Background: Connecdenns are GEFs for Rab35, a GTPase controlling endocytic recycling. Regulation of connecdenn function is unknown.
Results: The GEF activity of connecdenn 1 and 2 are autoinhibited, and this is regulated by Akt activation downstream of insulin stimulation.
Conclusion: Phosphorylation of connecdenn by Akt may provide a mechanism to control cargo recycling.
Significance: Signaling pathways impinge on membrane-trafficking pathways.
Keywords: cell biology, endocytosis, endosome, guanine nucleotide exchange factor (GEF), intracellular trafficking, membrane trafficking, Rab, DENN domain, Rab35, connecdenn
Abstract
Connecdenn 1/2 are DENN (differentially expressed in normal and neoplastic cells) domain-bearing proteins that function as GEFs (guanine nucleotide exchange factors) for the small GTPase Rab35. Disruption of connecdenn/Rab35 function leads to defects in the recycling of multiple cargo proteins from endosomes with altered cell function, yet the regulation of connecdenn GEF activity is unexplored. We now demonstrate that connecdenn 1/2 are autoinhibited such that the purified, full-length proteins have significantly less Rab35 binding and GEF activity than the isolated DENN domain. Both proteins are phosphorylated with prominent phosphorylation sites between residues 500 and 600 of connecdenn 1. A large scale proteomics screen revealed that connecdenn 1 is phosphorylated at residues Ser-536 and Ser-538 in an Akt-dependent manner in response to insulin stimulation of adipocytes. Interestingly, we find that an Akt inhibitor reduces connecdenn 1 interaction with Rab35 after insulin treatment of adipocytes. Remarkably, a peptide flanking Ser-536/Ser-538 binds the DENN domain of connecdenn 1, whereas a phosphomimetic peptide does not. Moreover, connecdenn 1 interacts with 14-3-3 proteins, and this interaction is also disrupted by Akt inhibition and by mutation of Ser-536/Ser-538. We propose that Akt phosphorylation of connecdenn 1 downstream of insulin activation regulates connecdenn 1 function through an intramolecular interaction.
Introduction
The Rab superfamily of small GTPases controls essentially every step of vesicle trafficking from vesicle formation to vesicle transport and, eventually, docking and fusion with the target membrane (1). Rab proteins regulate such a large range of functions by recruiting unique sets of effector proteins that mediate their downstream activities. Rabs cycle between active, GTP-bound, and inactive, GDP-bound forms, and in the active form they engage their effectors. Guanine nucleotide exchange factors (GEFs)6 activate Rabs by catalyzing the removal of GDP, allowing for exchange with GTP, and GTPase activating proteins (GAPs) turn off Rabs by enhancing their intrinsic rate of GTP hydrolysis (1).
Although GEFs and GAPs catalyze the nucleotide switch, additional control mechanisms are required to create the precise spatial and temporal activation that is characteristic of Rabs and other small GTPases. A key mechanism is modification of the catalytic activity of GEF and GAP proteins (2). The catalytic activity of many GEFs and GAPs is inhibited by intramolecular interactions that create steric hindrance and obstruct the substrate binding site, resulting in inefficient function of the catalytic domain (2). This autoinhibition has been documented in multiple GEFs including TIM, a GEF for RhoA (3), and intersectin, a GEF for Cdc42 (4). GEF proteins achieve full catalytic activity when the autoinhibition is relieved, which can be accomplished through multiple mechanisms. For example, phosphorylation of TIM releases the intramolecular interaction and leads to an increase in the nucleotide exchange rate (3). Phosphorylation can also have the opposite effect and reduce the GEF activity as observed in Dock6, a GEF for Rac1 and Cdc42 (5). Interaction with a binding partner can also lead to a change in GEF activity (2). For instance, the autoinhibition of intersectin is released when it interacts with its binding partner, neuronal Wiskott-Aldrich Syndrome protein (4). Recently, interaction with the 14-3-3 family of proteins has been implicated in regulation of GEF activity (6, 7). The majority of 14-3-3 interactions are phospho-dependent, and the 14-3-3 binding motifs contain either a phosphoserine or a phosphothreonine residue (8). A GEF protein is first phosphorylated and subsequently interacts with 14-3-3, thus creating a multipart control system. An example is seen in the regulation of β1 Pix, where PKA phosphorylates β1 Pix at two sites allowing 14-3-3 to bind, leading to reduced GEF activity (6).
GEF and GAP proteins serve as ideal loci for signaling cascades to impinge on membrane trafficking. For example, the GAP activity of AS160/TBC1D4, a GAP for Rab10, is regulated in response to insulin signaling (9). Insulin signaling activates Akt, which phosphorylates AS160 at four different residues blocking the enzymatic activity of AS160 (9). Inactivation of AS160 is required for the Rab10-dependent transport and plasma membrane fusion of intracellular storage vesicles that contain glucose transporter 4 (GLUT4), thus placing GLUT4 on the surface to mediate glucose uptake. Akt phosphorylation creates two 14-3-3 binding sites in AS160, and the interaction with 14-3-3 is required for inactivation of AS160 (10). Overall, by modifying the GAP activity of AS160, insulin signaling regulates membrane trafficking events.
Proteins bearing a DENN (differentially expressed in normal and neoplastic cells) domain form the largest family of Rab GEFs with 26 members identified to date (11–13). The GEF activity resides in the highly conserved DENN domain, and several DENN proteins have been paired with their target Rabs (14–16). The connecdenn (also known as DENND1) subfamily contains three members, connecdenn 1–3 (DENND1A-C) that all have enzymatic activity toward Rab35 (15, 17). The regions outside the DENN domain share low homology and contain various motifs for protein interactions (14, 17). In this study we demonstrate that connecdenn 1 and 2 are inhibited by an intramolecular interaction and that in connecdenn 1 this mechanism of regulation is controlled by Akt signaling downstream of insulin activation in adipocytes.
Experimental Procedures
Antibodies and Constructs
Monoclonal (M2) FLAG antibody was purchased from Sigma. Rat monoclonal HSC70 antibody was purchased from Enzo Life Science. Rabbit polyclonal phospho-Akt (Ser-473) antibody was from Cell Signaling Technology, and polyclonal Akt antibody was from New England Biolabs. Affinity-purified connecdenn 1 antibody was produced in-house as previously described (18). GST-14-3-3ϵ, wild-type, and K49E mutant (rat amino acids 1–255 in pGEX-6P1) were generous gifts from Dr. Philippe P. Roux, Université de Montréal. GST-Rab35 (human amino acids 1–201 in pGEX-6P1), GST-Rab35-S22N (human amino acids 1–201 in pGEX-6P1), FLAG-connecdenn 1 DENN domain (mouse amino acids 1–403 in pcDNA3-FLAG), FLAG-connecdenn 2 DENN domain (human amino acids 2–421 in pCMV-Tag2B), FLAG-connecdenn 1 full-length (mouse amino acids 2–1016 in pCMV-Tag2B), and FLAG-connecdenn 2 full-length (human amino acids 2–775 in pcDNA3-FLAG) were previously described (17, 18). FLAG-connecdenn 1 residues 1–500, FLAG-connecdenn 1 residues 1–600, FLAG-connecdenn 1 residues 1–800, and FLAG-connecdenn 1 residues 375–1016 were cloned by PCR in pCMV-Tag2B using FLAG-connecdenn 1 full-length as a PCR template. FLAG-connecdenn 1 S536E/S538E was generated using the QuikChange II site-directed mutagenesis kit from Agilent Technologies. Maltose-binding protein (MBP) peptides encoding residues 527–547 of connecdenn 1, wild-type, or S536E/S538E mutant were generated by oligo annealing into the pMal-c2X vector. The oligos encode a stop codon leading to MBP-peptides, whereas the control vector generates a MBP-lacZ fusion protein. All constructs were verified by sequence analysis. For the sequences of the oligonucleotides used to generate the constructs, see Table 1.
TABLE 1.
Oligonucleotides for production of connecdenn 1 constructs
CD 1, connecdenn 1; s, sense; as, antisense; FL, full-length.
| CD 1–01-s-BglII | GCG AGA TCT GGC TCC AGG ATC AAG CAA AAC |
| CD 1–500-as-XhoI | GCG CTC GAG TCA GAT TGG TCT CCG GTC TTC TCG |
| CD 1–600-as-XhoI | GCG CTC GAG TCA TTT GGG GGC CCG GAG ATC C |
| CD 1–800-as-XhoI | GCG CTC GAG TCA GCC TGA CCA GGC TGT GTT AAG |
| CD 1–1016-as-XhoI | GCG CTC GAG TCA CTC AAA GGT CTC CCA CTG |
| CD 1–377-s-BglII | GCG AGA TCT TCC GGT GAA GGT TTC AGC GAT G |
| CD 1-S536E/S538E-s | CGG ACA TTG AAA GAA GAA GAC GAG GCA GAA GGT GAT GAG |
| CD 1-FL-S536E/S538E-as | CTC ATC ACC TTC TGC CTC GTC TTC TTC TTT CAA TGT CCG |
| CD 1 527–547 WT-s | GATCC CCG CAG CCG TAT CGC ACC CTG AAA GAA AGC GAT AGC GCG GAA GGC GAT GAA ACC GAA AGC CCG TAA C |
| CD 1 527–547 WT-as | TCGAG TTA CGG GCT TTC GGT TTC ATC GCC TTC CGC GCT ATC GCT TTC TTT CAG GGT GCG ATA CGG CTG CGG G |
| CD 1 527–547 S536E/S538E-s | GATCC CCG CAG CCG TAT CGC ACC CTG AAA GAA GAA GAT GAA GCG GAA GGC GAT GAA ACC GAA AGC CCG TAA C |
| CD 1 527–547 S536E/S538E-as | TCGAG TTA CGG GCT TTC GGT TTC ATC GCC TTC CGC TTC ATC TTC TTC TTT CAG GGT GCG ATA CGG CTG CGG G |
In Vitro GDP/GTP Exchange Assays
GST-tagged Rab35 was expressed in Escherichia coli BL21. Pelleted bacteria were resuspended in phosphate-buffered saline (PBS) supplemented with protease inhibitors (0.83 mm benzamidine, 0.23 mm phenylmethylsulfonyl fluoride, 0.5 μg/ml aprotinin, and 0.5 μg/ml leupeptin) and sonicated. Triton X-100 was added to 1% final concentration, and samples were incubated for 30 min at 4 °C. Lysates were centrifuged at 30,700 × g for 15 min; the supernatant was then incubated with glutathione-Sepharose beads for 1 h at 4 °C. The beads were washed 3 times with PreScission protease cleavage buffer (20 mm Tris, 150 mm NaCl, 1 mm dithiothreitol, 1 mm EDTA, pH 7.0), and the purified fusion proteins were cleaved from the GST tag by overnight incubation with PreScission protease (GE Healthcare) at 4 °C. Cleaved Rab35 was then exchanged into GEF loading buffer (20 mm Tris, pH 7.5, 100 mm NaCl); samples were snap-frozen in liquid nitrogen and stored at −80 °C. All FLAG-tagged connecdenn constructs were expressed in HEK-293 cells. At 48 h post-transfection, cells were collected in 20 mm HEPES supplemented with protease inhibitors and sonicated. Triton X-100 was added to 1% final concentration, and the lysates were incubated for 30 min at 4 °C. Lysates were then centrifuged at 21,000 × g for 15 min, and the supernatants were incubated with 12.5 μl of protein G-Sepharose and 5 μg of monoclonal FLAG (M2) antibody for 3 h at 4 °C. Beads were washed in GEF incubation buffer (20 mm Tris, pH 7.5, 100 mm NaCl, and 5 mm MgCl2). Each immunoprecipitation was performed in duplicate; one sample was immediately added to the in vitro GDP/GTP exchange assays, whereas the second sample was resolved by SDS-PAGE and processed for Western blot.
For the GDP/GTP exchange assays, first, a 15 μm concentration of purified Rab35 was loaded with 30 μm GDP (Sigma) by incubation for 10 min at 30 °C in GEF loading buffer with 5 mm EDTA. To stabilize the loaded GDP, 10 mm MgCl2 was added, and samples were incubated for 10 min at 30 °C. Exchange reactions were carried out at room temperature in 90 μl of total volume containing 1.25 μm preloaded GTPase, immunoprecipitated GEFs, 0.5 mg/ml bovine serum albumin, 5 μm GTPγS (PerkinElmer Life Sciences), 0.2 mCi/mmol [35S]GTPγS (PerkinElmer Life Sciences), and 0.5 mm dithiothreitol in GEF incubation buffer. At the indicated time points, 15 μl of the reaction was removed, added to 1 ml of ice-cold wash buffer (20 mm Tris, pH 7.5, 100 mm NaCl, 20 mm MgCl2), and passed through nitrocellulose filters. The filters were washed with 5 ml of wash buffer and counted using a liquid scintillation counter (Beckman Coulter LS6500 scintillation counter).
Connecdenn Phosphorylation Assays
FLAG-tagged full-length connecdenn 1 and 2 or various deletion constructs of connecdenn 1 were expressed in HEK-293 cells. At 18 h post-transfection, cells were treated with okadaic acid (OA), Na3VO4, or DMSO. After 2 h cells were collected in lysis buffer (20 mm HEPES, 150 mm NaCl, 1 mm dithiothreitol, 1% Triton X-100, pH 7.4) supplemented with protease inhibitors and phosphatase inhibitors (5 mm sodium pyrophosphate, 500 nm OA, 1 mm Na3VO4, 10 mm NaF). The lysates were incubated for 15 min at 4 °C and centrifuged at 21,000 × g for 15 min. The supernatants were resolved by SDS-PAGE and processed for Western blotting. For autoradiography experiments, FLAG-tagged connecdenn 1 or 2 full-length constructs were expressed in HEK-293. At 18 h post-transfection, regular Dulbecco's modified Eagle's medium (DMEM) was replaced by phosphate-free DMEM, and 0.125 μCi of label-free [32]Pi (PerkinElmer Life Sciences) was added to each 15-cm plate of cells. After 2 h, cells were stimulated with either 250 nm OA or DMSO for 2 h. Cells were collected in lysis buffer supplemented with protease and phosphatase inhibitors. The lysates were incubated for 15 min at 4 °C and centrifuged at 234,000 × g for 15 min. The supernatants were incubated with 12.5 μl of Protein G-Sepharose (GE Healthcare) and 5 μg of monoclonal FLAG (M2) antibody for 1 h at 4 °C. The beads were washed, resolved by SDS-PAGE, and processed for autoradiography.
Affinity Selection Assays
Various FLAG-tagged constructs were expressed in HEK-293 cells. At 18 h post-transfection, cells were treated with either 250 nm OA or DMSO for 2 h or were left untreated. Cells were collected in lysis buffer supplemented with protease inhibitors and, when appropriate, phosphatase inhibitors. The lysates were incubated for 15 min at 4 °C and centrifuged at 234,000 × g for 15 min. At the same time, various GST fusion proteins were expressed in E. coli BL21. Pelleted bacteria were resuspended in PBS supplemented with protease inhibitors and sonicated. Triton X-100 was added to 1% final concentration, and the samples were incubated for 15 min at 4 °C. The samples were centrifuged at 30,700 × g for 15 min, and the supernatants were incubated with glutathione-Sepharose beads for 1 h at 4 °C. Beads were washed with lysis buffer, and the pre-coupled fusion proteins were incubated with lysates of HEK-293 cells for 1 h at 4 °C. Beads were washed with lysis buffer, resolved by SDS-PAGE, and processed for Western blotting. In other assays MBP-lacZ or MBP-connecdenn 1 peptides were expressed in E. coli BL-21. Pelleted bacteria were resuspended in lysis buffer supplemented with protease inhibitors and sonicated. The samples were centrifuged at 30,700 × g for 15 min, and the supernatants were incubated with amylose resin for 1 h at 4 °C. Beads were washed with lysis buffer, and the pre-coupled fusion proteins were incubated with lysates of HEK-293 cells expressing FLAG-DENN domain for 1 h at 4 °C. Beads were washed with lysis buffer, resolved by SDS-PAGE, and processed for Western blotting.
Treatment and Analysis of Adipocytes
Mouse 3T3-L1 pre-adipocytes were cultured in DMEM containing 10% bovine calf serum at 37 °C in 5% CO2 until reaching full confluency. To induce differentiation, two-day post-confluent 3T3-L1 pre-adipocytes were stimulated for 72 h with DMEM containing 10% fetal bovine serum (FBS) supplemented with 5 μg/ml insulin, 1 μm dexamethasone, and 0.5 mm isobutyl-1-methylxanthine. The medium was then replaced with DMEM containing 10% FBS supplemented with 1 μm insulin alone. After 48 h, the medium was replaced with DMEM supplemented with 10% FBS until the cells were utilized for experiments. 3T3-L1 adipocytes were serum-starved for 2 h then treated with 100 nm insulin for 30 min in the presence or absence of 10 μm MK2206, an Akt inhibitor administered 30 min before insulin treatment. Cells were collected in lysis buffer supplemented with protease inhibitors and phosphatase inhibitors, and the affinity selection assay was performed as described earlier.
Statistical Analysis
For in vitro GEF assays, data were plotted in GraphPad Prism, and curves were fitted by a nonlinear regression one-phase association (n = 2, mean ± S.D.). The amount of endogenous connecdenn 1 specifically bound to Rab35 S22N or 14-3-3 and FLAG-connecdenn 1 wild-type (WT) or mutant bound to 14-3-3 were quantified by densitometry using ImageJ. Statistical significance was determined using an unpaired t test in GraphPad Prism (n = 4, mean ± S.E.).
Results
GEF Activities of Connecdenn 1 and 2 Are Autoinhibited
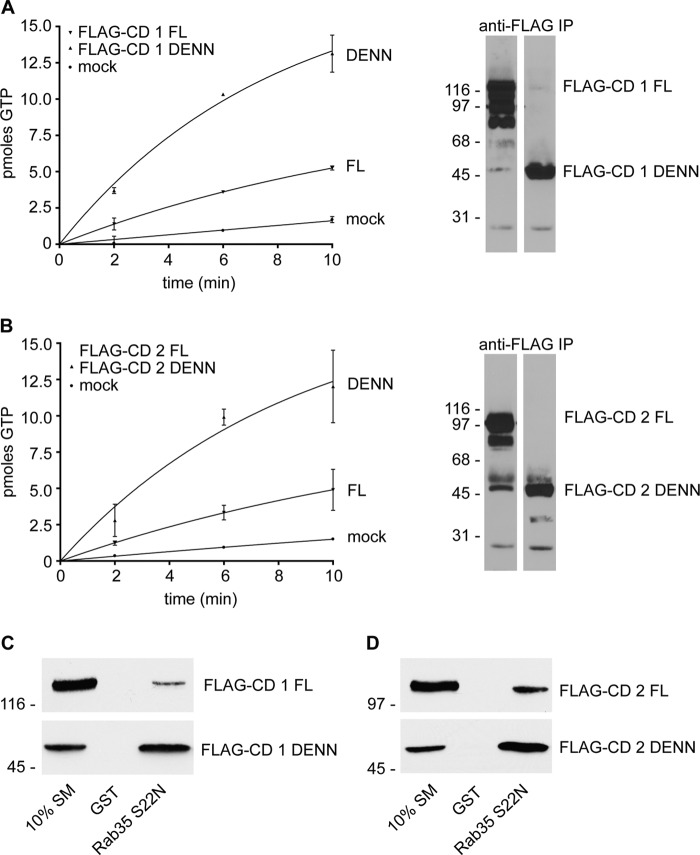
The enzymatic activity of many GEF proteins is autoinhibited (2). To test if the connecdenn family is regulated by this mechanism, we compared the catalytic activity of full-length connecdenn 1 and connecdenn 2 to their corresponding isolated DENN domains. We chose to investigate connecdenn 1 and 2 as they have the most robust GEF activity toward Rab35. The proteins were immunoprecipitated after overexpression in HEK-293 cells and added to purified Rab35 preloaded with GDP. The number of pmol of Rab35 loaded with GTP is two-three times greater when incubated with the isolated DENN domains as compared with full-length connecdenn 1 and connecdenn 2 (Fig. 1, A and B). The right panels of Fig. 1, A and B, show Western blots of a duplicate sample of the immunoprecipitated connecdenn proteins, demonstrating that there was an equal (or greater) amount of the full-length protein as compared with the DENN domain. The DENN domain is located at the N terminus in the connecdenn family; thus the C-terminal region plays an inhibitory role on the GEF activity of the DENN domain. We speculate that this inhibition results from autoinhibition, but we cannot rule out that a protein binding partner(s) that interacts with the C-terminal region of the connecdenns regulates the GEF activity.
FIGURE 1.
Isolated DENN domains of connecdenn 1 and 2 have greater GEF activity than the full-length proteins. A, FLAG-tagged full-length (FL) connecdenn 1 (CD 1) or its isolated DENN domain was immunoprecipitated from HEK-293 cell lysates along with immunoprecipitation (IP) from mock-transfected cells as a control. Aliquots of the immunoprecipitated proteins were processed for FLAG Western blot (right panel) or were added to the exchange reaction that contained [35S]GTPγS and purified Rab35 preloaded with GDP (left panel). The enzymatic activity based on pmol of Rab35 loaded with GTPγS over time is shown. B, as for A except with connecdenn 2 (CD 2). C, GST or GST-Rab35 S22N (constitutively GDP-bound mutant) were incubated with HEK-293 lysates expressing FLAG-tagged full-length connecdenn 1 or its isolated DENN domain. Specifically bound proteins were processed for Western blot with FLAG antibody. Starting material (SM) represents 10% of input. D, as for C except with connecdenn 2.
In general, inhibitory intramolecular interactions do not modify the enzymatic rate but instead block access of the GEF to its substrate (2). We thus compared the DENN domains to full-length connecdenn 1 and 2 for their ability to bind Rab35. Interestingly, Rab35-S22N (a constitutively GDP-bound mutant) interacts more strongly with the isolated DENN domains than the corresponding full-length proteins (Fig. 1, C and D). The change in substrate binding implies that the C-terminal regions create steric hindrance that obstructs the interaction between the DENN domains and Rab35. Thus, the low Rab35 binding of full-length connecdenn 1 and 2 parallels the low GEF activity.
Connecdenn 1 and 2 Are Phosphorylated
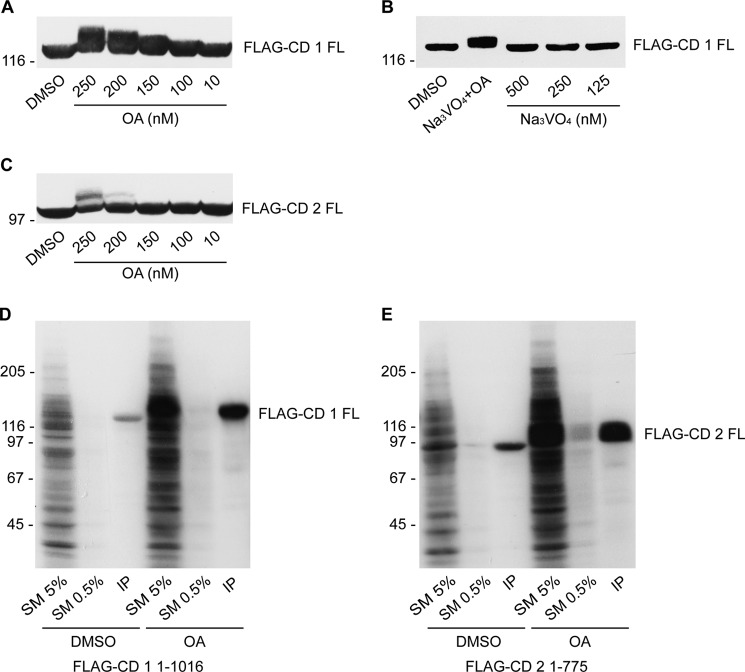
Phosphorylation is a well established mechanism to regulate GEF and GAP activities (2). Proteomic screens reveal that connecdenn 1 and 2 are highly phosphorylated both at steady state and in response to specific stimuli (19–21). In a large scale study examining global steady-state phosphorylation in nine different mouse tissues, a total of 15 distinct serine/threonine phospho-peptides from 8 different tissues were identified in connecdenn 1, and 10 phosphorylated serine/threonine residues in all nine tissues were detected in connecdenn 2 (19). Certain phosphopeptides were unique to a specific tissue, whereas the majority of phospho-peptides were detected in multiple tissues. To examine the phosphorylation further, we initially evaluated phosphorylation of connecdenn 1 in response to two phosphatase inhibitors: OA, a serine/threonine phosphatase inhibitor, and Na3VO4, a tyrosine phosphatase inhibitor. We observed an upward gel shift of overexpressed full-length connecdenn 1 in response to OA after 2 h of treatment with a minimum concentration of 150 nm (Fig. 2A), whereas treatment with Na3VO4 did not cause an upward shift (Fig. 2B). No shift was seen with DMSO (vehicle control). We also observed a shift of full-length connecdenn 2 in response to OA after 2 h of treatment with a minimum concentration of 200 nm (Fig. 2C). Thus, connecdenn 1 and 2 appear to be phosphorylated on the serine/threonine residue(s).
FIGURE 2.
Connecdenn proteins are phosphorylated on serine and threonine residues. A, HEK-293 cells expressing FLAG-tagged full-length connecdenn 1 (FLAG-CD 1 FL) were treated with DMSO (vehicle control) or with the indicated concentrations of OA for 2 h and then processed for Western blot with FLAG antibody. B, HEK-293 cells expressing FLAG-connecdenn 1 full-length were treated with DMSO or with 250 nm OA and 500 nm Na3VO4 or with the indicated concentrations of Na3VO4 alone for 2 h and then processed for Western blot with FLAG antibody. C, as in A except with full-length FLAG-tagged connecdenn 2 (FLAG-CD 2 FL). D, HEK-293 cells expressing FLAG-connecdenn 1 full-length were preincubated in phosphate-free DMEM media for 2 h with 0.125 μCi of label-free [32]Pi/15-cm plate and subsequently treated with 250 nm OA or DMSO for 2 h. FLAG-connecdenn 1 full-length was immunoprecipitated, resolved by SDS-PAGE, and subjected to autoradiography along with aliquots of the starting material (SM) equal to 5% or 0.5% of that added to the immunoprecipitation (IP). E, as in D but for FLAG-connecdenn 2 full-length.
To confirm that the reduced electrophoretic mobility was caused by phosphorylation, we measured incorporation of radioactive phosphate in response to OA. HEK-293 cells transfected with full-length FLAG-connecdenn 1 or 2 were incubated with [32]Pi in the presence of DMSO or OA. The connecdenn proteins were immunoprecipitated with anti-FLAG antibody, and the resulting autoradiograph reveals a robust increase of [32]Pi incorporation in the immunoprecipitated connecdenn proteins after OA treatment (Fig. 2, D and E). These data confirm that connecdenns are phosphoproteins.
Mapping Connecdenn 1 Phosphorylation
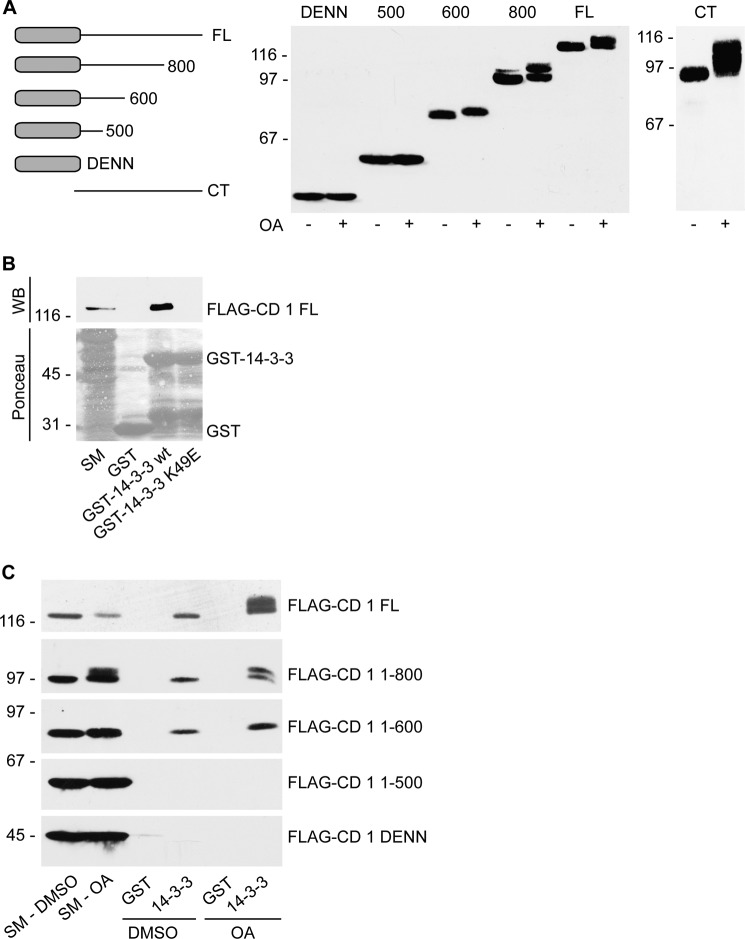
We next sought to map the phosphorylated region(s) of connecdenn 1 using a series of deletion constructs. We found that phosphorylation of connecdenn 1 occurs outside the DENN domain as a C-terminal construct lacking the DENN domain (residues 375–1016) is shifted in response to OA, whereas the DENN domain (residues 1–403) is not (Fig. 3A). Moreover, OA does not change the gel migration of connecdenn 1 1–500, suggesting that this construct does not contain any phospho-sites. In contrast, a 1–600-residue construct undergoes a substantial shift as do constructs 1–800 and full-length connecdenn 1 (Fig. 3A). Therefore, the region between amino acids 500 and 600 contains a phosphorylated residue(s). However, this analysis does not exclude the possibility of additional phosphorylation events C-terminal of residue 600.
FIGURE 3.
Connecdenn 1 is phosphorylated in the C terminus and phosphorylation promotes 14-3-3 binding. A, FLAG-tagged full-length (FL) connecdenn 1, deletion constructs with the indicated boundaries, the isolated DENN domain (residues 1–406), or the isolated C-terminal (CT) region (residues 375–1016) were expressed in HEK-293 cells. Cells were treated with either 250 nm OA or DMSO (vehicle control) for 2 h. Total cell lysates were processed for Western blot with FLAG antibody. B, GST, GST-14-3-3 wild-type (WT) of GST-14-3-3 K49E mutant were incubated with HEK-293 lysates expressing FLAG-tagged full-length connecdenn 1 (FLAG-CD 1 FL). Specifically bound proteins were processed for Western blot (WB) with FLAG antibody. Starting material (SM) equals 10% of the input. C, GST or GST-14-3-3 wild-type were incubated with HEK-293 lysates expressing FLAG tagged full-length connecdenn 1 or various C-terminal deletion constructs as described in A. Before lysis, cells were treated with either 250 nm OA or DMSO for 2 h. Specifically bound proteins were processed for Western blot with FLAG antibody. Starting material equals 10% of the input.
Connecdenn 1 Binds to 14-3-3 in a Phosphorylation-dependent Manner
Connecdenn 1 was identified as a binding partner of 14-3-3 in a large scale proteomic analysis of the 14-3-3 interactome in HEK-293 cells (22). We thus tested the interaction between 14-3-3 (using the ϵ isoform) and connecdenn 1 by performing a pulldown experiment with GST-14-3-3 wild type or a K49E mutant that ablates the binding of 14-3-3ϵ to its substrate Raf-1 (23). Fig. 3B shows that connecdenn 1 specifically interacts with the wild-type form of 14-3-3.
The majority of 14-3-3 binding sites contain a phosphoserine or phosphothreonine; however, there are also known 14-3-3 binding motifs that do not contain phosphorylated residues (24). Thus, we tested if the interaction between 14-3-3 and connecdenn 1 is phospho-dependent. We overexpressed full-length connecdenn 1 in HEK-293 cells and performed 14-3-3 pulldown experiments from cells treated with DMSO or OA. OA treatment increases the interaction of 14-3-3 with connecdenn 1, suggesting that phosphorylation regulates this interaction (Fig. 3C). There is basal 14-3-3 binding in DMSO-treated cells (Fig. 3, B and C), which could be attributed to phosphorylation of connecdenn 1 at steady-state conditions as observed in the autoradiography analysis of DMSO-treated cells (Fig. 2D) and as reported in phosphoproteomic screens.
To map the 14-3-3 binding site, we tested binding between 14-3-3 and different connecdenn 1 deletion constructs. The connecdenn 1 DENN domain and connecdenn 1 1–500 do not interact with 14-3-3, whereas connecdenn 1 1–600 binds to 14-3-3 (Fig. 3C). Therefore, there is a binding site within the 500–600-residue region, which matches the observation of a phosphorylation site(s) in the same region (Fig. 3A).
We also examined the changes in 14-3-3 binding of deletion constructs in response to OA. OA treatment results in a modest up-regulation of 14-3-3 binding for connecdenn 1 1–600 and connecdenn 1 1–800 (Fig. 3C). OA enhances binding of connecdenn 1 full-length to a greater degree, implying that there is a second 14-3-3 binding site (in addition to the site between 500–600) between residue 800 and the C terminus.
Akt-dependent Regulation of 14-3-3 and Rab35 Binding
We next sought to examine regulation of connecdenn 1 in a more physiological context. Rab35 has been previously connected to GLUT4 trafficking in that overexpression of TBC1D13, a GAP for Rab35, inhibits translocation of GLUT4 to the plasma membrane in response to insulin (25). Moreover, a phosphoproteomic study reported that connecdenn 1 is phosphorylated by Akt after insulin treatment in adipocytes (20). Interestingly, the Akt sites in connecdenn 1 correspond to residues Ser-536 and Ser-538 (20), and both 14-3-3 binding and OA-dependent phosphorylation of connecdenn 1 localize to amino acids between residues 500 and 600. We thus investigated if Akt regulates 14-3-3 binding to connecdenn 1. We found that the Akt inhibitor MK2206 significantly reduces 14-3-3 binding to endogenous connecdenn 1 in adipocytes stimulated with insulin (Fig. 4, A–C). Because 14-3-3 binding depends on phosphorylation (Fig. 3C), the decrease in 14-3-3 interaction after Akt inhibition suggests that Akt phosphorylates connecdenn 1 in insulin-stimulated adipocytes. Notably, 14-3-3 binding is reduced but not eliminated by Akt inhibition, likely due to the presence of Akt-independent 14-3-3 binding sites between residue 800 and the C terminus (Fig. 3C). We also tested if Akt phosphorylation influences Rab35 binding. Interestingly, inhibition of Akt significantly reduces the ability of connecdenn 1 to interact with Rab35 in insulin-stimulated cells (Fig. 5, A–C). Thus, Akt activity is required for 14-3-3 interaction and the ability of the DENN domain to interact with Rab35. Because Rab35 binding parallels GEF activity, our data suggest that Akt activity downstream of insulin stimulation could enhance connecdenn GEF activity and activation of Rab35. This is consistent with the observation that insulin-induced GLUT4 translocation is blocked by overexpression of the Rab35 GAP TBC1D13 (25).
FIGURE 4.
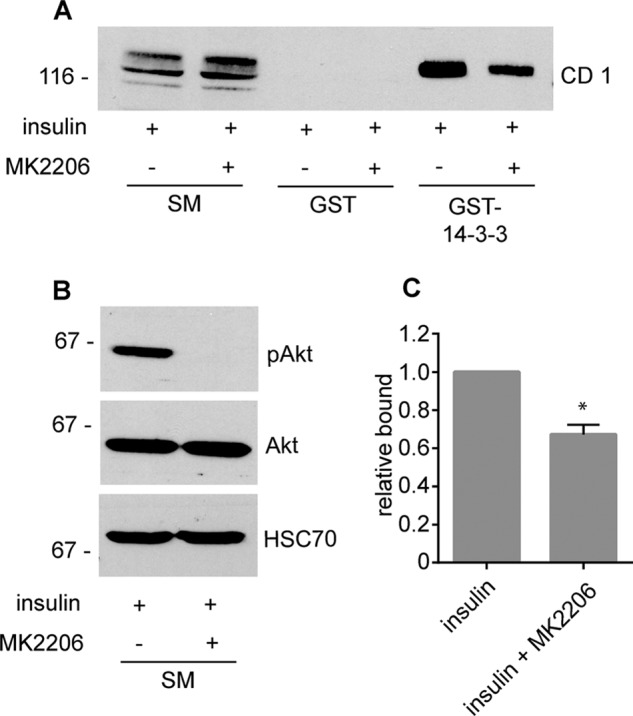
Akt inhibition reduces 14-3-3 binding. A, highly differentiated 3T3-L1 adipocytes were stimulated with 100 nm insulin for 30 min in the presence or absence of 10 μm MK2206, an Akt inhibitor administered 30 min before insulin treatment. After treatment, cell lysates were prepared and incubated with GST or GST-14-3-3 pre-coupled to glutathione-Sepharose beads, and specifically bound proteins were detected with an antibody recognizing endogenous connecdenn 1. SM, starting material. B, cell lysates prepared as in A were processed for Western blots with antibodies against the indicated proteins. C, quantification of the binding of connecdenn 1 to GST-14-3-3 as in A. The bar represents S.E. Statistical analysis employed an unpaired t test. p < 0.05.
FIGURE 5.
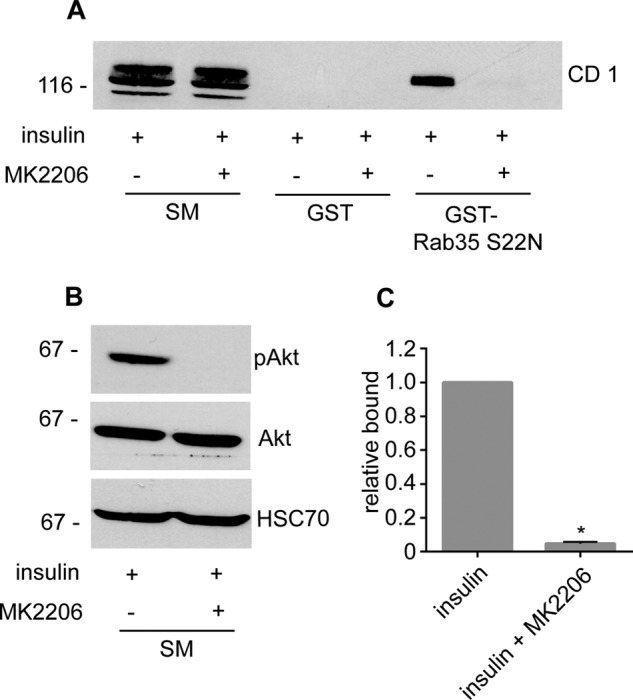
Akt inhibition reduces Rab35 binding. A, highly differentiated 3T3-L1 adipocytes were stimulated with 100 nm insulin for 30 min in the presence or absence of 10 μm MK2206, an Akt inhibitor administered 30 min before insulin treatment. After treatment, cell lysates were prepared and incubated with GST or GST-Rab35 S22N (constitutively GDP-bound mutant) pre-coupled to glutathione-Sepharose beads, and specifically bound proteins were detected with an antibody recognizing endogenous connecdenn 1. SM, starting material. B, cell lysates prepared as in A were processed for Western blots with antibodies against the indicated proteins. C, quantification of the binding of connecdenn 1 to GST-Rab35 S22N as in A. The bar represents S.E. Statistical analysis employed an unpaired t test. *, p < 0.05.
An Intramolecular Interaction within Connecdenn 1 Is Regulated by Phosphorylation of Ser-536/Ser-538
We next sought to explore the mechanism by which the GEF activity of connecdenn 1 is regulated. Given that Akt-mediated phosphorylation influences both 14-3-3 and Rab35 binding, we questioned if the Akt sites at Ser-536 and Ser-538 are involved in regulating connecdenn 1 auto-inhibition. We thus generated a MBP-peptide encoding amino acids 527–547, encompassing the two Akt sites. Intriguingly, this peptide showed weak but highly reproducible binding to the DENN domain of connecdenn 1, whereas there was no binding to a MBP control (Fig. 6A). In the endogenous protein, the peptide will have higher affinity for the DENN domain, as it is in the same molecule, creating avidity affects. Remarkably, a phosphomimetic mutation, with the two serines mutated to glutamic acid, did not bind the DENN domain (Fig. 6A). Thus, there is an intramolecular interaction between the DENN domain and the C-terminal region, mediated by a site that includes Ser-536 and Ser-538, and it is possible that Akt-mediated phosphorylation of these sites disrupts the intramolecular interaction. Mutation of these two sites also disrupts the interaction with 14-3-3 (Fig. 6B), suggesting that upon phosphorylation, 14-3-3 binds this site and stabilizes connecdenn 1 in an open conformation.
FIGURE 6.
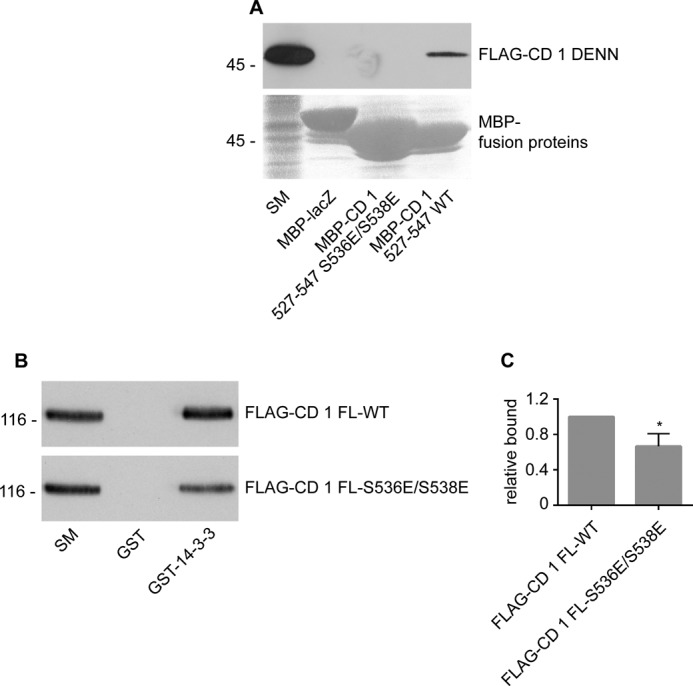
Ser-536 and Ser-538 contribute to interactions with the DENN domain and 14-3-3. A, FLAG-tagged connecdenn 1 DENN domain (FLAG-CD 1 DENN) was expressed in HEK-293 cells, and cell lysates were incubated with amylose resin coupled to MBP fused to lacZ or a connecdenn 1 peptide encoding residues 527–547, WT, or S536E/S538E mutant. Specifically bound proteins were processed for Western blot with FLAG antibody. Starting material (SM) equals 5% of the input. B, GST or GST-14-3-3 were incubated with HEK-293 lysates expressing FLAG tagged full-length connecdenn 1 (FLAG-CD 1) WT or S536E/S538E mutant. Specifically bound proteins were processed for Western blot with FLAG antibody. Starting material equals 10% of the input. C, quantification of the binding of connecdenn 1 to MBP-peptides as in B. The bar represents S.E. Statistical analysis was employed an unpaired t test. *, p < 0.05.
Discussion
The Rab family of small GTPases regulates multiple steps of membrane trafficking. The important physiological role of Rab proteins is reflected in their association with many diseases, including neurological disorders, infectious diseases, and cancers (1, 26). Consequently, cells have developed sophisticated, multilayered regulation of Rabs to ensure their correct temporal and spatial activation. One aspect of regulation is centered on GEF and GAP proteins. In all families of small GTPases, autoinhibition has emerged as an important mechanism of regulation of GEFs and GAPs. In a closed conformation, an inhibitory neighboring domain creates steric hindrance around the GTPase-binding site and reduces the catalytic activity. Specific signals can lead to rearrangement of domains, release of inhibition, and full catalytic activity. In this scenario, GEF and GAP proteins are not constitutively active but rather are turned on in response to stimuli ensuring precise activation and inactivation of the target small GTPases.
In this study we demonstrate that the GEF activities of two members of a DENN protein family are autoinhibited. It appears that through an intramolecular interaction, the C-terminal regions of connecdenn 1 and connecdenn 2 obstruct substrate binding and reduce GEF activity. At least in the case of connecdenn 1, this activity is mediated by interaction of the DENN domain with a site containing Ser-536 and Ser-538. Connecdenn 1 and 2 activate Rab35 during fast recycling; thus, regulation of these two GEFs is likely involved in trafficking of numerous cell surface receptors. We also envision that other DENN proteins are inhibited by intramolecular interactions (27).
Inherent to the idea of autoinhibition is the requirement for mechanisms to release the inhibition, such as interaction with a binding partner and phosphorylation. For instance, intersectin attains maximal enzymatic activity after binding to its partner, N-WASP, which changes the conformation of the SH3 domain and relieves the autoinhibition (4). In other cases phosphorylation changes GEF and GAP activities (2). For example, in TIM, the GEF activity is inhibited by an adjacent 22 amino acid α-helix linker that directly interacts with the catalytic domain (3). Phosphorylation of two tyrosine residues in the inhibitory helix leads to rearrangement of the helix and an increase in GEF activity (4). Phosphorylation can also lead to a decrease in the enzymatic activity of GEF and GAP proteins. For instance, phosphorylation of Dock6 suppresses the nucleotide exchange rate (5), and phosphorylation of AS160 also turns off its GAP activity (9). Here we show that connecdenn 1 and connecdenn 2 are phosphorylated on serine threonine residues in response to OA. In adipocytes, Akt inhibition results in reduced Rab35 binding to connecdenn 1, likely corresponding to reduced GEF activity. Consistently, whereas a peptide encoding Ser-536 and Ser-538, the two major Akt phosphorylation sites in connecdenn 1 (20), binds to the DENN domain, the same peptide but with phosphomimetic mutants of these residues fails to bind to the DENN domain. We envision that Akt-dependent phosphorylation disrupts auto-inhibition, allowing for enhanced interaction with Rab35 and increased GEF activity.
There are a number of cases where 14-3-3 proteins serve as an extra level of regulation of GEF proteins in addition to phosphorylation. 14-3-3 proteins are a family of highly conserved regulatory molecules, and the majority of 14-3-3 binding motifs depend on phosphorylation of serine/threonine (8). Connecdenn 1 has been reported as a binding partner of 14-3-3 in a proteomic study (22). We confirm this interaction biochemically by using a pulldown experiment and establish that there are two binding sites. Both binding sites are phospho-dependent as 14-3-3 binding increases in response to OA and insulin.
There are seven mammalian isoforms of 14-3-3 that function as obligatory dimers. Each dimer contains two adjacent binding pockets that simultaneously engage two binding sites (24). In some cases a single 14-3-3 dimer interacts with two different proteins and acts as an adaptor (28). However, it is more common for the substrate to contain two binding motifs that simultaneously interact with both 14-3-3 monomers (24). In some cases phosphorylation of a single site is sufficient to recruit 14-3-3, and the interaction with either of the two sites produces the same physiological outcome (29). There are many other cases where physiological outcome depends on which single binding motif or both motifs are phosphorylated and engage 14-3-3. Raf-1 kinase contains a total of three 14-3-3 binding sites that are phosphorylated by different kinases to regulate the enzymatic activity (30, 31). Depending on which two sites are phosphorylated, 14-3-3 dimers can lock Raf-1 in either an active or an inactive conformation (30, 31). 14-3-3 dimers have a rigid structure that enables them to induce conformational changes to the ligand when both binding sites are within one protein. This property allows 14-3-3 to regulate the substrate's enzymatic activity (24). Connecdenn 1 also contains two 14-3-3 binding sites, and we propose that the 14-3-3 dimer binds to both sites within one connecdenn 1 molecule. Inhibition of Akt reduces binding of both 14-3-3 and Rab35 to connecdenn 1, suggesting that Akt-dependent phosphorylation of connecdenn 1 enhances 14-3-3 binding and places connecdenn 1 in an open conformation. Consistently, mutation of Ser-536 and Ser-538 reduces 14-3-3 binding.
Rab35 is a key regulator of fast recycling and controls trafficking of numerous cell surface receptors (32). For example, disruption of Rab35 leads to a decrease in cell surface levels of cadherins and an increase in surface levels of integrins, which is associated with cancer progression (33). It is not surprising that Rab35 is highly regulated in order to achieve and preserve the correct levels of cell surface receptors. Here we show that two major GEFs for Rab35 that function on early endosomes are regulated by autoinhibition and phosphorylation. Moreover, 14-3-3 binds to connecdenn 1 and could be involved in regulation of GEF activity. Importantly, we demonstrate that Akt signaling is a positive regulator of Rab35 binding and perhaps GEF activity, thereby coupling cell signaling to Rab35-mediated trafficking. We also hypothesize that phosphorylation of connecdenn 1 occurs in multiple pathways upstream of Rab35-mediated trafficking and serves as a global mechanism for turning on Rab35 during the fast recycling pathways.
Author Contributions
G. K. and N. N. conceived and conducted all experiments except those in Fig. 1, A and B. A. L. M. conceived and conducted the experiments in Fig. 1, A and B. C. C., I. L., and M. S. I. provided assistance in conducting and interpreting experiments throughout. P. S. M. conceived and coordinated the study. P. S. M. and N. N. wrote the paper with contributions from G. K. All authors reviewed the results and approved the final version of the manuscript.
Acknowledgments
We thank Dr. Philippe Roux for the gift of 14-3-3 constructs, Dr. Xiang-Jiao Yang for the gift of 3T3-L1 pre-adipocytes, and Dr. David James for discussion. We also thank Jacynthe Philie and Drs. Mathilde Chaineau, Martine Girard, and Chanshuai Han for support.
This work was supported by Canadian Institutes of Health Research Grant MOP15396 (to P. S. M.). The authors declare that they have no conflicts of interest with the contents of this article.
- GEF
- guanine nucleotide exchange factor
- GAP
- GTPase activating protein
- GLUT4
- glucose transporter 4
- DENN
- differentially expressed in normal and neoplastic cell
- MBP
- maltose-binding protein
- GTPγS
- guanosine 5′-3-O-(thio)triphosphate
- OA
- okadaic acid.
References
- 1. Stenmark H. (2009) Rab GTPases as coordinators of vesicle traffic. Nat. Rev. Mol. Cell Biol. 10, 513–525 [DOI] [PubMed] [Google Scholar]
- 2. Cherfils J., Zeghouf M. (2013) Regulation of small GTPases by GEFs, GAPs, and GDIs. Physiol. Rev. 93, 269–309 [DOI] [PubMed] [Google Scholar]
- 3. Yohe M. E., Rossman K. L., Gardner O. S., Karnoub A. E., Snyder J. T., Gershburg S., Graves L. M., Der C. J., Sondek J. (2007) Auto-inhibition of the Dbl family protein Tim by an N-terminal helical motif. J. Biol. Chem. 282, 13813–13823 [DOI] [PubMed] [Google Scholar]
- 4. Hussain N. K., Jenna S., Glogauer M., Quinn C. C., Wasiak S., Guipponi M., Antonarakis S. E., Kay B. K., Stossel T. P., Lamarche-Vane N., McPherson P. S. (2001) Endocytic protein intersectin-l regulates actin assembly via Cdc42 and N-WASP. Nat. Cell Biol. 3, 927–932 [DOI] [PubMed] [Google Scholar]
- 5. Miyamoto Y., Torii T., Yamamori N., Ogata T., Tanoue A., Yamauchi J. (2013) Akt and PP2A reciprocally regulate the guanine nucleotide exchange factor Dock6 to control axon growth of sensory neurons. Sci. Signal. 6, ra15. [DOI] [PubMed] [Google Scholar]
- 6. Chahdi A., Sorokin A. (2008) Protein kinase A-dependent phosphorylation modulates β1Pix guanine nucleotide exchange factor activity through 14-3-3β binding. Mol. Cell. Biol. 28, 1679–1687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. O'Toole T. E., Bialkowska K., Li X., Fox J. E. (2011) Tiam1 is recruited to β1-integrin complexes by 14-3-3ζ where it mediates integrin-induced Rac1 activation and motility. J. Cell Physiol. 226, 2965–2978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yaffe M. B., Rittinger K., Volinia S., Caron P.R., Aitken A., Leffers H., Gamblin S. J., Smerdon S. J., Cantley L. C. (1997) The structural basis for 14–3-3:phosphopeptide binding specificity. Cell 91, 961–971 [DOI] [PubMed] [Google Scholar]
- 9. Sano H., Kane S., Sano E., Mîinea C. P., Asara J. M., Lane W. S., Garner C. W., Lienhard G. E. (2003) Insulin-stimulated phosphorylation of a Rab GTPase-activating protein regulates GLUT4 translocation. J. Biol. Chem. 278, 14599–14602 [DOI] [PubMed] [Google Scholar]
- 10. Ramm G., Larance M., Guilhaus M., James D.E. (2006) A role for 14-3-3 in insulin-stimulated GLUT4 translocation through its interaction with the RabGAP AS160. J. Biol. Chem. 281, 29174–29180 [DOI] [PubMed] [Google Scholar]
- 11. Levivier E., Goud B., Souchet M., Calmels T. P., Mornon J. P., Callebaut I. (2001) uDENN, DENN, and dDENN: indissociable domains in Rab and MAP kinases signaling pathways. Biochem. Biophys. Res. Commun. 287, 688–695 [DOI] [PubMed] [Google Scholar]
- 12. Levine T. P., Daniels R. D., Gatta A. T., Wong L. H., Hayes M. J. (2013) The product of C9orf72, a gene strongly implicated in neurodegeneration, is structurally related to DENN Rab-GEFs. Bioinformatics 29, 499–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang D., Iyer L. M., He F., Aravind L. (2012) Discovery of novel DENN Proteins: implications for the evolution of eukaryotic intracellular membrane structures and human disease. Front. Genet. 3, 283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Marat A. L., Dokainish H., McPherson P. S. (2011) DENN domain proteins: regulators of Rab GTPases. J. Biol. Chem. 286, 13791–13800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Allaire P. D., Marat A. L., Dall'Armi C., Di Paolo G., McPherson P. S., Ritter B. (2010) The Connecdenn DENN domain: a GEF for Rab35 mediating cargo-specific exit from early endosomes. Mol. Cell 37, 370–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yoshimura S., Gerondopoulos A., Linford A., Rigden D. J., Barr F. A. (2010) Family-wide characterization of the DENN domain Rab GDP-GTP exchange factors. J. Cell Biol. 191, 367–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Marat A. L., McPherson P. S. (2010) The connecdenn family, Rab35 guanine nucleotide exchange factors interfacing with the clathrin machinery. J. Biol. Chem. 285, 10627–10637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Allaire P. D., Ritter B., Thomas S., Burman J. L., Denisov A. Y., Legendre-Guillemin V., Harper S.Q., Davidson B. L., Gehring K., McPherson P. S. (2006) Connecdenn, a novel DENN domain-containing protein of neuronal clathrin-coated vesicles functioning in synaptic vesicle endocytosis. J. Neurosci. 26, 13202–13212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Huttlin E. L., Jedrychowski M. P., Elias J. E., Goswami T., Rad R., Beausoleil S. A., Villén J., Haas W., Sowa M. E., Gygi S. P. (2010) A tissue-specific atlas of mouse protein phosphorylation and expression. Cell 143, 1174–1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Humphrey S. J., Yang G., Yang P., Fazakerley D. J., Stöckli J., Yang J. Y., James D. E. (2013) Dynamic adipocyte phosphoproteome reveals that Akt directly regulates mTORC2. Cell Metab. 17, 1009–1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Weintz G., Olsen J. V., Frühauf K., Niedzielska M., Amit I., Jantsch J., Mages J., Frech C., Dölken L., Mann M., Lang R. (2010) The phosphoproteome of toll-like receptor-activated macrophages. Mol. Syst. Biol. 6, 371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Collins B. C., Gillet L. C., Rosenberger G., Röst H. L., Vichalkovski A., Gstaiger M., Aebersold R. (2013) Quantifying protein interaction dynamics by SWATH mass spectrometry: application to the 14-3-3 system. Nat. Methods 10, 1246–1253 [DOI] [PubMed] [Google Scholar]
- 23. Zhang L., Wang H., Liu D., Liddington R., Fu H. (1997) Raf-1 kinase and exoenzyme S interact with 14–3-3ζ through a common site involving lysine 49. J. Biol. Chem. 272, 13717–13724 [DOI] [PubMed] [Google Scholar]
- 24. Fu H., Subramanian R. R., Masters S. C. (2000) 14-3-3 proteins: structure, function, and regulation. Annu. Rev. Pharmacol. Toxicol. 40, 617–647 [DOI] [PubMed] [Google Scholar]
- 25. Davey J. R., Humphrey S. J., Junutula J. R., Mishra A. K., Lambright D. G., James D. E., Stöckli J. (2012) TBC1D13 is a RAB35 specific GAP that plays an important role in GLUT4 trafficking in adipocytes. Traffic 13, 1429–1441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ioannou M. S., Bell E. S., Girard M., Chaineau M., Hamlin J. N., Daubaras M., Monast A., Park M., Hodgson L., McPherson P. S. (2015) DENND2B activates Rab13 at the leading edge of migrating cells and promotes metastatic behavior. J. Cell Biol. 208, 629–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xu J., Fotouhi M., McPherson P. S. (2015) Phosphorylation of the exchange factor DENND3 by ULK in response to starvation activates Rab12 and induces autophagy. EMBO Rep. 16, 709–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Braselmann S., McCormick F. (1995) Bcr and Raf form a complex in vivo via 14–3- proteins. EMBO J. 14, 4839–4848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. She Q. B., Solit D. B., Ye Q., O'Reilly K. E., Lobo J., Rosen N. (2005) The BAD protein integrates survival signaling by EGFR/MAPK and PI3K/Akt kinase pathways in PTEN-deficient tumor cells. Cancer Cell 8, 287–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tzivion G., Luo Z., Avruch J. (1998) A dimeric 14–3-3 protein is an essential cofactor for Raf kinase activity. Nature 394, 88–92 [DOI] [PubMed] [Google Scholar]
- 31. Dhillon A.S., Yip Y. Y., Grindlay G. J., Pakay J. L., Dangers M., Hillmann M., Clark W., Pitt A., Mischak H., Kolch W. (2009) The C terminus of Raf-1 acts as a 14–3-3-dependent activation switch. Cell. Signal. 21, 1645–1651 [DOI] [PubMed] [Google Scholar]
- 32. Chaineau M., Ioannou M. S., McPherson P. S. (2013) Rab35: GEFs, GAPs and effectors. Traffic 14, 1109–1117 [DOI] [PubMed] [Google Scholar]
- 33. Allaire P. D., Seyed Sadr M., Chaineau M., Seyed Sadr E., Konefal S., Fotouhi M., Maret D., Ritter B., Del Maestro R. F., McPherson P. S. (2013) Interplay between Rab35 and Arf6 controls cargo recycling to coordinate cell adhesion and migration. J. Cell Sci. 126, 722–731 [DOI] [PubMed] [Google Scholar]