by IL-6 trans-signaling are associated with several important physiological and metabolic pathways including cell proliferation (VEGFA, IL6, AGT, PLA2G2A), response to hypoxia, immune response (HIF1A, ANGPTL4, SOD2), and glucose metabolism (PDK1, LDHA, ALDOC, HK2, GPI, ENO1, ENO2, PGK1). This was further confirmed by the observation that IL-6 trans-signaling, but not IL-6 alone, had a significant effect on HASM proliferation.
Several genes upregulated in HASM by IL-6 trans-signaling standout as having potential roles in airway diseases (Table 5). Most notable is VEGFA (vascular endothelial growth factor), a potent proangiogenic factor known to be upregulated by Th2 proinflammatory cytokines such as IL-4, IL-13, and IL-5 (71). In asthma, Vegf is one of the key factors that induce ASM cell proliferation and airway remodeling, resulting in thickening of the airways wall (34). The induction of VEGFA gene expression by IL-6 in ASM cells has been studied previously under classical IL-6 and IL-6 trans-signaling conditions, and those findings demonstrate that VEGF protein secretion is strongly induced under trans-signaling conditions and weakly induced under classical signaling conditions (1). Our data showing VEGFA gene expression is greater under trans-signaling conditions compared with classical IL-6 signaling are thus in agreement with this previous observation. In addition, our observation that IL-6 trans-signaling leads to greater HASM proliferation compared with classical IL-6 signaling suggests that Vegf activation could be driven by sustained IL-6 trans-signaling in the lung. IL-6 trans-signaling may contribute to asthma pathogenesis both by directly inducing ASM proliferation and indirectly by inducing the release of Vegf, which by paracrine effects may contribute to airway remodeling.
IL-6 trans-signaling activation of VEGFA expression could also have significant effects in other lung diseases. In lung cancer, Vegf promotes both angiogenesis and tumor progression and is a key target for anti-cancer therapies (40). VEGFA expression is also strongly linked to hypoxia (19). Interestingly, despite the normoxic conditions used in our experiments, we found that IL-6 trans-signaling resulted in an enrichment of genes associated with the hypoxia pathway (Table 3), including ANGPTL4 (48), HIF1A (62), APLN (70), and multiple glycolysis genes (Table 2) (42). Thus IL-6 trans-signaling appears to induce a pseudo-hypoxic gene expression pattern in HASM that is similar to the aerobic glycolysis state of the Warburg effect observed in cancer cells.
The most highly expressed gene under classical and trans-signaling conditions in this study was PLA2G2A, which encodes the acute phase protein (sPLA2) (IIA) (50). sPLA2 (IIA) is secreted by lung mast cells and macrophages and increased levels of this enzyme have been detected in BAL fluid of subjects with pneumonia, sarcoidosis, and ARDS (12, 20, 27, 35). Our results also suggest that sPLA2 (IIA) may also be secreted by HASM. In the lung, sPLA2 (IIA) can hydrolyze the sn-2 fatty acid from glycerophospholipids, resulting in the production of arachidonic acid, the precursor to inflammatory prostaglandins and the bronchoconstricting leukotrienes. Furthermore, sPLA2 (IIA) also causes lung surfactant degradation (26), which can result in increased air-liquid surface tension in the lung that can cause alveolar collapse-associated acute lung injury. sPLA2 (IIA) expression has also been observed in lung cancer, and knockdown of the PLA2G2A gene reduced the growth of lung cancer cells in vivo and in vitro (74). Interestingly, sPLA2 (IIA) has been implicated in the pathogenesis of rheumatoid arthritis (RA) (43), a disease associated with IL-6 trans-signaling (16). In a recent RA study, blockade of IL-6 trans-signaling by the anti-IL-6 receptor monoclonal antibody tocilizumab resulted in a 61% decline in the concentration of sPLA2 (IIA) in serum of treated subjects (43). Our qPCR data show that PLA2G2A mRNA expression is highly elevated (>26 fold) in HASM cells under IL-6 trans-signaling conditions. If sPLA2 (IIA) protein and activity levels are strongly correlated with PLA2G2A mRNA expression levels, we could then hypothesize that increased and sustained IL-6 trans-signaling in the lung could elevate the secretion of sPLA2 (IIA) in alveolus with two consequences. First, the increased production of arachidonic acid catalyzed by the metabolism of glycerophospholipids could lead to increased leukotriene production. Lahiri et al. (39) have already observed a synergy between IL-6 and IL-1β in the increase of PGE2 release from ASM and speculated that IL-6 was responsible for induction of phospholipase A2 activity. Second, elevation in sPLA2 (IIA) production would cause simultaneous destruction of lung surfactant leading to a loss of alveolar gas exchange. In light of these two potential consequences of IL-6 trans-signaling in the lung, we propose that anti-IL6R therapies could attenuate the deleterious effects of sPLA2 (IIA) expression in the lung.
Several genes with links to other lung diseases are also upregulated by IL-6 trans-signaling. First, the gene SLC39A8 encodes the zinc transporting protein known as ZIP8 (8), which is a major transporter of the toxic metal cadmium (Cd) (49). It has been hypothesized that ZIP8 expression is increased by cigarette smoke-induced inflammation and that the resulting increase of ZIP8 expression promotes the transport of Cd from cigarette smoke into lung epithelial cells. Interestingly, elevated ZIP8 mRNA and protein levels have been observed in the lungs of chronic smokers and physiological measures of Cd are linked to lung cancer and reduced lung function (8). A second notable gene is PTX, which encodes innate immunity protein pentraxin 3. Elevated levels of pentraxin 3 protein have been measured in the bronchial tissues of allergic asthmatic subjects and are primarily detected in ASM cells (75). Pentraxin 3 levels also become elevated in subjects with ARDS within 24 h of diagnosis, presumably as an activator of the local innate immune system in the lung (3). In addition, elevated levels of serum pentraxin 3 have been detected in subjects with sepsis and appear to be an important biomarker of sepsis severity (69). Given the strong association of the IL-6 pathway with these conditions, our data suggest that IL-6 trans-signaling could play an important role in elevating these markers of lung disease.
A major limitation of this study is that the observed gene expression is in an in vitro model and these results will have to be confirmed in vivo. IL-6 trans-signaling is driven by increased levels of IL-6 receptor shedding (60) and IL-6 receptor shedding levels are greatly enhanced by the IL-6 receptor 358Ala isoform (18). Thus confirmatory studies in subjects with inflammatory lung disease should be stratified by inheritance of the IL6R Asp358Ala coding variation (rs2228145). Additionally, to ensure that IL-6 trans-signaling is activated, subjects should be screened for elevated levels of lung or systemic IL-6 and sIL6R. In our previous study, subjects with severe asthma were nearly twice as frequently homozygous for the 358Ala IL-6 receptor isoform (∼28%) as were subjects with mild asthma (∼15%) (23). In addition, elevated levels of IL-6 have been observed in sputum and BAL of subjects with severe asthma (47, 51). Thus subjects with severe asthma who inherit the 358Ala IL-6 receptor may be the ideal genotypic group to confirm our gene expression results with respect to airway disease.
In summary, we have performed studies to determine the effect of IL-6 trans-signaling on human ASM cells by comparing intracellular signaling, transcription activation, gene expression profiles, and function effects (cell proliferation) by classical and IL-6 trans-signaling. Our data on human ASM cells clearly demonstrated a differential activation of signaling and gene expression profile by IL-6 trans-signaling compared with classical IL-6 signaling. These findings also establish IL-6 trans-signaling as a potential contributor to asthma pathogenesis, and future studies are needed to establish the effect of IL-6 trans-signaling on other cell types.
GRANTS
We acknowledge the support from the Wake Forest School of Medicine Office of Research for providing the critical Pilot Study funding this work and National Institutes of Health Grants HL109164, HL65899, HL089992, HL69167, AI8230, HL077916, and AG041265 for additional financial support.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
M.B.R., D.A.D., and G.A.H. conception and design of research; M.B.R., D.A.D., W.C., S.S., and G.A.H. performed experiments; M.B.R., J.C., W.C., and G.A.H. analyzed data; M.B.R., D.A.D., J.C., W.C., C.L., A.T.H., and G.A.H. interpreted results of experiments; M.B.R., W.C., and G.A.H. prepared figures; M.B.R., D.A.D., J.C., C.L., A.T.H., E.R.B., and G.A.H. drafted manuscript; M.B.R., D.A.D., J.C., C.L., A.T.H., E.R.B., and G.A.H. edited and revised manuscript; M.B.R., D.A.D., J.C., C.L., A.T.H., E.R.B., and G.A.H. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Abdoulaye Diallo, Carla Martin, and Lina Purcell for technical help in the laboratory. We also acknowledge the subjects who donated tissue for this study.
REFERENCES
- 1.Ammit AJ, Moir LM, Oliver BG, Hughes JM, Alkhouri H, Ge Q, Burgess JK, Black JL, Roth M. Effect of IL-6 trans-signaling on the pro-remodeling phenotype of airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 292: L199–L206, 2007. [DOI] [PubMed] [Google Scholar]
- 2.Balantic M, Rijavec M, Skerbinjek KM, Suskovic S, Silar M, Kosnik M, Korosec P. Asthma treatment outcome in children is associated with vascular endothelial growth factor A VEGFA polymorphisms. Mol Diagn Ther 16: 173–180, 2012. [DOI] [PubMed] [Google Scholar]
- 3.Balhara J, Koussih L, Zhang J, Gounni AS. Pentraxin 3: an immuno-regulator in the lungs. Front Immunol 4: 127, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bao S, Liu MJ, Lee B, Besecker B, Lai JP, Guttridge DC, Knoell DL. Zinc modulates the innate immune response in vivo to polymicrobial sepsis through regulation of NF-κB. Am J Physiol Lung Cell Mol Physiol 298: L744–L754, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beghe B, Hall IP, Parker SG, Moffatt MF, Wardlaw A, Connolly MJ, Fabbri LM, Ruse C, Sayers I. Polymorphisms in IL13 pathway genes in asthma and chronic obstructive pulmonary disease. Allergy 65: 474–481, 2010. [DOI] [PubMed] [Google Scholar]
- 6.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Statist Soc 57: 289–300, 1995. [Google Scholar]
- 7.Berta J, Kenessey I, Dobos J, Tovari J, Klepetko W, Jan AH, Hegedus B, Renyi-Vamos F, Varga J, Lorincz Z, Paku S, Ostoros G, Rozsas A, Timar J, Dome B. Apelin expression in human non-small cell lung cancer: role in angiogenesis and prognosis. J Thorac Oncol 5: 1120–1129, 2010. [DOI] [PubMed] [Google Scholar]
- 8.Besecker B, Bao S, Bohacova B, Papp A, Sadee W, Knoell DL. The human zinc transporter SLC39A8 Zip8 is critical in zinc-mediated cytoprotection in lung epithelia. Am J Physiol Lung Cell Mol Physiol 294: L1127–L1136, 2008. [DOI] [PubMed] [Google Scholar]
- 9.Black JL, Panettieri RA Jr, Banerjee A, Berger P. Airway smooth muscle in asthma: just a target for bronchodilation? Clin Chest Med 33: 543–558, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cordoba-Lanus E, de-Torres JP, Lopez-Aguilar C, Rodriguez-Perez MC, Maca-Meyer N, Montejo-de-Garcini A, Aguirre-Jaime A, Perez-Mendez L, Casanova C. Association of IL-6 gene polymorphisms and COPD in a Spanish population. Respir Med 102: 1805–1811, 2008. [DOI] [PubMed] [Google Scholar]
- 11.Daldegan MB, Teixeira MM, Talvani A. Concentration of CCL11, CXCL8 and TNF-alpha in sputum and plasma of patients undergoing asthma or chronic obstructive pulmonary disease exacerbation. Braz J Med Biol Res 389: 1359–1365, 2005. [DOI] [PubMed] [Google Scholar]
- 12.De Marino V, Gentile M, Granata F, Marone G, Triggiani M. Secretory phospholipase A2: a putative mediator of airway inflammation. Int Arch Allergy Immunol 1182–1184: 200–201, 1999. [DOI] [PubMed] [Google Scholar]
- 13.Eyries M, Siegfried G, Ciumas M, Montagne K, Agrapart M, Lebrin F, Soubrier F. Hypoxia-induced apelin expression regulates endothelial cell proliferation and regenerative angiogenesis. Circ Res 103: 432–440, 2008. [DOI] [PubMed] [Google Scholar]
- 14.Faffe DS, Whitehead T, Moore PE, Baraldo S, Flynt L, Bourgeois K, Panettieri RA, Shore SA. IL-13 and IL-4 promote TARC release in human airway smooth muscle cells: role of IL-4 receptor genotype. Am J Physiol Lung Cell Mol Physiol 285: L907–L914, 2003. [DOI] [PubMed] [Google Scholar]
- 15.Faffe DS, Flynt L, Mellema M, Moore PE, Silverman ES, Subramaniam V, Jones MR, Mizgerd JP, Whitehead T, Imrich A, Panettieri RA Jr, Shore SA. Oncostatin M causes eotaxin-1 release from airway smooth muscle: synergy with IL-4 and IL-13. J Allergy Clin Immunol 115: 514–520, 2005. [DOI] [PubMed] [Google Scholar]
- 16.Febbraio MA, Rose-John S, Pedersen BK. Is interleukin-6 receptor blockade the Holy Grail for inflammatory diseases? Clin Pharmacol Ther 87: 396–398, 2010. [DOI] [PubMed] [Google Scholar]
- 17.Forde PM, Ettinger DS. Targeted therapy for non-small-cell lung cancer: past, present and future. Expert Rev Anticancer Ther 13: 745–758, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galicia JC, Tai H, Komatsu Y, Shimada Y, Akazawa K, Yoshie H. Polymorphisms in the IL-6 receptor IL-6R gene: strong evidence that serum levels of soluble IL-6R are genetically influenced. Genes Immun 5: 513–516, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Goudar RK, Vlahovic G. Hypoxia, angiogenesis, and lung cancer. Curr Oncol Rep 10: 277–282, 2008. [DOI] [PubMed] [Google Scholar]
- 20.Granata F, Balestrieri B, Petraroli A, Giannattasio G, Marone G, Triggiani M. Secretory phospholipases A2 as multivalent mediators of inflammatory and allergic disorders. Int Arch Allergy Immunol 131: 153–163, 2003. [DOI] [PubMed] [Google Scholar]
- 21.Hamon Y, Jaillon S, Person C, Ginies JL, Garo E, Bottazzi B, Ghamrawi S, Urban T, Subra JF, Bouchara JP, Mantovani A, Jeannin P, Delneste Y. Proteolytic cleavage of the long pentraxin PTX3 in the airways of cystic fibrosis patients. Innate Immun 19: 611–622, 2013. [DOI] [PubMed] [Google Scholar]
- 22.Han B, Ma X, Zhang J, Zhang Y, Bai X, Hwang DM, Keshavjee S, Levy GA, McGilvray I, Liu M. Protective effects of long pentraxin PTX3 on lung injury in a severe acute respiratory syndrome model in mice. Lab Invest 92: 1285–1296, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hawkins GA, Robinson MB, Hastie AT, Li X, Li H, Moore WC, Howard TD, Busse WW, Erzurum SC, Wenzel SE, Peters SP, Meyers DA, Bleecker ER; National Heart, Lung, and Blood Institute-sponsored Severe Asthma Research Program (SARP). The IL6R variation Asp358Ala is a potential modifier of lung function in subjects with asthma. J Allergy Clin Immunol 130: 510–515, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He JQ, Foreman MG, Shumansky K, Zhang X, Akhabir L, Sin DD, Man SF, DeMeo DL, Litonjua AA, Silverman EK, Connett JE, Anthonisen NR, Wise RA, Paré PD, Sandford AJ. Associations of IL6 polymorphisms with lung function decline and COPD. Thorax 64: 698–704, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heron M, van Moorsel CH, Grutters JC, Huizinga TW, van der Helm-van Mil AH, Nagtegaal MM, Ruven HJ, van den Bosch JM. Genetic variation in GREM1 is a risk factor for fibrosis in pulmonary sarcoidosis. Tissue Antigens 77: 112–117, 2011. [DOI] [PubMed] [Google Scholar]
- 26.Hite RD, Seeds MC, Jacinto RB, Balasubramanian R, Waite M, Bass D. Hydrolysis of surfactant-associated phosphatidylcholine by mammalian secretory phospholipases A2. Am J Physiol Lung Cell Mol Physiol 275: L740–L747, 1998. [DOI] [PubMed] [Google Scholar]
- 27.Hite RD, Grier BL, Waite BM, Veldhuizen RA, Possmayer F, Yao LJ, Seeds MC. Surfactant protein B inhibits secretory phospholipase A2 hydrolysis of surfactant phospholipids. Am J Physiol Lung Cell Mol Physiol 302: L257–L265, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hughes JM, Arthur CA, Baracho S, Carlin SM, Hawker KM, Johnson PR, Armour CL. Human eosinophil-airway smooth muscle cell interactions. Mediators Inflamm 9: 93–99, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inata J, Hattori N, Yokoyama A, Ohshimo S, Doi M, Ishikawa N, Hamada H, Kohno N. Circulating KL-6/MUC1 mucin carrying sialyl Lewisa oligosaccharide is an independent prognostic factor in patients with lung adenocarcinoma. Int J Cancer 120: 2643–2649, 2007. [DOI] [PubMed] [Google Scholar]
- 30.Ishikawa N, Kohno N. Interstitial lung disease and lung cancer. Gan To Kagaku Ryoho 37: 6–9, 2010. [PubMed] [Google Scholar]
- 31.Ishikawa N, Hattori N, Yokoyama A, Kohno N. Utility of KL-6/MUC1 in the clinical management of interstitial lung diseases. Respir Investig 50: 3–13, 2012. [DOI] [PubMed] [Google Scholar]
- 32.Jahnz-Rozyk K, Plusa T, Mierzejewska J. Blood serum eotaxin and eosinophil cationic protein in asthmatic patients. Pol Merkur Lekarski 8: 319–321, 2000. [PubMed] [Google Scholar]
- 33.Jaillon S, Mancuso G, Hamon Y, Beauvillain C, Cotici V, Midiri A, Bottazzi B, Nebuloni M, Garlanda C, Frémaux I, Gauchat JF, Descamps P, Beninati C, Mantovani A, Jeannin P, Delneste Y. Prototypic long pentraxin PTX3 is present in breast milk, spreads in tissues, and protects neonate mice from Pseudomonas aeruginosa lung infection. J Immunol 191: 1873–1882, 2013. [DOI] [PubMed] [Google Scholar]
- 34.Kanazawa H. Role of vascular endothelial growth factor in the pathogenesis of chronic obstructive pulmonary disease. Med Sci Monit 13: RA189–RA195, 2007. [PubMed] [Google Scholar]
- 35.Kim DK, Fukuda T, Thompson BT, Cockrill B, Hales C, Bonventre JV. Bronchoalveolar lavage fluid phospholipase A2 activities are increased in human adult respiratory distress syndrome. Am J Physiol Lung Cell Mol Physiol 269: L109–L118, 1995. [DOI] [PubMed] [Google Scholar]
- 36.Kim KC, Lillehoj EP. MUC1 mucin: a peacemaker in the lung. Am J Respir Cell Mol Biol 39: 644–713, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koli K, Myllarniemi M, Vuorinen K, Salmenkivi K, Ryynanen MJ, Kinnula VL, Keski-Oja J. Bone morphogenic protein-4 inhibitor gremlin is overexpressed in idiopathic pulmonary fibrosis. Am J Pathol 169: 61–71, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kreiner-Moller E, Chawes BL, Vissing NH, Koppelman GH, Postma DS, Madsen JS, Olsen DA, Baty F, Vonk JM, Kerkhof M, Sleiman P, Hakonarsson H, Mortensen LJ, Poorisrisak P, Bisgaard H, Bønnelykke K. VEGFA variants are associated with pre-school lung function, but not neonatal lung function. Clin Exp Allergy 4311: 1236–45, 2013. [DOI] [PubMed] [Google Scholar]
- 39.Lahiri T, Laporte JD, Moore PE, Panettieri RA Jr, Shore SA. Interleukin-6 family cytokines: signaling and effects in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 280: L1225–L1232, 2001. [DOI] [PubMed] [Google Scholar]
- 40.Lauro S, Onesti CE, Righini R, Marchetti P. The use of bevacizumab in non-small cell lung cancer: an update. Anticancer Res 34: 1537–1545, 2014. [PubMed] [Google Scholar]
- 41.Li N, Huang HQ, Zhang GS. Association between SOD2 C47T polymorphism and lung cancer susceptibility: a meta-analysis. Tumour Biol 35: 955–959, 2014. [DOI] [PubMed] [Google Scholar]
- 42.Lunt SY, Vander Heiden MG. Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu Rev Cell Dev Biol 27: 441–464, 2011. [DOI] [PubMed] [Google Scholar]
- 43.McInnes IB, Thompson L, Giles JT, Bathon JM, Salmon JE, Beaulieu AD, Codding CE, Carlson TH, Delles C, Lee JS, Sattar N. Effect of interleukin-6 receptor blockade on surrogates of vascular risk in rheumatoid arthritis: MEASURE, a randomised, placebo-controlled study. Ann Rheum Dis 74: 694–702, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meyer N, Akdis CA. Vascular endothelial growth factor as a key inducer of angiogenesis in the asthmatic airways. Curr Allergy Asthma Rep 13: 1–9, 2013. [DOI] [PubMed] [Google Scholar]
- 45.Misior AM, Yan H, Pascual RM, Deshpande DA, Panettieri RA, Penn RB. Mitogenic effects of cytokines on smooth muscle are critically dependent on protein kinase A, and are unmasked by steroids and cyclooxygenase inhibitors. Mol Pharmacol 73: 566–574, 2008. [DOI] [PubMed] [Google Scholar]
- 46.Misior AM, Deshpande DA, Loza MJ, Pascual RM, Hipp JD, Penn RB. Glucocorticoid- and protein kinase A-dependent transcriptome regulation in airway smooth muscle. Am J Respir Cell Mol Biol 41: 24–39, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morjaria JB, Babu KS, Vijayanand P, Chauhan AJ, Davies DE, Holgate ST. Sputum IL-6 concentrations in severe asthma and its relationship with FEV1. Thorax 66: 537, 2011. [DOI] [PubMed] [Google Scholar]
- 48.Murata M, Yudo K, Nakamura H, Chiba J, Okamoto K, Suematsu N, Nishioka K, Beppu M, Inoue K, Kato T, Masuko K. Hypoxia upregulates the expression of angiopoietin-like-4 in human articular chondrocytes: role of angiopoietin-like-4 in the expression of matrix metalloproteinases and cartilage degradation. J Orthop Res 27: 50–57, 2009. [DOI] [PubMed] [Google Scholar]
- 49.Napolitano JR, Liu MJ, Bao S, Crawford M, Nana-Sinkam P, Cormet-Boyaka E, Knoell DL. Cadmium-mediated toxicity of lung epithelia is enhanced through NF-κB-mediated transcriptional activation of the human zinc transporter ZIP8. Am J Physiol Lung Cell Mol Physiol 302: L909–L918, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nevalainen TJ, Haapamaki MM, Gronroos JM. Roles of secretory phospholipases A2 in inflammatory diseases and trauma. Biochim Biophys Acta 1488: 83–90, 2000. [DOI] [PubMed] [Google Scholar]
- 51.Neveu WA, Allard JL, Raymond DM, Bourassa LM, Burns SM, Bunn JY, Irvin CG, Kaminsky DA, Rincon M. Elevation of IL-6 in the allergic asthmatic airway is independent of inflammation but associates with loss of central airway function. Respir Res 11: 28, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ohshimo S, Ishikawa N, Horimasu Y, Hattori N, Hirohashi N, Tanigawa K, Kohno N, Bonella F, Guzman J, Costabel U. Baseline KL-6 predicts increased risk for acute exacerbation of idiopathic pulmonary fibrosis. Respir Med 108: 1031–1039, 2014. [DOI] [PubMed] [Google Scholar]
- 53.Paplinska M, Hermanowicz-Salamon J, Nejman-Gryz P, Bialek-Gosk K, Rubinsztajn R, Arcimowicz M, Placha G, Gora J, Chazan R, Grubek-Jaworska H. Expression of eotaxins in the material from nasal brushing in asthma, allergic rhinitis and COPD patients. Cytokine 60: 393–399, 2012. [DOI] [PubMed] [Google Scholar]
- 54.Patel AM, Moreland LW. Interleukin-6 inhibition for treatment of rheumatoid arthritis: a review of tocilizumab therapy. Drug Des Devel Ther 4: 263–278, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Piairo P, Moura RS, Nogueira-Silva C, Correia-Pinto J. The apelinergic system in the developing lung: expression and signaling. Peptides 32: 2474–2483, 2011. [DOI] [PubMed] [Google Scholar]
- 56.Richards KL, Zhang B, Sun M, Dong W, Churchill J, Bachinski LL, Wilson CD, Baggerly KA, Yin G, Hayes DN, Wistuba II, Krahe R. Methylation of the candidate biomarker TCF21 is very frequent across a spectrum of early-stage nonsmall cell lung cancers. Cancer 117: 606–617, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rose-John S, Mitsuyama K, Matsumoto S, Thaiss WM, Scheller J. Interleukin-6 trans-signaling and colonic cancer associated with inflammatory bowel disease. Curr Pharm Des 15: 2095–2103, 2009. [DOI] [PubMed] [Google Scholar]
- 58.Sartini D, Morganti S, Guidi E, Rubini C, Zizzi A, Giuliante R, Pozzi V, Emanuelli M. Nicotinamide N-methyltransferase in non-small cell lung cancer: promising results for targeted anti-cancer therapy. Cell Biochem Biophys 67: 865–873, 2013. [DOI] [PubMed] [Google Scholar]
- 59.Savarimuthu Francis SM, Larsen JE, Pavey SJ, Duhig EE, Clarke BE, Bowman RV, Hayward NK, Fong KM, Yang IA. Genes and gene ontologies common to airflow obstruction and emphysema in the lungs of patients with COPD. PLoS One 63: e17442, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Scheller J, Ohnesorge N, Rose-John S. Interleukin-6 trans-signalling in chronic inflammation and cancer. Scand J Immunol 63: 321–329, 2006. [DOI] [PubMed] [Google Scholar]
- 61.Seeds MC, Grier BL, Suckling BN, Safta AM, Long DL, Waite BM, Morris PE, Hite RD. Secretory phospholipase A2-mediated depletion of phosphatidylglycerol in early acute respiratory distress syndrome. Am J Med Sci 343: 446–451, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sharp FR, Bernaudin M. HIF1 and oxygen sensing in the brain. Nat Rev Neurosci 5: 437–448, 2004. [DOI] [PubMed] [Google Scholar]
- 63.Smith LT, Lin M, Brena RM, Lang JC, Schuller DE, Otterson GA, Morrison CD, Smiraglia DJ, Plass C. Epigenetic regulation of the tumor suppressor gene TCF21 on 6q23-q24 in lung and head and neck cancer. Proc Natl Acad Sci USA 103: 982–987, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Smyth GK. Limma: linear models for microarray data. In: Bioinformatics and Computational Biology Solutions Using R and Bioconductor, edited by Gentleman R, Carey V, Dudoit S, Irizarry R, Huber W. New York: Springer, 397–420, 2005. [Google Scholar]
- 65.Sun X, Singleton PA, Letsiou E, Zhao J, Belvitch P, Sammani S, Chiang ET, Moreno-Vinasco L, Wade MS, Zhou T, Liu B, Parastatidis I, Thomson L, Ischiropoulos H, Natarajan V, Jacobson JR, Machado RF, Dudek SM, Garcia JG. Sphingosine-1-phosphate receptor-3 is a novel biomarker in acute lung injury. Am J Respir Cell Mol Biol 47: 628–636, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sun X, Elangovan EV, Mapes B, Camp SM, Sammani S, Saadat L, Ceco E, Ma SF, Flores C, Macdougall MS, Quijada H, Liu B, Kempf CL, Wang T, Chiang ET, Garcia JG. The NAMPT promoter is regulated by mechanical stress, STAT5, and ARDS-associated genetic variants. Am J Respir Cell Mol Biol 51: 660–667, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Uusitalo-Seppala R, Koskinen P, Leino A, Peuravuori H, Vahlberg T, Rintala EM. Early detection of severe sepsis in the emergency room: diagnostic value of plasma C-reactive protein, procalcitonin, and interleukin-6. Scand J Infect Dis 43: 883–890, 2011. [DOI] [PubMed] [Google Scholar]
- 68.Uusitalo-Seppala R, Peuravuori H, Koskinen P, Vahlberg T, Rintala EM. Role of plasma bactericidal/permeability-increasing protein, group IIA phospholipase A2, C-reactive protein, and white blood cell count in the early detection of severe sepsis in the emergency department. Scand J Infect Dis 44: 697–704, 2012. [DOI] [PubMed] [Google Scholar]
- 69.Uusitalo-Seppala R, Huttunen R, Aittoniemi J, Koskinen P, Leino A, Vahlberg T, Rintala EM. Pentraxin 3 PTX3 is associated with severe sepsis and fatal disease in emergency room patients with suspected infection: a prospective cohort study. PLoS One 81: e53661, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Visser YP, Walther FJ, Laghmani el H, Laarse A, Wagenaar GT. Apelin attenuates hyperoxic lung and heart injury in neonatal rats. Am J Respir Crit Care Med 182: 1239–1250, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wen FQ, Liu X, Manda W, Terasaki Y, Kobayashi T, Abe S, Fang Q, Ertl R, Manouilova L, Rennard SI. TH2 cytokine-enhanced and TGF-beta-enhanced vascular endothelial growth factor production by cultured human airway smooth muscle cells is attenuated by IFN-gamma and corticosteroids. J Allergy Clin Immunol 111: 1307–1318, 2003. [DOI] [PubMed] [Google Scholar]
- 72.Wu D, Zhou J, Bi H, Li L, Gao W, Huang M, Adcock IM, Barnes PJ, Yao X. CCL11 as a potential diagnostic marker for asthma? J Asthma 51: 847–854, 2014. [DOI] [PubMed] [Google Scholar]
- 73.Xia YC, Redhu NS, Moir LM, Koziol-White C, Ammit AJ, Al-Alwan L, Camoretti-Mercado B, Clifford RL. Pro-inflammatory and immunomodulatory functions of airway smooth muscle: emerging concepts. Pulm Pharmacol Ther 26: 64–74, 2013. [DOI] [PubMed] [Google Scholar]
- 74.Yu JA, Mauchley D, Li H, Meng X, Nemenoff RA, Fullerton DA, Weyant MJ. Knockdown of secretory phospholipase A2 IIA reduces lung cancer growth in vitro and in vivo. J Thorac Cardiovasc Surg 144: 1185–1191, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang J, Shan L, Koussih L, Redhu NS, Halayko AJ, Chakir J, Counni AS. Pentraxin 3 PTX3 expression in allergic asthmatic airways: role in airway smooth muscle migration and chemokine production. PLoS One 74: e34965, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang D, Ren WH, Gao Y, Wang NY, Wu WJ. Clinical significance and prognostic value of pentraxin-3 as serologic biomarker for lung cancer. Asian Pac J Cancer Prev 14: 4215–4221, 2013. [DOI] [PubMed] [Google Scholar]