Abstract
The basidiomycete Laetiporus sulphureus var. miniatus belongs to the Aphyllophorales, Polyporaceae, and grows on the needleleaf tree. The fruiting bodies of Laetiporus species are known to produce N-methylated tyramine derivatives, polysaccharides, and various lanostane triterpenoids. As part of our ongoing effort to discover biologically active compounds from wood-rotting fungi, an anti-inflammatory triterpene, LSM-H7, has been isolated from the fruiting body of L. sulphureus var. miniatus and identified as acetyl eburicoic acid. LSM-H7 dose-dependently inhibited the NO production in RAW 264.7 cells without any cytotoxicity at the tested concentrations. Furthermore it suppressed the production of proinflammatory cytokines, mainly inducible nitric oxide synthase, cyclooxygenase-2, interleukin (IL)-1β, IL-6 and tumor necrosis factor α, when compared with glyceraldehyde 3-phosphate dehydrogenase. These data suggest that LSM-H7 is a crucial component for the anti-inflammatory activity of L. sulphureus var. miniatus.
Keywords: Acetyl eburicoic acid, Anti-inflammation, Laetiporus sulphureus var. miniatus, Nitric oxide, Proinflammatory cytokine
Edible mushrooms have been well-characterized as precious food sources that are rich in vitamins, essential amino acids, fibers and minerals. Some of them have long being used as medicinal agents that exhibit profound health enhancing benefits. Many bioactive molecules of mushroom origin, such as polysaccharides, proteins, acids, terpenoids, and proteoglycans, have been characterized as being potentially beneficial to the immune system, cardiac system, and the nervous system [1,2,3,4].
Macrophages are the sentinel cells that display important role in host defensive system against microbial infections and metastasis by causing growth suppression and metastatic lysis. In response to regulating agents like lipopolysaccharide (LPS), interferon γ, or some other microbial agents, macrophages release interleukin (IL)-1β, tumor necrosis factor α (TNF-α), IL-1, IL-6, and nitric oxide to induce anti-inflammatory and tumoricidal activity [5]. In mammalian cells, Toll-like receptors (TLRs) play key roles in macrophages in helping to recognize microbial insults with subsequent immune responses [6]. These TLRs initiate a series of kinase phosphorylations and cause nuclear factor κB activation through association with MyD88 in conjunction with TNF receptor-associated factor 6. Several studies have demonstrated a pivotal role of TLRs in recognizing bioactive compounds obtained from fungi that exhibits various biological roles via TLR-mediated signal pathways [7,8,9].
The basidiomycete Laetiporus sulphureus var. miniatus, belongs to the Aphyllophorales, Polyporaceae, grows on the needleleaf tree, and is the most readily recognized among macrofungi due to its striking yellow or orange color [10]. It is widely distributed in Asia including in Korea and Japan. Laetiporus species produce N-methylated tyramine derivatives, polysaccharides, various lanostane triterpenoids, laetiporic acids, and other compounds [10]. L. sulphureus var. miniatus is known to produce a number of polysaccharides, lanostane triterpenoids, lanostanol glycosides, benzofuran glycosides, and acetylenic acid [11,12].
As part of our ongoing effort to discover biologically active compounds from wood-rotting fungi, we have isolated an anti-inflammatory triterpene, LSM-H7, from the fruiting body of L. sulphureus var. miniatus and identified its chemical structure as acetyl eburicoic acid, which is a powerful apoptosis inducer [13]. LSM-H7 potently inhibited the nitric oxide production with no cytotoxicity at given concentrations. Moreover it also suppressed the expression of proinflammatory cytokines in a dose-dependent manner. Herein, we report for the first time the anti-inflammatory activity of LSM-H7 on RAW 264.7 cells under the stimulation of TLR-4 ligand LPS.
MATERIALS AND METHODS
Chemicals
Dulbecco's modified Eagle's medium (DMEM) (WelGene Co., Daegu, Korea), fetal bovine serum (FBS) (WelGene), streptomycin and penicillin (Lonza, Walkersville, MD, USA), TRI reagent solution (AM9738; Applied Biosystems, Ambion, Austin, TX, USA), oligodT (Bioneer Co., Daejeon, Korea), inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), TNF-α, IL-6, and IL-1β primers were obtained from Bioneer. LPS (Escherichia coli 055:B5), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide (MTT), and other reagents and chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Isolation and structure identification
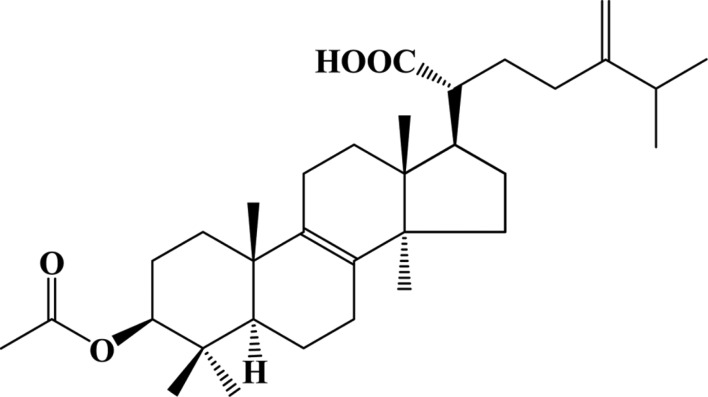
The fruiting bodies of L. sulphureus var. miniatus were collected at Mt. Odae, Gangwon Province, Korea, and they were ground and extracted with methanol at room temperature. The methanolic extract was concentrated under reduced pressure, followed by partitioning of the aqueous resultant between hexane and water. The hexane-soluble portion was concentrated under reduced pressure and subjected to silica gel column chromatography eluted with hexane : ethyl acetate (50 : 1~1 : 1, v/v, stepwise). An active fraction was chromatographed on a column of Sephadex LH-20 eluted with chloroform: methanol (1 : 1, v/v), followed by silica gel column chromatography eluted with hexane : ethyl acetate (20 : 1~1 : 1, v/v, stepwise). Finally, an active fraction was separated by a C18 Sep-pak cartridge eluted with a gradient (80% aqueous methanol to methanol) to afford compound LSM-H7. Compound LSM-H7 was identified as acetyl eburicoic acid by interpretation of 1H NMR, 13C NMR, 1H-1H COSY, HMQC, and HMBC spectra [13]. Acetyl eburicoic acid was previously reported from Laetiporus sulphureus as an apoptosis inducer. The chemical structure of LSM-H7 is depicted in Fig. 1.
Fig. 1. Structure of LSM-H7 (= acetyl eburicoic acid).
Cell culture
Murine macrophage cell line RAW 264.7, originating from American Type Culture Collection, was cultured in DMEM supplemented with 8% FBS and 100 IU/mL penicillin and 100 µg/mL streptomycin sulfate. The incubating conditions were humidified 5% CO2 at 37℃.
Nitric oxide (NO) assay
Nitric oxide was measured by a method based on the Griess reaction assay [6]. Briefly, RAW 264.7 cells were seeded in a 96-well plate and incubated with or without LPS (0.1 µg/mL) in the absence or presence of LSM-H7 at the indicated concentrations for 18 hr. The cell culture supernatant (100 µL) was mixed with Griess reagent (0.2% naphthylethylenediamine dihydrochloride and 2% sulphanilamide in 5% phosphoric acid) in DW at equal volumes and incubated for 5min at room temperature. The absorbance in each well was then analyzed at 540 nm in an enzyme-linked immunosorbent assay reader.
Cell viability (MTT) assay
To determine the cytotoxic effects of LSM-H7, a cell viability assay was done using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide reagent, which was added to the culture medium at a final concentration of 0.1 mg/mL. After 4 hr of incubation at 37℃ in 5% CO2, the resulting violet colored crystals were dissolved in 100 µL dimethyl sulfoxide and absorbance values were measured at 560 nm.
RNA extraction and quantitative real time polymerase chain reaction
RAW 264.7 cells were pretreated with or without LSM-H7 at the concentrations indicated for 30 min and then stimulated with LPS (0.1 µg/mL) for 18 hr. Total RNA was extracted using TRIZOL reagent (Invitrogen, Carlsbad, CA, USA) following the manufacturer's instructions. Total RNA (2 µg) was annealed with Oligo dT (Bioneer) for 10 min at 70℃ and cooled for 5 min on ice, reverse transcribed using reverse transcriptase pre-mix (Bioneer) in 20 µL of reaction mixture and run for 90 min at 42.5℃ using a thermal cycler. The reactions were terminated at 95℃ for 5 min to inactivate the reverse transcriptase. The reverse transcription polymerase chain reaction was performed using aliquots of cDNA obtained from reverse transcription reaction in a polymerase chain reaction (PCR) premix along with the sequence of primers as shown in Table 1. The PCR products were then electrophoresed in 1% agarose gel stained with ethidium bromide and visualized using Eagle Eyes image analysis software (Stratagene, LA Jolla, CA, USA). The intensity of band densities was normalized for corresponding glyceraldehyde 3-phosphate dehydrogenase, which is a housekeeping gene used as an RNA internal standard and the ratios were compared.
Table 1. The sequence of oligonucleotides used for PCR.

PCR, polymerase chain reaction; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; iNOS, inducible nitric oxide synthase; COX-2, cyclooxygenase 2; IL, interleukin; TNF-α, tumor necrosis factor α.
Statistical analysis
The results are presented as mean ± standard error. One way analysis of variance followed by Dunnett's t test was used for statistical analysis. p-values less than 0.001 and 0.05 were considered statistically significant.
RESULTS AND DISCUSSION
The understanding of herbal pharmacology has advanced tremendously alongside modern medicine during the last few decades. There have been reports on the overall advantageous effects of L. sulphureus var. miniatus. Our current findings have revealed for the first time that compound LSM-H7 from L. sulphureus var. miniatus suppresses inflammation in vitro.
LSM-H7 inhibited NO production and iNOS mRNA expression in a dose-dependent manner
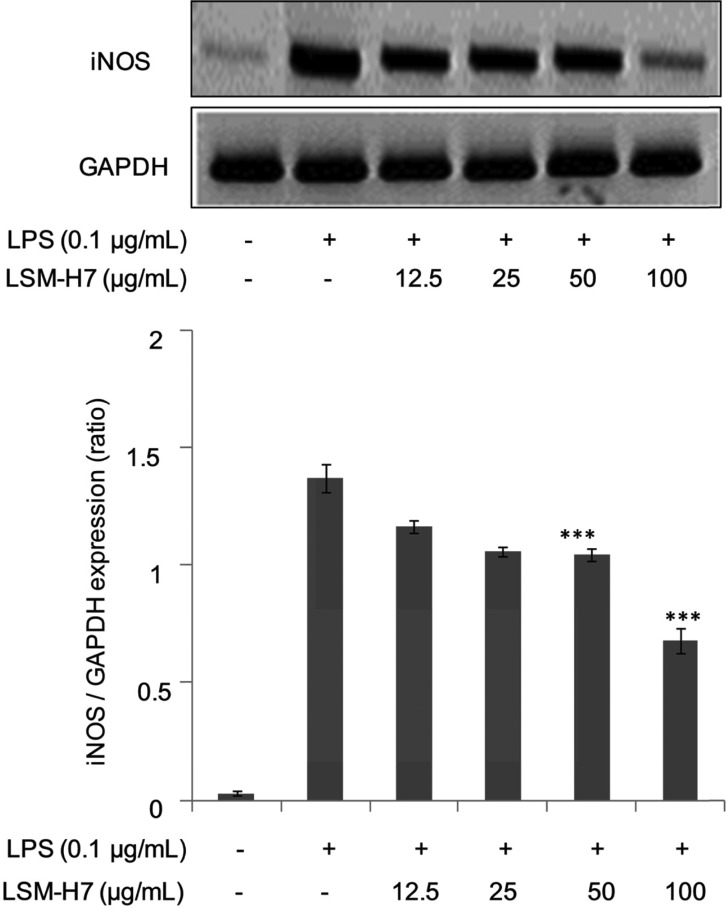
Bacterial LPSs are potent inducers of innate immunity [5]. In some mammals, any foreign insult triggers macrophages to synthesize inducible nitric oxide (NOS2). This enzyme uses NADPH and oxygen to act on L-arginine to produce large amounts of NO and citrulline. The sustained production of NO helps macrophages to kill invading pathogens [14]. The results shown in Fig. 2A demonstrate that LSM-H7 dose-dependently inhibited NO production, clearly indicating that it acts as a potent anti-inflammatory agent in counter acting the inflammation produced by bacterial LPS efficiently. We also observed in (Fig. 2B) that LSM-H7 did not show any cytotoxic effects over the dosage range selected for experimentation, further validating that this dosage can be used in future experimentation for advanced experiments involving LSM-H7. The definitive role of iNOS in host immunity is primarily attributable to production of large quantities of NO upon stimulation by proinflammatory cytokines. Mostly induction of elevated iNOS output occurs in an oxidative environment causing oxidative bursts of macrophages leading to anti-inflammatory and anti-metastatic activities [15]. Suppression of iNOS at the transcriptional level by the compound of interest here i.e. LSM-H7 proves its ability to mitigate inflammation (Fig. 3).
Fig. 2. LSM-H7 suppressed lipopolysaccharide (LPS) activated nitric oxide release. RAW 264.7 cells were preincubated with LSM-H7 for 30 min and then stimulated with LPS for 18 hr. Cell supernatant was mixed with equal amounts of Griess reagent and NO production was measured (A). Effects of LSM-H7 on cell viability were measured by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide assay (B). Values in bar graph are mean ± SEM of three independent experiments. ***p < 0.001 are considered significant compared to LPS only.
Fig. 3. LSM-H7 inhibited mRNA expression of inducible nitric oxide synthase (iNOS). For mRNA expression, RAW 264.7 cells were pretreated with LSM-H7 for 30 min and then stimulated with lipopolysaccharide (LPS) for 18 hr. Total RNA was extracted and mRNA expression of iNOS was determined by reverse transcription polymerase chain reaction. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as an internal control. Image is representative of three independent experiments. Values in bar graphs are mean ± SEM of three independent experiments. ***p < 0.001 are considered significant compared to LPS only.
Attenuation in the levels of COX-2 by LSM-H7
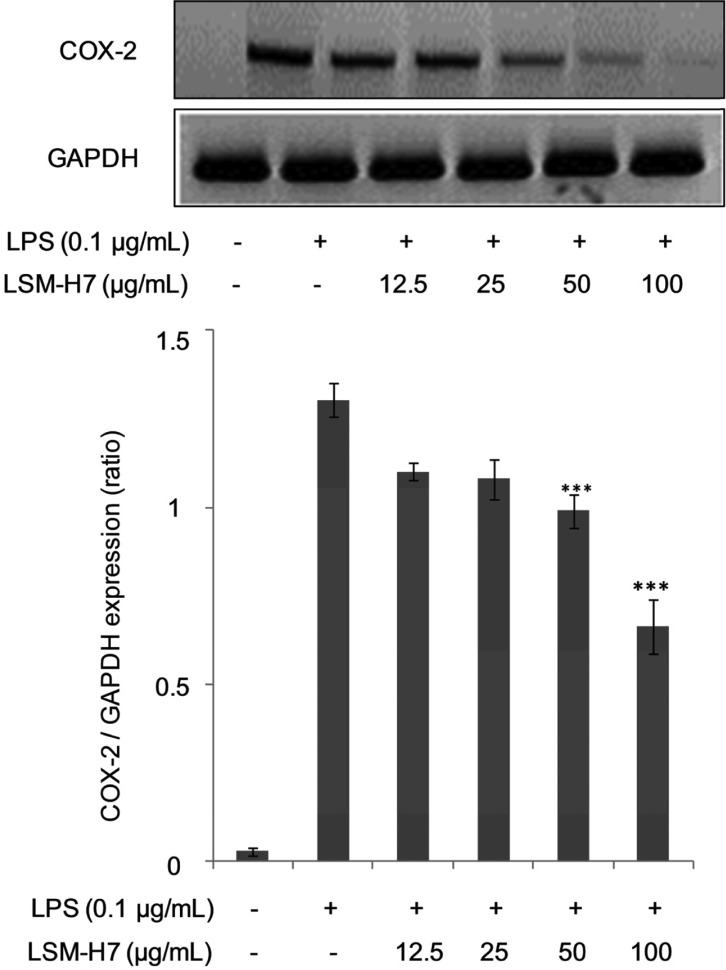
COX, which is also known as prostaglandin-endoperoxide synthase, is an enzyme responsible for the creation of important biological mediators i.e., prostanoids, prostaglandins, prostacyclin, and thromboxane [16]. Until now two forms of the COX enzyme have been identified i.e., COX-1, which is expressed in many cells and tissues, and COX-2, which is selectively induced by proinflammatory cytokines at the times of inflammation. The discovery of COX-2 has led to the hypothesis that any agent that should be termed as anti-inflammatory agent should bear the property to inhibit the levels of inducible COX-2 [17]. As can be seen from Fig. 4, LSM-H7 dose dependently attenuated the production of COX-2, further identifying it as a good anti-inflammatory agent.
Fig. 4. Attenuation in the levels of cyclooxygenase 2 (COX-2) by LSM-H7. RAW 264.7 cells were treated with LSM-H7 and stimulated with lipopolysaccharide (LPS) 30 min later. Total RNA was extracted and mRNA expression COX-2 cytokine was determined by reverse transcription polymerase chain reaction. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as an internal control. Image is representative of three independent experiments. Values in bar graph are mean ± SEM of three independent experiments. ***p < 0.001 are considered significant compared to LPS only.
Reduction in the mRNA levels of IL-1β under LSM-H7 action
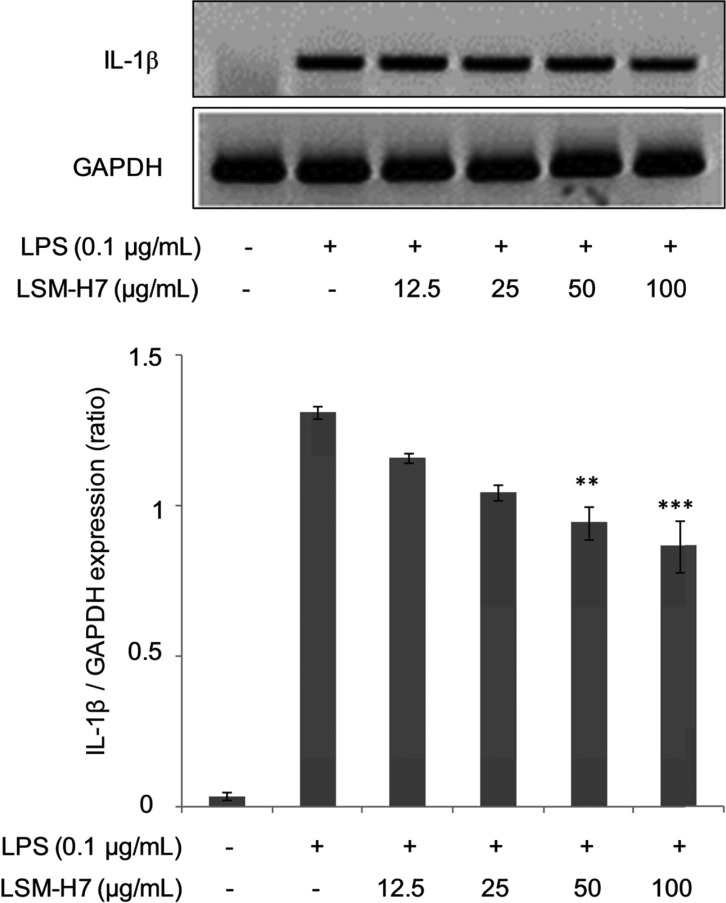
IL-1β, which is also termed as catabolin, is a cytokine protein. This cytokine is formed by activated macrophages as a proprotein, which is processed proteolytically to its active form by caspase 1 [18]. IL-1β is an important mediator of the inflammatory response that is being involved in a variety of cellular activities, like cellular proliferation, differentiation, and apoptosis. As being an important mediator of inflammatory response, the level of this cytokine in times of inflammation is always elevated. In the gene expression of proinflammatory cytokines by LSM-H7, we have shown that it inhibits the transcriptional expression of IL-1β indicating that LSM-H7 can potently act as an anti-inflammatory agent in suppressing inflammation (Fig. 5).
Fig. 5. Reduction in the mRNA levels of interleukin (IL)-1β under LSM-H7 action. IL-1β expression was determined when total RNA was extracted from RAW 264.7 cells under LSM-H7 treatment and lipopolysaccharide (LPS) stimulation. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as an internal control. Image is representative of three independent experiments. Values in bar graph are mean ± SEM of three independent experiments. ***p < 0.001 and **p < 0.05 are considered significant compared to LPS only.
Dose dependent decrease in the levels on IL-6 cytokine mediated by LSM-H7 compound
IL-6 is a type of an interleukin that acts both as pro-inflammatory cytokine and an anti-inflammatory myokine [19]. This cytokine is secreted by T cells and macrophages to mediate immune response, e.g., during microbial infection and after trauma leading to inflammation. IL-6 also acts in combating infection, as previous research shows the necessity of IL-6 to develop resistance against bacterium Streptococcus pneumoniae [20]. Fig. 6 shows that LSM-H7 inhibits the expression of IL-6 in a dose dependent manner confirming it to be potent inflammatory mitigator.
Fig. 6. Dose dependent decrease in the levels on interleukin (IL)-6 cytokine mediated by LSM-H7 compound. For mRNA expression of IL-6, RAW 264.7 cells were pretreated with LSM-H7 for 30 min and then stimulated with lipopolysaccharide (LPS) for 18 hr. Total RNA was extracted and mRNA expression of IL-6 was determined by reverse transcription polymerase chain reaction. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as an internal control. Image is representative of three independent experiments. Values in bar graphs are mean ± SEM of three independent experiments. ***p < 0.001 and **p < 0.05 are considered significant compared to LPS only.
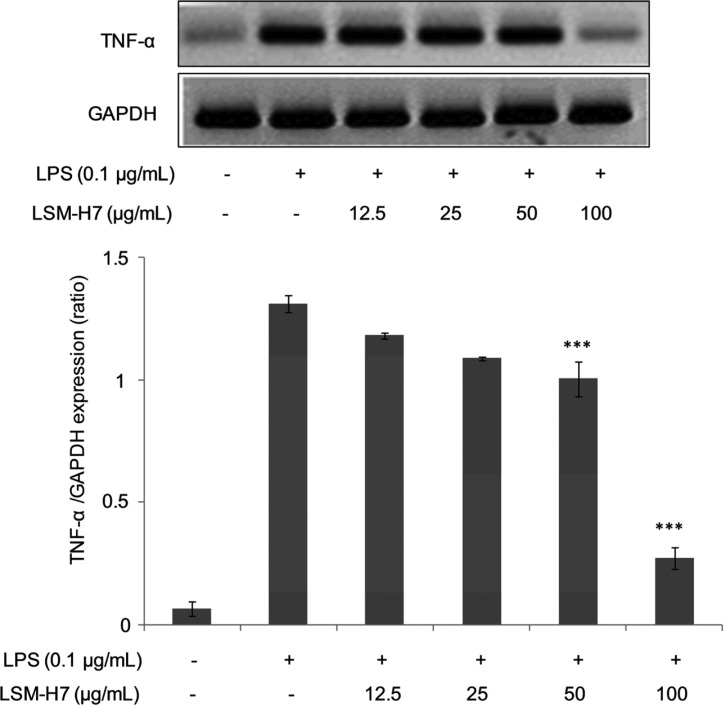
Diminution in TNF-α level in LPS stimulated RAW 264.7 cells under LSM-H7 action
Tumor necrosis factor (TNF, cachexin, or cachectin) is predominantly an adipokine involved in systemic inflammation and belongs to a group of cytokines that mediate acute phase reaction. Its main site of production is chiefly by activated macrophages; however, it can also be produced by neutrophils, eosinophils, mast cells, natural killer cells, lymphocytes and neurons [21,22]. Enormous quantities of TNF are released in response to bacterial LPS and IL-1 [17]. TNF-α along with IL-1 and IL-6 possess various functions like stimulating the release of corticotrophin releasing hormone, suppressing appetite, elevating the normal body temperature, stimulating macrophagal phagocytosis, increasing insulin resistance and so on [23]. Since TNF-α has been identified as a primary agent that is mainly produced by activated macrophages, so its levels in the presence of an anti-inflammatory agent should decrease. And this is the case we saw when LSM-H7 suppressed the TNF-α levels in our RAW 264.7 cell line as shown in Fig. 7.
Fig. 7. Diminution in tumor necrosis factor α (TNF-α) level in lipopolysaccharide (LPS) stimulated RAW 264.7 cells under LSM-H7 action. RAW 264.7 cells were treated with LSM-H7 and stimulated with LPS 30 min later. Total RNA was extracted and mRNA expression of TNF-α cytokine was determined by reverse transcription polymerase chain reaction. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as an internal control. Image is representative of three independent experiments. Values in bar graph are mean ± SEM of three independent experiments. ***p < 0.001 are considered significant compared to LPS only.
So all in all we concluded that LSM-H7 component of L. sulphureus, potently decreases the levels of nitric oxide and inhibits the expression of proinflammatory cytokines. The further validation of its anti-inflammatory properties requires more experimentation.
ACKNOWLEDGEMENT
This work was supported by a grant from the Korea Forest Service and by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2013062358), Republic of Korea.
References
- 1.Mattila P, Salo-Väänänen P, Könkö K, Aro H, Jalava T. Basic composition and amino acid contents of mushrooms cultivated in Finland. J Agric Food Chem. 2002;50:6419–6422. doi: 10.1021/jf020608m. [DOI] [PubMed] [Google Scholar]
- 2.Borchers AT, Krishnamurthy A, Keen CL, Meyers FJ, Gershwin ME. The immunobiology of mushrooms. Exp Biol Med (Maywood) 2008;233:259–276. doi: 10.3181/0708-MR-227. [DOI] [PubMed] [Google Scholar]
- 3.Yang N, Tong X, Xiang Y, Zhang Y, Liang Y, Sun H, Wang DC. Molecular character of the recombinant antitumor lectin from the edible mushroom Agrocybe aegerita. J Biochem. 2005;138:145–150. doi: 10.1093/jb/mvi109. [DOI] [PubMed] [Google Scholar]
- 4.Jeurink PV, Noguera CL, Savelkoul HF, Wichers HJ. Immunomodulatory capacity of fungal proteins on the cytokine production of human peripheral blood mononuclear cells. Int Immunopharmacol. 2008;8:1124–1133. doi: 10.1016/j.intimp.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 5.Dinarello CA. Proinflammatory cytokines. Chest. 2000;118:503–508. doi: 10.1378/chest.118.2.503. [DOI] [PubMed] [Google Scholar]
- 6.Yayeh T, Jung KH, Jeong HY, Park JH, Song YB, Kwak YS, Kang HS, Cho JY, Oh JW, Kim SK, et al. Korean red ginseng saponin fraction downregulates proinflammatory mediators in LPS stimulated RAW264.7 cells and protects mice against endotoxic shock. J Ginseng Res. 2012;36:263–269. doi: 10.5142/jgr.2012.36.3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akira S. Mammalian toll-like receptors. Curr Opin Immunol. 2003;15:5–11. doi: 10.1016/s0952-7915(02)00013-4. [DOI] [PubMed] [Google Scholar]
- 8.Medvedev AE, Piao W, Shoenfelt J, Rhee SH, Chen H, Basu S, Wahl LM, Fenton MJ, Vogel SN. Role of TLR4 tyrosine phosphorylation in signal transduction and endotoxin tolerance. J Biol Chem. 2007;282:16042–16053. doi: 10.1074/jbc.M606781200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsu HY, Hua KF, Lin CC, Lin CH, Hsu J, Wong CH. Extract of Reishi polysaccharides induces cytokine expression via TLR4-modulated protein kinase signaling pathways. J Immunol. 2004;173:5989–5999. doi: 10.4049/jimmunol.173.10.5989. [DOI] [PubMed] [Google Scholar]
- 10.Imazeki R, Hongo T. Coloured illustrations of mushrooms of Japan. Vol. II. Osaka: Hoikusha Press; 1998. pp. 141–142. [Google Scholar]
- 11.Hwang HS, Lee SH, Baek YM, Kim SW, Jeong YK, Yun JW. Production of extracellular polysaccharides by submerged mycelial culture of Laetiporus sulphureus var. miniatus and their insulinotropic properties. Appl Microbiol Biotechnol. 2008;78:419–429. doi: 10.1007/s00253-007-1329-6. [DOI] [PubMed] [Google Scholar]
- 12.Yoshikawa K, Bando S, Arihara S, Matsumura E, Katayama S. A benzofuran glycoside and an acetylenic acid from the fungus Laetiporus sulphureus var. miniatus. Chem Pharm Bull (Tokyo) 2001;49:327–329. doi: 10.1248/cpb.49.327. [DOI] [PubMed] [Google Scholar]
- 13.León F, Quintana J, Rivera A, Estévez F, Bermejo J. Lanostanoid triterpenes from Laetiporus sulphureus and apoptosis induction on HL-60 human myeloid leukemia cells. J Nat Prod. 2004;67:2008–2011. doi: 10.1021/np049762o. [DOI] [PubMed] [Google Scholar]
- 14.Mungrue IN, Husain M, Stewart DJ. The role of NOS in heart failure: lessons from murine genetic models. Heart Fail Rev. 2002;7:407–422. doi: 10.1023/a:1020762401408. [DOI] [PubMed] [Google Scholar]
- 15.Green SJ, Scheller LF, Marletta MA, Seguin MC, Klotz FW, Slayter M, Nelson BJ, Nacy CA. Nitric oxide: cytokine-regulation of nitric oxide in host resistance to intracellular pathogens. Immunol Lett. 1994;43:87–94. doi: 10.1016/0165-2478(94)00158-8. [DOI] [PubMed] [Google Scholar]
- 16.Kurumbail RG, Kiefer JR, Marnett LJ. Cyclooxygenase enzymes: catalysis and inhibition. Curr Opin Struct Biol. 2001;11:752–760. doi: 10.1016/s0959-440x(01)00277-9. [DOI] [PubMed] [Google Scholar]
- 17.Masters SL, Simon A, Aksentijevich I, Kastner DL. Horror autoinflammaticus: the molecular pathophysiology of autoinflammatory disease. Annu Rev Immunol. 2009;27:621–668. doi: 10.1146/annurev.immunol.25.022106.141627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferguson-Smith AC, Chen YF, Newman MS, May LT, Sehgal PB, Ruddle FH. Regional localization of the interferon-beta 2/B-cell stimulatory factor 2/hepatocyte stimulating factor gene to human chromosome 7p15-p21. Genomics. 1988;2:203–208. doi: 10.1016/0888-7543(88)90003-1. [DOI] [PubMed] [Google Scholar]
- 19.Auron PE, Webb AC, Rosenwasser LJ, Mucci SF, Rich A, Wolff SM, Dinarello CA. Nucleotide sequence of human monocyte interleukin 1 precursor cDNA. Proc Natl Acad Sci U S A. 1984;81:7907–7911. doi: 10.1073/pnas.81.24.7907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van der Poll T, Keogh CV, Guirao X, Buurman WA, Kopf M, Lowry SF. Interleukin-6 gene-deficient mice show impaired defense against pneumococcal pneumonia. J Infect Dis. 1997;176:439–444. doi: 10.1086/514062. [DOI] [PubMed] [Google Scholar]
- 21.Gahring LC, Carlson NG, Kulmar RA, Rogers SW. Neuronal expression of tumor necrosis factor alpha in the murine brain. Neuroimmunomodulation. 1996;3:289–303. doi: 10.1159/000097283. [DOI] [PubMed] [Google Scholar]
- 22.Walsh LJ, Trinchieri G, Waldorf HA, Whitaker D, Murphy GF. Human dermal mast cells contain and release tumor necrosis factor alpha, which induces endothelial leukocyte adhesion molecule 1. Proc Natl Acad Sci U S A. 1991;88:4220–4224. doi: 10.1073/pnas.88.10.4220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verstovsek S, Maccubbin D, Ehrke MJ, Mihich E. Tumoricidal activation of murine resident peritoneal macrophages by interleukin 2 and tumor necrosis factor alpha. Cancer Res. 1992;52:3880–3885. [PubMed] [Google Scholar]