Abstract
We collected and identified 5,721 ectomycorrhizal fruiting bodies (EcM) from Naejangsan National Park from June 2004 to 2013, belonging to 1 phylum, 1 class, 6 orders, 19 families, 40 genera, and 196 species. Of these, 2,249 individuals were identified as 89 species belonging to 11 genera in 7 families in the Agaricales; 1,511 were identified as 43 species belonging to 2 genera in 1 family in the Russulales; 1,132 were identified as 50 species belonging to 21 genera in 6 families in the Boletales; 793 were identified as 8 species belonging to 3 genera in 2 families in the Cantharellales; 29 were identified as 3 species belonging to 2 genera in 2 families in the Thelephorales; and 7 were identified as 3 species belonging to 1 genus in 1 family in the Gomphales. Thus, most of the EcMs identified belonged to the following 3 orders: Agaricales, Russulales, and Boletales. Russulaceae were most common (43 species), followed by Boletaceae (39 species), and Amanitaceae (27 species); most individuals were Russulaceae (1,511), followed by Hydnagiaceae (1,071) and Boletaceae (804). The monthly distribution showed that the greatest number of individuals and species of EcM, including the dominant ones, occur around July~September at an elevation of 200~299 m, diminishing markedly above 600 m. The greatest number of individuals and species, including the dominant ones, were collected in the period with average temperatures 25.0~26.9℃, lows of 21.0~22.9℃, and highs of 30.0~31.9℃, relative humidity > 76%, and rainfall > 400 mm.
Keywords: Boletaceae, Climatic factors, Ectomycorrhizal fruiting bodies (EcM), Naejangsan National Park, Russulaceae
Naejangsan National Park (NJNP) (35°24'~35°41' N, 126°49'~126°56' E) dominated by Quercus variabilis, Pinus thunbergii, and Carpinus laxiflora, which provide a habitat for higher fungi of various kinds, especially for ectomycorrhizal fruiting bodies (EcM) growing symbiotically with various temperateregion trees such as those of the families Pinaceae, Fagaceae, and Betulaceae [1,2]. The EcMs play vital roles in connecting the roots of these trees and aiding in water transpiration, nutrient absorption, growth, and pathogen protection [3,4]. In the forest, fruiting bodies of many types can be found, such as Russulales (Russula spp., Lactarius spp.), Boletales (Boletus spp., Suillus spp.), Cortinarius (Cortinarius spp.), Laccaria (Laccaria spp.), Pisolithus (Pisolithus spp.), Amanitas (Amanita spp.), Scleroderms (Scleroderma spp.), Strobilomyces (Strobilomyces spp.), and Cantharellus (Cantharellus spp.) [5].
Species diversity and community structure have been reported to be influenced by various factors, including human factors such as lumber extraction [6,7] and pollution [8,9], arboreal factors such as density of trees harboring ectomycorrhizal fungi [10,11,12], seasonal features of leaves (deciduous or evergreen) [13], and quality of fallen leaves [14], and soil factors such as soil organic content [15] and soil nitrogen and carbon contents [16,17], among others.
In particular, climate change has been shown to have strong effects on crowding and the richness index, due to the very small crowding structure and limited expansion of ectomycorrhizal fungi [18].
This study aimed to provide basic data on species diversity by investigating the distribution of EcMs in NJNP, including correlations with altitude and time of collection, and to determine the effects of climatic/environmental factors on the number and distribution of species and individuals of EcM.
MATERIALS AND METHODS
Study location and period
We primarily investigated areas near mountain trails that we judged to be suited for EcM growth, from Naejangsa to Ggachibong, Naejangsa to Bulchulbong, Baegyangsa Unmoomam to Sajabong, and Chonnam National University Training Center to Gatbawi.
At an average of 4 times per month in July, August, and September, and 2 times per month in April, May, June, and October, the Line Transect Method (searching 10m to either side of trails) was used, for a total of 191 times from April 2004 to October 2013 (30 times in 2004, 27 in 2005, 21 in 2006, 18 in 2007, 17 in 2008, 15 in 2009, 18 in 2010, 18 in 2011, and 27 in 2013).
Collection
Among the EcMs found during the investigation period, we performed on-the-spot identifications for those with readily discernible characteristics, such as Lepiota, Agaricales, Amanita, and Volva. Fruiting bodies that could not be identified in the field were placed in collection bags and transported to the Environmental Ecology Laboratory at Wonkwang University; collection location, date, and habitat were recorded for each specimen. At the laboratory, chemical reaction tests were performed using Melzer's solution, KOH, or guaiacol, and basidia, basidiospores, and cystidia were observed using microscopes, for species classification. Both foreign [19,20,21,22,23] and domestic references [24,25] were used for species classification, and the final classification was performed using the classification system in CABIes Index Fungorum (http://www.indexfungorum.org/).
Climatic data and data analysis
Monthly data collected at the Chungeup weather station were used for the study. For July, August, and September, weekly average data were used to calculate monthly average data, and in April, May, June, and October, 7-day data (including the investigation date) were used to calculate monthly data. All climatic factors (average, highest, and lowest temperatures, humidity, and rainfall) were divided into 8 stages. For data analysis, all EcM collected in a given month were compiled, analyzed by ANOVA, and compared using Duncan's test (ver. 12.0K; SPSS Inc., Chicago, IL, USA) to determine the species number of total ectomycorrhizal/dominant ectomycorrhizal fungi and numeric differences in occurrence.
RESULTS AND DISCUSSION
EcM distribution
We collected and identified a total of 5,721 EcM, belonging to 1 phylum, 1 class, 6 orders, 19 families, 40 genera, and 196 species. As shown in Table 1 and Supplementary Table 1, 2,249 individuals were identified as 89 species belonging to 11 genera in 7 families in the Agaricales; 1,511 were identified as 43 species belonging to 2 genera in 1 family in the Russulales; 1,132 were identified as 50 species belonging to 21 genera in 6 families in the Boletales; 793 were identified as 8 species belonging to 3 genera in 2 families in the Cantharellales; 29 were identified as 3 species belonging to 2 genera in 2 families in the Thelephorales; and 7 were identified as 3 species belonging to 1 genus in 1 family in the Gomphales. Most of the EcMs collected belonged to the following 3 orders: Agaricales, Russulales, and Boletales. Species in the Russulaceae were most common (43 species), followed by Boletaceae (39 species), Amanitaceae (27 species), Cortinariaceae (24), and Inocybaceae (17). Most individuals belonged to the Russulaceae (1,511 individuals), followed by the Hydnagiaceae (1,071), Boletaceae (804), Cantharellaceae (777), and Amanitaceae (701). Comparison with the EcM distribution determined after a wildfire [26] showed differences in species number, but the same ectomycorrhizal fungi remained dominant.
Table 1. The number of species and individuals of ectomycorrhizal fruiting bodies collected from 2004 to 2011, 2013 in Naejangsan National Park.

Distribution by investigation period
Analysis of EcM distribution by year showed that the highest number of species (Fig. 1) was collected in 2004 (147 species) followed by 2005 (142), and 2007 (101) the fewest were collected in 2009 (57). The most occurrence population distribution (Fig. 2) was 2008 (989 individuals) followed by 2005 (919), and 2007 (846), the least being 2006 (220).
Fig. 1. The number of ectomycorrhizal fruiting bodies species collected during the survey period.
Fig. 2. The number of ectomycorrhizal fruiting bodies individuals collected during the survey period.
Aggregated by month (Fig. 3), the highest number of species were collected in August (14 families, 31 genera, 136 species) followed by July (16 families, 32 genera, 130 species), and September (16 families, 29 genera, 117 species) in occurrence population number July was the most (2,352 individuals) followed by August (2,032), September (904); none were collected in April or November (Fig. 4).
Fig. 3. The number of ectomycorrhizal fruiting bodies species by month.
Fig. 4. The number of ectomycorrhizal fruiting bodies individuals by month.
The distribution of EcM species (Table 2) showed that Amanitaceae were common in August (23 species), Boletaceae in June/July (31), Cortinariaceae in September (12), Inocybaceae in July (11), and Russulaceae in August (38).
Table 2. Distribution of species of dominant EcMs by month in NJNP.

Numbers in parentheses are number of families.
EcM, ectomycorrhizal fruiting bodies; NJNP, Naejangsan National Park.
Occurrence population distribution showed that most Amanitaceae were collected in July (325 individuals), most Boletaceae in July (373), most Cortinariaceae in September (47), most Inocybaceae in July (90), and most Russulaceae in July (631) (Table 3).
Table 3. Distribution of individuals of dominant EcMs by month in NJNP.

Numbers in parentheses are number of families.
EcM, ectomycorrhizal fruiting bodies; NJNP, Naejangsan National Park.
Thus, it appears that numbers and species diversity of EcMs is highest from July~September, which is similar to the report that species diversity of fruiting bodies of ectomycorrhizal fungi is greatest from July~September [27].
Distribution by altitude
Analysis of species distribution by altitude (Fig. 5) showed that most species occurred at a height of 200~299 m (18 families, 38 genera, 185 species), followed by 500~599 m (17 families, 35 genera, 131 species), and 300~399 m (14 families, 23 genera, 85 species); few were found above 700 m (5 families, 6 genera, 12 species). Occurrence population distribution (Fig. 6) showed that most occurrences were found at an altitude of 200~299m (2,941 individuals), followed by 500~599 m (1,055), and 100~199 m (802); fewest were found above 700 m (28). Analysis of the dominant EcMs by species number (Table 4) and by individual numbers (Table 5) showed that most occurrences were found at an altitude of 200~299 m, and the fewest were found above 700 m; thus, species and individuals decrease as altitude increases. Most EcMs, by both numbers of species and individuals, were found at 200~299 m, gradually decreasing as altitude increased, with remarkably few fungi found above 600 m. These results are concordant with another report [28] that showed that altitude affects species diversity, and crowding composition.
Fig. 5. The number of ectomycorrhizal fruiting bodies species by altitude.
Fig. 6. The number of ectomycorrhizal fruiting bodies individuals by altitude.
Table 4. Distribution of species of dominant EcMs according to altitude in NJNP.

EcM, ectomycorrhizal fruiting bodies; NJNP, Naejangsan National Park.
Table 5. Distribution of individuals of dominant EcMs according to altitude in NJNP.
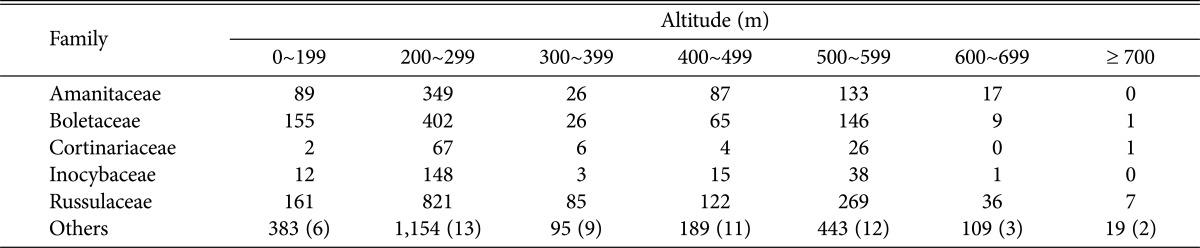
Numbers in parentheses are number of families.
EcM, ectomycorrhizal fruiting bodies; NJNP, Naejangsan National Park.
Distribution by climatic factors
Results for EcM/dominant EcM distribution by climatic factors are shown in Tables 6, 7, 8, 9, 10, 11, 12, 13, 14, 15.
Table 6. Duncan's multiple range test between mean air temperature and total EcMs.

EcM, ectomycorrhizal fruiting bodies.
a~cThe mean difference is significant at the 0.05 level.
Table 7. Duncan's multiple range test between mean air temperature and the number of species and individuals of dominant EcMs.

EcM, ectomycorrhizal fruiting bodies.
a~dThe mean difference is significant at the 0.05 level.
Table 8. Duncan's multiple range test between the highest air temperature and total EcMs.

EcM, ectomycorrhizal fruiting bodies.
a~dThe mean difference is significant at the 0.05 level.
Table 9. Duncan's multiple range test between maximum air temperature and the number of dominant EcM species and individuals.

EcM, ectomycorrhizal fruiting bodies.
Table 10. Duncan's multiple range test between the lowest air temperature and total EcMs.

EcM, ectomycorrhizal fruiting bodies.
a~dThe mean difference is significant at the 0.05 level.
Table 11. Duncan's multiple range test between the lowest air temperature and the number of dominant EcM species and individuals.

EcM, ectomycorrhizal fruiting bodies.
a~dThe mean difference is significant at the 0.05 level.
Table 12. Duncan's multiple range test between relative humidity and total EcMs.

EcM, ectomycorrhizal fruiting bodies.
a~eThe mean difference is significant at the 0.05 level.
Table 13. Duncan's multiple range test between relative humidity and the number of dominant EcM species and individuals.

EcM, ectomycorrhizal fruiting bodies.
a~dThe mean difference is significant at the 0.05 level.
Table 14. Duncan's multiple range test between rainfall and total EcMs.

EcM, ectomycorrhizal fruiting bodies.
a~dThe mean difference is significant at the 0.05 level.
Table 15. Duncan's multiple range test between rainfall and the number of dominant EcM species and individuals.

EcM, ectomycorrhizal fruiting bodies.
a~dThe mean difference is significant at the 0.05 level.
Species number of EcMs by average temperature (Table 6) shows significance at temperatures 25~26.9℃, whereas individual numbers were highest at 25~28.9℃; overall, significance was higher at 25~26.9℃. The number of dominant EcM species and individuals show significance for Amanitaceae, Boletaceae and Russulaceae at 25~26.9℃, for Inocybaceae at 23~26.9℃, and for Cortinariaceae at 21~22.9℃, especially, above 27℃, significance decreased across all EcMs (Table 7). In individual number, there was significance for Amanitaceae at 25~26.9℃, for Boletaceae at 27~28.9℃, for Cortinariaceae at 21~22.9℃, for Inocybaceae at 23~26.9℃, and for Russulaceae at 25~28.9℃.
EcM species number by the highest temperatures (Table 8) finds significance at 30.0~31.9℃ whereas individual number finds significance at 30.0~33.9℃, overall at 30.0~31.9℃. The number of dominant EcM species and individuals (Table 9) finds significance for Amanitaceae, Boletaceae, Inocybaceae, Russulaceae at 30~31.9℃, for Cortinariaceae at 26~27.9℃, significance gradually decreasing above 32℃. In the number of individuals, there was significance for Amanitaceae at 30~31.9℃, for Boletaceae at 30~33.9℃, for Cortinariaceae at 26~27.9℃, for Inocybaceae at 30~31.9℃, and for Russulaceae at 30~33.9℃.
EcM species number by the lowest temperatures (Table 10) finds significance at 21.0~22.9℃ whereas in number of individuals, significance is found at 21.0~24.9℃, overall significance was higher at 21.0~22.9℃. The number of dominant EcM species and individuals (Table 11) finds significance for Amanitaceae and Boletaceae at 21~24.9℃, for Inocybaceae, and Russulaceae at 21~22.9℃, and for Cortinariaceae at 15~17.9℃, while in number of individuals, significance is found for Amanitaceae, Boletaceae, and Russulaceae at 21~24.9℃, for Cortinariaceae at 18~20.9℃, and for Inocybaceae at 21~22.9℃.
These results show that species and individuals of most ectomycorrhizal and dominant EcMs number finds high significance at average temperatures 25.0~26.9℃, at the highest 30.0~31.9℃, and at the lowest 21.0~22.9℃, which is similar to the report [29] that significance of EcM is high at averages 25.0~26.9℃, at the highest 30.0~31.9℃, and at the lowest 22.0~23.9℃.
The EcM numbers of species and individuals by relative humidity (Table 12) finds significance at above 73.0%, overall, above 76.0%. The number of dominant EcM species and individuals finds significance for Amanitaceae, Cortinariaceae, and Inocybaceae at above 76%, for Boletaceae, and Russulaceae at above 73%, while in the number of individuals, significance is found for Amanitaceae, Boletaceae, and Russulaceae at above 73%, and for Cortinariaceae, and Inocybaceae at above 76% (Table 13).
Collectively, these results show that most EcMs and dominant EcMs have high species number and occurrence population when humidity is above 76%, although they differ by family, similar to a report [30,31] that temperature and humidity influence EcM population density.
The number of EcM species and individuals by rainfall (Table 14) finds significance only at > 400 mm/mon. Dominant ectomycorrhizal fungi species/individual number finds significance for Amanitaceae, Boletaceae, Inocybaceae, and Russulaceae at above 400.0mm/monthly average, while Cortinariaceae showed no significant differences between amounts of rainfall (Table 15).
Thus, most dominant EcMs have high occurrence population at an average monthly rainfall above 400.0 mm, although they differ by family, similar to the report [29,32] that showed that higher monthly rainfall increased the occurrence of fruiting bodies of ectomycorrhizal fungi.
ACKNOWLEDGEMENTS
This study was supported by a grant from Rural Development Administration (Project No. PJ01011103), Republic of Korea.
ELECTRONIC SUPPLEMENTARY MATERIAL
Supplementary data including one table can be found with this article online at http://www.mycobiology.or.kr/src/sm/mb-43-122-s001.pdf.
Detailed list of EcMs collected from 2004 to 2013 in NJNP
References
- 1.Smith SE, Read D. Mycorrhizal symbiosis. 3rd ed. London: Academic Press; 2008. [Google Scholar]
- 2.Van der Heijden MG, Bardgett RD, Van Straalen NM. The unseen majority: soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol Lett. 2008;11:296–310. doi: 10.1111/j.1461-0248.2007.01139.x. [DOI] [PubMed] [Google Scholar]
- 3.Baxter JW, Dighton J. Ectomycorrhizal diversity alters growth and nutrient acquisition of grey birch (Betula populifolia) seedlings in host-symbiont culture conditions. New Phytol. 2001;152:139–149. doi: 10.1046/j.0028-646x.2001.00245.x. [DOI] [PubMed] [Google Scholar]
- 4.Dahlberg A. Community ecology of ectomycorrhizal fungi: an advancing interdisciplinary field. New Phytol. 2001;150:555–562. [Google Scholar]
- 5.Natarajan K, Senthilarasu G, Kumaresan V, Rivière T. Diversity in ectomycorrhizal fungi of a dipterocarp forest in Western Ghats. Curr Sci. 2005;88:1893–1895. [Google Scholar]
- 6.Jones MD, Durall DM, Cairney JW. Ectomycorrhizal fungal communities in young forest stands regenerating after clearcut logging. New Phytol. 2003;157:399–422. doi: 10.1046/j.1469-8137.2003.00698.x. [DOI] [PubMed] [Google Scholar]
- 7.Heinonsalo J, Koskiahde I, Sen R. Scots pine bait seedling performance and root colonizing ectomycorrhizal fungal community dynamics before and during the 4 years after forest clear-cut logging. Can J For Res. 2007;37:415–429. [Google Scholar]
- 8.Parrent JL, Morris WF, Vilgalys R. CO2-enrichment and nutrient availability alter ectomycorrhizal fungal communities. Ecology. 2006;87:2278–2287. doi: 10.1890/0012-9658(2006)87[2278:canaae]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 9.Andrew C, Lilleskov EA. Productivity and community structure of ectomycorrhizal fungal sporocarps under increased atmospheric CO2 and O3. Ecol Lett. 2009;12:813–822. doi: 10.1111/j.1461-0248.2009.01334.x. [DOI] [PubMed] [Google Scholar]
- 10.Ishida TA, Nara K, Hogetsu T. Host effects on ectomycorrhizal fungal communities: insight from eight host species in mixed conifer-broadleaf forests. New Phytol. 2007;174:430–440. doi: 10.1111/j.1469-8137.2007.02016.x. [DOI] [PubMed] [Google Scholar]
- 11.Dickie IA, Dentinger BT, Avis PG, McLaughlin DJ, Reich PB. Ectomycorrhizal fungal communities of oak savanna are distinct from forest communities. Mycologia. 2009;101:473–483. doi: 10.3852/08-178. [DOI] [PubMed] [Google Scholar]
- 12.Peay KG, Kennedy PG, Bruns TD. Rethinking ectomycorrhizal succession: are root density and hyphal exploration types drivers of spatial and temporal zonation? Fungal Ecol. 2011;4:233–240. [Google Scholar]
- 13.Morris MH, Smith ME, Rizzo DM, Rejmánek M, Bledsoe CS. Contrasting ectomycorrhizal fungal communities on the roots of co-occurring oaks (Quercus spp.) in a California woodland. New Phytol. 2008;178:167–176. doi: 10.1111/j.1469-8137.2007.02348.x. [DOI] [PubMed] [Google Scholar]
- 14.Aponte C, García LV, Marañón T, Gardes M. Indirect host effect on ectomycorrhizal fungi: leaf fall and litter quality explain changes in fungal communities on the roots of cooccurring Mediterranean oaks. Soil Biol Biochem. 2010;42:788–796. [Google Scholar]
- 15.Kernaghan G. Mycorrhizal diversity: cause and effect? Pedobiologia (Jena) 2005;49:511–520. [Google Scholar]
- 16.Twieg BD, Durall DM, Simard SW, Jones MD. Influence of soil nutrients on ectomycorrhizal communities in a chronosequence of mixed temperate forests. Mycorrhiza. 2009;19:305–316. doi: 10.1007/s00572-009-0232-7. [DOI] [PubMed] [Google Scholar]
- 17.Kjøller R, Nilsson LO, Hansen K, Schmidt IK, Vesterdal L, Gundersen P. Dramatic changes in ectomycorrhizal community composition, root tip abundance and mycelial production along a stand-scale nitrogen deposition gradient. New Phytol. 2012;194:278–286. doi: 10.1111/j.1469-8137.2011.04041.x. [DOI] [PubMed] [Google Scholar]
- 18.Peay KG, Bruns TD, Kennedy PG, Bergemann SE, Garbelotto M. A strong species-area relationship for eukaryotic soil microbes: island size matters for ectomycorrhizal fungi. Ecol Lett. 2007;10:470–480. doi: 10.1111/j.1461-0248.2007.01035.x. [DOI] [PubMed] [Google Scholar]
- 19.Breitenbach J, Kränzlin F. Fungi of Switzerland. Vol. 1. Ascomycetes. Lucerne: Verlag Mykologia; 1984. [Google Scholar]
- 20.Breitenbach J, Kränzlin F. Fungi of Switzerland. Vol. 2. Nongilled fungi. Lucerne: Verlag Mykologia; 1986. [Google Scholar]
- 21.Breitenbach J, Kränzlin F. Fungi of Switzerland. Vol. 3. Boletes and Agarics (1st part). Strobilomycetaceae, Boletaceae, Paxillaceae, Gomphidiaceae, Hygrophoraceae, Tricholomataceae, Polyporaceae (lamellate) Lucerne: Verlag Mykologia; 1991. [Google Scholar]
- 22.Breitenbach J, Kränzlin F. Fungi of Switzerland. Vol. 4. Agarics (2nd part). Entolomataceae, Pluteaceae, Amanitaceae, Agaricaceae, Coprinaceae, Strophariaceae. Lucerne: Verlag Mykologia; 1995. [Google Scholar]
- 23.Breitenbach J, Kränzlin F. Fungi of Switzerland. Vol. 5. Agarics (3rd part). Cortinariaceae. Lucerne: Verlag Mykologia; 2000. [Google Scholar]
- 24.Park WH, Lee HD. Illustrated book of Korean medicinal mushrooms. Seoul: Kyohaksa; 2003. [Google Scholar]
- 25.Park WH, Lee JH. New wild fungi of Korea. Seoul: Kyohaksa; 2011. [Google Scholar]
- 26.Kim HJ, Chung JC, Jang SK, Jang KK. Distribution of ectomycorrhizal fruit bodies according to forest fire area. Korean J Ecol Environ. 2013;46:251–264. [Google Scholar]
- 27.Park YW, Koo CD, Lee HY, Ryu SR, Kim TH, Cho YG. Relationship between macrofungi fruiting and environmental factors in Songnisan National Park. Korean J Environ Ecol. 2010;24:657–679. [Google Scholar]
- 28.Bahram M, Põlme S, Kõljalg U, Zarre S, Tedersoo L. Regional and local patterns of ectomycorrhizal fungal diversity and community structure along an altitudinal gradient in the Hyrcanian forests of northern Iran. New Phytol. 2012;193:465–473. doi: 10.1111/j.1469-8137.2011.03927.x. [DOI] [PubMed] [Google Scholar]
- 29.Jang SK, Hur TC. Relationship between climatic factors and the distribution of higher fungi in Byeonsanbando National Park, Korea. Mycobiology. 2014;42:27–33. doi: 10.5941/MYCO.2014.42.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lange M. Fungus flora in August. Ten year observation in a Danish beech wood districts. Bot Tidsskr. 1978;73:21–54. [Google Scholar]
- 31.Eveling DW, Wilson RN, Gillespie ES, Bataillé A. Environmental effects on sporocarp counts over fourteen years in a forest area. Mycol Res. 1990;94:998–1002. [Google Scholar]
- 32.Jang SK. Distribution of higher fungi in Wolchulsan National Park. Korean J Mycol. 2014;42:9–20. doi: 10.5941/MYCO.2014.42.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Detailed list of EcMs collected from 2004 to 2013 in NJNP








