Background: Pregnancy promotes physiological adaptations throughout the body mediated by the female sex hormones.
Results: Pregnancy promotes switching of skeletal muscle to a glycolytic phenotype through the smoothelin-like protein 1 transcriptional cofactor.
Conclusion: Deletion of SMTNL1 is able to mimic the effect of pregnancy in mice.
Significance: Novel mechanism to explain insulin resistance during pregnancy.
Keywords: gene expression, pregnancy, proteomics, skeletal muscle, steroid hormone receptor, smoothelin-like protein 1
Abstract
Pregnancy promotes physiological adaptations throughout the body, mediated by the female sex hormones progesterone and estrogen. Changes in the metabolic properties of skeletal muscle enable the female body to cope with the physiological challenges of pregnancy and may also be linked to the development of insulin resistance. We conducted global microarray, proteomic, and metabolic analyses to study the role of the progesterone receptor and its transcriptional regulator, smoothelin-like protein 1 (SMTNL1) in the adaptation of skeletal muscle to pregnancy. We demonstrate that pregnancy promotes fiber-type changes from an oxidative to glycolytic isoform in skeletal muscle. This phenomenon is regulated through an interaction between SMTNL1 and progesterone receptor, which alters the expression of contractile and metabolic proteins. smtnl1−/− mice are metabolically less efficient and show impaired glucose tolerance. Pregnancy antagonizes these effects by inducing metabolic activity and increasing glucose tolerance. Our results suggest that SMTNL1 has a role in mediating the actions of steroid hormones to promote fiber switching in skeletal muscle during pregnancy. Our findings also bear on the management of gestational diabetes that develops as a complication of pregnancy in ∼4% of women.
Introduction
Skeletal muscle (SKM)3 shows plasticity and is able to adapt its contractile phenotype in response to physiological stress, including exercise training, hormonal shifts, aging, and pathological stress (1). Skeletal muscle plasticity has been studied extensively in endurance exercise training in both humans and animals and shown to promote the switching of SKM fiber content to a more oxidative phenotype characterized by an increase in the relative numbers of type I slow-twitch and type2a fast-twitch fibers and a decrease in type2b fast-twitch glycolytic fibers (2, 3). Alternatively, intermittent bursting effort promotes transformation of type2a/b fibers to a more glycolytic type2b phenotype, with fewer mitochondria and increased expression of the MHC2b isoform as well as enzymes associated with glycolysis. SKM is also the primary site for the caloric disposal of glucose and long chain fatty acids, which is profoundly affected by the composition and the metabolic and enzymatic properties of its fibers. Switching of SKM to an oxidative phenotype is associated with a decreased susceptibility to the development of insulin resistance. Exercise training alone can reverse obesity-induced insulin resistance by increasing the oxidative capacity of muscle through the enhancement of mitochondrial density and glucose transporter content (4, 5). Conversely, reduced oxidative capacity of SKM is associated with predisposition to the development of insulin resistance and increased weight gain.
Pregnancy is also a physiological state that can promote major changes in SKM, mainly mediated by the female sex steroid hormones estrogen and progesterone (6). Both progesterone receptors (PR) and estrogen receptors (ER) are expressed in SKM, but their function and relevance have yet to be defined (7). Some direct links between the effect of progesterone and estrogen on SKM come from studies on pregnancy-induced insulin resistance. The increased glucose requirements of the gravid uterus during pregnancy are thought to necessitate major adjustments in glucose production and utilization by maternal SKM, adipose, and other tissues. This is the reason why normal pregnancy is associated with the development of insulin resistance, which is thought of as a means by which the mother can supply the developing fetus with sufficient glucose for growth. However, in 2–4% of all pregnancies, this condition progresses to a type II diabetic state known as gestational diabetes and becomes a complication of pregnancy. At the mid-term of pregnancy both SKM and adipose tissues show insulin resistance (8), but the underlying molecular mechanisms that promote this response are unknown. These mechanisms have been speculated to be linked to increased circulating insulin levels promoted by an increase in the number and mass of pancreatic β cells induced by progesterone (9).
Smoothelin-like protein 1 (SMTNL1) plays a role in mediating exercise-induced adaptations in both smooth and SKM that are sex-dependent (10). For example, striated muscle in male smntl−/− mice exhibit an endurance phenotype, whereas female null mice exhibit a more glycolytic phenotype (11). Pregnancy was also found to induce the expression of SMTNL1 in tissues such as uterine and vascular smooth muscle and sex hormone-related tissues. In uterine smooth muscle, SMTNL1 plays a major role in pregnancy to promote adaptive responses, and this process is specifically mediated through interactions of SMTNL1 with the steroid hormone receptor PR-B. In vitro and in vivo SMTNL1 selectively binds PR and does not bind other steroid hormone receptors. This suggests that SMTNL1 is a bifunctional co-regulator of PR-B signaling and thus provides a molecular mechanism whereby PR-B is targeted to alter gene expression patterns to coordinately promote alterations in uterine smooth muscle function during pregnancy (12). Based on these observations we have speculated that SMTNL1 could function as a transcriptional regulator of SKM differentiation and plasticity; this putative role is evident by the nuclear localization of the protein upon phosphorylation at serine 301 by protein kinase G/A and by the presence of a number of regulatory transcription factor binding sites in the promoter region of smtnl1 (13). In this study we show that pregnancy induces switching of SKM to a glycolytic phenotype and this effect is mediated through SMTNL1. A range of proteomic, global gene array, and metabolic studies in wild type (WT) and smtnl1−/− mice support this hypothesis. The finding that deletion of SMTNL1 promotes fiber specific expression of both ER and PR suggests that these events are likely to be mediated through progesterone or estrogen during pregnancy. We suggest that in SKM, these events are natural adaptations of normal pregnancy and potentially infer evolutionary advantages to the mother by increasing her ability to store fat and her physical strength to carry the developing fetus.
Experimental Procedures
Antibodies
Semi-quantitative Western blotting (SQ-WB) was performed with antibodies specific for PR (Abcam), ERα (Santa Cruz), SMTNL1, SMTNL1S301A (Proteintech Inc.), MYPT1 (D. J. Hartshorne, University of Arizona), tubulin (Sigma), anti-Glut4 (Santa Cruz Biotechnology), anti-IRS1 (Cell Signaling Technology), anti-MHCI (14), anti-MHC2a, anti-MHC2b (14), and Developmental Studies Hybridoma Bank, University of Iowa). The specificity of MHC antibodies has been established before (10, 14).
Mouse Colony Maintenance and Pregnancy Studies
Congenic 129 SvEv smtnl1−/− mouse were created as described (10). Pregnancy and pseudo-pregnancy studies were conducted as described (11). For pregnancy studies 8-week-old mice were sacrificed at days 14–17. Animal studies were approved by the Duke University Institutional Animal Care and Use Committee. ERKO mice were housed, and tissue sections were dissected at NIEHS/National Institutes of Health, Research Triangle Park, NC. Procedures involving humans were approved by the University and Medical Center Institutional Review Board at East Carolina University.
Intraperitoneal Glucose Tolerance Test
An intraperitoneal glucose tolerance test was performed at 5–6-week-old animals using smtnl+/+ and smtnl−/− non-pregnant and 14-day pregnant animals. After a 12-h fast, mice were injected intraperitoneally with glucose (2 mg/kg body weight). Blood glucose levels were determined from tail vein blood at 0, 30, 60, and 120 min after the glucose injection by Ascensia Breeze Blood Glucose Monitoring System.
Microarray
RNeasy Lipid Tissue Mini kit (Qiagen) was used to isolate total RNA from the plantarus skeletal muscle of wild type and/or smtnl1−/− of pregnant (day 17) and/or non-pregnant mice according to the manufacturer's protocol, and RNA were stored in liquid N2 at −80 °C until further processing. The quantity and quality of RNA were assessed using a NanoDrop ND-1000 spectrophotometer and an Agilent Bioanalyzer and samples with an RNA Integrity number (RIN) >7 were only used for further analysis. For microarray hybridizations, 100 ng of total RNA was amplified and labeled using the MessageAmp Premier Kit (Ambion). Equal amounts of labeled cRNA were hybridized to the Affymetrix Mouse Genome 430 2.0 microarray (Affymetrix) according to the manufacturer's protocol. Partek Genomics Suite 6.4 (Partek Inc., St. Louis, MO) was used to perform data analysis. Robust multi-chip analysis normalization was done on the entire data set. Multi-way analysis of variance and -fold change were performed to select target genes that were differentially expressed between the different comparisons (i.e. WN versus KN, KN versus KP, WP versus KP, WN versus WP). Top differentially expressed genes were selected with a p value cut-off of 0.05 based on the analysis of variance test and -fold change cutoff of ≥2. Gene Ontology Enrichment analysis on the gene lists was performed with χ2 test and limited to functional groups with more than two genes. Hierarchical Clustering was performed on differentially expressed genes based on Average Linkage with Pearson's Dissimilarity. Additionally, the gene lists were analyzed using the GeneGo software for obtaining pathway maps, biological networks, and diseases relevant to the list. All microarray experimental results are available at the Duke Microarray facility website.
Biochemical Assays and Semi-quantitative Western blot Analysis
Tissue samples were frozen in liquid N2 at the time of harvest, stored at −80 °C, and homogenized as described (10). Densitometry of the blots was performed, and scans were analyzed by the Volume Analyze feature of the Molecular Analyst Software (Bio-Rad) and Image J. The density of the protein of interest was normalized to tubulin and plotted as relative numbers except for the Western blot analysis of SMTNL1Ser-301 phosphorylation when density data were also normalized to the SMTNL1 expression. All IP procedures using anti-ERα, -PR-B, and SMTNL1 antibodies were carried out as described (15).
Immunohistochemistry and Fiber Typing
Immunohistochemistry and fiber typing of mouse tissues were performed as described previously (10, 14). Human rectus abdominis samples of premenopausal patients of hysterectomy (non-pregnant) or C-sectioning (pregnant) and sections of vastus lateralis biopsies of age-matched healthy women were treated similarly. Images taken on a Zeiss LSM 510 confocal laser scanning microscope were processed using LSM 5 Examiner software program. For detection of glycogen, Periodic Acid Schiff staining (Sigma) was applied on mouse and human skeletal muscle tissues following the instructions of manufacturer, and the density was measured by Image J software and normalized to the data of WT non-pregnant tissues. A representative set of images of n = 5–7 experiments is shown in the figures.
Proteomic Studies
Plantaris samples of WT and SMTNL null mice in days 0 and 14 of pregnancy, for proteomic analysis as described in samples of WT and SMTNL null mice in days 0 and 14 of pregnancy, were homogenized in a glass homogenizer in sample buffer (5 m urea, 4% CHAPS, 1 mm DTT) and centrifuged at 15,000 × g for 15 min as described before (16). Samples were subjected parallel to multi-dimensional proteomic analysis using micro anion-exchange separation, one-dimensional SDS-PAGE, and LC-MALDI TOF-TOF MS/MS analysis. Proteins were visualized with silver-staining, and gels were dried. Proteins showing increased recovery between each condition were excised for identification by MALDI-TOF TOF mass spectrometry (supplemental Table S1). Changes in individual proteins were determined using a combination of densitometry and iTRAQ (17, 18) (supplemental Table S1).
Comprehensive Laboratory Animal Monitoring System (CLAMS)
Seven- to eight-week-old either pregnant or non-pregnant 129 WT and smtnl1−/− mice were fed a normal diet (5001 chow) and were individually housed for 1 week to acclimate them to individual housing. Mice were then placed into individual CLAMS (Columbus Instruments, Columbus, OH) cages and acclimated for 24 h. Data collection consisted of 48-h feeding/24-h fasting/24-h re-feeding phases. Mice were monitored over 4 days. File displays were collected every 20 min for oxygen consumption (VO2, volume of oxygen consumed; ml/kg/h), VCO2 (volume of carbon dioxide produced, ml/kg/h), RER (respiratory exchange ratio, the respiratory quotient of VCO2/VO2, indicative of macronutrient utilization), heat (kcal/h), accumulated food (g), accumulated drink (g), XY total activity (all horizontal beam breaks in counts), XY and Z activity, and substrate utilization, estimated by the RER in fasting or feeding conditions.
Statistical Analysis
Data were normalized by taking values obtained for WT males as 1 and by calculating a corresponding proportional value for WT females and both sexes of KO mice. Normalized data were analyzed by t tests (for two groups) or by general linear models (GLM, for >2 groups). Parametric statistical tests were used if the assumptions of such tests were met; otherwise, we log-transformed data for analyses. In GLMs, we tested all possible interaction terms and report here the final models obtained by excluding non-significant (p > 0.05) interactions. When any covariate or factor was significant in GLMs, we applied Tukey's HSD procedure to test for pairwise differences in group means. Tests were conducted in the R statistical environment (R Development Core Team 2008).
Results
Pregnancy and SMTNL1 Regulate Glycolytic Fiber Switching in Mice and Humans
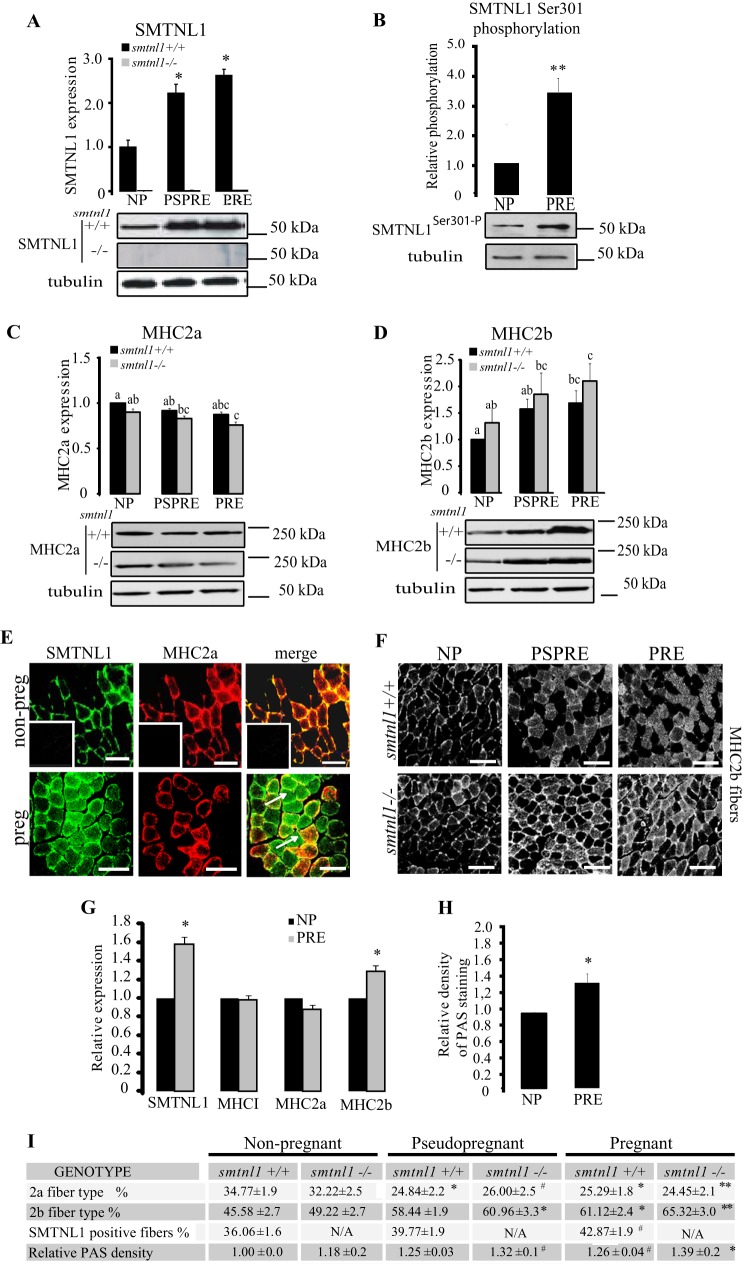
To investigate the sex-related differences in female smtnl1−/− mice, we conducted Western blot analysis and fiber typing experiments in pregnant animals. Because exercise induces fiber transformation in non-pregnant animals, pseudo-pregnant mice were also examined to discriminate between the physical effects of increased weight gain of the pregnant mother from the effects of hormonal regulation of SKM. Pseudo-pregnant mice proceed with the normal hormonal changes observed in fully pregnant animals but do not experience weight gain from the developing fetal mice in utero. Pregnancy and pseudo-pregnancy promote a 2.2-fold increase of SMTNL1 expression within the mixed fiber plantaris muscle compared with non-pregnant females (tmax day 16 ± 2) (Fig. 1A). Fig. 1B shows that pregnancy induces the phosphorylation (tmax day 12 ± 2) of SMTNL1 at Ser-301. Previously we demonstrated that in vivo SMTNL1 localization is highly regulated, and phosphorylation at Ser-301 promotes translocation from the cytosol to the nucleus (11), suggesting its potential role as transcriptional cofactor (12). Western analysis of MHC2a and -2b levels showed increased expression of MHC2b in response to both pregnancy and SMTNL1 deletion with concomitant reduction in MHC2a expression (Fig. 1, C and D). These expression changes were also mimicked in pseudo-pregnant mice, suggesting that these effects are regulated by the primary sex hormones rather than to physical stress arising from the developing fetal mice (Fig. 1, C and D). Detailed fiber typing of MHC isoform expression by immunohistochemistry (IHC) of SKM from non-pregnant WT females showed that SMTNL1 expression was confined only to type2a muscle fibers in murine plantaris muscle (Fig. 1E), which was also confirmed in human SKM (Fig. 1G). By day 13 of pregnancy, SMTNL1 expression was greatly increased, which implied that pregnancy promoted increased numbers of type2a fibers. However, IHC of MHC2a expression showed a decline in the mean proportion of type2a fibers (Fig. 1E). This phenomenon was more pronounced in SKM from pregnant female smtnl1−/− mice, which showed a >10% decline in type2a fibers relative to WT littermates (Fig. 1, E–I). Although SMTNL1 is not expressed in “bona fide” type2b fibers, co-staining experiments with anti-MHC2a and -MHC2b suggested that staining of SMTNL1 can also be found in fibers undergoing type2a/type2b transition (see arrows in Fig. 1E).
FIGURE 1.
Pregnancy induced a fiber type switch to a glycolytic phenotype in SKM, and it is enhanced by SMTNL1 deletion. A, expression of SMTNL1 in non-pregnant (NP), pregnant (PRE), and pseudopregnant (PSPRE) plantaris muscle by SQ-WB. B, phosphorylation of SMTNL1 Ser-301 in non-pregnant (NP) and pregnant (PRE) plantaris muscle. Means + SEM (n = 4–5/group), t test, *, p < 0.05. C and D, pregnancy reduces MHC2a expression (C) and increases expression of MHC2b (D) in SKM. Protein expression levels in non-pregnant, pregnant, and pseudopregnant by SQ-WB. n = 4–13/group, data are presented as the mean ± S.E., GLM with Tukey's test. Different letters indicate significant differences, p < 0.05. E, fiber typing by IHC and confocal microscopy of plantaris muscle. SMTNL1 (green) is expressed in type2a fibers (red) in control SKM. SMTNL1 expression shows localization in fibers different from type2a (arrows). Insets show secondary antibody controls. Scale bars = 20 μm. F, analysis of MHC2b expression (white) in plantaris muscle. Scale bar = 10 μm. G, SMTNL1, MHCI, MHC2a, and MHC2b expression in human SKM in pregnancy. H, quantitative Periodic Acid Schiff staining for glycogen content of human rectus abdominis sections. G and H, means ± S.E. (n = 4–5/group), t test. *, p < 0.05. I, quantitative fiber counting of plantaris muscle of non-pregnant, pregnant, and pseudopregnant (smtnl1+/+ and smtnl1−/− animals. PAS, Periodic Acid Schiff. Data are the means ± S.E. (n = 14–15/group), t test. #, p < 0.1; *, p < 0.05; **, p < 0.001.
If pregnancy and SMTNL1 deletion reduces MHC2a expression, this raises the question of whether these conditions concomitantly increase MHC2b levels and numbers of type2b fibers? Quantitative Western blot analysis and IHC with anti-MHC2b suggested that pregnancy and pseudo-pregnancy induced a 15–20% increase in proportion of type2b glycolytic fibers (Fig. 1, F and I). IHC examination of the expression of SMTNL1, MHC1, and MHC2a/2b in abdominis rectus muscle isolated from women undergoing hysterectomy (non-pregnant control) or C-section (as pregnant) suggested that pregnancy was also likely to promote similar fiber switching in humans (Fig. 1G). Pregnancy or pseudo-pregnancy did not affect the expression of MHCI, the marker of oxidative slow type 1 fibers. The decrease of protein expression of type2a marker MHC2a was only tendentious significant, whereas that of MHC2b increased by 20%, indicating that fibers switched from oxidative to the more glycolytic phenotype in human pregnant SKM. The increased type2b content in pregnancy is accompanied by a 24% increase in glycogen content (Fig. 1H). These data suggest that pregnancy promotes the transformation of SKM fiber type to a more glycolytic phenotype in mice and humans and that SMTNL1 may play a regulatory role in this process.
The Effects of SMTNL1 Deletion and Pregnancy on Global Gene Expression in Skeletal Muscle
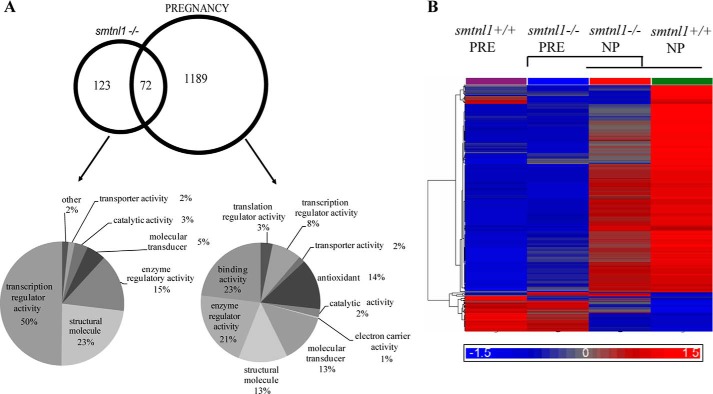
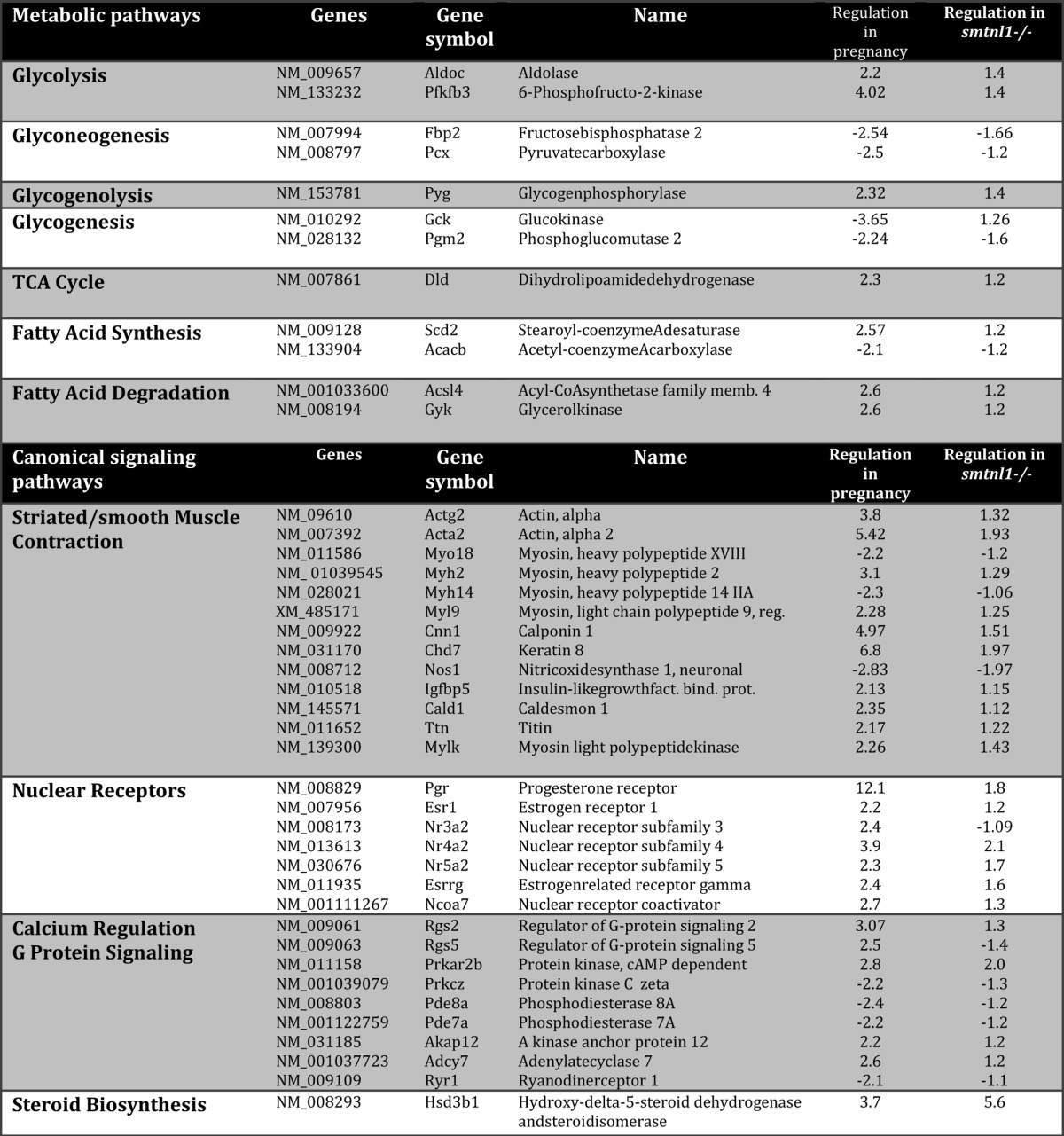
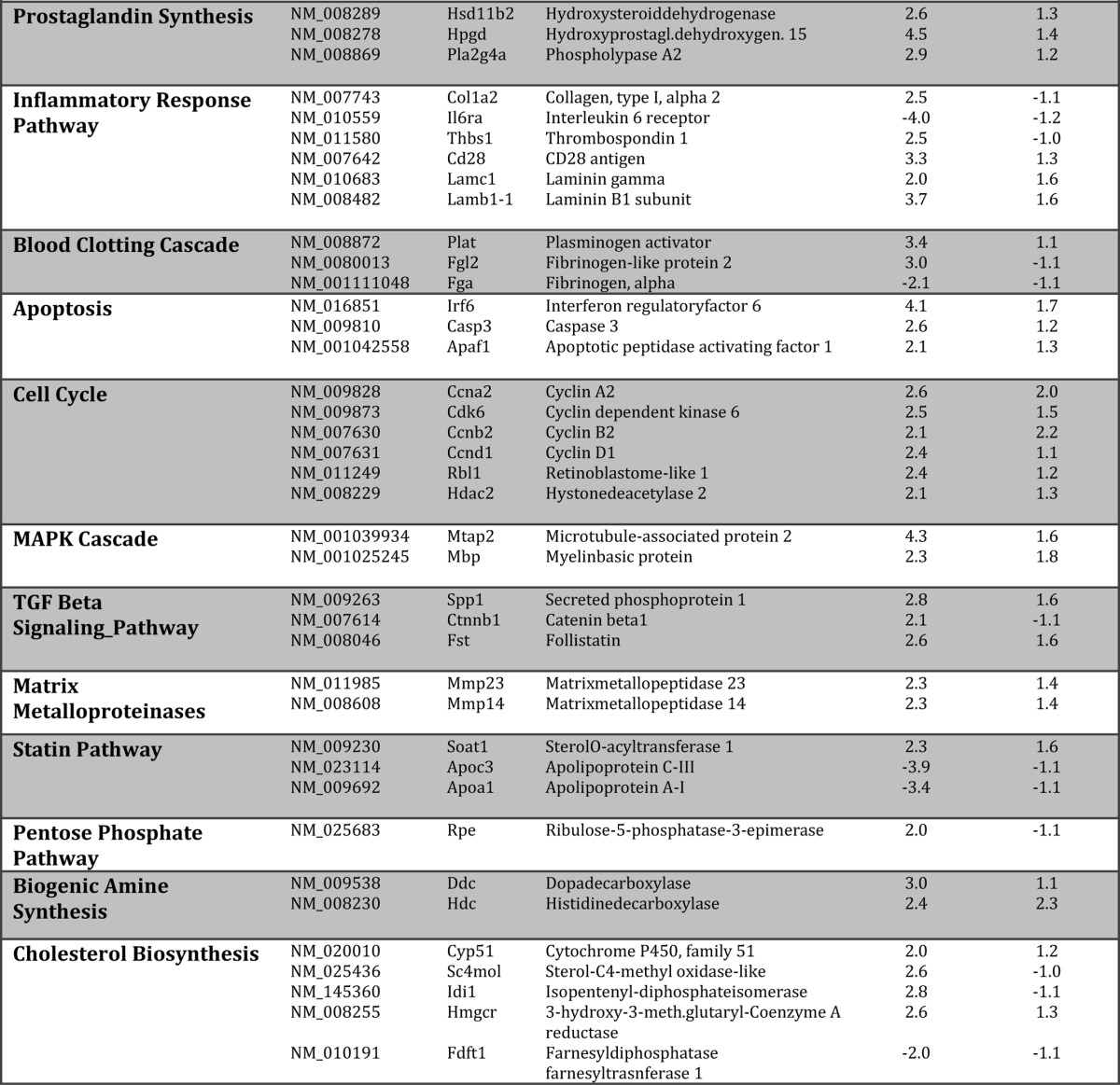
Given the observations that both SMTNL1 and pregnancy promote changes in the mean total fiber composition of SKM, we carried out global gene analysis to determine the extent of this switch in four experimental groups (smtnl1−/−, pregnant WT, and pregnant smtnl1−/− groups were compared with non-pregnant WT). A total of 1384 genes were differentially expressed between the groups. Specifically, the comparison of WT non-pregnant mice versus smtnl1−/− non-pregnant mice identified 195 genes, whereas the comparison of WT non-pregnant mice and WT pregnant mice resulted in 1261 genes expressed differently. The expression of 72 genes was related specifically to both pregnancy and SMTNL1 deletion (Fig. 2A). Complete lists of genes for all four comparisons are available at the Duke Microarray facility website. We also conducted Gene Ontology (GO) category enrichment analyses to determine the molecular function of GO terms (Fig. 2A). Based on their biological functions, these genes play a role in cytoskeleton organization, calcium binding, regulation of metabolic processes and steroid synthesis, immune function, and growth regulation. Fifty percent of the genes altered by SMTNL1 deletion were related to transcriptional regulators, and the remaining 50% included structural molecules (23%) and enzyme regulators (15%) (Fig. 2A). We performed hierarchical clustering on all differentially expressed genes using average linkage with Pearson's dissimilarity and present the number of induced and repressed genes in a heat map (Fig. 2B). Genes with significant changes in expression were assigned to different canonical pathways and subjected to GeneGo Analysis. Pregnancy was found to be largely related either to metabolism such as glycolysis-glyconeogenesis, fatty acid synthesis, or contractile proteins or signaling pathways involving prostaglandin and steroid synthesis and nuclear hormone receptor signaling (Table 1). SMTNL1 deletion caused mostly the up-regulation of signaling pathways of nuclear receptors and skeletal/smooth muscle contraction. The altered canonical pathways and the up- and down- regulated genes of signaling are listed in Table 1.
FIGURE 2.
The relationship between genes differentially regulated in the comparisons of pregnancy and SMTNL deletion and the ontology of the related genes. A, Venn diagram; the numbers within the intersections of the circles indicate the common genes between the different groups (NP, non-pregnant; PRE, pregnant) compared to the smtnl1+/+ non-pregnant data. The numbers outside the circles indicate the number of genes differentially regulated between the two groups as indicated. The molecular function of the pregnancy and smtnl1−/− deletion-related genes are represented. B, the color code for the signal strength is shown in the box at the bottom in which induced genes are indicated by red, and repressed genes are indicated by blue.
TABLE 1.
The involvement of pregnancy and SMTNL1 regulated genes in metabolic and canonical signaling pathways
Genes were analyzed using the GeneGo software for obtaining pathway maps, biological networks, and diseases relevant to the study.
Proteomic Analysis Confirms Switching of SKM to a Glycolytic Phenotype in Response to Pregnancy and SMTNL1 Deletion
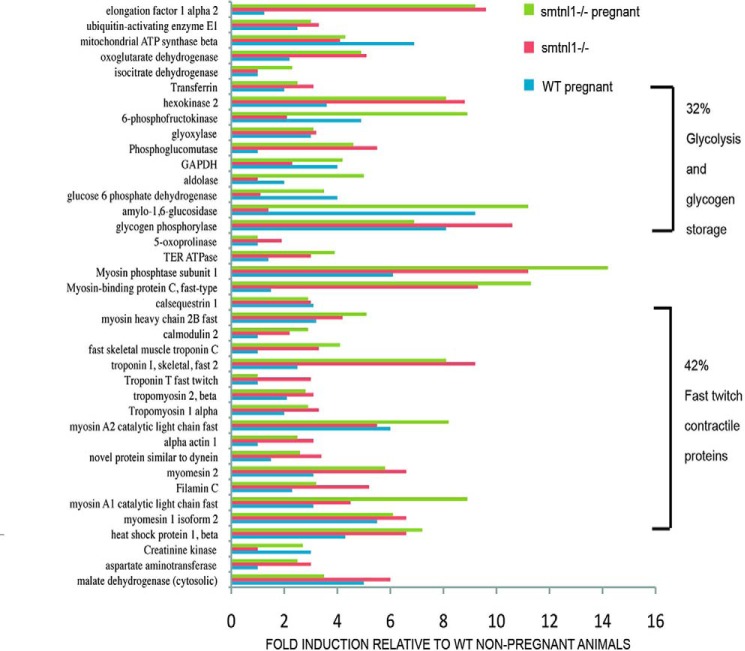
Multidimensional proteomic analysis showed that pregnancy promoted a striking and specific induction of several glycolytic enzymes and contractile proteins, the expression of which is specifically associated with type2b fibers (Fig. 3). In particular, 32% of the induced proteins are required for glycolysis (i.e. hexokinase, 6-phosphofructokinase, and aldolase), whereas others are associated with glycogen storage, e.g. glycogen phosphorylase and amylo-1,6-glucosidase. Additionally, ∼42% of the induced proteins were contractile proteins associated with fast twitch fibers such as MHC2b, fast twitch forms of troponins, fast twitch myosin light chains, and fast-type myosin-binding protein C, established markers of type2b fibers (supplemental Table S1 and Figshare/SMTNL1). Our proteomic data are consistent with the result of the microarray analysis and support the hypothesis that during pregnancy female mice adapt their skeletal muscle to a glycolytic phenotype. The finding that these pregnancy-induced adaptations are less emphasized in SMTNL1-deleted tissues suggests that the protein may regulate this process.
FIGURE 3.
Proteomic analysis shows that pregnancy and SMTNL1 deletion induces expression of glycolytic enzymes and contractile proteins associated with Type2b fibers. Plantaris muscle extracts were subjected to proteomic analysis prepared from smtnl1+/+ (WT), smtnl1+/+, pregnant (WP), smntl1−/− (KO), and smtnl1−/− pregnant (KOP) animals. The data show -fold induction of proteins in WP, KO, and KOP samples compared with WT. TER ATPase, transitional endoplasmic reticulum ATPase. See the related data in supplemental Table S1.
SMTNL1 Regulates the Expression of ERα and PR-B
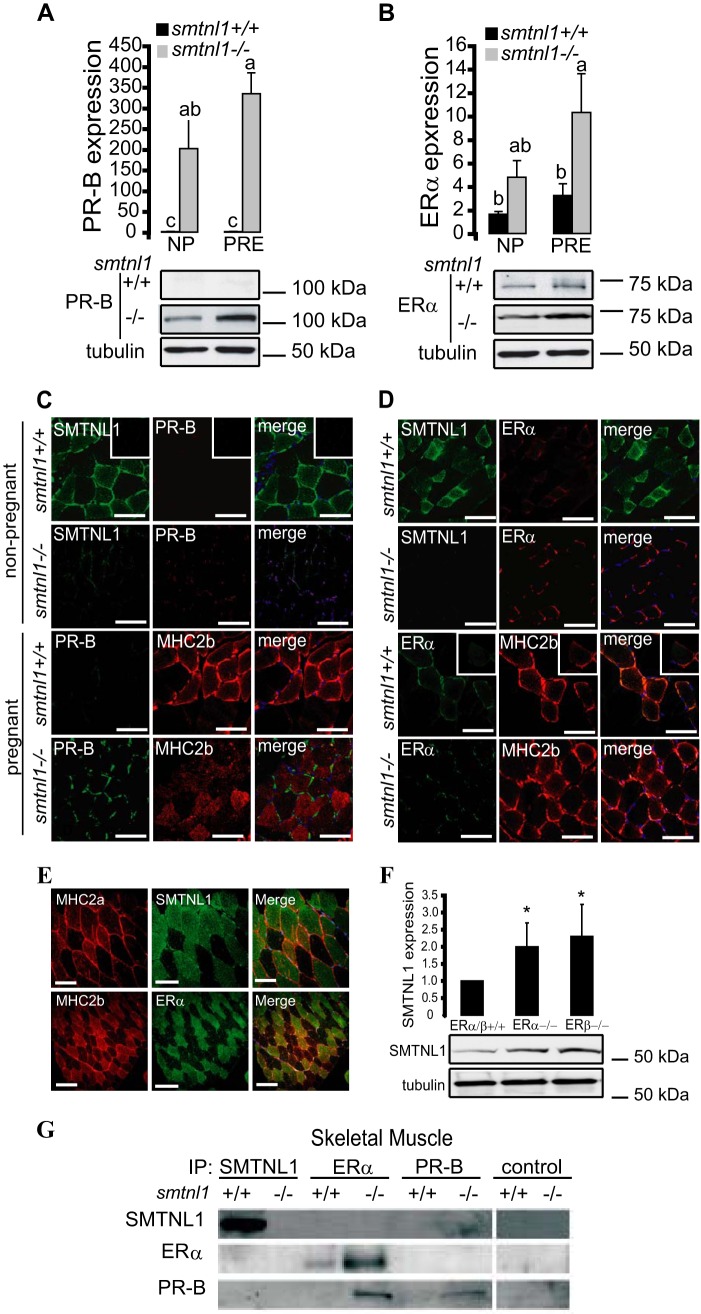
It has been established that SMTNL1 is expressed in smooth muscle as well as in reproductive tissues and that it is regulated through pregnancy (12). This observation supports links between the protein and steroid hormone action. Although both estrogen and progesterone clearly mediate adaptations in vascular and uterine smooth muscle, SKM is not generally recognized as a target of these hormones. We, therefore, examined PR and ER expression in SKM from smntl1−/− and WT mice. Western blot analysis of WT plantaris muscles showed that pregnancy induced a 1.5-fold increase in ERα expression by day 13. Remarkably, basal levels of ERα were induced 4–5-fold over WT levels in SKM isolated from non-pregnant smtnl1−/− and 10–15-fold over WT levels in pregnant smtnl1−/− mice (Fig. 4A). PR (A or B) was barely detectable in the muscle from either non-pregnant or pregnant WT mice using PR-B-specific antibody, but the PR-B isoform showed a dramatic increase in expression in SKM isolated from smtnl1−/− mice (Fig. 4A). In fiber typing experiments, PR-B expression was marginal in non-pregnant WT mice but showed discrete staining in all muscle fibers, including both type2a and type2b fibers in muscles isolated from pregnant animals (Fig. 4C). In contrast, ERα expression was confined only to type2b fibers in both non-pregnant and pregnant SKM (Fig. 4D). Notably, the expression of ERα was greatly enhanced in type2b fibers in muscles isolated from pregnant smtnl1−/− mice (Fig. 4D). A similar staining pattern was also observed in human SKM (Fig. 2E). Moreover, the deletion of either ERα or ERβ in mouse plantaris caused a significant increase in the protein expression level of SMTNL1 (Fig. 4F), suggesting the regulatory role of estrogen on SMTNL1 gene expression. The discrete expression of ERα in type2b fibers-only implies a role for estrogen in mediating the fiber switching process during pregnancy.
FIGURE 4.
The effects of pregnancy and SMTNL1 deletion on ER and PR expression in skeletal muscle. A and B, SMTNL1 deletion and pregnancy (PRE) induce expression of PR-B (A) and ERα (B) in SKM comparing to non-pregnant (NP) animals. Data are the mean ± S. E. for n = 4, GLM with Tukey's test; different letters indicate significant differences. p < 0.05. C, analysis of PR-B (red) in MHC2a (co-staining for SMTNL1 (green)) and MHC2b (red, staining for MHC2b) in plantaris. Scale bars = 20 μm. D, immunohistochemistry shows discrete expression of ERα (red) in type2b fibers in plantaris muscle. Scale bars = 20 μm. E, SMTNL1 and ERα localization in human skeletal muscle. Shown is localization of SMTNL1 (green) in type2a (red) and ERα (green) in type2b fibers (red) in human skeletal muscle by confocal microscopy. Scale bars represent 20 μm. F, SMTNL1 expression in plantaris of ERα−/− and ERβ−/− mice. Plantaris muscle of ERα/β+/+, ERα−/−, and ERβ−/− were lysed and analyzed by WB analysis using SMTNL1 antibody and anti-tubulin for loading control. Data were normalized, and values are relative changes compared with that obtained in ERα/β+/+. Data are the mean ± S.E., n = 4 each group. GLM: *, p > 0.05. G, SMTNL1 binds to PR-B but not to ERα in vivo in skeletal muscle. SMTNL, PR-B, and ERα were immunoprecipitated from skeletal muscle and uterine muscle (see positive control as shown in Ref. 12) extracts.
To investigate whether SMTNL1 mediates many of its cellular effects on skeletal muscle adaptation through direct interactions with either ER or PR, we carried out co-immunoprecipitation experiments from skeletal (Fig. 4G) muscle isolated from WT and smtnl1−/− mice. SMTNL1 co-precipitated with native PR-B but not ERα. In vivo interactions between these proteins were confirmed by repeating the IP experiments with antibodies to each of these proteins and Western blotting the IPs with anti-SMTNL1. Besides using SKM, we applied uterine smooth muscle extract as a positive control (data shown in our previous work in Bodoor et al. (12), as it expresses both ERα and PR under normal conditions). Smtnl1-deleted tissues did not present any interaction with SMTNL1 itself by IP. IP of PR-B or ERα from skeletal and uterine smooth muscle extracts prepared from smtnl1−/− mice showed an induction of both proteins. Similarly, although IP with ERα antibody demonstrated the presence of ERα and PR-B in the IP as expected, Western analysis with anti-SMTNL1 confirmed that it did not associate directly with ERα in vivo (Fig. 4G). Although our data do not support direct interactions of SMTNL1 with ERα, its increased expression observed after IP and Western analysis from smtnl1−/− mice may reflect that PR suppresses the expression of ERα at the transcriptional level. In accordance with this mechanism, it was reported that both ER and PR mutually regulate each others' expression through transcriptionally controlled feedback mechanisms (19).
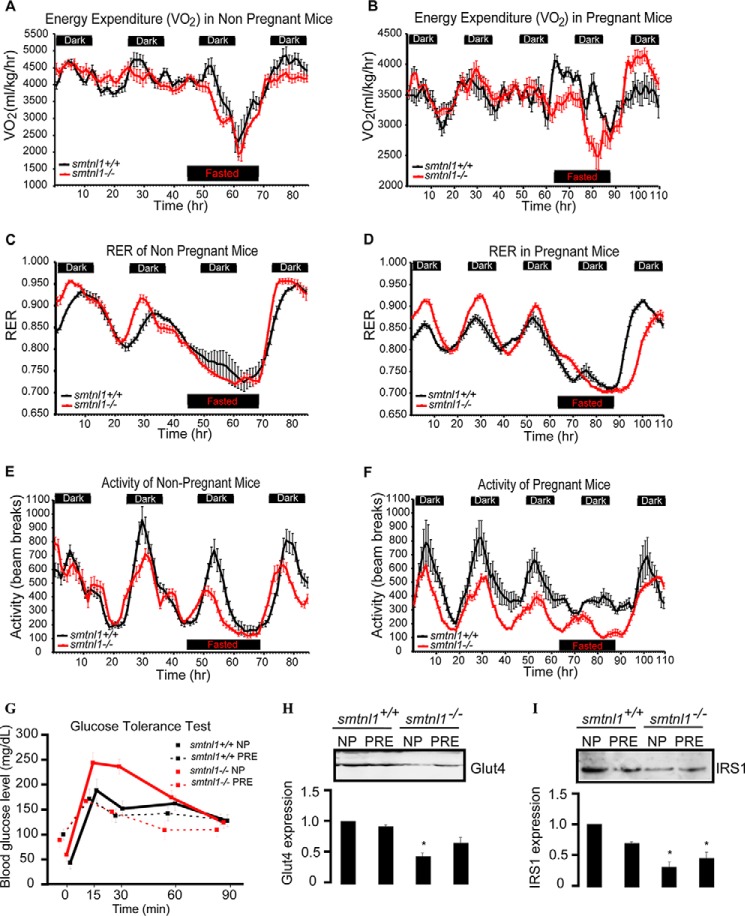
Pregnancy Induces Metabolic Activity and Reduces Oxygen Consumption
One objective of this study was to determine whether the effects of pregnancy and SMTNL1 deletion on SKM phenotype in mice resulted in any changes of energy expenditure and/or feeding behavior. Pregnant or non-pregnant WT and smtnl1−/− mice were housed in CLAMS apparatus, and metabolic measurements taken daily (Fig. 5, A–F). During the study there was no significant difference in food intake among the four experimental groups (pregnant and non-pregnant WT and smtnl1−/− mice; data not shown). Energy expenditure (VO2), or the amount of energy an individual uses daily to complete all regular body activities, of fed and fasted non-pregnant WT and smtnl1−/− mice was not significantly different among groups (Fig. 5, A and B). VO2 however, was significantly lower in the fed pregnant mice than in the fed non-pregnant mice. Interestingly, in fasted pregnant mice, both WT and smtnl1−/− animals showed a smaller decrease in VO2 than did those fed, suggesting an overall rescuing effect of pregnancy (Fig. 5, A and B). Normally, increases in the RER indicates increased utilization of carbohydrates for fuel. Conversely, decreased RER indicates a shift toward fatty acid utilization. The RER of both non-pregnant and pregnant smtnl1−/− mice was elevated in the fed state during the dark cycle (period of normal activity and feeding) compared with WT mice (Fig. 5, C and D), suggesting a hyper-metabolic shift toward carbohydrate utilization as fuel. RER was reduced in both the smtnl1−/− and WT pregnant animals compared to non-pregnant animals, demonstrating that the smtnl1−/− mice are able to efficiently shift substrate utilization (fats versus carbohydrates). RER data (Fig. 5, C and D) also revealed that there was an enhanced shift in substrate utilization in pregnant smtnl1−/− mice because the shift in metabolism between fatty acids and carbohydrates during the diurnal cycles was more pronounced in the smtnl1−/− pregnant mice. Non-pregnant smtnl1−/− mice showed a marked decrease in activity during the dark cycle compared with WT mice, and this difference was more pronounced in the pregnant pairings (Fig. 5, E and F). These findings suggest that although the smtnl1−/− mice can shift their metabolic pathways, their metabolism is less efficient than WT littermates as they preferentially utilize carbohydrates for fuel. This preference was enhanced when the smtnl1−/− mice become pregnant.
FIGURE 5.
Effect of pregnancy and SMTNL1 deletion on metabolic parameters of animals. A and B, energy expenditure in non-pregnant (A) and pregnant (B) mice. The volume of oxygen consumed, ml/kg/h (VO2) was measured over time. C and D, the RER is the respiratory quotient of the consumed oxygen and exhaled carbon dioxide (VO2/VCO2), was calculated versus time of non-pregnant (C) and pregnant (D) mice. Heat production was measured (kcal/h) in light and dark cycles. E and F, the activities of non-pregnant (E) and pregnant (F) animals were measured as the unit of beam breaks. G, changes in blood glucose levels (mean ± 1 S.E.) in smtnl1+/+ and -−/−, either pregnant or non-pregnant mice in increasing times followed by intraperitoneal glucose injection. H and I, expression of Glut4 (H) and IRS1 (I) in non-pregnant (NP) and pregnant (PRE) plantaris muscle. SMTNL1 deletion caused decreases in Glut4 and IRS1 expression and pregnancy partially rescued its effect. Protein expression levels were determined in non-pregnant and pregnant mice plantaris muscle by SQ-WB. n = 4–13/group. Data are presented as the mean ± S.E. Results are expressed as the means ± S.E. (n > 6 mice/group). #, p < 0.05; ***, p < 0.001 (repeated measures analysis of variance).
Effect of Pregnancy and SMTNL1 Deletion on Glucose Tolerance and on Insulin Signaling
To address the response to glucose administration, we performed an intraperitoneal glucose tolerance test. We analyzed data from the glucose tolerance test by repeated measures of analysis of variance. Pregnant mice had higher blood glucose levels at the start of the experiment (87.5 ± 29.95 mg/dl) than non-pregnant animals (57.0 ± 17.55 mg/dl, p = 0.007). Blood glucose levels increased sharply in smtnl1−/− non-pregnant mice, whereas the increase was more moderate in smtnl1−/− pregnant and WT mice (Fig. 5G). The interaction terms were significant both between genotype and time (F = 5.587, p < 0.001) and between status and time (F = 6.996, p < 0.001), confirming that the effect of time on blood glucose levels differed between individuals of different genotype and status. In the genotype-time interaction, the linear term was significant (F = 7.433, p = 0.014), indicating that the differences between KO and WT genotypes were similar at each measurement (Fig. 5G). WT pregnant mice also had slightly but not significantly lower blood glucose levels than WT non-pregnant mice between 15 and 60 min. These results show that smtnl1−/− non-pregnant mice have much lower glucose tolerance than smtnl1−/− pregnant or WT mice, indicating that pregnancy increases glucose tolerance in KO mice to the level of tolerance in WT mice. Impaired glucose tolerance is associated with pronounced insulin resistance and at cellular level occurs in tandem with changes in key steps in the insulin-signaling cascade that regulates glucose uptake by SKM. Insulin receptor substrate 1 (IRS1) and the insulin-sensitive glucose transporter 4 (Glut4) are believed to be key elements of insulin-signaling in SKM (20) To verify the importance of pregnancy and SMTNL1 deletion in the development of insulin resistance, SQ-WB analysis was conducted on non-pregnant and pregnant plantaris tissues of WT and smtnl1−/− mice, determining the protein expression of Glut4 and IRS1 (Fig. 5, H–I). SMTNL1 deletion decreased the expression of Glut4 and IRS1 by 53 and 58%, respectively. Pregnancy attenuated IRS1 expression significantly but had no effect on the Glut4 expression level in WT animals. However, pregnant smtnl1−/− animals exhibited an increase in both Glut4 and IRS1 expression in smtnl1−/− animals. Our results suggest that the impaired glucose tolerance of smtnl1−/− is the consequence of decreased Glut4 and IRS1 expression.
Discussion
Data presented herein suggest that pregnancy hormones such as progesterone and estrogen trigger the switching of SKM to a glycolytic phenotype through their respective nuclear receptors (Fig. 6). We hypothesize that SMTNL1 plays a negative regulatory role on the transcriptional activity of PR and ER in SKM and that protein kinase A- or G-mediated signaling can influence this process specifically through the phosphorylation of SMTNL1 at Ser-301 (11). Functionally, therefore, SMTNL1 acts as a transcriptional repressor of PR in vivo, and the phosphorylation of SMTNL1 specifically inhibits this function (12). Mechanistically this implies that the default function for SMTNL1 in non-pregnant animals is to repress PR function from promoting pregnancy-like adaptive responses in female SKM. Conversely, pregnancy (or adrenergic-induced signals) through Ser-301 phosphorylation relieves SMTNL1 inhibition of PR to promote appropriate changes in SKM gene expression resulting in switching to a more glycolytic phenotype. Intriguingly, protein kinase A-mediated signaling pathways have long been known to greatly affect both progesterone and estrogen signaling in vivo (21, 22). However, our study is the first to suggest that cyclic nucleotide-mediated signaling pathways in SKM may also be related to the regulation of gene expression by SMTNL1 acting through PR and ER.
FIGURE 6.
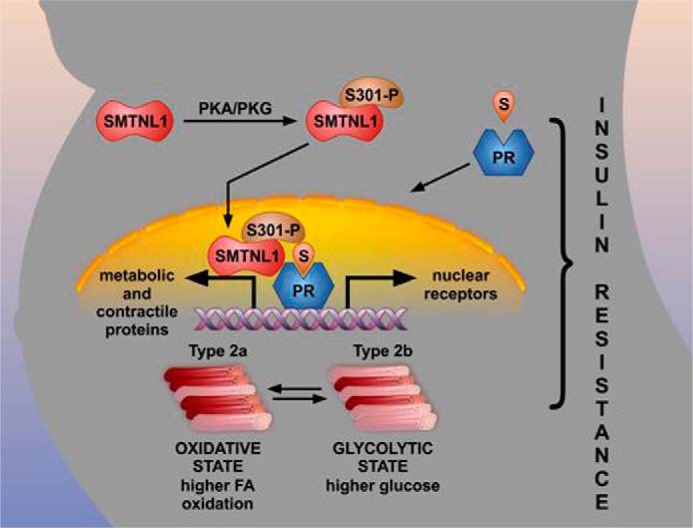
Schematic showing the molecular mechanism by which SMTNL1 regulates PR-B to coordinately promote skeletal muscle adaptations in response to pregnancy. In response to elevated steroid hormone, progesterone (S) levels SMTNL1 and PR-B enter the nucleus. In the non-pregnant state, SMTNL1 functions to repress PR-B activity; however, during pregnancy this inhibition is relieved and promotes activation of skeletal muscle-specific genes regulating the expression of metabolic and contractile proteins. This coordinated response adapts the mother's physiological state to support the weight gain originating from developing fetus. Switching to a glycolytic phenotype in skeletal muscle reduces the oxidative capacity of SKM, promoting increased storage of fatty acid (FA) and inducing an insulin-resistant state resulting in increased circulating glucose. PKA/PKG, protein kinase A/G.
The SMTNL1/PR-B Complex Presents a Homeostatic Feedback Mechanism Regulating the Expression of Each Other and of ERα
Expression studies in smtnl1−/− mice showed that PR-B and ER expression levels are strikingly regulated by intracellular levels of SMTNL1. Similarly, studies in ERα null mice showed that SMTNL1 expression was elevated in most tissues normally expressing the protein. Furthermore, SMTNL1 levels also closely followed progesterone levels throughout pregnancy. Together these findings suggest that SMTNL1 expression is closely linked to PR and ER and vice versa. The extent of SMTNL1 involvement in this feedback mechanism is most dramatic in smtnl1−/− mice with the observation that the expression of both PR and ER are greatly increased within specific muscle fibers in response to SMTNL1 deletion. The finding that ERα is specifically localized in type2b fibers suggests that these fibers are likely to be sensitive to estrogen when transitioned to glycolytic fibers. Interestingly, earlier work by others suggested that the expression of ER in SKM has a role in the development of muscle strength in humans (23, 24). Because prior work from our group showed that SMTNL1 only interacts with PR-B and-A and not with either isoform of ER (12), the altered ER expression observed in smtnl1−/− mice most likely must be mediated through PR via a homeostatic feedback mechanism (25), as PR and ER are known to mutually regulate the expression of each other. We, therefore, hypothesize that the differential localization and expression of SMTNL1, PR, and ER are key to the adaptation to pregnancy. We propose that under normal physiological conditions SMTNL1 is expressed in type2a fibers, where it represses the activity of PR. Because ER shows type2b-specific localization, this provides fiber-selective estrogen sensitivity. On one hand, circulating estrogen bound to ER might bind and repress the promoter of smtnl1, which provides an explanation to the low expression of SMTNL1 in type2b fibers in normal conditions and the increased SMTNL1 expression in ERα- or β-deleted animals (Fig. 4F). On the other hand, the increased number of type2b but a slight decreased number of 2a fibers detected in smtnl1−/− SKM could be explained by the release of PR repression by SMTNL1 in type2a fibers.
The Effects of Pregnancy on Metabolic Pathways Related to Insulin Resistance
Bioinformatics analysis of data from global gene expression and multidimensional proteomic analyses showed that pregnancy highly regulates sets of genes related to canonical pathways that play a role in glycolysis, gluconeogenesis, muscle contraction, and steroid biosynthesis as well as inflammatory responses, supporting our hypothesis of the manifestation of a glycolytic phenotype in pregnant SKM. The main profile of genes induced in smtnl1−/− deletion was transcriptional regulation, suggesting a primary role of SMTNL1 in gene expression as genes such as PR and nuclear receptor subfamily 3–4 are regulated. The majority of pathways regulated in pregnancy were related to the mediation of insulin resistance (26). During the progression of normal pregnancies, insulin resistance arises. Pronounced insulin resistance is associated with impaired glucose tolerance and with a high risk of gestational diabetes; however, the underlying mechanisms are not very well understood (27). Redundant control mechanisms operate to maintain normal glucose homeostasis in SKM. Insulin activates the insulin receptor tyrosine kinase, which activates signaling partners such as PKB/Akt and stimulates the translocation of GLUT4 vesicles to the plasma. IRS1 protein expression was increased in smtnl1−/− mice; moreover, pregnancy itself had a negative effect on IRS expression. Importantly, IRS expression is increased postpartum (20), and overexpression of the protein in a spontaneous gestational diabetes is associated with the reversal of insulin resistance (28). Pregnancy and SMTNL1 deletion are likely to regulate Glut4 expression through different pathways because smtnl1−/− tissues showed significantly lower levels of the Glut4 protein, an effect rescued by pregnancy. Our results showed that ER and PR expression levels had a robust increase in pregnant and smtnl1−/− tissues. Moreover, ER expression was related to a more efficient metabolic state, and ER KO animals presented overt insulin resistance (29). Although these findings strongly suggest a better metabolic status of SMTNL1 knock-out mice, we found impaired metabolism in smtnl1−/− mice. This controversy may be explained by the observation that circulating hormone levels influence the improvement of insulin resistance (26) and that the levels of circulating progesterone and estrogen decreased significantly during pregnancy (12).
How Might Pregnancy-induced Glycolytic Fiber Switching in Skeletal Muscle Contribute to Insulin Resistance
In Fig. 6 we suggest that pregnancy-induced fiber switching to a more glycolytic phenotype is a coordinated physiological response that may be at the heart of increased weight gain and generalized insulin resistance associated with normal pregnancy (Fig. 6). By virtue of its mass, SKM dictates the overall caloric disposal of both glucose and free fatty acids. If the oxidative capacity of SKM is increased through endurance exercise, one reduces the risk of developing insulin resistance, and exercise alone can reverse the symptoms of obesity-induced type II diabetes. This is because the primary sites of free fatty acid oxidation in mammals are oxidative type I and Type2a SKM fibers. Conversely, if the type2a content of SKM is reduced, the ability to oxidize free fatty acid is also reduced, thereby increasing storage of fatty acid (FA) as triglyceride in adipose tissue. When oxidative capacity is reduced, the storage of FA as triglyceride is further exacerbated in the presence of high carbohydrate levels. This is because humans and most mammals derive much of their stored triglyceride via pathways of de novo FA synthesis through the metabolism of glucose in adipose tissue and liver (30). Excess fat and carbohydrate in the diet, therefore, provides an optimal environment for excessive weight gain, especially in sedentary individuals. We hypothesize that switching to a glycolytic phenotype in pregnancy, with a reduced oxidative fiber content of SKM, therefore, provides a molecular basis to explain increased weight gain and insulin resistance in pregnant females. We suggest that this is a normal response to pregnancy and infers the evolutionary advantage of enabling the mother to increase circulating glucose levels for proper fetal development as well as store fat more efficiently to meet the caloric demands of lactation. Our results also suggest that switching to a glycolytic phenotype infers an advantage by increasing the mother's physical strength. Type2b muscle fibers are associated with strength and resistance training such as seen with weight lifting. An increase in the mother's physical strength would much better enable her to manage the increased weight of the fetus in the latter stages of pregnancy. Importantly, in ∼4% of all pregnancies, pregnancy-induced insulin resistance progresses to gestational diabetes. If pregnancy, therefore, promotes the transition to a more glycolytic phenotype as an evolutionary benefit, then this may render some women even more susceptible to developing gestational diabetes, especially under the influence of high energy Western diets.
Finally, considering all our understanding of muscle physiology and its importance to health and well being, one might ask why pregnancy-induced fiber switching has not already been reported in the literature. A search of the current literature suggests that this may be because the majority of muscle studies to date have been carried out in males only. The experimental bias of studies toward male over female physiology is likely attributable to the commonly held belief that the estrous cycle introduces hard-to-explain variability in physiological processes in female animals. Indeed, in studies of female physiology, ovariectomized females are typically used to remove the oestrus-related aspects of physiology from the inquiry. As a result, many normal physiological responses relevant to female physiology get purposefully ignored. Although further investigation is certainly warranted, particularly non-invasive studies of SKM in pregnant women, our findings could indicate that important aspects of women's health are being missed due to an experimental bias.
Author Contributions
B. L. designed, performed, and analyzed the experiments shown in Figs. 1 and in part in Figs. 2, 3, 4, and 6 and wrote the paper. K. B. analyzed the experiments in Fig. 2. S. A. performed the experiments in Figs. 1 and 5. D. H. W. assisted in the CLAMS study. D. L. performed the proteomic analysis. D. P. M. and R. S. helped in ER and PR studies. D. Z. provided MHC-specific antibodies. J. D. and R. C. H. provided human biopsies and performed the human study. T. R. performed CLAMS experiments. T. A. H. conceived and coordinated the study and wrote the paper.
Supplementary Material
Acknowledgments
We are grateful to Dr. Ferenc Erdődi (University of Debrecen, Debrecen, Hungary) for revising the paper and Dr. Kennet Korach (Reproductive and Developmental Toxicology Laboratory, Research Triangle Park, NC) for providing the ERKO mice as well as to Dr. Szabolcs Lengyel (Hungarian National Academy of Science, Ecology Research Center, Debrecen, Hungary) for helping in statistical analyses.
Note Added in Proof
Supplemental Table S1 was missing in the version of this article that was published as a Paper in Press on April 14, 2015. The supplemental table is now available.
This work was supported, in whole or in part, by National Institutes of Health Grant R01DK065954-05 (to T. A. H.). This work was also supported by Grants OTKA PD107898, TÁMOP-4.2.2.A-11/1/KONV-2012-0025, and TÁMOP 4.2.4.A/2-11-1-2012-0001, the University of Debrecen (RH/751/2015) (to B. L.), and by The King Hussein fellowship (to K. B.). The authors declare that they have no conflicts of interest with the contents of this article.

This article contains supplemental Table 1.
- SKM
- skeletal muscle
- smoothelin-like protein 1
- SMTNL1
- NP
- non-pregnant
- PR
- progesterone receptor
- ERα/β
- estrogen receptor α/β
- IHC
- immunohistochemistry
- SQ-WB
- semi quantitative Western blot analysis
- MHC1
- -2a, -2B, myosin heavy chain 1, 2a, 2b
- IRS
- insulin receptor substrate
- CLAMS
- comprehensive laboratory animal monitoring system
- GLM
- general linear models
- RER
- respiratory exchange ratio
- Glut4
- glucose transporter 4
- IRS1
- insulin receptor substrate 1
- IP
- immunoprecipitation
- WN
- WT non-pregnant
- KN
- KO-non-pregnant
- KP
- KO-pregnant.
References
- 1. Halayko A. J., Solway J. (2001) Molecular mechanisms of phenotypic plasticity in smooth muscle cells. J. Appl Physiol. 90, 358–368 [DOI] [PubMed] [Google Scholar]
- 2. Booth F. W., Thomason D. B. (1991) Molecular and cellular adaptation of muscle in response to exercise: perspectives of various models. Physiol. Rev. 71, 541–585 [DOI] [PubMed] [Google Scholar]
- 3. Fitts R. H. (2003) Effects of regular exercise training on skeletal muscle contractile function. Am. J. Phys. Med. Rehabil. 82, 320–331 [DOI] [PubMed] [Google Scholar]
- 4. Carey A. L., Kingwell B. A. (2009) Novel pharmacological approaches to combat obesity and insulin resistance: targeting skeletal muscle with “exercise mimetics.” Diabetologia 52, 2015–2026 [DOI] [PubMed] [Google Scholar]
- 5. Hamilton M. T., Booth F. W. (2000) Skeletal muscle adaptation to exercise: a century of progress. J. Appl. Physiol. 88, 327–331 [DOI] [PubMed] [Google Scholar]
- 6. Edwards D. P. (2005) Regulation of signal transduction pathways by estrogen and progesterone. Annu. Rev. Physiol. 67, 335–376 [DOI] [PubMed] [Google Scholar]
- 7. Barros R. P., Morani A., Moriscot A., Machado U. F. (2008) Insulin resistance of pregnancy involves estrogen-induced repression of muscle GLUT4. Mol. Cell. Endocrinol. 295, 24–31 [DOI] [PubMed] [Google Scholar]
- 8. Sugaya A., Sugiyama T., Yanase S., Shen X. X., Minoura H., Toyoda N. (2000) Expression of glucose transporter 4 mRNA in adipose tissue and skeletal muscle of ovariectomized rats treated with sex steroid hormones. Life Sci. 66, 641–648 [DOI] [PubMed] [Google Scholar]
- 9. Brănişteanu D. D., Mathieu C. (2003) Progesterone in gestational diabetes mellitus: guilty or not guilty? Trends Endocrinol. Metab. 14, 54–56 [DOI] [PubMed] [Google Scholar]
- 10. Wooldridge A. A., Fortner C. N., Lontay B., Akimoto T., Neppl R. L., Facemire C., Datto M. B., Kwon A., McCook E., Li P., Wang S., Thresher R. J., Miller S. E., Perriard J. C., Gavin T. P., Hickner R. C., Coffman T. M., Somlyo A. V., Yan Z., Haystead T. A. (2008) Deletion of the protein kinase A/protein kinase G target SMTNL1 promotes an exercise-adapted phenotype in vascular smooth muscle. J. Biol. Chem. 283, 11850–11859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lontay B., Bodoor K., Weitzel D. H., Loiselle D., Fortner C., Lengyel S., Zheng D., Devente J., Hickner R., Haystead T. A. (2010) Smoothelin-like 1 protein regulates myosin phosphatase-targeting subunit 1 expression during sexual development and pregnancy. J. Biol. Chem. 285, 29357–29366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bodoor K., Lontay B., Safi R., Weitzel D. H., Loiselle D., Wei Z., Lengyel S., McDonnell D. P., Haystead T. A. (2011) Smoothelin-like 1 protein is a bifunctional regulator of the progesterone receptor during pregnancy. J. Biol. Chem. 286, 31839–31851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ulke-Lemée A., Turner S. R., Mughal S. H., Borman M. A., Winkfein R. J., MacDonald J. A. (2011) Mapping and functional characterization of the murine smoothelin-like 1 promoter. BMC Mol. Biol. 12, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Akimoto T., Ribar T. J., Williams R. S., Yan Z. (2004) Skeletal muscle adaptation in response to voluntary running in Ca2+/calmodulin-dependent protein kinase IV-deficient mice. Am. J. Physiol. Cell Physiol. 287, C1311–C1319 [DOI] [PubMed] [Google Scholar]
- 15. Lontay B., Serfozo Z., Gergely P., Ito M., Hartshorne D. J., Erdodi F. (2004) Localization of myosin phosphatase target subunit 1 in rat brain and in primary cultures of neuronal cells. J. Comp. Neurol. 478, 72–87 [DOI] [PubMed] [Google Scholar]
- 16. Borman M. A., MacDonald J. A., Haystead T. A. (2004) Modulation of smooth muscle contractility by CHASM, a novel member of the smoothelin family of proteins. FEBS Lett. 573, 207–213 [DOI] [PubMed] [Google Scholar]
- 17. Schmidt C., Urlaub H. (2009) iTRAQ-labeling of in-gel digested proteins for relative quantification. Methods Mol. Biol. 564, 207–226 [DOI] [PubMed] [Google Scholar]
- 18. Shadforth I. P., Dunkley T. P., Lilley K. S., Bessant C. (2005) i-Tracker: for quantitative proteomics using iTRAQ. BMC Genomics 6, 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Clarke C. L., Sutherland R. L. (1990) Progestin regulation of cellular proliferation. Endocr. Rev. 11, 266–301 [DOI] [PubMed] [Google Scholar]
- 20. Kirwan J. P., Varastehpour A., Jing M., Presley L., Shao J., Friedman J. E., Catalano P. M. (2004) Reversal of insulin resistance postpartum is linked to enhanced skeletal muscle insulin signaling. J. Clin. Endocrinol. Metab. 89, 4678–4684 [DOI] [PubMed] [Google Scholar]
- 21. Coleman K. M., Dutertre M., El-Gharbawy A., Rowan B. G., Weigel N. L., Smith C. L. (2003) Mechanistic differences in the activation of estrogen receptor-α (ERα)- and ERβ-dependent gene expression by cAMP signaling pathway (s). J. Biol. Chem. 278, 12834–12845 [DOI] [PubMed] [Google Scholar]
- 22. Rowan B. G., Garrison N., Weigel N. L., O'Malley B. W. (2000) 8-Bromo-cyclic AMP induces phosphorylation of two sites in SRC-1 that facilitate ligand-independent activation of the chicken progesterone receptor and are critical for functional cooperation between SRC-1 and CREB binding protein. Mol. Cell. Biol. 20, 8720–8730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Onambele N. G., Skelton D. A., Bruce S. A., Woledge R. C. (2001) Follow-up study of the benefits of hormone replacement therapy on isometric muscle strength of adductor pollicis in postmenopausal women. Clin. Sci. 100, 421–422 [PubMed] [Google Scholar]
- 24. Skelton D. A., Phillips S. K., Bruce S. A., Naylor C. H., Woledge R. C. (1999) Hormone replacement therapy increases isometric muscle strength of adductor pollicis in post-menopausal women. Clin. Sci. 96, 357–364 [PubMed] [Google Scholar]
- 25. Bradshaw M. S., Tsai S. Y., Leng X. H., Dobson A. D., Conneely O. M., O'Malley B. W., Tsai M. J. (1991) Studies on the mechanism of functional cooperativity between progesterone and estrogen receptors. J. Biol. Chem. 266, 16684–16690 [PubMed] [Google Scholar]
- 26. Catalano P. M. (2010) Obesity, insulin resistance, and pregnancy outcome. Reproduction 140, 365–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Solomon C. G., Seely E. W. (2001) Brief review: hypertension in pregnancy: a manifestation of the insulin resistance syndrome? Hypertension 37, 232–239 [DOI] [PubMed] [Google Scholar]
- 28. Shao J., Yamashita H., Qiao L., Draznin B., Friedman J. E. (2002) Phosphatidylinositol 3-kinase redistribution is associated with skeletal muscle insulin resistance in gestational diabetes mellitus. Diabetes 51, 19–29 [DOI] [PubMed] [Google Scholar]
- 29. Bryzgalova G., Gao H., Ahren B., Zierath J. R., Galuska D., Steiler T. L., Dahlman-Wright K., Nilsson S., Gustafsson J. A., Efendic S., Khan A. (2006) Evidence that oestrogen receptor-α plays an important role in the regulation of glucose homeostasis in mice: insulin sensitivity in the liver. Diabetologia 49, 588–597 [DOI] [PubMed] [Google Scholar]
- 30. Towler M. C., Hardie D. G. (2007) AMP-activated protein kinase in metabolic control and insulin signaling. Circ. Res. 100, 328–341 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.