Background: DJ-1 is a ras-cooperating oncogene and activates the ERK pathway.
Results: DJ-1 directly binds to the kinase domain of c-Raf to stimulate its self-phosphorylation, followed by phosphorylation of MEK and ERK1/2 in EGF-treated cells.
Conclusion: DJ-1 activates the ERK pathway by directly binding to c-Raf but not to Ras.
Significance: DJ-1 is a positive regulator for the EGF/Ras/ERK pathway.
Keywords: extracellular-signal-regulated kinase (ERK); Parkinson disease (autosomal recessive, early onset) 7 (PARK7); protein kinase; Raf kinase; signal transduction
Abstract
DJ-1 is an oncogene and also a causative gene for familial Parkinson disease. DJ-1 has various functions, and the oxidative status of cysteine at position 106 (Cys-106) is crucial for determination of the activation level of DJ-1. Although DJ-1 requires activated Ras for its oncogenic activity and although it activates the extracellular signal-regulated kinase (ERK) pathway, a cell growth pathway downstream of Ras, the precise mechanism underlying activation of the ERK pathway by DJ-1 is still not known. In this study, we found that DJ-1 directly bound to the kinase domain of c-Raf but not to Ras and that Cys-106 mutant DJ-1 bound to c-Raf more weakly than did wild-type DJ-1. Co-localization of DJ-1 with c-Raf in the cytoplasm was enhanced in epidermal growth factor (EGF)-treated cells. Knockdown of DJ-1 expression attenuated the phosphorylation level of c-Raf in EGF-treated cells, resulting in reduced activation of MEK and ERK1/2. Although EGF-treated DJ-1 knock-out cells also showed attenuated c-Raf activation, reintroduction of wild-type DJ-1, but not C106S DJ-1, into DJ-1 knock-out cells restored c-Raf activation in a DJ-1 binding activity in a c-Raf-dependent manner. DJ-1 was not responsible for activation of c-Raf in phorbol myristate acetate-treated cells. Furthermore, DJ-1 stimulated self-phosphorylation activity of c-Raf in vitro, but DJ-1 was not a target for Raf kinase. Oxidation of Cys-106 in DJ-1 was not affected by EGF treatment. These findings showed that DJ-1 is a positive regulator of the EGF/Ras/ERK pathway through targeting c-Raf.
Introduction
The DJ-1 gene has been identified by us as a novel oncogene that transforms NIH3T3 cells in cooperation with the activated ras gene (1) and was later found to be a causative gene for familial Parkinson disease park7 (2). DJ-1 is a multifunctional protein that plays roles in anti-oxidative stress reaction, transcriptional regulation, regulation of signal transduction pathways, and reactions for chaperone and proteinase (3–5). DJ-1 is composed of 189 amino acids and contains three cysteine residues located at amino acid positions 46, 53, and 106 (Cys-106). Of the three cysteine residues, Cys-106 is highly sensitive to oxidative stress, and the oxidative state of Cys-106 determines the activation level of all of the DJ-1 functions (6–8). Excess oxidation of Cys-106 renders DJ-1 inactive, and such highly oxidized DJ-1 has been found in the brains of patients with Parkinson disease and Alzheimer disease (9, 10).
As for regulation of signal transduction pathways, DJ-1 activates the extracellular signal-regulated kinase (ERK) pathway (11–13) and PI3K/Akt pathway by inhibiting PTEN, a negative regulator for the Akt pathway (14, 15), and inhibits the apoptosis signaling kinase-1 (ASK1) pathway by directly binding to ASK1 itself or to Daxx, an activator for ASK1 (16, 17), thereby stimulating cell growth and inhibiting apoptosis. DJ-1 also reduces the expression level of DUSP1 phosphatase, an inhibitor for ERK1/2 and transcriptional target for p53, by inhibiting p53 activity, resulting in stimulation of ERK1/2 activity (18). Although oncogenic activity of DJ-1 requires activated Ras (1) and although Ras is a protein upstream of the ERK pathway, the mechanism underlying activation of the ERK pathway and Ras-dependent transformation by DJ-1 is not known.
When epidermal growth factor (EGF) binds to the epidermal growth factor receptor, the EGF receptor is activated by self-phosphorylation and transfers the EGF-triggering growth signal to Ras via adaptor proteins. GTP-activated Ras then transduces the growth signal to the ERK pathway comprising c-Raf, MEK, and ERK1/2 through a series of phosphorylation cascades. c-Raf is serine/threonine kinase and binds to GTP-bound Ras at its N-terminal region (19, 20). Without Ras signaling, c-Raf is phosphorylated at serines 43, 259, and 621 to be inactivated. When Ser-259 and Ser-621 of c-Raf are dephosphorylated by protein phosphatase 2A (21, 22) and when Ser-338 is phosphorylated after EGF stimulation (23), c-Raf is activated. Activated c-Raf then phosphorylates/activates MEK, and MEK phosphorylates/activates ERK1/2. Activated ERK1/2 is finally translocated from the cytoplasm to nucleus, in which ERK1/2 phosphorylates cell growth-related transcription factors, resulting in cell growth.
In this study, we found that DJ-1 directly binds to the kinase domain of c-Raf, but not to Ras, to stimulate phosphorylation activity of c-Raf at Ser-338 and that the C106S mutant DJ-1 activates c-Raf less than does wild-type DJ-1 in EGF-treated cells. DJ-1 knockdown and DJ-1 knock-out attenuated activation of c-Raf and its downstream MEK and ERK1/2 in cultured cells, and reintroduction of wild-type DJ-1, but not C106S DJ-1, into DJ-1 knock-out cells restored activation of the ERK pathway. These findings are the first findings showing the mechanism of activation of the ERK pathway by DJ-1.
Experimental Procedures
Cells
Establishment of cell lines from wild-type and DJ-1 knock-out mice, DJ-1+/+, and DJ-1−/− cells, respectively, and of DJ-1 knockdown NIH3T3 cells by shRNA targeting DJ-1, D2 cells, was described previously (24, 25). DJ-1+/+, DJ-1−/−, NIH3T3, D2, HeLa S3, and 293T cells were cultured in Dulbecco's modified Eagle's medium (DMEM) with 10% calf serum.
Plasmids
Nucleotide sequences of oligonucleotides used for construction of deletion mutants of c-Raf by PCR are shown in Table 1. PCR was carried out using the respective primers and pcDNA3-FLAG-c-Raf as a template for 5 min at 95 °C, 30 s at 95 °C, 30 s at 60 °C, and 32 cycles of 30 s at 72 °C. After PCR products had been cloned into pCR-TOPO (Invitrogen), plasmid DNAs obtained were digested with EcoRI and XhoI and the resultant fragments were inserted into EcoRI and XhoI sites of pcDNA3-FLAG. Deletion mutants of c-Raf, ΔCR3, ΔCR1, and CR3, harbored amino acid numbers spanning 1–306, 197–648, and 307–648, respectively, of c-Raf.
TABLE 1.
Nucleotide sequences of oligonucleotides used for construction of deletion mutants of c-Raf
| Plasmid name | Nucleotide sequence |
|---|---|
| pcDNA3-FLAG-c-Raf ΔCR3 | Sense, 5′-GGGAATTCATGGAGCACATACAGGGAGCT-3 |
| Antisense, 5′-GGCTCGAGTGACCAGCCTGTTGGGCTCAG-3′ | |
| pcDNA3-FLAG-c-Raf ΔC1 | Sense, 5′-GGGAATTCCCAAATTCCACTATTGGTGAT-3′ |
| Antisense, 5′-GGCTCGAGCTAGAAGACAGGCAGCCTCGG-3′ | |
| pcDNA3-FLAG-c-Raf CR3 | Sense, 5′-GGGAATTCCAGCCGAAAACCCCCGTGCCA-3′ |
| Antisense, 5′-GGCTCGAGCTAGAAGACAGGCAGCCTCGG-3′ |
Analysis of Expression Levels of ERK Pathway Proteins in EGF-treated Cells
NIH3T3 and D2 cells in 10-cm dishes were cultured in a medium with 0.1% calf serum for 6 h, treated with 50 ng/ml EGF for 1, 5, and 15 min, and harvested. HeLa S3 cells or DJ-1+/+ and DJ-1−/− cells in 10-cm dishes were transfected with 100 pmol of siRNA-targeting DJ-1 (si-DJ-1) and control siRNA (si-control) or with 3 μg of pcDNA3-WT DJ-1-FLAG and pcDNA3-C106S DJ-1-FLAG, respectively, by Lipofectamine 2000 (Invitrogen). Forty eight h after transfection, the cells were cultured in a medium with 0.1% calf serum for 6 h and then treated with 50 ng/ml EGF for 1, 5, and 15 min. Proteins were extracted from the cells in a buffer containing 50 mm Tris-HCl (pH 7.5), 150 mm NaCl, 5 mm EDTA, 0.5% Nonidet P-40, 100 μm 4-(2-aminoethyl) benzenesulfonyl fluoride hydrochloride, 2 μg/ml leupeptin, 1 μg/ml pepstatin A, and 10 μg/ml aprotinin for 20 min at 4 °C, loaded onto 10% SDS-polyacrylamide gels, and subjected to Western blotting. Antibodies used in this study were as follows: anti-c-Raf (1:1000, BD Biosciences); anti-phospho-c-Raf (Ser-338) (1:1000, Cell Signaling); anti-phospho-c-Raf (Ser-259) (1:1000, Cell Signaling); anti-MEK1/2 (1:1000, Cell Signaling); anti-phospho-MEK1/2 (Ser-217/Ser-221) (1:1000, Cell Signaling); anti-ERK1/2 (1:1000,Cell Signaling); anti-ERK1/2 (Thr-202/Tyr-204) (1:1000, CellSignaling); anti-actin (1:4000, Chemicon); anti-FLAG (1:4000, M2, Sigma); anti-DJ-1 (1:4000); and anti-oxidized DJ-1 (1:1000) antibodies. A rat anti-DJ-1 antibody was established by us as described previously (26). An anti-oxidized DJ-1 antibody was kindly provided by Dr. Y. Saito (27). After membranes had been reacted with primary antibodies, they were reacted with Alexa Fluor 680-conjugated anti-rabbit IgG (Molecular Probes) or IRDye 800-conjugated anti-mouse IgG (Rockland) antibodies and visualized by using an infrared imaging system (Odyssey, LI-COR). Nucleotide sequences used for si-DJ-1 and si-control were described previously (6).
Pulldown Assay
35S-Labeled c-Raf, its deletion mutants, and H-Ras were synthesized in vitro using the reticulocyte lysate of the TnT transcription-translation coupled system (Promega). Labeled proteins were mixed with GST, GST-DJ-1, or GST-C106S DJ-1 expressed in and prepared from Escherichia coli at 4 °C for 60 min in a buffer containing 150 mm NaCl, 5 mm EDTA, 50 mm Tris (pH 7.5), 0.05% bovine serum albumin, and 0.1% Nonidet P-40. After washing with the same buffer, the bound proteins were separated in a 10% polyacrylamide gel containing SDS and visualized by fluorography.
In Vivo Binding Assay
Proteins were extracted from HeLa S3 cells by the procedure described previously (28). Proteins were immunoprecipitated with a rabbit anti-DJ-1 antibody (1:500) or normal IgG, and the precipitates were analyzed by Western blotting with an anti-c-Raf, anti-phospho c-Raf (Ser-338), or anti-DJ-1 antibody (1:2000, 3E8, MBL, Nagoya, Japan) as described above. A rabbit anti-DJ-1 antibody was established by us as described previously (1). For ectopic expression systems, 293T cells were transfected with expression vectors for FLAG-c-Raf and DJ-1-HA by Lipofectamine 2000. Forty eight h after transfection, proteins were extracted from the cells and subjected to an in vivo binding assay as described above.
Indirect Immunofluorescence
HeLa S3 cells were cultured in a medium with 0.1% calf serum for 6 h and treated with 50 ng/ml EGF for 3 min. The cells were fixed with 4% paraformaldehyde at 4 °C for 15 min, then with 0.1% Triton X-100 at room temperature for 5 min, and reacted with mouse anti-c-Raf monoclonal (1:50, BD Biosciences), a rabbit anti-DJ-1 polyclonal (1:50), or rabbit anti-DJ-1 monoclonal (1:50, ab76008, abcam) antibody at 37 °C for 3 h. The cells were then reacted with an Alexa Fluor 488-conjugated anti-mouse IgG or with a rhodamine-conjugated anti-rabbit IgG at 37 °C for 1 h, and their nuclei were stained with DAPI. The cells were then observed under a fluorescent microscope (Biorevo BZ-9000, Keyence, Osaka, Japan).
In Vitro Kinase Assay
GST-wild-type, C106S DJ-1, wild-type, Y340E/Y341E, and Y340A/Y341A CR3 were expressed in and purified from E. coli, and GST-free DJ-1 and GST-free CR3 were prepared after GST-DJ-1 and GST-CR3 had been digested with PreScission protease (GE Healthcare). A recombinant CR3 fragment of c-Raf with Y340E/Y341E mutations was also purchased from R&D Systems. Fifteen ng of CR3 fragment was mixed with various amounts of DJ-1 on ice for 30 min and reacted with 10 μCi of [γ-32P]ATP at 22 °C for 20 min in a buffer containing 25 mm HEPES-KOH (pH 7.5), 30 mm NaCl, 10 mm MgCl2, and 1 mm DTT. After boiling the reaction mixture in a Laemmli buffer, proteins were separated on a 12% polyacrylamide gel and subjected to autoradiography.
Five hundred ng of wild-type, Y340E/Y341E, and Y340A/Y341A CR3 (WT CR3, E-CR3, and A-CR3, respectively) were reacted with various amounts of wild-type (WT) and C106S DJ-1 under the same condition as that described above. After boiling the reaction mixture in Laemmli buffer, proteins were separated on a 12% polyacrylamide gel and subjected to Western blotting with an anti-phospho c-Raf (Ser-338) antibody as described above. CR3 and DJ-1 were detected by Coomassie Brilliant Blue staining.
Separation of Phosphorylated Proteins in Phos-tag-containing Gels
The Phos-tag-containing gel was prepared by addition of 10 mm Phos-tag acrylamide AAL-107 (Wako Pure Chemicals, Osaka, Japan) and 10 mm MnCl2 into a 4.8% acrylamide separating gel. After separation of proteins on Phos-tag-containing gel, the gel was washed with a buffer containing 25 mm Tris, 192 mm glycine, and 1 mm EDTA and subjected to Western blotting.
Statistical Analyses
Data are expressed as means ± S.D. Statistical analyses were performed using one-way analysis of variance followed by unpaired Student's t test.
Results
Binding of DJ-1 to the Kinase Domain of c-Raf and Enhancement of Binding Activity of DJ-1 by EGF
The DJ-1 gene is a ras-cooperating oncogene (1), and DJ-1 activates the ERK pathway (11–13). We therefore examined the binding activity of DJ-1 to H-Ras and c-Raf. To do that, 35S-labeled wild-type c-Raf, wild-type H-Ras and G12V H-Ras, constitutively active H-Ras, were synthesized in vitro using a reticulocyte lysate and mixed with GST-DJ-1 and GST that had been expressed in and purified from E. coli, and pulldown assays were performed. As shown in Fig. 1A, neither wild-type H-Ras nor G12V H-Ras bound to GST-DJ-1 and GST. Wild-type c-Raf, however, bound to GST-DJ-1 more strongly than to GST, indicating that DJ-1 directly binds to c-Raf but not to H-Ras. Because Cys-106 in DJ-1 is crucial for DJ-1 activity, binding activity of C106S DJ-1, a substitution mutant of DJ-1 from cysteine to serine at Cys-106, to c-Raf was also examined by pulldown assays (Fig. 1B). The results showed that binding activity of C106S DJ-1 to c-Raf was ∼60% that of wild-type DJ-1. To examine the binding of DJ-1 to c-Raf in cells, 293T cells were transfected with various combinations of expression vectors for FLAG-c-Raf and DJ-1-HA. Forty eight h after transfection, proteins extracted from the cells were immunoprecipitated with an anti-FLAG antibody or with nonspecific IgG, and precipitates were analyzed by Western blotting with anti-FLAG and anti-HA antibodies. As shown in Fig. 1C, DJ-1-HA was precipitated with the anti-FLAG antibody but not with IgG, indicating complex formation of DJ-1-HA with FLAG-c-Raf. To examine endogenous binding of DJ-1 to c-Raf in cells, proteins extracted from HeLa S3 cells were immunoprecipitated with an anti-DJ-1 antibody or with IgG followed by Western blotting with anti-c-Raf and DJ-1 antibodies. The results showed an association of DJ-1 with c-Raf in HeLa S3 cells (Fig. 1D, 3rd and 4th lanes). Because c-Raf is activated by phosphorylation of Ser-338 in EGF-treated cells, the effect of EGF on binding activity of DJ-1 to c-Raf was then examined. HeLa S3 cells were first cultured in a medium with 0.1% calf serum for 6 h, then treated with 50 ng/ml EGF for 3 min, and subjected to co-immunoprecipitation assays using anti-DJ-1, anti-c-Ras, and anti-phospho c-Raf (Ser-338) antibodies. As shown in Fig. 1D, the association of DJ-1 with c-Raf and with phospho-c-Raf (Ser-338) was increased in EGF-treated cells compared with that in EGF-nontreated cells.
FIGURE 1.
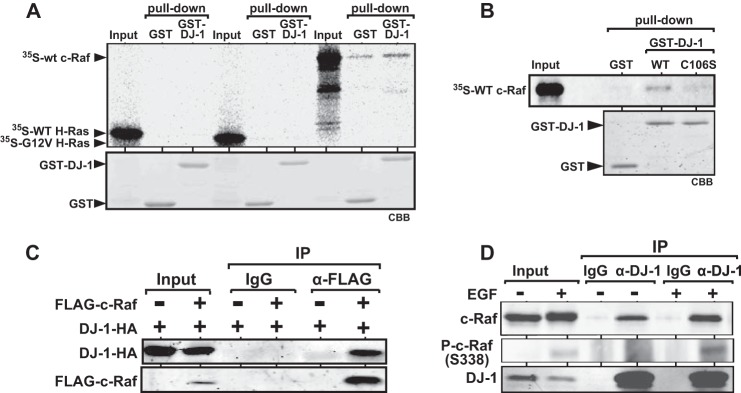
Direct binding of DJ-1 to c-Raf and stimulation of the association of DJ-1 with phosphorylated c-Raf by EGF. A, 35S-labeled c-Raf, wild-type H-Ras, and G12V H-Ras were reacted with GST-DJ-1 and GST, and pulldown experiments were carried out. Bound proteins were then detected by fluorography as described under “Experimental Procedures.” GST-DJ-1 and GST used were stained with Coomassie Brilliant Blue (CBB). B, 35S-labeled c-Raf was reacted with GST-DJ-1, GST-C106S DJ-1, and GST, and pulldown experiments were carried out as described in A. C, 293T cells were transfected with expression vectors for FLAG-c-Raf and DJ-1-HA. Forty eight h after transfection, proteins extracted from the cells were immunoprecipitated (IP) with an anti-FLAG antibody or with IgG, and precipitates were analyzed by Western blotting with anti-FLAG and anti-HA antibodies. D, HeLa S3 cells were cultured in a medium with 0.1% calf serum for 6 h and treated with or without 50 ng/ml EGF for 3 min. Proteins extracted from the cells were subjected to co-immunoprecipitation with an anti-DJ-1 antibody and Western blotting analysis with anti-c-Raf, anti-phospho-c-Raf (Ser-338), and anti-DJ-1 antibodies.
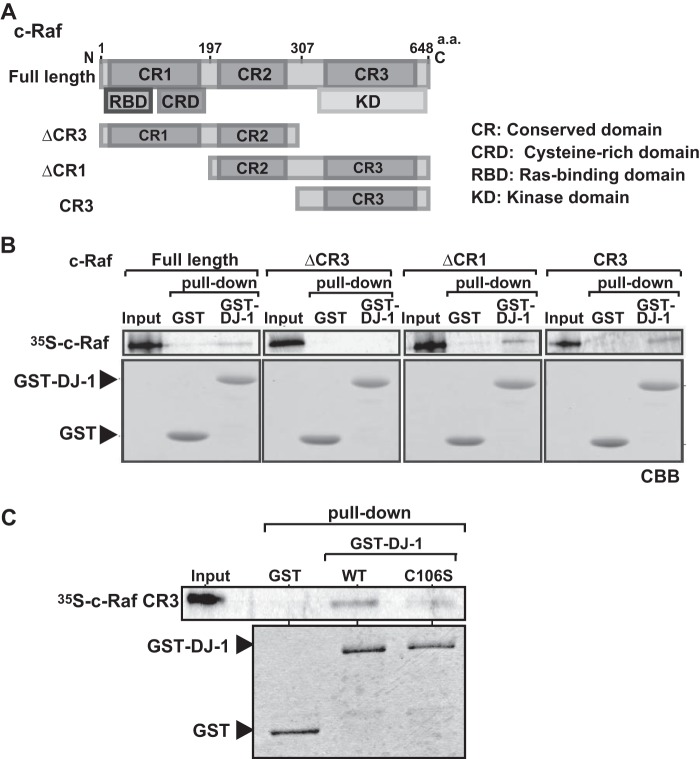
c-Raf is composed of several domains, including a Ras-binding domain, a cysteine-rich domain, a kinase domain, and three conserved domains (Fig. 2A). To identify the DJ-1-binding region in c-Raf, three deletion mutants, ΔCR3, ΔCR1, and CR3, were constructed, and in vitro pulldown assays using 35S-labeled c-Raf mutants, GST and GST-DJ-1, were carried out. As shown in Fig. 2B, ΔCR1 and CR3, but not ΔCR3, bound to GST-DJ-1 but not to GST, indicating that DJ-1 binds to CR3 containing the kinase domain. Binding activity of C106S DJ-1 to CR3 was then examined, and ∼60% of the binding activity of C106S compared with that of wild-type DJ-1 was obtained (Fig. 2C).
FIGURE 2.
Identification of the DJ-1-binding region in c-Raf. A, schematic drawing of deletion mutants of c-Raf. B, 35S-labeled full-sized c-Raf and its deletion mutants were reacted with GST-DJ-1 and GST, and pulldown experiments were carried out as described in the legend for Fig. 1A. C, 35S-labeled CR3 fragment of c-Raf was reacted with GST-DJ-1, GST-C106S DJ-1, and GST, and pulldown experiments were carried out as described in the legend for Fig. 1A. a.a., amino acids.
Co-localization of DJ-1 with c-Raf and Enhancement of Co-localization by EGF
HeLa S3 cells were first cultured in a medium with 0.1% calf serum for 6 h, treated with 50 ng/ml EGF for 3 min, and then stained with anti-DJ-1 and anti-c-Ras antibodies. Anti-DJ-1 antibodies used were rabbit anti-DJ-1 polyclonal (26) and rabbit anti-DJ-1 monoclonal (Abcam) antibodies, and these were designated as an anti-DJ-1 antibody-1 and anti-DJ-1 antibody-2, respectively. Cells were also stained with anti-mouse IgG and anti-rabbit IgG as controls. As shown in Fig. 3, DJ-1 was co-localized with c-Raf in the cytoplasm of EGF-nontreated cells, and its co-localization level was increased after EGF treatment. These results were consistent with enhanced binding activity of DJ-1 to c-Raf in EGF-treated cells as described in Fig. 1D.
FIGURE 3.

Enhanced co-localization of DJ-1 with c-Raf in EGF-treated cells. HeLa S3 cells were cultured in a medium with 0.1% calf serum for 6 h and treated with 50 ng/ml EGF for 3 min. The cells were stained with a mouse anti-c-Raf monoclonal, rabbit anti-DJ-1 polyclonal, or rabbit anti-DJ-1 monoclonal antibody. The cells were then reacted with Alexa Fluor 488-conjugated anti-mouse IgG or with a rhodamine-conjugated anti-rabbit IgG, and their nuclei were stained with DAPI. The cells were then observed under a fluorescent microscope.
Activation of c-Raf and Following ERK Pathway Proteins by DJ-1 in EGF-treated Cells
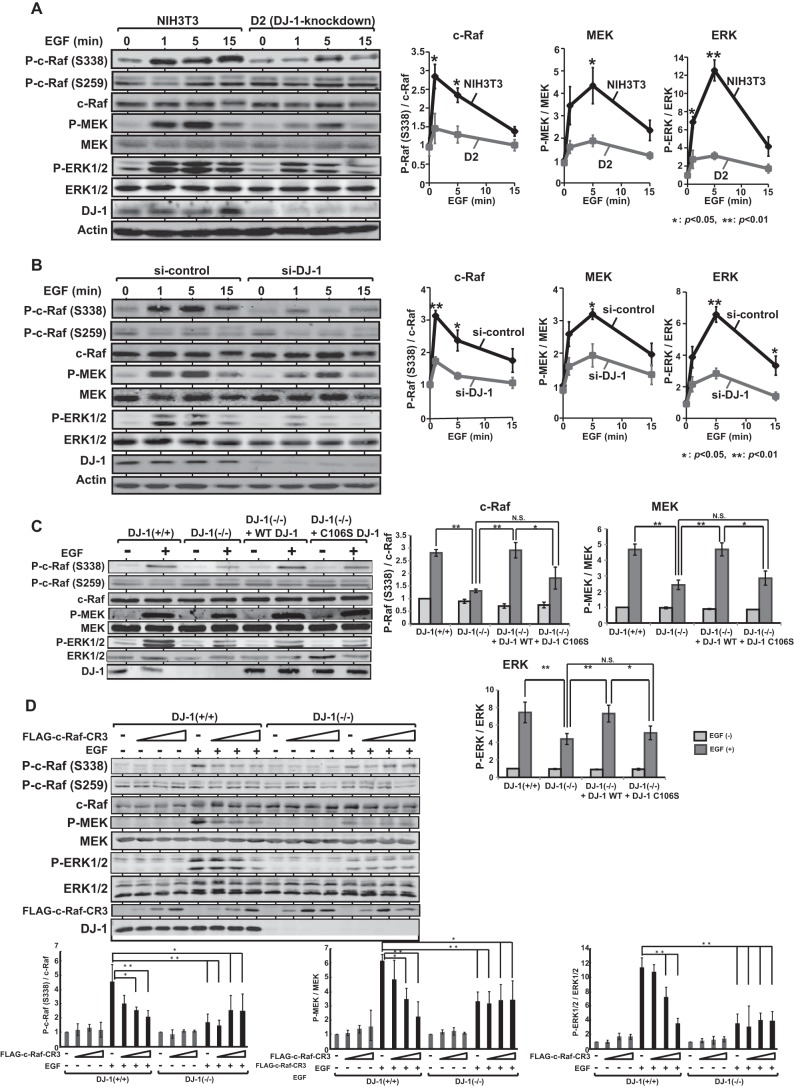
Serine 259 is phosphorylated in an inactive form of c-Raf, and EGF treatment in cells activates c-Raf by phosphorylating Ser-338 (21–23). Therefore, the effect of DJ-1 on activation of c-Raf in EGF-treated cells was examined. Mouse NIH3T3 and its DJ-1 knockdown D2 cells were first cultured in a medium with 0.1% calf serum for 6 h and treated with 50 ng/ml EGF for 1, 5, and 15 min. Expression levels of total c-Raf, phosphorylated c-Raf (Ser-338), and phosphorylated c-Raf (Ser-259) were then examined by Western blotting using proteins extracted from the cells, and the intensity of blotted bands was quantified. The results showed that although levels of phosphorylated c-Raf (Ser-338) were first increased at 1 min and decreased at 5 and 15 min after EGF treatment in both cell lines, the level of phosphorylated c-Raf (Ser-338), but not that of phosphorylated c-Raf (Ser-259), in NIH3T3 cells was much higher than that in DJ-1 knockdown D2 cells. The expression levels of phosphorylated MEK and phosphorylated ERK1/2, downstream kinases of c-Raf, were also examined. The levels of phosphorylated kinases peaked at 5 min after EGF treatment, and their levels in NIH3T3 cells were higher than those in D2 cells (Fig. 4A). Next, human HeLa S3 cells were transfected with siRNA-targeting DJ-1 (si-DJ-1) or with nonspecific siRNA (si-control). Forty eight h after transfection, the cells were treated with EGF as described above, and expression levels of phosphorylated ERK pathway proteins were examined. The results showed expression patterns similar to those observed in NIH3T3 and D2 cells (Fig. 4B). These results suggest that DJ-1 first activates c-Raf by phosphorylating Ser-338 and that activated c-Raf then activates downstream MEK and ERK1/2. To further confirm activity of DJ-1 in the ERK pathway, DJ-1+/+ and DJ-1−/− cells derived from wild-type and DJ-1 knock-out mice were cultured in a medium with 0.1% calf serum for 6 h and treated with EGF for 3 min, and expression levels of phosphorylated c-Raf (Ser-338), MEK, and ERK1/2 were examined. The same results as those for NIH3T3, HeLa S3, and their DJ-1 knockdown cells were obtained (Fig. 4C, 1st 4 lanes). Furthermore, when wild-type and C106S DJ-1 were re-introduced into DJ-1−/− cells, phosphorylation levels of c-Raf (Ser-338), MEK, and ERK1/2 in wild-type DJ-1 and C106S-introduced cells were restored to almost 100 and 60% of those in DJ-1+/+ cells, respectively (Fig. 4C, last 4 lanes).
FIGURE 4.
Activation of c-Raf by DJ-1 in EGF-treated cells. A, NIH3T3 and its DJ-1 knockdown D2 cells were cultured in a medium with 0.1% calf serum for 6 h and treated with 50 ng/ml EGF for 1, 5, and 15 min. Proteins extracted from the cells were analyzed by Western blotting with anti-phospho-c-Raf (Ser-338), anti-phospho-c-Raf (Ser-259), anti-c-Raf, anti-phospho-MEK, anti-MEK, anti-phospho-ERK1/2, anti-ERK1/2, anti-DJ-1, and anti-actin antibodies. Intensities of bands were quantified. The number of experiments (n) is 4. Significance: *, p < 0.05; **, p < 0.01. B, HeLa S3 cells were transfected with siRNA-targeting DJ-1 (si-DJ-1) or with nonspecific control RNA (si-control). Forty eight h after transfection, the cells were cultured in a medium with 0.1% calf serum for 6 h and treated with EGF for 1, 5 and 15 min, and expression levels of proteins were analyzed by Western blotting as described in the legend for A. n is 4. Significance: *, p < 0.05; **, p < 0.01. C, DJ-1+/+ cells, DJ-1−/− cells, and DJ-1−/− cells that had been transfected with expression vectors for wild-type DJ-1 or C106S DJ-1 were cultured in a medium with 0.1% calf serum for 6 h and treated with EGF for 3 min, and expression levels of proteins were analyzed by Western blotting as described in A. n is 4. Significance: *, p < 0.05; **, p < 0.01. N.S., nonsignificance. D, DJ-1+/+ and DJ-1−/− cells were transfected with various amounts of expression vectors for FLAG-CR3. Forty eight h after transfection, cells were cultured in a medium with 0.1% calf serum for 6 h and treated with or without EGF for 3 min, and expression levels of proteins were then examined by Western blotting as described in the legend for A. FLAG-CR3 was detected with an anti-FLAG antibody (M2, Sigma). The amounts of expression vectors for FLAG-CR3 transfected to 10-cm dishes were 5, 20, and 40 μg in lanes represented by triangle. n is 4. Significance: *, p < 0.05; **, p < 0.01.
Because DJ-1 binds to CR3, the kinase domain of c-Raf, it is possible that the excess amount of CR3 compared with the amount of full-sized c-Raf in cells inhibits activation activity of DJ-1 toward c-Raf as dominant negatives. To examine this, DJ-1+/+ and DJ-1−/− cells were transfected with various amounts of expression vectors for FLAG-CR3. Forty eight h after transfection, cells were cultured in a medium with 0.1% calf serum for 6 h and treated with or without EGF for 3 min, and total c-Raf, phosphorylated c-Raf (Ser-338), and phosphorylated c-Raf (Ser-259) were then examined by Western blotting. The expression levels of phosphorylated MEK and phosphorylated ERK1/2 were also examined. Although enhanced phosphorylated levels of c-Raf, MEK, and ERK1/2 by EGF in DJ-1+/+ cells were inhibited by transfected CR3 in a dose-dependent manner, phosphorylated levels of these proteins were not changed in DJ-1−/− cells (Fig. 4D). These results suggest that DJ-1 activates the ERK pathway in a c-Raf-binding activity-dependent manner.
It is known that c-Raf is activated with phosphorylation of Ser-338 after cells have been treated with phorbol myristate acetate (PMA)3 via protein kinase C (29, 30). To examine the effect of PMA on the role of DJ-1 in phosphorylation of c-Raf, DJ-1+/+ and DJ-1−/− cells were first cultured in a medium with 0.1% calf serum and treated with 50 ng/ml PMA for 1, 5, and 15 min, and phosphorylation levels of c-Raf (Ser-338), MEK, and ERK1/2 were examined. As shown in Fig. 5, there were no significant differences in phosphorylation levels of proteins in DJ-1+/+ and DJ-1−/− cells, indicating that DJ-1 reacts against the EGF-triggered ERK pathway.
FIGURE 5.
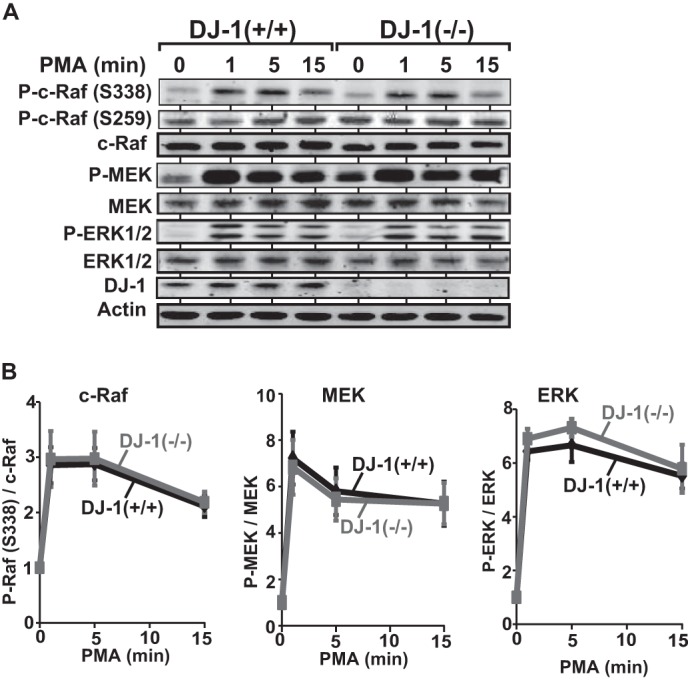
Effect of DJ-1 on activation of c-Raf in PMA-treated cells. A, DJ-1+/+ and DJ-1−/− cells were cultured in a medium with 0.1% calf serum for 6 h and treated with 50 ng/ml EGF for 1, 5, and 15 min. Expression levels of proteins were then analyzed by Western blotting as described in the legend for Fig. 4A. B, intensities of bands shown in A were quantified. n is 4.
Stimulation of Kinase Activity of c-Raf by DJ-1
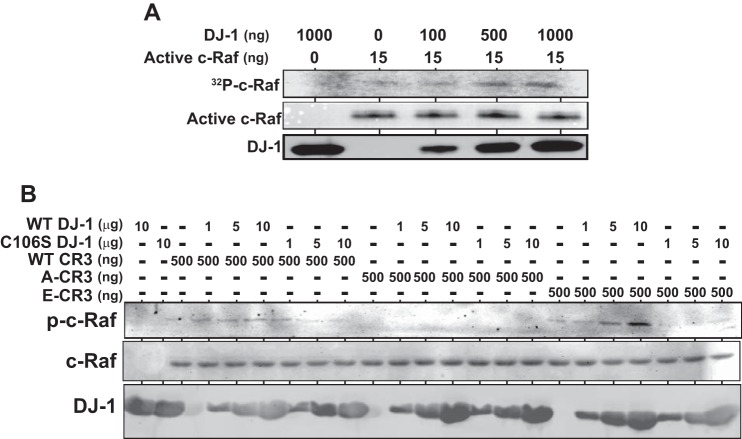
To examine the effect of DJ-1 on kinase activity of c-Raf, a constitutively active c-Raf fragment spanning amino acids 306–648 (CR3 fragment shown in Fig. 2A) with substitution mutations of Y340E and Y341E was reacted with purified DJ-1 and [γ-32P]ATP, separated on a polyacrylamide gel, and subjected to autoradiography. As shown in Fig. 6A, self-phosphorylation activity of c-Raf was increased by DJ-1 in a dose-dependent manner, indicating that DJ-1 enhances kinase activity of c-Raf by its direct binding.
FIGURE 6.
Stimulation of kinase activity of c-Raf by DJ-1. A, constitutively active form of the CR3 fragment of c-Raf (15 ng) was incubated with various amounts of recombinant DJ-1 and [γ-32P]ATP. 32P-Labeled CR3 was then separated on an SDS-polyacrylamide gel and subjected to fluorography. The CR3 fragment and DJ-1 used were detected by Western blotting with anti-c-Raf and anti-DJ-1 antibodies. B, constant amounts of recombinant wild-type, Y340E/Y341E, and Y340A/Y341A CR3 fragments of c-Raf (WT CR3, E-CR3, and A-CR3, respectively) were reacted with various amounts of wild-type and C106S DJ-1, and phosphorylation levels of phosphorylated CR3 designated as p-c-Raf were examined by Western blotting with an anti-phospho-c-Raf (Ser-338) antibody. Recombinant CR3 designated by c-Raf and DJ-1 were detected by the Coomassie Brilliant Blue staining.
Because binding activity of C106S DJ-1 to c-Raf is weaker than that of wild-type DJ-1 (Fig. 1B), the effect of the Cys-106 mutation in DJ-1 on kinase activity of c-Raf was next examined using recombinant wild-type and C106S DJ-1, and wild-type Y340E/Y341E, and Y340A/Y341A CR3 fragments of c-Raf (WT CR3, E-CR3, and A-CR3, respectively). A-CR3 works as a kinase-dead mutant. Constant amounts of c-Raf and CR3 were reacted with various amounts of wild-type and C106S DJ-1, and phosphorylation levels of CR3 fragments were examined by Western blotting with an anti-phospho-c-Raf (Ser-338) antibody. As shown in Fig. 6B, wild-type DJ-1 but not C106S DJ-1 slightly increased the phosphorylation level of wild-type CR3 and strongly increased the phosphorylation level of constitutively active CR3 but not that of kinase-dead CR3, suggesting that DJ-1 stimulates kinase activity of c-Raf in DJ-1 binding activity in a c-Raf-dependent manner.
Modification of DJ-1 in EGF-treated Cells
Because DJ-1 stimulates kinase activity of c-Raf, it is possible that DJ-1 is phosphorylated by c-Raf. Before testing this possibility, we used Phos-tag-containing gel to separate phosphorylated proteins from nonphosphorylated proteins without using anti-phosphorylated protein-specific antibodies. Because minus charge regions of phosphorylated proteins make a complex with Mn2+ in Phos-tag acrylamide AAL-107, the mobility of phosphorylated proteins became slower than that of nonphosphorylated proteins in a gel (31, 32). To confirm this technique, HeLa S3 cells were cultured in a medium with 0.1% calf serum and treated with 50 ng/ml EGF for 3 min. Proteins were prepared from the cells in the presence or absence of a phosphatase inhibitor and calf intestine alkaline phosphatase (CIAP), separated on the Phos-tag-containing gel or on normal polyacrylamide gel, and subjected to Western blotting. Proteins separated on the Phos-tag-containing gel were reacted with an anti-ERK1/2 antibody, and proteins separated on the non-Phos-tag-containing gel were reacted with anti-ERK1/2 and anti-phospho-ERK (Tyr-202 and Tyr-204). As shown in Fig. 7A, three ERK bands appeared in the Phos-tag-containing gel, and the upper two bands disappeared by reaction with CIAP, indicating that the upper two bands correspond to phosphorylated ERK1/2. To examine whether DJ-1 is phosphorylated after cells receive the EGF signal and oxidative stress, HeLa S3 cells were cultured in a medium with 0.1% calf serum for 6 h and treated with 50 ng/ml EGF for 3 min or with 1 mm H2O2 for 30 min, and proteins were separated on the Phos-tag-containing gel or on the non-Phos-tag-containing gel and analyzed by Western blotting with anti-DJ-1 and anti-ERK antibodies. The results showed that although phosphorylated ERK1/2 was distinguished from nonphosphorylated ERK1/2 in EGF, H2O2, and EGF + H2O2-treated cells, mobility of DJ-1 did not change (Fig. 7B). It was also found that the level of phosphorylated c-Raf (Ser-338) was reduced in H2O2-treated cells. These results suggest that DJ-1 is not phosphorylated by activated c-Raf and unknown kinases after EGF treatment.
FIGURE 7.
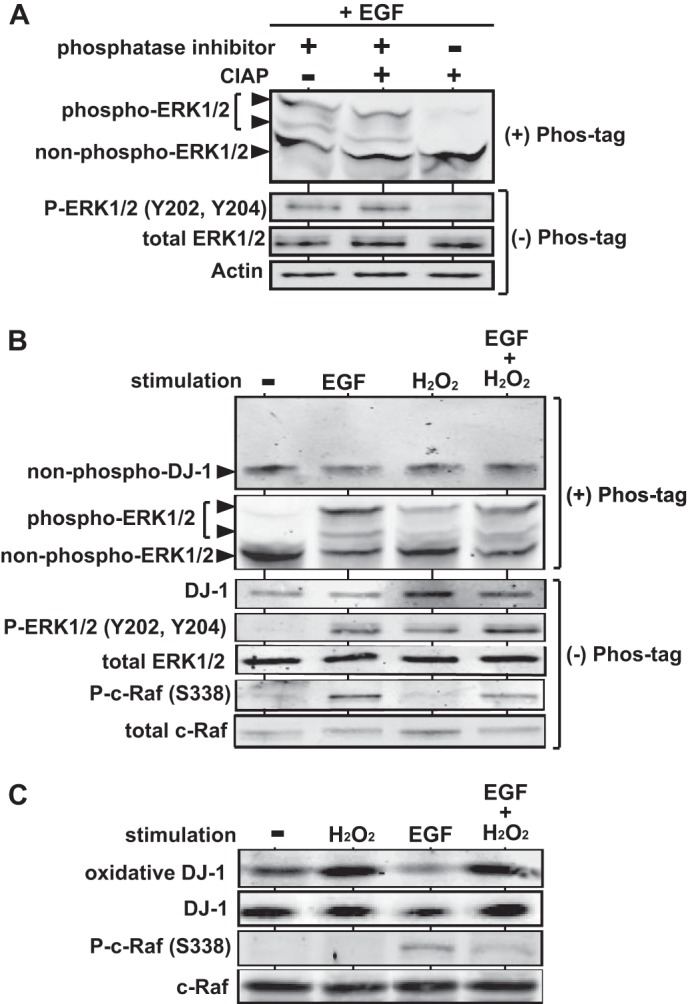
Effect of EGF treatment for cells on modification of DJ-1. A, HeLa S3 cells were cultured in a medium with 0.1% calf serum for 6 h and treated with 50 ng/ml EGF for 3 min. Proteins extracted from the cells were reacted with or without phosphatase inhibitor and CIAP, separated on Phos-tag- and non-Phos-tag-containing gels, and analyzed by Western blotting with anti-phospho-ERK1/2, anti-ERK1/2, and anti-actin antibodies. B, HeLa S3 cells were cultured in a medium with 0.1% calf serum for 6 h and treated with 50 ng/ml EGF for 3 min, with 1 mm H2O2 for 30 min, or with 50 ng/ml EGF for 3 min and 1 mm H2O2 for 30 min. Proteins extracted from the cells were then separated on Phos-tag- and non-Phos-tag-containing gels and analyzed by Western blotting with the respective antibodies. C, HeLa S3 cells were cultured in a medium with 0.1% calf serum for 6 h and treated with 50 ng/ml EGF for 3 min, with 1 mm H2O2 for 30 min, or with 50 ng/ml EGF for 3 min and 1 mm H2O2 for 30 min. Proteins extracted from the cells were then separated on Phos-tag- and non-Phos-tag-containing gels and analyzed by Western blotting with anti-oxidized DJ-1, anti-DJ-1, anti-phospho-c-Raf (Ser-338), and anti-c-Raf antibodies.
Because Cys-106 of DJ-1 is oxidized by oxidative stress (6, 7, 33, 34) and because C106S DJ-1 was shown to activate c-Raf less than does wild-type DJ-1 in this study, the oxidized DJ-1 level at Cys-106 was examined in EGF, H2O2, and EGF + H2O2-treated HeLa S3 cells using an anti-oxidized DJ-1 antibody. This antibody recognizes SO2H and SO3H, preferentially SO3H, forms of oxidized DJ-1 at Cys-106 (35). After cells had been treated with H2O2, the level of oxidized DJ-1 was increased, but phosphorylation of c-Raf (Ser-338) was not observed (Fig. 7C). Furthermore, the level of oxidized DJ-1 in EGF-treated cells was lower than that in nontreated cells, and the increased level of phosphorylated c-Raf by EGF was decreased by H2O2 treatment. These results suggest that oxidation of DJ-1 at Cys-106 with SO2H and SO3H forms is not necessary for DJ-1 to activate c-Raf in EGF-treated cells.
Discussion
Raf family proteins are serine/threonine kinases and include A-Raf, B-Raf, and c-Raf. These proteins have similar domain structures, including the Ras-binding domain and kinase domain at the proximal N and C terminus, respectively (19, 20). c-Raf is activated by EGF and PMA treatment in cells through phosphorylating Ser-338 via self-phosphorylation and protein kinase C (PKC), respectively (29, 30, 36). The PMA/PKC cascade does not include Ras. In this study, we first found that DJ-1 directly bound to the kinase domain of c-Raf but not to H-Ras (Figs. 1 and 2) and that binding activity of DJ-1 to c-Raf and co-localization of DJ-1 with c-Raf were enhanced by EGF treatment in cells (Figs. 1D and 3). Furthermore, DJ-1 enhanced phosphorylation of c-Raf at Ser-338, followed by activation of MEK and ERK1/2 (Fig. 4). Activation of c-Raf by DJ-1 depended on EGF but not on PMA (Fig. 5). These results suggest that DJ-1 directly targets c-Raf in the EGF/Ras/ERK signaling pathway, leading to cell growth.
Binding activity of C106S DJ-1 to c-Raf was reduced by 40% compared with that of wild-type DJ-1 (Fig. 1B). The phosphorylation levels of c-Raf at Ser-338 in wild-type DJ-1- and C106S DJ-1-transfected DJ-1−/− cells were restored to almost 100 and 60% of that in DJ-1+/+ cells, respectively (Fig. 4C), suggesting that stimulating activity of DJ-1 toward c-Raf phosphorylation depends on binding activity of DJ-1 to c-Raf. DJ-1 enhanced kinase activity of c-Raf in a system using recombinant c-Raf kinase domain and DJ-1 (Fig. 6), indicating that DJ-1 is able to activate c-Raf without other factors under these conditions. Although DJ-1 has several enzymatic activities, including protease (37–40) and glyoxalase activities (41–44), there is no report showing that DJ-1 is a protein kinase and that DJ-1 has a structural motif for kinase (3–5). These results therefore suggest that DJ-1 enhances self-phosphorylation activity of c-Raf without a role as kinase.
DJ-1 has been shown to undergo various modifications, including oxidation (6, 7, 33, 34), sumoylation (45), and S-nitrosylation (46, 47), and the effects of these modifications on activity of DJ-1 have been studied in detail (3–5). There are two reports showing phosphorylation of DJ-1 as follows: p53-dependent phosphorylation of DJ-1 by proteomic analysis (48) and p38-regulated/activated kinase-associated phosphorylation of a serine residue(s) of DJ-1 under an oxidative stress condition (49). However, the phosphorylated amino acid residue(s) has not been identified, and the role of phosphorylated DJ-1 has not been clarified. EGF treatment in cells did not induce phosphorylation of DJ-1 (Fig. 6B), suggesting that DJ-1 is not a substrate for c-Raf kinase or other c-Raf-induced kinases. Furthermore, EGF treatment did not stimulate oxidation of DJ-1 at Cys-106 with SO2H and SO3H forms (Fig. 6C). Cys-106 of DJ-1 is oxidized to SOH, SO2H, and SO3H forms. Although oxidation of Cys-106 with an SOH form is not ruled out due to specific recognition of Cys-106 with SO2H and SO3H forms by the anti-oxidized DJ-1 antibody used in this study, EGF treatment does not induce highly oxidized forms of DJ-1, suggesting that oxidation of Cys-106 with an SO2H form is not necessary for DJ-1 to activate c-Raf. DJ-1 enhances the ERK pathway upon various stimuli (11–13), and DJ-1 is an activated ras-cooperating oncogene (1). This study showed for the first time that DJ-1 activates the ERK pathway through direct activation of c-Raf.
Author Contributions
H. A. and S. M. M. I.-A. conceived and coordinated the study. H. A. wrote the paper. K. T.-N. and I. K.-O. designed, performed, and analyzed the experiments shown in Figs. 2–4 and 6. H. M. designed, performed, and analyzed the experiments shown in Figs. 1, 5, and 7. H. M. provided technical assistance. All authors reviewed the results and approved the final version of the manuscript.
Acknowledgment
We thank Dr. Yoshiro Saito for providing an anti-oxidized DJ-1 antibody.
This work was supported by grants-in-aid from the Ministry of Education, Culture, Sports, Science and Technology. The authors declare that they have no conflicts of interest with the contents of this article.
- PMA
- phorbol myristate acetate
- CIAP
- calf intestine alkaline phosphatase.
References
- 1. Nagakubo D., Taira T., Kitaura H., Ikeda M., Tamai K., Iguchi-Ariga S. M., Ariga H. (1997) DJ-1, a novel oncogene which transforms mouse NIH3T3 cells in cooperation with ras. Biochem. Biophys. Res. Commun. 231, 509–513 [DOI] [PubMed] [Google Scholar]
- 2. Bonifati V., Rizzu P., van Baren M. J., Schaap O., Breedveld G. J., Krieger E., Dekker M. C., Squitieri F., Ibanez P., Joosse M., van Dongen J. W., Vanacore N., van Swieten J. C., Brice A., Meco G., et al. (2003) Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science 299, 256–259 [DOI] [PubMed] [Google Scholar]
- 3. Kahle P. J., Waak J., Gasser T. (2009) DJ-1 and prevention of oxidative stress in Parkinson's disease and other age-related disorders. Free Radic. Biol. Med. 47, 1354–1361 [DOI] [PubMed] [Google Scholar]
- 4. Wilson M. A. (2011) The role of cysteine oxidation in DJ-1 function and dysfunction. Antioxid. Redox Signal. 15, 111–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ariga H., Takahashi-Niki K., Kato I., Maita H., Niki T., Iguchi-Ariga S. M. Neuroprotective function of DJ-1 in Parkinson's disease. Oxid. Med. Cell. Longev. 2013, 683920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Taira T., Saito Y., Niki T., Iguchi-Ariga S. M., Takahashi K., Ariga H. (2004) DJ-1 has a role in antioxidative stress to prevent cell death. EMBO Rep. 5, 213–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Canet-Avilés R. M., Wilson M. A., Miller D. W., Ahmad R., McLendon C., Bandyopadhyay S., Baptista M. J., Ringe D., Petsko G. A., Cookson M. R. (2004) The Parkinson's disease protein DJ-1 is neuroprotective due to cysteine-sulfinic acid-driven mitochondrial localization. Proc. Natl. Acad. Sci. U.S.A. 101, 9103–9108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Martinat C., Shendelman S., Jonason A., Leete T., Beal M. F., Yang L., Floss T., Abeliovich A. (2004) Sensitivity to oxidative stress in DJ-1-deficient dopamine neurons: an ES-derived cell model of primary Parkinsonism. PLoS Biol. 2, e327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bandopadhyay R., Kingsbury A. E., Cookson M. R., Reid A. R., Evans I. M., Hope A. D., Pittman A. M., Lashley T., Canet-Aviles R., Miller D. W., McLendon C., Strand C., Leonard A. J., Ariga H., Wood N. W., et al. (2004) The expression of DJ-1 (PARK7) in normal human CNS and idiopathic Parkinson's disease. Brain 127, 420–430 [DOI] [PubMed] [Google Scholar]
- 10. Choi J., Sullards M. C., Olzmann J. A., Rees H. D., Weintraub S. T., Bostwick D. E., Gearing M., Levey A. I., Chin L. S., Li L. (2006) Oxidative damage of DJ-1 is linked to sporadic Parkinson and Alzheimer diseases. J. Biol. Chem. 281, 10816–10824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lev N., Ickowicz D., Barhum Y., Lev S., Melamed E., Offen D. (2009) DJ-1 protects against dopamine toxicity. J. Neural. Transm. 116, 151–160 [DOI] [PubMed] [Google Scholar]
- 12. Gu L., Cui T., Fan C., Zhao H., Zhao C., Lu L., Yang H. (2009) Involvement of ERK1/2 signaling pathway in DJ-1-induced neuroprotection against oxidative stress. Biochem. Biophys. Res. Commun. 383, 469–474 [DOI] [PubMed] [Google Scholar]
- 13. He X., Zheng Z., Li J., Ben Q., Liu J., Zhang J., Ji J., Yu B., Chen X., Su L., Zhou L., Liu B., Yuan Y. (2012) DJ-1 promotes invasion and metastasis of pancreatic cancer cells by activating SRC/ERK/uPA. Carcinogenesis 33, 555–562 [DOI] [PubMed] [Google Scholar]
- 14. Kim R. H., Peters M., Jang Y., Shi W., Pintilie M., Fletcher G. C., DeLuca C., Liepa J., Zhou L., Snow B., Binari R. C., Manoukian A. S., Bray M. R., Liu F. F., Tsao M. S., Mak T. W. (2005) DJ-1, a novel regulator of the tumor suppressor PTEN. Cancer Cell 7, 263–273 [DOI] [PubMed] [Google Scholar]
- 15. Kim Y. C., Kitaura H., Taira T., Iguchi-Ariga S. M., Ariga H. (2009) Oxidation of DJ-1-dependent cell transformation through direct binding of DJ-1 to PTEN. Int. J. Oncol. 35, 1331–1341 [PubMed] [Google Scholar]
- 16. Junn E., Taniguchi H., Jeong B. S., Zhao X., Ichijo H., Mouradian M. M. (2005) Interaction of DJ-1 with Daxx inhibits apoptosis signal-regulating kinase 1 activity and cell death. Proc. Natl. Acad. Sci. U.S.A. 102, 9691–9696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Waak J., Weber S. S., Görner K., Schall C., Ichijo H., Stehle T., Kahle P. J. (2009) Oxidizable residues mediating protein stability and cytoprotective interaction of DJ-1 with apoptosis signal-regulating kinase 1. J. Biol. Chem. 284, 14245–14257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kato I., Maita H., Takahashi-Niki K., Saito Y., Noguchi N., Iguchi-Ariga S. M., Ariga H. (2013) Oxidized DJ-1 inhibits p53 by sequestering p53 from promoters in a DNA-binding affinity-dependent manner. Mol. Cell. Biol. 33, 340–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Daum G., Eisenmann-Tappe I., Fries H. W., Troppmair J., Rapp U. R. (1994) The ins and outs of Raf kinases. Trends Biochem. Sci. 19, 474–480 [DOI] [PubMed] [Google Scholar]
- 20. Brtva T. R., Drugan J. K., Ghosh S., Terrell R. S., Campbell-Burk S., Bell R. M., Der C. J. (1995) Two distinct Raf domains mediate interaction with Ras. J. Biol. Chem. 270, 9809–9812 [DOI] [PubMed] [Google Scholar]
- 21. Dhillon A. S., Pollock C., Steen H., Shaw P. E., Mischak H., Kolch W. (2002) Cyclic AMP-dependent kinase regulates Raf-1 kinase mainly by phosphorylation of serine 259. Mol. Cell. Biol. 22, 3237–3246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Abraham D., Podar K., Pacher M., Kubicek M., Welzel N., Hemmings B. A., Dilworth S. M., Mischak H., Kolch W., Baccarini M. (2000) Raf-1-associated protein phosphatase 2A as a positive regulator of kinase activation. J. Biol. Chem. 275, 22300–22304 [DOI] [PubMed] [Google Scholar]
- 23. Oehrl W., Rubio I., Wetzker R. (2003) Serine 338 phosphorylation is dispensable for activation of c-Raf1. J. Biol. Chem. 278, 17819–17826 [DOI] [PubMed] [Google Scholar]
- 24. Kim Y. C., Kitaura H., Iguchi-Ariga S. M., Ariga H. (2010) DJ-1, an oncogene and causative gene for familial Parkinson's disease, is essential for SV40 transformation in mouse fibroblasts through up-regulation of c-Myc. FEBS Lett. 584, 3891–3895 [DOI] [PubMed] [Google Scholar]
- 25. Takahashi-Niki K., Niki T., Taira T., Iguchi-Ariga S. M., Ariga H. (2004) Reduced anti-oxidative stress activities of DJ-1 mutants found in Parkinson's disease patients. Biochem. Biophys. Res. Commun. 320, 389–397 [DOI] [PubMed] [Google Scholar]
- 26. Maita C., Maita H., Iguchi-Ariga S. M., Ariga H. (2013) Monomer DJ-1 and its N-terminal sequence are necessary for mitochondrial localization of DJ-1 mutants. PLoS ONE 8, e54087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Saito Y., Hamakubo T., Yoshida Y., Ogawa Y., Hara Y., Fujimura H., Imai Y., Iwanari H., Mochizuki Y., Shichiri M., Nishio K., Kinumi T., Noguchi N., Kodama T., Niki E. (2009) Preparation and application of monoclonal antibodies against oxidized DJ-1. Significant elevation of oxidized DJ-1 in erythrocytes of early-stage Parkinson disease patients. Neurosci. Lett. 465, 1–5 [DOI] [PubMed] [Google Scholar]
- 28. Takahashi K., Taira T., Niki T., Seino C., Iguchi-Ariga S. M., Ariga H. (2001) DJ-1 positively regulates the androgen receptor by impairing the binding of PIASx α to the receptor. J. Biol. Chem. 276, 37556–37563 [DOI] [PubMed] [Google Scholar]
- 29. Barnard D., Diaz B., Clawson D., Marshall M. (1998) Oncogenes, growth factors and phorbol esters regulate Raf-1 through common mechanisms. Oncogene 17, 1539–1547 [DOI] [PubMed] [Google Scholar]
- 30. Xuan Y. T., Guo Y., Zhu Y., Wang O. L., Rokosh G., Messing R. O., Bolli R. (2005) Role of the protein kinase C-ϵ-Raf-1-MEK-1/2-p44/42 MAPK signaling cascade in the activation of signal transducers and activators of transcription 1 and 3 and induction of cyclooxygenase-2 after ischemic preconditioning. Circulation 112, 1971–1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kinoshita E., Kinoshita-Kikuta E., Takiyama K., Koike T. (2006) Phosphate-binding tag, a new tool to visualize phosphorylated proteins. Mol. Cell. Proteomics 5, 749–757 [DOI] [PubMed] [Google Scholar]
- 32. Kinoshita E., Kinoshita-Kikuta E., Koike T. (2009) Separation and detection of large phosphoproteins using Phos-tag SDS-PAGE. Nat. Protoc. 4, 1513–1521 [DOI] [PubMed] [Google Scholar]
- 33. Kinumi T., Kimata J., Taira T., Ariga H., Niki E. (2004) Cysteine-106 of DJ-1 is the most sensitive cysteine residue to hydrogen peroxide-mediated oxidation in vivo in human umbilical vein endothelial cells. Biochem. Biophys. Res. Commun. 317, 722–728 [DOI] [PubMed] [Google Scholar]
- 34. Mitsumoto A., Nakagawa Y., Takeuchi A., Okawa K., Iwamatsu A., Takanezawa Y. (2001) Oxidized forms of peroxiredoxins and DJ-1 on two-dimensional gels increased in response to sublethal levels of paraquat. Free Radic. Res. 35, 301–310 [DOI] [PubMed] [Google Scholar]
- 35. Saito Y., Miyasaka T., Hatsuta H., Takahashi-Niki K., Hayashi K., Mita Y., Kusano-Arai O., Iwanari H., Ariga H., Hamakubo T., Yoshida Y., Niki E., Murayama S., Ihara Y., Noguchi N. (2014) Immunostaining of oxidized DJ-1 in human and mouse brains. J. Neuropathol. Exp. Neurol. 73, 714–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zang M., Gong J., Luo L., Zhou J., Xiang X., Huang W., Huang Q., Luo X., Olbrot M., Peng Y., Chen C., Luo Z. (2008) Characterization of Ser338 phosphorylation for Raf-1 activation. J. Biol. Chem. 283, 31429–31437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Olzmann J. A., Brown K., Wilkinson K. D., Rees H. D., Huai Q., Ke H., Levey A. I., Li L., Chin L. S. (2004) Familial Parkinson's disease-associated L166P mutation disrupts DJ-1 protein folding and function. J. Biol. Chem. 279, 8506–8515 [DOI] [PubMed] [Google Scholar]
- 38. Koide-Yoshida S., Niki T., Ueda M., Himeno S., Taira T., Iguchi-Ariga S. M., Ando Y., Ariga H. (2007) DJ-1 degrades transthyretin and an inactive form of DJ-1 is secreted in familial amyloidotic polyneuropathy. Int. J. Mol. Med. 19, 885–893 [PubMed] [Google Scholar]
- 39. Chen J., Li L., Chin L. S. (2010) Parkinson disease protein DJ-1 converts from a zymogen to a protease by carboxyl-terminal cleavage. Hum. Mol. Genet. 19, 2395–2408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mitsugi H., Niki T., Takahashi-Niki K., Tanimura K., Yoshizawa-Kumagaye K., Tsunemi M., Iguchi-Ariga S. M., Ariga H. (2013) Identification of the recognition sequence and target proteins for DJ-1 protease. FEBS Lett. 587, 2493–2499 [DOI] [PubMed] [Google Scholar]
- 41. Lee J. Y., Song J., Kwon K., Jang S., Kim C., Baek K., Kim J., Park C. (2012) Human DJ-1 and its homologs are novel glyoxalases. Hum. Mol. Genet. 21, 3215–3225 [DOI] [PubMed] [Google Scholar]
- 42. Hasim S., Hussin N. A., Alomar F., Bidasee K. R., Nickerson K. W., Wilson M. A. (2014) A glutathione-independent glyoxalase of the DJ-1 superfamily plays an important role in managing metabolically generated methylglyoxal in Candida albicans. J. Biol. Chem. 289, 1662–1674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhao Q., Su Y., Wang Z., Chen C., Wu T., Huang Y. (2014) Identification of glutathione (GSH)-independent glyoxalase III from Schizosaccharomyces pombe. BMC Evol. Biol. 14, 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Toyoda Y., Erkut C., Pan-Montojo F., Boland S., Stewart M. P., Müller D. J., Wurst W., Hyman A. A., Kurzchalia T. V. (2014) Products of the Parkinson's disease-related glyoxalase DJ-1, d-lactate and glycolate, support mitochondrial membrane potential and neuronal survival. Biol. Open 3, 777–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Shinbo Y., Niki T., Taira T., Ooe H., Takahashi-Niki K., Maita C., Seino C., Iguchi-Ariga S. M., Ariga H. (2006) Proper SUMO-1 conjugation is essential to DJ-1 to exert its full activities. Cell Death Differ. 13, 96–108 [DOI] [PubMed] [Google Scholar]
- 46. Ito G., Ariga H., Nakagawa Y., Iwatsubo T. (2006) Roles of distinct cysteine residues in S-nitrosylation and dimerization of DJ-1. Biochem. Biophys. Res. Commun. 339, 667–672 [DOI] [PubMed] [Google Scholar]
- 47. Choi M. S., Nakamura T., Cho S. J., Han X., Holland E. A., Qu J., Petsko G. A., Yates J. R., 3rd, Liddington R. C., Lipton S. A. (2014) Transnitrosylation from DJ-1 to PTEN attenuates neuronal cell death in Parkinson's disease models. J. Neurosci. 34, 15123–15131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rahman-Roblick R., Hellman U., Becker S., Bader F. G., Auer G., Wiman K. G., Roblick U. J. (2008) Proteomic identification of p53-dependent protein phosphorylation. Oncogene 27, 4854–4859 [DOI] [PubMed] [Google Scholar]
- 49. Tang J., Liu J., Li X., Zhong Y., Zhong T., Liu Y., Wang J. H., Jiang Y. PRAK interacts with DJ-1 and prevents oxidative stress-induced cell death. Oxid. Med. Cell. Longev. 2014, 735618. [DOI] [PMC free article] [PubMed] [Google Scholar]