Abstract
The increased use of inhaled nicotine via e-cigarettes has unknown risks to lung health. Having previously shown that cigarette smoke (CS) extract disrupts the lung microvasculature barrier function by endothelial cell activation and cytoskeletal rearrangement, we investigated the contribution of nicotine in CS or e-cigarettes (e-Cig) to lung endothelial injury. Primary lung microvascular endothelial cells were exposed to nicotine, e-Cig solution, or condensed e-Cig vapor (1–20 mM nicotine) or to nicotine-free CS extract or e-Cig solutions. Compared with nicotine-containing extract, nicotine free-CS extract (10–20%) caused significantly less endothelial permeability as measured with electric cell-substrate impedance sensing. Nicotine exposures triggered dose-dependent loss of endothelial barrier in cultured cell monolayers and rapidly increased lung inflammation and oxidative stress in mice. The endothelial barrier disruptive effects were associated with increased intracellular ceramides, p38 MAPK activation, and myosin light chain (MLC) phosphorylation, and was critically mediated by Rho-activated kinase via inhibition of MLC-phosphatase unit MYPT1. Although nicotine at sufficient concentrations to cause endothelial barrier loss did not trigger cell necrosis, it markedly inhibited cell proliferation. Augmentation of sphingosine-1-phosphate (S1P) signaling via S1P1 improved both endothelial cell proliferation and barrier function during nicotine exposures. Nicotine-independent effects of e-Cig solutions were noted, which may be attributable to acrolein, detected along with propylene glycol, glycerol, and nicotine by NMR, mass spectrometry, and gas chromatography, in both e-Cig solutions and vapor. These results suggest that soluble components of e-Cig, including nicotine, cause dose-dependent loss of lung endothelial barrier function, which is associated with oxidative stress and brisk inflammation.
Keywords: tobacco, permeability, cell proliferation, sphingosine-1-phosphate, inflammation
cigarette smoking is the primary causative factor for chronic obstructive pulmonary disease (COPD), the third leading cause of death worldwide. We have shown that in addition to injuring the lung epithelium, soluble components of cigarette smoke (CS) can be directly injurious to lung endothelial cells by disrupting the lung endothelial barrier function (22). It is not known whether nicotine, the main component of CS, could be responsible for this effect. Furthermore, it is not known whether inhalation of the vapor released by electronic cigarettes (e-Cig) has similar effects as CS on lung endothelium. Given the increasing use of e-Cig, which results in inhalation of vapor produced by heating nicotine-containing liquid, it is important to define their biological effects on the lung endothelial barrier function.
Previous studies of nicotine in endothelial cells have generated diverse results, showing inhibition of human umbilical vein endothelial cell (HUVEC) proliferation (1), but enhanced proliferation of endothelial progenitor cells (35). Furthermore, high doses of nicotine were shown to inhibit cytokines required for neovascularization during bone healing (14, 26) and to affect the vasoreactivity of various vascular networks (8, 9, 16, 17). However, the effects of nicotine on lung barrier function are not known and, given that the loss of endothelial integrity contributes to lung inflammation and injury, they are important to define.
The mechanisms by which nicotine triggers systemic endothelial cell responses have been shown to involve increases in NO signaling molecules (18) and reactive oxygen species (ROS) (16), as well as generation of proapoptotic metabolites (28), events that would be expected to also impair lung endothelial barrier function. However, nicotine has been shown to have discrepant effects, either decreasing or increasing the expression of intracellular adhesion molecules in HUVEC (24, 25) via signaling pathways involving PKC, p38, and ERK1/2 MAPK (29, 33). Little is known about the direct effect of nicotine on the lung cell endothelial barrier and the mechanisms by which nicotine would exert such effects.
Endothelial cellular barrier is tightly regulated by the actomyosin cytoskeleton, whose contraction is governed by myosin light chain kinase (MLCK) and Rho kinase enzymatic activities. We have previously shown that CS extracts caused endothelial barrier dysfunction via oxidative stress, p38 MAPK activation, and ceramide release, which are generated upstream of Rho kinase activation and cellular contraction (22). In addition, CS-induced barrier dysfunction may be attributed to loss of intercellular tethering forces (22), which are typically reinforced by sphingosine-1-phosphate (S1P) signaling (10). We investigated whether nicotine, one of the hundreds of molecules present in CS extracts, is sufficient to alter lung endothelial barrier function by affecting cytoskeletal regulation.
Using measurements of endothelial monolayer barrier function in cultured primary cells via transcellular electrical cellular impedance sensing (ECIS) (27) and in vivo assessment of oxidative stress and extravasated inflammatory cells in the bronchoalveolar lavage, we show that nicotine and e-Cig solutions or vapor condensates cause dose-dependent cell injury manifested by decreased barrier function and decreased cell proliferation, via specific signaling pathways.
MATERIALS AND METHODS
Reagents and Pharmacological Inhibitors
Unless otherwise stated, all chemical reagents were purchased from Sigma-Aldrich (St. Louis, MO). Free radical scavenger and precursor to glutathione N-acetylcysteine (NAC; 0.5 M) and p38 MAPK inhibitor SB203580 (5 μM) were from Santa Cruz Biotechnology (Dallas, TX). ERK1/2 inhibitor PD98059 (50 μM) and JNK inhibitor SP600125 (50 μM) were from Calbiochem (San Diego, CA). The S1P analogs (S)-FTY720 phosphonate (1S), (S)-FTY720 enephosphonate (2S), (R)-FTY720 phosphonate (1R), and (R)-FTY720 enephosphonate (2R) (all solubilized in MeOH) were synthesized as previously described (13). Ceramide synthase inhibitor, fumonisin (FB1, 10 μM), was from Cayman Chemical (Ann Arbor, MI); and the serine palmitoyl transferase inhibitor, myriocin (Myr; 50 nM), was from Biomol International (Plymouth Meeting, PA). The neutral sphingomyelinase (nSMase) inhibitor GW4869 (GW; 20 μM) and acid sphingomyelinase (aSMase) inhibitor imipramine (Imi; 50 μM) were from Calbiochem. Nicotine solutions [Vanilla Dream (e-Cig 1), Kentucky Prime (e-Cig 2), and nicotine-free Kentucky Prime] were obtained from Sigma. E-Cig solutions for vaporization were purchased from World of Vapor (Indianapolis, IN). E-Cig used to generate vapor (iClear 16) was from Innokin (Shenzhen, China).
Cells
Primary rat lung endothelial cells (RLEC; from Dr. Troy Stevens, University of Southern Alabama) and human bronchial epithelial cell line Beas-2B (American Type Culture Collection, Manassas, VA) were cultured in DMEM with high glucose (DMEM-HG) from Invitrogen (Hercules, CA) containing 10% FBS and 1% penicillin/streptomycin. Primary mouse lung endothelial cells (MLEC; from Dr. Patty Lee, Yale University) were grown as RLEC but with 20% FBS. Primary human microvascular cells-lung derived (HMVEC-LBl; Lonza) were grown in Endothelium Cell Growth Basal Medium (EBM-2) with supplements (EGM-2MV SingleQuot Kit supplemented with growth factors). All cultures were maintained at 37°C with 5% CO2. Before treatments (2 h), the culture medium was replaced with basal media containing 2% FBS.
Transcellular Electrical Resistance Measurements
Electrical resistance across cell monolayers was measured using ECIS (Applied Biophysics, Troy, NY), as previously described (20). Cells were cultured on gold microelectrodes and the total resistance was measured in real time across monolayers and recorded continuously every 2–4 min over 24 h. Shown in the figures are select (e.g., 5-h and 20-h) time points as representative of changes induced by exposures studied. Experiments were conducted after cells were confluent [i.e., transendothelial electrical resistance (TER) achieved a steady state]. TER values (in ohms) for each time point were normalized to the initial resistance value (at the beginning of the recording) and plotted as normalized TER.
Animal Experiments
All experiments were performed according to Indiana University School of Medicine Institutional Animal Care and Use Committee guidelines and approved protocols. C57Bl/6 mice (4-mo-old females) were nebulized (nebulizer unit 2.5–4.0 VMD, Aerogen; Galway, Ireland) using either one dose of nicotine (2 μg) and harvested immediately, or two doses of e-Cig solution (1 μg each) and harvested after either 30 min or 24 h. Controls were nebulized with saline and harvested at similar time points.
Oxidative Stress Measurements
Mouse plasma and bronchoalveolar lavage fluid.
As a marker of oxidative damage, 8-hydroxydeoxyguanosine (8-OHdG) was quantified in plasma (1:10 dilution) or bronchoalveolar lavage fluid (BALF) using an OxiSelect Oxidative DNA Damage ELISA kit (Cell Biolabs; San Diego, CA). Total nitrotyrosine was determined in plasma (1:10) using competitive ELISA (Hycult; Uden, Netherlands) following the manufacturer's specifications.
Rat lung endothelial cells.
Cells were grown on gelatin-coated coverslips pretreated with nicotine (10 mM, 30 min) with or without NAC (0.5 M). ROS were detected using an Image-iT LIVE Green Reactive Oxygen Species Detection Kit (Invitrogen) following the manufacturer's instructions. Nuclei were stained using DAPI (Invitrogen). Photographs were captured using a Nikon 80i fluorescent microscope.
CS Extract and e-Cig Vapor Condensate
Aqueous CS extract was obtained from filtered research-grade cigarettes (2R4F) or nicotine-free cigarettes (1R5F) from the Kentucky Tobacco Research and Development Center (University of Kentucky, Lexington, KY) as previously described (22). A stock CS extract (100%) was prepared by bubbling smoke from two cigarettes into 20 ml of PBS at a rate of 1 cigarette/min to 0.5 cm above the filter, followed by pH adjustment to 7.4 and 0.2-μm filtration. A similar procedure was followed for air control (AC) extract preparation by bubbling ambient air. Treatments were performed with CS or AC extract concentrations ranging from 1% to 20% (vol:vol). Condensed e-Cig vapor was collected in a 25-ml side-armed Erlenmeyer flask placed under vacuum while connected to the e-Cig via Tygon tubing. The temperature of the heating coil inside the e-Cig was not measured. A vacuum trap was created to collect the postvaporized condensate of e-Cig solutions using a gel-loading tip as a constriction point. A total of 125 μl of condensate was collected from vaporization of 600 μl of e-Cig solution over ∼30 min and applied to cell cultures in indicated concentrations (vol:vol).
Ceramide Determination
Following treatments, the culture medium was washed with PBS and cells were collected in methanol, followed by lipid extraction utilizing a modified Bligh and Dyer method, and sphingolipid analyses were performed via combined liquid chromatography-tandem mass spectrometry using an AB-Sciex API4000 triple quadrupole mass spectrometer (Foster City, CA) interfaced with an Agilent 1100 series liquid chromatograph (Agilent Technologies, Wilmington, DE) as previously described (19). Ceramide analytes were ionized via positive ion electrospray ionization. Elution of the ceramides was detected by multiple reaction monitoring characteristic for 14:0, 16:0, 18:0, 18:1, 20:0, 24:0, and 24:1 ceramides. C17:0-ceramide was employed as an internal standard. All ceramide measurements were normalized by lipid phosphorus (Pi) (19).
Cell Proliferation and Toxicity Assays
MTT assay (Invitrogen) was used to determine cell proliferation/metabolic activity following the manufacturer's instructions. RLEC were plated in triplicate at 2,500 cells/ml for 18 h and the medium was then replaced with 2% FBS-containing medium with inhibitors or their vehicle controls for 2 h, followed by addition of nicotine and overnight incubation before assay. An assay using the Cell Counting Kit-8 (CCK-8; Dojindo, Rockville, MD) was performed on 1.6 × 104 cells/well plated on 96-well tissue culture plates and treated the next day as indicated. The kit's CCK-8 solution was added and the plate was read using a spectrophotometer at 450 nm. Cytotoxicity/LDH assay (Roche, Indianapolis, IN) was performed in HLMVEC plated in triplicate onto 96-well dishes at 20 × 105 or 50 × 105 cells/well.
Immunoblotting
Cells were grown in six-well dishes, washed with ice-cold PBS immediately after treatment, and collected by centrifugation. Cells were lysed in standard RIPA buffer with protease and phosphatase inhibitors (Complete and Phostop, respectively; Roche). Samples containing equal protein amounts as determined by bicinchoninic acid protein analysis (Pierce; Rockford, IL) were resolved using SDS-PAGE, transferred onto polyvinylidene difluoride using semidry transfer (Bio-Rad; Hercules, CA), and probed with the following primary antibodies (all from Cell Signaling, Beverly, MA, unless otherwise stated): phospho-p38 (1:500); total p38 (1:1,000); phospho-MLC (1:500); and phospho-myosin phosphatase target subunit 1 (phos-MYPT1, 1:500). Horseradish peroxidase-conjugated secondary antibodies to rabbit, rat, or mouse were from Amersham (Piscataway, NJ). Protein expression was detected using enhanced chemiluminescence (ECL-plus; Amersham), quantified by densitometry, and normalized by a housekeeping protein, vinculin (1:10,000; Calbiochem).
Nuclear Magnetic Resonance
Samples for NMR spectroscopy were prepared by dissolving 25 μl of e-Cig solution (World of Vapor) in 675 μl of [2H4] methanol from Cambridge Isotope Laboratories (Andover, MA). NMR spectra at 1H were acquired at 25°C on a Varian Inova 500 equipped with a 5-mm triple-resonance pulsed-field gradient probe. All spectra were acquired with 90° pulse with 8,192 complex points, 64 transients, and 3-s recycle delay.
Mass Spectrometry
One microliter of each sample was diluted with 199 μl of 50% acetone nitrile in water with 0.1% formic acid. The samples were analyzed using a Thermo Scientific Orbitrap Velos Pro hybrid ion trap-Orbitrap mass spectrometer through direct infusion using a syringe pump. The flow rate was 3 μl/min and the resolution was 60,000.
Gas Chromatography-Mass Spectrometry
All experiments used an Agilent 6890N gas chromatograph coupled with an Agilent 5975 mass spectrometer. The method utilized an oven program with an initial temperature of 40°C held for 1 min, a ramp of 20°C/min, and a final temperature of 300°C held for 1 min. The carrier gas was hydrogen, with a flow rate of 2.5 ml/min and a split ratio of 20:1. The inlet was set at 250°C. The mass spectrometer operated in electron ionization mode, with a scan range of m/z 50–550, and a solvent delay of 2 min. In an initial experiment to determine the ingredients of each sample, 25 mg of nicotine, nicotine-containing, and nicotine-free e-Cig solutions, and e-Cig condensed vapor were placed in a 25-ml volumetric flask and diluted to the mark with dichloromethane. The samples were filtered with a polytetrafluoroethylene syringe filter and analyzed. In a separate quantitation experiment, nicotine and quinoline were diluted with dichloromethane to produce four standard solutions: a 100 mg/ml nicotine, 1 mg/ml quinoline solution; a 10 mg/ml nicotine, 1 mg/ml quinoline solution; a 1 mg/ml nicotine, 1 mg/ml quinoline solution; and a 0.1 mg/ml nicotine, 1 mg/ml quinoline solution. The ratio of nicotine to quinoline in each standard was determined by peak integration, and this information was used to create a calibration curve. Approximately 50 mg of three condensed vapor samples was transferred into a 2-ml volumetric flask, spiked with 2 mg of quinolone, and diluted to the mark with dichloromethane. These samples were also analyzed via gas chromatography-mass spectrometry using the same method and compared against the calibration curve to determine the amount of nicotine in each sample.
Statistical Analysis
SigmaStat 3.5 (San Jose, CA) or Prism 6 (San Diego, CA) software was utilized for comparisons among groups by ANOVA as indicated, followed by intergroup comparisons with Tukey's post hoc testing. For experiments in which two conditions were being compared, a two-tailed Student's t-test was used. All data are expressed as means ± SE and statistically significant differences were considered if P < 0.05.
RESULTS
To investigate the contribution of nicotine in CS extract to the loss of lung endothelial barrier function, we compared the effect of soluble extract from nicotine-containing and nicotine-free cigarettes. Primary RLEC exposed to nicotine-containing CS extract (10% vol:vol) exhibited increased monolayer permeability as measured by ECIS in a time-dependent manner, with ∼40% decrease in TER at 5 h and ∼50% at 20 h (Fig. 1A), consistent with our previous report (22). In contrast, similar concentrations of nicotine-free CS extract had a markedly diminished effect, with no loss of barrier function at 5 h and only ∼ 30% loss of TER at 20 h (Fig. 1A). These results suggest that nicotine directly contributes to the damaging effect of soluble CS extract on lung endothelial barrier. We next tested whether exposure to nicotine itself decreases the endothelial barrier function. Upon incubation of primary RLEC with increasing concentrations of nicotine (up to 50 mM for up to 15 h), we noted significant time- and dose-dependent decreases in TER (Fig. 1B). Using a similar setup, monolayers of primary mouse lung endothelial cells or human lung microvascular endothelial cells challenged with nicotine exhibited similar time and dose-dependent decreases in TER (Fig. 1, C and D), indicating that nicotine effects are not species-specific.
Fig. 1.
Effect of nicotine on lung endothelial and epithelial barrier function. A: transcellular electrical resistance (TER) measured at the indicated time point normalized to TER at baseline (at the beginning of the measurement, before any treatment) in cells exposed to ambient air control extract (AC), nicotine-containing cigarette smoke extract (CS), or nicotine-free CS extract (all solutions were 10% vol:vol) measured by electrical cellular impedance sensing (ECIS) in primary lung microvascular endothelial cells. Values are means ± SE, n = 4–10, one-way ANOVA (with Tukey's post hoc testing for intergroup comparisons). B–D: normalized TER measured at the indicated time (hours) in primary lung rat microvascular endothelial cells (RLEC, B), primary mouse lung endothelial cells (C), and primary human lung microvascular endothelial cells (D) exposed to the indicated concentrations of nicotine. Values are means ± SE, n = 5–56, one-way ANOVA with Tukey's post hoc testing.
Similar to pure, analytical-grade nicotine solutions tested above, two separate nicotine-containing solutions (e-Cig 1 and e-Cig 2) used for vaporization in commercially available e-Cigs also triggered barrier dysfunction in RLEC, consistent with their purported nicotine concentration (Fig. 2A). Unexpectedly, barrier dysfunction was also induced by exposures to similar volumes of an e-Cig solution (e-Cig 2) that lacked nicotine (Fig. 2A). Of note, the nicotine-free e-Cig 2 was marketed as having the same flavor as nicotine-containing e-Cig 2 and shared the same manufacturer. Since vaporization of e-Cig solutions may generate different metabolites than the original solution due to heating, we investigated whether the condensed vapor isolated from an e-Cig affected the endothelial barrier. For similar volumes as nicotine solutions, the e-Cig vapor condensate was less potent on altering RLEC barrier function (Fig. 2A). The barrier disruptive effect of e-Cig solutions on human lung endothelial cells was nicotine dose related (Fig. 2B) and of similar magnitude to that caused by exposures to 3% CS extract, a relatively low concentration we have previously shown to not cause cell death (22). The vapor condensate of e-Cig also caused a dose-related loss of endothelial barrier that required a higher volume compared with nonvaporized solutions of e-Cig (Fig. 2C).
Fig. 2.
Effect of commercial electronic cigarette (e-Cig) solutions on lung endothelial barrier. A and B: normalized TER measured in cells (RLEC in A and human lung microvascular endothelial cells in B) exposed to nicotine (15 mM, 5 h), to CS extract (CSE with similar nicotine content), or to e-Cig extracts or condensed vapor (commercial preparation with the indicated nicotine content; 5 h). Values are means ± SE, n = 4–10, one-way ANOVA with Tukey's post hoc testing. C: normalized TER measured in human lung microvascular endothelial cells exposed to the indicated volume (microliters, μl) of e-Cig or condensed e-Cig vapor. Values are means ± SE n = 4–10, one-way ANOVA with Tukey's post hoc testing.
These results suggested that although nicotine in CS extracts is sufficient to trigger endothelial barrier dysfunction, the effects of e-Cig solutions and vapors are only in part nicotine-dependent. Since commercially available e-Cig extracts and vapors are not well regulated or biochemically defined, we determined the composition of these solutions compared with that of CS extract and analytical-grade nicotine solutions. NMR confirmed the presence of nicotine in e-Cig solutions marked as nicotine-containing and confirmed the lack of nicotine in nicotine-free e-Cig solutions tested (Fig. 3A). In addition, NMR detected the propylene glycol and glycerol in e-Cig solutions, and acrolein in e-Cig vapor condensate. These compounds, in particular nicotine, acrolein, and glycerol, were confirmed using high-resolution mass spectrometry (MS) (Fig. 3B) by comparing the monoisotopic mass of each compound with its theoretical monoisotopic mass (Table 1). MS could not detect propylene glycol, likely because of its poor ionization, but confirmed the lack of nicotine in nicotine-free e-Cig solutions and, demonstrating increased sensitivity compared with NMR, detected acrolein not only in condensed e-Cig vapor, but also in all e-Cig solutions tested. This finding suggested that heating e-Cig solutions produced vapor was not a necessary step to produce acrolein. We confirmed these results using a third complementary method, gas chromatography (GC) (Fig. 3C). Quantitative GC analysis determined that the condensed e-Cig vapor generated for our experiments lost up to four times the nicotine compared with the e-Cig (stock) solutions used for vaporization. These measurements suggested that the concentrations of nicotine used in these experiments are within the range of nicotine that is inhaled by the average smoker per cigarette (1–2 mg total nicotine).
Fig. 3.
Composition of e-Cig and condensed e-Cig vapor. A: spectra from indicated solutions analyzed with NMR [resonances are ± 0.05 parts per million (ppm)], which detected methanol solvent OH 4.87, s; 3.30, quintet; nicotine, H2 8.50, d; H6 8.44, dd; H4 7.85, dt; H5 7.42, dd; H9a 3.24, t; H7 3.20, dd; H9b 2.37, dd; H11a 2.26, m; HN-methyl 2.17, s; H10a 1.98, m; H10b 1.88, m; H11b 1.77, m; propylene glycol H2, 3.78, m; H1 3.42, d; H3 1.15, d; glycerol, H2 3.66, tt; and H1,3 3.57, dm. In some spectra, a small aldehyde singlet (presumed acrolein) is visible at 9.77 ppm. Spectra from noted molecules were obtained from high-resolution electrospray ionization-mass spectrometry (ESI-MS, B) or gas chromatography (C) analyses of indicated solutions.
Table 1.
Theoretical and detected monoisotopic mass of compounds identified by mass spectrometry in e-Cig
| Compound | Formula | Theoretical Monoisotopic Mass* | Detected Monoisotopic Mass |
|---|---|---|---|
| Nicotine | C10H14N2 | 163.12352 | 163.12157 |
| Acrolein | C3H4O | 57.03403 | 57.03297 |
| Glycerol | C3H8O3 | 93.05516 | 93.05386 |
e-Cig, e-cigarette extract.
Given the relatively high concentrations of nicotine applied to cells in cultures, we ensured that the nicotine effect on the endothelium was not due to cell toxicity/necrosis, as determined by lactate dehydrogenase (LDH) release (data not shown). However, nicotine exposure of RLEC significantly and dose-dependently decreased MTT activity, a test that reflects cellular metabolic activity and decreased CCK8 activity, a marker of cellular proliferation (Fig. 4, A and B). Nicotine-containing e-Cig solutions had similar inhibitory effect on endothelial proliferation as measured by CCK8 activity (Fig. 4C). These results indicated that nicotine and e-Cig even at nontoxic (nonlethal) exposure levels significantly impact the behavior of relevant primary cell cultures.
Fig. 4.
Effect of nicotine on proliferation of lung endothelial cells. Cell proliferation was determined with the metabolic activity indicator, MTT (A), or the cell division marker, CCK-8 (B), in primary rat lung microvascular endothelial cells exposed to increasing concentrations of nicotine or e-Cig (C) solutions. Values are means ± SE, n = 3, one-way ANOVA with Tukey's post hoc testing.
To investigate whether inhalation of nicotine and e-Cig solutions also trigger short-term (immediate, 30 min, or 24 h) pulmonary responses in vivo, we administered 1 μg of nicotine or 2 μg of e-Cig to mice via nebulization. These doses are equivalent to smoking one or two cigarettes, respectively. There was a trend toward a rapid increase in polymorphonuclear cells in the BALF at 24 h (Table 2) indirectly reflecting a permissive endothelial barrier for inflammatory cell extravasation. In addition, there was evidence of systemic oxidative and nitroxidative stress, indicated by increased 8-OHdG and nitrotyrosine levels in plasma in response to inhalation analytical-grade nicotine (Fig. 5, A and B). These changes were paralleled by increases in the oxidative stress marker 8-OHdG levels in the BALF (Fig. 5C). Oxidative stress tended to increase by ∼15% and ∼10% compared with saline vehicle in mice exposed to e-Cig solutions, as measured by 8-OHdG levels in plasma and BALF, respectively (data not shown). Overall, these studies indicate that even brief exposures of lungs to nicotine via inhalation are associated with pulmonary responses such as inflammation and oxidative stress, which may cause or be the result of altered lung endothelial barrier function. A direct oxidative stress-inducing effect of nicotine exposure was confirmed in cell cultures using a fluorescently-labeled ROS indicator and the ROS scavenger NAC (Fig. 5D).
Table 2.
Cells detected in bronchoalveolar fluid of mice exposed to inhaled e-Cig or saline control and collected at the indicated time
| Treatment | Time | Macrophages | Lymphocytes | PMN | Number |
|---|---|---|---|---|---|
| Saline | 30 min | 33,228 ± 9,264 | 106 ± 82 | 0 ± 0 | 3 |
| e-Cig 1 | 30 min | 28,278 ± 6,664 | 56 ± 34 | 0 ± 0 | 3 |
| Saline | 24 h | 87,128 ± 21,520 | 1,205 ± 399 | 0 ± 0 | 3 |
| e-Cig 1 | 24 h | 62,317 ± 13,064 | 2,122 ± 1,862 | 561 ± 427 | 3 |
Values are means ± SE. PMN, polymorphonuclear cells.
Fig. 5.
Oxidative stress induced by nicotine. A: nitrotyrosine levels from the plasma of C57Bl/6 mice nebulized with one dose of nicotine and harvested immediately. Levels of 8-OHdG in plasma (B) or bronchoalveolar lavage fluid (BALF, C) of C57Bl/6 mice nebulized with one dose of nicotine and collected immediately. Values are means ± SE, n = 3 per group, Student's t-test. D: detection of reactive oxygen species (ROS, green) in rat lung microvascular endothelial cells exposed to nicotine (10 mM for 30 min) with or without N-acetylcysteine (NAC, 0.5 M) using Image-iT LIVE Green Reactive Oxygen Species Detection Kit and DAPI staining of nuclei (blue).
To define the signaling pathways by which nicotine impairs lung endothelial barrier function, we focused on the mechanisms previously shown to be important in CS extract-induced endothelial permeability such as ROS, MAPK, and sphingolipid pathways, as well as cytoskeletal/cellular contractility effectors (22). ROS played a critical role in the upstream activation of signaling pathways induced by CS extract, which decreased the lung endothelial barrier in cell culture models (22). Despite increased ROS induced by nicotine in vivo and in vitro, treatment of RLEC with NAC, a potent ROS scavenger that attenuates CS extract-induced barrier dysfunction (Fig. 6A), failed to reduce the nicotine-induced loss of barrier in these cells. This result suggested that the mechanism by which high concentrations of nicotine-induced barrier dysfunction may be distinct from those engaged by whole CS extract (containing lower nicotine concentrations).
Fig. 6.
Signaling in nicotine-induced endothelial barrier dysfunction. A: normalized TER measured in rat lung endothelial cells exposed to CS (10%) or to nicotine (15 mM) for the indicated time (hours), and effect of the antioxidant N-acetylcysteine (NAC, 0.5 M, means ± SE, n = 2–12). B: p38 MAPK activation by nicotine in lung endothelium detected by Western blot analysis for phospho- and total p38 (α, β, γ, and δ isoforms) in rat lung microvascular endothelial cells (RLEC) exposed to ambient air control extract (AC), CS extract (CS), or nicotine solution at the indicated concentrations and time points. The blot is representative of n = 3. C: normalized TER measured in RLEC exposed to nicotine (15 mM) for the indicated time (hours), and effect of a p38 inhibitor (SB203580, 30 μM, means ± SE, n = 6–50), one-way ANOVA with Tukey's post hoc test. D: myosin light chain kinase activation detected by immunoblotting for phospho-myosin light chain (Ser19) of RLEC following exposure to nicotine solution (15 mM for 1 h) in the absence or presence of the antioxidant NAC (0.5 M), the ERK-MAPK inhibitor PD98059 (PD 50 μM), or the p38 inhibitor SB203580 (SB, 30 μM). E: myosin phosphatase inhibition detected by phosphorylation of myosin phosphatase target subunit 1 (MYPT1) in RLEC exposed to nicotine (15 mM) for the indicated time points in the presence of 3 μM Rho kinase inhibitor Y29632 (RhoKinh). F: normalized TER measured in RLEC exposed to nicotine (10 mM) for the indicated time, and effect of a Rho kinase inhibitor (Y29632, 3 μM). Values are means ± SE, n = 10, one-way ANOVA with Tukey's post hoc test.
At concentrations shown to cause barrier dysfunction, nicotine significantly activated p38 MAPK in RLEC, similar to CS extract, whereas nicotine-free CS extract (in similar concentrations) failed to induce phospho-p38 (Fig. 6B). Despite nicotine-dependent activation of p38 MAPK, treatment of RLEC with p38 inhibitor SB203580 (5 μM) did not reduce nicotine-induced endothelial permeability (Fig. 6C). Because neither the ERK inhibitor PD98059 (at 50 μM) nor the JNK inhibitor SP600125 (at 50 μM) attenuated nicotine-induced endothelial cell permeability (data not shown), we conclude that nicotine induces MAPK-independent alterations in the lung endothelial barrier.
Nicotine activated the actin-myosin apparatus, as measured by increased phosphorylation of MLC (Fig. 6D). Nicotine-induced MLC phosphorylation was partially inhibited by the ROS scavenger NAC and abolished by p38 inhibitor (Fig. 6D). MLC phosphorylation occurs by activation of the MLC kinase (MLCK) or by inhibition of MLC phosphatase. We investigated whether the myosin phosphatase target subunit 1, MYPT1, is inhibited (via phosphorylation) during nicotine exposure. Nicotine increased MYPT1 phosphorylation within 15 min of application, and for up to 60 min (Fig. 6E). Nicotine-induced MYPT1 phosphorylation was prevented by treatment with the Rho kinase inhibitor Y27632 (Fig. 6E). Unlike NAC or p38 inhibition, Rho kinase inhibition significantly attenuated nicotine-induced barrier dysfunction in RLEC (Fig. 6F), implicating a critical role for Rho kinase-induced MYPT1 inhibition for nicotine's effect on endothelial permeability.
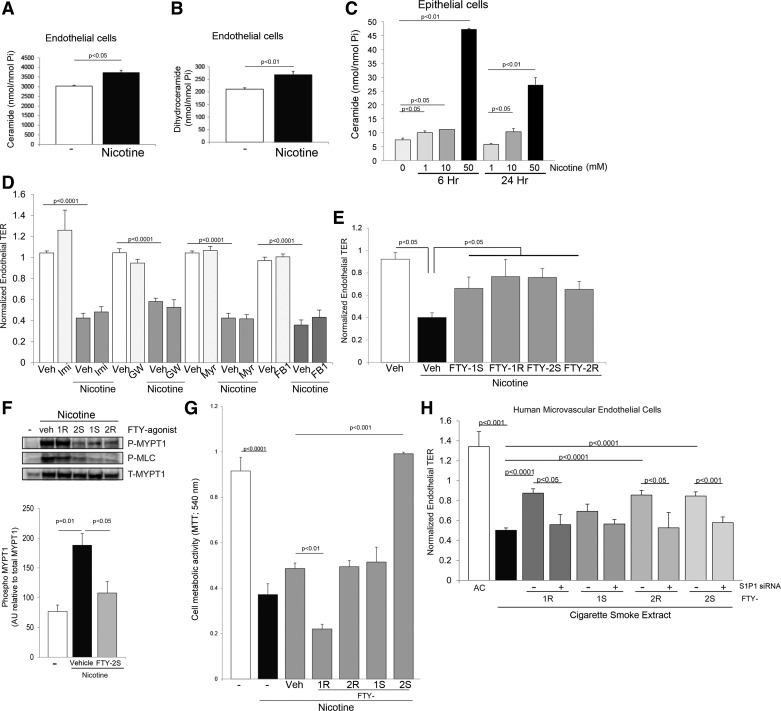
We have previously shown that Rho kinase was also a key mediator of endothelial permeability induced by CS extract, and that ceramides were involved in the upstream signaling leading to Rho kinase activation. We therefore interrogated the role of the sphingolipid pathway in nicotine-induced endothelial effects. First, nicotine exposure significantly increased Cer 16:0, total ceramides, and total dihydroceramides (precursors of ceramides in the de novo sphingolipid pathway) in RLEC (Fig. 7A). This effect was not cell type- or host species-specific, because incubation of cells in the human bronchial cell line Beas-2B with varying concentrations of nicotine also caused a significant and dose-dependent increase in total ceramides (Fig. 7B) and dihydroceramides (data not shown). Pharmacological inhibition of neutral sphingomyelinase, or any of the other enzymes involved in ceramide production such as acid sphingomyelinase, serine palmitoyltransferase, and ceramide synthase (with imipramine, myriocin, and fumonisin B1, respectively) did not attenuate the nicotine-induced decrease in TER (Fig. 7C). In contrast, treatment with analogs of S1P, a barrier-enhancing, downstream metabolite of ceramide, significantly attenuated nicotine-induced endothelial permeability in RLEC (Fig. 7D). We tested S1P analogs because the S1P molecule has a short duration of action and it is impractical to use with exposures that lead to a relatively slow onset of increased permeability. Indeed, concomitant treatment of S1P (5 μM) with nicotine did not attenuate permeability responses to nicotine (data not shown). Nonetheless, FTY720 mono-(1) or bi-(2) phosphonate enantiomers (R or S) of the S1P analog FTY720, significantly inhibited the decrease in lung endothelial permeability triggered by nicotine exposure (Fig. 7E). This effect was associated with decreased nicotine-induced MYPT1 and MLC phosphorylation (Fig. 7F), suggesting that FTY720 analogs affected, at least in part, actin cytoskeletal contraction. Because S1P is also a pro-proliferative signaling molecule, we investigated whether increased endothelial cell proliferation could explain improvement in the endothelial barrier induced by the S1P agonists. Interestingly, only the FTY-2S agonist significantly increased cell proliferation, as measured by the MTT assay (Fig. 7G), indirectly suggesting that cell proliferation is not the main mechanism by which S1P agonists exert barrier protective effects in response to nicotine. Because it is not known whether S1P could also ameliorate CS extract-induced permeability, we interrogated the effect of S1P agonists in primary human lung microvascular endothelial cells, along with its dependence on S1P receptor 1 (S1P1) signaling. All FTY agonists, with the exception of FTY-1S, significantly improved CS extract-induced endothelial permeability; this effect was abolished by knockdown of S1P1 with specific siRNA (Fig. 7H). These studies revealed that nicotine triggers selective signaling pathways that partially overlap those engaged by CS extract to disrupt the barrier and proliferative functions of endothelial cells (Fig. 8).
Fig. 7.
Role of sphingolipids in cellular responses to nicotine. Ceramide (A) and dihydroceramide (B) levels in RLEC following exposure to nicotine (15 mM for 4 h) and in human lung epithelial cells Beas-2B (C) following exposure to the indicated nicotine concentrations (mM) for the indicated time. Values are means ± SE, n = 3, Student's t-test. D: effect of ceramide synthesis inhibitors including that of ASMase with imipramine (Imi, 50 μM), of nSMase with GW4869 (GW, 15 μM), of the de novo pathway with myriocin (Myr, 50 nM); or of ceramide synthases in the recycling pathway with fumonisin B1 (FB1, 5 μM); or their respective vehicle controls dH2O (for FB1, Imi, and Myr) or DMSO (for GW). E: normalized TER of RLEC exposed to nicotine (15 mM for 5 h) and impact of S1P receptor agonists (FTY phosphonate analogs 1S, 1R, 2S, 2R, 10 μM) or vehicle (methanol). Values are means ± SE, n = 15–45, Student's t-test. F: myosin phosphatase inhibition MLCK activation and MLCK activation detected by phospho-Mypt1 (Thr 696) and phospho-MLC (Ser19) immunoblotting followed by densitometry in RLEC exposed to nicotine (15 mM for 20 min) and vehicle (methanol) or the indicated FTY720 analog (10 μM). Values are means ± SE, n = 3, one-way ANOVA with Tukey's post hoc test. G: cell proliferation measured with MTT in RLEC exposed to nicotine (15 mM) in the presence or absence of S1P receptor agonists (FTY phosphonate analogs 1S, 1R, 2S, 2R, 10 μM) or vehicle (methanol). Values are means ± SE, n = 3. H: TER of primary human lung microvascular cells exposed to CS (3%) or air extract (3%) and attenuated with S1P receptor agonists (FTY phosphonate analogs 1S, 1R, 2S, 2R, 10 μM) in the presence or absence of S1PR1-specific siRNA. Values are means ± SE, n = 4–14, ANOVA with Tukey's post hoc test.
Fig. 8.
Schematic of signaling events detected in lung endothelial cells exposed to nicotine. Arrows indicate activation and blocked lines indicate inhibition. Nicotine activates Rho kinase, which in turn inhibits the myosin phosphatase target subunit 1, MYPT1, enhancing phosphorylation of myosin light chains (MLC-P) to increase endothelial permeability. Rho kinase may have other targets in the cell to increase endothelial permeability because nicotine-induced oxidative stress (ROS)-dependent p38 MAPK activation also contributed to myosin light chain phosphorylation (MLC-P), but not sufficiently to alone increase permeability. Nicotine also increases the ceramide/sphingosine-1-phosphate (S1P) ratios, which may inhibit lung endothelial cell proliferation. Enhancing S1P signaling opposes the decreased cell proliferation and the increase in permeability induced by nicotine in part by inhibiting MLC phosphorylation and restoring the lung endothelial barrier function.
DISCUSSION
The results presented indicate that nicotine has dose-related deleterious pulmonary effects that result in loss of lung endothelial barrier function, acute lung inflammation, and decreased lung endothelial cell proliferation. These findings enhance our understanding of how CS exposure causes inflammation and further elucidate the pulmonary effects of nicotine inhalation.
The preservation of an intact endothelial barrier is determined by a balance of contracting cytoskeletal forces and the integrity of cell-cell contacts, both of which can be affected by exposure to soluble components of CS extract (22). In this work we identified that nicotine, which can be absorbed in the circulation as a component of CS or e-Cig, disrupts endothelial barrier by increasing actomyosin contractile signaling, primarily by Rho kinase-dependent phosphorylation and therefore inhibition of endothelial myosin phosphatase, causing increased MLC phosphorylation. Interestingly, although nicotine caused oxidative stress and activated p38 MAPK similarly to CS extract (22), neither p38 MAPK inhibition nor the ROS scavenger NAC were sufficient to restore barrier function following nicotine exposure, in contrast to their remarkable effect on CS-induced barrier dysfunction. These results suggest several possible explanations that include a threshold of MLC phosphorylation that is needed for barrier dysfunction, which is achievable by Rho kinase activation but not by p38 MAPK alone. Such a concept is supported by a recent report in which Rho kinase was found, under certain conditions, to activate p38, but not vice versa (34). Alternatively, nicotine-activated Rho kinase may have additional targets that cause barrier dysfunction besides MLC phosphorylation, as supported by the recent finding of a critical role for Rho kinase isoform 2 in regulating cellular junctional tension (2). Finally, at least theoretically, nicotine-activated p38 MAPK may have additional unexpected barrier-enhancing activities that counteract its MLC phosphorylation effects. Either inhibition of Rho kinase or enhancement of S1P to S1P1 signaling significantly counteracted the barrier-disruptive effects of nicotine (Fig. 8) and CS extract. The novel finding of a protective effect of S1P1 agonists on the CS/nicotine-disrupted endothelial barrier is not surprising given reports of similar S1P1-dependent protective effects of FTY phosphonates against lung endothelial permeability during sepsis or acute lung injury (23, 31, 32), or during synergistic conditions of cystic fibrosis transmembrane conductance regulator (CFTR) inhibition and CS extract exposure (3). FTY phosphonates acted at least in part by activating MYPT1 and inhibiting MLC phosphorylation, although an additional effect on intercellular tethering cannot be ruled out. That S1P augmentation did not recapitulate the effects of FTY phosphonates may be due to the short half-life of the molecule, or to complementary, S1P-independent mechanisms of action of FTY phsosphonates (7).
Using various pharmacological inhibitors of nicotinic receptors to test their involvement in nicotine's effects on the pulmonary endothelium, we could not identify a protective effect against barrier dysfunction (data not shown). However, it is possible that untested receptors and other mediators may regulate nicotine-altered endothelial barrier function. This may be true especially in response to high, cytotoxic nicotine exposure levels shown to inhibit prostaglandin and endothelin expression in bovine pulmonary endothelial cells (25), but these were not studied here. The concentrations of nicotine used in our cell culture studies were derived from detailed dose-response testing, were noncytotoxic, and induced significant effects at levels higher than those absorbed in the circulation by individuals who smoke, but which may be achieved in tissue levels with high nicotine concentrations, such as the lung (6, 30). The effect of nicotine on barrier function may be organ dependent because other studies have shown an improvement in the gut barrier function by cholinergic actions of nicotine on enteric glial cells (4).
Although many of the nicotine effects on the lung endothelium were dose-dependent, nicotine-independent deleterious effects of e-Cig solutions were also noted. We have identified acrolein as putative mediator for nicotine-independent toxicity on the basis of its presence in both e-Cig solution and vapor and on a large body of literature showing adverse pulmonary effects of acrolein, including on endothelial intercellular tethering molecules (12). The signaling effects on nicotine-free e-Cig vapors on the lung endothelial barrier remain to be investigated.
The noted dose-dependent antiproliferative effects of nicotine on lung endothelial cells may have implications in angiogenesis and in lung injury repair. Our results on primary lung endothelial cells are in contrast to pro-proliferative effects of nicotine on human umbilical vascular endothelial cells (11), systemic vasculature, or on lung cancer cells (15), suggesting cell type-specific effects of low-dose nicotine.
Intravital lung microcopy in animals with intact circulation (no pump-perfusion) demonstrated that the in vitro finding of CS extracts causing decreased endothelial barrier function was paralleled by increased lung inflammation in vivo, as measured by increased adherence of circulating leukocytes to the lung microvasculature within 20 min of CS inhalation, without pulmonary edema (21). This previous work led us to complement our investigations of nicotine in cell culture models with in vivo studies of acute lung and systemic effects of nebulized nicotine and e-Cig extracts, mimicking the inhalation of e-Cig vapors by humans. We found that nicotine and e-Cig extracts caused rapid oxidative and nitroxidative stress observed in the BALF and plasma as well as a trend toward greater neutrophil lung inflammation at 24 h following inhalation as measured by the relatively less sensitive method of BALF cytospins, rather than intravital microscopy. Although future studies will determine how these acute inflammatory lung responses translate into long-term effects of recurrent e-Cig exposures, we anticipate these will include dose-dependent sustained oxidative-stress and inflammatory lung damage with limitation of endothelial repair. In this context, ceramide/S1P balance may serve as an important rheostat of alveolar integrity, as was observed in experimental models of COPD (5). By augmenting barrier-enhancing and angiogenic S1P signaling via S1P1, such as was shown here with pharmacological agonists, one may be able to improve barrier function in vivo and potentially attenuate the chronic damage caused by e-Cig inhalation.
The clinical implications of this work related to the potential detrimental lung effects of exposure to e-Cig. Although further studies are needed to determine the usual levels of absorbed e-Cig vapor that are harmful to human lung health, our results in primary human and murine lung endothelial cells and in animal models indicate they are dose related.
GRANTS
Support for this study was provided by National Institutes of Health Grants RO1 HL-077328 and R21 DA-029249 to I. Petrache. The American Chemical Society Project SEED Program sponsored S. Law.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.S.S., C.P., B.D.R., R.B., J.G., and I.P. conception and design of research; K.S.S., S.X.C., S.L., M.J.V.D., C.P., M.J.J., W.C.H., E.S.K., X.L., M.W., W.D.K., C.J.C., B.D.R., R.B., and J.G. performed experiments; K.S.S., S.X.C., S.L., M.J.V.D., C.P., M.J.J., W.C.H., E.S.K., X.L., M.W., W.D.K., C.J.C., B.D.R., J.G., and I.P. analyzed data; K.S.S., C.P., W.C.H., M.W., W.D.K., B.D.R., J.G., and I.P. interpreted results of experiments; K.S.S., S.X.C., S.L., M.J.V.D., X.L., M.W., W.D.K., C.J.C., B.D.R., J.G., and I.P. prepared figures; K.S.S., B.D.R., J.G., and I.P. drafted manuscript; K.S.S., W.C.H., M.W., B.D.R., J.G., and I.P. edited and revised manuscript; K.S.S., S.X.C., S.L., M.J.V.D., C.P., M.J.J., W.C.H., X.L., M.W., W.D.K., C.J.C., B.D.R., J.G., and I.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Margie Albrecht, Alayna Hutchinson, and Yuan Gu for expert technical assistance. NMR spectra were acquired at the IUPUI School of Science NMR Center.
REFERENCES
- 1.An N, Andrukhov O, Tang Y, Falkensammer F, Bantleon HP, Ouyang X, Rausch-Fan X. Effect of nicotine and porphyromonas gingivalis lipopolysaccharide on endothelial cells in vitro. PloS One 9: e96942, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beckers CM, Knezevic N, Valent ET, Tauseef M, Krishnan R, Rajendran K, Corey Hardin C, Aman J, van Bezu J, Sweetnam P, van Hinsbergh VW, Mehta D, van Nieuw Amerongen GP. ROCK2 primes the endothelium for vascular hyperpermeability responses by raising baseline junctional tension. Vascul Pharmacol. First published April 11, 2015; doi: 10.1016/j.vph.2015..03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown MB, Hunt WR, Noe JE, Rush NI, Schweitzer KS, Leece TC, Moldobaeva A, Wagner EM, Dudek SM, Poirier C, Presson RG Jr, Gulbins E, Petrache I. Loss of cystic fibrosis transmembrane conductance regulator impairs lung endothelial cell barrier function and increases susceptibility to microvascular damage from cigarette smoke. Pulm Circ 4: 260–268, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheadle GA, Costantini TW, Bansal V, Eliceiri BP, Coimbra R. Cholinergic signaling in the gut: a novel mechanism of barrier protection through activation of enteric glia cells. Surg Infect (Larchmt) 15: 387–393, 2014. [DOI] [PubMed] [Google Scholar]
- 5.Diab KJ, Adamowicz JJ, Kamocki K, Rush NI, Garrison J, Gu Y, Schweitzer KS, Skobeleva A, Rajashekhar G, Hubbard WC, Berdyshev EV, Petrache I. Stimulation of sphingosine 1-phosphate signaling as an alveolar cell survival strategy in emphysema. Am J Respir Crit Care Med 181: 344–352, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dobrinas M, Choong E, Noetzli M, Cornuz J, Ansermot N, Eap CB. Quantification of nicotine, cotinine, trans-3′-hydroxycotinine and varenicline in human plasma by a sensitive and specific UPLC-tandem mass-spectrometry procedure for a clinical study on smoking cessation. J Chromatogr B Analyt Technol Biomed Life Sci 879: 3574–3582, 2011. [DOI] [PubMed] [Google Scholar]
- 7.Dudek SM, Camp SM, Chiang ET, Singleton PA, Usatyuk PV, Zhao Y, Natarajan V, Garcia JG. Pulmonary endothelial cell barrier enhancement by FTY720 does not require the S1P1 receptor. Cell Signal 19: 1754–1764, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.El-Mas MM, El-Gowilly SM, Gohar EY, Ghazal AR. Pharmacological characterization of cellular mechanisms of the renal vasodilatory effect of nicotine in rats. Eur J Pharmacol 588: 294–300, 2008. [DOI] [PubMed] [Google Scholar]
- 9.Galanzha EL, Chowhury P, Tuchim VV, Zharov VP. Monitoring of nicotine impact in microlymphatics of rat mesentery with time-resolved microscopy. Lymphology 38: 181–192, 2005. [PubMed] [Google Scholar]
- 10.Garcia JG, Liu F, Verin AD, Birukova A, Dechert MA, Gerthoffer WT, Bamberg JR, English D. Sphingosine 1-phosphate promotes endothelial cell barrier integrity by Edg-dependent cytoskeletal rearrangement. J Clin Invest 108: 689–701, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heeschen C, Jang JJ, Weis M, Pathak A, Kaji S, Hu RS, Tsao PS, Johnson FL, Cooke JP. Nicotine stimulates angiogenesis and promotes tumor growth and atherosclerosis. Nat Med 7: 833–839, 2001. [DOI] [PubMed] [Google Scholar]
- 12.Jang AS, Concel VJ, Bein K, Brant KA, Liu S, Pope-Varsalona H, Dopico RA Jr, Di YP, Knoell DL, Barchowsky A, Leikauf GD. Endothelial dysfunction and claudin 5 regulation during acrolein-induced lung injury. Am J Respir Cell Mol Biol 44: 483–490, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu X, Sun C, Valentine WJ, Shuyu E, Liu J, Tigyi G, Bittman R. Chiral vinylphosphonate and phosphonate analogues of the immunosuppressive agent FTY720. J Org Chem 74: 3192–3195, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma L, Zheng LW, Sham MH, Cheung LK. Effect of nicotine on gene expression of angiogenic and osteogenic factors in a rabbit model of bone regeneration. J Oral Maxillofac Surg 68: 777–781, 2010. [DOI] [PubMed] [Google Scholar]
- 15.Ma X, Jia Y, Zu S, Li R, Jia Y, Zhao Y, Xiao D, Dang N, Wang Y. α5 Nicotinic acetylcholine receptor mediates nicotine-induced HIF-1alpha and VEGF expression in non-small cell lung cancer. Toxicol Appl Pharmacol 278: 172–179, 2014. [DOI] [PubMed] [Google Scholar]
- 16.Mayhan WG, Sharpe GM. Chronic exposure to nicotine alters endothelium-dependent arteriolar dilatation: effect of superoxide dismutase. J Appl Physiol 86: 1126–1134, 1999. [DOI] [PubMed] [Google Scholar]
- 17.Mimura K, Tomimatsu T, Sharentuya N, Tskitishvili E, Kinugasa-Taniguchi Y, Kanagawa T, Kimura T. Nicotine restores endothelial dysfunction caused by excess sFlt1 and sEng in an in vitro model of preeclamptic vascular endothelium: a possible therapeutic role of nicotinic acetylcholine receptor (nAChR) agonists for preeclampsia. Am J Obstet Gynecol 202: 464 e461–e466, 2010. [DOI] [PubMed] [Google Scholar]
- 18.Park HS, Cho K, Park YJ, Lee T. Chronic nicotine exposure attenuates proangiogenic activity on human umbilical vein endothelial cells. J Cardiovasc Pharmacol 57: 287–293, 2011. [DOI] [PubMed] [Google Scholar]
- 19.Petrache I, Natarajan V, Zhen L, Medler TR, Richter AT, Cho C, Hubbard WC, Berdyshev EV, Tuder RM. Ceramide upregulation causes pulmonary cell apoptosis and emphysema-like disease in mice. Nat Med 11: 491–498, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petrache I, Verin AD, Crow MT, Birukova A, Liu F, Garcia JG. Differential effect of MLC kinase in TNF-alpha-induced endothelial cell apoptosis and barrier dysfunction. Am J Physiol Lung Cell Mol Physiol 280: L1168–L1178, 2001. [DOI] [PubMed] [Google Scholar]
- 21.Presson RG Jr, Brown MB, Fisher AJ, Sandoval RM, Dunn KW, Lorenz KS, Delp EJ, Salama P, Molitoris BA, Petrache I. Two-photon imaging within the murine thorax without respiratory and cardiac motion artifact. Am J Pathol 179: 75–82, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schweitzer KS, Hatoum H, Brown MB, Gupta M, Justice MJ, Beteck B, Van Demark M, Gu Y, Presson RG Jr, Hubbard WC, Petrache I. Mechanisms of lung endothelial barrier disruption induced by cigarette smoke: role of oxidative stress and ceramides. Am J Physiol Lung Cell Mol Physiol 301: L836–L846, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singleton PA, Dudek SM, Chiang ET, Garcia JG. Regulation of sphingosine 1-phosphate-induced endothelial cytoskeletal rearrangement and barrier enhancement by S1P1 receptor, PI3 kinase, Tiam1/Rac1, and alpha-actinin. FASEB J 19: 1646–1656, 2005. [DOI] [PubMed] [Google Scholar]
- 24.Speer P, Zhang Y, Gu Y, Lucas MJ, Wang Y. Effects of nicotine on intercellular adhesion molecule expression in endothelial cells and integrin expression in neutrophils in vitro. Am J Obstet Gynecol 186: 551–556, 2002. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki N, Ishii Y, Kitamura S. Effects of nicotine on production of endothelin and eicosanoid by bovine pulmonary artery endothelial cells. Prostaglandins Leukot Essent Fatty Acids 50: 193–197, 1994. [DOI] [PubMed] [Google Scholar]
- 26.Theiss SM, Boden SD, Hair G, Titus L, Morone MA, Ugbo J. The effect of nicotine on gene expression during spine fusion. Spine 25: 2588–2594, 2000. [DOI] [PubMed] [Google Scholar]
- 27.Tiruppathi C, Malik AB, Del Vecchio PJ, Keese CR, Giaever I. Electrical method for detection of endothelial cell shape change in real time: assessment of endothelial barrier function. Proc Natl Acad Sci USA 89: 7919–7923, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tithof PK, Elgayyar M, Schuller HM, Barnhill M, Andrews R. 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone, a nicotine derivative, induces apoptosis of endothelial cells. Am J Physiol Heart Circ Physiol 281: H1946–H1954, 2001. [DOI] [PubMed] [Google Scholar]
- 29.Ueno H, Pradhan S, Schlessel D, Hirasawa H, Sumpio BE. Nicotine enhances human vascular endothelial cell expression of ICAM-1 and VCAM-1 via protein kinase C, p38 mitogen-activated protein kinase, NF-kappaB, and AP-1. Cardiovasc Toxicol 6: 39–50, 2006. [DOI] [PubMed] [Google Scholar]
- 30.Urakawa N, Nagata T, Kudo K, Kimura K, Imamura T. Simultaneous determination of nicotine and cotinine in various human tissues using capillary gas chromatography/mass spectrometry. Int J Legal Med 106: 232–236, 1994. [DOI] [PubMed] [Google Scholar]
- 31.Wang L, Dudek SM. Regulation of vascular permeability by sphingosine 1-phosphate. Microvasc Res 77: 39–45, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang L, Sammani S, Moreno-Vinasco L, Letsiou E, Wang T, Camp SM, Bittman R, Garcia JG, Dudek SM. FTY720 (s)-phosphonate preserves sphingosine 1-phosphate receptor 1 expression and exhibits superior barrier protection to FTY720 in acute lung injury. Crit Care Med 42: e189–e199, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Y, Wang Z, Zhou Y, Liu L, Zhao Y, Yao C, Wang L, Qiao Z. Nicotine stimulates adhesion molecular expression via calcium influx and mitogen-activated protein kinases in human endothelial cells. Int J Biochem Cell Biol 38: 170–182, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Wu T, Xing J, Birukova AA. Cell-type-specific crosstalk between p38 MAPK and Rho signaling in lung micro- and macrovascular barrier dysfunction induced by Staphylococcus aureus-derived pathogens. Transl Res 162: 45–55, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu M, Liu Q, Sun J, Yi K, Wu L, Tan X. Nicotine improves the functional activity of late endothelial progenitor cells via nicotinic acetylcholine receptors. Biochem Cell Biol 89: 405–410, 2011. [DOI] [PubMed] [Google Scholar]