Background: FGF signaling controls development and regeneration of the prostate. However, its role in prostate stem cells is not clear.
Results: FGFR2 in basal prostate stem cells (P-bSCs) controls their self-renewal and differentiation. FGFR2 deficiency in P-bSCs impaired postnatal development of the prostate.
Conclusion: FGFR2 signaling maintains P-bSC stemness.
Significance: The findings suggest a novel avenue to manipulate P-bSC self-renewal and dormancy.
Keywords: cell signaling, fibroblast growth factor (FGF), fibroblast growth factor receptor (FGFR), prostate, stem cells, stem cell
Abstract
Prostate stem cells (P-SCs) are capable of giving rise to all three lineages of prostate epithelial cells, which include basal, luminal, and neuroendocrine cells. Two types of P-SCs have been identified in both human and mouse adult prostates based on prostasphere or organoid cultures, cell lineage tracing, renal capsule implantation, and expression of luminal- and basal-specific proteins. The sphere-forming P-SCs are from the basal cell compartment that express P63, and are therefore designated as basal P-SCs (P-bSCs). Luminal P-SCs (P-lSCs) express luminal cytokeratins and Nkx3.1. Herein, we report that the type 2 FGF receptor (FGFR2) signaling axis is crucial for preserving stemness and preventing differentiation of P-bSCs. FGFR2 signaling mediated by FGFR substrate 2α (FRS2α) is indispensable for formation and maintenance of prostaspheres derived from P63+ P-bSCs. Ablation of Fgfr2 in P63+ cells in vitro causes the disintegration of prostaspheres. Ablation of Fgfr2 in vivo reduces the number of P63-expressing basal cells and enriches luminal cells. This suggests a basal stem cell-to-luminal cell differentiation. In addition, ablation of Fgfr2 in P63+ cells causes defective postnatal development of the prostate. Therefore, the data indicate that FGFR2 signaling is critical for preserving stemness and preventing differentiation of P-bSCs.
Introduction
The prostate is an androgen-dependent male reproductive organ that is comprised of epithelial and stromal compartments. The epithelial compartment contains basal, luminal, and neuroendocrine cells. Androgen deprivation results in massive apoptosis in luminal epithelial cells and prostate atrophy. Only a limited number of cells in the epithelial compartment survive in the regressed state. After androgen replenishment, the surviving epithelial cells are actively engaged in proliferation and the prostate regenerates to its original state within 14 days. This regression/regeneration cycle can occur for more than 15 rounds. This suggests the existence of castration resistant prostate stem cells (P-SCs)2 that contribute to prostate regeneration (1–3). Two types of P-SCs have been identified in adult prostate, which are designated as basal P-SCs (P-bSCs) and luminal P-SCs (P-lSCs), respectively (47). P-bSCs that express basal cell cytokeratins and P63 have been identified based on prostasphere cultures, in vivo renal capsule regeneration, and lineage tracing assays in vivo, whereas P-lSCs are identified based on castration-resistant Nkx3.1 expression in regenerating prostate and organoid cultures (4–14). Both types of P-SCs give rise to both basal and luminal cells both in vivo and in vitro. Both lineages of cells have been shown capable of developing prostate cancers with distinct aggressiveness and molecular signatures upon loss of Pten function (9, 15, 16).
The fibroblast growth factor (FGF) family consists of 18 tyrosine kinase receptor-binding ligands regulating a broad spectrum of cellular processes. FGFs bind and activate the transmembrane tyrosine kinases encoded by four highly homologous genes, denoted Fgfr1, Fgfr2, Fgfr3, and Fgfr4 (17). Aberrant expression and activation of the FGF signaling axis is associated with many diseases that include developmental disorders and cancer (18). FGF receptor substrate 2α (FRS2α) is a proximal adaptor protein and substrate for the FGFR kinases. When it is phosphorylated after activation of the FGFR kinases, it recruits multiple downstream signaling amplifiers to the FGFR kinase, including upstream scaffolds for the MAP kinase and phosphatidylinositol (PI3) 3-kinase pathways. Ablation of Fgfr2 in mouse prostatic epithelial precursor cells compromises bud formation, branching morphogenesis, growth, and acquisition of androgen dependence of the prostate, whereas ablation of Frs2α impairs prostate branching morphogenesis and growth without affecting androgen dependence of the prostate (19, 20).
The FGF signaling axis regulates a wide range of processes in embryonic development, stem cell maintenance, and differentiation. FGF2 is required for sustaining self-renewal and pluripotency of human embryonic stem cells (21). Precise regulation of Fgfr expression is required during human embryonic stem cell specification (22). FGF signaling has also been implicated in a variety of tissue stem cell activities, including neural stem cells (23), bone marrow mesenchymal stem cells (24), and hematopoietic stem cells (25). Studies from our group have shown that the FGF signaling axis prevents differentiation of cardiac stem cells (26) and dental epithelial stem cells (27). Disruption of FGF signaling leads to premature differentiation of cardiac progenitor cells. However, thus far, the roles of FGF signaling in P-SC self-renewal and differentiation are still controversial. It has been reported that paracrine stimulation of prostate basal/stem cells with FGF10 results in multifocal adenocarcinoma (28). FGF7 (KGF) has also been shown to suppress α2β1 integrin function and promotes differentiation of the transient amplifying population in human prostatic epithelium (29). Herein we report that FGF signaling mediated by FGFR2/FRS2α-dependent pathways played a critical and specific role in self-renewal and differentiation of P-bSCs. Inhibition of the PI3K/AKT pathway suppressed P-bSC self-renewal activity in a reversible manner, whereas inhibition of ERK induced P-bSC differentiation and permanently abolished sphere-forming activity. Tissue specific ablation of Fgfr2 in prostate basal cells, which were capable of giving rise to all epithelial lineages of the prostate (30), reduced the numbers of P-bSCs and basal cells in the prostate. The results indicate that FGFR2 is critical for P-bSC self-renewal and differentiation both in vivo and in vitro and provides a novel avenue for control of P-bSC self-renewal by manipulation of FGF signaling.
Materials and Methods
Animals
Mice were housed under the Program of Animal Resources of the Institute of Biosciences and Technology in accordance with the principles and procedure of the Guide for the Care and Use of Laboratory Animals. All experimental procedures were approved by the Institutional Animal Care and Use Committee. Mice carrying loxP-flanked Frs2α, Fgfr1, Fgfr2 alleles, and the Nkx3.1cre and P63CreERT2 knock-in alleles were bred and genotyped as described (19, 31–34). Prostate tissues were harvested for the described analyses after the animals were euthanized by CO2 suffocation.
Inducible Gene Ablation
For inducible gene ablation, mice bearing Fgfr1f/f-P63CreERT2, Fgfr2f/f-P63CreERT2, Fgfr1/2f/f-P63CreERT2, and its wild type counterpart alleles were administered 100 mg/kg of tamoxifen (Sigma, 20 mg/ml stock solution in corn oil). For in vitro ablation, cells bearing the aforementioned alleles were treated with 4-hydroxytamoxifen (Sigma, diluted in alcohol at a stock concentration of 5 mm) at the indicated concentrations.
Prostasphere Cultures
The conditions for culturing and passaging prostaspheres were adapted by modification of published procedures (4). Briefly, prostates dissected from 6- to 8-week-old male mice were minced with a pair of steel scissors, followed by incubating with 1 mg/ml of collagenase (Sigma) in 10 ml of DMEM with 10% FBS at 37 °C for 90 min. Cells were washed with PBS, further digested with 0.25% trypsin/EDTA for 10 min at 37 °C, and passed several times through a 25-gauge syringe. After inactivation of trypsin by FBS, cells were passed through a 40-μm cell strainer, washed with Dulbecco's PBS (Sigma), and counted. Prostate cells (3 × 104) were suspended in 50 μl of the prostate epithelial growth medium (Lonza, Walkersville, MD) and then mixed with Matrigel (BD Biosciences) at a ratio of 1:1. The cell mixtures were plated around the rim of wells in a 12-well plate and allowed to solidify at 37 °C for 30 min. Then, 1 ml of prostate epithelial growth medium was added to each well, and the medium was replenished every other day. After plating for 8–10 days, the spheres with a diameter over 100 μm were scored. To harvest the spheres, the Matrigel was digested by incubation in 1 ml of 1 mg/ml of dispase solution (Invitrogen) at 37 °C for 30 min, followed by centrifugation. To subculture the spheres, the pellets were digested with 1 ml of 0.05% trypsin/EDTA (Invitrogen) for 5 min at 37 °C. After inactivation of trypsin by FBS, the cells were passed through a 40-μm filter, counted by a hemocytometer, and replated. FGF7 or FGF10 was added to the cell culture medium at a final concentration of 10 ng/ml. 4-Hydroxytamoxifen was added to the cell culture medium at a final concentration of 500 nm.
Flow Cytometry and Cell Sorting (FACS)
Dissociated single prostate cells were stained with FITC-conjugated anti-CD31, -CD45, and -Ter119 antibodies (eBioscience), phycoerythrin-conjugated anti-Sca-1 antibody (eBioscience), and Alexa 647-conjugated anti-CD49f antibody (Biolegend). All antibody incubations, washes, and flow cytometric analyses were performed in cell sorting buffer (2% FBS in PBS). FACS was conducted on a BD FACSAria I SORP, and cells were sorted into DMEM + 20% FBS. Primary antibody labeling for cell sorting was conducted by incubation for 20 min on ice with antibody dilution according to the manufacturer's suggestions in a volume of 100 μl/105-108 cells in cell sorting buffer. The cells were washed in 1 ml of ice-cold cell sorting buffer, resuspended in 0.5 ml of cell sorting buffer, and analyzed.
Histology
Prostaspheres were fixed with 4% paraformaldehyde/PBS solution for 30 min. The fixed spheres were pelleted and mixed with Histogel. After solidifying at room temperature, the Histogel pellets were serially dehydrated, embedded in paraffin, and sectioned as described (4). For general histology, slides were re-hydrated and stained with hematoxylin and eosin (H&E). For immunostaining, the antigens were retrieved by boiling in citrate buffer (pH 8.0) for 20 min. The source and concentration of primary antibodies were: mouse anti-p63 (1:150 dilution) from Santa Cruz (Santa Cruz, CA), rabbit anti-phosphorylated ERK (1:200 dilution), rabbit anti-phosphorylated AKT (1200 dilution), mouse anti-Ki67 (1:200 dilution), rabbit anti-β-catenin (1:200 dilution), and anti-cleaved caspase 3 (1:200 dilution) from Cell Signaling Technology. The ExtraAvidin Peroxidase System (Sigma) and fluorescence-conjugated secondary antibodies (Invitrogen) were used to visualize specifically bound antibodies. For immunofluorescence staining, the nuclei were counterstained with To-Pro 3 before being observed under a confocal microscope (Zeiss LSM 510).
Western Blotting
Prostaspheres were homogenized in RIPA buffer (50 mm Tris-HCl buffer, pH 7.4, 1% Nonidet P-40, 150 mm NaCl, 0.25% sodium deoxycholate, 1 mm EGTA, 1 mm PMSF), and the extracted proteins were harvested by centrifugation. Samples containing 30 μg of proteins were separated by SDS-PAGE and electroblotted onto PVDF membranes for Western analyses with the indicated antibodies. The dilutions of the antibodies were: anti-phosphorylated ERK1/2, 1:1000; anti-phosphorylated AKT, 1:1000; anti-ERK1/2, 1:1000; and anti-AKT, 1:1000 (Cell Signaling Technology). After being washed with TBST buffer to remove nonspecific antibodies, the membranes were then incubated with horseradish peroxidase-conjugated rabbit antibody at room temperature for 1 h. The specifically bound antibodies were visualized by using the ECL-Plus chemoluminescent reagents. The films were scanned with a densitometer for quantitation.
RNA Expression
Total RNA was isolated from prostaspheres using the TRIzol RNA isolation reagents (Life Technologies). The first-strand cDNAs were reverse transcribed from the RNA template using SuperScript III reverse transcriptase (Invitrogen) and random primers according to manufacturer's protocols. Real-time PCR analyses were carried out using the Fast SYBR Green Master Mix (Life Technologies) as instructed by the manufacturer. Relative abundance of mRNA was calculated using the comparative threshold (CT) cycle method and were normalized to β-actin as the internal control. The mean ± S.D. among at least three individual experiments are shown.
Statistical Analysis
Statistical analysis was performed using the two tailed t test, with significance set to p < 0.05. Error bars indicate standard deviation.
Results
FGF Signaling Promotes Prostasphere Formation
The FGF7/10-FGFR2 signaling axis is important in prostate development and maintenance of tissue homeostasis in the prostate (19, 35, 36). To determine whether FGF signaling was required for prostasphere formation, FGF7 or FGF10 was added to the culture medium at a concentration of 10 ng/ml. Both FGF7 and FGF10 significantly increased the number and size of prostaspheres (Fig. 1A). This indicated that FGF signaling promoted prostasphere formation and growth of individual spheres.
FIGURE 1.
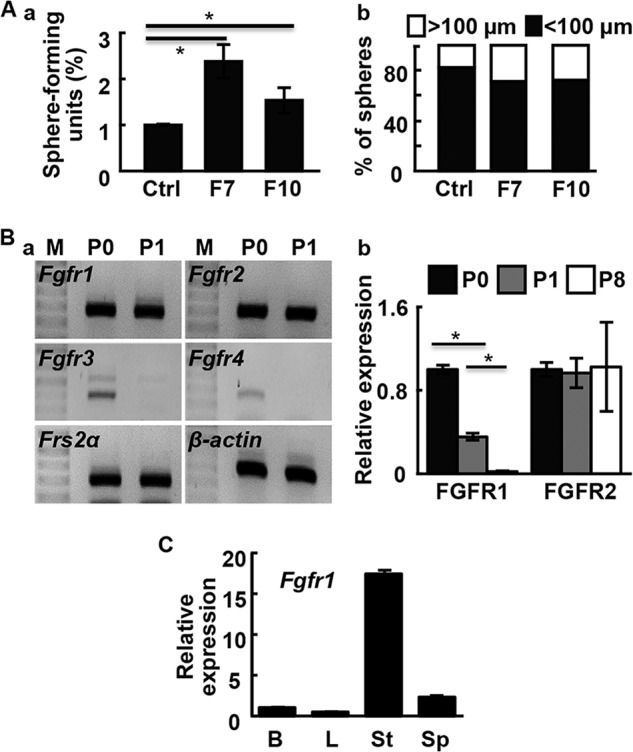
FGF promotes prostasphere formation and growth. A, FGF7 (F7) or FGF10 (F10) were added to the culture medium (10 ng/ml). Sphere numbers per 100 cells were calculated (a) and sphere sizes (b) were measured at day 10. B, RT-PCR (a) and real-time RT-PCR (b) analyses of the indicated gene expression in primary prostate cells (P0) and prostaspheres of the 1st (P1) and 8th (P8) generations. C, real-time RT-PCR analyses of Fgfr1 expression in primary prostate cells and prostaspheres. M, DNA molecular weight markers; B, basal epithelial cells; L, luminal epithelial cells; St, stromal cells; Sp, prostasphere cells; *, p ≤ 0.05.
To determine which FGFR isoform mediated the FGF signaling, we employed RT-PCR analyses to examine the expression of FGFR isoforms in prostaspheres and primary prostate cells. The results revealed that Fgfr1 and Fgfr2 were expressed in prostaspheres and primary prostate cells; Fgfr3 and Fgfr4 were detectable only in the primary prostate cells (Fig. 1B, a). In addition, Frs2α, an adaptor protein required for activation of the ERK and PI3K/AKT pathways by FGFR, was also expressed in prostaspheres (Fig. 1B, a). Although Fgfr2 expression remained stable in primary, P1, and P8 prostaspheres, Fgfr1 expression was reduced in P8 prostaspheres (Fig. 1B, b). Real-time RT-PCR analyses of primary prostate cells showed that Fgfr1 was predominantly expressed in stromal cells (Fig. 1C). However, a trace amount of Fgfr1 expression was detectable in basal cells and prostaspheres.
Inhibition of FGF Signaling Impairs Self-renewal of Prostaspheres
To determine whether FGF signaling was critical in prostasphere formation, spheres were treated with FGFR tyrosine kinase inhibitor 341608. The results showed that prostasphere formation was suppressed by 341608 (Fig. 2A). Real-time RT-PCR analyses revealed that the inhibitor concurrently increased expression of CK18 and AR and decreased expression of P63 (Fig. 2B). This suggested a transition from basal to luminal cells. To further test the role of FGFR signaling in basal cell maintenance, we treated the K5H2B/GFP reporter-bearing prostaspheres with FGFR inhibitors. The GFP+ cells were reduced in the inhibitor group (Fig. 2C). This indicated that inhibition of FGF signaling in prostasphere cells led to loss of CK5 expression and suggested that the cells differentiated.
FIGURE 2.
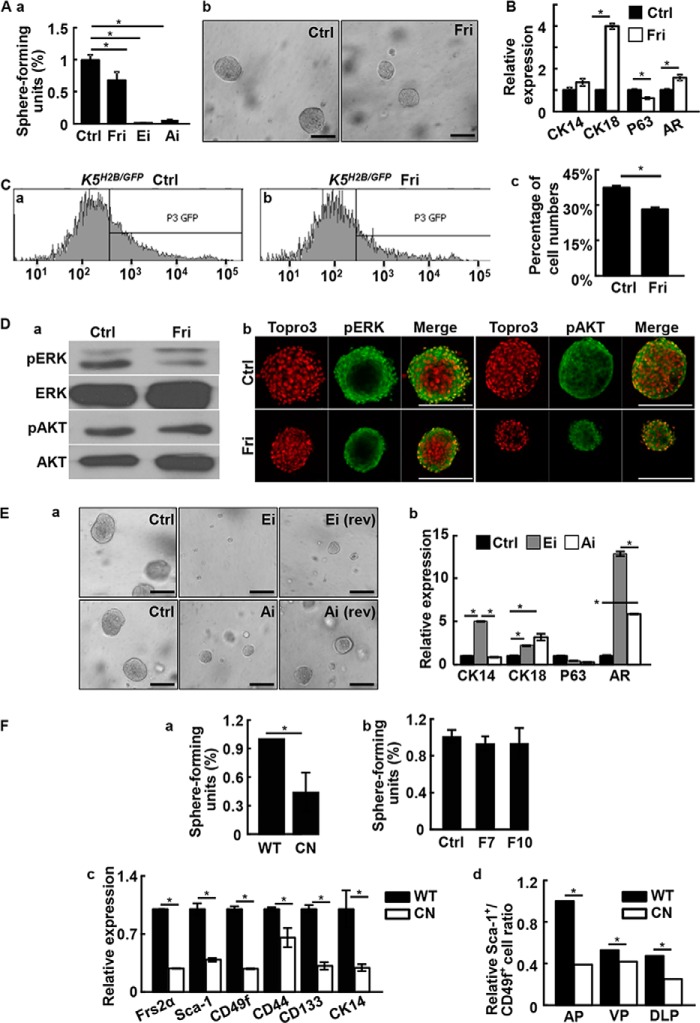
Inhibition of FGF signaling suppresses prostasphere formation. A, prostasphere cultures were carried out with or without the indicated inhibitor for 6 days. Spheres with a diameter greater than 100 μm were scored (a). Representative spheres were shown in b. B, real-time RT-PCR analyses of the indicated genes expression in prostaspheres with or without treating with 500 nm FGFR inhibitors. C, K5H2B/GFP spheres were treated with 500 nm FGFR inhibitors and GFP positive cells were detected by FACS (a and b). The quantitative data are shown in panel c. D, the prostasphere cultures were treated with 500 nm FGFR inhibitor and phosphorylation of ERK and AKT was analyzed with Western blot (a) or immunostaining (b) analyses. E, prostaspheres were cultured with or without 10 μm ERK or AKT inhibitors. Rev indicates that the inhibitors were removed 6 days later. Representative pictures are shown in panel a and real-time RT-PCR analyses of the indicated genes expression are shown in panel b. F, panel a, primary Frs2αcn and Frs2αf/f prostate cells were cultured in Matrigel and the spheres were quantitated at day 10. Panel b, prostate cells prepared from Frs2αcn prostate were cultured in the presence of absence of 10 ng/ml of FGF7 or FGF10. The sphere numbers were scored at day 10. Panel c, total RNA extracted from Frs2αf/f or Frs2αcn prostates were subjected to real-time RT-PCR of the indicated genes. Panel d, FACS analyses showing reduced P-bSCs in individual prostate lobes of Frs2αCN prostates. AP, anterior prostate; VP, ventral prostate; DLP, dorsolateral lobes; Ctrl, solvent control; Fri, FGFR inhibitor; pERK, phosphorylated ERK; pAKT, phosphorylated AKT; AR, androgen receptor; WT, Frs2αf/f; CN, Frs2αCN; data are normalized with β-actin loading control and expressed as mean ± S.D. from triplicate samples; *, p ≤ 0.05; scale bars, 100 μm.
We then employed Western blot and immunostaining to determine whether activation of the ERK or PI3K/AKT pathway, the two major signaling pathways downstream of the FGFR, was affected by inhibition of FGFR in the prostaspheres (Fig. 2D). Both analyses revealed that only phosphorylated ERK, but not phosphorylated AKT, was compromised by FGFRi treatment (Fig. 2D). To further clarify whether the two pathways contributed to prostasphere growth, SL327, an ERK inhibitor (ERKi), or LY294002, an AKT inhibitor (AKTi) was added to the sphere culture medium for 6 days. The results indicated that both ERKi and AKTi suppressed prostasphere growth (Fig. 2E, a). This was despite the fact that inhibition of FGFR signaling did not reduce phosphorylation of AKT. Interestingly, withdrawal of AKTi, but not ERKi, partially restored sphere formation. This suggested that the inhibition of prostaspheres due to AKTi was reversible, whereas that of ERKi was irreversible. The result was consistent with a previous report that suppressing AKT signaling compromised proliferation of prostate sphere-forming cells without affecting cell survival (37). However, both pathways had pro-differentiation functions in prostaspheres (Fig. 2E, b).
FRS2α is an adaptor bridging FGFR tyrosine kinases and downstream signaling pathways. To determine whether FRS2α was required for prostasphere formation, Frs2α alleles were tissue specifically ablated in prostate epithelial cells using Nkx3.1Cre as described (20). Ablation of Frs2α significantly reduced the sphere forming activity of primary prostate cells (Fig. 2F, a). In addition, FGF7 and FGF10 failed to promote sphere-forming activity in Frs2α null cells. This suggested that FRS2α was required for FGF7/10 to promote prostasphere formation and growth (Fig. 2F, b). RT-PCR analyses further demonstrated that ablation of Frs2α reduced expression of stem cell markers in the prostate. This included Sca-1, CD49f, CD44, and CD133, as well as the basal cell-associated CK14 (Fig. 2F, c). Furthermore, Sca-1+/CD49f+ cells were reduced in all lobes of Frs2α null prostates (Fig. 2F, d).
Ablation of FGF Signaling Specifically in P63-expressing Cells Suppresses Prostasphere Formation
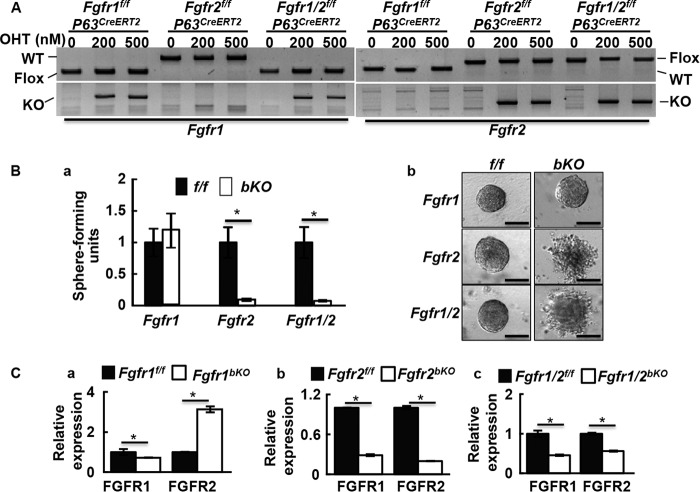
Because sphere-forming cells expressed basal cell marker P63, and P63-expressing cells were capable of giving rise to all cell types in the spheres, we then investigated whether FGFR1 and FGFR2 in P63+ basal cells played a role in prostasphere formation. Prostaspheres derived from mice bearing P63CreERT2 and either Fgfr1 floxed (Fgfr1f/f) or Fgfr2 floxed (Fgfr2f/f), or both (Fgfr1/2f/f) were treated with 4-hydroxytamoxifen to delete the Fgfr1f/f and/or Fgfr2f/f alleles. PCR analyses showed that the floxed sequences were deleted in the cells bearing Fgfr1f/f-P63CreERT2, Fgfr2f/f-P63CreERT2, and Fgfr1/2f/f-P63CreERT2 alleles (Fig. 3A), which are hereafter designated as Fgfr1bKO, Fgfr2bKO, and Fgfr1/2bKO, respectively. The reduction in sphere formation by deletion of Fgfr2 or both Fgfr1 and Fgfr2 indicated that intact Fgfr2 alleles were required for prostasphere formation (Fig. 3B, a). The deletion of Fgfr1 alone did not affect prostasphere formation. In addition, deleting Fgfr2, or both Fgfr1 and Fgfr2, but not Fgfr1, at day 1 of primary culture impaired sphere integrity (Fig. 3B, b). Real-time RT-PCR revealed that ablation of Fgfr1 resulted in elevated Fgfr2 expression. In contrast, the deletion of Fgfr2 in prostaspheres caused a concomitant decline in Fgfr1 expression (Fig. 3C).
FIGURE 3.
FGFR2 signaling is required for prostasphere formation. A, PCR analyses of gene ablation in prostaspheres. B, prostaspheres were cultured in the presence or absence of 4-hydroxytamoxifen to induce gene ablations. Relative sphere forming unites were calculated (a) and morphology of the spheres were shown (b). C, real-time RT-PCR analyses of Fgfr1 and Fgfr2 expression in the indicated cells with or without 4-hydroxytamoxifen treatment. Data are mean ± S.D. from three mice; OHT, 4-hydroxytamoxifen; *, p ≤ 0.05; scale bars, 100 μm.
Wnt signaling enhances the self-renewal of prostate basal/stem cells and promotes expansion of “triple positive” (CK5+, CK8+, p63+) prostate progenitor cells (38). The basal progenitor cells have high Wnt signaling activity that positively correlates with basal cell numbers (39). Interestingly, quantitative PCR revealed that ablation of Fgfr1 in p63-expressing cells increased and ablation of Fgfr2 reduced β-catenin at the mRNA level (Fig. 4A, a). Immunofluorescent staining further confirmed the finding at the protein level (Fig. 4B, a). We reported similar results in dental epithelial stem cells (27). Together with the data that Fgfr2 expression was increased in Fgfr1-deficient cells (Fig. 3C), the results indicated that FGFR2 enhanced Wnt signaling and enhanced self-renewal of P-bSCs. Real-time RT-PCR and immunofluorescence staining showed that expression of BCl-2 was decreased (Fig. 4A, b), whereas cleaved caspase 3 was increased upon Fgfr2 deletion or the Fgfr1/2 double deletion (Fig. 4B, b). Thus, the results suggested that loss of FGFR2 signals promoted P-bSC apoptosis. The loss of FGFR2 signaling induced the loss of both stemness and commitment to apoptosis. Whether the two have a causal link needs to be further investigated. Notably, real-time RT-PCR analyses showed that expression of Notch-1 was increased in the Fgfr1 ablated prostaspheres (Fig. 4C). Induction of Notch signaling in prostaspheres inhibited their proliferation and disrupted prostasphere formation, which eventually offset the increased Wnt signaling.
FIGURE 4.
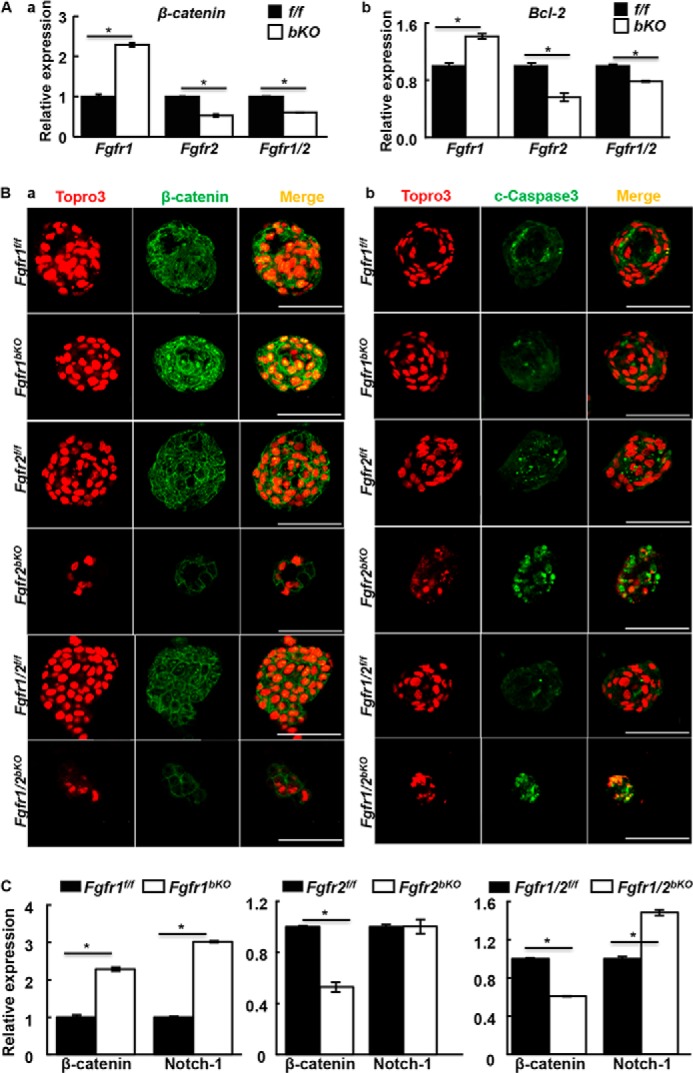
FGFR2 signaling in P-bSCs up-regulates the WNT pathway and suppresses apoptosis. A, the indicated prostaspheres treated with 4-hydroxytamoxifen at day 2 after inoculation were harvested at day 10. Expression of β-catenin or Bcl-2 was assessed by real-time RT-PCR. B, immunostaining with anti-β-catenin or anti-c-Caspase 3 antibodies for the same spheres. C, expression of Notch-1 was assessed by real-time RT-PCR. Ctrl, solvent control; OHT, 4-hydroxytamoxifen; c-Caspase3, cleaved caspase 3; *, p ≤ 0.05; scale bars, 100 μm.
FGFR2 Is Required for Maintenance of Basal Cell Homeostasis in Adult Prostates
To assess the effect of ablation of Fgfr1 or Fgfr2 in basal cells in the adult prostate, mice carrying Fgfr1f/f-P63CreERT2, Fgfr2f/f-P63CreERT2, or Fgfr1/2f/f-P63CreERT2 alleles were treated with tamoxifen for 5 consecutive days. Real-time RT-PCR analyses showed that expression of Fgfr1 and Fgfr2 in the dissociated basal cells was reduced 1 week later (Fig. 5A). Prostate cells were dissociated and basal and luminal cell populations were analyzed with a cell sorter (Fig. 5B). Double deletion of Fgfr1 and Fgfr2 reduced the number of basal cells by 38% and increased luminal cells by 14%. The total number of stromal cells remained the same. Ablation of Fgfr2 alone caused a similar decrease of 39% in basal cells, whereas luminal cells increased by 28%. P63 immunostaining further confirmed the reduction in basal cells in Fgfr2 or Fgfr1/2 conditional knock-out adult prostates (Fig. 5C) however, whether FGFR2 directly regulated expression of P63 remained to be determined. The single ablation of Fgfr1 had no impact on cellular composition of the prostate. Real-time RT-PCR analyses also showed that expression of P63 was not affected by Fgfr1 ablation in prostaspheres. However, expression of both CK14 and CK18 were increased, indicating more differentiated cells in the Fgfr1bKO prostaspheres (Fig. 5D). Furthermore, the sphere-forming activity of Fgfr2 or Fgfr1/Fgfr2-deficient basal cells was impaired (Fig. 5E). Together, the results suggested that ablation of Fgfr2, but not Fgfr1, in vivo impaired basal cell homeostasis and enhanced basal-to-luminal cell differentiation. Thus, modulation of FGF signaling in adult basal/progenitor cells can be a determinant in specification of cell lineage.
FIGURE 5.
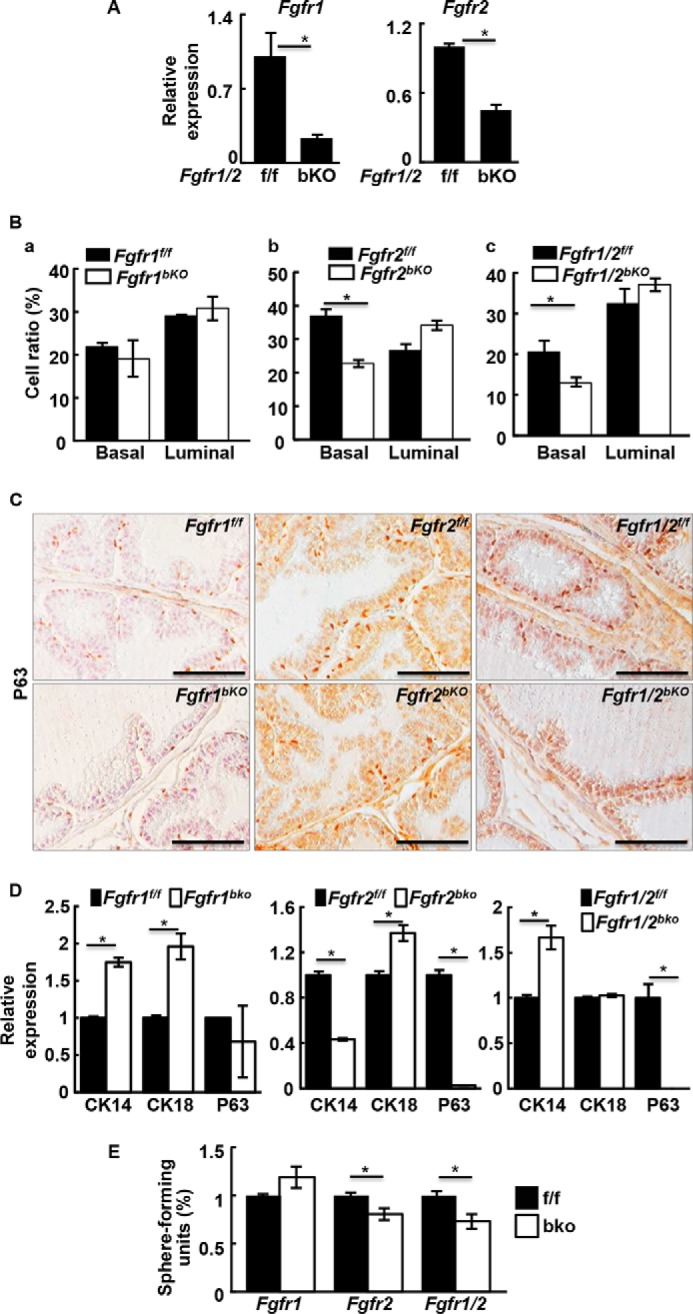
FGFR2 is required for maintaining basal cell homeostasis in the adult prostate. A and B, the indicated mice were injected intraperitonally with tamoxifen for 5 consecutive days at the age of 8 weeks. The prostates were harvested 1 week later and isolated basal cells were subjected to RT-PCR analyses (A) or FACS analyses for cellular compositions (B). C, immunostaining of P63+ cells in the indicated prostate with or without tamoxifen injection. Representative pictures from three mice are shown. D, real-time RT-PCR analyses showing expression of the indicated gene in Fgfr1bKO, Fgfr2bKO, and Fgfr1/2bKO spheres. E, sphere-forming analyses of FACS-fractionated basal cells from mice with the indicated genotype. f/f, homozygous floxed alleles; bKO, basal cell specific knock-out; data represent mean ± S.D. from three mice; *, p ≤ 0.05; scale bars, 100 μm.
To investigate whether FGF signaling in P63-expressing cells contributed to prostate development, 2-week-old mice bearing Fgfr1f/f-P63CreERT2, Fgfr2f/f-P63CreERT2, or Fgfr1/2f/f-P63CreERT2 alleles were injected intraperitoneally with tamoxifen to activate the Cre recombinase. Ablation of Fgfr2 reduced the size of the prostate by 20% at 6 weeks of age (Fig. 6A). In addition, the complexity of epithelial enfolding and the ductal network were also reduced (Fig. 6B). Consistent with our in vitro sphere assay data, ablation of Fgfr2 in prostates in vivo induced apoptosis, as shown by the reduction of anti-apoptotic protein Bcl-2 expression (Fig. 6C). The Fgfr2bKO prostate also had reduced basal cells and proliferating cells, as demonstrated by P63 and Ki67 immunostaining, respectively (Fig. 6D). Similarly, double ablation of Fgfr1 and Fgfr2 also reduced cell proliferation in developing prostate (Fig. 6E), as well as decreased prostasphere-forming P-bSCs (Fig. 6F). Ablation of Fgfr1 alone in basal cells had no effect (data not shown). The results demonstrated that FGFR2 in p63+ basal cells was required for prostate development. The results are in line with our previous reports that ablation of Fgfr2, but not Fgfr1 in prostate progenitor cells impairs prostate branching morphogenesis (19, 40).
FIGURE 6.
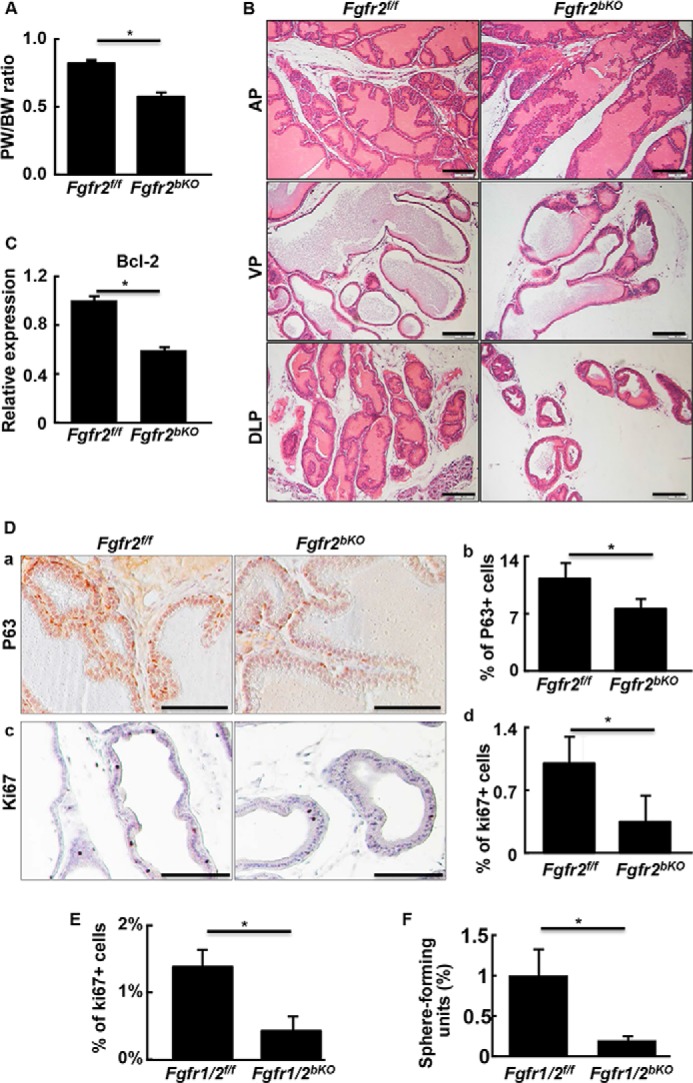
Disruption of Fgfr1 and Fgfr2 in P63 expressing cells perturbs prostate morphogenesis. A, prostate versus body weight ratio showing ablation of Fgfr2 in basal cells compromised prostate growth. B, prostate tissues from 6-week-old mice were dissected, sectioned, and H&E stained. C, real-time RT-PCR analyses showing reduced expression of Bcl-2 in Fgfr2bKO prostate. D, immunostaining showing reduced P63+ basal cells (a) and Ki67+ proliferating cells (c) in Fgfr2bKO prostate. Average cell numbers calculated from three samples are shown in panels b and d, respectively. E, prostate sections of 6-week-old Fgfr1/2bKO mice were immunostained with anti-Ki67 antibodies and the Ki67 positive cells were quantitated. F, sphere-forming efficiency of the indicated primary prostate cells was analyzed. Average numbers of positive cells were calculated from 3 individuals and expressed as mean ± S.D.; *, p ≤ 0.05; scale bars, 100 μm.
Discussion
Prostasphere culture is a method for study and quantitation of self-renewal of both human and mouse P-bSCs (4, 8, 41). In this report, we showed that FGF signals mediated by the FGFR2-FRS2α pathway are required for prostasphere formation, growth, and maintenance. This indicates that FGF signals mediated by the FGFR2 isotype in particular are required for self-renewal and maintenance of P-bSCs. In addition, we also demonstrated that FGFR2 signaling was required for maintenance of basal cell homeostasis in the prostate. Unlike the prostasphere culture that is suitable for P-bSCs, the recently reported organoid culture methods are suitable for luminal P-lSCs (12, 13). Interesting, constitutive activation of Notch signaling improves the sphere-forming activity of luminal cells in sphere culture conditions (37). We have addressed the hierarchy of P-bSCs and P-lSCs in the accompanying manuscript. However, whether FGF signaling has similar roles in P-bSCs and P-lSCs remains to be characterized.
FGF and heparin are routinely used to support prostasphere culture. However, how FGF signaling contributes to maintenance and self-renewal of P-bSC within the prostaspheres and prostate in vivo is not understood. We combined sphere culture in vitro and genetically altered mouse models in vivo to show that FGF signaling, mediated by FRS2α-dependent pathways, plays a critical role in self-renewal and differentiation of P-bSCs. Ablation of Fgfr2 or Frs2α compromised self-renewal of P-bSCs (19). Both AKT and ERK signaling cascades were implicated in prostasphere development. However, only phosphorylation of ERK was affected by inhibition of FGFR. This suggests that other pathways that phosphorylate and maintain activity of AKT may compensate for the absence of FGFR. This is consistent with earlier reports showing that during prostate development, activation of the MAP kinase pathway, but not the AKT pathway, by FGFR2 is mediated by FRS2α (20).
Inhibition of either the ERK or AKT pathway suppressed prostasphere formation. However, the fact that removal of AKTi allowed P-SCs to resume sphere formation indicated that inhibition of AKT reversibly suppressed proliferation of cells in the sphere and did not affect the stemness of P-SCs. In contrast, the P-bSCs failed to resume sphere formation after removal of ERKi. This indicated that inhibition of ERK caused loss of stemness of P-SCs. Similarly, ablation of FGFR2 in P-bSCs caused disruption of prostaspheres and increased differentiation of sphere cells. Together, the data demonstrates that loss of FGFR2 signaling abrogates stemness of P-bSCs.
Ablation of Fgfr2 in prostate progenitor cells at embryonic day 17.0 with Nkx3.1Cre causes defects in prostate branching morphogenesis (19). The adult mutant prostate exhibits a primitive epithelial ductal network and has less P63-expressing basal cells. Although relatively mild, ablation of Fgfr2 in basal cells with inducible P63CreERT2 in postnatal day 7 caused similar defects. Because Nkx3.1 is expressed in early prostate bud formation and P63CreERT2 was activated at day 7 after birth, the difference in extents of defects are likely due to timing of the Fgfr2 ablation.
WNTs are secreted ligands that bind to and activate their receptors to elicit regulatory signals via the canonical β-catenin dependent and non-canonical β-catenin-independent pathways (42). Reciprocal interactions between the FGF and WNT pathways have been shown in various studies. FGF elevates WNT signaling by inhibiting transcription of the WNT antagonists dkk1 and notum1a (43). PI3K, a downstream mediator of the FGF pathway, increases WNT signaling via phosphorylation of glycogen synthase kinase 3β, which, in turn, promotes MAPK phosphorylation. This is also a key component in the FGF signaling pathway. FGF signaling also regulates expression of WNT ligands (44) and LRP6-PPPS/TP kinase phosphorylation (45), as well as the localization of β-catenin (46). We reported previously that FGFR2 promotes WNT signaling in dental epithelial stem cells via down-regulation of WNT receptor inhibitors that included sFRP1, sFRP4, and Wif1, which was increased in Fgfr2 mutant CLs (27). In this study, we also showed that FGFR2 promoted β-catenin transcription and its nuclear localization. However, the molecular mechanism by which the cross-talk between these two pathways regulate P-bSC self-renewal remains to be addressed.
Expression and nuclear localization of β-catenin were increased in Fgfr1 null prostaspheres (Fig. 4). Notably real-time RT-PCR analyses showed that expression of Notch-1 was also increased in the same prostasphere by 2-fold. It has been reported that the WNT and Notch pathways have opposing roles on prostate progenitor cell proliferation and differentiation (38). The effect of increased WNT signaling likely was counterbalanced by elevated Notch-1, and therefore did not cause a detectable phenotype in Fgfr1bKO prostates.
The prostate epithelial compartment consists of basal epithelial cells that express cytokeratin 5/14 and luminal epithelial cells that express cytokeratin 8/18. Basal cells account for ∼10% of cells in the mature prostate epithelium. The origin of these two populations of epithelial cells and whether basal cells serve as a precursor for luminal cells or have their own separate function remains controversial. Cells with stem cell properties have been described in both epithelial compartments in different assay systems. In the accompanying manuscript (47), we report that prostasphere-forming P-bSCs reside in the basal compartment and that P-bSCs can give rise to P-lSCs, but not vice versa. Thus, it appears that P-bSCs sit at an earlier, more primitive, or higher position in the P-SC hierarchy than P-lSCs. In this report, we further determine the role of FGFR2 signaling in maintaining stemness and preventing differentiation of P-bSCs. Whether FGFR2 signaling also elicits similar activities in P-lSCs is interesting and will be characterized in our follow-up studies.
In conclusion, FGF signaling mediated by the FGFR2 isotype is required for preserving stemness and preventing differentiation of prostate stem cells. Manipulation of the FGF signaling axis to direct P-bSCs to remain in dormant or active stages may advance design of strategies to control prostate cancer relapse after conventional therapies.
Author Contributions
Y. H., T. H., J. L., C. W., L. A., P. Y., J. C., and C. J. performed the experiments and data analyses; J. X., Z. Z., W. L. M., and F. W. analyzed the data and contributed to manuscript writing.
Acknowledgments
We thank Drs. Michael Shen, Juha Patanen, and David Ornitz for sharing the Nkx3.1cre knock-in mice, Fgfr1floxed and Fgfr2floxed mice, respectively, Dr. Li Xin for constructive suggestions and discussions, Alon Azares for FACS cell sorting and data analysis, and Dr. Stefan Siwko for critical reading of the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grants CA96824 and DE023106 (to F. W.), CA140388 (to W. L. M., and F. W.), Cancer Prevention and Research Institution of Texas Grants CPRIT110555 (to F. W. and W. L. M.) and TAMU1400302 (to F. W.), Natural Science Foundation of Zhejiang Province of China Grant Y2110492, and National Natural Science Foundation of China Grants s81101712, 31371470, and 81270761 (to C. W.). The authors declare that they have no conflicts of interest with the contents of this article.
- P-SC
- prostate stem cell
- P-bSC
- basal prostate stem cell
- P-LSC
- luminal P-SC
- FRS2α
- FGF receptor substrate 2α
- ERKi
- ERK inhibitor
- AKTi
- AKT inhibitor.
References
- 1. English H. F., Santen R. J., Isaacs J. T. (1987) Response of glandular versus basal rat ventral prostatic epithelial cells to androgen withdrawal and replacement. Prostate 11, 229–242 [DOI] [PubMed] [Google Scholar]
- 2. Evans G. S., Chandler J. A. (1987) Cell proliferation studies in the rat prostate: II. the effects of castration and androgen-induced regeneration upon basal and secretory cell proliferation. Prostate 11, 339–351 [DOI] [PubMed] [Google Scholar]
- 3. Sugimura Y., Cunha G. R., Donjacour A. A. (1986) Morphological and histological study of castration-induced degeneration and androgen-induced regeneration in the mouse prostate. Biol. Reprod. 34, 973–983 [DOI] [PubMed] [Google Scholar]
- 4. Xin L., Lukacs R. U., Lawson D. A., Cheng D., Witte O. N. (2007) Self-renewal and multilineage differentiation in vitro from murine prostate stem cells. Stem Cells 25, 2760–2769 [DOI] [PubMed] [Google Scholar]
- 5. Goldstein A. S., Lawson D. A., Cheng D., Sun W., Garraway I. P., Witte O. N. (2008) Trop2 identifies a subpopulation of murine and human prostate basal cells with stem cell characteristics. Proc. Natl. Acad. Sci. U.S.A. 105, 20882–20887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Leong K. G., Wang B. E., Johnson L., Gao W. Q. (2008) Generation of a prostate from a single adult stem cell. Nature 456, 804–808 [DOI] [PubMed] [Google Scholar]
- 7. Goldstein A. S., Huang J., Guo C., Garraway I. P., Witte O. N. (2010) Identification of a cell of origin for human prostate cancer. Science 329, 568–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Garraway I. P., Sun W., Tran C. P., Perner S., Zhang B., Goldstein A. S., Hahm S. A., Haider M., Head C. S., Reiter R. E., Rubin M. A., Witte O. N. (2010) Human prostate sphere-forming cells represent a subset of basal epithelial cells capable of glandular regeneration in vivo. Prostate 70, 491–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Choi N., Zhang B., Zhang L., Ittmann M., Xin L. (2012) Adult murine prostate basal and luminal cells are self-sustained lineages that can both serve as targets for prostate cancer initiation. Cancer Cell 21, 253–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ousset M., Van Keymeulen A., Bouvencourt G., Sharma N., Achouri Y., Simons B. D., Blanpain C. (2012) Multipotent and unipotent progenitors contribute to prostate postnatal development. Nat. Cell Biol. 14, 1131–1138 [DOI] [PubMed] [Google Scholar]
- 11. Liu J., Pascal L. E., Isharwal S., Metzger D., Ramos Garcia R., Pilch J., Kasper S., Williams K., Basse P. H., Nelson J. B., Chambon P., Wang Z. (2011) Regenerated luminal epithelial cells are derived from preexisting luminal epithelial cells in adult mouse prostate. Mol. Endocrinol. 25, 1849–1857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chua C. W., Shibata M., Lei M., Toivanen R., Barlow L. J., Bergren S. K., Badani K. K., McKiernan J. M., Benson M. C., Hibshoosh H., Shen M. M. (2014) Single luminal epithelial progenitors can generate prostate organoids in culture. Nat. Cell Biol. 16, 951–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Karthaus W. R., Iaquinta P. J., Drost J., Gracanin A., van Boxtel R., Wongvipat J., Dowling C. M., Gao D., Begthel H., Sachs N., Vries R. G., Cuppen E., Chen Y., Sawyers C. L., Clevers H. C. (2014) Identification of multipotent luminal progenitor cells in human prostate organoid cultures. Cell 159, 163–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang X., Kruithof-de Julio M., Economides K. D., Walker D., Yu H., Halili M. V., Hu Y. P., Price S. M., Abate-Shen C., Shen M. M. (2009) A luminal epithelial stem cell that is a cell of origin for prostate cancer. Nature 461, 495–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lu T. L., Huang Y. F., You L. R., Chao N. C., Su F. Y., Chang J. L., Chen C. M. (2013) Conditionally ablated Pten in prostate basal cells promotes basal-to-luminal differentiation and causes invasive prostate cancer in mice. Am. J. Pathol. 182, 975–991 [DOI] [PubMed] [Google Scholar]
- 16. Wang Z. A., Mitrofanova A., Bergren S. K., Abate-Shen C., Cardiff R. D., Califano A., Shen M. M. (2013) Lineage analysis of basal epithelial cells reveals their unexpected plasticity and supports a cell-of-origin model for prostate cancer heterogeneity. Nat. Cell Biol. 15, 274–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McKeehan W. L., Wang F., Kan M. (1998) The heparan sulfate-fibroblast growth factor family: diversity of structure and function. Prog. Nucleic Acids Res. Mol. Biol. 59, 135–176 [DOI] [PubMed] [Google Scholar]
- 18. Corn P. G., Wang F., McKeehan W. L., Navone N. (2013) Targeting fibroblast growth factor pathways in prostate cancer. Clin. Cancer Res. 19, 5856–5866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lin Y., Liu G., Zhang Y., Hu Y. P., Yu K., Lin C., McKeehan K., Xuan J. W., Ornitz D. M., Shen M. M., Greenberg N., McKeehan W. L., Wang F. (2007) Fibroblast growth factor receptor 2 tyrosine kinase is required for prostatic morphogenesis and the acquisition of strict androgen dependency for adult tissue homeostasis. Development 134, 723–734 [DOI] [PubMed] [Google Scholar]
- 20. Zhang Y., Zhang J., Lin Y., Lan Y., Lin C., Xuan J. W., Shen M. M., McKeehan W. L., Greenberg N. M., Wang F. (2008) Role of epithelial cell fibroblast growth factor receptor substrate 2α in prostate development, regeneration and tumorigenesis. Development 135, 775–784 [DOI] [PubMed] [Google Scholar]
- 21. Xu C., Inokuma M. S., Denham J., Golds K., Kundu P., Gold J. D., Carpenter M. K. (2001) Feeder-free growth of undifferentiated human embryonic stem cells. Nat. Biotechnol. 19, 971–974 [DOI] [PubMed] [Google Scholar]
- 22. Dvorak P., Dvorakova D., Koskova S., Vodinska M., Najvirtova M., Krekac D., Hampl A. (2005) Expression and potential role of fibroblast growth factor 2 and its receptors in human embryonic stem cells. Stem Cells 23, 1200–1211 [DOI] [PubMed] [Google Scholar]
- 23. Zheng W., Nowakowski R. S., Vaccarino F. M. (2004) Fibroblast growth factor 2 is required for maintaining the neural stem cell pool in the mouse brain subventricular zone. Dev. Neurosci. 26, 181–196 [DOI] [PubMed] [Google Scholar]
- 24. Eom Y. W., Oh J. E., Lee J. I., Baik S. K., Rhee K. J., Shin H. C., Kim Y. M., Ahn C. M., Kong J. H., Kim H. S., Shim K. Y. (2014) The role of growth factors in maintenance of stemness in bone marrow-derived mesenchymal stem cells. Biochem. Biophys. Res. Commun. 445, 16–22 [DOI] [PubMed] [Google Scholar]
- 25. Itkin T., Ludin A., Gradus B., Gur-Cohen S., Kalinkovich A., Schajnovitz A., Ovadya Y., Kollet O., Canaani J., Shezen E., Coffin D. J., Enikolopov G. N., Berg T., Piacibello W., Hornstein E., Lapidot T. (2012) FGF-2 expands murine hematopoietic stem and progenitor cells via proliferation of stromal cells, c-Kit activation, and CXCL12 down-regulation. Blood 120, 1843–1855 [DOI] [PubMed] [Google Scholar]
- 26. Zhang J., Liu J., Liu L., McKeehan W. L., Wang F. (2012) The fibroblast growth factor signaling axis controls cardiac stem cell differentiation through regulating autophagy. Autophagy 8, 690–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chang J. Y., Wang C., Liu J., Huang Y., Jin C., Yang C., Hai B., Liu F., D'Souza R. N., McKeehan W. L., Wang F. (2013) Fibroblast growth factor signaling is essential for self-renewal of dental epithelial stem cells. J. Biol. Chem. 288, 28952–28961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Memarzadeh S., Xin L., Mulholland D. J., Mansukhani A., Wu H., Teitell M. A., Witte O. N. (2007) Enhanced paracrine FGF10 expression promotes formation of multifocal prostate adenocarcinoma and an increase in epithelial androgen receptor. Cancer Cell 12, 572–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Heer R., Collins A. T., Robson C. N., Shenton B. K., Leung H. Y. (2006) KGF suppresses {alpha}2{beta}1 integrin function and promotes differentiation of the transient amplifying population in human prostatic epithelium. J. Cell Sci. 119, 1416–1424 [DOI] [PubMed] [Google Scholar]
- 30. Pignon J. C., Grisanzio C., Geng Y., Song J., Shivdasani R. A., Signoretti S. (2013) p63-expressing cells are the stem cells of developing prostate, bladder, and colorectal epithelia. Proc. Natl. Acad. Sci. U.S.A. 110, 8105–8110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lin Y., Zhang J., Zhang Y., Wang F. (2007) Generation of an Frs2α conditional null allele. Genesis 45, 554–559 [DOI] [PubMed] [Google Scholar]
- 32. Trokovic R., Trokovic N., Hernesniemi S., Pirvola U., Vogt Weisenhorn D. M., Rossant J., McMahon A. P., Wurst W., Partanen J. (2003) FGFR1 is independently required in both developing mid- and hindbrain for sustained response to isthmic signals. EMBO J. 22, 1811–1823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yu K., Xu J., Liu Z., Sosic D., Shao J., Olson E. N., Towler D. A., Ornitz D. M. (2003) Conditional inactivation of FGF receptor 2 reveals an essential role for FGF signaling in the regulation of osteoblast function and bone growth. Development 130, 3063–3074 [DOI] [PubMed] [Google Scholar]
- 34. Lee D. K., Liu Y., Liao L., Wang F., Xu J. (2014) The prostate basal cell (BC) heterogeneity and the p63-positive BC differentiation spectrum in mice. Int. J. Biol. Sci. 10, 1007–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kan M., Uematsu F., Wu X., Wang F. (2001) Directional specificity of prostate stromal to epithelial cell communication via FGF7/FGFR2 is set by cell- and FGFR2 isoform-specific heparan sulfate. In Vitro Cell Dev. Biol. Anim. 37, 575–577 [DOI] [PubMed] [Google Scholar]
- 36. Lu W., Luo Y., Kan M., McKeehan W. L. (1999) Fibroblast growth factor-10: a second candidate stromal to epithelial cell andromedin in prostate. J. Biol. Chem. 274, 12827–12834 [DOI] [PubMed] [Google Scholar]
- 37. Kwon O. J., Valdez J. M., Zhang L., Zhang B., Wei X., Su Q., Ittmann M. M., Creighton C. J., Xin L. (2014) Increased Notch signalling inhibits anoikis and stimulates proliferation of prostate luminal epithelial cells. Nat. Commun. 5, 4416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shahi P., Seethammagari M. R., Valdez J. M., Xin L., Spencer D. M. (2011) Wnt and Notch pathways have interrelated opposing roles on prostate progenitor cell proliferation and differentiation. Stem Cells 29, 678–688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang B. E., Wang X. D., Ernst J. A., Polakis P., Gao W. Q. (2008) Regulation of epithelial branching morphogenesis and cancer cell growth of the prostate by Wnt signaling. PloS One 3, e2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yang F., Zhang Y., Ressler S. J., Ittmann M. M., Ayala G. E., Dang T. D., Wang F., Rowley D. R. (2013) FGFR1 is essential for prostate cancer progression and metastasis. Cancer Res. 73, 3716–3724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rybak A. P., He L., Kapoor A., Cutz J. C., Tang D. (2011) Characterization of sphere-propagating cells with stem-like properties from DU145 prostate cancer cells. Biochim. Biophys. Acta 1813, 683–694 [DOI] [PubMed] [Google Scholar]
- 42. Clevers H., Loh K. M., Nusse R. (2014) Stem cell signaling: an integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science 346, 1248012. [DOI] [PubMed] [Google Scholar]
- 43. Stulberg M. J., Lin A., Zhao H., Holley S. A. (2012) Crosstalk between Fgf and Wnt signaling in the zebrafish tailbud. Dev. Biol. 369, 298–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yin Y., White A. C., Huh S. H., Hilton M. J., Kanazawa H., Long F., Ornitz D. M. (2008) An FGF-WNT gene regulatory network controls lung mesenchyme development. Dev. Biol. 319, 426–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. C̆ervenka I., Wolf J., Mas̆ek J., Krejci P., Wilcox W. R., Kozubík A., Schulte G., Gutkind J. S., Bryja V. (2011) Mitogen-activated protein kinases promote WNT/β-catenin signaling via phosphorylation of LRP6. Mol. Cell. Biol. 31, 179–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Minor P. J., He T. F., Sohn C. H., Asthagiri A. R., Sternberg P. W. (2013) FGF signaling regulates Wnt ligand expression to control vulval cell lineage polarity in C. elegans. Development 140, 3882–3891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Huang Y., Hamana T., Liu J., Wang C., An L., You P., Chang J. Y., Xu J., McKeehan W. L., Wang F. (2015) Prostate sphere-forming stem cells are derived from the P63-expressing basal compartment. J. Biol. Chem. 290, 17745–17752 [DOI] [PMC free article] [PubMed] [Google Scholar]